Abstract
Introduction
Acellular nerve allografts (ANAs) yield less consistent favorable outcomes compared with autografts for long gap reconstructions. We evaluated whether a hybrid ANA can improve 6-cm gap reconstruction.
Methods
Rat sciatic nerve was transected and repaired with either 6-cm hybrid or control ANAs. Hybrid ANAs were generated using a 1-cm cellular isograft between 2.5-cm ANAs, whereas control ANAs had no isograft. Outcomes were assessed by graft gene and marker expression (n = 4; at 4 weeks) and motor recovery and nerve histology (n = 10; at 20 weeks).
Results
Hybrid ANAs modified graft gene and marker expression and promoted modest axon regeneration across the 6-cm defect compared with control ANA (P < 0.05), but yielded no muscle recovery. Control ANAs had no appreciable axon regeneration across the 6-cm defect.
Discussion
A hybrid ANA confers minimal motor recovery benefits for regeneration across long gaps. Clinically, the authors will continue to reconstruct long nerve gaps with autografts.
Keywords: acellular nerve allograft, autograft, nerve regeneration, peripheral nerve, senescence
Damage to a peripheral nerve can result in a gap causing loss of function. For injuries in which direct nerve repair is not possible and transfer is not indicated, a nerve autograft remains the standard of care to bridge the gap. However, autografts have disadvantages for the patient, including donor site morbidity and increased operating time.1–3 Therefore, alternatives to nerve autografts are desirable, such as nerve guidance conduits and acellular tissue scaffolds, including acellular nerve allografts (ANAs). Multiple groups, including our own, have demonstrated that ANAs hold promise to act as nerve autograft substitutes.4,5 However, although adequate for short gaps (<3 cm), ANAs have limited clinical data to support their use to reconstruct longer nerve gaps (>3–5 cm) to provide functional recovery, especially motor, comparable with the autograft standard.6–10 In addition, multiple animal studies have demonstrated that acellular nerve scaffolds, such as ANAs, do not facilitate comparable axon regeneration to isografts4,5 and fail to consistently facilitate axon regeneration across longer gaps (>3 cm) or promote motor recovery.11,12 Overall, these issues present concerns regarding the use of ANAs to treat long gap clinical nerve injuries.
A key aspect to the design of commercially available nerve alternatives is to avoid or remove innate cells and other immunogenic components to avoid tissue rejection. Therefore, acellular nerve scaffolds rely on host cell migration from the nerve ends to repopulate the scaffold during regeneration.13,14 This repopulation is a dynamic process involving stromal cells, including macrophages as well as Schwann cells, to generate a microenvironment conducive to functional nerve regeneration.13–21 Although this process is adequate for short acellular scaffolds and ANAs (<3 cm), recent animal studies by our group have demonstrated that long ANAs (6 cm), although repopulated with cells, contain a cellular population imbalance, consisting of increased populations of cells expressing markers of senescence and stress, when compared with short ANAs or injured nerve.12 In addition, these long ANAs have delayed revascularization compared with short ANAs or long isografts (6 cm).22–24 These factors are hypothesized to yield a poor regenerative environment for axon growth. Therefore, strategies to improve the regenerative environment of long ANAs may improve nerve regeneration.
Cell transplantation therapies using non-immunogenic sources of cells can repopulate short empty conduits and acellular scaffolds and improve the regenerative environment, thus promoting axon regeneration.25 A small selection of cell transplantation therapies to treat nerve injuries have included obtaining Schwann cells directly from patients for retransplantation26–28 as well as numerous stem cell transplant procedures (for a more extensive review see Szynkaruk et al.3 and Johnson et al.25). Although cell transplantation therapies continue to hold great promise to generate the diverse range of the cells needed for nerve regeneration,29 these approaches are still being developed and translated to the clinic. An alternative approach to provide a diverse range of cells for nerve regeneration with immediate translational potential consists of techniques to incorporate endogenous nerve or cellular grafts directly to acellular grafts, such as ANAs. Previously, our group demonstrated in a rat model that small quantities of nerve harvested during surgery at the injury site incorporated either within or serially along empty conduits improved regenerative outcomes across the defect (<3 cm) compared with a single empty conduit spanning the defect.30,31 This stepping stone approach could hold promise for long ANAs as this both reduces the acellular region and provides an early additional source of cells to repopulate the ANA, which in turn may improve the regenerative environment and reduce cellular replicative burden from the nerve ends.11,12,32
In an effort to improve long ANAs (6 cm), we evaluated the impact of interposing a short, cellular nerve isograft (stepping stone) between short, acellular nerve grafts (<3 cm each). This approach generated long (6 cm) hybrid ANAs. We hypothesized that incorporating cells into a long ANA via a cellular nerve graft could overcome the deficiencies in nerve regeneration across a 6-cm nerve gap.
METHODS
All materials used in this study were purchased from Sigma-Aldrich (St. Louis, M issouri), unless otherwise specified.
Animals and Experimental Design
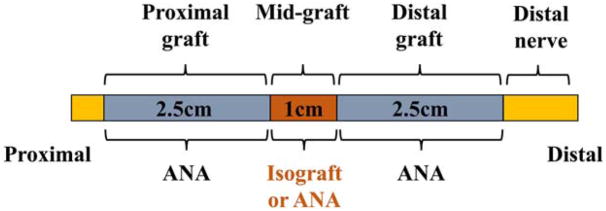
The sciatic nerves of adult male Lewis rats (250 g, 8–10 weeks old; Charles River Laboratories, Wilmington, Massachusetts) were transected and then immediately repaired with 6-cm hybrid ANAs or control ANAs (Fig. 1). To generate ANAs, Sprague–Dawley rats (250 g, Charles River Laboratories) were killed to obtain donor sciatic nerve (~3–4.5 cm in length), which was processed to generate acellular nerve grafts. The Sprague–Dawley (SD; RT-1b MHC) rat strain is MHC incompatible with Lewis (RT-11 MHC) yielding an appropriate allograft donor. Sciatic nerve allografts harvested from donor rats were chemically processed and decellularized using a series of detergents, as previously described.12 To provide hybrid ANAs (6 cm), a 1-cm nerve isograft obtained from adult male Lewis rats was coapted between two 2.5-cm ANAs. We chose an isograft rather an autologous piece of nerve due to constraints in securing a long nerve graft within the rat sciatic nerve injury model. The grafted nerves need to loop and insert into an under-the-skin “pocket” around the femur requiring the nerve ends to remain their original length after transection.11 The sigmoidal nature of this path does not interfere with regeneration.33 To provide control ANAs (6 cm), a 1-cm ANA was coapted between two 2.5-cm ANAs. Control ANAs contained a short ANA (1 cm) between two 2.5-cm ANAs rather than a single long (6 cm) ANA to control for possible suture line effects. However, previously we did not observe that an additional suture line to “daisy-chain” nerve grafts in series had an impact on nerve regeneration.34 Animals were randomized to 2 study arms (A and B), where 2 end-points were designated for study: 4 weeks (study A) and 20 weeks (study B). Animal numbers for each arm of the study were chosen based on previous studies.11,12,34,35
FIGURE 1.
Diagram of the surgical model. To generate hybrid ANAs (6 cm), a 1-cm isograft was coapted between two 2.5-cm ANAs. To generate control ANAs (6 cm), a 1-cm ANA was coapted between two 2.5-cm ANAs. [Color figure can be viewed at wileyonlinelibrary.com]
In the first set of studies (study A), 8 rats were randomized to the same 2 groups (n = 4 per group) consisting of either a hybrid ANA or control ANA. These rats were used to assess changes to the environments due to having an isograft placed between short ANAs. At 4 weeks postoperatively, chosen because it takes approximately 10 weeks before axons cross a 3-cm ANA,11,12 nerve sections from each graft segment (ANA vs. isograft) and distal nerve were quantified using immunohistochemistry (IHC) and quantitative real-time polymerase chain reaction (qRT-PCR) analyses. The expression of stress and senescence markers was evaluated by staining sections for senescence-associated β-galactosidase (SA-β-gal) activity and p16INK4A expression.12,32,36–38 The gene expression of select neurotrophic factors (Gdnf and Bdnf) was analyzed using qRT-PCR, where Gdnf and Bdnf expression are known to have a major role in promoting nerve regeneration.39–41
In the second set of studies (study B), 20 experimental rats were assigned to 2 groups (n = 10 per group) and underwent similar procedures as in study A. At 20 weeks postoperatively, evoked contractile muscle force and wet muscle mass were evaluated and the entire sciatic nerve, including the graft, was harvested en bloc for histomorphometric analysis. This end-point was chosen based on previous studies, which demonstrated that 20 weeks was an acceptable end-point to measure the final regenerative outcome after surgical reconstructions in rat nerve injury models.11,42–45
Surgical procedures and perioperative care measures were conducted in compliance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care, the institutional animal care and use committee of Washington University, and the National Institutes of Health. All animals were housed in a central animal care facility and provided with food (PicoLab Rodent Diet 20; Purina Mills Nutrition International, St. Louis, Missouri) and water ad libitum. Animals were monitored daily postoperatively for signs of infection and/or distress.
Surgical Procedures
Surgical procedures were performed as described elsewhere11 under aseptic conditions and with the aid of an operating microscope under magnifications of 10× to 25×. One surgeon (the first author Y.Y.) performed all operations. Anesthesia was provided by subcutaneous delivery of ketamine (75 mg/kg; Fort Dodge Animal Health, Fort Dodge, Iowa) and dexmedetomidine (0.5 mg/kg; Pfizer Animal Health, Exton, Pennsylvania). The right sciatic nerve was exposed using a muscle-splitting technique. For donor ANAs, the dissection was extended proximally and distally to allow harvest of ~3–4 cm of nerve. These sciatic nerves were transferred to aseptic tubes to undergo acellular processing or used immediately as fresh nerve isografts. These grafts were assessed for homogeneity of the length of the graft, including similar diameters and lack of branch points from the graft. Donor animals were then killed by pentobarbital overdose (Somnasol 150 mg/kg; Delmarva Laboratories, Des Moines, Iowa). In experimental rats, the recipient sciatic nerve was transected 5 mm proximal to the trifurcation of the sciatic nerve. The defect was reconstructed with a hybrid or control ANA secured to the proximal and distal nerve stumps using 4 interrupted 9-0 nylon epineurial sutures at each suture line (including the additional suture line in groups 2 and 4). For both groups, the two 2.5-cm ANAs were first secured to the 1-cm ANA or isograft using four 9-0 nylon epineurial sutures to yield a 6-cm graft, then attached to the proximal and distal nerve ends. During the grafting procedures, care was taken to maintain nerve alignment and protect the nerve epineurial membrane. The grafted nerves were shaped like a loop and inserted into an under-the-skin “pocket” around the femur, as performed previously.11,12 All microsurgical operations to reconstruct the defect were performed using 16× overall magnification. The muscle and skin were closed in a layered fashion. Atipamezole solution (0.1 mg/kg; Zoetis, Florham Park, New Jersey) was administered for anesthesia reversal. The animals underwent recovery on a warming pad and were monitored for postoperative complications before return to a central animal care facility. Postoperative pain was managed using buprenorphine SR (0.05 mg/kg; ZooPharm, Windsor, Colorado) every 8–12 hours as needed. At the appropriate endpoint, the animals were either anesthetized for regenerative outcome evaluation (described next) or killed using pentobarbital intraperitoneal injection, and the sciatic nerve and ANA obtained for analysis, as described in the experimental design.
Gene Expression Analysis
Harvested nerves were cut into 3 spatial nerve sections along the length of the grafts (proximal graft: 2.5-cm ANA; midgraft: 1-cm isograft or ANA; distal graft: 2.5-cm ANA) as well as the distal nerve (1 cm in length) and stored in RNAlater solution (QIA-GEN, Valencia, California) at −80°C until RNA extraction. The gene expression levels of the neurotrophic factors of Gdnf and Bdnf in these spatial nerve sections (n = 4 per group) were analyzed by qRT-PCR. Total RNA was extracted by using Trizol kit (QIAGEN) according to the manufacturer’s instructions. RNA was then reverse transcribed into complementary DNA strands (cDNAs) using the protocol described in the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, California). Gene expression of Gdnf and Bdnf were analyzed using commercially available primers (TaqMan, Life Technologies). Amplification of each target cDNA was done using a thermocycler (Step One Plus, Applied Biosystems) with normalization to a housekeeping gene (β-actin), and the data analyzed on Step One software, version 2.2.2 (Applied Biosystems).
Histological Analysis of Cellular Stress and Senescence
Tissue was assessed for markers of stress and senescence using SA-β-gal staining and IHC. Tissue was flash frozen in OCT compound (VWR) on dry ice and sectioned longitudinally into 20-μm-thick slices on a cryostat (Leica, Buffalo Grove, Illinois). SA-β-gal staining was performed immediately after sectioning using the detection kit as previously described.11,12 For IHC, tissue was postfixed in 4% paraformaldehyde, blocked with 2% normal goat serum diluted with 0.3% Triton-X in phosphate-buffered saline, then labeled with an appropriate p16 primary antibody (p16INK4A, 1:500 dilution; Abcam) followed by labeling with the appropriate secondary antibody conjugated to the fluorophore Alexa 555 (1:500 dilution; Life Technologies). After staining, sections were imaged at 20× using the Nanozoomer HT (Hamamatsu, Bridgewater, New Jersey). For each sample, 4–8 sections were analyzed for average intensity using ImageJ (NIH, Bethesda, Maryland). For SA-β-gal staining, areas were defined positive if staining was at least 2 standard deviations greater than the average intensity for uninjured, normal nerve. The total positive staining area was normalized to the total area of the tissue to calculate a percentage positive for SA-β-gal. For IHC, each tissue area was measured for fluorescence median intensity using standardized exposure times and orientation. Uninjured, normal nerve was always included for sample staining as a control and for normalization of staining intensity.
Muscle Force and Muscle Mass Evaluation
An aspect of functional recovery (muscle contractile force production and muscle recovery from atrophy) was assessed by measuring evoked motor response and wet muscle mass in the extensor digitorum longus (EDL) muscle. Animals were anesthetized as before and immobilized in an automated functional assessment station (FAST System, Red Rock Laboratories, St. Louis, Missouri) to measure muscle force, as previously described,35,46,47 where muscle forces was evaluated using a 5-N load cell (S100; Strain Measurement Devices, Inc., Meriden, Connecticut), with an accuracy of 2 mV/V or 0.05 mN). Immediately after muscle force measurements, all animals were killed and muscle and nerve harvested. The gastrocnemius, tibialis anterior, and EDL muscles were harvested from the experimental and contralateral limbs to measure relative wet muscle mass. Muscles were weighed and the experimental muscle mass was normalized to the contralateral side for each animal to account for size and mass differences in rats over the 20-week recovery period.35
Axon Regeneration Analysis
After muscle force and mass analysis, harvested nerves were fixed in 3% glutaraldehyde in 0.1-mol/L phosphate buffer. The sciatic nerve and ANA was processed and assessed for evidence of nerve regeneration by histomorphometric analyses, as described elsewhere.49 Briefly, an observer blinded to the experimental groups postfixed the specimens in osmium tetroxide, embedded the samples in epoxy resin, sectioned 1-μm-thick sections using an ultramicrotome, and counterstained cut sections using 1% toluidine blue. Analyses of microscopic images were performed with an automated digital image analysis system linked to histomorphometry software to quantify the total myelinated axon number.48
Statistical Analyses
Data are reported as mean with standard deviation. Statistical analyses were performed using Statistica software (StatSoft, Inc., Tulsa, Oklahoma). Data were tested for normality graphically and using the Kolmogorov–Smirnov test. A 2-tailed t-test was performed to assess statistical differences between the 2 groups. P < 0.05 was considered significant in all statistical tests performed.
RESULTS
Characterization of Regenerative Environment of Hybrid ANAs
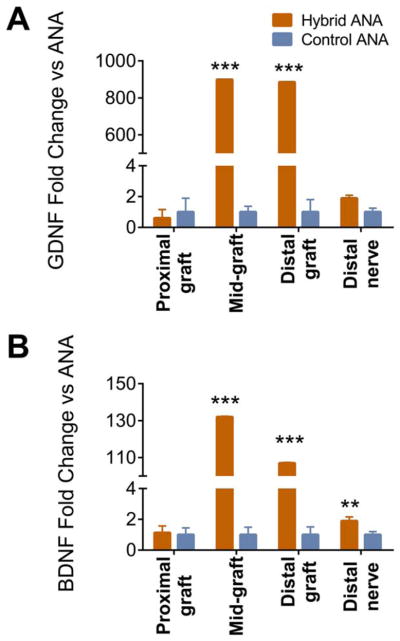
To understand whether an interposed isograft within a long ANA could improve the regenerative environment of long ANAs before substantial axon regeneration, the expression of select neurotrophic factors (Gdnf and Bdnf) and markers of senescence were analyzed with a 4-week end-point within the proximal, middle, and distal graft regions. For either experimental group, there were no relative differences in Gdnf or Bdnf expression in the proximal graft (Fig. 2). Conversely, both Gdnf and Bdnf expression levels were highly elevated in the middle and distal graft in the hybrid ANA compared with the control ANA. This increase in neurotrophic factor expression even held to the distal nerve, where there was a moderate increase in Bdnf expression in the distal nerve of the hybrid ANA compared with control ANA.
FIGURE 2.
Gdnf and Bdnf expression within ANAs 4 weeks after defect reconstruction. Hybrid and control ANAs were divided into regions to evaluate Gdnf (A) and Bdnf (B) expression. Hybrid ANAs contained regions with elevated gene expression. Data represented as mean ± standard deviation (n = 4 per group). **P < 0.01 and ***P < 0.001 vs. control ANA. [Color figure can be viewed at wileyonlinelibrary.com]
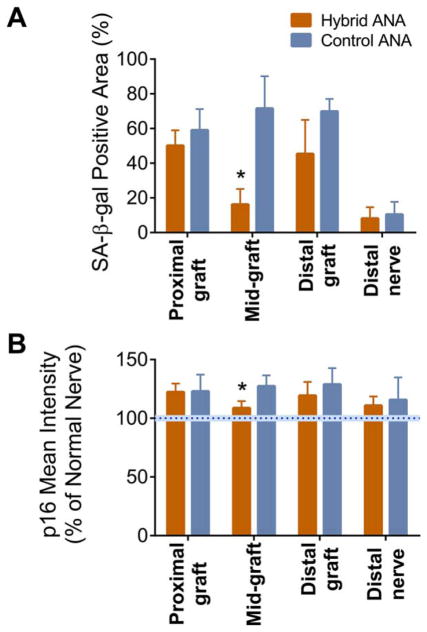
At this same 4-week end-point, these graft regions were assessed for markers of senescence. SA-β-gal staining indicated that graft regions composed of ANAs had relatively intense staining, with these regions averaging ~50% or greater positive area for SA-β-gal activity (Fig. 3A). Both experimental groups contained no statistical differences between either group in SA-β-gal activity within the proximal and distal graft. Conversely, the hybrid ANA midgraft contained a significantly decreased SA-B-gal-positive area compared to the control ANA midgraft control ANA (~16 ± 9% vs. ~72 ± 19%). Both experimental groups contained lower (~20%) SA-β-gal–positive areas in the distal nerve, with no statistical difference between groups.
FIGURE 3.
Evaluation of markers of cellular senescence within ANAs 4 weeks after defect reconstruction. Hybrid and control ANAs were divided into regions to evaluate staining for SA-β-gal activity (A) and p16 expression (B) (intensity normalized to uninjured nerve indicated by blue dotted bar). The hybrid ANAs’ midgraft region had decreased senescence markers. Data represent mean ± standard deviation (n = 4 per group). *P < 0.05 vs. control ANA. [Color figure can be viewed at wileyonlinelibrary.com]
Comparing an additional marker of senescence (p16 expression), similar patterns emerged as with SA-β-gal activity (Fig. 3B). Neither experimental group had statistically different p16 expression levels in proximal and distal graft regions, where both regions were elevated compared with p16 expression in normal nerve. However, the hybrid ANA contained significantly lower p16 expression in the midgraft compared with control ANA. The distal nerve in either group had relatively normal p16 expression, with no statistical differences between groups. Overall, these data demonstrate that an isograft interposed between ANAs has a modest impact on the regenerative environment of these long ANAs.
Evaluation of Nerve Regeneration Using Hybrid ANAs
The impact of interposing a short isograft in series between short ANAs (generating a hybrid ANA) on nerve regeneration was evaluated at 20 weeks using histology and motor recovery metrics. Qualitatively, hybrid ANAs had a similar macroscopic appearance to control ANAs during the tissue harvest. The harvested ANAs were visually similar in appearance to previous results using 6-cm ANAs to reconstruct rat sciatic nerve defects.11
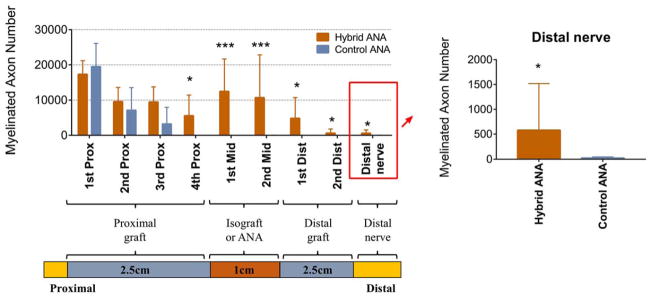
Histological cross-sections were taken along the length of the graft and distal nerve with a higher number of spatial segments assessed in the proximal graft (Figs. 4 and 5), as we previously determined that axons arrest within the first 2 cm of 6-cm ANAs.11,12 For perspective, the normal rat sciatic nerve contains ~8,000 myelinated axons.49 The number of myelinated axons within the proximal nerve graft was robust (greater than normal nerve) in both experimental groups, indicating that axons regenerated into the ANA grafts. Both groups (hybrid ANA vs. control ANA) contained no statistical differences in myelinated axon numbers in the first 3 spatial graft segments (~1.5-cm length of ANA). However, by the fourth spatial segment within the proximal graft (~2 cm distal from the proximal nerve coaptation site), the control ANA demonstrated virtually no myelinated axons (<50 fibers) and an “arrest” of axon regeneration within the ANA. Conversely, the hybrid ANA contained a significantly greater number of myelinated axons at this segment (~5,500 myelinated axons).
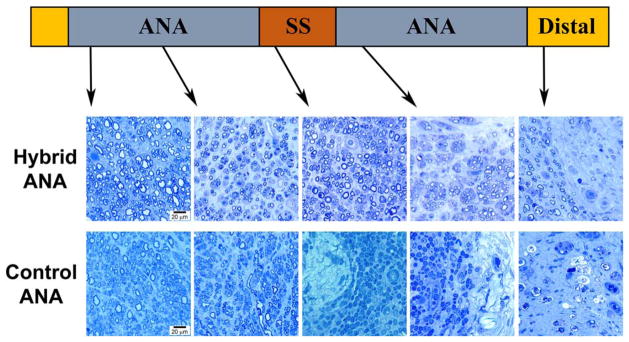
FIGURE 4.
Histological images of nerve regeneration throughout and across the grafts at 20 weeks after defect reconstruction. Toluidine blue–counterstained nerve cross-sections show myelinated axons in all sections of the hybrid ANA with a gradual decline in number as axons proceed distally. Control ANA shows little to no myelinated axons reaching the midgraft region or distal nerve. Scale bar = 20 μm. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.
Histomorphometric analysis of axonal regeneration at 20 weeks after defect reconstruction. Hybrid ANAs facilitated greater numbers of myelinated axons across the defect compared with control ANAs. Data represent mean ± SD (n = 10 per group). *P < 0.05 and ***P < 0.001 vs. control ANA. [Color figure can be viewed at wileyonlinelibrary.com]
Within the midgraft and distal graft a similar pattern followed, where the hybrid ANA contained myelinated axons, whereas the control ANA contained little to no myelinated axons within the graft regions (<50 fibers). Although myelinated axon numbers remained high in the hybrid ANA midgraft region, axon numbers decreased in the distal graft region. Overall, the hybrid ANA facilitated modest axon regeneration across the long defect, where ~600 myelinated axons reached the distal nerve, whereas the control ANA had almost no myelinated axons reach the distal nerve. However, considering aspects of motor recovery, neither experimental group achieved measurable muscle force production, nor were any differences in wet muscle mass observed (data not shown). Overall, these results demonstrate that hybrid ANA promoted improved axon regeneration within the graft compared with control ANA, and a very small number of myelinated axons cross the long 6-cm defect. However, no evidence of motor recovery was observed using the hybrid ANA.
DISCUSSION
Our work has demonstrated that a hybrid ANA containing an isograft had an impact on the regenerative environments of the adjacent ANAs, where the primary impact to the overall hybrid ANA was an isolated region (the isograft). In addition, although incorporating an isograft between ANAs improved axon regeneration within and across the long hybrid ANA compared with the control ANA, the overall motor regenerative outcome was unfavorable, as no muscle force or mass recovered.
Previously, our group validated that a “stepping stone” approach to reconstruct short nerve defects (<3 cm) improves regenerative outcomes compared with a nerve autograft substitute alone.30,31 The current study assessed whether this approach has merit to improve nerve regeneration across long defects. Based on the outcome of the studies, we conclude this approach is not an effective strategy to reconstruct ≥6-cm defects. Although we observed axons crossing the defect bridged by 6-cm hybrid ANAs, the number of myelinated axons in the distal nerve was low relative to normal nerve or 6-cm isografts (~600 myelinated axons using 6-cm hybrid ANA vs. ~4,000 myelinated axons using a 6-cm isograft,11 whereas normal rat sciatic nerve contains ~8,000 myelinated axons49). In addition, we observed no potential for motor functional recovery in the hybrid ANA despite axons crossing the defect, as indicated by no change in muscle mass atrophy and no production of contractile muscle force. In an earlier study we determined that a long, 6-cm isograft provided a moderate level of motor functional recovery, as extensor digitorum longus contractile muscle force was ~50% of its uninjured muscle force capacity.11 As only modest numbers of myelinated axons crossed the 6-cm hybrid ANA, the observed lack of motor recovery corresponded appropriately.
Characterization of hybrid the ANA early regenerative environment (4 weeks) taken with the regenerative outcome (20 weeks) revealed insights as to the critical aspects of nerve regeneration across long nerve defects. ANAs initially contain few or no growth factors, so as to encourage nerve regeneration due to a lack of cells. One goal of our hybrid ANA approach was to increase growth factor availability by incorporating cells within an ANA. Incorporating cells via nerve within a long ANA (hybrid ANA) increased neurotrophic factor expression in regions of the hybrid ANAs, where increased growth factor availability is known to improve nerve regeneration for acellular scaffolds.34,35,50,51 Indeed, Gdnf and Bdnf expression were highly elevated in the middle and distal graft in the hybrid ANA compared with the control ANA corresponding to increased axon regeneration.
Although ANAs initially have no cells, our previous work demonstrated that long ANAs (6 cm) become repopulated with a majority of Schwann cells expressing senescence markers.12 An additional goal of our hybrid ANA approach was to introduce cells to an ANA, which may reduce the accumulation of senescent cells via a reduction in cellular replicative burden. However, incorporating cells via a nerve within a long ANA had only a moderate impact on the accumulation of senescence markers throughout the long grafts, as only the midgraft (cellular isograft region) had decreased expression of senescence and stress markers. As hybrid ANAs only moderately improved axon regeneration across these long grafts, this result suggests that the increased expression of senescence markers may have impacted nerve regeneration. This result coincides with previous work demonstrating that an accumulation of cells expressing markers of senescence precedes and is associated with inferior axon regeneration.11,12 Overall, the findings characterizing the environment of hybrid ANAs indicate that both an increase in neurotrophic factors and a reduction in the accumulation of senescence markers may be necessary to yield robust axon regeneration and motor functional recovery.
In conclusion, the interposition of a cellular graft (isograft) to generate a hybrid ANA moderately increased axonal regeneration across the graft but had minimal impact in improving aspects of motor recovery. Our results clarify that, not only do constructs such as ANAs have a length limit, but previous successful approaches (i.e., “stepping stone”) used for improving short defect reconstructions have limitations when applied to long defect reconstructions. Our findings disprove our original hypothesis. The fact that a 1-cm cellular nerve graft (isograft) in series with acellular grafts would not robustly support axon regeneration demonstrates the challenge in improving the ability of nerve substitutes to support nerve regeneration across long (>5 cm) nerve gaps. Although this was an animal study, evidence from this work strongly suggests that the use of a short piece of nerve (1 cm) between ANAs to generate a hybrid ANA is unlikely to be an effective strategy in human clinical use to reconstruct long (>5 cm) defects. In our clinical setting, we will continue to reconstruct long nerve defects with autografts.
Acknowledgments
This work was supported in part by grants from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (R01 NS086773 and P30 NS057105) to Washington University. The content is solely the responsibility of the authors and does not represent the views of the National Institutes of Health or Washington University.
Abbreviations
- ANA
acellular nerve allograft
- Bdnf
brain-derived neurotrophic factor
- EDL
extensor digitorum longus
- Gdnf
glial-derived neurotrophic factor
- IHC
immunohistochemistry
- qRT-PCR
quantitative real-time polymerase chain reaction
- SA-β-gal
senescence-associated β-galactosidase
- SC
Schwann cell
- SD
Sprague–Dawley
References
- 1.Mackinnon SE. Surgical management of the peripheral nerve gap. Clin Plast Surg. 1989;16:587–603. [PubMed] [Google Scholar]
- 2.Mackinnon SE. Technical use of synthetic conduits for nerve repair. J Hand Surg. 2011;36:183. doi: 10.1016/j.jhsa.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Szynkaruk M, Kemp SW, Wood MD, Gordon T, Borschel GH. Experimental and clinical evidence for use of decellularized nerve allografts in peripheral nerve gap reconstruction. Tissue Eng B Rev. 2013;19:83–96. doi: 10.1089/ten.TEB.2012.0275. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–799. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 5.Hoben G, Yan Y, Iyer N, Newton P, Hunter DA, Moore AM, et al. Comparison of acellular nerve allograft modification with Schwann cells or VEGF. Hand. 2015;10:396–402. doi: 10.1007/s11552-014-9720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YH, Chung MS, Gong HS, Chung JY, Park JH, Baek GH. Sural nerve autografts for high radial nerve injury with nine centimeter or greater defects. J Hand Surg. 2008;33:83–86. doi: 10.1016/j.jhsa.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Karabeg R, Jakirlic M, Dujso V, Obradovic G, Arslanagic S. Outcomes of ulnar nerve grafting. Med Arch. 2013;67:39–41. doi: 10.5455/medarh.2013.67.39-41. [DOI] [PubMed] [Google Scholar]
- 8.Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32:1–14. doi: 10.1002/micr.20975. [DOI] [PubMed] [Google Scholar]
- 9.Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg. 2012;37:2340–2349. doi: 10.1016/j.jhsa.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Salomon D, Miloro M, Kolokythas A. Outcomes of immediate allograft reconstruction of long-span defects of the inferior alveolar nerve. J Oral Maxillofac Surg. 2016;74:2507–2514. doi: 10.1016/j.joms.2016.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Saheb-Al-Zamani M, Yan Y, Farber SJ, Hunter DA, Newton P, Wood MD, et al. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol. 2013;247C:165–177. doi: 10.1016/j.expneurol.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poppler LH, Ee X, Schellhardt L, Hoben GM, Pan D, Hunter DA, et al. Axonal growth arrests after an increased accumulation of schwann cells expressing senescence markers and stromal cells in acellular nerve allografts. Tissue Eng A. 2016;22:949–961. doi: 10.1089/ten.tea.2016.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundborg G, Hansson HA. Nerve regeneration through preformed pseudosynovial tubes. A preliminary report of a new experimental model for studying the regeneration and reorganization capacity of peripheral nerve tissue. J Hand Surg. 1980;5:35–38. doi: 10.1016/s0363-5023(80)80041-4. [DOI] [PubMed] [Google Scholar]
- 14.Whitlock EL, Myckatyn TM, Tong AY, Yee A, Yan Y, Magill CK, et al. Dynamic quantification of host Schwann cell migration into peripheral nerve allografts. Exp Neurol. 2010;225:310–319. doi: 10.1016/j.expneurol.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundborg G, Hansson HA. Regeneration of peripheral nerve through a preformed tissue space. Preliminary observations on the reorganization of regenerating nerve fibres and perineurium. Brain Res. 1979;178:573–576. doi: 10.1016/0006-8993(79)90716-9. [DOI] [PubMed] [Google Scholar]
- 16.Williams LR, Longo FM, Powell HC, Lundborg G, Varon S. Spatial-temporal progress of peripheral nerve regeneration within a silicone chamber: parameters for a bioassay. J Comp Neurol. 1983;218:460–470. doi: 10.1002/cne.902180409. [DOI] [PubMed] [Google Scholar]
- 17.Hall SM. Regeneration in cellular and acellular autografts in the peripheral nervous system. Neuropathol Appl Neurobiol. 1986;12:27–46. doi: 10.1111/j.1365-2990.1986.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 18.Hall SM. The effect of inhibiting Schwann cell mitosis on the re-innervation of acellular autografts in the peripheral nervous system of the mouse. Neuropathol Appl Neurobiol. 1986;12:401–414. doi: 10.1111/j.1365-2990.1986.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 19.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harbor Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cattin AL, Burden JJ, van Emmenis L, Mackenzie FE, Hoving JJ, Garcia Calavia N, et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattin AL, Lloyd AC. The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol. 2016;39:38–46. doi: 10.1016/j.conb.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Farber SJ, Hoben GM, Hunter DA, Yan Y, Johnson PJ, Mackinnon SE, et al. Vascularization is delayed in long nerve constructs compared with nerve grafts. Muscle Nerve. 2016;54:319–321. doi: 10.1002/mus.25173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Best TJ, Mackinnon SE, Midha R, Hunter DA, Evans PJ. Revascularization of peripheral nerve autografts and allografts. Plast Reconstr Surg. 1999;104:152–160. [PubMed] [Google Scholar]
- 24.Best TJ, Mackinnon SE, Evans PJ, Hunter D, Midha R. Peripheral nerve revascularization: histomorphometric study of small- and large-caliber grafts. J Reconstr Microsurg. 1999;15:183–190. doi: 10.1055/s-2007-1000090. [DOI] [PubMed] [Google Scholar]
- 25.Johnson PJ, Wood MD, Moore AM, Mackinnon SE. Tissue engineered constructs for peripheral nerve surgery. Eur Surg Acta Chir Austr. 2013:45. doi: 10.1007/s10353-013-0205-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levi AD, Guenard V, Aebischer P, Bunge RP. The functional characteristics of Schwann cells cultured from human peripheral nerve after transplantation into a gap within the rat sciatic nerve. J Neurosci. 1994;14:1309–1319. doi: 10.1523/JNEUROSCI.14-03-01309.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi AD. Characterization of the technique involved in isolating Schwann cells from adult human peripheral nerve. J Neurosci Methods. 1996;68:21–26. doi: 10.1016/0165-0270(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 28.Levi AD, Burks SS, Anderson KD, Dididze M, Khan A, Dietrich WD. The use of autologous schwann cells to supplement sciatic nerve repair with a large gap: first in human experience. Cell Transplant. 2016;25:1395–1403. doi: 10.3727/096368915X690198. [DOI] [PubMed] [Google Scholar]
- 29.Lavasani M, Thompson SD, Pollett JB, Usas A, Lu A, Stolz DB, et al. Human muscle-derived stem/progenitor cells promote functional murine peripheral nerve regeneration. J Clin Invest. 2014;124:1745–1756. doi: 10.1172/JCI44071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda T, Mackinnon SE, Best TJ, Evans PJ, Hunter DA, Midha RT. Regeneration across ‘stepping-stone’ nerve grafts. Brain Res. 1993;618:196–202. doi: 10.1016/0006-8993(93)91266-u. [DOI] [PubMed] [Google Scholar]
- 31.Francel PC, Francel TJ, Mackinnon SE, Hertl C. Enhancing nerve regeneration across a silicone tube conduit by using interposed short-segment nerve grafts. J Neurosurg. 1997;87:887–892. doi: 10.3171/jns.1997.87.6.0887. [DOI] [PubMed] [Google Scholar]
- 32.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 33.Kawamura DH, Hadlock TA, Fox IK, Brenner MJ, Hunter DA, Mackinnon SE. Regeneration through nerve isografts is independent of nerve geometry. J Reconstr Microsurg. 2005;21:243–249. doi: 10.1055/s-2005-871751. [DOI] [PubMed] [Google Scholar]
- 34.Yan Y, Wood MD, Hunter DA, Ee X, Mackinnon SE, Moore AM. The effect of short nerve grafts in series on axonal regeneration across isografts or acellular nerve allografts. J Hand Surg. 2016;41:e113–121. doi: 10.1016/j.jhsa.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Wood MD, MacEwan MR, French AR, Moore AM, Hunter DA, Mackinnon SE, et al. Fibrin matrices with affinity-based delivery systems and neurotrophic factors promote functional nerve regeneration. Biotechnol Bioeng. 2010;106:970–979. doi: 10.1002/bit.22766. [DOI] [PubMed] [Google Scholar]
- 36.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- 37.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113:3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 38.Litaker JR, Pan J, Cheung Y, Zhang DK, Liu Y, Wong SC, et al. Expression profile of senescence-associated beta-galactosidase and activation of telomerase in human ovarian surface epithelial cells undergoing immortalization. Int J Oncol. 1998;13:951–956. doi: 10.3892/ijo.13.5.951. [DOI] [PubMed] [Google Scholar]
- 39.Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003;183:610–619. doi: 10.1016/s0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 40.Hoke A, Gordon T, Zochodne DW, Sulaiman OA. A decline in glial cell-line-derived neurotrophic factor expression is associated with impaired regeneration after long-term Schwann cell denervation. Exp Neurol. 2002;173:77–85. doi: 10.1006/exnr.2001.7826. [DOI] [PubMed] [Google Scholar]
- 41.Willand MP, Rosa E, Michalski B, Zhang JJ, Gordon T, Fahnestock M, et al. Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience. 2016;334:93–104. doi: 10.1016/j.neuroscience.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 42.Gordon T, Hendry M, Lafontaine CA, Cartar H, Zhang JJ, Borschel GH. Nerve cross-bridging to enhance nerve regeneration in a rat model of delayed nerve repair. PLoS One. 2015;10:e0127397. doi: 10.1371/journal.pone.0127397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendry JM, Alvarez-Veronesi MC, Snyder-Warwick A, Gordon T, Borschel GH. Side-to-side nerve bridges support donor axon regeneration into chronically denervated nerves and are associated with characteristic changes in Schwann cell phenotype. Neurosurgery. 2015;77:803–813. doi: 10.1227/NEU.0000000000000898. [DOI] [PubMed] [Google Scholar]
- 44.Placheta E, Wood MD, Lafontaine C, Frey M, Gordon T, Borschel GH. Macroscopic in vivo imaging of facial nerve regeneration in Thy1-GFP rats. JAMA Facial Plast Surg. 2015;17:8–15. doi: 10.1001/jamafacial.2014.617. [DOI] [PubMed] [Google Scholar]
- 45.Placheta E, Wood MD, Lafontaine C, Liu EH, Hendry JM, Angelov DN, et al. Enhancement of facial nerve motoneuron regeneration through cross-face nerve grafts by adding end-to-side sensory axons. Plast Reconstr Surg. 2015;135:460–471. doi: 10.1097/PRS.0000000000000893. [DOI] [PubMed] [Google Scholar]
- 46.Hegedus J, Jones KE, Gordon T. Development and use of the incremental twitch subtraction MUNE method in mice. Clin Neurophysiol Suppl. 2009;60:209–217. doi: 10.1016/s1567-424x(08)00022-6. [DOI] [PubMed] [Google Scholar]
- 47.Major LA, Hegedus J, Weber DJ, Gordon T, Jones KE. Method for counting motor units in mice and validation using a mathematical model. J Neurophysiol. 2007;97:1846–1856. doi: 10.1152/jn.00904.2006. [DOI] [PubMed] [Google Scholar]
- 48.Hunter DA, Moradzadeh A, Whitlock EL, Brenner MJ, Myckatyn TM, Wei CH, et al. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. J Neurosci Methods. 2007;166:116–124. doi: 10.1016/j.jneumeth.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood MD, Kemp SW, Liu EH, Szynkaruk M, Gordon T, Borschel GH. Rat-derived processed nerve allografts support more axon regeneration in rat than human-derived processed nerve xenografts. J Biomed Mater Res A. 2014;102:1085–1091. doi: 10.1002/jbm.a.34773. [DOI] [PubMed] [Google Scholar]
- 50.Wood MD, Kim H, Bilbily A, Kemp SW, Lafontaine C, Gordon T, et al. GDNF released from microspheres enhances nerve regeneration after delayed repair. Muscle Nerve. 2012;46:122–124. doi: 10.1002/mus.23295. [DOI] [PubMed] [Google Scholar]
- 51.Marquardt LM, Ee X, Iyer N, Hunter D, Mackinnon SE, Wood MD, et al. Finely tuned temporal and spatial delivery of gdnf promotes enhanced nerve regeneration in a long nerve defect model. Tissue Eng A. 2015;21:2852–2864. doi: 10.1089/ten.tea.2015.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]