Abstract
Cardiovascular disease is a leading cause of morbidity and mortality worldwide, and a key barrier to improved outcomes is medication non-adherence. The aim of this study is to review the role of mobile health (mHealth) tools for improving medication adherence in patients with cardiovascular disease. We performed a systematic search for randomized controlled trials that primarily investigated mHealth tools for improving adherence to cardiovascular disease medications in patients with hypertension, coronary artery disease, heart failure, peripheral arterial disease, and stroke. We extracted and reviewed data on the types of mHealth tools used, preferences of patients and healthcare providers, the effect of the mHealth interventions on medication adherence, and the limitations of trials. We identified 10 completed trials matching our selection criteria, mostly with <100 participants, and ranging in duration from 1 to 18 months. mHealth tools included text messages, Bluetooth-enabled electronic pill boxes, online messaging platforms, and interactive voice calls. Patients and healthcare providers generally preferred mHealth to other interventions. All 10 studies reported that mHealth interventions improved medication adherence, though the magnitude of benefit was not consistently large and in one study was not greater than a telehealth comparator. Limitations of trials included small sample sizes, short duration of follow-up, self-reported outcomes, and insufficient assessment of unintended harms and financial implications. Current evidence suggests that mHealth tools can improve medication adherence in patients with cardiovascular diseases. However, high-quality clinical trials of sufficient size and duration are needed to move the field forward and justify use in routine care.
Keywords: mHealth, Telehealth, Medication adherence, Cardiovascular disease
Introduction
Poor medication adherence and persistence are associated with increased re-hospitalization, morbidity, mortality, and increased healthcare costs.1 The World Health Organization (WHO) has identified medication non-adherence as a priority preventable healthcare problem, and a key barrier to improving clinical outcomes.2 Medication non-adherence has been reported to occur in >60% patients with cardiovascular disease, the leading cause of morbidity and mortality worldwide.3,4
Reasons for poor medication use are multifactorial and include, but are not limited to, poor medication adherence (the extent to which a patient acts in accordance with the prescribed interval and dose of a dosing regimen) and persistence (the duration of time from initiation to discontinuation of therapy).5 Various aetiologies are reported for medication non-adherence and non-persistence. While medication non-adherence may be attributed to forgetfulness, communication barriers, socio-economic factors, and motivation,3,6 medication non-persistence may result from financial hardships, fear of side effects, false beliefs about disease or treatment, and lack of understanding about medication purpose.7 As a result, a multi-modal approach regarding medication intake is needed to improve outcome among patients with chronic conditions.
To improve medication adherence, there has been recent interest in the use of behavioural interventions, such as calendar pill boxes or pill blister packaging, in-office patient counselling, and follow-up telephone calls from care providers.8 A tool with surging interest is mobile health (mHealth).9–11 To date, no standardized definition of mHealth has been established. mHealth, electronic Health (eHealth), and telehealth are terms that have been used interchangeably.12 WHO has defined mHealth technology as a medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices.13 In addition, the American Telemedicine Association (ATA) considers mHealth as a form of telemedicine.14
Use of cell phones, particularly smartphones, has increased in recent years. Recent estimates suggest that 90% of American adults own a cell phone and 64% own a smartphone.15 It is estimated that a quarter of the world's adult population, surpassing 2 billion, will be using smartphones in 2016.16 As such, field of mHealth is heavily focused on smartphone-based or smartphone-linked interventions. The widespread adoption of smartphones provides a unique opportunity for researchers and clinicians to utilize mHealth as a means for delivering healthcare in an automated, personalized, and cost-efficient manner.
Previous studies have reviewed the use of mHealth as a tool to improve medication adherence in patients with various chronic medical conditions such as acquired immunodeficiency syndrome, diabetes, and chronic pulmonary obstructive disease.17,18 A recent systematic review of controlled trials and observational studies suggested that there is a potential for mHealth technologies to facilitate adherence to chronic disease management (improvement on adherence behaviour in 56% of trials), although more evidence is needed to support the effectiveness of mHealth technologies.17 In addition, a recent meta-analysis of randomized controlled trials demonstrated that mobile phone text messaging approximately doubles the odds of medication adherence among patients with chronic conditions, but suggested cautious interpretation of these reports, as more investigations should be performed to determine definite influences of mHealth technologies on clinical outcomes as well as patient populations who mostly benefit from these interventions.19
Although previous reviews have explored the literature regarding the applicability of mHealth for patients with chronic conditions, to our knowledge, no studies have specifically reviewed randomized controlled trials, which provide higher quality evidence, for enhancing medication adherence in patients with cardiovascular disease by mHealth. In this study, we aim to fill this literature gap by reviewing evidence for mHealth interventions that target medication adherence in patients with cardiovascular disease, with attention to the types of mHealth tools used, preferences of patients and healthcare providers, the effect of the mHealth interventions on medication adherence, and the limitations of trials.
Methods
Data source and search strategy
We searched MEDLINE using PubMed for the time range from 1 January 1966 to 11 December 2015 for studies that evaluated medication adherence in response to mHealth interventions in patients with cardiovascular disease. We searched ‘text words’ for most entries and MeSH headings for major entries with relevant keywords (Table 1).
Table 1.
Search strategy
| Set# | Search string | Results |
|---|---|---|
| 1 | mhealth[tw] OR mobile health[tw] OR telehealth[tw] OR eHealth[tw] electronic health[tw] OR digital health[tw] OR mobile app*[tw] OR mobile phone*[tw] OR cell phone*[tw] cellular phone*[tw] OR smartphone*[tw] OR tablet*[tw] OR smart phone*[tw] OR iPhone* OR iPad* OR android OR handheld*[tw] OR phone call*[tw] OR short messag*[tw] OR sms[tw] OR multimedia message*[tw] OR mms[tw] OR text messag*[tw] OR Telemedicine[mh] OR Mobile applications[mh] OR Reminder Systems[mh] | 87 694 |
| 2 | cardiovascular[tw] OR cardia*[tw] OR heart*[tw] OR coronary*[tw] OR myocard*[tw] OR angina*[tw] OR infarct*[tw] OR ischem*[tw] OR arrhythmia*[tw] OR hyperten*[tw] OR hyperlipidemia[tw] OR heart failure[tw] OR stroke*[tw] OR cerebrovasc*[tw] OR peripheral arterial disease*[tw] OR peripheral vascular disease*[tw] OR peripheral artery disease[tw] OR Cardiovascular Diseases[mh] OR Acute Coronary Syndrome[mh] OR Stroke[mh] | 2 970 688 |
| 3 | adheren*[tw] OR Medication Adherence[mh] | 132 356 |
| 4 | (#1 AND #2 AND #3) | 418 |
| 5 | (#1 AND #2 AND #3) Filters: English | 390 |
Study selection and data collection
Inclusion criteria
We included completed randomized controlled trials on patients with cardiovascular disease that evaluated the effect of mHealth interventions on adherence to cardiovascular disease medications for the treatment of hypertension, ischaemic heart disease, myocardial infarction, acute coronary syndrome, heart failure, stroke, and peripheral arterial disease. We considered trials for inclusion if the intervention was consistent with the definition of mHealth by WHO or ATA. Trials with results related to patient- or investigator-reported medication adherence were included in this review. Only trials that considered medication adherence as the primary endpoint were included.
Exclusion criteria
We excluded studies that evaluated the use of mHealth technology for adherence to non-cardiovascular medications and for other co-morbidities in patients with cardiovascular disease. We excluded studies that were not randomized or lacked an appropriate control group such as studies reporting pre- and post-intervention results in a single group following the use of an mHealth intervention. We also excluded studies other than English language, studies using regular phone calls alone, those terminated before the completion of study, or if other endpoints such as medication persistence or patient satisfaction were primarily evaluated. There were no restrictions with regard to patients' age, sex, and ethnicity and study location and date.
Data extraction
Two authors (Y.G. and S.K.) independently screened the titles and abstracts of search results. Irrelevant records were excluded and full-text articles of relevant results were reviewed for final inclusion of studies. Discordances were resolved by consensus or by discussion with a third reviewer (S.S.M). One reviewer (Y.G.) extracted data on the types of mHealth tools used, preferences of patients and healthcare providers, the effect of the mHealth interventions on medication adherence, and the limitations of trials.
Results
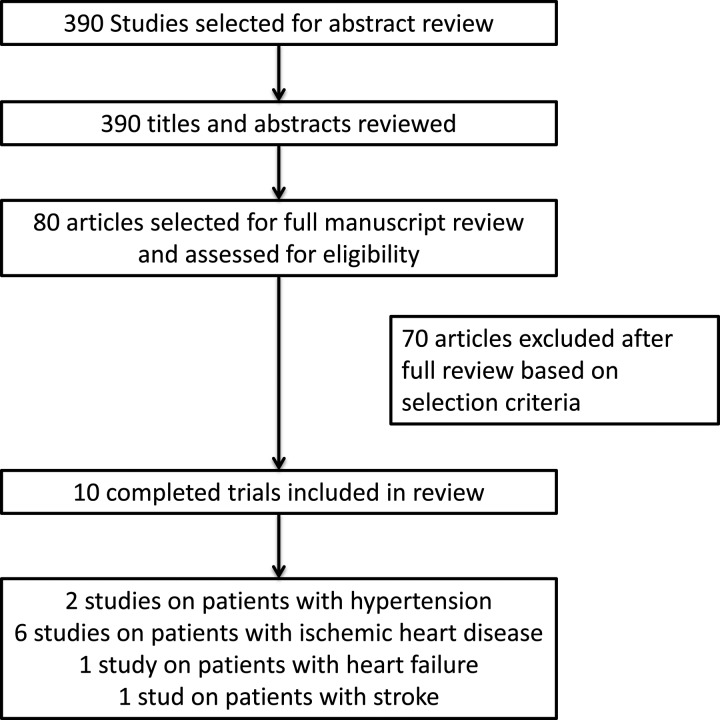
We found 390 records using the search strategy noted in Table 1. After reviewing the title and abstracts, 80 studies were selected for full-text review. Finally, we excluded 71 records and identified 10 trials20–29 matching our inclusion criteria (Figure 1). For simplification purposes, trials were classified and tabulated according to the type of cardiovascular disease.
Figure 1.
Flow chart of the literature review search.
Duration of trials ranged from 1 to 18 months. Sample size ranged from 20 to 21 752 individuals. Five studies had a sample size <100. Eight out of the nine studies used text messaging independently or as a part of the intervention. Three studies used electronic medication tray/electronic pill boxes with timestamps, three studies used smartphone apps, and one study used interactive voice response calls. Patients and healthcare providers responded positively to mHealth and generally preferred it to other tools.
Outcome measures
All 10 of the included trials examined medication adherence as primary endpoint. Medication adherence, if self-reported, was calculated using the Morisky Medication Adherence Scale (MMAS).26 In addition to MMAS, two trials23,27 assessed medication adherence by electronic monitoring devices described as Medication Event Monitoring System (MEMS), which record date and time of each medication removal. McGillicuddy et al.27 calculated adherence score, using a modified method as previously described by Russell et al.30 A dose taken within the 3- or 6-h window resulted in a full or half score for that dosing time, respectively. Quilici et al.20 measured patients' adherence to aspirin using arachidonic acid-induced platelet aggregation (AA-Ag) testing after intervention.
Type of mHealth tools
All studies except two trials21,28 used text messaging independently or in combination with other interventions. The content of text messages included health education, reminders to take prescribed medications, and interactive automated messages, modified according to the patient population, culture, language, education, and social environment.
A number of trials passively sent regular reminders to patients in order to improve medication adherence. Park et al.23 used short messaging service (SMS) to remind and/or educate participants with coronary heart disease about the use of cardiovascular medications. Investigators customized the text messages and invited text message replies by the patients. Quilici et al.20 used daily SMS reminders for aspirin adherence in patients who underwent percutaneous coronary intervention and stenting following acute coronary syndromes. Fang et al.24 used text messaging along with Micro Letter, an online open-access platform messenger service. It sent information related to disease condition and medication adherence to patients at regular intervals under supervision of a nurse and doctor. This information included text messages, images, and media content related to the disease. Investigators assessed self-reported medication adherence.
Two trials used smarted systems to actively send text messages to non-adherent patients. McGillicuddy et al.27 used a wireless GSM (Global System for Mobile communication) electronic medication tray (Figure 2), a wireless Bluetooth-enabled blood pressure monitoring device, and a smartphone. The electronic tray reminded patients by blinking from a specified dose compartment at the prescribed time. If not attended to in 30 min, it would ring for 30 min, and if it still was not attended to after that period, then this resulted in a text message reminder or an automated phone call to the patient. If blood pressure measurements recorded and transmitted through the blood pressure monitoring device were out of the normal range, the study coordinator was alerted. In another trial, Goldstein et al.29 used two interventions that they classified into ‘telehealth’ and ‘mHealth’. The telehealth arm included an electronic pill box, which had pill compartments with reminder alarms and timestamps. Reminder alarms were either activated or inactivated depending on the arm of the study. Each container was connected to a telephone line, and data were transferred using a built-in modem to a central server. In the mHealth arm, a smartphone (iPhone) was used with a medication adherence application (iRx Reminder LLC), which provided medication reminders and acted as a passive medication-taking log. Participants could respond to the reminder as documenting the number of pills taken or to snooze for a later time.
Figure 2.

Wireless global system for mobile communication electronic medication tray. From McGillicuddy et al.31
Two trials used mHealth tools other than text messaging. Vollmer et al.21 used interactive voice response calls. This involved automated phone calls plus personalized reminder letters, additional mailed materials, live outreach calls, and electronic medical record (EMR)-based feedback to the primary care providers when patients were due or overdue for a refill. EMR-based feedback was delivered to the primary care provider if patients were >90 days overdue. Tian et al.28 evaluated a smartphone-based electronic decision support system (EDSS) used by community health workers (CHW) in resource-limited settings in China, India, and Tibet. The CHWs were provided a 1-day EDSS orientation followed by delivery of the intervention to high-risk patients monthly for 1 year using a mobile cardiovascular management programme via an Android-based app. The app consisted of prompts regarding blood pressure, current medication use, medical history, contraindications to cardiac medications, and lifestyle habits that aided in the decision support. CHWs received suggestions relating to two lifestyle modifications (smoking cessation and/or low salt diet) and two medications (aspirin and/or blood pressure lowering agents) based on patient's assessment. Usual care/control received medications in primary healthcare centres without additional interventions.
Results by study population
Hypertension
mHealth improved medication adherence to anti-hypertensive medications and reduced blood pressure in two randomized controlled trials.
McGillicuddy et al.27 conducted a study including 20 participants followed up for 3 months. They used a prototype mHealth medication and blood pressure self-management system, assessing its feasibility, acceptability, and outcomes. A modified adherence score, as previously described by Russell et al.,30 was used to evaluate whether patients take medications in the recommended timeframe. Adherence score significantly increased from baseline to 3 months in the mHealth compared with the standard of care arm and accompanied with clinical improvement. Systolic blood pressure (SBP) improved from 138 to 122 mmHg and diastolic blood pressure (DBP) from 88 to 81 mmHg in the mHealth arm, and in the standard care arm, SBP increased from 132 to 139 mmHg and DBP from 76 to 79 mmHg at 3 months. Limitations of the study included the small sample size with recruitment from one transplant centre, and a question of bias due to the financial incentive involved with participation. The intervention arm also received mHealth devices for free.
The SimCard Study28 was a year-long cluster-randomized controlled trial of 2086 (47 villages: 27 in China and 20 in India) ‘high-risk’ cardiovascular patients, defined as age >40 years old with self-reported coronary heart disease, stroke, diabetes, or measured SBP ≥160 mmHg. The control group had access to free medications in primary health centres. Compared with control group, the intervention had a 25.5% (P < 0.001) higher net increase in the use of anti-hypertensive medications. Secondary outcomes showed significant difference in aspirin use (net difference 17.1%, P < 0.001). SBP also improved significantly (−2.7 mmHg, P = 0.04). Results from this multi-site trial suggested that mobile technology would be potentially helpful to improve medication adherence among resource-limited populations. However, since CHW served a pivotal role in the intervention, the generalizability and applicability of results to healthcare settings with few human resources may be limited. This trial was not able to distinguish the effectiveness of different intervention components, as multiple interventions were used.
Ischaemic heart disease
We found six trials that evaluated medication adherence among patients with coronary heart disease, the details of which are provided in Table 2. All trials showed significant improvements in medication adherence. Sample sizes of trials were different (with 60–21 752 patients), with durations ranging from 30 days to 18 months. Studies were done in the USA, UK, France, Malaysia, and China.
Table 2.
Randomized controlled trials including patients with ischaemic heart disease that used mHealth to improve medication adherence
| Study | Design | Population | Study intervention | Results | Limitations |
|---|---|---|---|---|---|
| Quilici et al. (2013)20 | RCT 1 month |
n = 546
Patients with ACS status post-PCI shown to be aspirin-responsive |
Daily SMS reminders for aspirin adherence vs. control; 1 month after hospital discharge admitted to Antiplatelet Monitoring Unit to compare adherence and platelet function | SMS reminders improved aspirin adherence as reported by patients (OR [95% CI]: 0.37 [0.15–0.90]; P = 0.02) and as shown by platelet testing (OR [95% CI]: 0.43 [0.22–0.86]; P = 0.01) |
|
| Vollmer et al. (2014)21 | RCT 1 year |
n = 21 752
Patients with CVD ± type 2 diabetes and suboptimal medication adherence |
Arm 1: Interactive voice recognition phone calls (IVR) Arm 2: IVR-enhanced (IVR+) phone calls, letters, EMR-feedback, mailed materials Control: Usual care |
Both phone interventions significantly increased adherence to statins (IVR+ equal to IVR) and ACEIs/ARBs (IVR+ more than IVR) compared with usual care (1.6–3.7%). |
|
| Wald et al. (2014)22 | RCT 6 months |
n = 301
Patients prescribed blood pressure and/or lipid lowering meds |
Automated daily TM for 2 weeks, alternate days for 2 weeks, then weekly with the goal to assess patients' adherence vs. control (no text) | Lower non-adherence rates among TM group 14/150 (9%) vs. control 38/151 (25%) (95% CI: 7–24), P < 0.001. Non-adherence defined as taking <80% of prescribed regimen |
|
| Park et al. (2014)23 | RCT 30 days |
n = 90
Patients with ACS status post-PCI at time of discharge |
Arm 1: TM for medication and reminders Arm 2: Educational TM Control: No TM Adherence monitored MEMS for statin and antiplatelet |
TM patients had higher percentage of correct doses taken (P = 0.02), taken on schedule (P = 0.01), percentage number of doses (P = 0.01) |
|
| Fang and Li (2015)24 | RCT 6 months |
n = 280
Patients with CAD confirmed by CT or angiography |
Arm 1: SMS Arm 2: SMS + Micro Lettera Control: Phone only |
Intervention groups had higher cumulative adherence; SMS + Micro Letter (OR [95% CI]: 0.069 [0.032–0.151], P=<0.001), SMS only (OR [95% CI]: 0.339 [0.183–0.629]) |
|
| Khonsari et al. (2015)25 | RCT 8 weeks |
n = 62
Patients post-hospital discharge following ACS |
Automated SMS-based reminders on medication adherence vs. control (no text) | Medication adherence and heart functional status were higher in SMS group (P < 0.001); control group had 4.09 times greater risk of low adherence (95% CI: 1.82–9.18) |
|
AA-Ag, arachidonic acid-induced platelet aggregation; ACEI/ARB, angiotensin-converting enzyme/angiotensin receptor blocker; ACS, acute coronary syndrome; CAD, coronary artery disease; CI, confidence interval; DBP: diastolic blood pressure; EMR, electronic medical record; IVR, interactive voice response calls; LDL-C: low-density lipoprotein cholesterol; MEMS, Medication Event Monitoring System; NNT, number needed to treat; OR, odds ratio; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; SMS, short messaging service; TM, text messaging.
aMicro Letter platforms provide users in China with access to news and other information via open-access Kik Messenger-like programs.
The largest of the studies was that done by Vollmer et al.,21 which took place in three large health maintenance organizations in the USA. Participants were 40 years or older with diabetes mellitus or atherosclerotic cardiovascular disease and had suboptimal medication adherence. Although the intervention had a positive effect, it was a modest one: adherence to statins and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers significantly increased from 1.6 to 3.7% compared with usual care (P < 0.05).
Heart failure
We found one study examining mHealth for medication adherence in heart failure. Goldstein et al.29 performed a randomized controlled trial including 60 participants with a follow-up of 28 days. This study compared the application of telehealth (using an ePill box) vs. mHealth (using a smartphone) regarding medication adherence and patients' acceptance of devices. The study had four arms involving possession of one of the two devices (either ePill box or smartphone) with or without active programmed reminders (active vs. passive medication-taking system). Participants rated their device based on factors such as helpfulness, quality of life, willingness to recommend the device to a friend, and satisfaction. Overall adherence was 78%. No significant difference in medication adherence was observed between the telehealth and mHealth groups regardless of the reminder systems. Adherence was 80% for ePill box, 76% for smartphone, 79% for active medication reminder arms, and 78% for passive medication reminder devices; however, patients preferred the mHealth approach and rated the smartphone higher than the ePill box (mean score 48.7 vs. 33.4; P < 0.001). The study had some limitations, including the lack of baseline data for adherence before intervention, inability of the smartphone application to capture overdoses, inconsistency between methods of ascertaining adherence between devices (bin opening vs. self-report), small sample size, and short duration.
Heart failure management is multifaceted and requires close monitoring of physical parameters such as weight, blood pressure, and volume status as well as cardiac rehabilitation. We encountered a number of studies involving the use of telehealth that examined these parameters. However, telehealth, but not mHealth, interventions have been used extensively in the management of heart failure. These interventions were not the focus of this review and are reviewed elsewhere.32
Stroke
We found one trial on patients with stroke. The SMS4Stroke study33 was a parallel-group, assessor-blinded, randomized controlled superiority trial with 200 participants. In addition to usual care, the intervention arm received reminder SMS messages for 2 months containing personalized prescription-tailored daily medication reminders with twice-weekly health information. Medication adherence was measured at recruitment and after 2 months in both groups. The increase in MMAS adherence score was minor in the control group (0.1), compared with the intervention group (0.8). On univariate analysis, the mean MMAS score was 0.65 (0.0–1.0) points higher in the intervention group compared with the control group. Multivariable analysis showed that mean difference in adherence score between the intervention group and the standard group was 0.54 (95% CI: 0.22–0.85; P = <0.01) when adjusted for potential confounders.
Peripheral arterial disease
mHealth has been used in patients with peripheral arterial disease, but we could not find any trials specifically examining medication adherence.
Discussion
Integrating technology to interface with patients through hybrid forms of mHealth, eHealth, and telehealth is currently a major initiative of clinical research. The ubiquity of mobile devices with diverse capabilities provides an opportunity to improve health outcomes. Mobile technology has the potential to make healthcare better, faster, less costly, and more accessible. mHealth has shown promising early results in improving medication adherence. It has the potential to improve the quality of care, provide access to multiple resources, and become more efficient by allowing monitoring and real-time analysis of health data while also enabling patients to learn and become more engaged in self-management of their condition through highly personalized tools.
Making comparisons across studies is challenging because of inherent differences in their designs, outcome measures, and the multitude of ways that adherence was assessed. However, among the studies reviewed, all demonstrated positive outcomes in one or more of their objectives. Recent interest has also been seen in smart watches because they are potentially as multifunctional as cell phones. Sailer et al.34 investigated requirements of a smart watch-based medication reminder system and presented an early prototype to improve medication adherence. Nevertheless, no trials have been conducted yet using smart watches. Although various types of technology would allow clinicians to choose the best tool based on patients' preference, it seems necessary to examine benefits and drawbacks of each method before recommending them under the umbrella term of mHealth.
Across the included trials, outcomes were measured in terms of medication adherence by multiple methods such as pharmacy refill rates, electronic medication tray with Bluetooth devices transmitting pill taking logs, blood levels, mobile apps with reminders, and patient self-documentation about completing the required instructions assessed on scales such as MMAS. The effect of mHealth interventions on other parameters of healthcare involving lifestyle and behaviour modification, including daily monitoring of weight, blood pressure, heart rate, and smoking, as well as recording and transmitting electrocardiogram, has also been assessed in studies with promising results.35 In this review, encouraging among all studies was the positive response of patients and healthcare providers to the mHealth interventions. However, in some instances, patient preference for one tool was not necessarily the determinant factor for the observed positive outcome. Goldstein et al.29 compared different tools including pillboxes and smartphones. Patients preferred the smartphone-based approach despite similar outcomes in all arms. This preference without significant change in outcome warrants further attention with well-designed clinical trials.
Park et al.23 saw improvement in both control and intervention arms and attributed this to Hawthorne bias (observer bias), which is a change in participants' behaviour, as they are aware of being observed. Although a challenge to differentiating effects in experimental studies, it is inherently a positive effect that suggests clinical practice can include closer monitoring implemented through digital means.
Financial implications are a concern prior to initiating any intervention. Cost information is essential for insurance companies to consider financing such healthcare. Among the trials screened, there were no studies evaluating the cost of improvement of medication adherence using mHealth. Studies involving direct phone calls from the provider or other healthcare staff to the patient did not evaluate financial implications for the extra time spent.21
There is paucity of data on how mHealth interventions have been developed, including whether any input is considered by users or patients. Differentiating components of the intervention, including text messages vs. reminders, improving knowledge vs. reinforcing behaviour change, being part of a study altering behaviour vs. continued closer monitoring, is pivotal to improving outcomes. No comparative studies testing different technologies alone or in combination with traditional interventions have yet been published.
Adherence to long-term treatment in chronic diseases is poor.36 The growing literature regarding the use of mHealth in non-cardiovascular chronic illnesses could inform mHealth application in patients with cardiovascular disease, who require consistent care. A meta-analysis of 16 trials, including patients with chronic diseases such as human immunodeficiency virus (HIV) infection, asthma, allergic rhinitis, diabetes mellitus, epilepsy, and cardiovascular disease, demonstrated that text messaging could improve medication adherence.36 Although mHealth has been effective in increasing adherence to anti-tuberculosis drugs and antiretroviral therapies in HIV patients,19 paucity of evidence regarding effectiveness of mHealth for other populations calls for further well-designed studies.
The American Heart Association (AHA)37 recently released a scientific statement where they reviewed studies integrating the use of mHealth for cardiovascular disease prevention, targeting weight loss, smoking cessation, blood glucose management, blood pressure control, and lipid management. The authors raised concerns about the present state of the mHealth for cardiovascular disease, strongly advocating for further high-quality clinical research in this area. mHealth apps, unlike medications, are commonly developed in a short period without formal testing in clinical trials. Many studies have failed to adhere to the principle of intention to treat and have relied on patient self-reporting. Further rigorous research is needed to support the use of mHealth in cardiovascular disease, and it seems reasonable to assume that producing this evidence must be a shared responsibility of healthcare providers, consumers, and producers.
The greatest growth of mobile phone use has been in low- and middle-income countries, yet the body of research regarding mHealth has largely ignored these settings.38 The combined effect of chronic non-communicable and communicable diseases in developing world results in ‘dual burden’ of illnesses39 that call for the use of effective interventions such as mHealth technologies. mHealth is an especially attractive strategy given the potential for low-cost scalability and reproducibility of interventions. However, more studies are needed in developing countries to measure the effectiveness of mHealth use in improving health-related quality measures, such as disability-adjusted life-years.40
In this systematic review, we examined trials that evaluated medication adherence as the primary outcome. However, adherence should be considered one part of the cascade from prescription to persistence, and it is unlikely that mHealth or an isolated intervention for adherence will make a significant impact on hard outcomes without consideration of other steps in this pathway. More investigations are needed to explore multiple aspects of medication-taking behaviour, such as concordance (negotiated agreement between the patient and the physician or other healthcare professional),41 preference, satisfaction, and persistence.42
As with any new technology, mHealth for cardiovascular medication adherence is a work in progress. Absence of substantial evidence does not mean absence of effectiveness. Awareness of this new tool is rising. However, we are in need of stronger evidence from well-designed and implemented studies with larger sample sizes, and longer duration of intervention and follow-up, focusing on motivation, education, closed loop relationship between the healthcare provider and patient, and evaluation of cost-effectiveness.
Acknowledgement
We thank Lyn Camire, MA, ELS, Department of Medicine, MedStar Union Memorial Hospital, for editorial assistance.
Conflict of interest: M.J.B. reports grants from AHA, NHLBI, FDA, and Aetna Foundation; and personal fees from Pfizer, Luitpold Pharmaceuticals, Novartis, Aralez, and ISIS/Akcea, outside the submitted work. S.S.M. reports grants from the PJ Schafer Cardiovascular Research Fund, American Heart Association, Aetna Foundation, and Google, outside the submitted work; has received honoraria from the American College of Cardiology for dyslipidemia-related educational activities; and is listed as a co-inventor on a pending patent filed by Johns Hopkins University for a novel method of LDL-C estimation.
References
- 1. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Adherence to long-term therapies: evidence for action. World Health Organization; 2003. http://www.who.int/chp/knowledge/publications/adherence_introduction.pdf. (7 January 2016). [Google Scholar]
- 3. Baroletti S, Dell'Orfano H. Medication adherence in cardiovascular disease. Circulation 2010;121:1455–1458. [DOI] [PubMed] [Google Scholar]
- 4. National Institutes of Health. Morbidity & mortality: 2012 chart book on cardiovascular, lung, and blood diseases. National Institutes of Health; 2015. https://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook_508.pdf. (5 August 2015). [Google Scholar]
- 5. Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 2013;51(Suppl. 3):S11–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care 2005;43:521–530. [DOI] [PubMed] [Google Scholar]
- 7. McHorney CA, Spain CV. Frequency of and reasons for medication non-fulfillment and non-persistence among American adults with chronic disease in 2008. Health Expect 2011;14:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schneider PJ, Murphy JE, Pedersen CA. Impact of medication packaging on adherence and treatment outcomes in older ambulatory patients. J Am Pharm Assoc (2003) 2008;48:58–63. [DOI] [PubMed] [Google Scholar]
- 9. Boulos MN, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online 2011;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clifford GD, Clifton D. Wireless technology in disease management and medicine. Ann Rev Med 2012;63:479–492. [DOI] [PubMed] [Google Scholar]
- 11. Hayakawa M, Uchimura Y, Omae K, Waki K, Fujita H, Ohe K. A smartphone-based medication self-management system with realtime medication monitoring. Appl Clin Inform 2013;4:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fatehi F, Wootton R. Telemedicine, telehealth or e-health? A bibliometric analysis of the trends in the use of these terms. J Telemed Telecare 2012;18:460–464. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. mHealth: new horizons for health through mobile technologies. World Health Organization; 2011. http://www.who.int/goe/publications/goe_mhealth_web.pdf. (7 January 2016). [Google Scholar]
- 14. Telemedicine frequently asked questions (FAQs). American Telemedicine Association; 2015. http://www.americantelemed.org/about-telemedicine/faqs-Vha3gvlVhBc. (15 October 2015). [Google Scholar]
- 15. Pew Research Center. Mobile technology fact sheet. Pew Research Center; 2015. http://www.pewinternet.org/fact-sheets/mobile-technology-fact-sheet/ (5 August 2015). [Google Scholar]
- 16. 2 billion consumers worldwide to get smart(phones) by 2016. emarketer com; 2015. http://www.emarketer.com/Article/2-Billion-Consumers-Worldwide-Smartphones-by-2016/1011694. (15 August 2015). [Google Scholar]
- 17. Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res 2015;17:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anglada-Martinez H, Riu-Viladoms G, Martin-Conde M, Rovira-Illamola M, Sotoca-Momblona J, Codina-Jane C. Does mHealth increase adherence to medication? Results of a systematic review. Int J Clin Pract 2015;69:9–32. [DOI] [PubMed] [Google Scholar]
- 19. Thirumurthy H, Lester RT. M-health for health behaviour change in resource-limited settings: applications to HIV care and beyond. Bull World Health Organ 2012;90:390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quilici J, Fugon L, Beguin S, Morange PE, Bonnet J-L, Alessi M-C, Carrieri P, Cuisset T. Effect of motivational mobile phone short message service on aspirin adherence after coronary stenting for acute coronary syndrome. Int J Cardiol 2013;168:568. [DOI] [PubMed] [Google Scholar]
- 21. Vollmer WM, Owen-Smith AA, Tom JO, Laws R, Ditmer DG, Smith DH, Waterbury AC, Schneider JL, Yonehara CH, Williams A. Improving adherence to cardiovascular disease medications with information technology. Am J Manag Care 2014;20(SP17):SP502–SP510. [PMC free article] [PubMed] [Google Scholar]
- 22. Wald DS, Bestwick JP, Raiman L, Brendell R, Wald NJ. Randomised trial of text messaging on adherence to cardiovascular preventive treatment (INTERACT trial). PLoS One 2014;9:e114268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park LG, Howie-Esquivel J, Chung ML, Dracup K. A text messaging intervention to promote medication adherence for patients with coronary heart disease: a randomized controlled trial. Patient Educ Couns 2014;94:261–268. [DOI] [PubMed] [Google Scholar]
- 24. Fang R, Li X. Electronic messaging support service programs improve adherence to lipid-lowering therapy among outpatients with coronary artery disease: an exploratory randomised control study. J Clin Nurs 2016;25:664–671. [DOI] [PubMed] [Google Scholar]
- 25. Khonsari S, Subramanian P, Chinna K, Latif LA, Ling LW, Gholami O. Effect of a reminder system using an automated short message service on medication adherence following acute coronary syndrome. Eur J Cardiovasc Nurs 2015;14:170–179. [DOI] [PubMed] [Google Scholar]
- 26. Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 27. McGillicuddy JW, Gregoski MJ, Weiland AK, Rock RA, Brunner-Jackson BM, Patel SK, Thomas BS, Taber DJ, Chavin KD, Baliga PK. Mobile health medication adherence and blood pressure control in renal transplant recipients: a proof-of-concept randomized controlled trial. JMIR Res Protoc 2013;2:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tian M, Ajay V, Dunzhu D, Hameed S, Li X, Liu Z, Li C, Chen H, Cho K, Li R. A cluster-randomized controlled trial of a simplified multifaceted management program for individuals at high cardiovascular risk (SimCard trial) in rural Tibet, China, and Haryana, India. Circulation 2015;132:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldstein CM, Gathright EC, Dolansky MA, Gunstad J, Sterns A, Redle JD, Josephson R, Hughes JW. Randomized controlled feasibility trial of two telemedicine medication reminder systems for older adults with heart failure. J Telemed Telecare 2014;20:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Russell CL, Conn VS, Ashbaugh C, Madsen R, Hayes K, Ross G. Medication adherence patterns in adult renal transplant recipients. Res Nurs Health 2006;29:521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGillicuddy JW, Gregoski MJ, Weiland AK, Rock RA, Brunner-Jackson BM, Patel SK, Thomas BS, Taber DJ, Chavin KD, Baliga PK, Treiber FA. Mobile health medication adherence and blood pressure control in renal transplant recipients: a proof-of-concept randomized controlled trial. JMIR Res Protoc 2013;2:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maric B, Kaan A, Ignaszewski A, Lear SA. A systematic review of telemonitoring technologies in heart failure. Eur J Heart Fail 2009;11:506–517. [DOI] [PubMed] [Google Scholar]
- 33. Kamal AK, Shaikh Q, Pasha O, Azam I, Islam M, Memon AA, Rehman H, Akram MA, Affan M, Nazir S. A randomized controlled behavioral intervention trial to improve medication adherence in adult stroke patients with prescription tailored Short Messaging Service (SMS)-SMS4Stroke study. BMC Neurol 2015;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sailer F, Pobiruchin M, Wiesner M, Meixner G. An approach to improve medication adherence by smart watches. Stud Health Technol Inform 2015;210:956. [Google Scholar]
- 35. Leon N, Surender R, Bobrow K, Muller J, Farmer A. Improving treatment adherence for blood pressure lowering via mobile phone SMS-messages in South Africa: a qualitative evaluation of the SMS-text Adherence SuppoRt (StAR) trial. BMC Fam Pract 2015;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thakkar J, Kurup R, Laba T-L, Santo K, Thiagalingam A, Rodgers A, Woodward M, Redfern J, Chow CK. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Internal Med 2016;176:340–349. [DOI] [PubMed] [Google Scholar]
- 37. Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B. Current science on consumer use of mobile health for cardiovascular disease prevention a scientific statement from the American Heart Association. Circulation 2015;132:1157–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Peiris D, Praveen D, Johnson C, Mogulluru K. Use of mHealth systems and tools for non-communicable diseases in low-and middle-income countries: a systematic review. J Cardiovasc Transl Res 2014;7:677–691. [DOI] [PubMed] [Google Scholar]
- 39. Boutayeb A. The double burden of communicable and non-communicable diseases in developing countries. Trans R Soc Trop Med Hyg 2006;100:191–199. [DOI] [PubMed] [Google Scholar]
- 40. Kahn JG, Yang JS, Kahn JS. ‘Mobile’ health needs and opportunities in developing countries. Health Aff 2010;29:252–258. [DOI] [PubMed] [Google Scholar]
- 41. Cortet B, Bénichou O. Adherence, persistence, concordance: do we provide optimal management to our patients with osteoporosis? Joint Bone Spine 2006;73:e1–e7. [DOI] [PubMed] [Google Scholar]
- 42. Barbosa CD, Balp M-M, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 2012;6:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]