Abstract
Objective:
The aim of the Advanced Approach to Arterial Stiffness study was to compare arterial stiffness measured simultaneously with two different methods in different age groups of middle-aged and older adults with or without metabolic syndrome (MetS). The specific effects of the different MetS components on arterial stiffness were also studied.
Methods:
This prospective, multicentre, international study included 2224 patients aged 40 years and older, 1664 with and 560 without MetS. Patients were enrolled in 32 centres from 18 European countries affiliated to the International Society of Vascular Health & Aging. Arterial stiffness was evaluated using the cardio-ankle vascular index (CAVI) and the carotid–femoral pulse wave velocity (CF-PWV) in four prespecified age groups: 40–49, 50–59, 60–74, 75–90 years. In this report, we present the baseline data of this study.
Results:
Both CF-PWV and CAVI increased with age, with a higher correlation coefficient for CAVI (comparison of coefficients P < 0.001). Age-adjusted and sex-adjusted values of CF-PWV and CAVI were weakly intercorrelated (r2 = 0.06, P < 0.001). Age-adjusted and sex-adjusted values for CF-PWV but not CAVI were higher in presence of MetS (CF-PWV: 9.57 ± 0.06 vs. 8.65 ± 0.10, P < 0.001; CAVI: 8.34 ± 0.03 vs. 8.29 ± 0.04, P = 0.40; mean ± SEM; MetS vs. no MetS). The absence of an overall effect of MetS on CAVI was related to the heterogeneous effects of the components of MetS on this parameter: CAVI was positively associated with the high glycaemia and high blood pressure components, whereas lacked significant associations with the HDL and triglycerides components while exhibiting a negative association with the overweight component. In contrast, all five MetS components showed positive associations with CF-PWV.
Conclusion:
This large European multicentre study reveals a differential impact of MetS and age on CAVI and CF-PWV and suggests that age may have a more pronounced effect on CAVI, whereas MetS increases CF-PWV but not CAVI. This important finding may be due to heterogeneous effects of MetS components on CAVI. The clinical significance of these original results will be assessed during the longitudinal phase of the study.
Keywords: aging, arterial stiffness, cardio-ankle vascular index, large artery, metabolic syndrome, pulse wave velocity
INTRODUCTION
Changes in body composition in men and women occur with aging with exponential loss of lean tissues [1]. The prevalence of cardiovascular risk factors and metabolic syndrome (MetS) dramatically increases with age at least until the age of 60 years [2]. This is observed in both developed [3] and even more in developing countries [4]. Therefore, MetS is becoming a pandemic disease with major consequences on public health.
The influence of the different metabolic and haemodynamic components of MetS on arterial health has been assessed in different clinical studies. A relationship has been demonstrated between the presence of MetS and progression of arterial stiffness of the aorta and other large arteries [5,6]. In a previous longitudinal study, an acceleration of arterial aging over a period of 7 years was shown as a function of the number of components of the MetS [7]. In addition, the presence of nonalcoholic fatty liver disease has also been shown to be associated with increased pulse wave velocity (PWV) especially when this condition was associated with MetS [8]. However, it is unknown whether MetS as a whole and its different components affect arterial stiffness to the same extent in younger and elderly individuals.
Carotid–femoral pulse wave velocity (CF-PWV) has been considered as the ‘gold standard’ [9–12] measurement of arterial stiffness, due to the large number of studies identifying PWV as an independent predictor of total mortality and cardiovascular events [13–16]. However, despite the importance of PWV, this method presents a number of limitations and sources of inaccuracy. First, the determination of the transit distance travelled by the pressure waves using body surface measurements may not reliably represent the true length of the arterial segment, especially with obesity and when the arteries become more tortuous with age. Second, CF-PWV is not a simple unidirectional path length for the pulse wave; therefore, determination of the actual travelled path length is somewhat approximate [11]. Moreover, several confounding factors (physiological and technical) for PWV have been reported. The most significant physiological factors affecting PWV are blood pressure (BP) and heart rate, whereas technical confounders include the algorithm of the used device and the considered arterial pathway (carotid–femoral, brachial–ankle etc.) [10–12].
The cardio-ankle vascular index (CAVI) [17,18] assesses arterial mechanical and elastic properties by means of the beta stiffness index, which is relatively independent of BP levels at the time of the measurement. The use of CAVI for cardiovascular risk assessment was first introduced in Asia [17–20], and its use has gradually increased in recent years in Europe [21].
In this report, we present the baseline data of the TRIPLE A-Stiffness study, which is a multicentre longitudinal study, performed in a large European population. The analysis of the baseline data presented herein aimed to establish the values of arterial stiffness measured simultaneously with CF-PWV and CAVI in four different age groups of middle-aged and older adults according to the presence or absence of MetS.
METHODS
Study design and patients
This is an international multicentre prospective longitudinal study with three scheduled visits at baseline and after 2 and 5 years of follow-up. In the present report, we present the analysis of the baseline data.
Patients aged 40 years and older were recruited in 32 outpatient centres participating in the TRIPLE A-Stiffness study network from 18 countries. All were followed in outpatient clinics for prevention check-up and/or monitoring of cardiovascular risk factors. To assess the influence of age on the effects of MetS on arterial stiffness, four age groups were prespecified: Group 1: 40–49 years, Group 2: 50–59 years, Group 3: 60–74 years and Group 4: at least 75 years.
Noninclusion criteria were factors potentially impairing the quality of CAVI or PWV measurements or render PWV recording unreliable, namely: known significant peripheral arterial disease, ankle–brachial index less than 0.9 (even unilateral), limb amputation; history of vascular surgery of the carotid artery, femoral artery or aorta; BMI more than 40 kg/m2; atrial fibrillation and/or other major arrhythmia; and pregnancy.
Informed written consent was obtained in all included patients. The study is registered at www.clinicaltrials.gov with the ID number: NCT02318628.
Investigators collected the data from clinical examination, BP, PWV and CAVI. Measurements were performed after 5–10-min rest to obtain a steady haemodynamic state. At the baseline visit, the following tasks were performed:
-
(1)
Blood sample for bioassays or reporting of the results of the laboratory examination performed at ±12 weeks of the visit date.
-
(2)
Physical examination, disease history and treatments.
-
(3)
Assessment of MetS components.
-
(4)
Measurement of SBP, DBP and pulse pressure using a validated automatic electronic device.
-
(5)
ECG.
-
(6)
Measurement of PWV and CAVI as described below.
Metabolic syndrome assessment
MetS was defined using the National Cholesterol Education Program – Adult treatment Panel revised version (NCEP-R) definition [22,23]; all patients were assessed for the presence of MetS requiring the association of three of the following five criteria:
-
(1)
abdominal obesity with waist circumference more than 102 cm in men and more than 88 cm in women
-
(2)
triglycerides at least 150 mg/dl, or presence of a specific treatment for lipid abnormalities
-
(3)
HDL cholesterol less than 40 mg/dl in men and less than 50 mg/dl in women, or presence of a specific treatment for lipid abnormalities
-
(4)
SBP at least 130 or DBP at least 85 mmHg, or presence of antihypertensive treatment
-
(5)
fasting glucose at least 100 mg/dl, or presence of drug treatment for increased glucose.
Blood pressure measurements
BP was measured in clinic according to the European Society of Hypertension guidelines using validated equipment that meets certification criteria [24]. Two or more readings were averaged. If the first two readings differed by more than 15 mmHg, additional readings were obtained and averaged. The average values of the BP measurements were reported in the electronic case report form. Ambulatory BP monitoring (ABPM) was also performed in those patients who agreed. The ABPM data are currently under analysis.
Pulse wave velocity
Measurements of CF-PWV were performed using a validated automatic device [Complior (ALAM Medical, Pantin, France), Sphygmocor (AtCor Medical, Sydney, Australia) and PulsePen (Diatecne, Milan, Italy)]. Because of the use of several devices, normalization of the measurement values was performed according to the European Experts recommendations [12].
Cardio-ankle vascular index
The cardio-ankle vascular index, CAVI, was measured and automatically calculated using the VaSera system (Fukuda Denshi Co, Japan) as per the manufacturer's recommendations. CAVI requires placement of ECG electrodes on both wrists, a microphone for phonocardiography on the sternum, and four BP cuffs wrapped around the four limbs. The upper arm and ankle pulse waves, as well as BP are measured. CAVI is disregarded if the ankle–brachial index (ABI) is less than 0.9 [17].
Ankle–brachial index
ABI was automatically obtained from the CAVI measurements performed with the VaSera device (Fukuda Denshi Co). It is calculated bilaterally as the ratio of SBP in the ankle to the SBP in the arm [17].
Plasma measurements
Glycaemia, total cholesterol, triglycerides, LDL cholesterol, HDL cholesterol, serum creatinine, creatinine clearance (modification of diet in renal disease) and haemoglobin A1c were measured ±12 weeks of the study visit.
Statistical analysis
Descriptive values are expressed as mean ± SD, median and interquartile range, or number or percentage. For the comparisons among groups with or without MetS, the Wilcoxon rank-sum test was used for continuous variables and the chi-square test for discrete variables. Effects of each MetS component on sex-adjusted and age-adjusted CF-PWV and CAVI values were assessed with the Tukey–Kramer post-hoc test. Interaction between presence of MetS and age group on CF-PWV or CAVI was assessed with multivariate trend analysis of variance and Tukey–Kramer post-hoc test. The same test was used for studying the interaction between waist circumference component and age group on CF-PWV or CAVI.
CF-PWV and CAVI variations according to age, sex and the five components of the MetS were conducted using multivariate analysis. In the final multivariate model, only the variables found to be significant at the P less than 0.10 level were retained.
Pearson's correlation was used to study the relationship between age and CF-PWV or CAVI and between age-adjusted and sex-adjusted values of CAVI and CF-PWV. The Hotelling–Williams test was used for comparison of the correlation coefficients between age and CF-PWV vs. age and CAVI.
A P value less than 0.05 was regarded as statistically significant. Statistical analyses were performed using the NCSS 9 statistical software package (Kaysville, Utah, USA).
RESULTS
General characteristics of the population
A total of 2348 patients were enrolled in 32 centres from 18 countries. A total of 124 patients were excluded from the present analysis due to either missing information on elements for the NCEP-2005 MetS classification or lacking CAVI measurements. Following classification by the NCEP-R 2005 MetS definition, 1664 patients (74.8%) were classified with MetS and 560 without.
The main clinical characteristics of the entire population and the two subgroups according to the presence of MetS are shown in Table 1. Mean age of this population was 60 ± 11 years (53% women). Patients with MetS were older, had more often a family history of cardiovascular disease (CVD) and presented a higher prevalence of cardiovascular risk factors, stroke, myocardial infarction, angina, heart failure and renal failure. Patients with MetS had higher PWV and CAVI values, with this difference being maintained after adjustment for age and sex for PWV (P < 0.0001) but not for CAVI (P = 0.40)
TABLE 1.
Baseline characteristics of the study population
| All | MetS-no | MetS-yes | |
| Number | 2224 | 560 | 1664 |
| Age (years) | 60 ± 11 | 57 ± 11 | 61 ± 11* |
| Women (%) | 53% | 54% | 52% |
| Waist circumference (cm) | 101 ± 13 | 91 ± 12 | 104 ± 12* |
| Glycaemia (mg/dl) | 108 ± 34 | 93 ± 19 | 114 ± 36* |
| HDL (mg/dl) | 56 ± 26 | 62 ± 18 | 54 ± 28* |
| Triglycerides (mg/dl) | 144 ± 82 | 101 ± 43 | 159 ± 87* |
| SBP (mmHg) | 140 ± 18 | 135 ± 17 | 142 ± 18* |
| DBP (mmHg) | 85 ± 11 | 83 ± 11 | 85 ± 11* |
| PP (mmHg) | 55 ± 14 | 52 ± 13 | 56 ± 14* |
| HR (b/min) | 69 ± 11 | 68 ± 11 | 69 ± 11** |
| Antidiabetic med. | 20% | 2% | 26%* |
| Hypolipidaemic med. | 49% | 3% | 65%* |
| Antihypertensive med. | 75% | 45% | 85%* |
| Antihypert. drugs (number) | 1.68 ± 1.32 | 0.77 ± 1.05 | 1.99 ± 1.26* |
| Family history CVD | 40% | 38% | 40% |
| Obesity | 50% | 22% | 59%* |
| Hypertension | 81% | 60% | 89%* |
| Dyslipidaemia | 74% | 42% | 85%* |
| Diabetes | 25% | 5% | 32%* |
| Stroke | 3.5% | 2.1% | 3.9%** |
| Myocardial infarction | 7.6% | 2.0% | 9.5%* |
| Angina | 13% | 4% | 16%* |
| Heart failure | 9.2% | 2.9% | 11.4%* |
| Renal Failure | 5.4% | 2.9% | 6.3%*** |
| CF-PWV (m/s) | 9.33 ± 2.51 | 8.42 ± 2.09 | 9.65 ± 2.57* |
| CAVI (arbitrary units) | 8.32 ± 1.34 | 8.06 ± 1.36 | 8.41 ± 1.32* |
MetS-no, absence of metabolic syndrome; MetS-yes, presence of metabolic syndrome. Comparison between MetS-no and MetS-yes. CAVI, cardiac-ankle vascular index; CF-PWV, carotid–femoral pulse wave velocity; CVD, cardiovascular disease; HR, heart rate; PP, pulse pressure; WC, waist circumference.
*P < 0.001.
**P < 0.05.
***P < 0.01.
Relationship between age and arterial stiffness
Figure 1 shows the effects of age on arterial stiffness measured with CF-PWV and CAVI in men and women. A stronger correlation coefficient was observed with CAVI (r2 = 0.37; P < 0.0001), y = 0.07 + 3.87 than with PWV (r2 = 0.14; P < 0.0001), y = 0.09 + 4.39. Comparison of the correlation coefficients using the Hotelling–Williams test showed that the age/CAVI relationship was statistically stronger (P < 0.001) than the age/PWV relationship. Effects of age on arterial stiffness did not differ between men and women.
FIGURE 1.
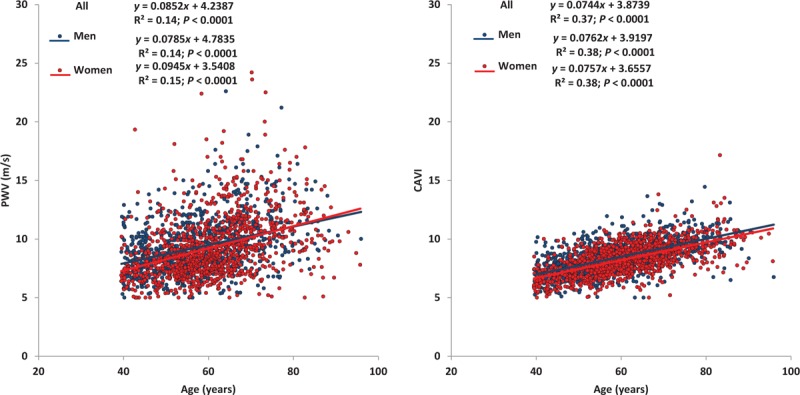
Evolution of pulse wave velocity and cardio-ankle vascular index values with age.
Metabolic syndrome and arterial stiffness
Age-adjusted and sex-adjusted values for CF-PWV but not CAVI were higher in presence of MetS: for CF-PWV, values were 9.57 ± 0.06 vs. 8.65 ± 0.10 m/s, (mean ± SEM; MetS vs. no MetS; P < 0.001); for CAVI, values were 8.34 ± 0.03 vs. 8.29 ± 0.04 (P = 0.40), respectively. The analysis by sex also showed differential effects of MetS on CF-PWV and CAVI (Fig. 2). Both measurements of arterial stiffness were lower in women than in men although the sex-effect was stronger with CAVI (see below for multivariate analyses).
FIGURE 2.
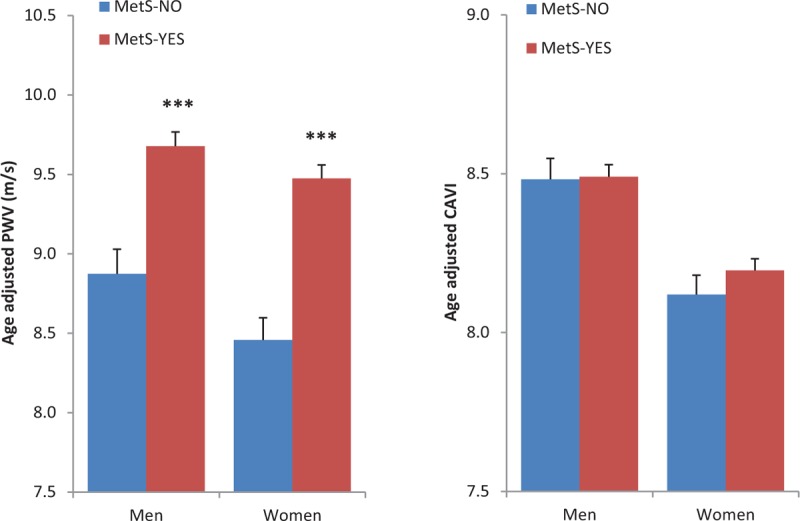
Effects of metabolic syndrome and sex-adjusted on age-adjusted pulse wave velocity and cardio-ankle vascular index values.
Figure 3 shows the effects of MetS on sex-adjusted PWV and CAVI values in the four predefined age-groups. For PWV, mean values in patients with vs. without MetS age group were 8.50 ± 0.15 vs. 7.65 ± 0.19 m/s in the less than 50 age group, 8.98 ± 0.11 vs. 8.23 ± 0.18 m/s in the 50–59 age group, 10.25 ± 0.10 vs. 9.09 ± 0.20 m/s in the 60–74 age group and 11.23 ± 0.20 vs. 10.06 ± 0.38 m/s in the at least 75 age group. For CAVI, the same analysis yielded the following values: 7.24 ± 0.06 vs. 7.02 ± 0.08 in the less than 50 age group, 7.98 ± 0.05 vs. 7.99 ± 0.08 in the 50–59 age group, 8.85 ± 0.04 vs. 8.86 ± 0.09 in the 60–74 age group and 9.84 ± 0.08 vs. 9.69 ± 0.17 in the at least 75 age group (values are the mean ± SEM). The interaction term between age group/MetS on PWV or CAVI was not statistically significant (P = 0.14 for PWV and P = 0.38 for CAVI).
FIGURE 3.
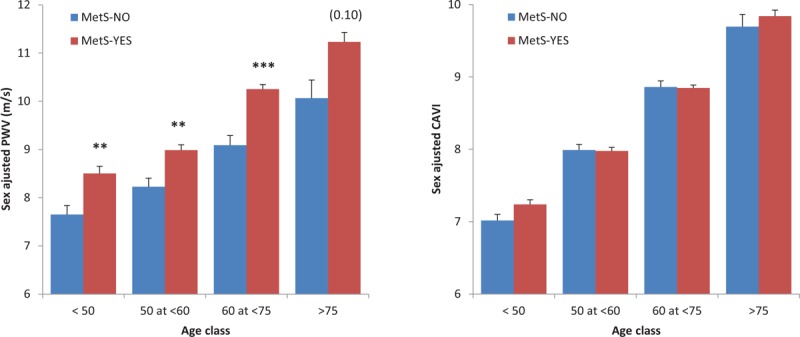
Effects of metabolic syndrome on sex-adjusted pulse wave velocity and cardio-ankle vascular index values according to age groups.
Effects of metabolic syndrome components on pulse wave velocity and cardio-ankle vascular index
Figure 4 shows the effects of each of the five MetS components on age-adjusted and sex-adjusted PWV and CAVI values. The presence of any of these five components was associated with higher PWV values (all P < 0.0001). By contrast, the effects were much more heterogeneous with regard to CAVI. Glycaemia and BP components were associated with higher CAVI values (P < 0.0001 and <0.02, respectively) whereas the HDL or triglyceride component did not influence CAVI. Finally, presence of high waist circumference was associated with lower age-adjusted and sex-adjusted CAVI values.
FIGURE 4.
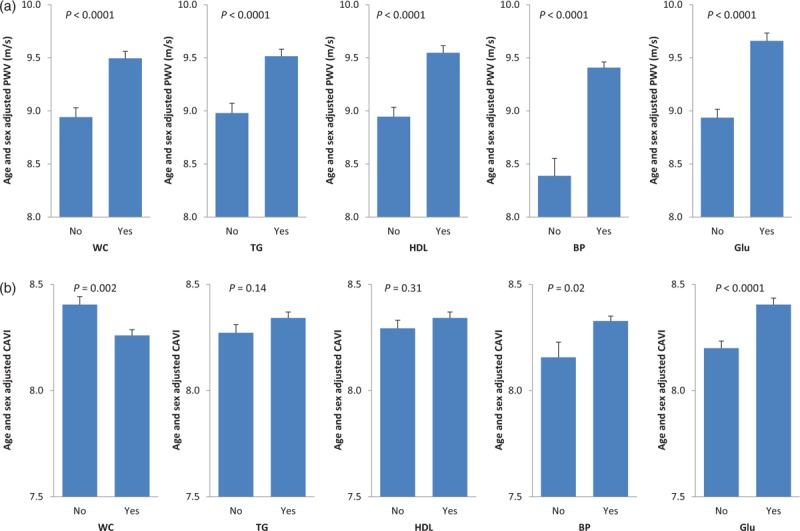
Effects of each metabolic syndrome component on sex-adjusted and age-adjusted pulse wave velocity (a) and cardio-ankle vascular index (b) values.
As there are several interactions between the different MetS components, the arterial effects of the five MetS components were also tested using a multiple regression analysis including age and sex. The results of this analysis are shown in Table 2. Older age and presence of the BP, glycaemia and HDL components were independent determinants of high CF-PWV. Sex and the two remaining components of the MetS (waist circumference and triglycerides) did not show an independent effect on PWV. On the other hand, older age, male sex, presence of the BP and glycaemia components and absence of the overweight component (i.e. lower waist circumference) were all independent determinants of higher CAVI values.
TABLE 2.
Multivariate analysis in explaining pulse wave velocity and cardio-ankle vascular index variations according to age, sex and each of the five metabolic syndrome components
| PWV | R2 (%) | Reg Coeff ± SEM | |
| Age (years) | 11.2 | 0.076 ± 0.005 | <0.0001 |
| Women | 0.2 | −0.19 ± 0.11 | 0.09 |
| BP (yes) | 1.0 | 0.81 ± 0.19 | <0.0001 |
| Glu (yes) | 1.4 | 0.57 ± 0.11 | <0.0001 |
| HDL (yes) | 0.7 | 0.42 ± 0.12 | 0.0003 |
| WC (yes) | – | – | 0.22 |
| TG (yes) | – | – | 0.29 |
| Model | 13.7 |
| CAVI | R2 (%) | Reg Coeff ± SEM | |
| Age (years) | 35.6 | 0.074 ± 0.002 | <0.0001 |
| Women | 1.2 | −0.24 ± 0.05 | <0.0001 |
| BP (yes) | 0.2 | 0.18 ± 0.08 | 0.03 |
| Glu (yes) | 1.2 | 0.25 ± 0.05 | <0.0001 |
| WC (yes) | 1.1 | −0.25 ± 0.05 | <0.0001 |
| TG (yes) | – | – | 0.29 |
| HDL (yes) | – | – | 0.49 |
| Model | 36.6 |
Yes means presence of this MetS component. BP, blood pressure; Glu, glucose; MetS, metabolic syndrome; Reg Coeff, regression coefficient; TG, triglycerides; WC, waist circumference.
The interaction between each of the five MetS components was subsequently tested within the four age groups on the effects on PWV and CAVI. The only significant age/MetS component interaction regarded the effects of waist circumference on CAVI (P = 0.04). To further investigate this interaction, the effects of waist circumference on CAVI were analysed according to both sex and age groups (Fig. 5). The age/waist circumference interaction on CAVI was found to be strong in women with lower CAVI values in the presence of high waist circumference in the older group of patients.
FIGURE 5.
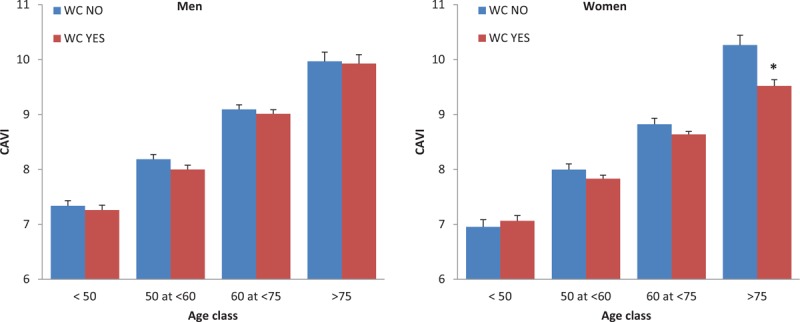
Effects of the waist circumference on cardio-ankle vascular index values according to sex and age groups.
All of the aforementioned differences in the determination of CAVI and PWV led as expected to a low correlation between the age-adjusted and sex-adjusted values of these variables (Fig. 6).
FIGURE 6.
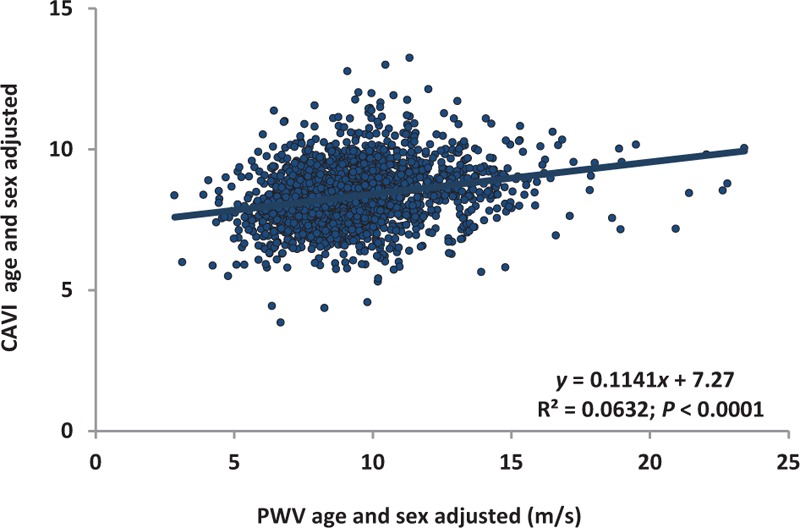
Relationship between age-adjusted and sex-adjusted pulse wave velocity values vs. cardio-ankle vascular index values.
DISCUSSION
This large European multicenter study reports the values of arterial stiffness measured simultaneously with two different methods, namely CF-PWV and CAVI, in four different age groups of patients aged 40 and older with or without MetS.
Measuring arterial stiffness with carotid–femoral pulse wave velocity and cardio-ankle vascular index
Although CF-PWV and CAVI both represent measurements of arterial stiffness, their fundamental principles differ, for mainly two reasons:
-
(1)
PWV is based on the Moens–Korteweg/Bramwell–Hill Eqs. [25] and measures the velocity of the pulse waves between two arterial sites. It is well known that this velocity is dependent on both arterial structure/function but also on distension pressure at the time of the measurement [26–28], thereby explaining a more pronounced influence of BP variations during PWV assessment. On the other hand, CAVI corresponds to the β index, which reflects the slope of the relationship between changes in pressure and changes in volume [17–20], thus explaining why this index is much less dependent on acute pressure variations at the time of the measurements. The present results showing a less pronounced effect of BP levels on CAVI than on CF-PWV goes along previous observations on the same topic [18,21,29]. Recently, Spronck et al.[30] concluded that CAVI as it is currently implemented differs from the β0 stiffness index and is inherently BP-dependent, thus potentially leading to erroneous conclusions in arterial stiffness; these conclusions have been debated in a recent reply by Shirai et al.[31] who showed that the two arterial mathematical methods (CAVI and β0) were not different and concluded that the influence of BP levels on CAVI was very weak. The results of the current study confirm the relative ‘pressure-independency’ of the CAVI. It is however important to clarify that both CF-PWV and CAVI are influenced by the prevalence of hypertension, as chronic hypertension modifies the arterial structure and function increasing arterial stiffness. Therefore, the relative BP-independence of CAVI as compared with CF-PWV refers to the BP at the time of measurement but not the chronic BP levels over time.
-
(2)
CF-PWV measures arterial stiffness primarily of the descending aorta, whereas CAVI estimates stiffness of a more general territory including ascending aorta, aortic arch, descending aorta and lower limb arteries [32].
These two differences may explain why the correlation between CF-PWV and CAVI, although statistically significant, showed a weak regression coefficient (r2 = 0.18). Our results corroborate those of previous studies [21] performed in smaller number of patients and reporting low correlation coefficients between CF-PWV and CAVI.
Effects of age and sex on cardio-ankle vascular index and carotid–femoral pulse wave velocity
Several studies have established that age is associated with arterial stiffness in normal patients and patients with various conditions for both CF-PWV and CAVI. Moreover, several experts suggested considering normal values of arterial stiffness according to age [12]. The results of our study showed in this specific population that both CF-PWV and CAVI were correlated with age although with a stronger correlation coefficient with CAVI (r2 = 0.37) than with CF-PWV (r2 = 0.14). Furthermore, in the current study, effects of sex (higher arterial stiffness in men than in women) were detected with CAVI but not with CF-PWV. The fact that CAVI explores a larger vascular territory as stated above could explain such differences between the two methods.
Effects of metabolic syndrome on cardio-ankle vascular index and carotid–femoral pulse wave velocity
Our results indicate differential effects according to the different MetS components as well as according to the different methods for evaluating vascular stiffness, with important implications for risk stratification of patients with and without MetS.
Previous studies have shown that MetS is associated with increased cardiovascular organ damage and acceleration of age-related arterial changes such as arterial stiffness mainly assessed with PWV [6,7]. The current study corroborates these previous reports and show that patients with MetS exhibited significantly higher CF-PWV in all age groups. In contrast, CAVI was not altered in the presence of MetS, thereby further reinforcing that these two methods reflect different aspects of arterial stiffness. In the current study, certain MetS components were found to have different, even opposite, effects on CF-PWV and CAVI. Thus, whereas all five MetS components were positively associated with age-adjusted and sex-adjusted CF-PWV, only high glycaemia and high BP components exhibited a positive association with CAVI. Significantly, the lipidaemia criteria (low HDL and high triglyceride components) were not associated with CAVI, and even more unexpectedly, high waist circumference was associated with lower CAVI values. The multivariate analysis confirmed these differences in the effects of MetS components, with high-BP and high-glycaemia as the common independent predictor for increased PWV and CAVI.
The explanation for the discordant effects of waist circumference on CF-PWV and CAVI remains to be established. Although previous studies have suggested that overweight/obesity is not associated with increased CAVI [33], this is the first report to raise the notion of opposite effects of waist circumference on CF-PWV and CAVI. It should however be acknowledged that we cannot exclude that abdominal obesity may lead to an over-estimation of the distance in the CF-PWV formula, leading to an overestimation of the impact of waist circumference on arterial stiffness by CF-PWV [12]. However, the different centres participating in the study used the appropriate method for distance measurements to avoid this caveat.
The role of overweight and waist circumference in MetS remains a matter of debate. In the International Diabetes Federation definition, the presence of central obesity is an obligatory criterion for the presence of MetS [34], although this is not the case for most other guidelines and consensus articles [22,23]. Moreover, the waist circumference thresholds differ according to the various classifications and different ethnic origins [23]. Although the present analysis was not designed to answer this question, the ongoing longitudinal component of the current study should provide answers to these questions by assessing the long-term evolution of CAVI and CF-PWV values but also by recording the cardiovascular events in this population.
Interest of cardio-ankle vascular index for the evaluation of arterial stiffness
In Europe, CF-PWV is used in several specialized centres and recommended by scientific societies as the gold standard to assess arterial stiffness [9–12]. The results of this study show that CAVI provides complementary information to that provided by PWV. Clinical studies now show that CAVI could represent a useful tool for the assessment of arterial health in large multicenter studies, owing to several advantages related to feasibility, reproducibility and facility of use, with little or no observer bias [17,21,35].
Beyond these practical aspects, the interest of CAVI is also related to the minimal impact of BP values at the time of the measurements, which allows a better assessment of the intrinsic elastic properties of the vascular system. This may be of major interest in some subgroups of patients especially in very old patients in whom BP levels are very often influenced by comorbidities and poor general condition [36].
Limitations of the study: Some parameters of clinical interest were not taken into account in the current study, namely: the cohorts included patients both with and without prevalent CVD; cardiac function was unknown and pharmacological treatments were not taken into consideration. No specific sampling of C reactive protein or other vascular biomarkers was performed. However, this also implies a generalizability of the results to unselected patient populations.
In conclusion, the current large European multicentre study shows the differential impact of MetS and age on CAVI and CF-PWV. Age had a more pronounced effect on CAVI, whereas MetS increased CF-PWV but not CAVI. This important finding is probably due to the heterogeneous effects of the MetS components on CAVI. The clinical significance of these original results will be assessed during the longitudinal phase of the study.
ACKNOWLEDGEMENTS
The study was carried out thanks to the valuable contribution of numerous investigators. We would like to express our sincere thanks and gratitude to Siranush Aroyan, Olga Paulava, Olga Bourko, Tatiana Nechesova, Bojan Jelakovic, Jelena Kos, Tajana Željković Vrkić, Vedran Premužić, Mario Laganović, Ivan Pećin, Zoran Miovski, Alena Krajcoviechiva, Pavel Sulc, Anna Kearney-Schwartz, Alexandra Yannoutsos, Anastasios Kollias, Marilena Zeniodi, Endre Kolossvary, Reka Onody, Ildiko Szabo, Szabolcs Lengyel, Mauro Zamboni, Gabrielle Comellato, Andrea Rossi, Nicola di Daniele, Manfredi Tesauro, Valentina Rovella, Selim Berkinbaev, Aisulu Musagalieva, Amina Rakisheva, Andrejs Erglis, Marina Berzina, Milana Zabunova, Marija Tokmanceva, Gita Rancane, Jelizaveta Burca, Danuta Czarnecka, Tomasz Grodzicki, Jerzy Gazowski, Alsu Zairova, Oksana Mikhailova, Olesya Dogotar, Elena Sharanova, Oxana Rotar, Asiiat Alieva, Alexandr Orlov, Maria Boyarivova, Nadejda Zvartau, Daniela Tasic, Dacha Dragisic, Manuel A. Gomez Marcos, Ruth Marti Lluch, Maria Garcia Gil, Oksana Rekovets, Svitlana Kushnir, Anna Dobrokhod, Olena Torbas, Yuriy Rudyck.
We thank Mr Pierre Pothier for language review and stimulating discussions.
Sponsoring: The study was carried by the International Society of Vascular Health – ISVH as main sponsor. Cosponsorings were obtained from: Fukuda Denshi Co Ltd., Japan which provided the CAVI devices for certain investigation centres; e-CoreLab, France which supported the study follow-up, electronic case report forms and data management; BPLab, Russia which provided 24-h ABPM devices to certain investigation centres.
Conflicts of interest
There are no conflicts of interest.
Reviewer's Summary Evaluation
Reviewer 2
This study assesses the association of stiffness indices measured as carotid-femoral pulse wave velocity (cfPWV, pressure dependent) and cardio-ankle vascular index (CAVI, relatively pressure independent) with metabolic syndrome (Mets). Baseline results highlight the difference in sensitivity of cfPWV and CAVI to age and blood pressure as well as to components of Mets. A future follow up study in the same cohort aims to confirm whether CAVI gives additional prognostic information in relation to intrinsic vascular aging in Mets, which is not obtained by cfPWV. The effect of correcting CAVI for a potential theoretical pressure dependency due to the variation in the ’reference’ pressure, as required in the computation of CAVI, has not been explicitly assessed.
Footnotes
Abbreviations: ABPM, ambulatory blood pressure monitoring; BP, blood pressure; CAVI, cardio-ankle vascular index; CF-PWV, carotid–femoral pulse wave velocity; MetS, metabolic syndrome; NCEP, National Cholesterol Education Program; PP, pulse pressure; PWV, pulse wave velocity
REFERENCES
- 1.Genton L, Karsegard VL, Chevalley T, Kossovsky MP, Darmon P, Pichard C. Body composition changes over 9 years in healthy elderly subjects and impact of physical activity. Clin Nutr 2011; 30:436–442. [DOI] [PubMed] [Google Scholar]
- 2.Akbulut G, Koksal E, Bilici S, Acar Tek N, Yildiran H, Karadag MG, Sanlier N. Metabolic syndrome (MS) in elderly: a cross sectional survey. Arch Gerontol Geriatr 2011; 53:e263–e266. [DOI] [PubMed] [Google Scholar]
- 3.Pannier B, Thomas F, Eschwege E, Bean K, Benetos A, Leocmach Y, et al. Cardiovascular risk markers associated with the metabolic syndrome in a large French population: the ‘SYMFONIE’ study. Diabetes Metab 2006; 32:467–474. [DOI] [PubMed] [Google Scholar]
- 4.Temmar T, Watfa G, Joly L, Kearney-Schwartz A, Youcef M, Bensalah S, et al. Elderly Algerian women lose their sex-advantage in terms of arterial stiffness and cardiovascular profile. J Hypertens 2013; 31:2244–2250. [DOI] [PubMed] [Google Scholar]
- 5.Henry RM, Kostense PJ, Spijkerman AM, Dekker JM, Nijpels G, Heine RJ, et al. Arterial stiffness increases with deteriorating glucose tolerance status: the Hoorn Study. Circulation 2003; 107:2089–2095. [DOI] [PubMed] [Google Scholar]
- 6.Scuteri A, Najjar SS, Orru M, Usala G, Piras MG, Ferrucci L, et al. The central arterial burden of the metabolic syndrome is similar in men and women: the SardiNIA Study. Eur Heart J 2010; 31:602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safar ME, Thomas F, Blacher J, Nzietchueng R, Bureau JM, Pannier B, Benetos A. Metabolic syndrome and age-related progression of aortic stiffness. J Am Coll Cardiol 2006; 47:72–75. [DOI] [PubMed] [Google Scholar]
- 8.Salvi P, Ruffini R, Agnoletti D, Magnani E, Pagliarani G, Comandini, et al. Increased arterial stiffness in nonalcoholic fatty liver disease: the Cardio-GOOSE study. J Hypertens 2010; 28:1699–1707. [DOI] [PubMed] [Google Scholar]
- 9.Asmar R. Factors influencing pulse wave velocity. Arterial stiffness and pulse wave velocity. Clinical applications Editions scientifiques et médicalesParis:Elsevier SAS; 1999. 57–86. Chap. IV. [Google Scholar]
- 10.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert Consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 11.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 2015; 66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values’. The Reference Values for Arterial Stiffness Collaboration. Eur Heart J 2010; 31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999; 99:2434–2439. [DOI] [PubMed] [Google Scholar]
- 14.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension 2002; 39:10–15. [DOI] [PubMed] [Google Scholar]
- 15.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 2001; 37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 16.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 2006; 113:657–663. [DOI] [PubMed] [Google Scholar]
- 17.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter: cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006; 13:101–107. [DOI] [PubMed] [Google Scholar]
- 18.Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, et al. Contradictory effects of β1- and α1-aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)-the independency of CAVI from blood pressure. J Atheroscler Thromb 2011; 18:49–55. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T, Shimada M, Ishida H, Matsuda N, Fujiu A, Ando Y, Nitta K. Relation of stiffness parameter beta to carotid arteriosclerosis and silent cerebral infarction in patients on chronic hemodialysis. Int Urol Nephrol 2009; 41:739–745. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi k, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: theory and applications. J Hypertens 2015; 33:1742–1757. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Sanchez L, Garcia-Ortiz L, Patino-Alonso M, Recio-Rodriguez J, Frontera G, Ramos R, et al. MARK group. The association between the cardio ankle vascular index and other parameters of vascular structure and function in Caucasian adults: the MARK study. J Atheroscler Thromb 2015; 22:901–911. [DOI] [PubMed] [Google Scholar]
- 22.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. AHA/NHLBI Scientific Statement. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 24.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. Authors/Task Force. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 25.Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc London Series B 1926; 93:298–306. [Google Scholar]
- 26.Nichols WW, O’Rourke MF, Vlachopoulos C. McDonald's blood flow in arteries. Theoretical, experimental and clinical principles. 6th ed.London:Hodder Arnold; 2011. [Google Scholar]
- 27.Salvi P, Palombo C, Salvi GM, Labat C, Parati G, Benetos A. Left ventricular ejection time, not heart rate, is an independent correlate of aortic pulse wave velocity. J Appl Physiol 2013; 115:1610–1617. [DOI] [PubMed] [Google Scholar]
- 28.Spronck B, Heusinkveld MHG, Vanmolkot FH, Op’t Roodt J, Hermeling E, Delhass T, et al. Pressure-dependence of arterial stiffness: potential clinical implications. J Hypertens 2015; 33:330–338. [DOI] [PubMed] [Google Scholar]
- 29.Benetos A. Assessment of arterial stiffness in an older population: the interest of the cardio-ankle vascular index (CAVI). Eur Heart J Suppl 2017; 19:B11–B16. [Google Scholar]
- 30.Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens 2017; 35:98–104. [DOI] [PubMed] [Google Scholar]
- 31.Shirai K, Shimizu K, Takata M, Suzuki K. Independency of the cardio-ankle vascular index from blood pressure at the time of measurement. J Hypertens 2017; 35:1521–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asmar R. Principles & usefulness of the cardio ankle vascular index (CAVI). A new global arterial stiffness index. Eur Heart J Suppl 2017; 19:B4–B10. [Google Scholar]
- 33.Park HE, Choi SY, Kim HS, Kim MK, Cho SH, Oh BH. Epicardial fat reflects arterial stiffness: assessment using 256-slice multidetector coronary computed tomography and cardio-ankle vascular index. J Atheroscler Thromb 2012; 19:570–576. [DOI] [PubMed] [Google Scholar]
- 34.Alberti KG, Zimmet P, Shaw J. IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet 2005; 366:1059–1062. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Cordes M, Recio-Rodriguez JI, García-Ortiz L, Hanssen H, Schmidt-Trucksäss A. Diurnal variation of arterial stiffness in healthy individuals of different ages and patients with heart disease. Scand J Clin Lab Invest 2014; 74:155–162. [DOI] [PubMed] [Google Scholar]
- 36.Benetos A, Gautier S, Labat C, Salvi P, Valbusa F, Marino F, et al. Mortality and cardiovascular events are best predicted by low central/periph-eral pulse pressure amplification but not by high blood pressure levels in elderly nursing home subjects: the PARTAGE. J Am Coll Cardiol 2012; 60:1503–2151. [DOI] [PubMed] [Google Scholar]


