Abstract
Aims
Transcatheter aortic valve implantation (TAVI) is an increasingly common intervention for patients with aortic stenosis deemed high risk for major cardiac surgery, but identifying those who will benefit can be challenging. Frailty reflects physiological reserve and may be a useful prognostic marker in this population. We performed a systematic review and meta-analysis of the association between frailty and outcomes after TAVI.
Methods and Results
Five databases were searched between January 2000 and May 2015. From 2623 articles screened, 54 were assessed for eligibility. Ten cohort studies (n = 4592) met the inclusion criteria of reporting a measure of frailty with early (≤30 days) or late (>30 days) mortality and procedural complications following TAVI as defined by the Valve Academic Research Consortium (VARC). Frailty was associated with increased early mortality in four studies (n = 1900) (HR 2.35, 95% CI 1.78–3.09, P < 0.001) and increased late mortality in seven studies (n = 3159) (HR 1.63, 95% CI 1.34–1.97, P < 0.001). Objective frailty tools identified an even higher risk group for late mortality (HR 2.63, 95% CI 1.87–3.70, P < 0.001). Frail individuals undergoing TAVI have a mortality rate of 34 deaths per 100 patient years, compared with 19 deaths per 100 patient years in non-frail patients. There was limited reporting of VARC procedural outcomes in relation to frailty, preventing meta-analysis.
Conclusion
Frailty assessment in an already vulnerable TAVI population identifies individuals at even greater risk of poor outcomes. Use of objective frailty tools may inform patient selection, but this requires further assessment in large prospective registries.
Keywords: TAVI, Frailty, Aortic stenosis, Prognosis, Risk factors, Ageing
Introduction
Aortic stenosis is the most common valvular disease in the Western World, affecting 1 in 8 individuals over the age of 75 years. The incidence of functionally important disease is rising in line with the ageing population, providing challenges for conventional valve replacement surgery.1 Patients over 80 years old undergoing elective cardiac surgery have more operative complications and a 10% mortality rate at 30 days; therefore, decisions around intervention in older patients are complex.2 Transcatheter aortic valve implantation (TAVI) has become a widespread and viable alternative for patients considered high risk for conventional surgery. Population modelling suggests in excess of 91 000 people fall into this category across North America each year.1 The Society of Thoracic Surgeons (STS)3 and EuroSCORE4 tools are often used to guide treatment based on the predicted risk of poor outcomes, but these scoring systems have not been designed or formally tested in TAVI populations. The application of such scores in elderly patients suitable for conventional surgery has also been questioned.5,6 Many believe that a holistic approach through frailty assessment may improve the decision-making process.
Frailty is a multimodal concept describing loss of strength, endurance, and physiological reserve across multiple systems that increases vulnerability for developing dependency or death.7 It becomes more common with age but is a very distinct concept of biological rather than chronological years; indeed, the majority of individuals over 85 years old are not frail. Common models focus on the development of a phenotype or the gradual accumulation of deficits over time, but there is no clear consensus on the best form of measurement.7–9 Within non-cardiac surgical cohorts, frailty is predictive of mortality, post-operative complications, and institutionalization.10–13 It is plausible that such measures applied to high-risk patients undergoing TAVI may improve the discrimination of current risk assessment tools for important patient outcomes. In this systematic review, we evaluate the effect of preoperative frailty on important patient outcomes after TAVI.
Methods
Search strategy
We conducted a systematic literature review of Medline, EMBASE, and CINAHL databases between 1 January 2000 and 1 June 2015 using the key search terms of frailty (and its synonyms) and TAVI (and its synonyms) (see Supplementary material online, Appendix). Reference and forward citation searching via the Web of Science (Thomson Reuters) was performed on papers meeting the criteria for inclusion. Hand-searching using the primary search terms was performed within the three most commonly identified journals from the initial search. This was repeated using the Google Scholar search engine.
Eligibility criteria
We included any primary peer-reviewed paper where a measure of frailty was defined by the authors prior to TAVI, and where this was related to at least one of the predefined post-TAVI outcomes. No other assessments were adjudicated to represent frailty unless stipulated as a determinant of frailty by the authors of a study. No restrictions were placed on the age of study participants, specific vascular route, or operator technique by which TAVI was performed. Results in all languages were considered, using translation services where required to adjudicate eligibility.
The primary outcome was all-cause mortality after TAVI, either reported in the short (≤30 days) or long term (>30 days). Secondary outcomes comprised procedural complications as defined by the Valve Academic Research Consortium (VARC) standardized endpoint definitions. These include cardiovascular mortality, myocardial infarction, major stroke, bleeding, acute kidney injury requiring dialysis, and numerous other vascular complications.14 Any measures of functional capacity or patient independence after TAVI were sought as secondary outcomes where the relationship to a pre-TAVI frailty measure was presented. Review articles and non-peer-reviewed material (such as conference proceedings and poster abstracts) were excluded.
Data extraction
All extracted abstracts and full-text articles meeting the inclusion criteria were assessed between three researchers (A.A., A.V., and C.H.), such that two people independently reviewed each submission. Disagreements were resolved by consensus including the third reviewer. For each study meeting the inclusion criteria, a standardized data extraction form was developed to record study design, TAVI population demographics, assessed risk of the population (STS and EuroSCORE), specific frailty measure, length to follow-up, and any data related to the primary and/or secondary outcomes. Where the relationship between frailty and outcome was qualitatively but not quantitatively expressed, primary authors were contacted in an attempt to gain additional primary data. Where the same study appeared to be reported across more than one article, only the most complete submission was included, with the aim of maximizing the volume of frailty data included.
Quality and bias assessment
No validated quality assessment tool has been widely established to assess observational studies that are not designed to directly compare two groups. The Newcastle–Ottawa scale was used to provide a structured assessment of sample selection (four points), comparability (two points), and outcomes (three points).15 This gives a maximum score of 9 points. Studies were independently assessed by two reviewers and disagreement resolved by consensus: ≥7 points considered high quality for frailty reporting and <7 moderate or low quality. Publication bias was assessed in the primary end point with the greatest number of studies by creating a funnel plot and using Egger's regression test.16 We then corrected for asymmetry using the trim-and-fill method to determine an adjusted effect size.17
Data synthesis and analysis
All included studies were observational cohorts with respect to frailty. Meta-analysis was performed when at least three studies reported a comparable end point to generate a meta-estimate. Given the wide number of frailty tools available, significant heterogeneity was expected across the studies, and therefore a random-effects model (maximum likelihood approach) was chosen to calculate summary effect estimates.18 Statistical analysis was performed using the metafor statistical package within R version 3.1.3 (http://www.r-project.org) and GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA). A P-value of <0.05 was considered statistically significant.
Results
Search results and patient characteristics
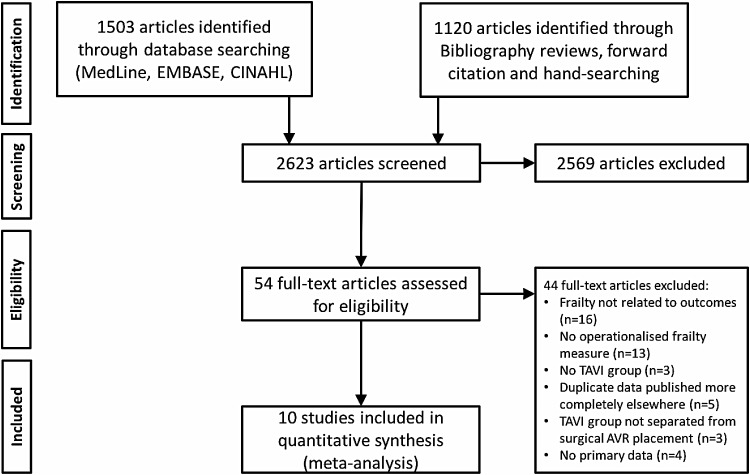
We identified 2623 abstracts from our initial search, resulting in 54 articles for full-text review to assess eligibility. Ten studies from Europe and North America met the full inclusion criteria (Figure 1). These comprised 4592 patients undergoing TAVI in whom a frailty measure was made prior to surgery. The mean age was 80–86 years, 34–53% of participants were men, and the STS-predicted 30-day mortality rates where available were between 6.3 and 16.6%. In those studies detailing the access route chosen for TAVI, the femoral approach was the most common, although this ranged from 47 to 100% of cases. The proportion of TAVI patients identified as frail varied greatly across the included studies, from 5 to 83% (Table 1).
Figure 1.
Flow diagram of reviewed studies.
Table 1.
Contextual details of included studies
| Author, year | Country | Definition of frailty | n | Mean age (years) | Male gender (%) | Proportion frail (%) | TAVI access route | 30-day mortality (%) | 1-year mortality (%) |
|---|---|---|---|---|---|---|---|---|---|
| Ewe, 201020 | The Netherlands/Italy | Fried criteria based on gait speed, grip strength, weight loss, physical activity, and exhaustion | 147 | 80 | 43 | 32.7 | Femoral 51%, apical 49% | 6.8 | 15.0 |
| Stortecky, 201235 | Switzerland | Frailty index based on geriatric assessment of cognition, nutrition, timed get-up-and-go, ADLs, and disability. Scored 0–7 with ≥3 considered frail | 100 | 84 | 40 | 49 | Femoral 85%, apical 14%, subclavian 1% | 8.0 | 19.0 |
| Rodés-Cabau, 201236 | Canada | Subjective assessment of multidisciplinary team | 339 | 81 | 45 | 25.1 | Femoral 48%, apical 52% | 10.6 | – |
| Kamga, 201319 | Belgium | ISAR score (self-reported functional dependence, recent hospitalization, impaired memory, difficulties with vision and polypharmacy) SHERPA score (age, ADLs, cognitive decline, falls, and self-perceived health) |
30 | 86 | 53 | ISAR: 83.3% moderate or high risk SHERPA: 73.3% moderate or high risk |
Femoral 100% | – | 26.7 |
| Zahn, 201337 | Germany | Presumed subjective assessment (limited detail) | 1318 | 82 | 42 | 17.7 | Femoral 88%, apical 9%, subclavian 2%, aortic 1% | – | 19.9 |
| Puls, 201438 | Germany | Katz index of ADLs (score <6 frail) | 300 | 82 | 34 | 48 | Femoral 47%, apical 53% | 11.3 | 28 |
| Seiffert, 201439 | Germany | Subjective assessment guided by CHSA clinical frailty scale29 score ≥6 | 347a | 81 | 52 | 4.6 | – | – | 24.2 |
| Capodanno, 201440 | Italy | Geriatric status scale based on ADLs, cognition, continence, and mobility. Scored 0–3 with ≥2 labelled frail | 1256b | 82 | 42 | 24.4 | – | 6.1 | – |
| Debonnaire, 201541 | The Netherlands/Italy | Presumed subjective assessment | 511 | 82 | 38 | 19.2 | Femoral 52%, apical 48% | 5.7 | 15.7 |
| Green, 201542 | USA | Frailty score composed of serum albumin, grip strength, gait speed, and ADLs. Scored between 0 and 12 with ≥6 considered frail | 244 | 86 | 52 | 45.1 | Femoral 49%, others presumed apical | 8.6 | 23.5 |
ADLs, activities of daily living.
Observed mortality data refer to the whole study population including frail and non-frail individuals.
aOnly the Bonn subgroup that received frailty assessment considered from this multicentre study.
bOnly the development cohort of this study included. The validation data set does not contain frailty related outcome data.
Definitions of frailty
Frailty was identified by authors as either subjective (four studies) or objective (six studies). Subjective frailty was based on the judgement of a clinical team without reporting use of a specific tool. Objective frailty was determined by use of a tool specifically with the purpose of defining frailty, such as activity of daily living assessments, comprehensive geriatric assessment, and frailty indices. With the exception of one small study of 30 patients by Kamga et al.,19 frailty data were available as a dichotomized variable when related to outcomes, even where it had been measured on a continuous scale.
Frailty and mortality
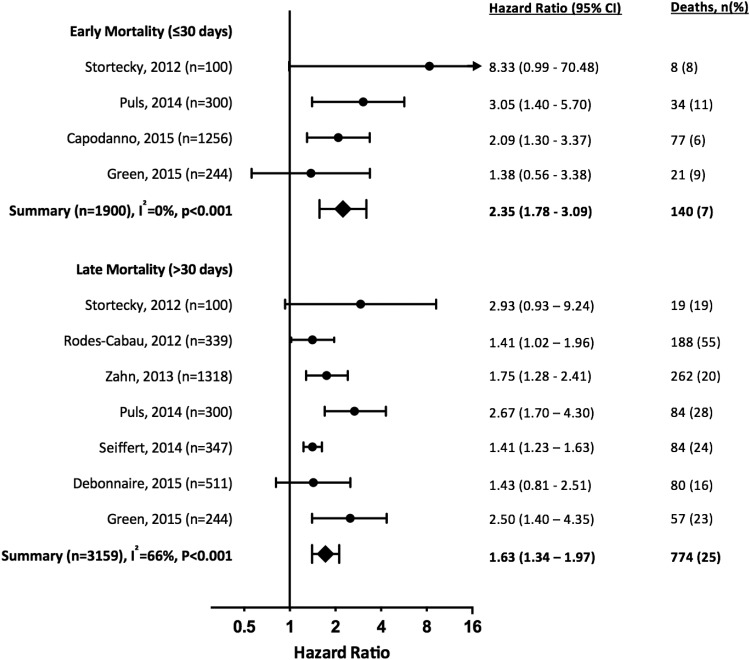
Four studies (n = 1900) reported frailty (using objective measures) and early (≤30 days) mortality after TAVI (Table 2 and Figure 2), identifying greater than doubling of the risk of early death amongst patients identified as frail (HR 2.35, 95% CI 1.78–3.09, P < 0.001). All papers reported unadjusted univariate analyses for the association between frailty and mortality. There was no significant heterogeneity between studies (I2 = 0%, P = 0.33).
Table 2.
Early (≤30 days) outcomes related to frailty in included studies
| Author, year | Outcome(s) related to frailty | Adjustment | Effect estimatea | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|---|---|
| Stortecky, 201235 | 30-day MACCE | Nil | 4.78 | 0.96 | 23.77 | 0.05 |
| 30-day MAACE (per unit increase in frailty index) | Nil | 1.66 | 1.14 | 2.44 | 0.01 | |
| 30-day all-cause mortality | Nil | 8.33 | 0.99 | 70.48 | 0.03 | |
| 30-day all-cause mortality (per unit increase in frailty index) | Nil | 2.18 | 1.32 | 3.61 | 0.002 | |
| Puls, 201438 | All-cause mortality | Nil | 3.05 | 1.4 | 5.7 | 0.003 |
| Procedural myocardial infarction | Nil | 1.08 | 0.15 | 7.59 | 0.94 | |
| Procedural major stroke | Nil | 0.98 | 0.41 | 2.33 | 0.95 | |
| Procedural TIA | Nil | 1.08 | 0.07 | 17.16 | 0.95 | |
| Life-threatening or disabling bleeding | Nil | 0.86 | 0.45 | 1.62 | 0.63 | |
| Major bleeding | Nil | 2.17 | 0.84 | 5.62 | 0.11 | |
| Minor bleeding | Nil | 1.50 | 1.05 | 2.16 | 0.03 | |
| Renal failure requiring dialysis | Nil | 2.01 | 1.09 | 3.70 | 0.02 | |
| Capodanno, 201440 | All-cause mortality | Nil | 2.09 | 1.30 | 3.37 | 0.003 |
| Green, 201542 | All-cause mortality | Nil | 1.34 | 0.59 | 3.04 | 0.48 |
| Cardiovascular mortality | Nil | 1.22 | 0.47 | 3.14 | 0.68 | |
| Major stroke | Nil | 0.61 | 0.06 | 6.63 | 0.68 | |
| Major bleeding | Nil | 1.74 | 0.69 | 4.42 | 0.24 | |
| Major vascular complications | Nil | 1.42 | 0.49 | 4.11 | 0.52 | |
| Permanent pacemaker insertion | Nil | 1.02 | 0.46 | 2.26 | 0.97 | |
| Renal failure requiring dialysis | Nil | 1.57 | 0.60 | 4.07 | 0.36 |
MAACE, major adverse cardiovascular and cerebral events.
aWhere not presented directly by authors, relative risk ratios calculated from two-by-two tables for those with and without frailty.
Figure 2.
Risk of early (≤30 days after TAVI) and late (>30 days) mortality in studies suitable for meta-analysis ordered by date of publication. Summary meta-estimate calculations based on random-effects model analysis.
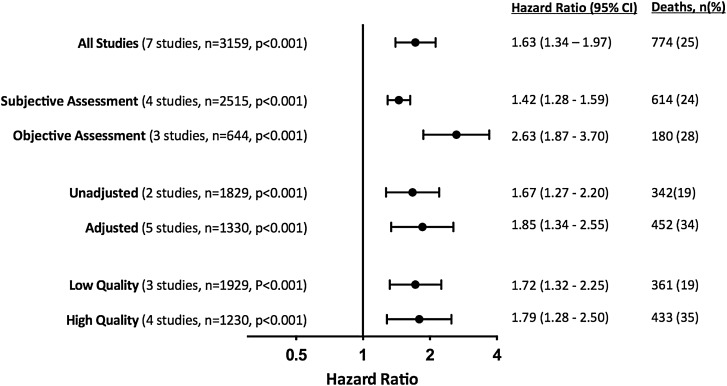
Seven studies (n = 3159) quantified the relationship between frailty and late mortality >30 days after TAVI, with every study completing at least 1 year of follow-up (Table 3 and Figure 2). All reported an increased risk of death amongst frail patients, with an overall effect size of HR 1.63 (95% CI 1.34–1.97, P < 0.001). The was only marginally increased by restricting analysis to studies undertaking adjustment for potential confounders (5 studies, HR 1.85, 95% CI 1.34–2.55, P < 0.001) or including only studies of higher quality for frailty reporting (4 studies, HR 1.79, 95% CI 1.28–2.50, P < 0.001). There was moderate heterogeneity (I2 = 66%, P = 0.01), which was reduced by performing a sensitivity analysis by the type of frailty measure used (Figure 3 and Supplementary material online, Figure S1). The mortality risk for frail patients was greater amongst those studies using an objective measure (HR 2.63, 95% CI 1.87–3.70, P < 0.001) rather than subjective assessment (HR 1.42, 95% CI 1.28–1.59, P < 0.001).
Table 3.
Late (≥30 days) outcomes related to frailty in included studies
| Author, year | Outcome(s) related to frailty | Adjustment | Effect estimatea | Lower 95% CI | Upper 95% CI | P-value |
|---|---|---|---|---|---|---|
| Ewe, 201020 | MACCE defined as composite of death, nonfatal stroke, heart failure, or nonfatal myocardial infarction (mean follow-up of 9.1 months) | Logistic EuroSCORE, peripheral vascular disease, previous CABG, baseline LVEF | 4.20 | 2.00 | 8.84 | <0.001 |
| Stortecky, 201235 | 1-year MACCE | Nil | 4.89 | 1.64 | 14.6 | 0.003 |
| 1-year MACCE | STS score | 4.17 | 1.37 | 12.72 | 0.01 | |
| 1-year MACCE | Logistic EuroSCORE | 4.48 | 1.48 | 13.53 | 0.01 | |
| 1-year MACCE (per unit increase in frailty index) | Nil | 1.80 | 1.33 | 2.45 | <0.001 | |
| 1-year all-cause mortality | Nil | 3.68 | 1.21 | 11.19 | 0.02 | |
| 1-year all-cause mortality | STS score | 2.93 | 0.93 | 9.24 | 0.07 | |
| 1-year all-cause mortality | Logistic EuroSCORE | 3.29 | 1.06 | 10.15 | 0.04 | |
| 1-year all-cause mortality (per unit increase in frailty index) | Nil | 1.80 | 1.31 | 2.47 | <0.001 | |
| Rodés-Cabau, 201236 | All-cause mortality (mean follow-up of 42 ± 15 months) | Atrial fibrillation, cerebrovascular disease, COPD, eGFR, pulmonary hypertension | 1.41 | 1.02 | 1.96 | 0.034 |
| Late all-cause mortality (excluding mortality within 30 days of TAVI) | Age, atrial fibrillation, COPD, eGFR | 1.52 | 1.07 | 2.17 | 0.021 | |
| Kamga, 201319 | 1-year all-cause mortality (per 1 unit increase in SHERPA score) | Unclear but likely gender, BMI, pulmonary hypertension, diabetes | 2.74 | 1.39 | 5.39 | 0.004 |
| Zahn, 201337 | 1-year mortality | Nil | 1.50 | 1.19 | 1.89 | <0.001 |
| Puls, 201438 | All-cause mortality (median follow-up of 537 days) | Age and sex | 2.67 | 1.7 | 4.3 | <0.0001 |
| Seiffert, 201439 | 1-year mortality | Age and sex | 1.41 | 1.23 | 1.63 | <0.001 |
| Debonnaire, 201541 | 1-year mortality | Nil | 1.29 | 0.80 | 2.06 | 0.29 |
| Green, 201542 | 1-year all-cause mortality (frailty dichotomized) | Nil | 2.18 | 1.27 | 3.75 | 0.005 |
| 1-year all-cause mortality (frailty dichotomized) | Stepwise inclusion of variablesb with entry/stay criteria of 0.1/0.1 and a maximum of one covariate for every 10 events | 2.5 | 1.40 | 4.35 | 0.002 | |
| 1-year all-cause mortality (per unit increase in frailty score) | Nil | 1.12 | 1.02 | 1.22 | 0.01 | |
| Poor outcome (death or poor quality of lifec) at 6 months | Stepwise inclusion of variablesb as above | 2.21 | 1.09 | 4.46 | 0.03 | |
| Poor outcome (death or poor quality of lifec) at 1 year | Stepwise inclusion of variablesb as above | 2.40 | 1.14 | 5.05 | 0.02 |
MACCE, major adverse cardiovascular and cerebral events; CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; BMI, body mass index; TIA, transient ischaemic attack; STS, Society of Thoracic Surgeons.
aWhere not presented directly by authors, relative risk ratios calculated from two-by-two tables for those with and without frailty.
bCandidate variables: age, sex, body mass index, access route, STS score, diabetes, hypertension, angina, heart failure, New York Heart Association Class IV, coronary artery disease, previous coronary angioplasty, previous CABG, cerebrovascular disease, peripheral vascular disease, previous balloon aortic valvuloplasty, permanent pacemaker, renal disease, liver disease, chronic pulmonary disease, aortic valve mean gradient, ejection fraction, moderate or severe mitral regurgitation.
cPoor quality of life defined as Kansas City Cardiomyopathy Questionnaire Overall Summary score <45 or a decrease of ≥10 points on serial testing before and after TAVI.
Figure 3.
Risk of late (>30 days after TAVI) mortality amongst frail patients. Summary meta-estimates presented grouped by type of frailty assessment used (subjective vs. objective), adjustment for confounders (unadjusted vs. adjusted) and study quality with regard to frailty reporting (high vs. low). All summary meta-estimate calculations based on random-effects model analysis. Individual study level data are presented in Supplementary material online, Figure S1.
Five studies provided the absolute number of deaths by frailty status allowing combined incidence estimations. This calculation totalled 3629 TAVI patients (24.6% frail) followed for the equivalent of 2717 patient years. Amongst those with frailty, 34 deaths/100 patient years were observed, against 19 deaths/100 patient years in non-frail individuals (Table 4). Two studies could not be included in the meta-analysis due to frailty being reported as a continuous variable,19 or because only a composite end point of MACCE (major adverse cardiovascular or cerebrovascular event) rather than all-cause mortality was reported.20 However, both studies did report significant associations of frailty with poorer outcomes including late mortality.
Table 4.
Comparisons of mortality in frail and non-frail patients after TAVI
| Author, year | Zahn, 201337 | Puls, 201438 | Capodanno, 201440 | Debonnair, 201541 | Green, 201542 | Overall |
|---|---|---|---|---|---|---|
| Frail (n) | 233 | 144 | 306 | 98 | 110 | 891 |
| Frail deaths (n) | 70 | 80 | 30 | 20 | 36 | 236 |
| Non-frail (n) | 1085 | 156 | 950 | 413 | 134 | 2738 |
| Non-frail deaths (n) | 217 | 37 | 47 | 60 | 21 | 382 |
| Follow-up period | Mean 12.9 months | Median 537 days | 30 days | 1 year (censored) | 1 year (censored) | – |
| Frail years of follow-up | 250 | 212 | 25 | 98 | 110 | 695 |
| Non-frail years of follow-up | 1166 | 230 | 78 | 413 | 134 | 2021 |
| Death rate/100 frail patient years | 28 | 38 | 120 | 20 | 33 | 34 |
| Death rate/100 non-frail patient years | 19 | 16 | 60 | 15 | 16 | 19 |
Significance value for difference between bold values: P<0.001.
Frailty and VARC outcomes
There was wide variation in the reporting of secondary outcomes across the included studies, with only three studies reporting comparable outcomes in relation to frailty. Meta-analysis of these end points was therefore not possible. VARC outcome measures ≤30 days after TAVI were reported in relation to frailty status in only two of the included studies, totalling 544 patients (Table 2). Both used objective tools, and reported increased effect estimates for the risk of major bleeding and renal failure requiring dialysis in frail patients, but only the latter complication reached significance in the paper by Puls et al. (OR 2.23, 95% CI 1.12–4.47, P = 0.02). Both studies reported no increase in the risk of stroke amongst frail individuals after TAVI.
Quality and risk of bias
Six studies met our frailty-defined criteria for high quality (Newcastle–Ottowa scale score ≥7), and four were considered moderate or low in quality (see Supplementary material online, Table S1). No study scored maximum points. All those considered of lower quality did not include adjustment for potential confounders of the relationship between frailty and outcomes. Publication bias was observed amongst the seven studies reporting late mortality (Egger's test for asymmetry P = 0.02). Adjustment by the trim-and-fill method (see Supplementary material online, Figure S2 funnel plot) had no effect on the size estimate, which remained statistically significant (HR 1.59, 95% CI 1.33–1.90, P < 0.001 vs. HR 1.63, 95% CI 1.34–1.97, P < 0.001 before adjustment).
Discussion
In this systematic review and meta-analysis, we explored the relationship between pre-procedure frailty and outcomes after TAVI in 10 studies from Europe and North America comprising 4592 patients. We have made several important observations. First, the measurement of frailty detects a population at double the risk of both early and late mortality after TAVI. Second, using objective measures of frailty appears to identify an even more vulnerable group than ‘end-of-the-bed’ subjective assessment. However, it is worth acknowledging that such subjective frailty assessment still provides important discrimination of risk within a population already considered at ‘high risk’ for conventional surgery. Third, VARC complication rates in relation to frailty status are not well reported, with only very limited data to suggest increased risk of dialysis requirement and bleeding risk in frail patients. However, these observations were not suitable for meta-analysis and are subject to competing risk bias from the increased early mortality observed amongst those with frailty.
A recent review by Puri et al.21 has emphasized the potential value of frailty assessment in TAVI candidates. Through the process of systematic review and meta-analysis, we have further clarified the growing body of research in this area and have numerically quantified the mortality risk of frailty identified by both objective and subjective measures. Established methods for determining those most likely to benefit from TAVI over medical management or conventional surgical aortic valve replacement are lacking. The PARTNER randomized controlled trial of high-risk severe aortic stenosis patients, demonstrated improved survival with TAVI, but 43% of patients had still died within 2 years of intervention compared with 68% with standard medical care. The stroke rate of 13.8% in the TAVI cohort was also more than double that of medically managed patients,22–25 although rates are falling as procedural techniques improve.26 TAVI as an intervention may therefore have population-level survival benefits over medical management, but the severe aortic stenosis population is heterogeneous and individual risk is likely to vary greatly.
Mortality prediction using traditional risk assessment tools such as the STS mortality score and logistic EuroSCORE was commonly reported amongst the reviewed papers. It is possible to directly compare these figures to observe early (≤30 days) mortality in six of the included studies (see Supplementary material online, Table S2). This comparison highlights the poor correlation of predictive scores with actual outcomes in this population, which is perhaps unsurprising given these tools were developed in younger cohorts excluding TAVI. Others have also identified the weakness of existing risk scores.5,6 It is noteworthy that these predictive algorithms only provide prognostic estimates for early surgical outcomes, which may not be the most important end point after TAVI. In such complex older patients approaching the end of life, quality of life after intervention may be more important than survival or avoidance of procedural complications. A systematic review by Kim et al.27 of function and quality of life after TAVI reported mixed patient outcomes, with improvements in physical function amongst survivors not matched by changes in psychological and general health measures.
Frailty has gained traction within surgical and cardiovascular literature as a potential metric for the currently unmeasured risk of older patients undergoing complex interventions.10–13 Whilst this may be seen as positive for the holistic care of older patients, there is wide variation in definitions and measurement. In this review, the six studies that sought to objectively measure frailty each used different tools, varying from functional scales to composite scores including nutrition, cognition, and mobility. In the absence of trial data with randomization based on frailty, it is not possible to infer which elements of these measures will carry the most prognostic weight. However, it is notable that all the tools used included some estimation of participation in activities of daily living. It is possible that such measures are particularly sensitive to procedural risk in severe aortic stenosis populations as impairments may reflect established heart failure at the time of consideration for TAVI.
There remains no consensus on the optimum approach to frailty assessment. The majority of studies included in this review considered frailty as a dichotomized variable for the purpose of outcome analysis. This reflects the phenotypic model of frailty and is perhaps attractive as a simple clinical concept.8 However, forcing a continuous variable into a binary form limits the consideration of a ‘pre-frail’ status and may be open to criticism for the potentially arbitrary nature of the threshold used to define frailty. Dichotomous phenotypic frailty assessment may also suffer from saturation amongst the highest-risk populations and therefore provide limited discrimination compared with an index of deficits.28 A formal frailty index, such as that first described by Rockwood et al.,29 may better reflect the accumulation of markers of frailty over time. Three of the included studies do present some outcome data per unit change in the chosen frailty index, but given the differences in the structure of these scales, meta-estimation of a combined effect size was not possible or logical.
Although the included studies comprise 4592 patients undergoing TAVI, there are even larger published population registries in America, the UK, France, Germany, Italy, and Belgium. Unfortunately, there is currently no systematic measurement of frailty within any of these cohorts of consecutive patients.30–34 It is likely that these registries will be used to produce future TAVI-specific surgical risk assessment tools similar to STS and EuroSCORE, and therefore inclusion of frailty measurement would provide a valuable opportunity to test effectiveness in large populations.
Limitations
Several limitations of our review should be considered. First, there are no studies randomized by frailty status, and so it is likely that patient selection in the observational cohort studies included in our meta-analysis was already influenced by underlying and unmeasured frailty. This is inevitable given the nature of TAVI as a treatment reserved for high-risk aortic stenosis patients requiring valve replacement. Whilst this selection bias may limit interpretation of frailty measurement in a broader aortic stenosis population, the results are representative of real-world TAVI cohorts. Studies evaluating frailty and outcomes in patients referred for TAVI, but in whom the procedure was felt too high risk by their multidisciplinary team, would be informative, but to our knowledge, no such studies have been reported. Second, we have only included studies where frailty was defined by the researchers. It is possible that other data exist including similar measurements without specific use of the term frailty. However, such studies would be less likely to report outcomes directly related to these measures without acknowledging the concept of frailty. Third, the meta-estimate for early mortality is based on a small number of studies, without adjustment for potential confounders. We were limited by the infrequent reporting of standardized VARC complications in relation to frailty status, and these interpretations are open to competing risk bias. Therefore, whilst the observations of the effect of frailty on early outcomes are important, further work is required in this area. It is in this light that the addition of objective frailty measures to ongoing large TAVI registries would be helpful.
Conclusions
We demonstrate that frailty is associated with poorer early and late outcomes in TAVI patients. Objective frailty tools identify an even more vulnerable population at greater than double the late mortality risk of non-frail patients. There is currently a lack of consistency in frailty measures and clarity in reporting against standardized early VARC outcomes. Given the ongoing uncertainty in appropriate patient selection for TAVI, randomized controlled trials should consider including patients based on an objective assessment of frailty status.
Supplementary material
Funding
A.A. is supported by a Clinical Research Fellowship from Chest, Heart and Stroke Scotland (RES/Fell/A163), and N.L.M. is supported by an Intermediate Clinical Research Fellowship from the British Heart Foundation (FS/10/024/28266).
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We would like to acknowledge the support of our certified librarian Sheila Fisken in the preparation of the search strategy. A.A., A.M., and S.S. are members or associated members of the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative, who provided systematic review training.
References
- 1. Osnabrugge RLJ, Mylotte D, Head SJ, Mieghem NMV, Nkomo VT, LeReun CM, Bogers JJC, Piazza N, Kappetein AP. Aortic stenosis in the elderly. J Am Coll Cardiol 2013;62:1002–1012. [DOI] [PubMed] [Google Scholar]
- 2. Vasques F, Lucenteforte E, Paone R, Mugelli A, Biancari F. Outcome of patients aged ≥80 years undergoing combined aortic valve replacement and coronary artery bypass grafting: a systematic review and meta-analysis of 40 studies. Am Heart J 2012;164:410–418. [DOI] [PubMed] [Google Scholar]
- 3. O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand ST, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg 2009;88:S23–S42. [DOI] [PubMed] [Google Scholar]
- 4. Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, Lockowandt U. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 5. Rosenhek R, Iung B, Tornos P, Antunes MJ, Prendergast BD, Otto CM, Kappetein AP, Stepinska J, Kaden JJ, Naber CK, Acarturk E, Gohlke-Barwolf C. ESC Working Group on Valvular Heart Disease Position Paper: assessing the risk of interventions in patients with valvular heart disease. Eur Heart J 2012;33:822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osswald BR, Gegouskov V, Badowski-Zyla D, Tochtermann U, Thomas G, Hagl S, Blackstone EH. Overestimation of aortic valve replacement risk by EuroSCORE: implications for percutaneous valve replacement. Eur Heart J 2009;30:74–80. [DOI] [PubMed] [Google Scholar]
- 7. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Robledo LMG, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 9. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 10. Partridge JSL, Harari D, Dhesi JK. Frailty in the older surgical patient: a review. Age Ageing 2012;41:142–147. [DOI] [PubMed] [Google Scholar]
- 11. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, Fried LP. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010;210:901–908. [DOI] [PubMed] [Google Scholar]
- 12. Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr 2009;48:78–83. [DOI] [PubMed] [Google Scholar]
- 13. Robinson TN, Wallace JI, Wu DS, Wiktor A, Pointer LF, Pfister SM, Sharp TJ, Buckley MJ, Moss M. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg 2011;213:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodes-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403–2418. [DOI] [PubMed] [Google Scholar]
- 15. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute; http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (1 March 2016). [Google Scholar]
- 16. Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 18. Kelley GA. Statistical models for meta-analysis: a brief tutorial. World J Methodol 2012;2:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamga M, Boland B, Cornette P, Beeckmans B, De Meester C, Chenu P, Gurne O, Renkin J, Kefer J. Impact of frailty scores on outcome of octogenarian patients undergoing transcatheter aortic valve implantation. Acta Cardiol 2013;68:599–606. [DOI] [PubMed] [Google Scholar]
- 20. Ewe SH, Marsan NA, Pepi M, Delgado V, Tamborini G, Muratori M, Ng ACT, van der Kley F, de Weger A, Schalij MJ, Fusari M, Biglioli P, Bax JJ. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J 2010;160:1113–1120. [DOI] [PubMed] [Google Scholar]
- 21. Puri R, Iung B, Cohen DJ, Rodés-Cabau J. TAVI or No TAVI: identifying patients unlikely to benefit from transcatheter aortic valve implantation. Eur Heart J 2016 Jan 26. pii: ehv756. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22. Leon MB, Smith CR, Mack M, Miller C, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, for the PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 23. Smith CR, Leon MB, Mack MJ, Miller C, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, for the PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 24. Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB, for the PARTNER Trial Investigators. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696–1704. [DOI] [PubMed] [Google Scholar]
- 25. Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, Fontana GP, Dewey TM, Thourani VH, Pichard AD, Fischbein M, Szeto WY, Lim S, Greason KL, Teirstein PS, Malaisrie SC, Douglas PS, Hahn RT, Whisenant B, Zajarias A, Wang D, Akin JJ, Anderson WN, Leon MB, for the PARTNER Trial Investigators. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686–1695. [DOI] [PubMed] [Google Scholar]
- 26. Hamm CW, Arsalan M, Mack MJ. The future of transcatheter aortic valve implantation. Eur Heart J 2016;37:803–810. [DOI] [PubMed] [Google Scholar]
- 27. Kim CA, Rasania SP, Afilalo J, Popma JJ, Lipsitz LA, Kim DH. Functional status and quality of life after transcatheter aortic valve replacement: a systematic review. Ann Intern Med 2014;160:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Theou O, Brothers TD, Pena FG, Mitnitski A, Rockwood K. Identifying common characteristics of frailty across seven scales. J Am Geriatr Soc 2014;62:901–906. [DOI] [PubMed] [Google Scholar]
- 29. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gilard M, Eltchaninoff H, Iung B, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Lefevre T, Himbert D, Tchetche D, Carrié D, Albat B, Cribier A, Rioufol G, Sudre A, Blanchard D, Collet F, Dos Santos P, Meneveau N, Tirouvanziam A, Caussin C, Guyon P, Boschat J, Le Breton H, Collart F, Houel R, Delpine S, Souteyrand G, Favereau X, Ohlmann P, Doisy V, Grollier G, Gommeaux A, Claudel J, Bourlon F, Bertrand B, Van Belle E, Laskar M, for the FRANCE 2 Investigators. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med 2012;366:1705–1715. [DOI] [PubMed] [Google Scholar]
- 31. Ussia GP, Barbanti M, Petronio AS, Tarantini G, Ettori F, Colombo A, Violini R, Ramondo A, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, Poli A, De Carlo M, Napodano M, Fiorina C, De Marco F, Antoniucci D, de Cillis E, Capodanno D, Tamburino C, on behalf of the CoreValve Italian Registry Investigators. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur Heart J 2012;33:969–976. [DOI] [PubMed] [Google Scholar]
- 32. Bosmans JM, Kefer J, De Bruyne B, Herijgers P, Dubois C, Legrand V, Verheye S, Rodrigus I, for the Belgian TAVI Registry Participants. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: results of the Belgian national registry. Interact Cardiovasc Thorac Surg 2011;12:762–767. [DOI] [PubMed] [Google Scholar]
- 33. Moat NE, Ludman P, de Belder MA, Bridgewater B, Cunningham AD, Young CP, Thomas M, Kovac J, Spyt T, MacCarthy PA, Wendler O, Hildick-Smith D, Davies SW, Trivedi U, Blackman DJ, Levy RD, Brecker SJD, Baumbach A, Daniel T, Gray H, Mullen MJ. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130–2138. [DOI] [PubMed] [Google Scholar]
- 34. Hamm CW, Mollmann H, Holzhey D, Beckmann A, Veit C, Figulla H, Cremer J, Kuck K, Lange R, Zahn R, Sack S, Schuler G, Walther T, Beyersdorf F, Bohm M, Heusch G, Funkat A, Meinertz T, Neumann T, Papoutsis K, Schneider S, Welz A, Mohr FW, for the GARY-Executive Board. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J 2014;35:1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stortecky S, Schoenenberger AW, Moser A, Kalesan B, Jüni P, Carrel T, Bischoff S, Schoenenberger C, Stuck AE, Windecker S, Wenaweser P. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv 2012;5:489–496. [DOI] [PubMed] [Google Scholar]
- 36. Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Osten M, Feindel CM, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson M, Thompson CR, Wood D, Toggweiler S, Gurvitch R, Lichtenstein SV, Doyle D, DeLarochellière R, Teoh K, Chu V, Bainey K, Lachapelle K, Cheema A, Latter D, Dumesnil JG, Pibarot P, Horlick E. Long-term outcomes after transcatheter aortic valve implantation: insights on prognostic factors and valve durability from the Canadian multicenter experience. J Am Coll Cardiol 2012;60:1864–1875. [DOI] [PubMed] [Google Scholar]
- 37. Zahn R, Gerckens U, Linke A, Sievert H, Kahlert P, Hambrecht R, Sack S, Abdel-Wahab M, Hoffmann E, Schiele R, Schneider S, Senges J. Predictors of one-year mortality after transcatheter aortic valve implantation for severe symptomatic aortic stenosis. Am J Cardiol 2013;112:272–279. [DOI] [PubMed] [Google Scholar]
- 38. Puls M, Sobisiak B, Bleckmann A, Jacobshagen C, Danner BC, Hünlich M, Beißbarth T, Schöndube F, Hasenfuß G, Seipelt R, Schillinger W. Impact of frailty on short- and long-term morbidity and mortality after transcatheter aortic valve implantation: risk assessment by Katz Index of activities of daily living. EuroIntervention 2014;10:609–619. [DOI] [PubMed] [Google Scholar]
- 39. Seiffert M, Sinning J, Meyer A, Wilde S, Conradi L, Vasa-Nicotera M, Ghanem A, Kempfert J, Hammerstingl C, Ojeda FM, Kim W, Koschyk DH, Schirmer J, Baldus S, Grube E, Mollmann H, Reichenspurner H, Nickenig G, Blankenberg S, Diemert P, Treede H, Walther T, Werner N, Schnabel RB. Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol 2014;103:631–640. [DOI] [PubMed] [Google Scholar]
- 40. Capodanno D, Barbanti M, Tamburino C, D'Errigo P, Ranucci M, Santoro G, Santini F, Onorati F, Grossi C, Covello RD, Capranzano P, Rosato S, Seccareccia F, on behalf of the OBSERVANT Research Group. A simple risk tool (the OBSERVANT score) for prediction of 30-day mortality after transcatheter aortic valve replacement. Am J Cardiol 2014;113:1851–1858. [DOI] [PubMed] [Google Scholar]
- 41. Debonnaire P, Fusini L, Wolterbeek R, Kamperidis V, van Rosendael P, van der Kley F, Katsanos S, Joyce E, Tamborini G, Muratori M, Gripari P, Bax JJ, Marsan NA, Pepi M, Delgado V. Value of the ‘TAVI2-SCORe’ versus surgical risk scores for prediction of one year mortality in 511 patients who underwent transcatheter aortic valve implantation. Am J Cardiol 2015;115:234–242. [DOI] [PubMed] [Google Scholar]
- 42. Green P, Arnold SV, Cohen DJ, Kirtane AJ, Kodali SK, Brown DL, Rihal CS, Xu K, Lei Y, Hawkey MC, Kim RJ, Alu MC, Leon MB, Mack MJ. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol 2015;116:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.