Abstract
Objective:
To evaluate the impact of olmesartan alone or combined with one to three antihypertensive drugs on 24-h blood pressure variability (BPV) and on distribution of BP reduction in a pooled individual data analysis of 10 double-blind, randomized, ambulatory BP monitoring (ABPM) studies.
Methods:
ABPMs were performed before and after 6–12 weeks of treatment with placebo (n = 119), active control monotherapy [n = 1195, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), dihydropyridine calcium channel blockers (DCCBs)] olmesartan monotherapy (n = 1410), active control dual combination [n = 79, DCCB + thiazide diuretic (TD)], olmesartan dual combination (n = 637, DCCB or TD), and triple combination therapy (n = 102, DCCB+TD). 24-h BPV was calculated as unweighted or weighted SD of the mean BP, and average real variability. BP control was assessed by smoothness index and treatment-on-variability index.
Results:
The greatest effect on 24-h systolic BPV/diastolic BPV was observed under olmesartan triple [−2.6/−1.9; −1.9/−1.3; −1.4/−1.3 mmHg] and active control dual combination [−1.8/−1.4; −1.9/−1.5; −1.2/−1.1 mmHg]. Smoothness indexes and treatment-on-variability indexes were significantly (P = 0.0001) higher under olmesartan dual (1.53/1.22, 1.67/1.29, 2.05/1.59), olmesartan triple (2.47/1.85, 2.80/2.06, 3.64/2.67), or active control dual combination (1.70/1.26, 1.85/1.33, 2.29/1.65) than under monotherapies (control: 0.86/0.73, 0.80/0.65, 1.01/0.82; olmesartan: 1.02/0.86, 0.95/0.78, 1.23/1.00). They were also greater in patients receiving high-dose olmesartan monotherapy or high-dose olmesartan dual combination than in the corresponding low-dose group.
Conclusion:
Olmesartan plus a DCCB and/or a TD produces a larger, more sustained, and smoother BP reduction than placebo and monotherapies, a desirable feature for a more effective prevention of the cardiovascular consequences of uncontrolled hypertension.
Keywords: ambulatory blood pressure monitoring, arterial hypertension, blood pressure, blood pressure variability, olmesartan, smoothness index, treatment-on-variability index, trough-to-peak ratio
INTRODUCTION
Increased values of blood pressure (BP) variability (BPV), either in the short (over 24 h) or in the long term (visit-to-visit), are regarded as important determinants of hypertensive organ damage [1–4] and cardiovascular outcomes over and above the prognostic impact of an elevated BP mean level [5–10]. Cardiovascular protection by antihypertensive treatment should aim not only at achieving control of the average BP values, but also at stabilizing BPV in the long term. Indeed, preclinical and clinical evidence suggests that treatment-related reduction of BPV may contribute to cardiovascular protection [11–13].
One of the easiest and most widely used approach to the assessment of the effect of antihypertensive therapy on BPV is the calculation of the SD of 24-h average BP values, obtained noninvasively by automated, oscillometric, intermittent ambulatory BP monitoring (ABPM) [14]. More recently, new parameters such as the weighted SD (the average of the day-time and night-time SD weighted for the duration of the day-time and night-time interval) and the average real variability (ARV, the average of the absolute differences between consecutive BP readings) have been proposed to capture 24-h BPV, because of their stronger association with the increased cardiovascular risk than unweighted SD [7,15–18]. Other approaches account for the combined effect of treatment on BP mean level and BPV and include the smoothness index and the treatment-on-variability index (TOVI). The smoothness index is computed by dividing the mean of the hourly BP reductions and its SD over the 24-h, and thus it incorporates information on both the average degree of BP reduction and its distribution throughout the recording period [19]. A large smoothness index usually indicates a consistent average BP reduction associated with a small variability among hours, and thus a superior cardiovascular protection and an improved prevention of target-organ damage [19,20]. The TOVI is computed as the ratio between the changes in 24-h mean BP and the 24-h SD of BP during treatment [21]. It represents an alternative measure of the effects of antihypertensive treatment on both mean BP levels and BPV, combining information on the reduction of 24-h average BP values and on the accompanying changes in short-term absolute 24-h BPV during treatment. Preliminary evidence suggest that the TOVI, although providing conceptually different information from the smoothness index, is as effective as the smoothness index in differentiating the size and the duration of the antihypertensive effect of different treatments [21].
Olmesartan is a highly potent and selective long-acting angiotensin II receptor blocker (ARB). Extensive clinical evidence from several large well design trials and clinical practice setting has documented the antihypertensive efficacy and good tolerability profile of oral olmesartan, given alone or in combination with a dihydropyridine calcium-channel blocker and/or a thiazide diuretic [22,23]. Moreover, data provided by ABPM studies also indicate that treatment with olmesartan is associated with a sustained and smooth 24-h BP control, particularly during the final hours of dosing, and with a slight buffering effect on 24-h BPV [24–26].
In the present study, we aimed at evaluating in greater detail the impact of treatment with olmesartan alone or in combination with one or two other antihypertensive drugs on 24-h BP control, and particularly on BPV and on 24-h distribution of BP reduction, through calculation of smoothness index, TOVI, and trough-to-peak ratio (TPR). This objective could be achieved through a pooled individual analysis of ABPM data from ten double-blind, randomized, parallel group studies [27–36].
METHODS
Study design and populations
Individual data from ten double-blind, randomized, parallel group studies were pooled together for the purpose of this analysis. Criteria for study selection and inclusion were availability of 24-h ABPM data and the investigation of two or more treatment arms of interest, as detailed below. Summary of the study designs and treatments for the included studies are summarized in Table 1. Further information may be found in the original publications of the individual studies [27–36]. For all trials an ABPM was available at baseline, after a 2–4 week placebo run-in or wash-out from previous antihypertensive treatment, and at the end of 6–12 weeks of active treatment with placebo, an active reference drug alone (azelnidipine 8 or 16 mg, losartan 50 mg, valsartan 80 mg, irbesartan 150 mg, candesartan 8 mg, ramipril 2.5–10 mg, daily) or in dual combination (amlodipine 10 mg plus hydrochlorothiazide 25 mg daily), olmesartan alone (2.5–80 mg daily), in dual combination with a dihydropyridine calcium-channel blocker (olmesartan 10 mg plus azelnidipine 8 mg, olmesartan 20 mg plus azelnidipine 16 mg or olmesartan 40 mg plus amlodipine 10 mg, daily) or with a thiazide diuretic (olmesartan 40 mg plus hydrochlorothiazide 25 mg daily), or in triple combination with a dihydropyridine calcium-channel blocker and a thiazide diuretic (olmesartan 40 mg plus amlodipine 10 mg plus hydrochlorothiazide 25 mg daily).
TABLE 1.
Summary of the main characteristics of the studies included in the pooled individual data analysis
| Study number | Main inclusion criteria | Original primary endpoint | Type of run-in | Duration of run-in (weeks) | Study treatments | Duration of double-blind treatment (weeks) | ABPM devicea | Number of patients included in the analysis for each treatment group | Overall number of patients included in the analysis for each study |
| CS866AZ-A-J201[data on file] | Age ≥20 years24-h BP ≥135/80 mmHgSitting office BP ≥140/90 mmHg | Changes in average 24-h BP with treatment | Placebo | 4–6 | Olmesartan 10 mg odAzelnidipine 8 mg odOlmesartan 10 mg and Azelnidipine 8 mg od | 8 | A&D TM2431 | 323331 | 96 |
| SE-866/06[27] | Age 18–75 yearsSitting office DBP 100–114 mmHg24-h DBP ≥84 mmHg and 30% of daytime DBP ≥90 mmHg | Safety and tolerability after multiple dosing | Placebo | 2 | PlaceboOlmesartan 20 mg odOlmesartan 80 mg od | 6 | Spacelabs 90207 | 262322 | 71 |
| CS-866/204[27] | Age ≥18 yearsSitting office DBP 100-115 mmHgDaytime DBP ≥90 mmHg | Change of average 24-h DBP | Placebo | 2–3 | PlaceboOlmesartan 5 mg odOlmesartan 20 mg odOlmesartan 80 mg odOlmesartan 2.5 mg bidOlmesartan 10 mg bidOlmesartan 40 mg bid | 8 | Spacelabs 90207 | 41413941454248 | 297 |
| CS-866/411[28,29] | Age ≥18 yearsSitting office DBP 100–115 mmHgDaytime DBP 90–120 mmHg | Change of sitting office DBP | Placebo | 4 | Olmesartan 20 mg odLosartan 50 mg odValsartan 80 mg odIrbesartan 150 mg od | 8 | Spacelabs 90207 | 130128120131 | 509 |
| SE-866/25[30,31] | Sitting office DBP 100–120 mmHg and SBP >150 mmHg24-h DBP ≥84 mmHg and 30% of daytime DBP ≥90 mmHg | Change of daytime DBP | Placebo | 2 | Olmesartan 20 mg odCandesartan 8 mg od | 8 | Spacelabs 90207 | 280272 | 552 |
| SE-866/11[27] | Whites aged ≥18 yearsSitting office DBP 100–114 mmHg24-h DBP ≥84 mmHg and 30% of daytime DBP ≥90 mmHg | Change of average 24-h DBP | Placebo | 2 | PlaceboOlmesartan 2.5 mg odOlmesartan 5 mg odOlmesartan 10 mg od | 12 | Spacelabs 90207 | 52525353 | 210 |
| CS-OLM-CLIN-1-12-03[32] | Age 65–89 yearsSitting office DBP 90–109 mmHg and/or SBP 140–179 mmHg | Percentage of normalized (office BP <140/90 mmHg) or responder patients (SBP reduction ≥20 mmHg or DBP reduction ≥10 mmHg) | Placebo | 2 | Olmesartan 10 mg odUp-titration to olmesartan 20 mg odUp-titration to olmesartan 40 mg odRamipril 2.5 mg odUp-titration to ramipril 5 mg odUp-titration to ramipril 10 mg od | 12 | Various(mainly Spacelabs 90202, 90207 or 90217 and A&D TM2420, 2421, 2430, Accutracker II) | 671031124879147 | 556 |
| MEIN/03/OLM-HYP/001[33] | Age 65–89 yearsSitting office DBP 90-109 mmHg and/or SBP 140–179 mmHg | Percentage of normalized patients (office DBP <90 mmHg) | Placebo | 2 | Olmesartan 10 mg odUp-titration to olmesartan 20 mg odUp-titration to olmesartan 40 mg odRamipril 2.5 mg odUp-titration to ramipril 5 mg odUp-titration to ramipril 10 mg od | 12 | Various(mainly Spacelabs 90202, 90207 or 90217 and A&D TM2420, 2421, 2430, Accutracker II) | 47194827 | 69 |
| CS866AZ-A-J301[34] | Age ≥20 years24-h BP ≥135/80 mmHgSitting office BP ≥140/90 mmHg | Changes in sitting office BP during treatment | Placebo | 4 | Olmesartan 20 mg odAzelnidipine 16 mg odOlmesartan 10 mg and azelnidipine 8 mg odOlmesartan 20 mg and azelnidipine 16 mg od | 12 | A&D TM2431 | 197198204204 | 803 |
| CS8635-A-U301[35,36] | Age 18-80 yearsSitting office BP ≥140/100 mmHg orSitting office BP ≥160/90 mmHg | Change of sitting office DBP | None (wash-out) | 2 | PlaceboOlmesartan 40 mg and amlodipine 10 mg odOlmesartan 40 mg and HCTZ 25 mgAmlodipine 10 mg and HCTZ 25 mgOlmesartan 40 mg and amlodipine 10 mg and HCTZ 25 mg | 12 | Spacelabs 90207 | 01019779102 | 379 |
bid, twice daily; BP, blood pressure; od, once daily.
aAccutracker II (SunTech Medical Inc., Morrisville, North Carolina, USA); A&D devices (A&D Company Ltd., Tokyo, Japan); Spacelabs devices (Spacelabs Healthcare, Snoqualmie, Washington, USA).
All studies enrolled study participants of both sexes, aged 18 years or older. The main inclusion criterion was a diagnosis of untreated or treated uncontrolled mild or moderate essential arterial hypertension, defined as an office sitting SBP at least 140 mmHg and/or a DBP at least 90 mmHg. In some studies, an elevated ABP was also required (24-h average SBP ≥135 mmHg and/or DBP ≥80 mmHg). Given the wide geographical location of the studies, study participants of different ethnic origin were included in the various trials (mainly Whites, African-Americans and Asians). The studies were conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki, and the respective protocols were approved by the Ethics Committee of the study centers. Written informed consent was sought and collected from all eligible patients prior to their inclusion into the study.
Ambulatory blood pressure monitoring methodology
ABPM was performed in all studies according to current guidelines [37]. Each ABPM started in the morning and had to record values for at least 24 h. Measurements were performed at intervals of 15–30 min throughout the whole monitoring period. Different devices were used in the different studies. However, all of them measured BP through the oscillometric principle and were clinically validated according to current standards (see Table 1 for details on the type of device used in the individual studies). In all studies, cuff sizes appropriate to study participant's arm were used, namely standard adult cuffs for arm circumferences ranging between 24 and 32 cm and large adult cuffs for arm circumferences ranging between 32 and 42 cm. Cuffs were placed on the nondominant arm and study participants were instructed to keep their arm still and remain motionless during the cuff's automatic inflation. After fitting the device in the outpatient clinic, study participants were sent back to their usual activities and asked to return 24 h later to have the device unfitted and the recording terminated.
Statistical analysis
Before being analyzed, each ABPM recording was scanned to automatically identify possible artifacts, by applying specific validated editing criteria [37]. All measurements not complying with such criteria and not clinically justified were marked as not valid and not included in the calculation of ABPM parameters. Only ABPMs with at least 22 h of recording, no occurrence of consecutive 2 h without valid readings, and at least 70% of expected number of valid single readings, were included in the analysis. This was carried out according to the intention-to-treat principle, including only study participants with valid ABPM recordings at baseline and at the end of the double-blind treatment period, even in presence of major protocol violations. The efficacy variables considered for the present individual pooled data analysis were: average of all BP values collected over the 24 h, the day (0700 to 2300 h) and the night (2300 to 0700 h); 24 h, day and night unweighted SD, defined as the SD of the 24 h, day or night mean BP value [14]; the 24-h weighted SD, defined as the SD of the average of all BP values during the day and the night, with weights corresponding to the duration of the daytime and night-time [15]; the ARV, for the 24-h and for the day and night, defined as the mean of the successive absolute differences between adjacent BP values [16]; 24 h, day and night variation coefficient, calculated by dividing the unweighted SD and ARV by the corresponding mean BP value; 24-h variation coefficient for weighted SD, calculated by dividing the weighted SD by the mean BP value; TPR, computed in each study participants by dividing the BP changes at trough (last 2 h of the monitoring period) by those at peak (average of the adjacent 2 h with the maximal BP reduction between the second and 8 h from the drug intake [38]; smoothness index, obtained by dividing the mean of hourly BP reductions and the SD of the average hourly differences [19]; TOVI, defined by the ratio between the mean of the 24-h BP reductions with treatment and the 24-h weighted SD, or the 24-h ARV, during treatment [21]. All variables were separately assessed for SBP and DBP.
Treatments were grouped as follows: placebo, active control monotherapy, olmesartan monotherapy, active control dual combination therapy, olmesartan dual combination therapy, and olmesartan triple combination therapy. Main demographic and clinical baseline characteristics were summarized and compared by treatment groups by analysis of variance for multiple comparisons or χ2 test. Differences between baseline and treatment values were computed and compared among treatment groups for mean BP values, unweighted and weighted SD, ARV, and corresponding variation coefficients by means of analysis of covariance for multiple comparisons with adjustment for baseline value and for confounding factors (age, sex, BMI, and region). Among treatment comparisons were carried out for smoothness index and TOVI with analysis of variance for multiple comparisons with adjustment for confounding factors (age sex, BMI, and region). Given the nonnormal distribution of individual TPRs, this measure of BP control was summarized by boxplots, showing median values, interquartile range (25th and 75th percentile) and the lowest and highest value within 1.5 interquartile of the lower (25th percentile) and higher quartile (75th percentile), respectively. TPRs were compared across groups by a nonparametric Kruskal–Wallis test. A multivariate regression analysis with 24-h BPV as dependent variable and 24-h mean BP value, group of treatment, region, age, BMI, cardiovascular disease, and sex as independent variables was run to seek for possible determinants of BPV. The level of statistical significance was kept at 0.05 throughout the whole study. Analyses on clinically meaningful subgroups with adequate size (men vs. women, older vs. younger, and obese patients) were also carried out to verify differences in the effect of treatment on BP control and BPV. Data are shown as mean (or median in case of TPR) ± SD or 95% confidence intervals for continuous variables and as absolute (n) and relative (%) frequencies for discrete variables.
RESULTS
Baseline demographic and clinical data
Overall 3542 of the 4213 patients (84.1%) undergoing ABPM in the 10-selected studies had valid recordings at baseline and at the end of the study and were, thus, included in the intention-to-treat analysis. The distribution of the number of study participants in the various study groups was unbalanced, with olmesartan monotherapy and active control monotherapy being the most represented groups (39.8 and 33.7% of the total sample, respectively). The olmesartan dual combination therapy group accounted for 18.0% of the population, with 84.8% of patients of this group treated with a combination including a dihydropyridine calcium-channel blocker. The average doses of olmesartan used as mono, dual combination, and triple combination therapy were 23.4 ± 18.8 mg, 22.5 ± 12.5 mg, and 40.0 ± 0.0 mg, respectively. The average dose of azelnidipine used in combination with olmesartan was 11.7 ± 4.0 mg. The rates of patients receiving high-dose treatment were 17.2% in the olmesartan monotherapy group (olmesartan 40 or 80 mg daily), 31.1% in the active control monotherapy group (azelnidipine 16 mg or ramipril 10 mg), and 63.1% in the olmesartan dual combination treatment group (olmesartan 20 mg plus azelnidipine 16 mg, olmesartan 40 mg plus amlodipine 10 mg or hydrochlorothiazide 25 mg). All patients in the active control dual combination therapy and in the olmesartan triple combination therapy were treated with a high-dose regimen.
As shown in Table 2, comparison of the baseline characteristics of the patients of the six different treatment groups showed a statistical heterogeneity. Such differences were accounted for in the adjusted analyses presented in the next paragraphs. The whole study sample aged on average 57.3 ± 11.7 years, with 31.7% of patients falling in the elderly category. Men were prevalent over women (60.0%). The average BMI was 28.1 ± 4.8 kg/m2, with 27.5% of patients being obese (BMI ≥30 kg/m2). Most of the recruited patients originated from Europe (41.2%) and the most represented ethnic group was the White one (64.9%). The prevalence of cardiovascular diseases (coronary heart disease, heart failure, and diabetes) was relatively low (14.9%). As expected office BPs (158.1 ± 12.7/99.6 ± 7.5 mmHg) were higher than 24-h average BPs (149.2 ± 13.6/91.5 ± 9.5 mmHg). Baseline values for other ABP indices assessed in the study are reported in Supplementary Table 1.
TABLE 2.
Demographic and clinical characteristics of the study population by type of treatment
| Placebo (n = 119) | Active control monotherapy (n = 1195) | Olmesartan monotherapy (n = 1410) | Active control dual combination (n = 79) | Olmesartan dual combination therapy (n = 637) | Olmesartan triple combination therapy (n = 102) | P value | All (n = 3542) | |
| Age (years) | 53.0 ± 10.7 | 58.0 ± 12.1 | 57.4 ± 12.0 | 56.2 ± 10.7 | 56.5 ± 10.2 | 56.9 ± 11.4 | 0.0001a | 57.3 ± 11.7 |
| Elderly | 18 (15.1) | 436 (36.5) | 477 (33.8) | 17 (21.5) | 146 (22.9) | 28 (27.5) | 0.0001a | 1122 (31.7) |
| Male sex | 63 (52.9) | 702 (58.7) | 855 (60.6) | 49 (62.0) | 408 (64.1) | 49 (48.0) | 0.015b | 2126 (60.0) |
| Height (cm) | 169.5 ± 10.1 | 168.2 ± 9.9 | 168.4 ± 9.4 | 171.7 ± 11.4 | 165.9 ± 9.4 | 168.6 ± 11.0 | 0.0001a | 168.0 ± 9.7 |
| Body weight (kg) | 79.4 ± 13.3 | 80.3 ± 17.3 | 78.7 ± 15.4 | 95.9 ± 23.3 | 77.0 ± 19.5 | 90.3 ± 19.9 | 0.0001a | 79.7 ± 17.4 |
| BMI (kg/m2) | 27.6 ± 3.6 | 28.2 ± 4.7 | 27.6 ± 4.1 | 32.4 ± 6.5 | 27.8 ± 5.7 | 31.7 ± 5.9 | 0.0001a | 28.1 ± 4.8 |
| Obese | 35 (29.4) | 333 (27.9) | 330 (23.4) | 51 (64.6) | 169 (26.5) | 57 (55.9) | 0.0001a | 975 (27.5) |
| Region | ||||||||
| Europe | 78 (65.6) | 585 (49.0) | 795 (56.4) | – | – | – | 0.0001a | 1458 (41.2) |
| USA | 41 (34.5) | 379 (31.7) | 386 (27.4) | 79 (100.0) | 198 (31.1) | 102 (100.0) | 1185 (33.5) | |
| Japan | – | 231 (19.3) | 229 (16.2) | – | 439 (68.9) | – | 899 (25.4) | |
| Race | ||||||||
| White | 10.5 (88.2) | 852 (71.3) | 1061 (75.2) | 62 (78.5) | 145 (22.8) | 75 (73.5) | 0.0001a | 2300 (64.9) |
| Asian | 1 (0.8) | 242 (20.3) | 239 (17.0) | – | 444 (69.7) | 3 (2.9) | 929 (26.2) | |
| Hispanic | 8 (6.7) | 53 (4.4) | 70 (5.0) | – | – | – | 131 (3.7) | |
| African-American | 5 (4.2) | 47 (3.9) | 38 (2.7) | 15 (19.0) | 46 (7.2) | 23 (22.5) | 174 (4.9) | |
| Other | – | 1 (0.1) | 2 (0.1) | 2 (2.5) | 2 (0.3) | 1 (1.0) | 8 (0.2) | |
| Coronary heart disease | 4 (3.4) | 37 (3.1) | 43 (3.0) | 1 (1.3) | 9 (1.4) | 6 (5.9) | 0.090 | 100 (2.8) |
| Heart failure | 1 (0.8) | – | 5 (0.4) | 1 (1.3) | – | – | 0.027b | 7 (0.2) |
| Diabetes mellitus | 9 (7.6) | 139 (11.6) | 149 (10.6) | 9 (11.4) | 113 (17.7) | 23 (22.5) | 0.0001a | 442 (12.5) |
| Cardiovascular disease | 13 (10.9) | 173 (14.5) | 187 (13.3) | 10 (12.7) | 119 (18.7) | 27 (26.5) | 0.0001a | 529 (14.9) |
| Sitting office SBP (mmHg) | 158.2 ± 12.9 | 158.3 ± 11.7 | 157.3 ± 12.6 | 163.8 ± 16.2 | 157.5 ± 13.6 | 166.3 ± 13.5 | 0.0001a | 158.1 ± 12.7 |
| Sitting office DBP (mmHg) | 102.6 ± 5.1 | 99.7 ± 7.8 | 100.1 ± 7.4 | 99.1 ± 9.0 | 98.0 ± 7.4 | 98.8 ± 7.3 | 0.0001a | 99.6 ± 7.5 |
| Sitting office HR (bpm) | 75.9 ± 8.5 | 73.3 ± 9.0 | 73.9 ± 9.5 | 73.6 ± 11.2 | 71.8 ± 9.2 | 75.9 ± 11.4 | 0.0001a | 73.4 ± 9.4 |
| 24-h SBP (mmHg) | 148.7 ± 10.5 | 148.9 ± 13.9 | 147.6 ± 13.3 | 148.0 ± 13.0 | 154.2 ± 13.0 | 146.3 ± 13.4 | 0.0001a | 149.2 ± 13.6 |
| 24-h DBP (mmHg) | 93.8 ± 6.0 | 91.0 ± 9.7 | 91.1 ± 9.2 | 88.9 ± 10.3 | 94.0 ± 9.7 | 86.2 ± 9.5 | 0.0001a | 91.5 ± 9.5 |
| 24-h HR (mmHg) | 79.9 ± 9.7 | 74.9 ± 10.1 | 75.4 ± 10.3 | 77.0 ± 10.9 | 71.9 ± 9.2 | 77.0 ± 10.5 | 0.0001a | 74.8 ± 10.2 |
Data are shown as means (± SD) or as absolute (n) and relative (%) frequencies. P values for differences across the study groups are also reported. HR, heart rate.
aP < 0.0001.
bP < 0.05.
Mean blood pressure values
Average baseline-adjusted ABP reductions by treatment group are shown in Fig. 1, whereas unadjusted changes are reported in Supplementary Table 2. All active drugs, but not placebo, reduced SBP and DBP during the whole 24 h, and the reduction was well maintained during the day and during the night, with larger effects during the waking hours.
FIGURE 1.
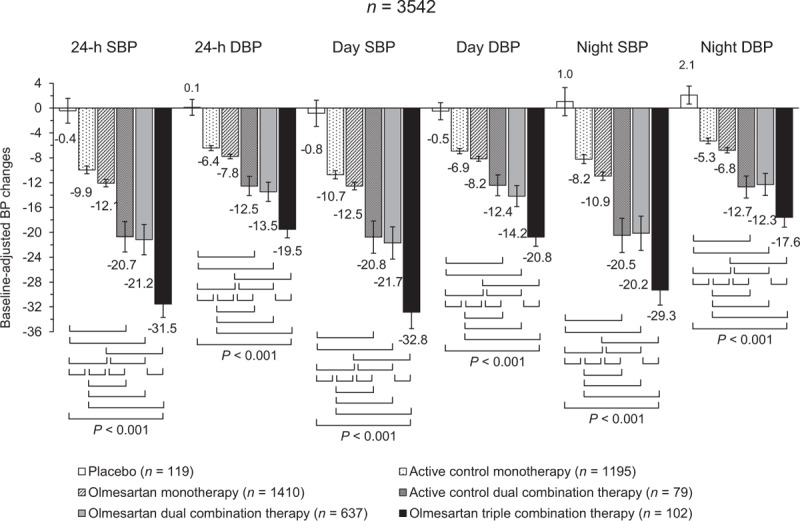
Adjusted 24 h, day and night SBP and DBP mean changes (95% confidence interval) from baseline after double blind treatment with placebo (n = 119), active control monotherapy (n = 1195), olmesartan monotherapy (n = 1410), active control dual combination therapy (n = 79), olmesartan dual combination therapy (n = 637), and olmesartan triple combination therapy (n = 102). The statistical significance of differences between individual pairs of treatments is indicated by the P value. Changes are adjusted for baseline value, age, sex, BMI, and region. BP, blood pressure.
Mean reductions in SBP and DBP were significantly (P < 0.001) greater after combination treatment than with monotherapy treatments, the magnitude of the reduction progressively increasing with the intensity of the combination treatment. BP drops under olmesartan monotherapy were significantly (P < 0.001) larger than under active control monotherapy and the greatest under high-dose treatment (Supplementary Table 3). No significant differences were observed between olmesartan dual combination therapy and active control dual combination therapy; however, when only high-dose olmesartan dual combination therapy was compared with active control dual combination therapy, a slightly larger 24-h BP reduction was observed. In addition, no difference in 24-h BP reduction was observed between patients treated with an olmesartan dual combination treatment including a dihydropyridine calcium-channel blocker [n = 540; SBP: −20.7 (−21.7, −19.8)/DBP: −12.4 (−12.9, −11.8) mmHg] or a thiazide diuretic [n = 97; SBP: −21.3 (−24.0, −18.7)/DBP: −12.5 (−14.2, −10.9) mmHg; P = 0.678 and P = 0.839 between treatments].
The effect of treatment on the whole 24 h was evident and confirmed for each hour in which the 24 h were subdivided, both for SBP and DBP (Fig. 2). Most of the efficacy displayed by each drug treatment in the first hours was well maintained in the hours farthest from the last drug intake.
FIGURE 2.
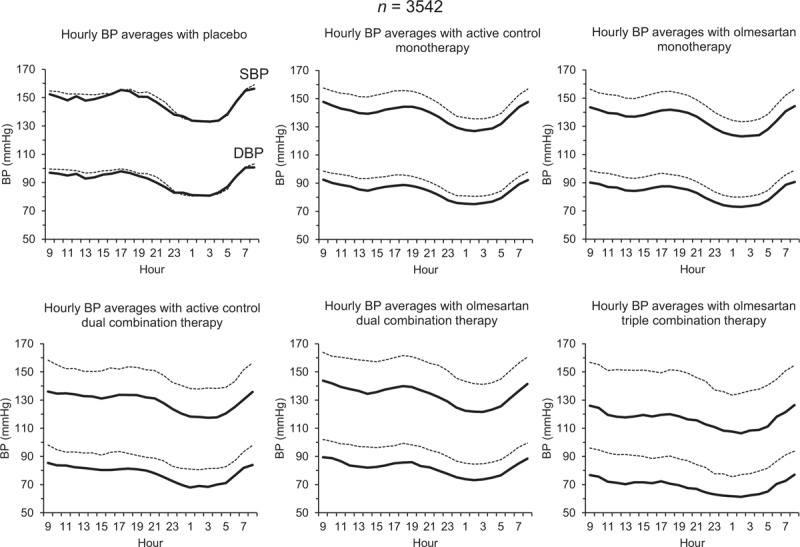
Average hourly SBP and DBP values at baseline (dashed lines) and at the end of the double-blind treatment (continuous lines) in patients treated with placebo (n = 119), active control monotherapy (n = 1195), olmesartan monotherapy (n = 1410), active control dual combination therapy (n = 79), olmesartan dual combination therapy (n = 637), and olmesartan triple combination therapy (n = 102). BP, blood pressure.
Blood pressure variability
As shown in Fig. 3 (adjusted changes) and in Supplementary Table 2 (unadjusted changes), placebo had no effect on BPV, small effects were observed under monotherapies, whereas the greatest effect was observed in the combination groups, and in particular in case of treatment with an active control dual combination and an olmesartan triple combination therapy. In all cases, no relevant differences in the magnitude and direction of the effect on BPV were observed with the different methods (unweighted SD, weighted SD, or ARV).
FIGURE 3.
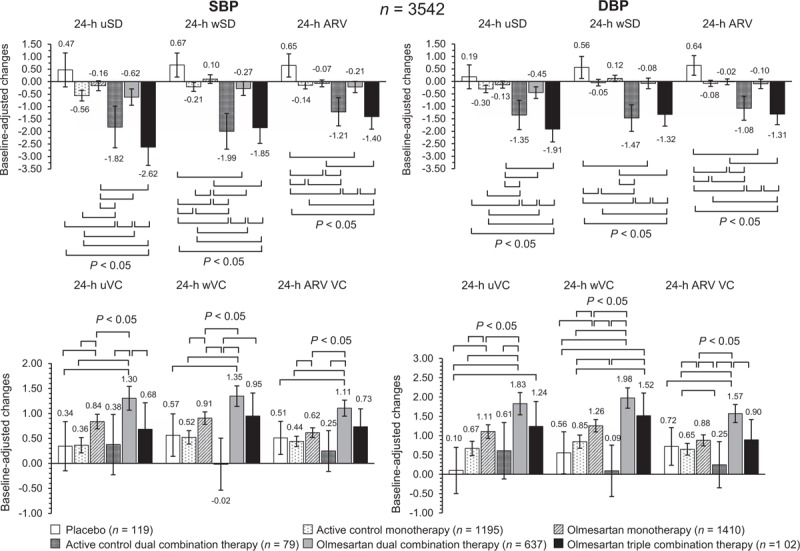
Adjusted average changes (95% confidence interval) in 24-h SBP and DBP variability from baseline after double blind treatment with placebo (n = 119), active control monotherapy (n = 1195), olmesartan monotherapy (n = 1410), active control dual combination therapy (n = 79), olmesartan dual combination therapy (n = 637), and olmesartan triple combination therapy (n = 102). BPV is shown as uSD, wSD, or ARV, and as uVC, wVC, or variation coefficient of average real variability. The statistical significance of differences between individual pairs of treatments is indicated by the P value. Changes are adjusted for baseline value, age, sex, BMI, and region. ARV VC, variation coefficient of average real variability; uVC, unweighted variation coefficient; uSD, unweighted SD; wVC, weighted variation coefficient; wSD, weighted SD.
In the olmesartan, dual combination treatment group, large BPV reductions were observed under the high than under the low dose (Supplementary Table 3) and no difference between the olmesartan dual combination including a dihydropyridine calcium-channel blocker [n = 540; ARV SBP: −0.17 (−0.44, 0.09)/ARV DBP: −0.16 (−0.40, 0.07) mmHg] and that including a thiazide diuretic [n = 97; ARV SBP: 0.001 (−0.75, 0.75)/ARV DBP: 0.03 (−0.63, 0.68) mmHg; P = 0.686 and P = 0.613 between treatments].
Changes in ABP mean and BPV with treatment were weakly, though yet significantly (P = 0.0001) correlated to each other (Fig. 4). A multivariate regression analysis (Table 3) indicated mean BP changes as the main determinants of changes in BPV, for all estimates. A smaller contribution was attributed to the type of treatment (for unweighted SBP), the geographic area (all measures of SBP variability), BMI (weighted SD and ARV of SBP), and sex (weighted SD of DBP).
FIGURE 4.
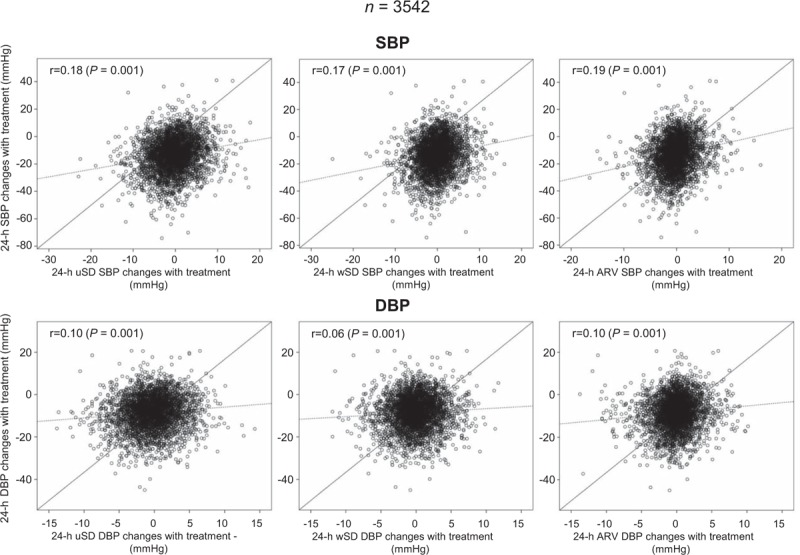
Correlation between 24-h average SBP or DBP changes with treatment and changes in 24-h SBP or DBP variability with treatment. BP variability is shown as uSD, wSD, or ARV. The correlation coefficient (r) and its statistical significance (P) are shown inside each panel. The continuous line refers to the identity line, the dashed line to the line of best fit (or trend line). ARV, average real variability; uSD, unweighted SD; wSD, weighted SD.
TABLE 3.
Determinants of changes in blood pressure variability assessed by multivariate regression analysis in the two study populations
| 24-h unweighted SD | P value | 24-h weighted SD | P value | 24-h ARV | P value | |
| SBP | ||||||
| 24-h BP changes | 0.212 (0.059, 0.083) | 0.0001a | 0.201 (0.047, 0.067) | 0.0001a | 0.216 (0.039, 0.055) | 0.0001a |
| Type of treatment | 0.055 (0.058, 0.331) | 0.005b | 0.034 (−0.014, 0.217) | 0.084 | 0.033 (−0.013, 0.163) | 0.094 |
| Region | 0.058 (0.117, 0.510) | 0.002b | 0.066 (0.133, 0.466) | 0.0001a | 0.073 (0.127, 0.381) | 0.0001a |
| Age | 0.021 (−0.005, 0.020) | 0.233 | 0.021 (−0.004, 0.017) | 0.228 | 0.021 (−0.003, 0.013) | 0.226 |
| BMI | 0.020 (−0.012, 0.048) | 0.242 | 0.036 (0.002, 0.053) | 0.037c | 0.037 (0.002, 0.040) | 0.034c |
| CV disease | 0.015 (−0.222, 0.574) | 0.386 | 0.011 (−0.226, 0.448) | 0.518 | 0.007 (−0.203, 0.311) | 0.680 |
| Sex | −0.025 (−0.507, 0.065) | 0.130 | −0.025 (−0.425, 0.058) | 0.137 | −0.023 (−0.313, 0.056) | 0.172 |
| DBP | ||||||
| 24-h BP changes | 0.103 (0.026, 0.053) | 0.0001a | 0.061 (0.009, 0.033) | 0.001b | 0.094 (0.018, 0.039) | 0.0001a |
| Type of treatment | −0.001 (−0.102, 0.095) | 0.942 | −0.025 (−0.145, 0.030) | 0.200 | −0.024 (−0.127, 0.029) | 0.220 |
| Region | 0.023 (−0.054, 0.234) | 0.223 | 0.028 (−0.033, 0.222) | 0.147 | 0.033 (−0.012, 0.217) | 0.079 |
| Age | −0.026 (−0.016, 0.002) | 0.146 | −0.020 (−0.013, 0.003) | 0.259 | −0.020 (−0.012, 0.003) | 0.267 |
| BMI | 0.013 (−0.014, 0.030) | 0.461 | 0.019 (−0.008, 0.031) | 0.268 | 0.021 (−0.007, 0.028) | 0.224 |
| CV disease | 0.006 (−0.239, 0.342) | 0.729 | 0.0001 (−0.260, 0.256) | 0.987 | −0.004 (−0.262, 0.200) | 0.795 |
| Sex | −0.031 (−0.404, 0.012) | 0.065 | −0.039 (−0.402, −0.033) | 0.021c | −0.021 (−0.272, 0.059) | 0.207 |
ARV, average real variability; BP, blood pressure; CV, cardiovascular.
aP < 0.0001.
bP < 0.01.
cP < 0.05.
The small changes in BPV observed with treatment were largely overridden by the effect on mean BP, as documented by higher variation coefficient of BP after treatment (Fig. 3 and Supplementary Table 2 and 3).
Results obtained for day and night BPV were in line with those observed for the 24 h (Supplementary Figure 1 and Supplementary Table 2).
Smoothness index, treatment-on-variability index, and trough-to-peak ratio
All active treatments had a significantly greater smoothness index than placebo. Treatment with olmesartan monotherapy resulted in smoothness indexes significantly larger than under active control, whereas treatment with active control dual combination therapy or olmesartan dual or triple combination therapy allowed smoothness indexes significantly larger than under corresponding monotherapies (Fig. 5 values adjusted for confounding factors and Supplementary Table 2 unadjusted values). Smoothness indexes were also greater in patients receiving high-dose olmesartan monotherapy than those treated with low-dose olmesartan monotherapy (Supplementary Table 3). They were also higher in patients treated with the high than with the low dose of olmesartan dual combination therapy and similar to those obtained in patients receiving a high-dose dual combination therapy (Supplementary Table 3).
FIGURE 5.
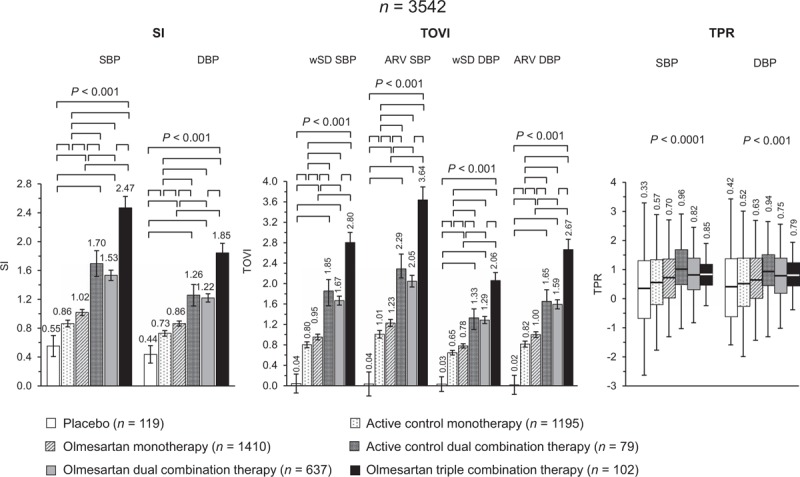
Adjusted average (95% confidence interval) SI and TOVI, and boxplots of TPR after double blind treatment with placebo (n = 119), active control monotherapy (n = 1195), olmesartan monotherapy (n = 1410), active control dual combination therapy (n = 79), olmesartan dual combination therapy (n = 637), and olmesartan triple combination therapy (n = 102). Data are shown for SBP and DBP. The statistical significance of differences between individual pairs of treatments is indicated by the P value. SI and TOVI data are adjusted for age, sex, BMI and region. ARV, average real variability; SI, smoothness index; TOVI, treatment on variability index; TPR, trough-to-peak ratio; wSD, weighted SD.
Despite some negligible discrepancy in the absolute values, the TOVI showed the same trend of smoothness index. No major differences could be seen between the behavior of both measures under different treatments, except that the placebo effect was negligible when evaluated through the TOVI.
Also TPRs were larger under active control dual combination therapy and olmesartan dual and triple combination therapy than under corresponding monotherapies, and larger under olmesartan monotherapy than under active control monotherapy. However, a substantial placebo effect was observed with this index.
Effect of treatment in specific subgroups
As shown in Table 4, also in subgroups of patients according to sex, age, and presence of obesity, mean reductions in SBP and DBP were significantly larger after combination than after monotherapy treatments, the magnitude of the reduction progressively increasing with the intensity of the combination. BP-lowering effect of treatment tended to be greater in women than in men and in younger vs. older patients. A consistent antihypertensive effect was observed also in presence of obesity.
TABLE 4.
Adjusted changes in 24-h average values, average real variability, smoothness index, and treatment on variability index by type of treatment in specific subgroups of patients
| Placebo | Active control monotherapy | Olmesartan monotherapy | Active control dual combination | Olmesartan dual combination therapy | Olmesartan triple combination therapy | P value | |
| 24-h SBP | |||||||
| Male sex (n = 2126) | −0.6 (−3.3, 2.1) | −9.3 (−10.1, −8.5) | −12.0 (−12.8, −11.3) | −19.9 (−23.0, −16.9) | −19.2 (−20.5, −18.1) | −28.7 (−31.7, −25.7) | 0.0001a |
| Female sex (n = 1416) | −0.4 (−3.4, −2.6) | −10.9 (−11.9, −9.9) | −12.1 (−13.1, −11.1) | −21.9 (−26.0, −17.8) | −24.4 (−26.1, −22.7) | −34.9 (−37.9, −31.8) | 0.0001a |
| Older patients (≥65 years) (n = 1122) | 4.4 (−0.8, 9.6) | −9.6 (−10.7, −8.6) | −11.5 (−12.6, −10.5) | −19.9 (−25.3, −14.6) | −19.8 (−21.9, −17.7) | −28.8 (−33.0, −24.5) | 0.0001a |
| Younger patients (<65 years) (n = 3430) | −1.4 (−3.6, 0.7) | −10.1 (−10.9, −9.3) | −12.4 (−13.1, −11.6) | −20.9 (−23.6, −18.1) | −21.6 (−22.6, −20.5) | −32.5 (−35.0, −30.0) | 0.0001a |
| Obese (n = 975) | 3.8 (−7.6, −0.1) | −8.9 (−10.2, −7.7) | −11.6 (−12.9, −10.4) | −21.2 (−24.2, −18.1) | −22.8 (−24.6, −20.9) | −33.1 (−36.1, −30.2) | 0.0001a |
| 24-h DBP | |||||||
| Male sex (n = 2126) | 0.2 (−1.6, 2.0) | −6.2 (−6.8, −5.7) | −7.9 (−8.4, −7.4) | −12.7 (−14.7, −10.7) | −12.9 (−13.7, −12.1) | −19.1 (−21.1, −17.1) | 0.0001a |
| Female sex (n = 1416) | −0.1 (−1.9, 1.8) | −6.7 (−7.3, −6.1) | −7.6 (−8.2, −7.0) | −12.3 (−14.8, −9.8) | −14.5 (−15.5, −13.4) | −20.1 (−22.0, −18.2) | 0.0001a |
| Older patients (≥65 years) (n = 1122) | 2.9 (−0.03, 5.9) | −5.7 (−6.3, −5.1) | −6.8 (−7.4, −6.2) | −10.7 (−13.8, −7.6) | −12.2 (−13.4, −10.9) | −16.9 (−19.4, −14.5) | 0.0001a |
| Younger patients (<65 years) (n = 3430) | −3.7 (−5.5, −2.0) | −6.8 (−7.3, −6.3) | −8.6 (−9.1, −8.1) | −11.3 (−13.2, −9.5) | −13.1 (−13.9, −12.4) | −18.7 (−20.4, −16.9) | 0.0001a |
| Obese (n = 975) | −0.9 (−3.3, 1.5) | −5.8 (−6.6, −5.1) | −7.9 (−8.8, −7.2) | −13.4 (−15.4, −11.4) | −15.1 (−16.3, −13.9) | −20.6 (−22.5, −18.7) | 0.0001a |
| 24-h ARV SBP | |||||||
| Male sex (n = 2126) | 0.43 (−0.22, 1.07) | −0.04 (−0.23, 0.16) | −0.02 (−0.20, 0.16) | −1.01 (−1.74, −0.28) | −0.03 (−0.31, 0.25) | −1.20 (−1.92, −0.47) | 0.003b |
| Female sex (n = 1416) | 0.90 (0.24, 1.55) | −0.30 (−0.52, −0.08) | −0.14 (−0.35, 0.08) | −1.55 (−2.45, −0.65) | −0.52 (−0,88, −0.15) | −1.68 (−2.36, −0.99) | 0.0001a |
| Older patients (≥65 years) (n = 1122) | 0.36 (−1.00, 1.72) | 0.05 (−0.23, 0.33) | −0.19 (−0.46, 0.08) | −1.33 (−2.76, 0.09) | −0.11 (−0.68, 0.46) | −1.51 (−2.63, −0.39) | 0.003b |
| Younger patients (<65 years) (n = 3430) | 0.76 (0.30, 1.23) | −0.28 (−0.45, −0.11) | −0.01 (−0.16, 0.15) | −1.13 (−1.72, −0.55) | −0.22 (−0.45, 0.01) | −1.33 (−1.87, −0.80) | 0.0001a |
| Obese (n = 975) | 0.29 (−0.39, 0.98) | −0.14 (−0.36, 0.08) | −0.07 (−0.29, 0.16) | −0.73 (−1.29, −0.16) | 0.07 (−0.26, 0.40) | −1.36 (−1.89, −0.82) | 0.0001a |
| 24-h ARV DBP | |||||||
| Male sex (n = 2126) | 0.50 (−0.03, 1.03) | 0.01 (−0.15, 0.16) | −0.04 (−0.18, 0.11) | −0.96 (−1.56, −0.36) | 0.04 (−0.19, 0.27) | −1.10 (−1.70, −0.51) | 0.0001a |
| Female sex (n = 1416) | 0.80 (0.21, 1.39) | −0.19 (−0.39, 0.01) | 0.02 (−0.17, 0.21) | −1.28 (−2.08, −0.47) | −0.33 (−0.66, −0.01) | −1.56 (−2.17, −0.95) | 0.0001a |
| Older patients (≥65 years) (n = 1122) | 0.34 (−0.77, 1.45) | −0.07 (−0.29, −0.16) | −0.19 (−0.41, 0.04) | −1.48 (−2.64, −0.32) | −0.08 (−0.54, 0.38) | −1.79 (−2.69, −0.88) | 0.0001a |
| Younger patients (<65 years) (n = 3430) | 0.79 (0.39, 1.19) | −0.12 (−0.26, 0.03) | 0.07 (−0.06, 0.21) | −0.96 (−1.47, −0.45) | −0.07 (−0.27, 0.13) | −1.12 (−1.59, −0.66) | 0.0001a |
| Obese (n = 975) | 0.96 (0.36, 1.55) | −0.18 (−0.37, 0.01) | 0.21 (0.00, 0.41) | −0.82 (−1.31, −0.33) | 0.06 (−0.23, 0.35) | −1.14 (−1.61, −0.67) | 0.0001a |
| SI SBP | |||||||
| Male sex (n = 2126) | 0.67 (0.47, 0.86) | 0.83 (0.78, 0.89) | 0.99 (0.94, 1.05) | 1.73 (1.51, 1.95) | 1.40 (1.31, 1.48) | 2.18 (1.96, 2.40) | 0.0001a |
| Female sex (n = 1416) | 0.43 (0.21, 0.65) | 0.91 (0.84, 0.98) | 1.05 (0.98, 1.12) | 1.64 (1.34, 1.95) | 1.76 (1.64, 1.88) | 2.78 (2.56, 3.01) | 0.0001a |
| Older patients (≥65 years) (n = 1122) | 0.68 (0.32, 1.03) | 0.82 (0.75, 0.89) | 0.96 (0.89, 1.03) | 1.54 (1.18, 1.92) | 1.40 (1.26, 1.55) | 2.47 (2.18, 2.76) | 0.0001a |
| Younger patients (<65 years) (n = 3430) | 0.52 (0.36, 0.69) | 0.89 (0.83, 0.95) | 1.04 (0.99, 1.09) | 1.73 (1.53, 1.94) | 1.58 (1.49, 1.66) | 2.47 (2.28, 2.66) | 0.0001a |
| Obese (n = 975) | 0.65 (0.36, 0.93) | 0.89 (0.80, 0.98) | 1.05 (0.05, 1.14) | 1.68 (1.44, 1.92) | 1.74 (1.59, 1.88) | 2.56 (2.33, 2.78) | 0.0001a |
| SI DBP | |||||||
| Male sex (n = 2126) | 0.52 (0.36, 0.68) | 0.71 (0.66, 0.78) | 0.86 (0.81, 0.90) | 1.31 (1.13, 1.50) | 1.17 (1.09, 1.24) | 1.74 (1.56, 1.93) | 0.0001a |
| Female sex (n = 1416) | 0.34 (0.16, 0.52) | 0.75 (0.69, 0.81) | 0.87 (0.81, 0.93) | 1.17 (0.92, 1.41) | 1.32 (1.22, 1.42) | 1.96 (1.78, 2.15) | 0.0001a |
| Older patients (≥65 years) (n = 1122) | 0.49 (0.21, 0.78) | 0.68 (0.62, 0.74) | 0.78 (0.72, 0.84) | 1.06 (0.76, 1.36) | 1.13 (1.01, 1.25) | 1.74 (1.50, 1.97) | 0.0001a |
| Younger patients (<65 years) (n = 3430) | 0.44 (0.30, 0.58) | 0.75 (0.70, 0.80) | 0.91 (0.86, 0.95) | 1.31 (1.13, 1.48) | 1.25 (1.19, 1.32) | 1.88 (1.72, 2.04) | 0.0001a |
| Obese (n = 975) | 0.52 (0.29, 0.76) | 0.74 (0.67, 0.82) | 0.90 (0.82, 0.98) | 1.20 (1.01, 1.40) | 1.35 (1.24, 1.46) | 1.88 (1.69, 2.06) | 0.0001a |
| TOVI SBP | |||||||
| Male sex (n = 2126) | 0.13 (−0.18, 0.44) | 0.94 (0.84, 1.03) | 1.21 (1.13, 1.30) | 2.32 (1.97, 2.67) | 1.84 (1.70, 1.97) | 3.18 (2.83, 3.53) | 0.0001a |
| Female sex (n = 1416) | −0.07 (−0.43, 0.29) | 1.11 (0.99, 1.23) | 1.25 (1.13, 1.37) | 2.25 (1.76, 2.74) | 2.41 (2.21, 2.61) | 4.15 (3.78, 4.52) | 0.0001a |
| Older patients (≥65 years) (n = 1122) | −0.11 (−0.73, 0.50) | 0.89 (0.76, 1.01) | 1.15 (1.03, 1.27) | 1.96 (1.32, 2.60) | 1.77 (1.51, 2.02) | 3.22 (2.73, 3.73) | 0.0001a |
| Younger patients (<65 years) (n = 3430) | 0.06 (−0.20, 0.31) | 1.08 (0.99, 1.17) | 1.26 (1.18, 1.35) | 2.37 (2.05, 2.70) | 2.15 (2.02, 2.27) | 3.78 (3.49, 4.08) | 0.0001a |
| Obese (n = 975) | 0.44 (−0.02, 0.89) | 1.02 (0.88, 1.17) | 1.23 (1.08, 1.39) | 2.37 (1.99, 2.74) | 2.38 (2.15, 2.59) | 3.98 (3.62, 4.34) | 0.0001a |
| TOVI DBP | |||||||
| Male sex (n = 2126) | 0.02 (−0.24, 0.27) | 0.79 (0.71, 0.86) | 1.01 (0.94, 1.08) | 1.75 (1.46, 2.04) | 1.50 (1.39, 1.81) | 2.52 (2.23, 2.81) | 0.0001a |
| Female sex (n = 1416) | 0.02 (−0.26, 0.29) | 0.86 (0.77, 0.95) | 0.98 (0.89, 1.07) | 1.49 (1.12, 1.85) | 1.77 (1.61, 1.92) | 2.84 (2.56, 3.12) | 0.0001a |
| Older patients (≥65 years) (n = 1122) | −0.02 (−0.50, 0.46) | 0.73 (0.63, 0.83) | 0.89 (0.79, 0.99) | 1.33 (0.83, 1.84) | 1.39 (1.19, 1.59) | 2.39 (1.99, 2.79) | 0.0001a |
| Younger patients (<65 years) (n = 3430) | 0.04 (−0.17, 0.24) | 0.86 (0.79, 0.94) | 1.05 (0.99, 1.12) | 1.72 (1.47, 1.98) | 1.66 (1.56, 1.76) | 2.75 (2.51, 2.98) | 0.0001a |
| Obese (n = 975) | 0.24 (−0.11, 0.59) | 0.83 (0.72, 0.94) | 1.04 (0.92, 1.16) | 1.63 (1.34, 1.92) | 1.77 (1.59, 1.94) | 2.77 (2.49, 3.04) | 0.0001a |
Data are shown as means and 95% confidence interval. P values for differences across the study groups are also reported.
ARV, average real variability SI, smoothness index; TOVI, treatment-on-variability index.
aP < 0.0001.
bP < 0.05.
As for the whole study population, the greatest effect on BPV, smoothness index, and TOVI was observed in the combination groups, and in particular in case of treatment with an active control dual combination and an olmesartan triple combination therapy. No systematic differences in the effect of treatment on BPV indices were observed within each subgroup (men or women, older or younger, and obese patients).
DISCUSSION
Our large pooled individual data analysis of double-blind, randomized, parallel group ABPM studies investigated the effect of olmesartan mono or combination therapy on 24-h BP control, with particular regard to the distribution of the treatment effect and its impact on BPV. Changes in mean 24-h BP were larger under olmesartan monotherapy than under placebo and active control monotherapies, which were based mainly on drugs acting on the renin–angiotensin-aldosterone system. Effects of single-drug treatment were maximal under high dose. In addition, olmesartan administered in combination with one or two other antihypertensive drugs, allowed a superior 24-h BP control than placebo or monotherapies (also including olmesartan). The effect of a combination containing olmesartan was the largest under high dose and no difference in the effect was observed between a dual combination of olmesartan with a dihydropyridine calcium-channel blocker or a thiazide diuretic. The antihypertensive effect achieved with olmesartan or comparators was well maintained during each hour of the 24 h, including the waking period and the night sleep. Treatment with any active drug not only reduced BP mean values but also BPV, in the whole study population as well as in subgroups of patients which are known to be particularly resistant to treatment such as men, elderly or obese patients. The decreasing effect of treatment on BPV is not surprising as it is well known that the degree of decrease in BPV is largely dependent on the extent of BP drop with treatment. Indeed, when we adjusted BPV changes for treatment effect on mean BP, by computing the variation coefficient, the effect of treatment on BPV was almost completely abolished. A multivariate analysis confirmed that the reduction in 24-h BPV with treatment is mainly to be attributed to a reduction in 24-h mean BP level.
Several studies based on 24-h ABPM have shown that antihypertensive drugs decrease ambulatory BPV, a reduction which is proportional to the decrease in mean BP values, suggesting that the effect of antihypertensive treatment on short-term BPV may be largely dependent on the BP lowering, per se [39,40]. In our study we found a collinearity between the reduction in average BP and BPV and in a multivariate analysis the major determinant of the change in BPV was the change in mean BP. However, the relationship was weak (correlation coefficient ranging between 0.06 and 0.19) and thus an independent effect of treatment on BPV cannot be excluded, particularly in case of combination treatments including a dihydropyridine calcium-channel blocker and/or a diuretic. Indeed, the fact that smoothness index and TOVI values, both incorporating information on the effect on mean BP and BPV, were the lowest under placebo and the largest under combination treatment, may suggest that treatment reduced BP but also effectively counteracted the increased BP variability associated with arterial hypertension. The behavior of smoothness index and TOVI in our study also confirms what has been documented in a previous pooled analysis based on another ARB, telmisartan, that these indices provide a robust measure of average BP reduction combined with the assessment of the treatment effect on BPV [21]. Furthermore, although in our study TOVI was characterized by higher values than smoothness index, the differences were only minor, and thus the two estimates may be regarded as interchangeable in their ability to differentiate between treatments. Interestingly, olmesartan always allowed achieving a persistent and smooth BP control over the 24 h, as documented by high values of smoothness index and TOVI in olmesartan-treated patients. The effect was stronger for the dual combination treatment, particularly in the group receiving high drug doses, and even more for the triple combination than for olmesartan monotherapy, indicating a gradual increase of BP lowering and smoothing efficacy.
The duration of BP control was evaluated in our study also by the well known and established TPR. This index exceeded the threshold for adequate BP control (≥0.50) in all treatment groups and was higher in the combination arms. However, a clear differentiation among treatment arms could not be pointed out, as in the case of smoothness index and TOVI. In addition, the TPR was characterized by a substantial placebo effect (0.33 for SBP and 0.42 for DBP), confirming that this popular method for the assessment of the duration of treatment effect is not as sensitive as smoothness index and TOVI for evaluating the 24-h BP-lowering coverage. This is not surprising, as the calculation of TPR concentrates only on two short time intervals, thus potentially missing valuable information relative to the remaining part of the 24 h. It is also affected by variations occurring either spontaneously or resulting from patients’ activities, which make the TPR a rather imprecise estimate of the overall entity and homogeneity of the BP-lowering effect [38].
The largest reduction of BPV with treatment was observed in our study in patients taking a two or three drug combination treatment, particularly those combinations including a long-acting ARB (olmesartan) and a dihydropyridine calcium-channel blocker (amlodipine or azelnidipine) and/or a thiazide diuretic (hydrochlorothiazide). Such a result could be expected as consistent with literature reporting calcium-channel blockers, mainly belonging to the dihydropyridine class, as the most effective antihypertensive agents in reducing BPV, whereas ARBs and ACE inhibitors (the prevalent drug classes included in the monotherapy group of our study), have a modest effect on this parameter [11,41–43]. The finding that the BPV-lowering effect of olmesartan was enhanced in combination with a dihydropyridine calcium-channel blocker and a thiazide diuretic is in line with the results of a recent study, where the combinations of calcium-channel-blockers with diuretics or ARBs on top of other treatments resulted in lower 24-h BPV vs. other combinations [39]. All this evidence taken together seems to indicate these combinations based on long-acting agents, as the most efficient treatments in lowering BPV in the short term.
In our pooled analysis, short-term BPV was estimated following three popular approaches (unweighted SD, weighted SD, and ARV). Although some numeric differences could be observed across the three methods, the trend of the effect was similar regardless of the methodology employed. Based on our results, we cannot figure out which is the best approach for the evaluation of the effect of treatment on BPV. However, we may hypothesize that the weighted SD and the ARV are superior to the unweighted SD, which incorporates the circadian rhythm of BP. Further studies are demanded to specifically address this methodological question.
We should emphasize that the present study has some limitations. The study is based on a post hoc analysis of 10 studies with some differences in designs and inclusion criteria. Although heterogeneity across the different studies and treatments for baseline BPs and patients’ characteristics were taken into account during statistical comparisons we cannot exclude that such differences may have influenced the results. The fact that we performed an individual data analysis, rather than a summary data meta-analysis, allowed us to adjust the results for possible confounding factors and may reinforce the robustness of our estimates, although the persistence of some bias cannot be excluded. Another limitation of our analysis is that the number of patients in each treatment arm varied widely (from 79 to 1410). However, we weighed such heterogeneity in the size of the study groups by an appropriate analytical approach. The duration of treatment also differed across the studies (from 6 to 12 weeks), although the ranges are compatible with the time required for the considered drugs to develop their activity almost completely. Finally, the number of study participants included in this pooled analysis was less than that presented in the original studies, because we applied more stringent quality criteria for the selection of ABPM data. Such an approach was compulsory, owing to the fact that a reliable assessment of BPV and BP control through smoothness index and TOVI may be achieved only when a consistent number of valid readings and hours is available over the 24 h [37].
In conclusion, the present pooled analysis confirms that olmesartan in combination with a dihydropyridine calcium channel blocker and/or thiazide diuretic is effective in producing a larger, more sustained and smoother BP reduction than placebo and monotherapies. Along with a more potent BP reduction the combination treatment also has a beneficial effect on BPV, although such effect is still largely dependent on that on the mean BP level. The achievement of a more homogeneous and sustained BP control, with reduced BP variations, may represent a desirable feature of a given antihypertensive drug treatment, because it may help in preventing the cardiovascular consequence associated with uncontrolled arterial hypertension.
ACKNOWLEDGEMENTS
All authors meet the criteria for authorship for this manuscript and gave final approval to the version to be published. S.O. conceived and performed the post hoc analysis, and wrote the manuscript; K.K., G.B., and G.P. critically revised the results of the analysis and the manuscript.
The work was financially supported by Daiichi-Sankyo Europe GmbH through an unconditional and unrestricted grant.
Conflicts of interest
S.O. received a grant for the data analysis and the preparation of the manuscript. K.K., G.B., and G.P. have occasionally received grants for lectures by the manufacturer of olmesartan.
Supplementary Material
Reviewers’ Summary Evaluations
Reviewer 1
The paper by Omboni and co-workers gives a comprehensive overview about the effects of olmesartan alone or combined with 1–2 drugs, compared to placebo, active monotherapy or active dual therapy, on 24-h BP reduction and 24-h BP variability. BP variability was evaluated by several indexes providing firm evidence regarding the effect of BP reduction on a better BP variability control. This posthoc analysis involves a very large number of subjects and the authors have correctly applied stringent quality criteria for the selection of ABPM data. I think that the paper is interesting as it is well written, easy to follow and well balanced.
Reviewer 2
Whether blood pressure variability, in addition to the absolute value of blood pressure, influences life prognosis is a topic that is still being debated. In this study, the authors compare six different treatment groups. While there are different background characteristics among the groups, the number of the subjects in the study is large, and this paper successfully provides comprehensive knowledge by using indicators of several 24-h blood pressure variability such as the variation coefficient and the smoothness index and treatment-on-variability index.
Footnotes
Abbreviations: ABPM, ambulatory blood pressure monitoring; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ARV, average real variability; BP, blood pressure; BPV, blood pressure variability; TOVI, treatment-on-variability index; TPR, trough-to-peak ratio
REFERENCES
- 1.Mancia G, Parati G, Hennig M, Flatau B, Omboni S, Glavina F, et al. ELSA Investigators. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens 2001; 19:1981–1989. [DOI] [PubMed] [Google Scholar]
- 2.Sega R, Corrao G, Bombelli M, Beltrame L, Facchetti R, Grassi G, et al. Blood pressure variability and organ damage in a general population: results from the PAMELA study (Pressioni Arteriose Monitorate E Loro Associazioni). Hypertension 2002; 39:710–714. [DOI] [PubMed] [Google Scholar]
- 3.Shintani Y, Kikuya M, Hara A, Ohkubo T, Metoki H, Asayama K, et al. Ambulatory blood pressure, blood pressure variability and the prevalence of carotid artery alteration: the Ohasama study. J Hypertens 2007; 25:1704–1710. [DOI] [PubMed] [Google Scholar]
- 4.Madden JM, O’Flynn AM, Fitzgerald AP, Kearney PM. Correlation between short-term blood pressure variability and left-ventricular mass index: a meta-analysis. Hypertens Res 2016; 39:171–177. [DOI] [PubMed] [Google Scholar]
- 5.Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension 2000; 36:901–906. [DOI] [PubMed] [Google Scholar]
- 6.Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, et al. Long-term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension 2007; 49:1265–1270. [DOI] [PubMed] [Google Scholar]
- 7.Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes Investigators. Prognostic value of reading-to-reading blood pressure variability over 24 h in 8938 subjects from 11 populations. Hypertension 2010; 55:1049–1057. [DOI] [PubMed] [Google Scholar]
- 8.Stolarz-Skrzypek K, Thijs L, Li Y, Hansen TW, Boggia J, Kuznetsova T, et al. Short-term blood pressure variability in relation to outcome in the International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome (IDACO). Acta Cardiol 2011; 66:701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010; 375:895–905. [DOI] [PubMed] [Google Scholar]
- 10.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016; 354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. ASCOT-BPLA and MRC Trial Investigators. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol 2010; 9:469–480. [DOI] [PubMed] [Google Scholar]
- 12.Xie HH, Shen FM, Xu LP, Han P, Miao CY, Su DF. Reduction of blood pressure variability by combination therapy in spontaneously hypertensive rats. J Hypertens 2007; 25:2334–2344. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Agnoletti D, Safar ME, Blacher J. Effect of antihypertensive agents on blood pressure variability: the Natrilix SR versus candesartan and amlodipine in the reduction of systolic blood pressure in hypertensive patients (X-CELLENT) study. Hypertension 2011; 58:155–160. [DOI] [PubMed] [Google Scholar]
- 14.Mancia G, Ferrari A, Gregorini L, Parati G, Pomidossi G, Bertinieri G, et al. Blood pressure and heart rate variabilities in normotensive and hypertensive human beings. Circ Res 1983; 53:96–104. [DOI] [PubMed] [Google Scholar]
- 15.Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka-Jaszcz K, et al. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens 2007; 25:2058–2066. [DOI] [PubMed] [Google Scholar]
- 16.Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens 2005; 23:505–511. [DOI] [PubMed] [Google Scholar]
- 17.Mena LJ, Maestre GE, Hansen TW, Thijs L, Liu Y, Boggia J, et al. International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (IDACO) Investigators. How many measurements are needed to estimate blood pressure variability without loss of prognostic information? Am J Hypertens 2014; 27:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stolarz-Skrzypek K, Thijs L, Richart T, Li Y, Hansen TW, Boggia J, et al. Blood pressure variability in relation to outcome in the International Database of Ambulatory blood pressure in relation to Cardiovascular Outcome. Hypertens Res 2010; 33:757–766. [DOI] [PubMed] [Google Scholar]
- 19.Parati G, Omboni S, Rizzoni D, Agabiti-Rosei E, Mancia G. The smoothness index: a new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens 1998; 16:1685–1691. [DOI] [PubMed] [Google Scholar]
- 20.Rizzoni D, Muiesan ML, Salvetti M, Castellano M, Bettoni G, Monteduro C, et al. The smoothness index, but not the trough-to-peak ratio predicts changes in carotid artery wall thickness during antihypertensive treatment. J Hypertens 2001; 19:703–711. [DOI] [PubMed] [Google Scholar]
- 21.Parati G, Dolan E, Ley L, Schumacher H. Impact of antihypertensive combination and monotreatments on blood pressure variability: assessment by old and new indices. Data from a large ambulatory blood pressure monitoring database. J Hypertens 2014; 32:1326–1333. [DOI] [PubMed] [Google Scholar]
- 22.Scott LJ, McCormack PL. Olmesartan medoxomil: a review of its use in the management of hypertension. Drugs 2008; 68:1239–1272. [DOI] [PubMed] [Google Scholar]
- 23.Tocci G, Paneni F, Passerini J, Volpe M. Triple combination therapy to improve blood pressure control: experience with olmesartan-amlodipine-hydrochlorothiazide therapy. Expert Opin Pharmacother 2012; 13:2687–2697. [DOI] [PubMed] [Google Scholar]
- 24.Kreutz R. Olmesartan/amlodipine: a review of its use in the management of hypertension. Vasc Health Risk Manag 2011; 7:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omboni S, Malacco E, Mallion JM, Fabrizzi P, Volpe M. Olmesartan vs. ramipril in elderly hypertensive patients: review of data from two published randomized, double-blind studies. High Blood Press Cardiovasc Prev 2014; 21:1–19. [DOI] [PubMed] [Google Scholar]
- 26.Omboni S, Malacco E, Mallion JM, Volpe M, Zanchetti A. Study Group. Twenty-four hour and early morning blood pressure control of olmesartan vs. ramipril in elderly hypertensive patients: pooled individual data analysis of two randomized, double-blind, parallel-group studies. J Hypertens 2012; 30:1468–1477. [DOI] [PubMed] [Google Scholar]
- 27.Püchler K, Laeis P, Stumpe KO. Blood pressure response, but not adverse event incidence, correlates with dose of angiotensin II antagonist. J Hypertens 2001; 19 (Suppl):S41–S48. [DOI] [PubMed] [Google Scholar]
- 28.Oparil S, Williams D, Chrysant SG, Marbury TC, Neutel J. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich) 2001; 3:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith DH, Dubiel R, Jones M. Use of 24-h ambulatory blood pressure monitoring to assess antihypertensive efficacy: a comparison of olmesartan medoxomil, losartan potassium, valsartan, and irbesartan. Am J Cardiovasc Drugs 2005; 5:41–50. [DOI] [PubMed] [Google Scholar]
- 30.Brunner HR, Stumpe KO, Januszewicz A. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24-h ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Investig 2003; 23:419–430. [DOI] [PubMed] [Google Scholar]
- 31.Brunner HR, Arakawa K. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil in achieving 24-h blood pressure reductions and ambulatory blood pressure goals. Clin Drug Investig 2006; 26:185–193. [DOI] [PubMed] [Google Scholar]
- 32.Malacco E, Omboni S, Volpe M, Auteri A, Zanchetti A. ESPORT Study Group. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens 2010; 28:2342–2350. [DOI] [PubMed] [Google Scholar]
- 33.Mallion JM, Omboni S, Barton J, Van Mieghem W, Narkiewicz K, Panzer PK, et al. Study Group. Antihypertensive efficacy and safety of olmesartan and ramipril in elderly patients with mild to moderate systolic and diastolic essential hypertension. Blood Press Suppl 2011; 1:3–11. [DOI] [PubMed] [Google Scholar]
- 34.Ogihara T, Saruta T, Shimada K, Kuramoto K. A randomized, double-blind, four-arm parallel-group study of the efficacy and safety of azelnidipine and olmesartan medoxomil combination therapy compared with each monotherapy in Japanese patients with essential hypertension: the REZALT study. Hypertens Res 2009; 32:1148–1154. [DOI] [PubMed] [Google Scholar]
- 35.Izzo JL, Jr, Chrysant SG, Kereiakes DJ, Littlejohn Iii T, Oparil S, Melino M, et al. 24-h efficacy and safety of triple-combination therapy with olmesartan, amlodipine, and hydrochlorothiazide: the TRINITY ambulatory blood pressure substudy. J Clin Hypertens (Greenwich) 2011; 13:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther 2010; 32:1252–1269. [DOI] [PubMed] [Google Scholar]
- 37.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 2014; 32:1359–1366. [DOI] [PubMed] [Google Scholar]
- 38.Omboni S, Parati G, Zanchetti A, Mancia G. Calculation of trough:peak ratio of antihypertensive treatment from ambulatory blood pressure: methodological aspects. J Hypertens 1995; 13:1105–1112. [DOI] [PubMed] [Google Scholar]
- 39.Mancia G, Omboni S, Parati G, Ravogli A, Villani A, Zanchetti A. Lack of placebo effect on ambulatory blood pressure. Am J Hypertens 1995; 8:311–315. [DOI] [PubMed] [Google Scholar]
- 40.Parati G, Ochoa JE, Lombardi C, Bilo G. Blood pressure variability: assessment, predictive value, and potential as a therapeutic target. Curr Hypertens Rep 2015; 17:537. [DOI] [PubMed] [Google Scholar]
- 41.Levi-Marpillat N, Macquin-Mavier I, Tropeano AI, Parati G, Maison P. Antihypertensive drug classes have different effects on short-term blood pressure variability in essential hypertension. Hypertens Res 2014; 37:585–590. [DOI] [PubMed] [Google Scholar]
- 42.Hocht C, Del Mauro JS, Bertera FM, Taira CA. Drugs affecting blood pressure variability: an update. Curr Pharm Des 2015; 21:744–755. [DOI] [PubMed] [Google Scholar]
- 43.Eguchi K. Effects of antihypertensive therapy on blood pressure variability. Curr Hypertens Rep 2016; 18:75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


