Abstract
Oxytocin (OT) is a potential treatment for multiple neuropsychiatric disorders. Since OT is a peptide, delivery by the intranasal (IN) route is the preferred method in clinical studies. Although studies have shown increased cerebrospinal fluid (CSF) OT levels following IN administration, this does not clearly demonstrate that the peripherally administered OT is entering the CSF. For example, it has been suggested that peripheral delivery of OT could lead to central release of endogenous OT. It is also unknown whether the IN route provides for more efficient entry of the peptide into the CSF compared to the IV route which requires blood brain barrier (BBB) penetration. To address these questions, we developed a sensitive and specific quantitative mass spectrometry assay that distinguishes labelled (d5-deuterated) from endogenous (d0) OT. We administered d5-oxytocin (80 IU) to 6 nonhuman primates via IN and IV routes as well as IN saline as a control condition. We measured plasma and CSF concentrations of administered and endogenous OT before (t=0) and after (t=10, 20, 30, 45, 60 minutes) d5-oxytocin dosing. We demonstrate CSF penetrance of d5, exogenous OT delivered by IN and IV administration. Peripheral administration of d5-OT did not lead to increased d0, endogenous OT in the CSF. This suggests that peripheral administration of OT does not lead to central release of endogenous OT. We also did not find that IN administration offered an advantage compared to IV administration with respect to achieving greater CSF concentrations of OT.
Introduction
OT is a nine amino acid peptide, synthesized in the magnocellular neurons of the paraventricular (PVN), supraoptic (SON), and accessory (AN) magnocellular nuclei of the hypothalamus and released into the bloodstream from the nerve terminals of these nuclei in the posterior pituitary(1). Peripherally, OT stimulates uterine contraction during parturition and milk let-down during lactation. Neurons from these nuclei also project centrally to forebrain regions(2) modulating neurocircuitry related to learning and memory, anxiety, fear, social approach and reward (3). In human behavioral and neuroimaging studies, peripheral delivery of OT modulates measures of behavioral and neural response (4–7). Several clinical studies were conducted to test the effect of intranasal OT as a treatment for numerous neuropsychiatric disorders including autism, schizophrenia, addiction, anxiety and depression [for review, see: (8)]. Delivering OT via the intranasal (IN) route, rather than the intravenous (IV) route, provides for easier, more efficient administration and protection from systemic toxicity (9). Importantly, the IN route is thought to be a “privileged pathway” (10), bypassing the BBB, conferring an advantage for central penetrance for molecules such as OT that cross the BBB with difficulty, ~.002% (11), though this latter study did not use labelled OT.
Despite these previous studies, important questions remain with respect to CSF penetrance of the peptide after IV and IN administration. Further, comparing these routes of administration, it is not known whether IN delivery of OT offers an advantage compared to IV, by perhaps bypassing the BBB. Lastly, the effect of administered OT on central or peripheral endogenous OT concentrations is unknown. Addressing these questions will aid development of OT as a potential treatment for neuropsychiatric disorders.
CNS penetrance of IN or IV OT has not been clearly demonstrated. Several studies have shown elevations in CSF OT following peripheral administration (12–18). However, none of these studies determined whether elevation in CSF OT was due to the administered peptide crossing into the CSF or whether there was an increase in OT due to stimulated release of central OT, via a feed forward mechanism, as has been suggested(19). Intraperitoneally delivered OT increased c-Fos expression in OT synthesizing neurons of the SON and PVN, perhaps leading to release of central OT (20). Similar results of increased c-Fos expression in PVN and other areas (area postrema and dorsal motor nucleus of vagus of the medulla) were also found with IN delivery of OT (21).
In studies examining the endogenous OT system in patients with neuropsychiatric disorders (22), peripheral (serum) OT concentrations are a surrogate measure for endogenous CNS OT. However, little is known about the relationship between peripheral and central OT concentrations and some contend that they are separately regulated (23). Indeed, the recent human study measuring CSF OT (18) and a nonhuman primate study (15) did not find a relationship between CSF and plasma OT. However, a recent rodent study (17) found that plasma and brain extracellular fluid concentrations were positively correlated. One problem interpreting these results was the use of different methods across studies to measure OT in plasma and CSF. Further, the assays commonly employed have limitations, with the enzyme-linked immunosorbent assay (ELISA) having limited specificity and the radioimmunoassay (RIA), limited sensitivity (24).
To address these issues, we developed and validated a highly sensitive and specific quantitative mass spectrometric assay that is able to distinguish endogenous (d0) and administered d5-deuterated OT. We employed this novel assay to measure endogenous and administered d5 OT in plasma and CSF after administering d5 OT to nonhuman primates. We examined whether, 1) administered OT via the IN and IV routes reaches the CSF, 2) endogenous CSF OT concentrations are affected following peripheral administration of OT, 3) IN OT leads to higher CSF concentrations of OT than IV administration, and 4) endogenous, d0 OT concentrations in plasma and CSF are correlated.
Materials and Methods
Establishing a novel liquid chromatography tandem mass spectrometry (LC-MS/MS) method for quantification of endogenous and deuterated oxytocin We developed and validated a liquid chromatography tandem mass spectrometry (LC-MS/MS) assay for accurate quantification of endogenous OT and exogenous d5-deuterated OT (d5 OT, Sigma Aldrich Chemicals, Supplementary Figure 1) in monkey plasma and CSF collected during our nonhuman primate d5-OT IV and IN administration studies. This method differentially quantifies endogenous d0 OT and administered d5 OT from 10–500 ng/L linear ranges. To prepare calibrators and quality control samples, human plasma, which contains <5 ng/L d0 OT, was fortified with known amounts of d0 (Cayman Chemical Company, Supplementary Figure 1) and d5 OT. Monkey CSF contains >10 ng/L d0 OT, therefore, CSF analytical calibrators and quality controls were prepared by fortifying a mixture of artificial CSF:blank human plasma (aCSF:plasma, 90:10, v/v) with d0 OT and d5 OT. Calibrators were prepared by fortifying 1 mL human plasma or 0.75 mL aCSF:plasma to contain d0 and d5 OT at the following concentrations: 10, 25, 50, 100, 250 and 500 ng/L; quality control samples were prepared at 30, 120 and 400 ng/L. D10 OT (Cayman Chemical Company, Supplementary Figure 1) was added to all calibrators, quality control and authentic samples as an internal standard at 50 ng/L to compensate for recovery and matrix effects. Plasma and CSF samples were clarified via acetonitrile precipitation and solid phase extraction (Strata X Drug B, 60mg/6mL, Phenomenex, Inc.) before LC-MS/MS analysis. Plasma and CSF samples were precipitated with 2 mL acetonitrile, supernatants collected after centrifugation were dried under nitrogen at 40°C until <1 mL solution remained and diluted with 5.5 mL 0.2M ammonium acetate buffer, pH 3.0. Solid phase extraction columns were conditioned with: methanol, water and 0.2M ammonium acetate buffer, pH 3.0 (2 mL each) before application of diluted sample supernatants that were loaded onto columns via gravity. Columns were washed with 0.2M ammonium acetate buffer, pH 3.0, water and acetonitrile (2 mL each). Following drying for 5 min with 275 kPa compressed air, analytes were eluted with 2 mL 2% ammonium hydroxide in methanol. Eluates were dried completely under nitrogen at 37°C, reconstituted in 125 μL mobile phase A:mobile phase B (95:5, v/v) and transferred into glass auto-sampler vials. Plasma samples were injected onto a Shimadzu Nexera ultra-high pressure liquid chromatograph coupled to an LCMS 8050 mass spectrometer (Shimadzu Scientific Instruments, Inc.) operated in positive electrospray mode. CSF samples were injected onto a Shimadzu LC-20xr high pressure liquid chromatograph coupled to a 6500+ QTRAP mass spectrometer (SCIEX) operated in positive electrospray mode. D0, d5 and d10 OT were analyzed via gradient elution with A) 0.1% formic acid in water and B) 0.1% formic acid in acetonitrile at 0.5 mL/min on a Kinetex C18 column (50 × 2.1 mm, 2.6 μm particle; Phenomenex, Inc.) with a 50 μL injection volume.
The following multiple reaction monitoring (MRM) mass transitions were monitored for quantifying the two compounds: d0) 1007 to 723, 1007 to 285, d5) 1012 to 723, 1012 to 290 while monitoring 1017 to 723 and 1017 to 295 for the d10 OT internal standard (quantification MRMs in bold, additional MRMs are for verifying each analyte’s identity). Lowest limits of quantification were 10 ng/L with upper limits of quantification of 500 ng/L for plasma and CSF methods. Recoveries and matrix effects presented in Supplementary Table 1 were: 89.5–97.6% and 116.3–209.9%, respectively, (n = 10) for human plasma; recoveries and matrix effects were: 88.5–92.7% and 138.0–181.6%, respectively (n = 3) for monkey plasma. Recoveries and matrix effects were: 90.4–111.0% and 247.7–539.4%, respectively (n = 6) for aCSF:plasma; recoveries and matrix effects were: 104.8–115.7% and 211.2–463.1% for d5 OT in monkey CSF (n = 3). Assay performance for quality control samples fortified into human and monkey plasma was similar, with all measured concentrations within ±20% of target; similarly quality control samples fortified into aCSF:plasma and monkey CSF achieved accuracy within ±20% of d5 OT target concentrations (See Supplementary Tables 2 and 3). For human plasma: intra-batch accuracy and imprecision were 92.8–106.2% of target and 5.6–9.0% coefficient of variation (N=5); inter-batch accuracy and imprecision were 92.0–98.9% of target and 5.5–8.3% coefficient of variation (N=25). For aCSF:plasma: intra-batch accuracy and imprecision were 93.4–107.5% of target and 2.7–9.8% coefficient of variation (N=5); inter-batch accuracy and imprecision were 96.1–105.3% of target and 6.2–10.4% coefficient of variation (N=20). Plasma samples quantifying >500 ng/L were diluted with blank human plasma and re-analyzed to fall within the assay linear range, and concentrations corrected for dilution factor. This highly sensitive, specific and accurate LC-MS/MS method provides acceptable performance for quantifying d0 and d5 OT in rhesus monkey plasma and CSF following d5 OT administration.
Administration of labelled OT to nonhuman primates and measurement of endogenous and administered oxytocin in plasma and CSF
Setting
The study was conducted in the National Institutes of Health (NIH) Intramural Research Program and carried out under a protocol approved by the Animal Care and Use Committee of the National Institute of Mental Health. Procedures adhered to applicable United States federal and local laws, regulations and standards, including the Animal Welfare Act (AWA 1990), Regulations (PL 89-544; USDA 1985), and Public Health Service (PHS) Policy (PHS 2002). All experimental procedures were performed under anesthesia, and all efforts were made to minimize suffering.
Subjects and Procedure
Male adult (N = 6) rhesus monkeys (Macaca Mulatta) (6.2–11.5 kg) participated in this study. All animals were pair housed. There were 3 DRUG conditions: IV and IN deuterated OT (d5 OT) [Sigma Aldrich Chemicals], and IN saline (control condition) administered in a within-subject counterbalanced order over a 3-week period (i.e., one session per week). The d5 OT dose was 80 IU similar to previous studies(12). Blood and CSF samples were taken immediately prior to and at 10, 20, 30, 45 and 60 min post d5 OT/saline administration.
Oxytocin
For IN administration, d5 OT (80 IU) was dissolved in 1 mL normal saline, drawn up in a syringe to which an IN mucosal atomization device (LMA MAD Nasal, Teleflex Medical; Research Triangle Park, NC) was attached. In the experiment, 0.5 mL solution or saline was administered to each nostril. Anesthetized animals were placed in a supine position with their head tilted back approximately 45 degrees with the chin up so the spray could better reach the epithelium. During each spray in one nostril, the other nostril and the mouth were held closed to allow the solution to reach the respiratory and olfactory epithelia as was done in a previous animal study (25). For the IV condition, 1 mL normal saline containing 80 IU d5 OT was injected into a peripheral vein in the leg.
Before all d5 OT or saline administration sessions, each monkey received an intramuscular (IM) dose of ketamine (10 mg/kg) and dexdomitor (0.01 mg/kg) in the home cage and upon sedation was immediately transferred to an adjacent treatment room for blood and CSF collection. Glycopyrrolate (0.01 mg/kg, i.m.) was also administered to reduce brachial secretions. Animals were closely monitored prior to, during, and after anesthesia until they could safely sit upright on their own. Post-procedure analgesics such as ketoprofen (2 mg/kg) were administered based upon veterinary consultation.
Blood and CSF samples
Blood samples (5 mL) were drawn from the femoral or peripheral vein and CSF (1.5 mL) was obtained from the cisterna magna using a sterile single-use needle. CSF collection typically took less than 60 seconds. Following collection, blood samples were immediately centrifuged at 4°C, plasma was then separated and frozen at −80°C within 20 minutes of sample collection. CSF samples were immediately frozen on dry ice and stored at −80°C.
Blood Contamination of CSF samples
Blood contamination of CSF samples was determined by color matching CSF samples with a standard curve of 8 saline samples fortified with blood concentrations ranging from 0.1% to 20%. The maximum blood contamination of any CSF sample was 1%. The approximate quantity of d5 OT in each CSF sample at each time point that was due to blood contamination was calculated by the following formula:
where 0.7 is a correction for the fraction of OT that is not protein bound (26) and the d5 OT plasma concentration is divided by 2 to approximate the d5 OT blood concentration. This quantity was subtracted from all non-zero CSF d5 OT concentrations at the corresponding time point. A negative value would indicate that the measured d5 OT was entirely due to blood contamination of CSF. Only one negative value was found using this calculation and that was in the IV OT condition for one monkey at T=10 (Tmax). Analyses excluding this time point were unchanged. Blood contamination could not be similarly calculated for CSF d0 OT as d0 OT plasma concentrations were less than the assay’s lowest limit of quantification.
Missing Samples
In total, missing plasma samples for each condition were: IN Saline (n=0), IV d5 OT (n=7), IN d5 OT (n=3). Missing CSF samples per condition were: IN Saline (n=4), IV d5 OT (n=10) and IN d5 OT (n= 4). One monkey was not dosed with IV d5 OT due to a medical complication after initiating anesthesia resulting in 12 missing samples: 6 CSF and 6 plasma. All other missing samples were due to technical difficulties with cisternal taps or blood draws.
Statistical and Pharmacokinetics Analyses
Four main outcome measures were d0 OT and d5 OT concentrations in plasma and CSF. Each outcome measure was normalized using log10 resulting in skewness of <1 and >−1. The normalized outcome measures were used in all linear mixed model analyses. The residuals from all mixed linear model analyses were examined for normality with the Shapiro-Wilk Test (p value ≥0.05). Nonparametric tests were used when results from analyses were not normally distributed.
The effect of IN and IV routes of administration of d5 OT on d5 concentrations in plasma and CSF was analyzed separately for each route using a linear mixed model where the dependent variable was plasma or CSF d5 OT and fixed effects were TIME (0,10,20,30,45, and 60 min); monkey ID was added as a random effect. Pairwise comparisons between time points T=0 and each of the 5 subsequent time points were Bonferroni-corrected.
A comparison of IN and IV routes of administration of d5 OT on d5 OT concentrations in plasma and CSF was also analyzed using a linear mixed model: dependent variables were the difference (IV-IN) plasma or CSF d5 OT concentrations; fixed effects was TIME (0, 10, 20, 30, 45, 60 minutes); monkey ID was added as a random effect. For a significant main effect of TIME, post hoc analyses were Bonferroni-corrected to examine pairwise differences between T=0 and each subsequent time point.
A mixed linear model was employed to determine whether baseline (t=0) d0 CSF concentrations were significantly different across DRUG conditions. A mixed linear model also was utilized to measure the effect of administration of d5 OT on endogenous (d0) OT plasma and CSF concentrations. Two separate analyses of DRUG ROUTE were conducted: 1) comparison between IN d5 OT and IN SALINE conditions and 2) comparison of IV and IN d5 OT conditions. Dependent variables were either plasma or CSF d0 OT; fixed effects were TIME (0, 10, 20, 30, 45, 60 minutes), DRUG ROUTE (IN Saline/IN d5 OT or IN d5 OT/IV d5 OT) and TIME x DRUG ROUTE interaction; a random factor was added for monkey ID. For a significant TIME x DRUG ROUTE interaction, post hoc analyses were Bonferroni-corrected pairwise comparisons between DRUG ROUTE conditions.
Correlations between 1) plasma and CSF d0 OT, 2) plasma and CSF d5 OT, 3) plasma d5 OT and CSF d0 0T and 4) plasma d5 OT and plasma d0 OT comparisons were conducted separately for each route of administration via linear mixed model with one of the variables in the pair as the dependent measure and one as a covariate. Pharmacokinetic parameters for each monkey were calculated for d5 OT in CSF and plasma via non-compartmental pharmacokinetics analysis. These parameters were maximum concentration (Cmax), time to maximum concentration (Tmax), and area under the concentration-time curve between 0 and 60 min post dose (AUC0–60). The latter parameter was calculated using the linear trapezoidal method. Comparison of the pharmacokinetic parameters of d5 OT in CSF and plasma for each monkey was conducted with paired t-tests (2-tailed) for Cmax, Tmax and AUC0–60. For d0 CSF, AUC0–60 was compared across the 3 DRUG conditions with a mixed linear model with DRUG condition as a repeated measure and monkey as a random factor.
Absolute % bioavailability of d5 OT after IN administration was calculated as 100 x (AUCIN/AUCIV). Drug targeting efficiency percentage (%DTE), a measure of the relative exposure of the CSF to the drug following IN administration of d5 OT vs IV administration of d5 OT was calculated with the following equation:
The value of %DTE can range from −∞ to ∞, and the values higher than 100% indicate more efficient drug delivery to the CNS following IN administration as compared to IV administration (10).
All analyses were conducted with IBM SPSS (Version 20, Armonk, New York) with the exception of the pharmacokinetic analyses which were computed with EXCEL.
Results
Endogenous, d0 OT plasma concentrations
All d0 OT plasma concentrations were less than the lowest limit of quantification for the assay (10 ng/L) at all time points for all three DRUG conditions for all monkeys. Therefore, this outcome measure was not used in the planned analyses. In the IN saline condition, no d5 OT was detected in CSF or plasma at any time point.
Evidence of administered d5 OT in CSF and plasma after IN and IV administration of d5 OT
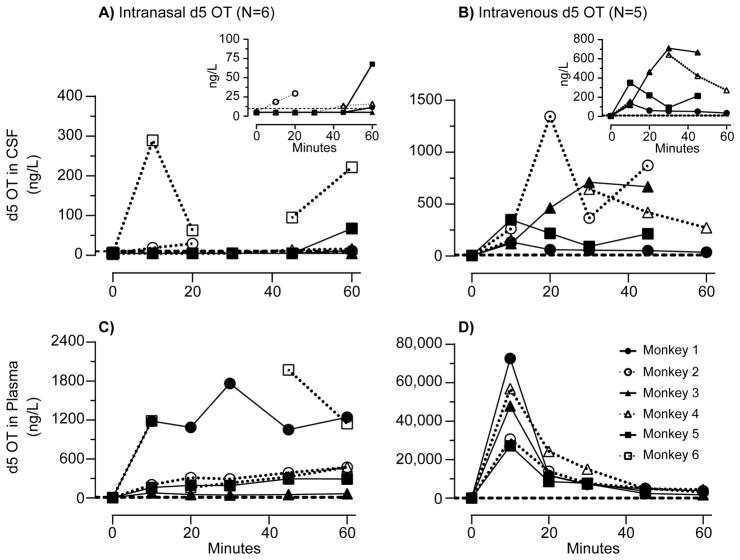
After IN and IV administration, d5 OT was identified in CSF and plasma (Figure 1).
Figure 1.
d5 oxytocin (OT) concentrations: Time course of d5 oxytocin (OT) concentrations in monkey cerebrospinal fluid (CSF, panels A and B) and plasma (panels C and D) after intranasal and intravenous administration of 80 IU d5 OT (N = 6). Dashed line indicates the limit of quantification (10 ng/L). Insets magnify time course at lower concentrations of d5 OT in CSF.
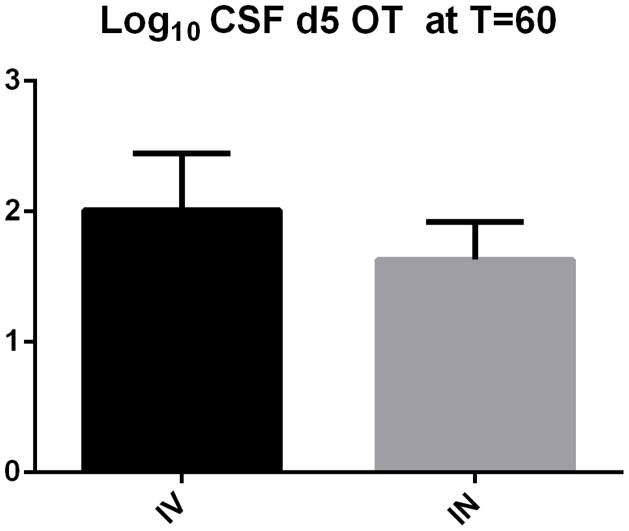
For IN administration there was a significant main effect of TIME on both CSF [F(5,19.80=2.78, p=0.047] and plasma [F(5,21.89)=107.17, p<0.001] d5 OT concentrations. CSF and plasma d5 OT levels at the 60 minute time point were each significantly different from zero [CSF, p= 0.03 (Figure 2); plasma: p<0.005].
Figure 2.
Significant elevations in Log 10 CSF d5 OT concentrations (SEM) at T= 60 minutes after IV (p<0.005) and IN (p=0.030) administration of 80 IU d5 OT.
For IV administration, there was a significant main effect of TIME on both CSF [F(5,15.59)=54.39, p<0.001] and plasma [F(5,17.24)=1123.6, p<0.001]. For CSF (Figure 2) and plasma, the pairwise comparisons between T0 and T60 as well as all other time points were significant (p<0.005).
IV administration yielded significantly higher d5 OT CSF concentrations than IN administration. There was a main effect of TIME [F(5, 12.32)= 18.58, p<0.001] on the difference between IV and IN CSF d5 OT concentrations. Pairwise comparisons between T=0 and T= 10, 20, 30 and 45 minutes were all significantly different (p<0.005). There was no significant difference between T=0 and T=60.
In plasma, there was also a main effect of TIME [ F(5, 18.25)=8652, p<0.001] on the difference between IV and IN plasma d5 OT concentrations. Pairwise comparisons between difference in plasma concentrations of d5 OT (IV-IN) were significantly different at T=0 and all subsequent 5 time points (p<0.005), with concentrations greater in the IV compared to IN condition.
Endogenous d0 OT concentrations after IN and IV administration of d5 OT and IN administration of Saline
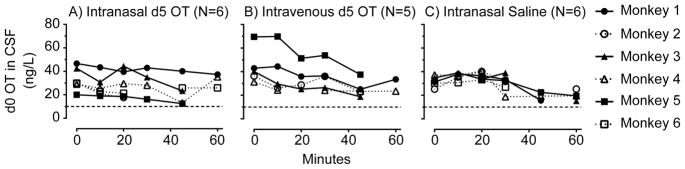
There was no difference in baseline (t=0) endogenous (d0) OT CSF concentrations across the three DRUG conditions [F(2,10.1)=2.07, p=0.177]. Time courses of d0 OT CSF concentrations after IN and IV administration of d5 OT and IN Saline are shown in Figure 3.
Figure 3.
d0 oxytocin (OT) concentrations: Time course of d0 oxytocin (OT) in monkey cerebrospinal fluid (CSF) at baseline and after A) intranasal d5 OT, B) intravenous d5 OT and C) intranasal saline administration (N = 5–6). Dashed line indicates lowest limit of quantification (10 ng/L).
Compared to IN Saline, there was no effect of IN d5 OT administration on CSF d0 levels [F(1,17.42) = 0.007, p=0.933]. There was a significant main effect of TIME [F(5, 34.96)= 3.66, p=0.009] but no significant effect of d5 OT on TIME [F(5,35.71)=2.35 p=0.06].
Comparing IN and IV d5 OT administration, there was no significant effect of DRUG ROUTE administration on CSF endogenous d0 OT concentrations [F(1,42.22)=3.29, p=0.077]. There was no significant effect of TIME on endogenous d0 OT CSF concentrations [F(5,40.41)=2.43, p=0.051] and no ROUTE x TIME interaction [F(5,39.93)=.42, p=0.835].
The effect of IN d5 OT vs IN saline or the effect of IV d5 OT vs IN d5 OT on plasma d0 OT concentrations could not be determined because all plasma d0 OT concentrations were below the assay’s lowest limit of quantification (10 ng/L).
Correlations between plasma and CSF endogenous and exogenous OT concentrations
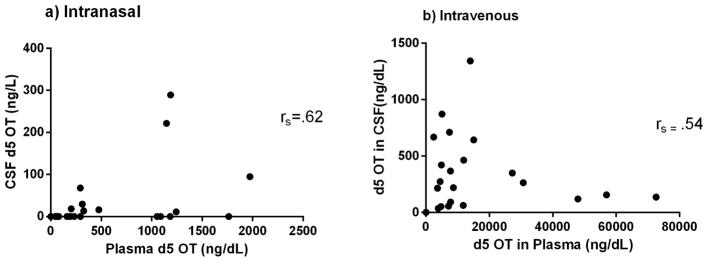
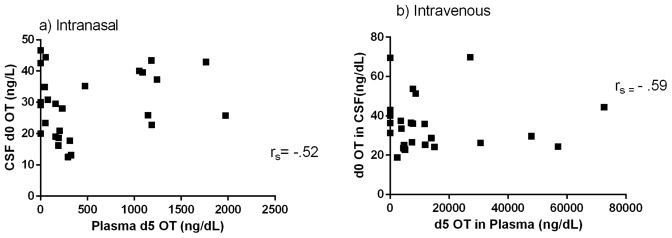
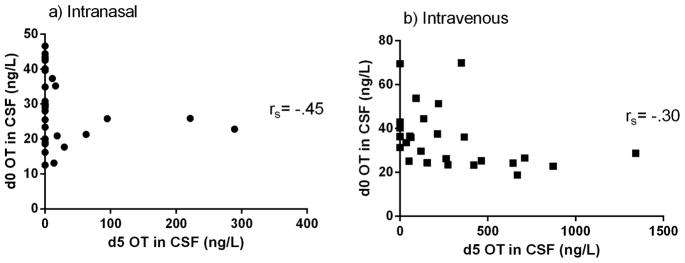
There was a significant correlation between CSF d5 OT and plasma d5 OT in the IN [t=4.52, df=4, p=0.011] and IV [t=3.09, df=4, p=0.037] conditions (Figure 4). There was a significant correlation between CSF d0 OT and plasma d5 OT in the IN [t=−2.95, df=5, p=0.032] condition and no significant correlation in the IV condition [t=.021, df=4, p=0.985] (Figure 5). There were no significant correlations between endogenous and administered OT in CSF in either the IN [t=−1.78, df=3, p=0.172] or the IV [t=1.40, df=4, p=.233] conditions (Figure 6). None of these correlations survived correction for multiple testing.
Figure 4.
Correlation of plasma d5 OT levels and CSF d5 OT levels with Spearman’s rho (rs) after a) IN and b) IV d5 OT delivery.
Figure 5.
Correlation of plasma d5 OT levels and CSF d0 OT levels with Spearman’s rho (rs) after a) IN and b) IV d5 OT delivery.
Figure 6.
Correlation of CSF d5 OT levels and CSF d0 OT levels with Spearman’s rho (rs) after a) IN and b) IV d5 OT delivery.
Correlation of d0 plasma and CSF OT was not possible, as all plasma d0 OT concentrations were below the assay’s lowest limit of quantification. Nor was the correlation of plasma d0 concentrations with administered D5 OT possible. Correlations were not done for the IN saline condition as d5 OT was not detected in plasma or CSF in this condition.
Pharmacokinetic Calculations for d5 OT administered IV and IN
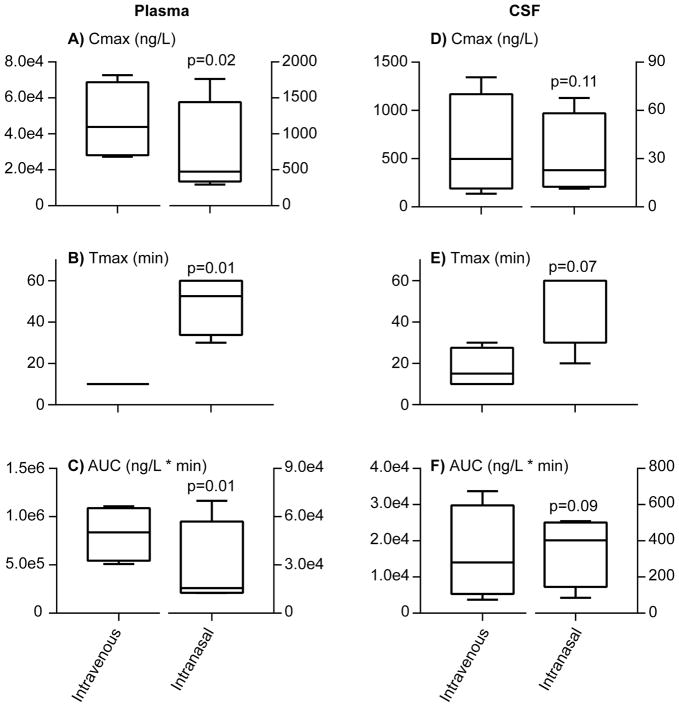
Figure 7 shows d5 OT pharmacokinetics in plasma and CSF after IV and IN administration. Bioavailability post IN administration was similar, albeit low for plasma and CSF indicating that the IN route did not confer greater availability for the nose to brain route compared to the nose to blood route. There was no significant difference between Tmax, Cmax and AUC0–60 for d5 OT in CSF when administered by IN compared to IV routes. For plasma d5 OT, however, there was a significant difference in Tmax, Cmax and AUC 0–60 between IV and IN routes of administration with larger concentrations and earlier time to peak concentration for the IV route. DTE%’s were 33.6, 50.5, 154.6 and 205.4 (data available only for 4 monkeys). The mean DTE was 111% with a standard deviation of 69%. For d0 OT in CSF, there was no significant difference between AUC0–60 between the 3 DRUG ROUTE conditions [F (2, 9.70) =0.886, p=0.443].
Figure 7.
Pharmacokinetic parameters: Boxplots depicting median and ranges for maximum concentrations (Cmax; panels A and D), times of maximum concentration occurrence (Tmax; panels B and E) and areas under the curve (AUC0 to 60min; panels C and F) of d5 oxytocin (OT) in monkey plasma and cerebrospinal fluid after intravenous and intranasal d5 OT administration (80 IU; N=4–6). P-values for paired t-test administration route comparisons are noted in each panel.
Discussion
This study demonstrates for the first time, CSF penetrance of exogenous OT administered peripherally (IN and IV) to nonhuman primates. We also found, 1) no evidence of a feed-forward effect on endogenous CSF OT concentrations after peripheral OT administration; 2) no evidence of a “privileged” nose to brain route with IN compared to IV delivery; and 3) development and validation of a sensitive and specific mass spectrometric assay that distinguishes between administered d5 OT and endogenous d0 OT concentrations in plasma and CSF.
These results extend previous studies reporting elevations in CSF OT after IN delivery (13–16) by measuring, separately, endogenous (d0) and administered (d5) OT in plasma and CSF after d5 OT dosing. This allowed us to differentiate between peripherally administered OT crossing into the CSF as opposed to leading to central release of OT.
Crucially, the LC-MS/MS assay we developed, validated and employed during our study was able to discriminate between d5 and d0 (endogenous) OT. Administration of deuterium-labeled (d5) OT combined with LC-MS/MS data acquired with unit mass resolution allowed us to distinguish between d0 OT (endogenous) and d5 OT (administered IV or IN) and quantify d0 and d5 OT to 10 ng/L concentrations in plasma and CSF with high accuracy and reproducibility due to inclusion of a d10 OT internal standard to compensate for recovery and matrix effects. This allowed us to determine whether IV or IN d5 OT crosses the BBB and also allowed us to determine the bioavailability of OT in plasma and CSF following IN administration. Previous methods measuring d0 OT via ELISA or RIA employed antibodies that bind to portions of the OT molecule and would not have been able to distinguish between d0 and d5 OT. Furthermore, antibodies can cross-react with various unknown sample components compromising specificity (i.e. vasopressin).
Simultaneous quantification of d0 and d5 OT, allowed us to determine that d0 OT plasma and CSF concentrations are not increased after d5 OT up to 60 minutes post-d5 OT administration. It is possible that stimulated release occurred outside the 60 minute sampling period or locally in the brain without manifesting as an elevation in d0 CSF levels.
A feed forward mechanism was suggested (19, 20, 23) as a process that may contribute to elevations in CSF OT after peripheral OT administration. Specifically, exogenous peptide, gaining access to the CNS or acting peripherally could stimulate hypothalamic nuclei to increase production of OT. The results reported herein, however, do not support that administration of d5 OT led to increased endogenous OT levels. Endogenous CSF OT concentrations were unaffected by IN administration of d5 OT compared to IN saline or IN d5 OT compared to IV d5 OT. In particular, the inclusion of a placebo condition supports the notion that the administration of OT does not affect endogenous OT concentrations in CSF as does the absence of a significant correlation between plasma d5 OT and CSF d0 OT. We could not measure endogenous d0 OT in plasma as all concentrations were below the assay limit of quantification, therefore, we cannot rule out the possibility of a feed forward effect on plasma levels of d0 OT, especially if elevations remained below the level of detection of the assay. We would expect, however, that if there were a significant feed forward effect of OT administration, d0 OT plasma concentrations would have increased, rising above the limit of quantification and be quantifiable during the course of the 60 minute sampling period.
The data presented here also allow for determination of whether the IN route is a “privileged pathway” for access to the CSF, namely direct passage of the drug from the nasal cavity to the CSF via the olfactory or trigeminal nerves, bypassing the BBB. There was no significant difference in pharmacokinetic parameters, Tmax, Cmax and AUC0–60 between the IN and IV routes of administration (Figure 4). The equivalent bioavailability in both compartments after IN administration suggests that IN delivery is not associated with an improvement in bioavailability compared to the blood to CSF pathway. This suggests either that the route to the CSF after IN delivery is across the BBB or that direct routes, via the trigeminal and or olfactory nerves do not result in more efficient transport into CSF. The brain targeting efficiency index (%DTE) (10) is a measure of relative accumulation of the drug in the brain following IN compared to IV administration. There does not appear to be a larger relative accumulation of OT in CSF in the IN condition relative to IV, as equivalence of the two routes would be 100% and 2 of the 4 monkeys were below equivalence. There was large inter-monkey variability for %DTE’s reflecting wide variability of CSF and plasma OT concentrations after IN administration. Although the average is just above 100%, the large standard deviation prevents concluding that IN delivery is the more efficient pathway for OT compared to IV.
Although the IN route does not appear to confer an advantage to reaching the CSF, the significant difference in pharmacokinetic parameters between IV and IN for the plasma affirms that a potential benefit of IN administration is avoidance of unnecessary systemic exposure to the drug with increased risk of potential side effects. In this scenario, compared to IV administration, the less invasive IN delivery would result in lower plasma concentrations that are one-tenth of that measured after IV administration. Of course, more research is needed on the effect of the magnitude of dose and chronicity of dosing on the endogenous OT system.
Lastly, this study sought to address important, open questions in studies that examine OT as a potential treatment for neuropsychiatric disorders. The rationale for using OT as a treatment for these disorders is based on its role to modulate neurocircuitry that is altered in disease. However, the extent to which the neurobehavioral effects of OT reported in preclinical and clinical studies are centrally mediated is unknown. Although these data indicate that IN (and IV) OT passes into the CSF, it is not known if this central access is responsible for the neurobehavioral effects reported to date with IN or IV OT. Indeed, the highly variable extent and time course of CSF penetrance, after IN administration in particular, raises the question of whether the central effect of OT as is observed in imaging studies (27), is mediated centrally or possibly by some peripheral mechanism as has been demonstrated for OT in feeding behaviors (28). One way to probe this mechanism would be to use the OT antagonist, atosiban, (used routinely in clinical practice to treat preterm labor), which does not pass the BBB. Blocking the peripheral effect of administered OT with an antagonist, could help to determine if there is a contribution of peripheral pathways to the observed neurobehavioral effects observed with IN or IV OT administration.
Further, the highly variable time course of d5 OT in the CSF calls into question the optimal interval between OT dosing and experimental procedures particularly for the IN route of administration. Indeed, in the IN condition, the only time point when d5 OT CSF concentrations were different from 0 was at 60 minutes post administration (Figure 2). Therefore, these data suggest that a reasonable interval (between administration and experimental procedures) is 60 minutes if the experimental aim includes presence of the administered OT in the CSF. Our results are limited to male monkeys; there is the possibility that the pharmacokinetics of OT in females might differ as suggested by a previous study where clearance rates were found to be lower for nonpregnant females compared to males, though differences were not significant (29).
While the endogenous OT system has been shown to be altered in various neuropsychiatric diseases including addiction (5, 30), the relationship between CSF and brain concentrations of both endogenous and administered OT is unknown. It is unknown if and where administered OT (via the IN and IV routes) is found in the brain. Further, it is unknown whether the extent of brain areas reached differs as a function of route of administration, as was found in rodents (21). As the results of this study measure only CSF, not brain levels of the administered peptide, further studies should be done investigating the brain penetrance of labelled OT after IN and IV administration. Quantification of endogenous plasma OT would also be important to examine the relationship between peripheral and central endogenous OT pools especially in the context of stress and psychiatric illness. As such, further research to increase sensitivity in the LC-MS/MS assay to enable quantification of endogenous plasma OT concentrations is important.
CSF sample contamination with blood ranged from 0–1% and CSF d5 OT concentrations were not higher in samples with more blood contamination. For example, monkey 6, with the highest CSF d5 OT levels after IN administration (Figure 1A), had contamination levels of <.1%; conversely monkeys with d5 OT concentrations in CSF below the limits of quantification had 5–10 fold higher levels of contamination. Also, it is unlikely that CSF d0 OT levels were contaminated by d0 OT from blood as plasma concentrations of d0 OT were below limits of quantification (10 ng/L), so it is unlikely that even the maximum blood contamination (1%) would have appreciably elevated d0 OT concentrations.
In conclusion, our results indicate that IN and IV administered OT reached the CSF within one hour after OT administration, there was no significant alteration in endogenous OT concentrations above 10 ng/L in the CSF, and there was no increased bioavailability of OT in the CSF after IN compared to IV delivery. The use of this sensitive and specific assay to measure and differentiate between endogenous d0 OT and administered d5 OT will further research into this peptide as a potential treatment for numerous neuropsychiatric disorders.
Supplementary Material
Supplementary Table 1: A) Oxytocin (OT) recovery as percentages and B) OT matrix effects as percent change in human plasma, monkey plasma, 10% human plasma in artificial cerebrospinal fluid and monkey cerebrospinal fluid.
Supplementary Table 2: Oxytocin (OT) inter- and intra-assay accuracy (percentage of expected concentration)
Supplementary Table 3: Oxytocin (OT) inter- and intra-assay imprecision (percent coefficients of variation)
Chemical structures of A) d0 oxytocin, B) d5 oxytocin and C) d10 oxytocin. NOTE: “d” denotes deuterium.
Acknowledgments
The authors thank Ms. Karen Smith, National Institutes of Health Library for bibliographic assistance.
Funding:
The work was supported by a Bench-to-Bedside (B2B) Grant (PI: Lee) funded by the National Institutes of Health (NIH) Office of Behavioral and Social Sciences Research (OBSSR), by NIH intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: Leggio), jointly supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the Intramural Research Program (IRP) of the National Institute on Drug Abuse (NIDA) and ZIA MH002928-01 (Unit on Learning and Decision Making; PI: Averbeck). FA is partially supported by grant number 1UH2TR000963 (PIs: Akhlaghi and Leggio) from the National Center for Advancing Translational Sciences (NCATS), NIH.
Footnotes
Author contributions:
MRL and BBA conceived of and designed the study; KBS, MAH, XXD designed and validated the oxytocin assay for oxytocin; MRL and AC conducted the monkey study; MRL, FA, KBS and BBA analyzed the data; MRL, BBA, LL and KBS wrote the manuscript.
Disclosures: The authors have no conflicts to report.
References
- 1.Ju G, Liu S, Tao J. Projections from the hypothalamus and its adjacent areas to the posterior pituitary in the rat. Neuroscience. 1986;19(3):803–28. doi: 10.1016/0306-4522(86)90300-3. [DOI] [PubMed] [Google Scholar]
- 2.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Stoop R. Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Current opinion in neurobiology. 2014;29:187–93. doi: 10.1016/j.conb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature reviews Neuroscience. 2011;12(9):524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18(1):1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- 6.Wei D, Lee D, Cox CD, Karsten CA, Penagarikano O, Geschwind DH, et al. Endocannabinoid signaling mediates oxytocin-driven social reward. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(45):14084–9. doi: 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mottolese R, Redoute J, Costes N, Le Bars D, Sirigu A. Switching brain serotonin with oxytocin. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(23):8637–42. doi: 10.1073/pnas.1319810111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114–26. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Vyas TK, Shahiwala A, Marathe S, Misra A. Intranasal drug delivery for brain targeting. Curr Drug Deliv. 2005;2(2):165–75. doi: 10.2174/1567201053586047. [DOI] [PubMed] [Google Scholar]
- 10.Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–40. doi: 10.1016/j.jconrel.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 11.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain research. 1983;262(1):143–9. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 12.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–6. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 13.Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proceedings of the National Academy of Sciences of the United States of America. 2012;109(3):959–64. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dal Monte O, Noble PL, Turchi J, Cummins A, Averbeck BB. CSF and blood oxytocin concentration changes following intranasal delivery in macaque. PloS one. 2014;9(8):e103677. doi: 10.1371/journal.pone.0103677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman SM, Samineni S, Allen PC, Stockinger D, Bales KL, Hwa GG, et al. Plasma and CSF oxytocin levels after intranasal and intravenous oxytocin in awake macaques. Psychoneuroendocrinology. 2016;66:185–94. doi: 10.1016/j.psyneuen.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA. Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology. 2014;45:49–57. doi: 10.1016/j.psyneuen.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38(10):1985–93. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Scientific reports. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ermisch A, Ruhle HJ, Landgraf R, Hess J. Blood-brain barrier and peptides. J Cereb Blood Flow Metab. 1985;5(3):350–7. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- 20.Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, et al. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addiction biology. 2010;15(4):448–63. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- 21.Maejima Y, Rita RS, Santoso P, Aoyama M, Hiraoka Y, Nishimori K, et al. Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas. Neuroendocrinology. 2015;101(1):35–44. doi: 10.1159/000371636. [DOI] [PubMed] [Google Scholar]
- 22.Rutigliano G, Rocchetti M, Paloyelis Y, Gilleen J, Sardella A, Cappucciati M, et al. Peripheral oxytocin and vasopressin: Biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res. 2016;241:207–20. doi: 10.1016/j.psychres.2016.04.117. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nature reviews Neuroscience. 2006;7(2):126–36. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 24.Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73(5):393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–96. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Fabian M, Forsling ML, Jones JJ, Lee J. The release, clearance and plasma protein binding of oxytocin in the anaesthetized rat. J Endocrinol. 1969;43(2):175–89. doi: 10.1677/joe.0.0430175. [DOI] [PubMed] [Google Scholar]
- 27.Wigton R, Radua J, Allen P, Averbeck B, Meyer-Lindenberg A, McGuire P, et al. Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci. 2015;40(1):E1–22. doi: 10.1503/jpn.130289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki Y, Maejima Y, Suyama S, Yoshida M, Arai T, Katsurada K, et al. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. American journal of physiology Regulatory, integrative and comparative physiology. 2015;308(5):R360–9. doi: 10.1152/ajpregu.00344.2014. [DOI] [PubMed] [Google Scholar]
- 29.Leake RD, Weitzman RE, Fisher DA. Pharmacokinetics of oxytocin in the human subject. Obstetrics and gynecology. 1980;56(6):701–4. [PubMed] [Google Scholar]
- 30.Lee MR, Rohn MC, Tanda G, Leggio L. Targeting the oxytocin system to treat addictive disorders: rationale and progress to date. CNS drugs. 2016;30(2):109–23. doi: 10.1007/s40263-016-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: A) Oxytocin (OT) recovery as percentages and B) OT matrix effects as percent change in human plasma, monkey plasma, 10% human plasma in artificial cerebrospinal fluid and monkey cerebrospinal fluid.
Supplementary Table 2: Oxytocin (OT) inter- and intra-assay accuracy (percentage of expected concentration)
Supplementary Table 3: Oxytocin (OT) inter- and intra-assay imprecision (percent coefficients of variation)
Chemical structures of A) d0 oxytocin, B) d5 oxytocin and C) d10 oxytocin. NOTE: “d” denotes deuterium.