Abstract
Aims
Cardiac disease is one of the leading causes of indirect maternal death, and myocardial infarction (MI) is one of its most common aetiologies. The objectives of this systematic review and meta-analysis were to characterize the incidence of pregnancy-associated MI (PAMI), as well as the maternal mortality and the case-fatality rates due to PAMI.
Methods and results
Articles were obtained by searching electronic databases, bibliographies and conference proceedings with no language or date restrictions. Two reviewers independently selected population-based cohort and case-control studies reporting on incidence, mortality and case-fatality rates for pregnancy-associated MI. Meta-analysis was performed to estimate pooled maternal incidence, mortality and case-fatality rates. Meta-regression was performed to explore heterogeneity. Based on 17 included studies, the pooled incidence of PAMI and maternal mortality from PAMI were 3.34 (2.09–4.58) and 0.20 (0.10–0.29) per 100 000 pregnancies, respectively. The case-fatality rate was 5.03% (3.78–6.27%). Country/region (meta-regression P = 0.006) and years of study (meta-regression P = 0.04) were potential explanations for the observed heterogeneity in the pooled incidence estimates of maternal MI and its associated mortality, with more recent studies and those conducted in the USA revealing the highest rates.
Conclusion
This article provides a global estimate of the incidence, mortality rate, and case fatality rate of pregnancy-associated MI. We identified higher rates of PAMI in the USA (relative to Canada and European countries) and rising rates over time. Further research regarding this population is needed, especially given rising maternal age and the increasing prevalence of cardiovascular risk factors.
Keywords: Incidence , Myocardial infarction , Pregnancy , Maternal , Morbidity , Mortality
Introduction
Pregnancy is considered a ‘cardiovascular stress test’1 that can unmask underlying cardiovascular disease through progressive haemodynamic changes2 which may lead to clinical decompensation and potentially life-threatening disease. While hypertension, sepsis, and haemorrhage remain the leading global causes of maternal death,3 cardiac disease has become the leading cause of maternal mortality in high income countries in recent years4,5 and has gained increasing relevance even in low and middle-income countries.6,7
Myocardial infarction (MI) is a major cause of morbidity and mortality for pregnant and postpartum women. A recent review of cause-specific maternal mortality in Canada showed coronary artery disease to be the leading etiology.4 In the recent UK Confidential Enquiries, ischaemic heart disease and maternal MI were the most common causes of maternal mortality due to cardiac disease.5
Despite this rising risk of maternal mortality and morbidity due to MI, there is an unclear understanding of the true incidence of pregnancy-associated MI (PAMI). In order to allocate resources and design appropriate programs, it is critical to understand the true burden of disease due to PAMI. Understanding the interaction between morbidity and mortality as related to pregnancy is also critical to informing maternal health programmes. Declines in overall maternal mortality observed with improvements in health care have been matched with an increasing proportion of cases of morbidity; a phenomenon described as the obstetric transition.8 In order to address questions related to maternal morbidity, the World Health Organization (WHO) convened a Maternal Morbidity Working Group (MMWG) to address the knowledge gaps regarding maternal morbidity.9,10 As part of this process, the MMWG identified the need for systematic reviews to research current estimates and evidence of maternal conditions of high priority. To date, there have been no systematic reviews that summarize the population-based data on pregnancy-associated MI. Our primary objective for this systematic review and meta-analysis was to characterize the overall incidence of PAMI. We also sought to determine the overall incidence of maternal mortality as well as the case fatality rates due to PAMI.
Methods
Search strategy
We performed this systematic review in accordance with the MOOSE guidelines for reporting of observational studies.11 We identified all potentially relevant articles by searching MEDLINE (1946 through March 2016), EMBASE (1980 through March 2016), PubMed (1960-2016), CENTRAL (Cochrane Central Register of Controlled Trials), and Web of Science Core Collection (1899–2015). This search was enhanced by scanning the bibliographies of identified articles and review articles, as well as by reviewing the conference proceedings from the first four Cardiac Problems in Pregnancy Congresses (2010, Valencia, Spain; 2012, Berlin, Germany; 2014, Venice, Italy; 2016, Las Vegas, USA). We also explored relevant data available in the grey literature by searching publications and reports available via national organizations including Statistics Canada; Health Canada; the Centre for Maternal and Child Health (CEMACH, UK) and the Centers for Disease Control (CDC, USA). There were no language or date restrictions.
As per the strategy recommended for systematic reviews of observational studies,11 we searched the electronic databases using three comprehensive search themes (regarding the population/exposure, outcomes and study design) which were then combined using the Boolean operator ‘AND’. This detailed search strategy is outlined in Supplementary material online, Appendix S1.
Study selection
We included population-based single and multi-centre cohort studies (reporting on the incidence of MI and/or mortality and case fatality rates due to MI in pregnant or postpartum women) as well as case control studies (reporting on case fatality rates). Studies describing only MI occurring outside of pregnancy and the postpartum period (defined as up to 6 weeks after delivery) were excluded. We excluded case reports and case series, since they cannot provide measures of pregnancy-associated MI incidence. Two of the authors (PG and MN) independently screened all abstracts for eligibility. The full text articles of eligible abstracts were then independently reviewed by both authors for inclusion. Discrepancies were resolved by consensus between the two reviewers.
Data extraction and quality assessment
Data were independently extracted by PG and MN, with disagreements resolved by consensus. The following data were extracted: author, publication year, study sample size, cohort demographics, study setting, total number of pregnancies, number of PAMIs, timing of MI (antepartum defined as conception until admission for delivery, peripartum defined as 24–48 h around delivery, postpartum defined as up to 6 weeks after delivery) and number of deaths due to PAMI. The primary outcome was the incidence of PAMI, determined as the number of PAMIs divided by the number of pregnancies. We also determined the rates of maternal mortality (defined as number of maternal deaths/number of total pregnancies) and case fatality (defined as number of maternal deaths/number of PAMI).
Study quality was assessed using an assessment tool based on general quality criteria developed for observational studies12 and tailored to our research question. Our quality assessment addressed whether the research question was clearly stated, the study sample selection was clearly described, study subject demographics were clearly described, inclusion and exclusion criteria were pre-specified and applied uniformly to all participants, MI diagnosis criteria were clearly defined and potentially important baseline risk factors that may influence MI incidence were identified.13 These quality elements were adjudicated as good, if clearly described and given a score of 1, or as poor if unclear, inadequately or not described and given a score of 0. These quality factors were evaluated individually and aggregated for a total quality score that could range from zero to six (out of a total score of six).
Data synthesis and analysis
The incidence of pregnancy-associated MI, along with 95% CIs, was identified for each study. For small proportions (when the numerator is too small), the calculated lower limit of a confidence interval may fall below zero based on a Gaussian distribution. To ensure that all CIs were between 0 and 1, the Wilson score interval was calculated using a binomial distribution.14 This has been shown to be suitable for studies with small sample size and/or extreme probabilities.15 Weights for the individual studies were calculated using the inverse of the variance method. To obtain a pooled estimate of the incidence of PAMI, a fixed effect model (using the method of Mantel and Haenszel) was initially performed. The Q-statistic was calculated to assess for significant heterogeneity between the included studies. If significant heterogeneity was observed using the fixed effect model, we performed a meta-analysis using a random effects model (using the method of DerSimonian & Laird) to obtain a pooled estimate of PAMI. We used meta-regression to explore potential factors associated with heterogeneity that might affect the incidence of PAMI. These were defined a priori and included country/region of study, time period of study, timing of PAMI and study quality (individual quality factors and total quality score).
All analyses were performed using STATA 13.0 (Statacorp, College Station, TX). A two-sided P-value less than 0.05 was considered statistically significant for meta-analyses and a P-value of less than 0.1 was considered statistically significant during meta-regression.
Results
Identification of studies
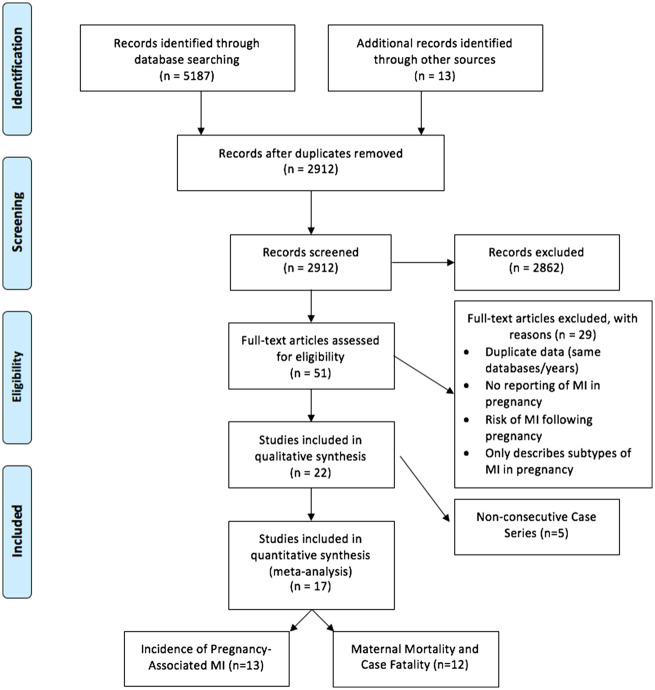
Figure 1 shows the study selection process. Following title/abstract screening, 51 papers remained for full text review. Of these, 17 studies comprising 75 570 508 women, met the inclusion criteria.4,5,16–29 Of the 34 excluded studies, seven reported on non-original data, 13 were not population-based, five were non-consecutive case series, three reported on cohorts with a history of prior ischemia or cardiomyopathy and six reported only on specific subtypes of MIs. Six studies which analyzed data from the same database–the US-based Nationwide Inpatient Sample (NIS) of the Healthcare Cost and Utilization Project (HCUP)–were identified,19,22,30–33 with overlapping years of study. In order to avoid duplication of data sampling, three studies were selected for inclusion in our analysis (covering the years of 2000–2002, 2004–2006, and 2008–2010), and a sensitivity analysis was performed evaluating the effects of substituting the excluded studies in their place (see Supplementary material online, Appendix S2). Such substitutions did not significantly alter the primary or secondary outcomes reported.
Figure 1.
Article flow diagram.
Of the 17 included articles, 13 reported on the primary outcome of PAMI incidence,16–18,21–27,29,31,33 eight of which also reported on mortality and/or case-fatality rates. The other four studies4,5,20,28 did not describe the incidence of PAMI but reported on mortality (total 12 studies) and/or case fatality (total 9 studies) rates due to PAMI.
Details of included studies
Table 1 shows details of the studies that met our inclusion criteria and reported on the incidence, maternal mortality and/or case fatality related to PAMI. The periods of study ranged from 196020 to 2011,34 and the study sample sizes ranged from 358 87421 to 12 670 12631 patients. Of the 17 study cohorts, eight were based in the United States (USA),16,17,20,22,23,26,31,33 three in Canada,4,25,29 two in the United Kingdom (UK),5,18 two in the Netherlands,21,28 one in Sweden,27 and one in Taiwan.24 No studies from low and middle income countries (LMICs) were identified. All included studies acquired their samples from either national or regional patient registries/databases or data collected from multiple centres.
Table 1.
Characteristics of included studies and their outcomes
| Study characteristics |
Outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Study setting | Sample size | Country | Study period | Pregnancy timing | Outcomes reported | MI incidence (per 100 000 pregnancies) | Mortality rate (per 100 000 pregnancies) | Case-fatality rate (%) |
| Bateman et al. 2013(a) | Medicaid database | 854 823 | USA | 2000–2007 | Antepartum postpartum | MI incidence | 7.60 (5.97–9.69) | — | — |
| Bateman et al. 2013(b) | Database | 2 233 630 | USA | 2007–2011 | Peripartum | MI incidence; mortality; case-fatality | 2.60 (2.01–3.36) | 0.13 (0.05–0.40) | 6.25 (1.31–17.20) |
| Bush et al. 2013 | OKOSS database | 3 444 507 | UK | 2005–2010 | Antepartum postpartum | MI incidence; mortality; case-fatality | 0.72 (0.49–1.07) | 0.00 (0.00–0.11) | 0.00 (0.00–30.85) |
| Cantwell et al. 2011 | Maternal mortality enquiry | 2 294 372 | UK | 1994–2008 | Antepartum peripartum postpartum | Mortatlity | — | 0.48 (0.27–0.87) | — |
| Grotegut et al. 2014 | National inpatient sample | 12 628 746 | USA | 2008–2010 | Peripartum postpartum | MI incidence | 3.05 (2.74–3.35) | — | — |
| Hibbard 1975 | State database | 3 194 000 | USA | 1960–1968 | Antepartum peripartum postpartum | Mortality | — | 0.28 (0.15–0.54) | — |
| Huisman et al. 2013 | Multicenter | 358 874 | Netherlands | 2004–2006 | Peripartum postpartum | MI incidence; mortality; case-fatality | 2.79 (1.51–5.13) | 0.28 (0.05–1.58) | 10 (0.25–44.50) |
| James et al. 2006 | National inpatient sample | 12 595 624 | USA | 2000–2002 | Antepartum postpartum | MI incidence; mortality; case-fatality | 6.82 (6.38–7.29) | 0.35 (0.26–0.47) | 5.12 (3.75–6.82) |
| Kuklina et al. 2010 | National inpatient sample | 12 670 176 | USA | 2004–2006 | Peripartum postpartum | MI incidence | 5.77 (5.49–6.05) | — | — |
| Ladner et al. 2005 | State database | 5 393 228 | USA | 1991–2000 | Antepartum peripartum postpartum | MI incidence; mortality; case-fatality | 2.80 (2.39–3.28) | 0.22 (0.13–0.39) | 7.95 (4.17–13.47) |
| Lin et al. 2011 | Registry | 1 132 064 | Taiwan | 1999–2003 | Peripartum | MI incidence | 2.92 (2.08–4.09) | — | — |
| Macarthur et al. 2006 | CIHI discharge database | 10 032 375 | Canada | 1970–1998 | Peripartum | MI incidence; mortality; case-fatality | 1.14 (0.95–1.36) | 0.02 (0.01–0.07) | 1.75 (0.21–6.19) |
| Mulla et al. 2015 | Hospital discharge database | 1 573 740 | USA | 2004–2007 | Antepartum peripartum postpartum | MI incidence; mortality; case-fatality | 6.54 (5.40–7.94) | 0.64 (0.34–1.17) | 9.71 (4.75–17.13) |
| Rusen et al. 2004 | National registry | 1 054 828 | Canada | 1997–2000 | Antepartum peripartum postpartum | Mortatlity; case fatality | — | 0.38 (0.15–0.98) | 12.90 (3.63–29.83) |
| Salonen Ros et al. 2001 | National registry | 1 003 489 | Sweden | 1987–1995 | Antepartum peripartum postpartum | MI incidence | 0.60 (0.27–1.30) | — | — |
| Schutte et al. 2010 | Maternal mortality enquiry | 2 557 208 | Netherlands | 1993–2005 | Antepartum peripartum postpartum | Mortality | — | 0.20 (0.08–0.46) | — |
| Wen et al. 2005 | CIHI discharge database | 2 548 824 | Canada | 1991–2000 | Peripartum | MI incidence; mortality; case-fatality | 1.22 (0.86–1.73) | 0.08 (0.02–0.29) | 6.45 (0.79–21.42) |
MI, myocardial infarction.
Study quality assessment
Table 2 displays the quality assessment of the included studies. The mean total quality score for the included studies was 4.6 out of six, with a range of three to six (indicating a range from moderate to excellent study quality). For all studies the research question, MI definition, and inclusion/exclusion criteria were clearly described. Seven studies had poor/inadequate description of subject demographics for their sample, such as the age and ethnicity of women included in the study. There was inconsistent and variable reporting of the prevalence of potentially important risk maternal factors such as body mass index, medical comorbidities (such as diabetes and hypertension) and pregnancy complications that may influence PAMI incidence.
Table 2.
Quality indicators of included studies
| Authoryear | Research question described | Sample described | Demographics described | Inclusion/exclusion criteria | MI clearly defined | Risk factors described | Overall quality score |
|---|---|---|---|---|---|---|---|
| Hibbard et al. 1975 | Yes | No | No | Yes | Yes | No | 3 |
| Salonen Ros et al. 2001 | Yes | Yes | No | Yes | Yes | No | 4 |
| Rusen et al. 2004 | Yes | No | No | Yes | Yes | No | 3 |
| Ladner et al. 2005 | Yes | No | Yes | Yes | Yes | Yes | 5 |
| Wen et al. 2005 | Yes | Yes | No | Yes | Yes | No | 4 |
| James et al. 2006 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Macarthur et al. 2006 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Schutte et al. 2010 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Kuklina et al. 2010 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Cantwell et al. 2011 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Lin et al. 2011 | Yes | No | No | Yes | Yes | No | 3 |
| Bateman et al. 2013 (a) | Yes | No | No | Yes | Yes | No | 3 |
| Bateman et al. 2013 (b) | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Bush et al. 2013 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Huisman et al. 2013 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Grotegut et al. 2014 | Yes | No | No | Yes | Yes | Yes | 4 |
| Mulla et al. 2015 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
MI, myocardial infarction.
Incidence of myocardial infarction in pregnancy
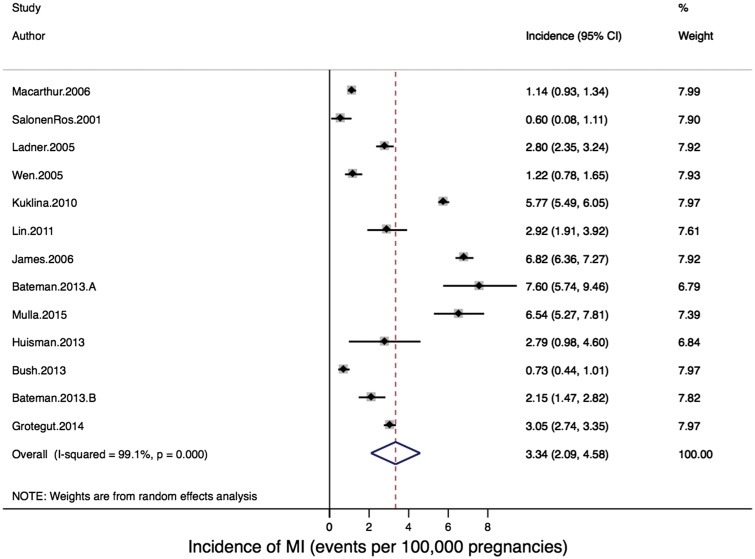
Thirteen studies reported on the primary outcome of pregnancy-associated MI incidence, including a total cohort of 66 470 100 pregnancies. The reported incidence of pregnancy-associated MI ranged from 0.6027 to 7.6017 per 100 000 pregnancies (Figure 2). When individual studies were combined in meta-analysis, there was significant heterogeneity: Q-statistic, P < 0.001; I2 = 98.5%, P < 0.001. The pooled estimate of pregnancy-associated MI using a random effects model was 3.34 per 100 000 pregnancies (95% CI: 2.09–4.58 per 100 000 pregnancies; Figure 2).
Figure 2.
Forest plot of incidence of pregnancy-associated myocardial infarction.
In meta-regression two factors were associated with significant heterogeneity: country/region of study (meta-regression P = 0.006) and study period (meta-regression P = 0.04) (Table 3). In stratified analysis, studies conducted in the USA reported the highest incidence proportions of pregnancy-associated MI (4.87/100 000 pregnancies), while studies done in Canada (1.15/100 000 pregnancies) and Europe (0.84/100 000 pregnancies) reported the lowest proportions. Similarly, in stratified analysis the studies examining cohorts between 2000 and 2009 reported the highest overall incidence of pregnancy-associated MI (4.39/100 000 pregnancies). Meta-regression evaluating differences by timing of PAMI during pregnancy (reported in 9 of the 13 included studies) did not explain the observed heterogeneity (meta-regression P = 0.54), with similar rates of PAMI described during the antepartum (1.68/100 000 pregnancies), peripartum (1.10/100 000 pregnancies) and postpartum periods (1.11/100 000 pregnancies). A sensitivity analysis restricted to the five papers which reported on risk factors for PAMI18,22,23,25,26 revealed an incidence and range of PAMI which was very similar to the entire cohort of studies (3.56 events/100 000 pregnancies, range 0.73–6.82). Finally, none of the ‘study quality’ assessment components, nor the aggregate study quality score, were identified as significant sources of heterogeneity (P = 0.84, see Table 3).
Table 3.
Stratified meta-analysis and meta-regression of pregnancy-associated MI incidence and maternal mortality by methodological and clinical source
| Potential variable | Cohort stratifications | MI incidence (per 100 000 pregnancies) | P-value | Maternal mortality (per 100 000 pregnancies) | P-value |
|---|---|---|---|---|---|
| Country/region | Canada | 1.15 (0.96–1.34) | 0.006 | 0.06 (–0.04–0.15) | 0.29 |
| Europe | 0.84 (0.29–1.40) | 0.19 (–0.04–0.42) | |||
| Taiwan | 2.92 (1.91–3.92) | N/A | |||
| USA | 4.87 (3.42–6.33) | 0.28 (0.17–0.38) | |||
| Study period | 1970–1989 | 0.92 (0.40–1.44) | 0.04 | 0.13 (–0.12–0.39) | 0.38 |
| 1990–1999 | 2.27 (1.06–3.49) | 0.17 (0.08–0.26) | |||
| 2000–2009 | 4.39 (2.56–6.23) | 0.28 (0.07–0.49) | |||
| Study quality | Range 3–6 (0–6) | Variable | 0.84 | Variable | 0.94 |
| Pregnancy timing | Antepartum | 1.68 (–0.14–3.50) | 0.54 | N/A | |
| Peripartum | 1.10 (0.43–1.77) | N/A | N/A | ||
| Postpartum | 1.11 (0.00–2.22) | N/A |
MI, myocardial infarction.
Maternal mortality and case-fatality due to pregnancy-associated myocardial infarction
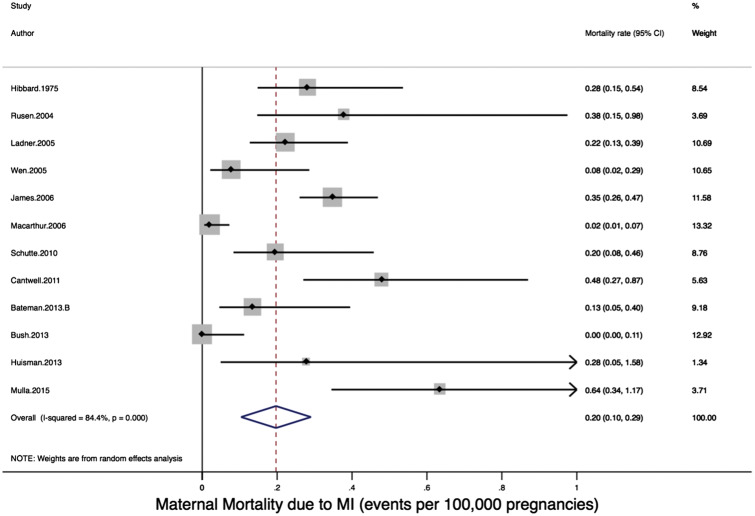
Twelve studies reported on the secondary outcome of mortality rate due to PAMI, with a total cohort of 47 281 210 pregnancies. The rate of maternal mortality due to MI ranged from 0.0018 to 0.6426 per 100 000 pregnancies (Figure 3). In meta-analysis, there was significant heterogeneity: Q-statistic, P < 0.001; I2 = 84.4%, P < 0.001. The pooled estimate of mortality rate due to pregnancy-associated MI using a random effects model was 0.20 per 100 000 pregnancies (95% CI: 0.10–0.29 per 100 000 pregnancies; Figure 3). In meta-regression, one factor was identified which may have contributed to the heterogeneity seen: country/region of study (meta-regression P = 0.29, P-value for USA-based studies vs. Canada/Europe combined = 0.09) (Table 3). In stratified analysis, studies conducted in the USA reported the highest rates of maternal mortality due to pregnancy-associated MI (0.28/100 000 pregnancies), while studies done in Canada (0.06/100 000 pregnancies) and Europe (0.19/100 000 pregnancies) reported lower rates. Examining cohorts by period of study did not explain the observed heterogeneity (meta-regression P = 0.38), although studies examining cohorts between 2000 and 2009 reported higher overall rates of maternal mortality due to PAMI (0.28/100 000 pregnancies) relative to earlier cohorts (0.13–0.17/100 000 pregnancies). None of the ‘study quality’ assessment components, nor the aggregate study quality score, were identified as significant sources of heterogeneity (P = 0.94, see Tables 2 and 3). Timing of MI during pregnancy was not consistently reported in the studies reporting on maternal mortality due to PAMI and was not evaluated as a cause of heterogeneity.
Figure 3.
Forest plot of incidence of maternal mortality due to pregnancy-associated myocardial infarction.
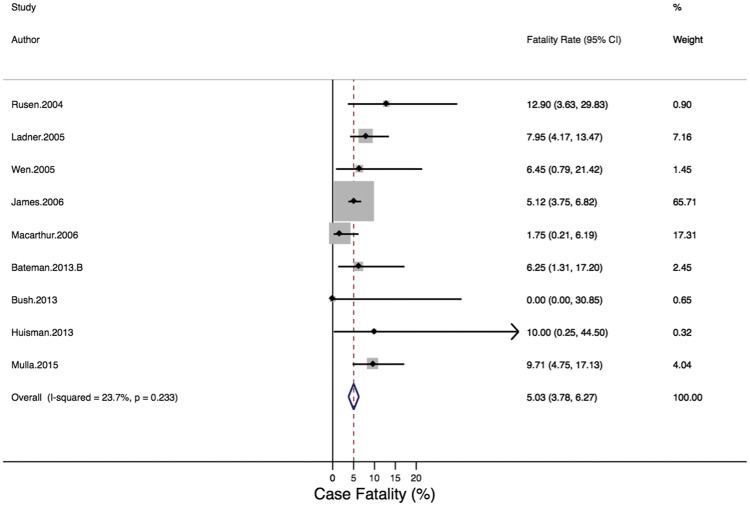
Nine studies (all cohort studies) reported on the secondary outcome of case-fatality rate due to PAMI, with a total cohort of 39 235 630 pregnancies. The case-fatality rate due to maternal MI ranged from 0.00%18 to 12.90%4 (Figure 4). When individual studies were combined in a meta-analysis, there was no significant heterogeneity: Q-statistic, P = 0.29; I2 = 23.7%, P =0.233. The case-fatality rates did not vary significantly by country/region or by period of study. The pooled estimate of case-fatality due to pregnancy-associated MI using a fixed effect model was 5.03% (95% CI: 3.78–6.27%; Figure 4).
Figure 4.
Forest plot of case-fatality rate due to pregnancy-associated myocardial infarction.
Discussion
Our study is the first systematic review and meta-analysis characterizing the incidence of pregnancy-associated MI. We determined an estimated pooled incidence of PAMI of 3.34 (2.09–4.58) per 100 000 pregnancies, based on 13 population-based studies reporting on over 66 million pregnancies in six countries. The maternal mortality rate due to PAMI among this global population of women was 0.20 (0.10–0.29) per 100 000 pregnancies, with a case fatality of 5.03% (3.78–6.27%). Our pooled estimate of the incidence of PAMI falls within the range of recently reported population-based studies. The most commonly referenced studies from the USA. in recent years reported rates of maternal MI of 2.8–6.8/100 000 pregnancies,22,23 and a recent multicenter study from the UK identified a rate of maternal MI of 0.72/100 000.18 However, there was significant heterogeneity in the incidence of pregnancy-associated MI across the population-based studies identified. Given the few number of countries included in the review and the fact that no data from LMIC countries was identified, it is unlikely that there is one ‘true’ global incidence of PAMI. Rather, differences over time and between regions will reflect true variation in this important maternal health outcome. The summary estimate of 3.34 PAMI events per 100 000 pregnancies is therefore a ‘weighted average’ of PAMI incidence. We explored the potential sources of heterogeneity by meta-regression, in which we observed a significantly higher reported incidence of maternal MI among U.S-based study cohorts (incidence of PAMI: 4.98/100 000 pregnancies) and in one study from Taiwan (2.92/100 000 pregnancies). This is much higher than the rates observed in similar developed countries (Canada and Europe) of 0.84–1.15 MIs/100 000 pregnancies.
The reasons for this apparent difference in national rates of PAMI are speculative, including differential access to medical care or population-based differences in the risk status of the maternal populations studied. Although the populations studied were from HIC, the nature of the study populations, particularly with reference to maternal age, racial mix, obesity, and other medical comorbidities which might predispose to MI, were poorly elucidated in the included studies (as various maternal comorbidities and pregnancy complications were inconsistently described in only 5 of 13 studies) due to their database methodology. As such, we were not able to assess whether the American maternal population varies with respect to known MI risk factors or comorbidities relative to other regions.
It is also possible that reporting tendencies may have biased these findings. In the US-based studies, several of which were based on the National Inpatient Sample of the US-Healthcare Cost and Utilization Project (HCUP) Database, a higher sensitivity to report on PAMI may have occurred. This finding of higher maternal risk in American women, however, is concordant with recent estimates of maternal mortality (up until 2015)35 which revealed a higher overall maternal mortality rate in the USA (14 per 100 000 pregnancies) relative to Canada (7/100 000), the UK (9/100 000), the Netherlands (7/100 000) and Taiwan (14/100 000).36 The influence of improved identification and documentation of maternal deaths on estimates which are intended to be internationally comparable remains under debate. The country/region-specific rates of maternal MI identified in our study mirror the regional differences in maternal mortality–thus providing supportive evidence that these differences are a real phenomenon.
We identified that pregnancy-associated myocardial infarction is becoming more common, paralleling the widespread phenomenon of increasing mean maternal age due to delayed maternity,37 as well as the global rise in obesity and metabolic syndrome.38 Increasing maternal age also confers a higher likelihood of accumulating medical comorbidities such as hypertension, diabetes, and dyslipidemia, thereby increasing cardiovascular risk. This increasing incidence of maternal MI was noted in the recent study by Ladner et al.,23 which reported that the rate of maternal MI in California increased from 1 in 73 400 to 1 in 24 600 between the years 1991–2000. Similarly, in the latest periodic review on maternal mortality in the UK, maternal mortality dropped slightly relative to the previous triennium (after rising progressively over the previous 9 years) but maternal mortality due to cardiac disease continued to rise (from 1.01 deaths per million pregnancies in 1985–1987 to 2.31 deaths per million pregnancies in 2006–2008).5
We found that the rate of maternal MI appears to be highest antepartum (1.68/100 000 pregnancies), with the subsequent incidence equally distributed across the peripartum (1.10/100 000) and postpartum (1.11/100 000) periods. Although pregnancy stage was not identified as a significant source of heterogeneity in our study, it is noteworthy that the antepartum interval is long (up to 40 weeks) relative to the peripartum (1–2 days) and postpartum (6 weeks) periods. This supports the clinical impression that while many maternal MIs may occur during the antepartum period, the latter two intervals confer the greatest day-to-day risk of pregnancy-associated MI. Mechanisms for an increased ‘day to day’ risk during the peripartum and postpartum periods might include the increased metabolic demands on the heart during labour, the use of uterotonic medications,16 as well as a dramatic return of cardiac preload following delivery. A recent case series suggested that the mechanism of maternal MI may also vary by the timing of the events, with atherosclerosis and thrombosis being most common among antepartum MI events while coronary artery dissection is the most common mechanism for postpartum MIs.39 Studies such as this are subject to publication bias, however, and may not reflect the true spectrum of pathology manifested in an obstetric population. The mechanism of MI was not consistently reported in these population-based database studies, and as such whether the mechanism of pregnancy-associated MI truly varies with the timing in pregnancy will require detailed evaluation of consecutive events in a large cohort/population and application of standard diagnostic criteria.
One of the strengths of our study is the inclusion of multiple population-based databases to synthesize all the existing worldwide published data supplemented by the search of multiple relevant national data sources. We included studies from six countries on three continents and limited our work to studies of general obstetric populations, rather than high-risk groups, in order to make the results widely generalizable. Despite the wide search, we were unable to identify any studies from LMICs. As we believe that PAMI is a significant global cause of maternal morbidity and mortality, more direct data from LMICs would be very useful to guide planning and resource allocation for maternal cardiac care in all regions; especially in light of reports of increasing incidence of non-communicable diseases. Our study has several other limitations. The rate of PAMI may have been overestimated in some population-based studies using large databases which rely on discharge diagnostic coding for the relevant (primary) outcome. Although this methodology has been validated as useful for population-based evaluation of rare outcomes,40 and the specificity of the diagnosis of myocardial infarction in database analyses may be as high as 97%,41 it is possible that the incidence of PAMI was overestimated in database studies relative to multicenter studies (in which the outcomes were more adjudicated).18 On the other hand, not all studies that we included reported on MI incidence during all time periods of pregnancy (antepartum, peripartum, and postpartum). If the timing-specific rates of MI were combined, we might arrive at a higher overall incidence of PAMI of up to 3.89/10 000 pregnancies (Table 3). Another limitation is that there is little detail regarding the mechanism of and risk factors for PAMI in the large databases required to evaluate the rare event of pregnancy-associated MI. Finally, a number of American studies that we initially identified were based on the same Nationwide Inpatient Sample database, resulting in overlapping cohorts of patients and necessitating the exclusion of studies with otherwise useful data.
Conclusions
Our study provides a comprehensive and global estimate of the incidence, mortality and case fatality rates of pregnancy-associated myocardial infarction. We identified regional/national differences and an increasing incidence of PAMI over time. Given the ongoing trend of increasing maternal age, as well as the rising prevalence of obesity and diabetes, further attention to and research regarding this population is needed. This is especially true in the context of the Sustainable Development Goals (SDG) which seek to end preventable maternal mortality.42 Both the Ending Preventable Maternal Mortality Strategy and the UN Secretary General’s Global Strategy for Women’s, Children’s, and Adolescent’s Health bring further nuance and detail to the vision of maternal health and stress the need for improved data and improving health systems.43,44 This is particularly relevant for maternal health, among which data on health outcomes and co-morbidities have been poorly documented or scarce. Research and collection of empirical data on maternal and perinatal outcomes should be strengthened, especially in LMICs.
Supplementary material
Supplementary material is available at European Heart Journal–Quality of Care and Clinical Outcomes online.
Funding
Funding was provided by the Bill and Melinda Gates Foundation and the WHO's Department of Reproductive Health and Research.
Conflict of interest: The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated.
Supplementary Material
References
- 1. Williams D. Pregnancy: a stress test for life. Curr Opin Obstetr Gynecol 2003;15:465–471. [DOI] [PubMed] [Google Scholar]
- 2. Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation 2014;130:1003–1008. [DOI] [PubMed] [Google Scholar]
- 3.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2(6):e323–333. [DOI] [PubMed] [Google Scholar]
- 4. Rusen ID. ea. Special Report on Maternal Mortality and Severe Morbidity in Canada. Ottawa: Minister of Public Works and Government Services Canada; 2004. [Google Scholar]
- 5. Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, Harper A, Hulbert D, Lucas S, McClure J, Millward-Sadler H, Neilson J, Nelson-Piercy C, Norman J, O'Herlihy C, Oates M, Shakespeare J, de Swiet M, Williamson C, Beale V, Knight M, Lennox C, Miller A, Parmar D, Rogers J, Springett A. Saving mothers' lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118(Suppl 1):1–203. [DOI] [PubMed] [Google Scholar]
- 6.Campanharo FF, Cecatti JG, Haddad SM, Parpinelli MA, Born D, Costa ML, Mattar R; Brazilian Network for Surveillance of Severe Maternal Morbidity Study Group. The Impact of Cardiac Diseases during Pregnancy on Severe Maternal Morbidity and Mortality in Brazil. PLoS One 2015;10(12):e0144385. [DOI] [PMC free article] [PubMed]
- 7. Mocumbi AO, Sliwa K, Soma-Pillay P. Medical disease as a cause of maternal mortality: the pre-imminence of cardiovascular pathology. Cardiovasc J Africa 2016;27:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Souza J, Tuncalp O, Vogel J, Bohren M, Widmer M, Oladapo O. Obstetric transition: the pathway towards ending preventable maternal deaths. BJOG 2014;121:1–4. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Evaluating the Quality of Care for Severe Pregnancy Complications: The WHO Near-Miss Approach for Maternal Health. Geneva: WHO; 2011. [Google Scholar]
- 10. Say L, Souza J, Pattinson R. Maternal near miss–towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol 2009;23:287–296. [DOI] [PubMed] [Google Scholar]
- 11. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 12. National Heart L, and Blood Institute (NIH). Quality assessment tool for observational cohort and cross-sectional studies. 2016. http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort (14 December 2016).
- 13.Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7(12):1272–1278. [DOI] [PubMed]
- 14. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Statis Assoc 1927;22:209–212. [Google Scholar]
- 15. Agresti A, Coull BA. Approximate is better than “Exact” for interval estimation of binomial proportions. Am Statist 1998;52:119–126. [Google Scholar]
- 16. Bateman BT, Huybrechts KF, Hernandez-Diaz S, Liu J, Ecker JL, Avorn J. Methylergonovine maleate and the risk of myocardial ischemia and infarction. Am J Obstet Gynecol2013;209(5):459–459. [DOI] [PMC free article] [PubMed]
- 17. Bateman BT, Mhyre JM, Hernandez-Diaz S, Huybrechts KF, Fischer MA, Creanga AA, Callaghan WM, Gagne JJ. Development of a comorbidity index for use in obstetric patients. Obstetr Gynecol 2013;122:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bush N, Nelson-Piercy C, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M; UKOSS. Myocardial infarction in pregnancy and postpartum in the UK. Eur J Prev Cardiol 2013;20(1):12–20. [DOI] [PubMed] [Google Scholar]
- 19. Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstetr Gynecol 2012;120:1029–1036. [DOI] [PubMed] [Google Scholar]
- 20. Hibbard LT. Maternal mortality due to cardiac disease. Clin Obstetr Gynecol 1975;18:27–36. [DOI] [PubMed] [Google Scholar]
- 21. Huisman CM, Zwart JJ, Roos-Hesselink JW, Duvekot JJ, van Roosmalen J. Incidence and predictors of maternal cardiovascular mortality and severe morbidity in the netherlands: a prospective cohort study. Plos One 2013;8(2):e56494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation 2006;113:1564–1571. [DOI] [PubMed] [Google Scholar]
- 23. Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstetr Gynecol 2005;105:480–484. [DOI] [PubMed] [Google Scholar]
- 24.Lin YS, Tang CH, Yang CY, Wu LS, Hung ST, Hwa HL, Chu PH. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol 2011;107(2):325–330. [DOI] [PubMed] [Google Scholar]
- 25. Macarthur A, Cook L, Pollard JK, Brant R. Peripartum myocardial ischemia: a review of Canadian deliveries from 1970 to 1998. Am J Obstetr Gynecol 2006;194:1027–1033. [DOI] [PubMed] [Google Scholar]
- 26. Mulla Zd Fau-Wilson B, Wilson B Fau-Abedin Z, Abedin Z Fau-Hernandez LL, Hernandez Ll Fau-Plavsic SK, Plavsic SK. Acute myocardial infarction in pregnancy: a statewide analysis. J Registr Manage 2015;42:12–17. [PubMed] [Google Scholar]
- 27. Salonen Ros H, Lichtenstein P, Bellocco R, Petersson G, Cnattingius S. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology 2001;12:456–460. [DOI] [PubMed] [Google Scholar]
- 28. Schutte JM, de Jonge L, Schuitemaker NW, Santema JG, Steegers EA, van Roosmalen J. Indirect maternal mortality increases in the Netherlands. Acta obstetricia et gynecologica Scandinavica 2010;89:762–768. [DOI] [PubMed] [Google Scholar]
- 29.Wen SW, Huang L, Liston R, Heaman M, Baskett T, Rusen ID, Joseph KS, Kramer MS; Maternal Health Study Group, Canadian Perinatal Surveillance System. Severe maternal morbidity in Canada, 1991–2001. CMAJ 2005; 173(7):759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995-2006. BJOG 2011;118:345–352. [DOI] [PubMed] [Google Scholar]
- 31. Kuklina EV, Callaghan WM. Cardiomyopathy and other myocardial disorders among hospitalizations for pregnancy in the United States: 2004-2006. Obstetr Gynecol 2010;115:93–100. [DOI] [PubMed] [Google Scholar]
- 32. Lima FV, Parikh PB, Zhu J, Yang J, Stergiopoulos K. Association of cardiomyopathy with adverse cardiac events in pregnant women at the time of delivery. 2015;3:257–266. [DOI] [PubMed] [Google Scholar]
- 33. Grotegut CA, Chisholm CA, Johnson LN, Brown HL, Heine RP, James AH. Medical and obstetric complications among pregnant women aged 45 and older. PLoS One 2014;9:e96237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bateman BT, Huybrechts KF, Hernandez-Diaz S, Liu J, Ecker JL, Avorn J. Methylergonovine maleate and the risk of myocardial ischemia and infarction. Am J Obstetr Gynecol 2013;209:459.e1–459.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization. Trends in maternal mortality: 1990 to 2015: estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division; 2015. http://www.who.int/reproductivehealth/publications/monitoring/maternal-mortality-2015/en/ (14 December 2016)
- 36. Hogan MC, Foreman KJ, Naghavi M, Ahn SY, Wang M, Makela SM, Lopez AD, Lozano R, Murray CJ. Maternal mortality for 181 countries, 1980-2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 2010;375:1609–1623. [DOI] [PubMed] [Google Scholar]
- 37. Mills TA, Lavender T. Advanced maternal age. Obstetr Gynaecol Reproduc Med 21:107–111. [Google Scholar]
- 38. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9:13–27. [DOI] [PubMed] [Google Scholar]
- 39. Elkayam U, Jalnapurkar S, Barakkat MN, Khatri N, Kealey AJ, Mehra A, Roth A. Pregnancy-associated acute myocardial infarction: a review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014;129:1695–1702. [DOI] [PubMed] [Google Scholar]
- 40. Schoenman JA, Sutton JP, Kintala S, Love D, Maw R. The value of hospital discharge databases. Agency Healthcare Res Quality 2005. [Google Scholar]
- 41. Petersen LA, Wright S, Normand S-LT, Daley J. Positive predictive value of the diagnosis of acute myocardial infarction in an administrative database. J Gen Int Med 1999;14:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. United Nations. Goal 3: Ensure healthy lives and promote well-being for all at all ages. 2015http://www.un.org/sustainabledevelopment/health/.
- 43. World Health Organization. Strategies Toward Ending Preventable Maternal Mortality (EPMM). Geneva: WHO; 2015. http://who.int/reproductivehealth/topics/maternal_perinatal/epmm/en/ (14 December 2016). [Google Scholar]
- 44. United Nations Secretary-General. The Global Strategy for Women’s, Children’s and Adolescents' Health (2016-2030). New York: United Nations; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.