Abstract
Flow regimes are a major driver of community composition and structure in riverine ecosystems, and flow regulation by dams often induces artificially-stable flow regimes downstream. This represents a major source of hydrological alteration, particularly in regions where biota is adapted to strong seasonal and interannual flow variability. We hypothesized that dam-induced hydrological stability should increase the availability of autochthonous resources at the base of the food web. This, in turn, should favour herbivorous over detritivorous strategies, increasing the diversity of primary consumers, and the food-web width and length. We tested this hypothesis by studying the longitudinal variation in food-web structure in a highly-seasonal Mediterranean river affected by an irrigation dam. We compared an unregulated reach to several reaches downstream of the dam. Hydrological and sedimentological stability increased downstream of the dam, and altered the type and quantity of available resources downstream, prompting a change from a detritus-based to an algae-based food web. The fraction of links between top and intermediate species also increased, and the food web became longer and wider at the intermediate trophic levels. Food-web structure did not recover 14 km downstream of the dam, despite a partial restitution of the flow regime. Our results advance the notion that hydrologic alteration affects riverine food webs via additions/deletions of taxa and variation in the strength and distribution of food-web interactions. Thus, flow regulation by dams may not only impact individual facets of biodiversity, but also food-web level properties across river networks.
Keywords: Food-web interactions, Hydrologic alteration, Mediterranean-climate rivers, Stream-flow regulation, Water stability
Graphical abstract
Highlights
-
•
Flow regulation increased flow stability in a highly-variable Mediterranean river.
-
•
The detritus-based food web shifted into an algae-based food web.
-
•
Changes in consumers and their interactions widened and lengthened the food chain.
-
•
Flow regime alteration may impact river food webs beyond their individual components.
1. Introduction
Discharge might be considered a ‘master variable’ that structures riverine habitat, influences water quality, and controls population and community dynamics and many ecosystem processes (Death and Winterbourn, 1995, Bunn and Arthington, 2002). Flow regimes control channel morphology and size, habitat diversity (riffles and pools) and substrate stability, which together influence the abundance, distribution, and diversity of organisms (Power et al., 1995, Nilsson and Svedmark, 2002). Flow variation is positively associated with allochthonous inputs of matter and energy (Tank et al., 2010), with the amount and seasonality of organic matter transport and accumulation (Uehlinger, 2000, Artigas et al., 2009), and with hydrologic connectivity (Jaeger et al., 2014, Ruhí et al., 2015). Because riverine communities are adapted to natural flow variability, flow alteration poses a major risk for the stability and functioning of aquatic ecosystems, changing both abiotic and biotic parameters (Poff and Zimmerman, 2010, Carlisle et al., 2011).
Dams occur worldwide (Nilsson et al., 2005) regulating most of the discharge in the northern hemisphere (Dynesius and Nilsson, 1994) and threatening some of the world's most biodiverse rivers (Winemiller et al., 2016). Depending on the purpose of the reservoir (e.g., hydropower, drinking water supply, irrigation) and on the river hydrology (i.e., permanent or intermittent), dams may change hydrological patterns in different ways. A permanent river may present episodes of downstream drought when it is affected by a hydropower dam (López-Moreno et al., 2009), while an intermittent river may show a reduction in hydrologic drought severity if the dam provides water for irrigation during dry periods (Batalla et al., 2004, Lobera et al., 2017). From a physical standpoint, large and small flow regulation structures can significantly alter flood frequency (Poff et al., 1997, Haxton and Findlay, 2008), thermal regimes (Caissie, 2006), and sediment loads downstream (Tena et al., 2011). From a biological perspective, the effects of flow regulation on riparian and riverine diversity are dependent on reservoir storage size (Power et al., 1996, Dudgeon, 2000), as well as on the composition of primary producers and consumers (Munn and Brusven, 1991, Morley et al., 2008). However, most research so far has concentrated on responses at the population or community levels (e.g., Stanford and Richard Hauer, 1992), with studies considering interactions among organisms focusing mostly on large river systems (e.g., Cross et al., 2013). This is despite the fact that small rivers are intrinsically more variable than larger ones (Sabo and Post, 2008, Sabo et al., 2010), and thus may be disproportionally affected by flow regulation.
Mediterranean rivers present high seasonal and interannual hydrological variation, with marked flow reduction in summer, and floods in autumn and spring (Gasith and Resh, 1999). Dry periods are common in Mediterranean rivers and may represent a selective pressure (Lytle and Poff, 2004). Mediterranean freshwater communities have evolved under constant flow variation, favouring traits that confer resistance and resilience to drought (Bonada et al., 2006). When intermittent rivers become regulated for human supply and irrigation, novel conditions for this adapted biota are created, increasing hydrological stability via dampened flood frequency and drought severity (Batalla et al., 2004, Döll et al., 2009). This flow stability can in turn change the flux of materials and energy (Abril et al., 2015); can act as a dissolved nitrogen (N) sink, causing relevant N cycling discontinuities (von Schiller et al., 2016); can enhance organic carbon processing (Aristi et al., 2014); and can favour biofilm biomass growth, reducing biofilm spatial heterogeneity and habitat quality (Belmar et al., 2013, Ponsatí et al., 2015). Altogether, these effects can ripple through the food web (Power et al., 2013) and result in altered trophic links, energy pathways, and food-web dynamics (Vander Zanden et al., 1999).
The study of food-web structure provides insights into how energy and matter flow through ecosystems (Baird and Ulanowicz, 1989, McIntyre et al., 2007). Food webs arise from community composition and interactions among taxa (Post, 2002); thus, food-web structure is sensitive to changes in biodiversity (in the form of local extinction and colonization) as well as to changes in the sign or strength of interactions that exist among organisms (Post and Takimoto, 2007). However, riverine food-web structure also integrates exogenous disturbance, community history and resource availability and type (i.e., allochthonous vs. autochthonous) (Post, 2002). Thus, understanding how food-web structure responds to anthropogenic disturbance can shed light into designing effective conservation strategies (McCann, 2007, Harvey et al., 2017).
Here we investigated to what extent food-web structure in an intermittent Mediterranean stream was affected by the presence of an irrigation dam that altered the river ecosystem by reducing the downstream flow variability. We also aimed to see whether food-web structure recovered downstream, in parallel with the progressive recovery of hydrological conditions due to the inputs of intermittent tributaries. To address these questions, we selected sites differing in flow regime but sharing the regional pool of species, and we studied local food webs via gut content analyses and food-web structure metrics. We predicted that: (1) Dam-induced hydrological stability should increase the availability of autochthonous resources downstream of the dam (Ponsatí et al., 2015). Because autochthonous resources generally have lower C:N and C:P ratios (Frost et al., 2002), and higher protein and lipid content than terrestrial matter (Lamberti, 1996), autochthony should favour herbivory over detritivory. (2) This shift should increase primary consumer diversity, increasing food-web width (via a higher diversity of trophic pathways) and food-chain length (FCL). This result would be in agreement with the dynamic stability or ‘disturbance’ hypothesis of FCL (Pimm and Lawton, 1977, Sabo et al., 2010). (3) Finally, we hypothesized that these effects should be reduced downstream, as flow variability is often progressively recovered with increasing distance from the dam (Batalla et al., 2004). Research on this topic may help anticipating the effects of increasing flow regulation by dams on riverine food webs, a critical question given the steep increase in dam building across the globe (Zarfl et al., 2016).
2. Materials and methods
2.1. Study location
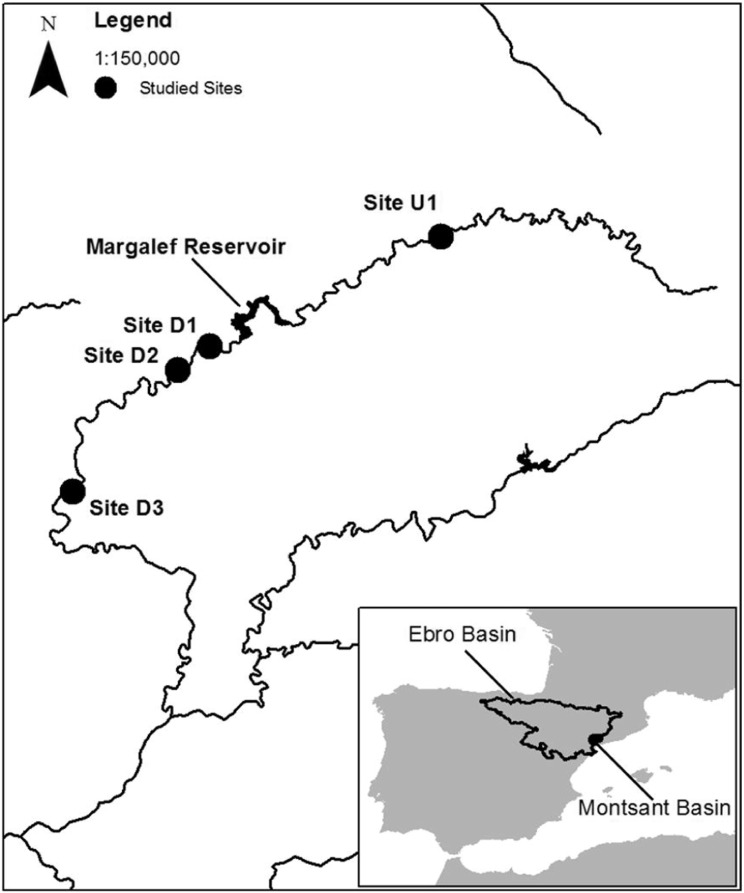
A survey was conducted during May 2012 in the Montsant River, an intermittently-flowing tributary of the Ebro River (NE Iberian Peninsula). Basal resources, invertebrate, and vertebrate samples were collected from four river segments (Fig. 1). These were an upstream site (U1) located 12.3 km upstream of the Margalef reservoir, and three sites downstream of the dam: D1 (1.3 km downstream of the dam), D2 (3.4 km downstream of the dam), and D3 (14.2 km downstream of the dam). Sampling was carried out in spring, as Mediterranean-climate river communities peak in species richness during that season (Gasith and Resh, 1999). This way, riverine food webs could be depicted in their full complexity. The sampling reaches were 100-m long, all including one pool (central part of the site) and two riffles (upstream and downstream of the pool). The Montsant River is classified as a mineralized, low-mountain Mediterranean river (R-M2) by the EU Water Framework Directive 2000/60/EC (European Union Council, 2000), and is considered a near-pristine by the riverine-riparian bioassessment index ECOSTRIMED (Bonada et al., 2006). The Montsant River is naturally intermittent, with dry periods during summer and sudden floods during spring and autumn (Mate et al., 2013). The Margalef reservoir (built in 1995 for irrigation; 3 hm3 nominal volume, 33.2 m dam height, 0.3 years residence time) laminates floods and provides permanently-flowing conditions, reducing downstream flow variability.
Fig. 1.
Locations of the study sites in the Montsant River, upstream (U1), and downstream (D1, D2, D3) of Margalef reservoir. Studied sites coordinates UTM, (x,y); U1 (824,850, 4,582,650); D1 (816,050, 4,578,550); D2 (814,850, 4,577,650); D3 (810,850, 4,573,050).
2.2. Hydrological characterization
Streamflow time series (1970–2012) at each site were obtained using the distributed hydrological model TETIS (Francés et al., 2007), a model designed to specifically suit the hydrological cycle in Mediterranean rivers (Medici et al., 2008). The presence of reservoirs was included in the model, together with topographical, geological, soil, and land use information, considering the presence of the reservoir and bypasses. The model was calibrated and validated in the watershed with a dataset of 13 years of daily streamflow at the inflow of the Siurana Reservoir (reservoir at the same basin, Nash-Sutcliffe efficiency = 0.67; see Ruhí et al., 2016, for details), and delivered daily flow series for the 15 years prior to the study (1998–2012). We analyzed these series with the Discrete Fast Fourier Transform [DFFT; (Sabo and Post, 2008)], and we quantified flow variability by adding up the number of daily high- and low-flow events over the 15 year series, with events being defined as flows falling beyond the 1 ± standard deviation threshold in the distribution of residuals or ‘anomalies’ (Sabo and Post, 2008).
2.3. Environmental characterization
Electrical conductivity (μS cm− 1), pH, and water temperature (°C) were measured in situ using hand-held probes, three times during the sampling day (WTW, Weilheim, Germany). Three water samples in each reach were collected for nutrient analyses (nitrate (NO3−, μg L− 1), nitrite (NO2 −, μg L− 1), ammonium (NH4+, μg L− 1), phosphate (PO43, μg L− 1), and for dissolved organic carbon analyses (DOC, μg L− 1). The water samples were filtered in 0.7 μm GF/F filters (Whatman Int. Ltd., Maidstone, UK) and kept at − 20 °C until analyzed. Phosphate concentration was determined colorimetrically using a spectrophotometer (Alliance-AMS Smartchem 140, AMS, Frepillon, France), after Murphy and Riley (1962). Nitrite, nitrate, and ammonium concentrations were determined on a Dionex ICS-5000 ion chromatograph (Dionex Co., Sunnyvale, USA; Hach Company, 2002). DOC concentration was determined on a Shimadzu TOC-V CSH coupled to a TNM-1 module (Shimadzu Co., Kyoto, Japan).
2.4. Streambed mapping
Basal resources available to primary consumers (coarse benthic organic matter (CBOM), fine benthic organic matter (FBOM), biofilm patches and macrophytes) were identified in situ using an underwater viewer (30 × 30 cm). The relative cover of these basal resources was recorded every 30 cm along ten cross-sectional transects at each reach. Roots and organic matter were also included in the mapping. Identification and mapping included the different patches of epilithic biofilms, macrophytes and bryophytes, and detritic organic matter. Biofilms were separated by their color, mucilage, macrocolonies of cyanobacteria, and macroalgae presence.
2.5. Biofilm collection and identification
Five stones for each of the three most representative biofilm patches (i.e., % cover) at each reach were randomly collected. Two subsamples were taken for the taxonomic analysis, one for diatoms and another for the non-diatom algae and cyanobacteria. Algae were scraped off to a final area of 2–10 cm2, and preserved in 4% formalin until analysis. Five replicates of 2–10 cm2 were taken for chlorophyll analysis, stored in the dark and frozen in the field (− 20 °C) until analysis.
Diatom cells were cleaned in boiling hydrogen peroxide, and cleaned frustules were mounted on permanent slides using Naphrax (r.i. 1.74; Brunel Microscopes Ltd., Chippenham, Wiltshire, UK). Up to 400 valves were counted on each slide by performing random transects under light microscopy (Nikon Eclipse 80i; Nikon, Tokyo, Japan) using Nomarski differential interference contrast optics at a magnification of 1000 × (see Appendix S1 for taxonomic keys). The non-diatom algal fraction was determined after counting 50 random microscope fields per aliquot (Tornés and Sabater, 2010). We also collected coarse benthic organic matter (CBOM) and fine benthic organic matter (FBOM) using a sediment corer (314 cm2, 3 replicates/reach). Samples were frozen (− 20 °C) and processed to obtain ash free dry weight (AFDW, in g/m2). Values were transformed to carbon weight after Margalef (1986).
2.6. Macrophyte and bryophyte collection and identification
For species identification we collected macrophytes and bryophytes when present. For biomass analysis, an area of 90 cm2 was collected for each species, and samples were preserved in zip-lock bags at 4 °C in the field until analysis.
2.7. Macroinvertebrate and vertebrate sampling and gut content analysis
At each sampling site, ten Surber sample-units (30 × 30 cm square; mesh aperture 500 μm) were collected, integrating the different microhabitats present in riffle (six samples) and pool habitats (four samples). These benthic samples were preserved in 4% formaline.
Vertebrates (fishes and frogs) were captured via electrofishing, using a 3-pass depletion method along the same section of the river (100 m), after closing it with blocking nets. All vertebrate individuals were measured and weighted, and up to twenty individuals of each fish species and size class were euthanized with an overdose of anaesthetic (MS-222) and frozen for gut content analysis (protected or vulnerable species were not euthanized).
Gut content analysis was used to determine feeding links among species. Twenty individuals of each taxon, developmental stage (e.g., larval instar, pupa or adult) and size class (in the case of vertebrates) were randomly selected at each site, stomach tracts were extracted, and gut contents were carefully removed and classified as animal or non-animal material under a dissecting microscope at 80 × magnification. Invertebrate non-animal contents were measured in volume and transformed to dry weight using derived volume-mass ratio transformations (see Appendix S2), and vertebrate non-animal material were dried at 60 °C during 24 h and weighed (g dry mass). Given the low biomass of non-animal material in invertebrate gut contents, groups of four invertebrate stomachs of the same taxon, size and site were pooled together for subsequent non-animal analysis; meanwhile, each vertebrate stomach was a sample. Non-animal material was stained with Rose Bengal and classified as detritus, bacteria, vegetal material, fungi, non-filamentous algae, filamentous algae, or diatom, using a phase-contrast microscope (600 ×). For each sample, fifty random fields were counted in order to assign categorical abundance values to each group. Animal material was identified to the lowest taxonomic level possible. Individuals were counted, and the first twenty-five individuals of each taxa were measured using an ocular reticule (± 0.1 mm). All chironomids were identified to genus level. Biomass (mg dry weight) was calculated using published length-mass relationships (e.g., Burgherr and Meyer, 1997, Benke et al., 1999).
2.8. Network structure properties
Food-web structure properties at each site were calculated using the Network3D (Yoon et al., 2004, Williams, 2010) and the Cheddar (Hudson et al., 2013) software. Network3D provided species, link, and omnivory properties analysis, and Cheddar provided the fraction of links between trophic levels, food chain properties, and consumer-prey asymmetries. These network metrics included: species richness (number of species present in the food-web), fraction of top species (number of species not preyed upon), intermediate species (consumer species preyed upon) and basal species, link density (number of links per species), connectance (number of realized links out of all possible trophic links), mean food-chain length and maximum food-chain length, omnivory (species that consume at two or more trophic levels), vulnerability (number of consumers using a given resource), generality (number of resources per consumer), and associated normalized standard deviations. For taxa with no stomach content, links were established using the available literature (see Table S1). Best link options and strengths were informed based on trophic position estimates obtained via C and N stable isotope analyses in the same catchment (Ruhí et al., 2016).
2.9. Data analysis
A Permutational Multivariate ANOVA (PERMANOVA) was used to test for differences in basal resource structure (biomass) across sites. Because 10 cross-sectional transects were made per site, ‘transect’ was treated as a random factor and nested within ‘site’ (fixed factor). In order to detect differences on a given variable across sites, we employed a Permutational Analysis of Variance (PERANOVA) following the same design as for the PERMANOVA, and pair-wise PERMANOVA tests were used to compare pairs of sites. In all cases, Euclidian distances were computed on fourth-root transformed data. We ran PERMANOVA and PERANOVA, using 999 permutations on PRIMER-E 6 v.6.1.11 and PERMANOVA + v.1.0.1 (PRIMER-E Ltd., Plymouth, UK).
3. Results
3.1. Hydromorphological characterization
Mean annual streamflow did not differ significantly among the studied sites (PERANOVA, pseudo-F3,891 = 2.5817, p = 0.075). However, flow variability was higher upstream (site U1) than downstream of the dam. The lowest variability was observed at site D1, where the number of low and high flow events was drastically reduced (relative to the upstream site). Flow variability progressively recovered in the downstream sites D2 and D3 (Table 1). The streambed substrata consisted of pebbles (> 80%) and cobbles at all sites, with sediment diameter size decreasing downstream (Table 1). Stream width and depth also changed longitudinally, with channel width being reduced immediately downstream of the dam.
Table 1.
Hydromorphological and water variables at each study site. The number of low and high flow days integrates the 15 years prior to the study and were obtained with DFFT analysis of TETIS model outputs.
| Environmental variables | Site U1 | Site D1 | Site D2 | Site D3 |
|---|---|---|---|---|
| Basin area (km2) | 40.7 | 97.6 | 113.1 | 141.4 |
| Basin regulation area (%) | 0 | 95.0 | 82.0 | 65.5 |
| Observed intermittence | Yes | No | No | No |
| Number of low flows | 72 | 30 | 64 | 73 |
| Number of high flows | 70 | 28 | 47 | 70 |
| Channel width (m) | 6.0 ± 3.1 | 4.0 ± 1.4 | 3.4 ± 1.0 | 5.1 ± 2.3 |
| Mean rock diameter (mm) | 50.6 ± 3.5 | 45.5 ± 2.6 | 40.3 ± 2.0 | 31.5 ± 1.6 |
| Pebbles substratum (%) | 82.1 | 80.0 | 93.4 | 98.0 |
| Cobbles substratum (%) | 16.1 | 20.0 | 6.6 | 2.0 |
| Light | Exposed | Shaded | Shaded | Exposed |
| T (°C) | 11.9 | 13.7 | 13.4 | 13.1 |
| Conductivity (μS cm− 1) | 365. 7 ± 0.6 | 412.0 ± 3.0 | 432.0 ± 0.0 | 485.0 ± 0.0 |
| DO (mg O2 L− 1) | 9.6 ± 0.4 | 12.1 ± 0.0 | 10.6 ± 0.0 | 7.8 ± 0.0 |
| DOC (mg C L− 1) | 1.8 ± 0.1 | 3.0 ± 0.1 | 2.4 ± 0.3 | 1.7 ± 0.2 |
| PO43 − (μg P L− 1) | 12.6 ± 0.7 | 8.0 ± 0.0 | 8.0 ± 0.0 | 5.2 ± 1.4 |
| NO2− (μg N L− 1) | 3.0 ± 5.3 | 5.8 ± 0.5 | 5.9 ± 1.6 | 5.0 ± 3.5 |
| NO3− (μg N L− 1) | 2.5 ± 0.8 | 533.4 ± 4.1 | 377.2 ± 4.5 | 27.5 ± 5.7 |
| NH4+ (μg N L− 1) | 4.5 ± 6.1 | 1.4 ± 0.7 | 0.9 ± 0.5 | 0.9 ± 0.3 |
| TDN (μg N L− 1) | 157.5 ± 7.7 | 686.1 ± 18.4 | 442 ± 83.9 | 177.5 ± 23.5 |
3.2. Basal resource characterization
Dominant basal resources differed across sites (PERANOVA, Macrophyte: pseudo-F3,555 = 19.051, p = 0.001; Algae: pseudo-F3,555 = 18.151, p = 0.001), with macrophytes dominating at site U1 and different algal patches dominating at all other sites (Table 2). Denuded tree roots substrata were restricted to impact sites (Table 2). Main basal resources were organic matter (CBOM and FBOM), diatoms (see Table S2), cyanobacteria and non-diatom algae (filamentous and non-filamentous algae, see Table S3), bryophytes (Fontinalis antipyretica and Rhynchostegium riparioides), and macrophytes (Groenlandia densa, Lemna minor, Mentha aquatica, Potamogeton coloratus, Ranunculus aquatilis, Ranunculus repens, and Rorippa nasturtium-aquaticum). Macrophytes and CBOM were the greatest contributors to total basal resource biomass at site U1 (Table 3). Macrophyte biomass at site U1 was significantly higher than at the other sites (PERANOVA Pair-wise test, p < 0.005). Bryophyte biomass slightly increased downstream, but there were not significant differences among sites (PERANOVA, pseudo-F3,555 = 1.654, p = 0.169). Algal biomass increased at site D1, presenting no difference with D2 but significantly decreased downstream (site D3, PERANOVA Pair-wise test, p < 0.01). Benthic organic matter (CBOM and FBOM) did not differ among sites (PERANOVA, CBOM: pseudo-F3,8 = 1.195, p = 0.37; FBOM: pseudo-F3,8 = 3.515, p = 0.078).
Table 2.
Stream relative cover proportion (%) at each site.
| Substrate | Site U1 | Site D1 | Site D2 | Site D3 |
|---|---|---|---|---|
| Root | – | – | 4.7 ± 16.7 | 3.4 ± 14.4 |
| Algae | 26.5 ± 34.9 | 83.9 ± 32.2 | 75.9 ± 32.1 | 60.1 ± 42.7 |
| Bryophyte | 2.6 ± 11.4 | 8.7 ± 21.6 | 9.9 ± 17.1 | 20.3 ± 30.6 |
| Macrophyte | 70.9 ± 38.0 | 6.4 ± 19.9 | 9.5 ± 19.8 | 16.2 ± 33.4 |
Table 3.
Coarse benthic organic matter (CBOM), fine benthic organic matter (FBOM), algae, bryophyte, and macrophyte biomass at each site (mean ± SD).
| Basal resource | Site U1 | Site D1 | Site D2 | Site D3 |
|---|---|---|---|---|
| CBOM (g m− 2) | 67.9 ± 92.7 | 72.6 ± 20.6 | 132.5 ± 28.0 | 76.3 ± 37.1 |
| FBOM (g m− 2) | 14.3 ± 12.0 | 106.8 ± 82.9 | 180.2 ± 129.2 | 62.8 ± 69.0 |
| Algae (g m− 2) | 0.2 ± 0.2 | 1.5 ± 0.9 | 1.2 ± 0.7 | 0.8 ± 0.6 |
| Bryophyte (g m− 2) | 2.9 ± 12.7 | 4.9 ± 13.0 | 6.4 ± 12.1 | 6.6 ± 11.3 |
| Macrophyte (g m− 2) | 64.3 ± 34.7 | 1.0 ± 3.5 | 1.5 ± 3.5 | 6.7 ± 18.8 |
3.3. Consumer characterization
A total of eight vertebrate taxa (Pelophylax perezi, Natrix maura, Parachondrostoma miegii, Barbus haasi, Luciobarbus graellsii, Gobio lozanoi, Anguilla anguilla, and Salmo truta) and 62 invertebrate taxa (see Table S4) were observed in the study sites. Invertebrate species richness was similar among sites, whereas community diversity decreased with dam impacts and recovered downstream (Table 4). Invertebrate composition differed between upstream and downstream sites. The upstream site presented 9 exclusive taxa - the most abundant being Nemoura sp. - and shared 20 taxa with the downstream sites (including the highly-abundant Ancylus fluviatilis and Orthocladiinae species). Downstream sites showed 41 taxa absent from site U1, with Caenis sp. and Ephemerella sp. being the most abundant ones. Sites D1 and D2 shared 20 taxa (see Table S4). Orthocladiinae was the most abundant invertebrate group in all sites. When this group was not considered in the analyses, invertebrate abundance decreased by the dam (site D1) and recovered downstream (Table 4).
Table 4.
Invertebrate and vertebrate community structure at each study site. Feeding strategies were assigned after Tachet et al. (2002). Invertebrate sizes are reported in mg of dry weight. “Prey Size of Vertebrate Predators” shows the mean and the range of invertebrate prey consumed by vertebrates.
| Site U1 | Site D1 | Site D2 | Site D3 | |
|---|---|---|---|---|
| Invertebrate richness | 28 | 33 | 28 | 26 |
| Invertebrate density (ind/m2) | 2140 | 4864 | 2034 | 9332 |
| Non-Orthocladiinae invertebrate abundance (ind/m2) | 936 | 282 | 1548 | 4458 |
| Vertebrate richness | 2 | 6 | 5 | 6 |
| Vertebrate density (ind/m2) | 0.03 | 2.05 | 2.06 | 2.24 |
| Community diversity (H′) | 3.2 | 2.1 | 2.7 | 3.0 |
| Percentage of invertebrate feeding strategies (%) | ||||
| Scraper | 42.7 | 36.4 | 35.7 | 38.5 |
| Shredder | 25.0 | 18.2 | 10.7 | 15.4 |
| Predator | 7.1 | 21.2 | 28.6 | 15.4 |
| Deposit feeder | 10.7 | 12.1 | 10.7 | 15.4 |
| Filter feeder | 7.1 | 6.1 | 7.1 | 7.7 |
| Piercer | 7.1 | 6.1 | 7.1 | 7.7 |
| Invertebrate size | ||||
| Mean (mg/ind) | 1.4 ± 0.1 | 0.6 ± 0.3 | 1.0 ± 0.3 | 0.3 ± 0.02 |
| Range (mg) | 0.2·10− 3–40.1 | 7.4·10− 3–371.5 | 7.0·10− 3–208.2 | 1.6·10− 3–45.9 |
| Size of invertebrate predators | ||||
| Mean (mg/ind) | 3.1 ± 0.7 | 38.0 ± 28.6 | 33.5 ± 15.1 | 15.2 ± 5.6 |
| Range (mg) | 0.2–12.2 | 0.7–208.2 | 0.04–208.2 | 0.05–45.9 |
| Prey size of invertebrate predators | ||||
| Mean (mg/ind) | 0.2 ± 0.02 | 0.2 ± 0.05 | 0.3 ± 0.06 | 0.26 ± 0.06 |
| Range (mg) | 1.8·10− 3–1.2 | 2.7·10− 3–0.8 | 0.2·10− 3–2.8 | 2.91·10− 3–1.3 |
| Invertebrate predator-prey mass ratio | 41.7 ± 10.3 | 1541.0 ± 828.4 | 1206.7 ± 641.3 | 130.2 ± 22.0 |
| Prey size of vertebrate predators | ||||
| Mean (mg/ind) | 97.3 ± 158.3 | 1.94 ± 10.19 | 0.1 ± 1.9 | 0.3 ± 2.3 |
| Range (mg) | 89.2·10− 4–341.1 | 3.2·10− 4–132.7 | 0.3·10− 4–98.4 | 3.5·10− 4–42.7 |
Fish were not present in the upstream site, where the water snake Natrix maura and the frog Pelophylax perezi were the only aquatic vertebrates present. Fish occurred at all downstream sites, with most fish species being common at all three sites except Anguilla anguilla, which was only present in D1 and D2. Vertebrate densities were relatively high at impact sites (Table 4).
3.4. Consumer diet description
Macroinvertebrate diet was composed (in decreasing biomass) of invertebrates, diatoms, detritus, vegetal material, dead animal material, filamentous algae, fungi, and non-filamentous algae (see Table S5). Vertebrates were largely herbivorous at all sites, although insectivore taxa were also abundant at sites D1 and D2. Vertebrate diets included terrestrial invertebrates at site U1, where P. perezi based 75.3% of its whole diet weight on Gryllotalpa gryllotalpa and Formicidae; and at site D2, where G. lozanoi, B. haasi and L. graellsii based 84.6%, 46.2% and 0.3% of their respective diet weights on Formicidae, other Hymenoptera, terrestrial Coleoptera, Araneae, and adult Chironomidae. In the studied sites, only a marginal case of piscivory was observed in site D2, where A. anguilla preyed on G. lozanoi (see Table S5).
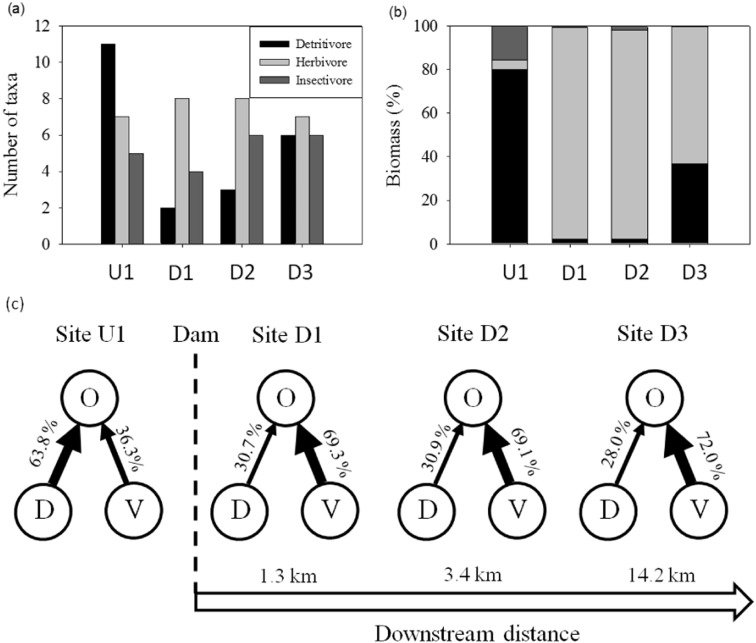
Richness and biomass of detritivore invertebrates decreased drastically by the dam, and recovered downstream (Fig. 2a). Herbivore invertebrates replaced the detritivore invertebrates at all impact sites (Fig. 2b). Orthocladiinae, the most abundant among the widespread taxa, presented a detritivore diet only at site U1, and shifted to herbivore strategies at the other sites by significantly reducing the ingested detritus fraction (PERANOVA, pseudo-F3,76 = 5.672, p = 0.01) (Fig. 2c; Table S5). This diet shift was observed for the freshwater limpet Ancylus fluviatilis (Planorbidae, Gastropoda) between sites U1 and D3 (t-test, F30 = 13.947, p = 0.01) (see Table S1). Chironomidae (Diptera) were the most abundant and recurrent prey (64.5% in vertebrates, 44% in total invertebrates, 83% in insectivorous invertebrates).
Fig. 2.
Macroinvertebrate feeding strategies at each study site. (a) richness of each feeding strategy; (b) proportion of biomass of each feeding strategy; (c) Orthocladiinae diet shifts between detritus + dead animal material (“D”) and vegetal material (including diatoms, algae and fungi; “V”), upstream and downstream of the dam. Percentages represent the contribution of each resource to Orthocladiinae diet (O).
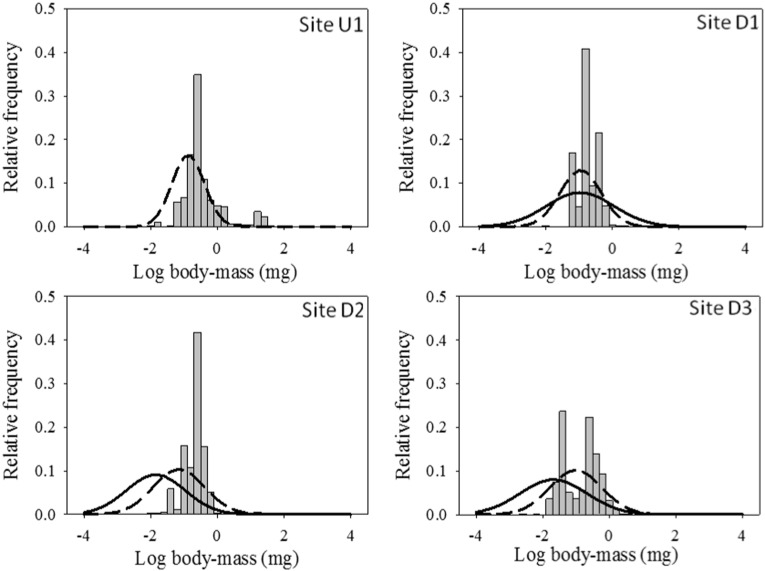
Invertebrate body size spectra differed among sites (Fig. 3). Mean invertebrate size was smaller downstream of the dam, in spite of larger individuals occurring in those sites (Table 4). Prey size of invertebrate predators was similar among sites (Table 4 and Fig. 3; PERANOVA, pseudo-F3,182 = 0.386, p = 0.77), but in site U1 the range of their prey size was wider. Invertebrate predator size was one order of magnitude higher downstream of the dam, and the body mass ratio of invertebrate predator-prey (Table 4) was higher at sites D1 and D2 and decreased further downstream (site D3), presenting similar values to those found in the upstream site. The size range of fish prey was highest at site D1, and decreased downstream (Fig. 4 and Table 4).
Fig. 3.
Macroinvertebrate body size distribution at the different study sites. Gray bars represent invertebrate body size availability, discontinuous lines represent invertebrate body size consumed by invertebrates, and continuous lines represent invertebrate body size consumed by fish.
Fig. 4.
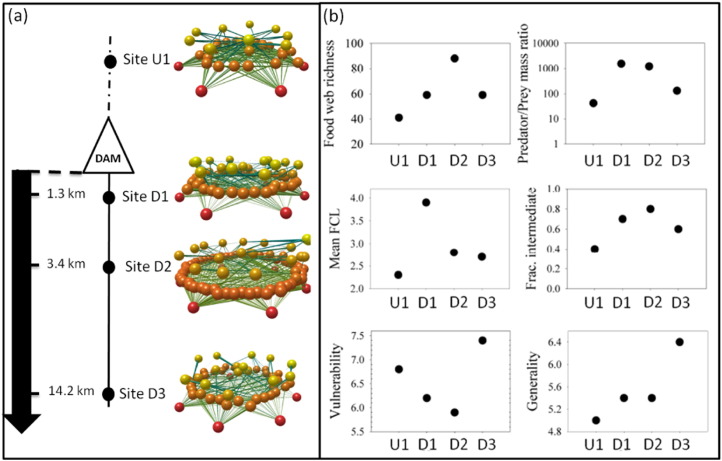
(a) Food-web diagrams representing basal resources (red), intermediate consumers (orange) and top predators (yellow), and the interactions among them. Diagrams were produced with the Network 3D software (Williams, 2010, Yoon et al., 2004). (b) Food-web structure metrics at each study site: Food-web richness considering all taxa, including those present only in gut contents; mean predator:prey mass ratio (mg/mg); mean food-chain length (an average of the different food chains across all the taxa in each food web); fraction of intermediate taxa (consumer taxa being preyed upon); vulnerability (number of consumers per taxa); and generality (number of resources per taxa).
3.5. Network properties
The number of nodes and trophic links (see Table S6) in the food webs increased downstream of the dam (Table 5). The maximum number of nodes and trophic links was recorded at site D2, where the lowest connectance values and the highest resource:consumer ratios were observed (Table 5). The upstream site had a high fraction of top-level species, and of direct trophic interactions between top predators and basal resources occurred (Table 5). The sites downstream of the dam presented a large fraction of intermediate species and a wider food web (Fig. 4, Table 5).
Table 5.
Food-web structure metrics at each site. Invertebrate terrestrial prey were excluded from this analysis, but considered in the rest of the study. SD, standard deviation.
| Site U1 | Site D1 | Site D2 | Site D3 | |
|---|---|---|---|---|
| Species properties | ||||
| Number of nodes (S) | 41 | 59 | 88 | 59 |
| Number of trophic links (L) | 170 | 283 | 434 | 322 |
| Fraction top level | 0.4 | 0.2 | 0.2 | 0.2 |
| Fraction intermediate | 0.4 | 0.7 | 0.8 | 0.6 |
| Fraction basal | 0.2 | 0.1 | 0.08 | 0.1 |
| Ratio resources:consumers | 0.7 | 0.8 | 0.9 | 0.9 |
| Link properties (complexity) | ||||
| Link density | 4.1 | 4.8 | 4.9 | 5.5 |
| Connectance | 0.10 | 0.08 | 0.06 | 0.09 |
| Fraction of links between | ||||
| Top and intermediate | 0.15 | 0.13 | 0.18 | 0.20 |
| Top and basal | 0.26 | 0.10 | 0.10 | 0.11 |
| Intermediate | 0.12 | 0.22 | 0.20 | 0.14 |
| Intermediate and basal | 0.46 | 0.55 | 0.52 | 0.55 |
| Chain properties | ||||
| Mean chain length | 2.3 | 3.9 | 2.8 | 2.7 |
| Median chain length | 2 | 3 | 3 | 3 |
| SD chain length | 0.9 | 0.9 | 1.0 | 0.9 |
| Maximum chain length | 4 | 5 | 5 | 5 |
| Omnivory properties | ||||
| Degree of omnivory | 0.12 | 0.17 | 0.11 | 0.20 |
| Consumer-prey asymmetries | ||||
| Generality | 5.0 | 5.4 | 5.4 | 6.4 |
| Vulnerability | 6.8 | 6.2 | 5.9 | 7.4 |
| SD standardised generality | 0.7 | 0.8 | 1.3 | 0.9 |
| SD standardised vulnerability | 1.7 | 1.8 | 2.3 | 1.8 |
Mean food chain was longest at site D1, where median and maximum FCL also peaked (Table 5). The maximum degree of omnivory was observed at sites D1 and D3, and the number of resources per consumer (generality) increased in all sites downstream of the dam, peaking in D3 (Table 5). The standard deviation of the number of consumers per resource (vulnerability) exceeded that of generality in all sites, indicating a greater variability in the number of consumers than of resources for a given species (Table 5).
4. Discussion
The effects of flow regulation by dams on riverine habitat and organisms have long been studied (e.g., Brittain and Saltveit, 1989, Poff and Zimmerman, 2010, Ponsatí et al., 2015), but impacts at the higher levels of biological organization (e.g., food webs) have received relatively less attention (but see Power et al., 1996, and Cross et al., 2013). Here we described longitudinal variation in food-web topology in a dam-regulated intermittent Mediterranean river, and found a positive association between dam-induced flow stability and resource quality, herbivory (over detritivory), and food-chain length and width. The impacts of regulation on food-web structure persisted downstream, despite a partial recovery of the flow regime.
4.1. Flow stability and herbivory
Dam-induced hydrological stability promoted the growth of algae over macrophytes, via flood suppression and increased riparian shading (Spink et al., 1993, Janauer and Dokulil, 2006). The higher nutritional quality of algae relative to detritus (Bowen, 1987, Stelzer and Lamberti, 2002), and the associated increase in algal production downstream of dams, can turn detritus-based into algae-based food webs (Power et al., 2013). In our study, the abundant Orthocladiinae and A. fluviatilis shifted diets accordingly. Several studies have shown that dams vastly reduce the frequency of high flows, favouring less dynamic hydromorphological conditions downstream (Batalla et al., 2004, Döll et al., 2009). This reduction in the frequency and intensity of floods often results in the terrestrialitzation of fluvial systems. This occurred in the Montsant River, where reduction in river width allowed terrestrial vegetation to encroach in part of the streambed. Taken together, these abiotic and biotic changes, ultimately controlled by the flow regime, influenced the type of basal resources in the riverine food web.
4.2. Terrestrialitzation and predatory interactions
Terrestrialization occurred in the regulated sites, and was manifested by a higher influence of terrestrial vegetation over the river channel, including the colonization of terrestrial plants on the streambed. This habitat change was associated with stream channel narrowing, and enabled predatory fish to complement their diets with terrestrial invertebrates (particularly in site D2). Terrestrial invertebrates can represent a substantial energy source for stream communities (Nakano et al., 1999a), and this subsidy could have favoured higher fish densities in site D2 (as in Woodward and Hildrew, 2002a). Predation on terrestrial prey could reduce fish pressure on freshwater invertebrates, thus increasing their densities and the fraction of links among intermediate species. These patterns are consistent with the decrease in omnivory and food-web widening observed in these sites. High fish densities supported by terrestrial prey can produce top-down effects if terrestrial intakes are interrupted (as in Nakano et al., 1999b). Thus, dam-induced terrestrial subsidization could decrease long-term stability of the subsidized riverine food web.
4.3. Food-web widening and lengthening
Dam-induced hydromorphological stability promoted autochthonous production and decreased allochthonous inputs at the base of the food webs. The availability of higher-quality basal resource downstream of the dam increased the richness and abundance of primary consumers, widening the food web. Several network metrics (species richness, number of links, mean FCL, and vulnerability) reflected horizontal and vertical changes in food-web structure. FCL results from community membership, available resources, predator-prey interactions, disturbance, and ecosystem size (Post, 2002). Therefore, although the barrier effect of the dam probably contributed to limiting fish population at the upstream reach (Power and Dietrich, 2002), surface water drying in that site also likely reset the community (Power et al., 2008). Drying limited the presence of viable fish populations upstream of the dam. This likely explains the commonly-observed shorter FCL in intermittent sites (McHugh et al., 2010, Sabo et al., 2010). In turn, the change from a detritus-based to an algal-based food web may enhance the abundance and richness of primary consumers. This could increase the abundance of predators and of the interactions among them (i.e., intraguild predation), lengthening FCL downstream of the dam (Ruhí et al., 2016). Of special interest is the decline in connectance in sites D1 and D2, probably related to increases in species richness and generality, in food-chain length (Schmid-Araya et al., 2002, Woodward and Hildrew, 2002a, Woodward and Hildrew, 2002b), and in body size disparity between invertebrates in the bottom vs. top of the food web (Schmid-Araya et al., 2002).
In addition to increased invertebrate richness and density, and decreased individual sizes, invertebrate predators downstream of the dam shifted from being dominated by Plecoptera to being exclusively represented by Odonata. Odonata can be adapted to coexist with fish (Pierce, 1988), and have passive ‘sit-and-wait’ foraging strategies (Tachet et al., 2002). Their elongated masks reduce reactive distances and differences in movement speed between predators and prey, allowing these large-bodied predators to capture smaller prey. This is coherent with the relatively higher predator:prey size ratios observed in the impact sites.
Composition shifts were also observed for vertebrates, with main differences being explained by fish richness and abundance. Unlike in the naturally-intermittent upstream site, downstream of the dam perennial flow sustained fish populations; accordingly, fish predator densities were higher there. Fish predation may have kept at bay amphibian larvae in the impact sites, as described by Hecnar and M'Closkey (1997) from lentic habitats.
4.4. Longitudinal patterns in food-web structure
Further downstream of the dam, small intermittent tributaries joined the regulated main stem. This restored the frequency of high and low-flow events observed in D3, but not their magnitude. The partial restoration of the flow regime was associated with an increase in river width, which reversed the terrestrialization observed just below the dam. There was a decrease of local allochthonous inputs at the food-web base, and an increase in light penetration. These changes favoured macrophyte abundance at the most downstream site, although algal-based sources still largely dominated. The reduction in FCL relative to the impacted (hydrologically more stable) upstream reaches indicates that other constraints like extinction-colonization dynamics could be limiting (Post, 2002). In this site (D3), macroinvertebrate body size partially recovered and size range was reduced. Schmid-Araya et al. (2002) reported that body-size disparity among organisms at the bottom vs. top of the food web could influence connectance. In our case, the reduction of size range allowed an increase in connectance. Additionally, a high number of predators feeding on a given prey is reflected in a higher vulnerability value, a property often associated with keystone species (Calizza et al., 2015). The relatively more unstable conditions in sites U1 and D3 could contribute to the high vulnerability values observed in those sites.
5. Concluding remarks
Our results illustrate how flow regulation by dams can alter food-web structure in intermittent rivers, not only via changes in community composition but also via changes in the relative importance of autochthonous production vs. allochthonous inputs. In the studied Mediterranean river, flow regulation increased basal autochthony and that led to wider and longer food webs. The recovery of network structure downstream of the dam was only partial. Thus, our study advances the notion that serial discontinuity may present cumulative effects on food webs, and impacts of flow regulation by dams may persist even if the physical template is locally restored. Our results emphasize that dam-induced flow alteration can impact the higher levels of biological organization. This is relevant in the light of the steep increase in dam planning and building globally, especially in developing, highly-biodiverse regions, where water resource and hydropower demand is escalating.
Acknowledgements
We thank Lydia Ponsatí, Roberto Merciai, Jose Andres Lopez and Joan Font for their help in the field, Adriana Hecht for fish gut content analyses and Joan Pere Casas-Ruiz for his helpful comments on the earlier version of the manuscript. This study was funded by the European Union's Seventh Programme through the GLOBAQUA project (grant agreement No 603629).
Editor: D. Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2017.12.296.
Appendix A. Supplementary data
Supplementary material
References
- Abril M., Muñoz I., Casas-Ruiz J.P., Gómez-Gener L., Barceló M., Oliva F., Menéndez M. Effects of water flow regulation on ecosystem functioning in a Mediterranean river network assessed by wood decomposition. Sci. Total Environ. 2015;517:57–65. doi: 10.1016/j.scitotenv.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Aristi I., Arroita M., Larrañaga A., Ponsatí L., Sabater S., von Schiller D., Elosegi A., Acuña V. Flow regulation by dams affects ecosystem metabolism in Mediterranean rivers. Freshw. Biol. 2014;59:1816–1829. [Google Scholar]
- Artigas J., Romaní A.M., Gaudes A., Muñoz I., Sabater S. Organic matter availability structures microbial biomass and activity in a Mediterranean stream. Freshw. Biol. 2009;54:2025–2036. [Google Scholar]
- Baird D., Ulanowicz R.E. The seasonal dynamics of the Chesapeake Bay ecosystem. Ecol. Monogr. 1989;59:329. [Google Scholar]
- Batalla R.J., Gómez C.M., Kondolf G.M. Reservoir-induced hydrological changes in the Ebro River basin (NE Spain) J. Hydrol. 2004;290:117–136. [Google Scholar]
- Belmar O., Bruno D., Martínez-Capel F., Barquín J., Velasco J. Effects of flow regime alteration on fluvial habitats and riparian quality in a semiarid Mediterranean basin. Ecol. Indic. 2013;30:52–64. [Google Scholar]
- Benke A.C., Huryn A.D., Smock L.A., Wallace J.B. Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J. North Am. Benthol. Soc. 1999:308–343. [Google Scholar]
- Bonada N., Dallas H., Rieradevall M., Prat N., Day J. A comparison of rapid bioassessment protocols used in 2 regions with Mediterranean climates, the Iberian peninsula and South Africa. J. North Am. Benthol. Soc. 2006;25:487–500. [Google Scholar]
- Bowen S.H. Composition and nutritional value of detritus. In: Moriarty D.J.W., Pullin R.S.V., editors. Detritus and Microbial Ecology in Aquaculture. ICLARM Conference Proceedings 14. International Center for Living Aquatic Resources Management; Manila, Philippines: 1987. pp. 192–216. [Google Scholar]
- Brittain J.E., Saltveit S.J. A review of the effect of river regulation on mayflies (Ephemeroptera) Regul. Rivers Res. Manag. 1989;3:191–204. [Google Scholar]
- Bunn S.E., Arthington A.H. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manag. 2002;30:492–507. doi: 10.1007/s00267-002-2737-0. [DOI] [PubMed] [Google Scholar]
- Burgherr P., Meyer E.I. Regression analysis of linear body dimensions vs. dry mass in stream macroinvertebrates. Arch. Hydrobiol. 1997;139:101–112. [Google Scholar]
- Caissie D. The thermal regime of rivers: a review. Freshw. Biol. 2006;51:1389–1406. [Google Scholar]
- Calizza E., Costantini M.L., Rossi L. Effect of multiple disturbances on food web vulnerability to biodiversity loss in detritus-based systems. Ecosphere. 2015;6:1–20. [Google Scholar]
- Carlisle D.M., Wolock D.M., Meador M.R. Alteration of streamflow magnitudes and potential ecological consequences: a multiregional assessment. Front. Ecol. Environ. 2011;9:264–270. [Google Scholar]
- Cross W.F., Baxter C.V., Rosi-Marshall E.J., Hall R.O., Kennedy T.A., Donner K.C., Kelly H.A.W., Seegert S.E.Z., Behn K.E., Yard M.D. Food-web dynamics in a large river discontinuum. Ecol. Monogr. 2013;83:311–337. [Google Scholar]
- Death R.G., Winterbourn M.J. Diversity patterns in stream benthic invertebrate communities: the influence of habitat stability. Ecology. 1995;76:1446. [Google Scholar]
- Döll P., Fiedler K., Zhang J. Global-scale analysis of river flow alterations due to water withdrawals and reservoirs. Hydrol. Earth Syst. Sci. 2009;13:2413–2432. [Google Scholar]
- Dudgeon D. Large-scale hydrological changes in tropical Asia: prospects for riverine biodiversity. Bioscience. 2000;50:793. [Google Scholar]
- Dynesius M., Nilsson C. Fragmentation and flow regulation of river systems in the northern third of the world. Science. 1994;266:753–762. doi: 10.1126/science.266.5186.753. [DOI] [PubMed] [Google Scholar]
- European Union Council Directive 2000/60/EC of the European Parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Communities. 2000;L327:1–72. [Google Scholar]
- Francés F., Vélez J.I., Vélez J.J. Split-parameter structure for the automatic calibration of distributed hydrological models. J. Hydrol. 2007;332:226–240. [Google Scholar]
- Frost P.C., Stelzer R.S., Lamberti G.A., Elser J.J. Ecological stoichiometry of trophic interactions in the benthos: understanding the role of C:N:P ratios in lentic and lotic habitats. J. North Am. Benthol. Soc. 2002;21:515–528. [Google Scholar]
- Gasith A., Resh V.H. Streams in Mediterranean climate regions: abiotic influences and biotic responses to predictable seasonal events. Annu. Rev. Ecol. Syst. 1999;30:51–81. [Google Scholar]
- Harvey E., Gounand I., Ward C.L., Altermatt F. Bridging ecology and conservation: from ecological networks to ecosystem function. J. Appl. Ecol. 2017;54:371–379. [Google Scholar]
- Haxton T.J., Findlay C.S. Meta-analysis of the impacts of water management on aquatic communities. Can. J. Fish. Aquat. Sci. 2008;65:437–447. [Google Scholar]
- Hecnar S., M'Closkey R. The effects of predatory fish on amphibian species richness and distribution. Biol. Conserv. 1997;79(2-3):123–131. [Google Scholar]
- Hudson L.N., Emerson R., Jenkins G.B., Layer K., Ledger M.E., Pichler D.E., Thompson M.S.A., O'Gorman E.J., Woodward G., Reuman D.C. Cheddar: analysis and visualisation of ecological communities in R. Methods Ecol. Evol. 2013;4:99–104. [Google Scholar]
- Jaeger K.L., Olden J.D., Pelland N. a. Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1–6. doi: 10.1073/pnas.1320890111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janauer G., Dokulil M. Macrophytes and algae in running waters. In: Ziglio G., Siligardi M., Flaim G., editors. Biological Monitoring of Rivers. Applications and Perpectives. 2006. pp. 89–110. (Brussels, Belgium) [Google Scholar]
- Lamberti G.A. The role of periphyton in benthic food webs. In: Stevenson R., Bothwell M., Lowe R., editors. Algal Ecology. 1996. pp. 533–572. (San Diego) [Google Scholar]
- Lobera G., Muñoz I., López-Tarazón J.A., Vericat D., Batalla R.J. Effects of flow regulation on river bed dynamics and invertebrate communities in a Mediterranean river. Hydrobiologia. 2017;784:283–304. [Google Scholar]
- López-Moreno J.I., Vicente-Serrano S.M., Begueria S., Garcia-Ruiz J.M., Portela M.M., Almeida A.B. Dam effects on droughts magnitude and duration in a transboundary basin: the lower river tagus, pain and Portugal. Water Resour. Res. 2009;45:1–13. [Google Scholar]
- Lytle D.A., Poff N.L. Adaptation to natural flow regimes. Trends Ecol. Evol. 2004;19:94–100. doi: 10.1016/j.tree.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Margalef R. Omega; 1986. Ecologia. [Google Scholar]
- Mate I., Barrull J., Salicrú M., Ruiz-Olmo J., Gosàlbez J. Habitat selection by southern water vole (Arvicola sapidus) in riparian environments of Mediterranean mountain areas: a conservation tool for the species. Acta Theriol. (Warsz). 2013;58:25–37. [Google Scholar]
- McCann K. Protecting biostructure. Nature. 2007;446:29. doi: 10.1038/446029a. [DOI] [PubMed] [Google Scholar]
- McHugh P.A., McIntosh A.R., Jellyman P.G. Dual influences of ecosystem size and disturbance on food chain length in streams. Ecol. Lett. 2010;13:881–890. doi: 10.1111/j.1461-0248.2010.01484.x. [DOI] [PubMed] [Google Scholar]
- McIntyre P.B., Jones L.E., Flecker A.S., Vanni M.J. Fish extinctions alter nutrient recycling in tropical freshwaters. Proc. Natl. Acad. Sci. 2007;104:4461–4466. doi: 10.1073/pnas.0608148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici C., Butturini A., Bernal S., Vázquez E., Sabater F., Vélez J., Francés F. Modelling the non-linear hydrological behaviour of a small Mediterranean forested catchment. Hydrol. Process. 2008;22:3814–3828. [Google Scholar]
- Morley S.a., Duda J.J., Coe H.J., Kloehn K.K., McHenry M.L. Benthic invertebrates and periphyton in the Elwha River basin: current conditions and predicted response to dam removal. Northwest Sci. 2008;82:179–196. [Google Scholar]
- Munn M.D., Brusven M.A. Benthic macroinvertebrate communities in nonregulated and regulated waters of the clearwater river, Idaho, USA. Regul. Rivers Res. Manag. 1991;6:1–11. [Google Scholar]
- Murphy J., Riley J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962;27:31–36. [Google Scholar]
- Nakano S., Miyasaka H., Kuhara N. Terrestrial–aquatic linkages: riparian arthropod inputs alter trophic cascades in a stream food web. Ecology. 1999;80:2435–2441. [Google Scholar]
- Nakano S., Miyasaka H., Kuhara N. Terrestrial-aquatic linkages: riparian arthropod inputs alter trophic cascades in a stream food web. Ecology. 1999;80:2435–2441. [Google Scholar]
- Nilsson C., Svedmark M. Basic principles and ecological consequences of changing water regimes: riparian plant communities. Environ. Manag. 2002;30:468–480. doi: 10.1007/s00267-002-2735-2. [DOI] [PubMed] [Google Scholar]
- Nilsson C., Reidy C.A., Dynesius M., Revenga C. Fragmentation and flow regulation of the world's large river systems. Science. 2005;308(80):405–408. doi: 10.1126/science.1107887. [DOI] [PubMed] [Google Scholar]
- Pierce C.L. Predator avoidance, microhabitat shift, and risk-sensitive foraging in larval dragonflies. Oecologia. 1988;77:81–90. doi: 10.1007/BF00380929. [DOI] [PubMed] [Google Scholar]
- Pimm S.L., Lawton J.H. Number of trophic levels in ecological communities. Nature. 1977;268:329–331. [Google Scholar]
- Poff N.L., Zimmerman J.K.H. Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshw. Biol. 2010;55:194–205. [Google Scholar]
- Poff N.L., Allan J.D., Bain M.B., Karr J.R., Prestegaard K.L., Richter B.D., Sparks R.E., Stromberg J.C. The natural flow regime: a paradigm for river conservation and restoration. Bioscience. 1997;47:769–784. [Google Scholar]
- Ponsatí L., Acuña V., Aristi I., Arroita M., García-Berthou E., von Schiller D., Elosegi A., Sabater S. Biofilm responses to flow regulation by dams in Mediterranean rivers. River Res. Appl. 2015;31:1003–1016. [Google Scholar]
- Post D.M. The long and short of food-chain length. Trends Ecol. Evol. 2002;17:269–277. [Google Scholar]
- Post D.M., Takimoto G. Proximate structural mechanisms for variation in food-chain length. Oikos. 2007;116:775–782. [Google Scholar]
- Power M.E., Dietrich W.E. Food webs in river networks. Ecol. Res. 2002;17:451–471. [Google Scholar]
- Power M.E., Sun A., Parker G., Dietrich W.E., Wootton J.T. Hydraulic food-chain models. Bioscience. 1995;45:159–167. [Google Scholar]
- Power M.E., Dietrich W.E., Finlay J.C. Dams and downstream aquatic biodiversity: potential food web consequences of hydrologic and geomorphic change. Environ. Manag. 1996;20:887–895. doi: 10.1007/BF01205969. [DOI] [PubMed] [Google Scholar]
- Power M.E., Parker M.S., Dietrich W.E. Seasonal reassembly of a river food web: floods, droughts, and impacts of fish. Ecol. Monogr. 2008;78:263–282. [Google Scholar]
- Power M.E., Holomuzki J.R., Lowe R.L. Food webs in Mediterranean rivers. Hydrobiologia. 2013;719:119–136. [Google Scholar]
- Ruhí A., Holmes E.E., Rinne J.N., Sabo J.L. Anomalous droughts, not invasion, decrease persistence of native fishes in a desert river. Glob. Chang. Biol. 2015;21:1482–1496. doi: 10.1111/gcb.12780. [DOI] [PubMed] [Google Scholar]
- Ruhí A., Muñoz I., Tornés E., Batalla R.J., Vericat D., Ponsatí L., Acuña V., von Schiller D., Marcé R., Bussi G., Francés F., Sabater S. Flow regulation increases food-chain length through omnivory mechanisms in a Mediterranean river network. Freshw. Biol. 2016;61:1536–1549. [Google Scholar]
- Sabo J.L., Post D.M. Quantifying periodic, stochastic, and catastrophic environmental variation. Ecol. Monogr. 2008;78:19–40. [Google Scholar]
- Sabo J.L., Finlay J.C., Kennedy T., Post D.M. The role of discharge variation in scaling of drainage area and food chain length in rivers. Science. 2010;330(80):965–967. doi: 10.1126/science.1196005. [DOI] [PubMed] [Google Scholar]
- Schmid-Araya J.M., Schmid P.E., Robertson A., Winterbottom J., Gjerlov C., Hildrew A.G. Connectance in stream food webs. J. Anim. Ecol. 2002;71:1056–1062. [Google Scholar]
- Spink A.J., Murphy K.J., Smith S.M., Westlake D.F. Effects of eutrophication on Ranunculus and Potamogeton. J. Aquat. Plant Manag. 1993;31:113–117. [Google Scholar]
- Stanford J.A., Richard Hauer F. Mitigating the impacts of stream and lake regulation in the flathead river catchment, Montana, USA: an ecosystem perspective. Aquat. Conserv. Mar. Freshwat. Ecosyst. 1992;2:35–63. [Google Scholar]
- Stelzer R.S., Lamberti G.A. Ecological stoichiometry in running waters: Periphyton chemical composition and snail growth. Ecology. 2002;83:1039–1051. [Google Scholar]
- Tachet H., Richoux P., Bournaud M., Usseglio-Polatera P. CNRS éditions; Paris: 2002. Invertébrés d'Eau Douce. 2nd correc. ed. [Google Scholar]
- Tank J.J.L., Rosi-Marshall E.J.E., Griffiths N.a., Entrekin S.a., Stephen M.L. A review of allochthonous organic matter dynamics and metabolism in streams. J. North Am. Benthol. Soc. 2010;29:118–146. [Google Scholar]
- Tena A., Batalla R.J., Vericat D., López-Tarazón J.a. Suspended sediment dynamics in a large regulated river over a 10-year period (the lower Ebro, NE Iberian Peninsula) Geomorphology. 2011;125:73–84. [Google Scholar]
- Tornés E., Sabater S. Variable discharge alters habitat suitability for benthic algae and cyanobacteria in a forested Mediterranean stream. Mar. Freshw. Res. 2010;61:441. [Google Scholar]
- Uehlinger U. Resistance and resilience of ecosystem metabolism in a flood-prone river system. Freshw. Biol. 2000;45:319–332. [Google Scholar]
- Vander Zanden M.J., Shuter B.J., Lester N., Rasmussen J.B. Patterns of food chain length in lakes: a stable isotope study. Am. Nat. 1999;154:406–416. doi: 10.1086/303250. [DOI] [PubMed] [Google Scholar]
- von Schiller D., Aristi I., Ponsatí L., Arroita M., Acuña V., Elosegi A., Sabater S. Regulation causes nitrogen cycling discontinuities in Mediterranean rivers. Sci. Total Environ. 2016;540:168–177. doi: 10.1016/j.scitotenv.2015.07.017. [DOI] [PubMed] [Google Scholar]
- Williams R.J. Microsoft Research; Cambridge, UK: 2010. Network3D Software. [Google Scholar]
- Winemiller K.O., McIntyre P.B., Castello L., Fluet-Chouinard E., Giarrizzo T., Nam S., Baird I.G., Darwall W., Lujan N.K., Harrison I., Stiassny M.L.J., Silvano R.A.M., Fitzgerald D.B., Pelicice F.M., Agostinho A.A., Gomes L.C., Albert J.S., Baran E., Petrere M., Zarfl C., Mulligan M., Sullivan J.P., Arantes C.C., Sousa L.M., Koning A.A., Hoeinghaus D.J., Sabaj M., Lundberg J.G., Armbruster J., Thieme M.L., Petry P., Zuanon J., Vilara G.T., Snoeks J., Ou C., Rainboth W., Pavanelli C.S., Akama A., van Soesbergen A., Saenz L. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science. 2016;351(80):128–129. doi: 10.1126/science.aac7082. [DOI] [PubMed] [Google Scholar]
- Woodward G., Hildrew A.G. Food web structure in riverine landscapes. Freshw. Biol. 2002;47:777–798. [Google Scholar]
- Woodward G., Hildrew A.G. Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol. 2002;71:1063–1074. [Google Scholar]
- Yoon I., Williams R., Levine E., Yoon S., Dunne J., Martinez N. Webs on the Web (WOW): 3D visualization of ecological networks on the WWW for collaborative research and education. In: Erbacher R.F., Chen P.C., Roberts J.C., Groehn M.T., Boerner K., editors. Proceedings of the IS&T/SPIE Symposium on Electronic Imaging. 2004. pp. 124–132. [Google Scholar]
- Zarfl C., Lumsdon A.E., Berlekamp J., Tydecks L., Tockner K. A global boom in hydropower dam construction. Aquat. Sci. 2016;77:161–170. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material