Abstract
Aims
Clopidogrel has, for long time, been accepted as the standard treatment for patients who have undergone a percutaneous coronary intervention (PCI). The introduction of prasugrel—and more recently, ticagrelor—has introduced a decision-making problem for clinicians and governments worldwide: to use the cheaper clopidogrel or the more effective, and also more expensive prasugrel or ticagrelor. We aim to give helpful contributions to this debate by analysing the cost-effectiveness of clopidogrel, prasugrel, and ticagrelor compared with each other.
Methods and results
We modified a previously developed Markov model of cardiac disease progression. In the model, we followed up cohorts of patients who have recently had a PCI until 100 years or death. Possible events are revascularization, bleeding, acute myocardial infarction, and death. Our analysis shows that ticagrelor is cost-effective in 77% of simulations at an incremental cost-effectiveness ratio of €7700 compared with clopidogrel. Ticagrelor was also cost-effective against prasugrel at a cost-effectiveness ratio of €7800. Given a Norwegian cost-effectiveness threshold of €70 000, both comparisons appear to be clearly cost-effective in favour of ticagrelor.
Conclusion
Ticagrelor is cost-effective compared with both clopidogrel and prasugrel for patients who have undergone a PCI.
Keywords: Percutaneous coronary intervention, Economic evaluation, Cost-effectiveness, Antiplatelets
Introduction
Each year, >10 000 Norwegians have a percutaneous coronary intervention (PCI) after either a myocardial infarction (MI) or angina. Antiplatelet therapy with 12 months use of the antiplatelet drug clopidogrel has, for decades, been considered the standard treatment after a PCI in order to prevent MI and death. Recently, two new antiplatelet drugs, prasugrel and ticagrelor, have been introduced. Both of these two drugs have proved to be efficacious with regards to, for example, MIs, but some concerns have also been raised with regards to increased risk of bleeding. 1 , 2 Hence, there is uncertainty as to the risk–benefit trade-off. Prices of the drugs are also an issue. Clopidogrel is no longer patented and prices have decreased considerably. The more newly developed prasugrel and ticagrelor are considerably more expensive; for instance, these cost >20 times as much in the UK ( http://www.nice.org.uk/guidance/ta236/resources/ta236-acute-coronary-syndromes-ticagrelor-costing-template ), and in Norway, these are >4 times as expensive ( http://legemiddelverket.no/Blaa_resept_og_pris/Helseoekonomiske%20rapporter/Documents/2012-2011/Brilique_Akutt-Koronarsyndrom_2011.pdf ). Similar differences are seen all across Europe.
In 2014, >36 000 patients in Norway used either clopidogrel, prasugrel, or ticagrelor. Of these, 71% used clopidogrel, 24% used ticagrelor, and 5% used prasugrel. 3 Hence, it seems that clopidogrel still is the preferred antiplatelet therapy among doctors.
Given the risk–benefit trade-off generated by the fact that each of the three available drugs are significantly better than at least one of the other drugs, and considerable cost difference, there is great uncertainty with regards to which of the three antiplatelet drugs offer the greatest value for money. Although some cost-effectiveness analyses have been conducted comparing each of the new drugs with clopidogrel, 4 no health economic evaluation has compared the cost-effectiveness of all these drugs with each other.
Our objective was to compare the cost-effectiveness of different antiplatelet drugs for patients who have undergone PCI.
Methods
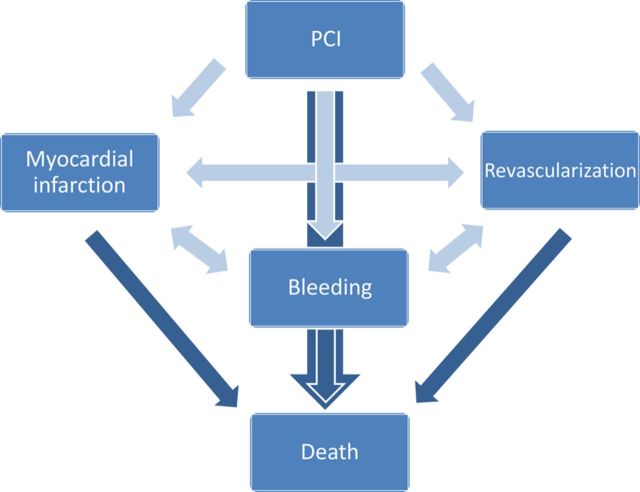
We modified a previously developed probabilistic Markov model (MOCCA—Model Of Cost-effectiveness of CArdiac Disease) to fit the current research question. 5 The model applies a lifelong healthcare payer perspective after a PCI operation, including risk of MI, major bleeding, new revascularization (PCI or coronary artery bypass graft), and death ( Figure 1 ).
Figure 1.
Model structure.
The model was built to model half-year cycles from the age of 50 onwards to 105 years old. In our base case analysis, we analysed 60 year olds until aged 100, hence 80 cycles. Within-cycle correction was done using Simpsons 1/3rd methods, as recommended by Elbasha and Chhatwal. 6
Efficacy data of prasugrel and ticagrelor compared with clopidogrel were based on the two licensing phase III randomized controlled trials including 13 608 and 18 624 participants, respectively. 1 , 2 Outcomes included significant reductions in risk of MI for both drugs, increased risk of bleeding and reduced risk of revascularization with prasugrel, and reduced overall mortality with ticagrelor ( Table 1 ). Due to different reporting in trials, effect on revascularization in our model was based on data on urgent target vessel revascularization from TRITON Thrombolysis in Myocardial Infarction Study Group (TIMI) 38 and recurrent ischaemia from PLATO invasive, as has also been done in a meta-analysis of these drugs. 7
Table 1.
Effect of new drugs compared with clopidogrel
| Hazard ratio | Confidence interval | Distribution | Source | |
|---|---|---|---|---|
| Prasugrel vs. clopidogrel | ||||
| Urgent target vessel revascularization | 0.66 | 0.54–0.81 | Log-normal (−0.4155, 0.1034) | TRITON TIMI-38 1 |
| Death from cardiovascular causes | 0.89 | 0.70–1.12 | Log-normal (−0.1165, 0.1199) | TRITON TIMI-38 1 |
| Non-fatal MI | 0.76 | 0.67–0.85 | Log-normal (−0.2744, 0.0607) | TRITON TIMI-38 1 |
| Major TIMI bleeding | 1.32 | 1.03–1.68 | Log-normal (0.2776, 0.1248) | TRITON TIMI-38 1 |
| Ticagrelor vs. clopidogrel | ||||
| Recurrent ischaemia | 0.93 | 0.82–1.05 | Log-normal (−0.0726, 0.0631) | PLATO invasive 2 |
| Death from cardiovascular causes | 0.79 | 0.69–0.91 | Log-normal (−0.2357, 0.0706) | PLATO invasive 2 |
| Non-fatal MI | 0.84 | 0.75–0.95 | Log-normal (−0.1744, 0.0603) | PLATO invasive 2 |
| Major TIMI bleeding | 1.03 | 0.93–1.15 | Log-normal (0.0296, 0.0542) | PLATO invasive 2 |
Costs of all three antiplatelet drugs are based on current prices from the Norwegian Medicines Agency: 8 €207 per year for clopidogrel (300 mg loading dose, thereafter 75 mg per day), €509 per year for prasugrel (60 mg loading dose, thereafter 10 mg per day), and €817 per year for ticagrelor (180 mg loading dose, thereafter 90 mg per day). Costs of treatment [acute myocardial infarction (AMI), revascularization and bleeding] were based on items from the original publication, 5 but all costs of services were based on fees for 2015 ( Table A2 ). We have chosen a Norwegian healthcare sector perspective for our analyses, as recently suggested in a Norwegian white paper on prioritizing. 9 Costs are converted into Euros (€) using average rate of 2014 (€1 = NOK 8.35).
Effectiveness is in our base case analysis measured as life years gained (LYG), which is one of the two acceptable measures in Norwegian cost-effectiveness analyses. 10 The other acceptable measure of effect in Norwegian health economic evaluations is quality-adjusted life years (QALYs). All weights were based on EQ-5D data, health states as derived in a Norwegian study by Pettersen et al.11 and events as used in a previous Norwegian economic evaluation. 12
Incremental cost-effectiveness ratios (ICERs) are regarded as cost-effective if below NOK 588 000 (€70 000) per LYG or QALYs, as recommended by the Norwegian Directorate of Health. 13 Recently, it was suggested in a white paper that the Norwegian threshold should rather be NOK 250 000 (€30 000) to better be in line with the opportunity cost. 9 We compared our results also with this suggested threshold. All costs and health benefits were discounted at 4% as recommended in Norwegian guidelines for health economic evaluations. 10
We incorporated probability distributions on all parameters in the model that were considered uncertain ( Table 1 , A1, and A2 ). Choice of distribution types was based largely on knowledge of how data of different types are usually distributed, as recommended by Briggs et al . 14 Uncertainty within each distribution was based on confidence intervals, where available. All results shown are based on probabilistic analyses, meaning that we sampled 10 000 times from all probability distributions in the model and calculated means of model outcomes based on these samples.
When modelling disease progression with different health states, there is often a possibility of double counting. In our model, and in the trials we based the difference in effectiveness on, there are possibilities that patients were classified as having both MI and revascularization or both MI and death. In addition to our base case simulation, we therefore performed scenario analyses with effect on fewer outcomes in the model. We did analyses eliminating either effect on revascularization or mortality or both, due to the possibility of overlap between these outcomes and AMI.
Risk of revascularization and bleeding was assumed to be the same in the first half year and in later half-year periods due to lack of data. Since these risks are likely to be lower in later half-year periods after the initial PCI, we performed separate analyses with these probabilities being 0 in all years post the first year.
We also conducted analyses where the model was run for only 5 years to explore whether results differ between a model based mostly on data (5 years) and a model based on assumptions with regards to extrapolation.
To explore whether cost-effectiveness would be different across ages, we also performed simulations for 50-, 70- and 80-year olds.
We performed one deterministic sensitivity analysis reducing the prices of the drugs not considered cost-effective in our base case analysis, to see how low these prices had to be for the drugs to be cost-effective.
Results
Sixty-year-old patients undergoing PCI had a life expectancy of 11.96 years (discounted) if treated with clopidogrel the first year. The treatment with prasugrel increased the life expectancy to 12.32 years, while ticagrelor resulted in 12.70 years. Prasugrel was cost-effective compared with clopidogrel, and ticagrelor was cost-effective compared with both prasugrel and clopidogrel ( Table 2 ).
Table 2.
Lifetime costs and effects (incrementals compared with strategy above)
| Lifetime cost (€) | Incremental cost (€) | Life expectancy | Life years gained | Incremental cost-effectiveness ratio | |
|---|---|---|---|---|---|
| Clopidogrel | 19 929 | 11.96 | |||
| Prasugrel | 22 649 | 2720 | 12.32 | 0.36 | 7505 €/LYG |
| Ticagrelor | 25 612 | 2963 | 12.70 | 0.38 | 7820 €/LYG |
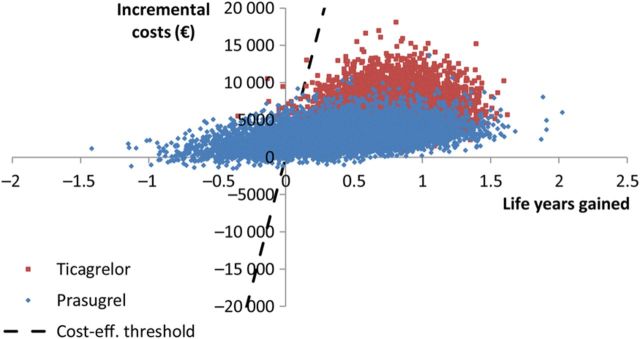
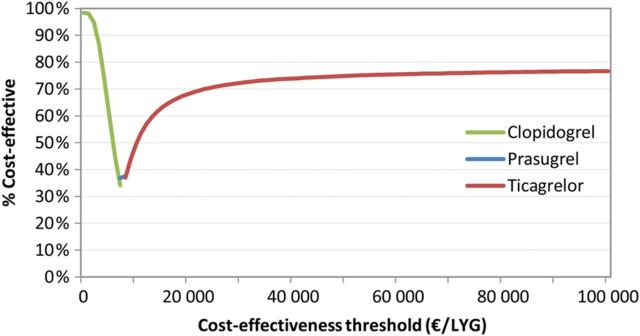
Monte Carlo simulations show that at an assumed Norwegian cost-effectiveness threshold of EUR 70 000 per life year gained, 76, 24, and 0.1% of simulations indicated that ticagrelor, prasugrel, and clopidogrel were cost-effective, respectively ( Figure 2 ). If we rather compared with the suggested threshold of EUR 30 000, these percentages would be 72, 27, and 0.4%. The cost-effectiveness acceptability frontier gives that clopidogrel is cost-effective for cost-effectiveness thresholds lower than €7505 per LYG, prasugrel is cost-effective for threshold between €7505 per LYG and €7820 per LYG, and that ticagrelor is cost-effective for threshold above €7820 per LYG ( Figure 3 ).
Figure 2.
Scatter plot of simulations (incrementals compared with clopidogrel).
Figure 3.
Cost-effectiveness acceptability frontier.
When including only efficacy data on MI and bleeding, prasugrel dominated the other two drugs with both lowest costs and highest effectiveness. When including efficacy on revascularization as well as bleeding and MI, clopidogrel was the most effective and least costly. When including efficacy only on death, bleeding, and MI, ticagrelor was the most effective and cost-effective. When excluding the probability of bleeding and revascularization after the first year, analyses gave that ticagrelor was the most effective and cost-effective.
In analyses of expected value of perfect information on parameters, we found that the relative effect on death of ticagrelor and prasugrel compared with clopidogrel was the only parameter with any value of gathering new evidence. The per person expected value of partial perfect information was €3220 and €580 for prasugrel and ticagrelor, respectively, indicating that for each person in Norway who experience a PCI, up to €3220 could be used to reduce the decision uncertainty, if this was completely removed by the research.
In sensitivity analyses without discounting health, life expectancy with clopidogrel, prasugrel, and ticagrelor was 17.52, 18.23, and 19.05, respectively. Applying discounted costs to these life expectancies gave ICERs of 3840 €/LYG of prasugrel compared with clopidogrel and 3620 €/LYG of ticagrelor compared with prasugrel.
When incorporating health-related quality of life in the model, expected discounted remaining QALYs were 9.54, 9.82, and 10.12 for clopidogrel, prasugrel, and ticagrelor, respectively. The cost-utility of prasugrel compared with clopidogrel then becomes 9531 €/QALY. Ticagrelor compared with prasugrel gives a cost-utility of 9987 €/QALY.
We explored the impact of reducing price of clopidogrel and prasugrel. Not even reducing prices to €0,- would make prasugrel or clopidogrel cost-effective, because the main differences in costs between the strategies are those resulting from reduction of clinical events.
In simulations of 50-, 70-, and 80 year-olds, we also found that ticagrelor was cost-effective compared with the other two drugs. Probabilities of ticagrelor being cost-effective were 76, 78, and 79% for 50-, 70-, and 80-year olds, respectively. Probability of clopidogrel being cost-effective was <0.3% in all cases.
We performed a separate simulation with only 5-year time horizon. In these analyses, ticagrelor was also the most cost-effective option, but the probability of being cost-effective was lower at 61%.
Discussion
Our base case analysis based on efficacy data on four different outcomes showed that ticagrelor is clearly cost-effective compared with prasugrel and clopidogrel. Simulations gave a 77% that ticagrelor is cost-effective, given the current evidence base. Considering that only 24% of users of these drugs use ticagrelor, it may be time for a change in practice.
Interpretation of the analyses did not change for different suggested cost-effectiveness thresholds. Results were also extremely robust with regards to choice of starting point for the model. Having a PCI at 50, 60, 70, or 80 did not change conclusions; ticagrelor is cost-effective and clopidogrel is clearly not.
In a recent review of cost-effectiveness, four studies were found that evaluated prasugrel vs. clopidogrel and two studies that evaluated ticagrelor vs. clopidogrel. 15 None of these six evaluations had more than a 2-year perspective, so our 40-year perspective is probably not all that comparable, yet all these analyses found the analysed new drug to be cost-effective compared with clopidogrel. Cost-effectiveness analyses are usually reckoned to not be transferable across jurisdictions, although recent evidence may indicate otherwise. 16 That taken into account, in addition to our analyses showing that ticagrelor is cost-effective even if the prices of the two other drugs were €0,- are clear indications that ticagrelor is likely to be cost-effective regardless of jurisdiction.
Remaining life expectancy for patients who have recently undergone a PCI is reasonable to assume to be shorter than for the general population. Our analyses indicate a remaining life expectancy of 17.5 years with current treatment strategy (clopidogrel) for 60-year olds, compared with an average of 24.5 years for the general population at the same age. This indicates that our model gives results around what was reasonable to assume.
Limitations
We used a cycle length of 6 months in our model analyses. In this way, we distinguished clearly between those events patients are at risk of getting during the first days and months after a PCI, compared with risks later on. We acknowledge that a shorter cycle length is even more accurate, and might have been preferable.
We chose a 40-year perspective when modelling 60-year-old patients who had undergone a PCI. By the end of this period, 99.99% would be dead; hence, all relevant aspects should have been included, as for instance recommended by SMDM-ISPOR. 17 On the other hand, modelling such a long period is largely based on assumptions. We, therefore, conducted a separate simulation with only a 5-year perspective, although results differ, conclusions do not; ticagrelor is still the most cost-effective.
A limitation of our analysis is the lack of longer follow-up data on revascularization and bleeding. Here, we assumed that rates were similar in later periods compared with the first half year.
When creating models of disease progression, choice of which health states to include is essential and may very well influence results. In our model, we included AMI, revascularization, bleeding, and death, mainly because these trials have indicated that the most pronounced differences between the drugs are on these outcomes. We might also have incorporated other health states, for instance stroke, but chose not to do so, because P -values of 0.22 and 0.93 indicate that if there is a difference, it is probably not that substantial. 1 , 2 In analyses exploring whether eliminating any of the included health states would affect the results, we found that all three drugs evaluated could be considered cost-effective, depending on which health states that was included. All scenarios analysed here can be justified based on reasoning related to double counting of events and hence be used by pharmaceutical companies when applying for reimbursement. We therefore urge governmental institutions appraising reimbursement applications to be aware of the potential impact of structural uncertainty when making decisions on reimbursement.
The present model is based solely on life years as health outcome. In other model of patients receiving PCI, outcomes such as avoided revascularizations and QALYs have also been presented. We did not use revascularizations as outcome because it would not capture the time aspect. QALYs could have been used, because there might be a lower QALY with bleeding compared with AMI. This impact would, however, be only for a limited time after each episode, making the impact on the ICER minimal.
Conclusions
Ticagrelor is clearly cost-effective compared with prasugrel and clopidogrel for a Norwegian setting. Results can be easily modified by pharmaceutical companies who want to prove their drug being the most cost-effective.
Funding
The study was performed as part of regular work at the Oslo University Hospital. Funding to pay the Open Access publication charges for this article was provided by Oslo University Hospital through University of Oslo.
Conflict of interest: D.A. reports personal fees from Astra-Zeneca, personal fees from Sanofi-Aventis, outside the submitted work.
Appendix
Table A1.
Probabilities and proportions
| Probability | Expectation | Distribution details | Source |
|---|---|---|---|
| AMI first 6 months after primary PCI | 0.0639 | Beta ( r = 823, n = 12 880) | SCAAR 18 |
| Revascularisation first 6 months after primary PCI | 0.01978 | Beta ( r = 232, n = 11 730) | WDHR 19 |
| Bleeding first 6 months after primary PCI | 0.00068 | Beta ( a = 11.620, b = 17 027) | Meta-analysis 20 |
| Death first 6 months | 0.0316 | Log-normal (1.6190, 0.0569) a | SCAAR 18 |
| AMI in later half-year periods | 0.01614 | Beta ( r = 507, n = 31 409) | SCAAR 18 |
| Revascularisation in later half-year periods | Assumed same as first half-year period | ||
| Bleeding in later half-year periods | Assumed same as first half-year period | ||
| Death in later half-year periods | 0.01028 | Log-normal(0.4856, 0.0489) a | SCAAR 18 |
| Mortality during PCI operation | 0.005 | Beta ( a = 30.710, b = 6111.3) | Feiring Heart Clinic 21 |
| Mortality during CABG operation | 0.008 | Beta ( a = 37.567, b = 4658.3) | Feiring Heart Clinic 21 |
| Bleeding mortality | 0.00193 | Beta ( r = 26, n = 13 457) | NOKC report 22 |
a Distribution of a rate ratio which is multiplied by mortality rate to give age-dependence.
Table A2.
Cost items
| Cost item | Expectation | Distribution details | Source |
|---|---|---|---|
| Cost per half year of ACE inhibitor | 1110.06 | No | NoMA 8 |
| Cost per ground ambulance turn-out | 11000.00 | Gamma (4, 0.00036) | NorCaD 23 |
| Cost per half year of ASA use | 145.13 | No | NoMA 8 |
| Cost per half year of beta-blocker | 341.76 | No | NoMA 8 |
| Half year cost of clopidogrel | 865.64 | No | NoMA 8 |
| Cost per loading dose of clopidogrel (300 mg) | 47.07 | No | NoMA 8 |
| Cost per DRG-point | 35127.00 | No | DRG price list 24 |
| Cost per GP lab test with ECG and cholesterol | 141.00 | No | GP tariff 25 |
| Cost per GP visit | 289.00 | No | GP tariff 25 |
| Cost per half year of prasugrel | 3413.13 | No | NoMA 8 |
| Cost per loading dose of prasugrel (60 mg) | 112.14 | No | NoMA 8 |
| Cost per half year of statin | 338.48 | No | NoMA 8 |
| Half year cost og ticagrelor treatment | 2124.53 | No | NoMA 8 |
| Cost per loading dose of ticagrelor (180 mg) | 23.27 | No | NoMA 8 |
| Number of GP lab tests per half year with ECG and cholesterol | 1.0 | Gamma (4, 4) | NorCaD 23 |
| Number of GP visits per half year when asymptomatic | 1.0 | Gamma (4, 4) | NorCaD 23 |
| Number of GP visits with STEMI at intervention hospital | 1.0 | Gamma (4, 4) | NorCaD 23 |
| Number of ambulances when STEMI at intervention hospital | 1.0 | Gamma(4, 4) | NorCaD 23 |
| Number of DRG 112e when STEMI at intervention hospital | 0.5 | Beta ( r = 50, n = 100) | NorCaD 23 |
| Number of DRG 112f when STEMI at intervention hospital | 0.5 | Beta ( r = 50, n = 100) | NorCaD 23 |
| Number of ambulances with STEMI at other hospitals | 2.8 | Gamma (2.8, 1) | NorCaD 23 |
| Number of DRG 112e when STEMI at other hospitals | 0.45 | Beta ( r = 45, n = 100) | NorCaD 23 |
| Number of DRG 112f when STEMI at other hospitals | 0.45 | Beta ( r = 45, n = 100) | NorCaD 23 |
| Number of DRG 121 when STEMI at other hospitals | 0.5 | Beta ( r = 50, n = 100) | NorCaD 23 |
| Number of DRG 122 when STEMI at other hospitals | 1.4 | Gamma (4.2, 3) | NorCaD 23 |
| Number of GP visits when STEMI without PCI facilities | 1.0 | No | NorCaD 23 |
| Weight for DRG 112e | 1.19 | Gamma (4, 3.3613) | DRG price list 24 |
| Weight for DRG 112f | 1.46 | Gamma (4, 2.7397) | DRG price list 24 |
| Weight for DRG 121 | 1.51 | Gamma (4, 2.6490) | DRG price list 24 |
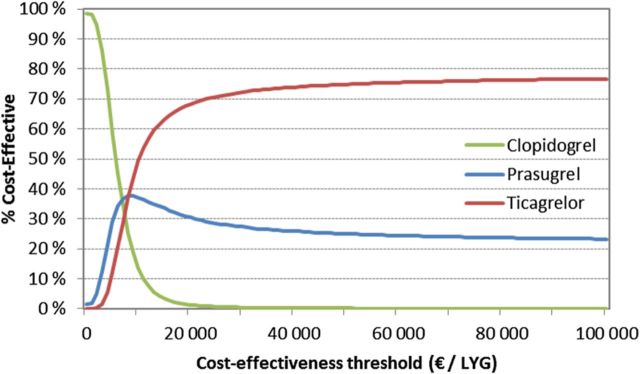
Figure A1.
Cost-effectiveness acceptability curve.
References
- 1. Wiviott SD , Braunwald E , McCabe CH , Montalescot G , Ruzyllo W , Gottlieb S , Neumann F-J , Ardissino D , De Servi S , Murphy SA , Riesmeyer J , Weerakkody G , Gibson CM , Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes . New Engl J Med 2007. ; 357 : 2001 – 2015 . [DOI] [PubMed] [Google Scholar]
- 2. Wallentin L , Becker RC , Budaj A , Cannon CP , Emanuelsson H , Held C , Horrow J , Husted S , James S , Katus H , Mahaffey KW , Scirica BM , Skene A , Steg PG , Storey RF , Harrington RA. Plato Investigators, Freij A, Thorsen M Ticagrelor versus clopidogrel in patients with acute coronary syndromes . New Engl J Med 2009. ; 361 : 1045 – 1057 . [DOI] [PubMed] [Google Scholar]
- 3. Norwegian prescription registry: Norwegian Institute of Public Health; [cited 2015] . www.reseptregisteret.no (9 September 2015) .
- 4. Friedewald WT , Levy RI , Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge . Clin Chem 1972. ; 18 : 499 – 502 . [PubMed] [Google Scholar]
- 5. Wisløff T , Atar D , Sonbo Kristiansen I . Cost effectiveness of drug-eluting stents as compared with bare metal stents in patients with coronary artery disease . Am J Ther 2013. ; 20 : 596 – 601 . [DOI] [PubMed] [Google Scholar]
- 6. Elbasha EH , Chhatwal J . Theoretical foundations and practical applications of within-cycle correction methods . Med Decis Making 2015. . [DOI] [PubMed] [Google Scholar]
- 7. Verdoia M , Schaffer A , Barbieri L , Cassetti E , Piccolo R , Galasso G , Marino P , Sinigaglia F , De Luca G. Benefits from new ADP antagonists as compared with clopidogrel in patients with stable angina or acute coronary syndrome undergoing invasive management: a meta-analysis of randomized trials . J Cardiovasc Pharmacol 2014. ; 63 : 339 – 350 . [DOI] [PubMed] [Google Scholar]
- 8. Norwegian Medicines Agency [cited 2015] . www.legemiddelverket.no (29 September 2015) .
- 9. Åpent og rettferdig – prioriteringer i helsetjenesten, Helse-og omsorgsdepartementet (2014) .
- 10. Helsedirektoratet. Økonomisk evaluering av helsetiltak-en veileder. 2012 11/2012. Report No.: IS-1985 .
- 11. Pettersen KI , Kvan E , Rollag A , Stavem K , Reikvam A . Health-related quality of life after myocardial infarction is associated with level of left ventricular ejection fraction . BMC Cardiovasc Disord 2008. ; 8 : 28 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wisløff T , Hagen G , Klemp M . Economic evaluation of warfarin, dabigatran, rivaroxaban, and apixaban for stroke prevention in atrial fibrillation . Pharmacoeconomics 2014. ; 32 : 601 – 612 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. The Norwegian Directorate of Health . Er det mulig å beregne verdien av liv og helse? In: Veidirektoratet , eds. Oslo, Norway; : Statens vegvesen; ; 2014. . [Google Scholar]
- 14. Briggs AH , Claxton K , Sculpher M . Making decision models probabilistic . In: Gray A , Briggs AH , (eds). Decision Modelling for Health Economic Evaluation . Oxford University; Press; ; 2006. . [Google Scholar]
- 15. Gialama F , Miloni E , Maniadakis N . Cost effectiveness of treatments for non-ST-segment elevation acute coronary syndrome . Pharmacoeconomics 2014. ; 32 : 1063 – 1078 . [DOI] [PubMed] [Google Scholar]
- 16. Boehler CE , Lord J . Mind the gap! A multilevel analysis of factors related to variation in published cost-effectiveness estimates within and between countries . Med Decis Making 2015. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts M , Russell LB , Paltiel AD , Chambers M , McEwan P , Krahn M . Conceptualizing a model: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-2 . Med Decis Making 2012. ; 32 : 678 – 689 . [DOI] [PubMed] [Google Scholar]
- 18. Long-term outcome of DES vs BMS implanted in Sweden 2003–2004 : Hearing before the FDA(12/7/2006, 2006) . [Google Scholar]
- 19. Kaltoft A , Jensen LO , Maeng M , Tilsted HH , Thayssen P , Bottcher M , Lassen JF , Krusell LR , Rasmussen K , Hansen KN , Pedersen L , Johnsen SP , Sørensen HT , Thuesen L . 2-year clinical outcomes after implantation of sirolimus-eluting, paclitaxel-eluting, and bare-metal coronary stents: results from the WDHR (Western Denmark Heart Registry) . J Am Coll Cardiol 2009. ; 53 : 658 – 664 . [DOI] [PubMed] [Google Scholar]
- 20. Antithrombotic Trialists' Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients BMJ 2002. ; 324 : 71 – 86 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mølstad P . Survival after percutaneous coronary intervention and coronary artery bypass grafting in a single centre . Scand Cardiovasc J 2007. ; 41 : 214 – 220 . [DOI] [PubMed] [Google Scholar]
- 22. Wisløff T , Ringerike T , Klemp M. A . systematic review and economic evaluation of prasugrel compared to clopidogrel after PCI . 2011 2011. Report No.: 5 . [PubMed] [Google Scholar]
- 23. Wisløff T , Selmer R , Halvorsen S , Kristiansen IS . Norwegian Cardiovascular Disease Model (NorCaD)–a simulation model for estimating health benefits and cost consequences of cardiovascular interventions . 2008 2008. Report No.: 23 . [Google Scholar]
- 24. Norwegian Directorate of Health . [Activity based financing 2015] In Norwegian. 2014 . [Google Scholar]
- 25. Association TNM . [Normal Tariff for GPs and emergency care 2014-2015] In Norwegian. 2014 . [Google Scholar]