Abstract
To assess whether Tie2-mediated vascular stabilization ameliorates neovascular age-related macular degeneration (AMD), we investigated the impact of adeno-associated virus-mediated gene therapy with cartilage oligomeric matrix protein angiopoietin-1 (AAV2.COMP-Ang1) on choroidal neovascularization (CNV), vascular endothelial growth factor (VEGF), and hypoxia-inducible factor (HIF) in a mouse model of the disease. We treated mice with subretinal injections of AAV2.COMP-Ang1 or control (AAV2.AcGFP, AAV2.LacZ, and phosphate-buffered saline). Subretinal AAV2 localization and plasmid protein expression was verified in the retinal pigment epithelium (RPE)/choroid of mice treated with all AAV2 constructs. Laser-assisted simulation of neovascular AMD was performed and followed by quantification of HIF, VEGF, and CNV in each experimental group. We found that AAV2.COMP-Ang1 was associated with a significant reduction in VEGF levels (29–33%, p < 0.01) and CNV volume (60–70%, p < 0.01), without a concomitant decrease in HIF1-α, compared to all controls. We concluded that a) AAV2 is a viable vector for delivering COMP-Ang1 to subretinal tissues, b) subretinal COMP-Ang1 holds promise as a prospective treatment for neovascular AMD, and c) although VEGF suppression in the RPE/choroid may be one mechanism by which AAV2.COMP-Ang1 reduces CNV, this therapeutic effect may be hypoxia-independent. Taken together, these findings suggest that AAV2.COMP-Ang1 has potential to serve as an alternative or complementary option to anti-VEGF agents for the long-term amelioration of neovascular AMD.
Keywords: Age-related macular degeneration, Choroidal neovascularization, Vascular endothelial growth factor, Cartilage oligomeric matrix protein, Angiopoietin-1, Adeno-associated virus, Subretinal injection
1. Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness among older Americans (Bressler, 2004; Congdon et al., 2004; Friedman et al., 2004; Pascolini et al., 2004). Neovascular AMD is a late form of the disease accountable for the majority of visual impairment connected with AMD (Ferris et al., 1984). The hallmark of neovascular AMD is choroidal neovascularization (CNV), which is typified by the growth and penetration of new blood vessels from the choroid through Bruch’s membrane and into the sub-retinal pigment epithelium (sub-RPE) and subretinal space, leading to fluid leakage, bleeding, and scarring of the macula (Nowak, 2006). Vascular endothelial growth factor (VEGF), a potent endothelial mitogen and vascular permeability factor, is the principal driver of angiogenesis in CNV (Funk et al., 2009; Sivaprasad and Hykin, 2013). The introduction of VEGF inhibitor drugs has been one of the greatest success stories of translational research, revolutionizing the treatment paradigm for neovascular AMD by slowing the progression of disease and stabilizing or reversing visual loss for the majority of patients (Brown et al., 2009; Campbell et al., 2012a; Comparison of Age-related Macular Degeneration Treatments Trials Research et al., 2012; Heier et al., 2012; Rosenfeld et al., 2006; Sivaprasad and Hykin, 2013; Solomon et al., 2014; Stewart, 2012).
Unfortunately, anti-VEGF agents are far from risk-free, and there is room for improvement in their safety, efficacy, applicability, and ease-of-use. Often requiring indefinite monthly or bimonthly intraocular injections (Sivaprasad and Hykin, 2013), these drugs can be costly and produce a spectrum of ophthalmic complications such as infection, bleeding, and retinal detachment (Gragoudas et al., 2004; Heier et al., 2012; Rosenfeld et al., 2006). Pharmacologically, chronic blockade of VEGF may hamper its neurotrophic benefits to the retina (Lazarovici et al., 2006; Nishijima et al., 2007; Romano et al., 2012; Saint-Geniez et al., 2009; Wang et al., 2004; Yodoi et al., 2009). Anti-VEGF administration also seems to increase risk of stroke and thromboembolic events (Bressler, 2004; Campbell et al., 2012b). Most importantly, a sizeable subset of AMD is refractory to conventional anti-VEGF regimens (Broadhead et al., 2014; Brown et al., 2009; Calvo et al., 2015; Rosenfeld et al., 2006; Shin et al., 2013; Tozer et al., 2013; Tranos et al., 2013). These limitations of VEGF blockers prompt the need to continue to investigate alternative or complementary strategies for neovascular AMD.
One possible target for therapeutic exploitation may be the Ang1-Tie2 signaling cascade. Like the VEGF-VEGFR system, it is an endothelial specific tyrosine kinase ligand-receptor system, which is a key player in vascular homeostasis, including vessel remodeling, maturation, and stabilization (Augustin et al., 2009; Das and McGuire, 2003; Fukuhara et al., 2010; Thurston et al., 2000). In pre-clinical studies, angiopoetin-1 (Ang1) has been shown to diminish CNV (Nambu et al., 2004, 2005; Wang et al., 2013) and counter VEGF by preventing vessel hyperpermeability (Baffert et al., 2006; Thurston et al., 2000, 1999). Cartilage oligomeric matrix protein-angiopoietin-1 (COMP-Ang1) was engineered to enhance solubility, yield, potency, and stability while mimicking the anti-leakage activity of native Ang1 (Cho et al., 2004; Fuxe et al., 2011).
To our knowledge, only one previous study has tested COMP-Ang1 in an AMD model, reporting that intravitreal injection of recombinant COMP-Ang1 protein is equivalent to the VEGF inhibitor fusion protein aflibercept in suppressing CNV formation, and superior to it in suppressing vascular leakage (Lee et al., 2014).
To build on this work, we hypothesized that the continuous expression of COMP-Ang1 via adeno-associated virus type 2 (AAV2)-mediated gene transfer would improve treatment sustainability. The sawtooth pattern of drug delivery entailed by the short half-life of recombinant protein (Zhu et al., 2011) creates a peak-trough problem with drug availability (Zhang et al., 2015) which could be circumvented by long-acting gene therapy with viral vectors (Campochiaro, 2011; Colella and Auricchio, 2010; Roy et al., 2010). The efficacy of Ad.COMP-Ang1 therapy is limited by its transient expression (i.e. 1 month duration) and intense immunogenicity (Campochiaro, 2011;Wang et al., 2004; Zhang et al., 2012). The intraocular humoral and cellular response to the nonpathogenic AAV, conversely, is minimal and benign (Amado et al., 2010; Cheng et al., 2013; Daya and Berns, 2008; Dismuke et al., 2013; Roy et al., 2010; Wang et al., 2004; Zhang et al., 2012). In human studies, the AAV2 serotype was shown to be safe for human retinal disorders, and effective for up to 3.5 years (Roy et al., 2010; Simonelli et al., 2010; Zhang et al., 2012).
Furthermore, because the pathology of AMD predominantly occurs in the subretinal tissues instead of the vitreous (Nowak, 2006), we surmised that localized subretinal delivery of COMP-Ang1 would improve treatment. With specificity for the outer retina and the RPE/choroid complex (Cronin et al., 2012; Muhlfriedel et al., 2013; Zhang et al., 2012, 2015), a subretinal route for AAV2 offers stronger and more diffuse transfection of these layers, along with a milder immune reaction, compared to an intravitreal route (Campochiaro, 2011; Li et al., 2008). Targeting this space may also mitigate the danger of medication-induced global retinal toxicity linked with the intravitreal approach (Campochiaro, 2011; Grunwald et al., 2014; Rofagha et al., 2013; Zhang et al., 2015).
Finally, because of our laboratory’s recent discovery that AAV2.COMP-Ang1 has a stabilizing effect on the ocular vasculature in diabetic retinopathy mediated in part through its suppression of hypoxia-induced VEGF secretion (Cahoon et al., 2015), we conjectured that there may be a similar link between COMP-Ang1, VEGF, and hypoxia inducible factor (HIF) in neovascular AMD.
In our experiments on a laser-induced mouse model of AMD, we sought to test the utility of subretinal AAV2.COMP-Ang1 in reducing CNV volume, as well as initiate exploration into the molecular underpinnings of this treatment.
2. Materials and methods
2.1. Animals
Age-matched (12 week old) male C57Bl6 mice (The Jackson Laboratory, Bar Harbor, ME) were randomly allocated into one of five experimental groups: AAV2.COMP-Ang1, AAV2.AcGFP (Aequorea coerulescens green fluorescent protein), AAV2.LacZ (β-galactosidase), phosphate-buffered saline (PBS), or no injection control.
For all in vivo injection and laser procedures, mice were placed under general anesthesia with an intraperitoneal injection of ketamine/ xylazine (Vedco, Saint Joseph, MO) at a dose of 90 mg/ 10 mg per kg body weight. Topical application of 1% tropicamide (Bausch & Lomb, Tampa, FL) dilated the pupils and provided local anesthesia, respectively. For in vivo fundoscopy and optical coherence tomography (OCT), mice were anesthetized by an initial inhalation of 3% isoflurane/O2 mixture in a closed canister at a flow rate of 1.0 Lpm. Pupils were dilated with a 1% tropicamide solution and afterwards hydrated periodically with saline solution to prevent corneal desiccation. For ex vivo assays requiring harvesting of the globes, mice were euthanized with carbon dioxide (CO2), and both eyes were enucleated.
The protocol was approved by the Institutional Animal Care and Use Committee of the University of Utah, and all animal experiments were performed in accordance with guidelines from the Association of Research in Vision and Ophthalmology’s Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. AAV2 vector construction
The plasmids pAAV.COMP-Ang1 and pAAV.AcGFP were created by ligating the COMP-Ang1 cDNA from pCMV-dhfr2-COMP-Ang1 (Koh Laboratory of Regenerative Medicine, Korea Advanced Institute of Science and Technology, Daejeon) into pAAV-MCS (Agilent Technologies, Santa Clara, CA), while pAAV.AcGFP was created by the same technique with AcGFP cDNA from pIRES2-AcGFP1 (Clontech Laboratories, Mountain View, CA). The pAAV.LacZ plasmid was obtained from Stratagene (La Jolla, CA). The AAV viral vector was next converted to serotype 2 (Vector Core Gene Therapy Center, University of Massachusetts, Worcester, MA). Lastly, cassettes from the plasmids were integrated into the AAV2 vectors, driven by the CMV promoter, to generate the experimental treatment AAV2.COMP-Ang1 and its sham controls AAV2.AcGFP and AAV2.LacZ.
2.3. Subretinal treatment injections
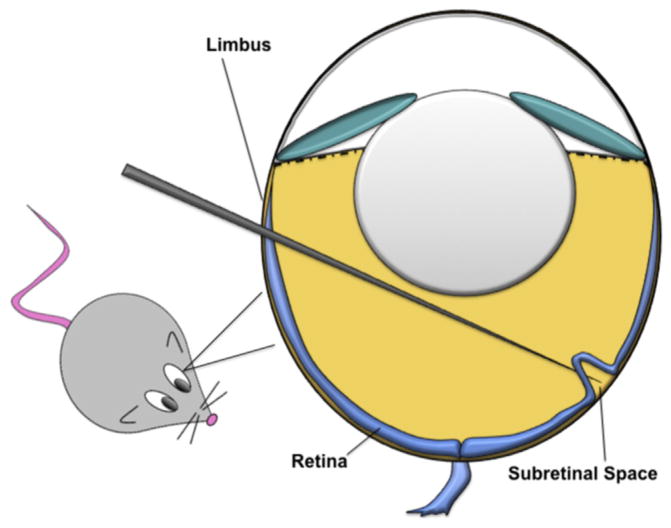
Solutions of viral particles were prepared at the following titers: AAV2.COMP-Ang1 (3 × 1011 viral units/mL); AAV2.AcGFP (3 × 1011 viral units/mL); AAV2.LacZ (5 × 1011 viral units/mL). Anesthetized mice were placed under a stereo microscope. A small incision was made behind the limbus with a 30.5-gauge needle. A blunt 33-gauge microsyringe (Hamilton Company, Reno, NV) was then inserted into the posterior chamber at the incision, passed through the vitreous to the posterior pole at the opposite wall of the globe, and then entered into the subretinal space (Fig. 1) to deposit 1 μL of AAV2 solution or PBS. Care was taken to avoid damaging the lens. Visualization of partial retinal detachment around the injection site by fundus examination at the conclusion of injection confirmed successful subretinal delivery.
Fig. 1.
Schematic diagram depicting subretinal injection in a mouse model.
2.4. Optical coherence tomography
To affirm the safety and efficacy of subretinal injections in vivo, the posterior segments of mice were imaged bilaterally with OCT (Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany) at 3 and 4 weeks after subretinal injection.
2.5. Funduscopy and X-Gal staining and light microscopy
To verify short-term AAV2 transfection in vivo, the retinochoroidal tissue in both eyes of mice from the AAV2.AcGFP group was funduscopically examined at one month after subretinal injection with the fluorescein angiography (FA) modality (Spectralis HRA + OCT, Heidelberg Engineering) for autofluorescence.
To reaffirm transfection ex vivo, retinas from the AAV2.LacZ group were harvested at the same one month endpoint and stained with X-gal (InvivoGen, San Diego, CA) as per manufacturer’s instructions. The RPE/choroid was then dissected, prepared for flat mount, and observed under brightfield illumination for blue LacZ signal.
2.6. Anti-FLAG immunoprecipitation and western blot
To verify long-term plasmid COMP-Ang1 transduction, globes from the AAV2.COMP-Ang1, AAV2.LacZ, PBS, and no injection groups were harvested three months after initial subretinal injection. After isolating the RPE/choroid/sclera complex from the remaining structures, the RPE/choroid tissue was dissected from the sclera. The RPE/choroid specimens were next put in 400 μl of radioimmunoprecipitation assay (RIPA) buffer (Sigma–Aldrich, St. Louis, MO) containing a protease and phosphatase inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), placed on ice, and subjected to homogenization with a sonic dismembranator (Fisher Scientific, Pittsburg, PA). Protein concentration was estimated by bicinchoninic acid assay (BCA), and overall protein levels were compared by running protein lysate samples on sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), blotting, and probing with anti-GAPDH antibody (1:3000, Abcam, Cambridge, MA). Another set of protein lysate samples were immuno-precipitated with anti-FLAG M2 affinity gel (Sigma–Aldrich) as per the manufacturer’s instructions. Eluted protein samples were then run on 12% SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. After staining with anti-FLAG biotinylated M2 antibody (Sigma–Aldrich), the proteins were incubated with streptavidin horseradish peroxidase substrate (HRP, Abcam) and detected by transillumination (FOTO/Analyst Electronic Imaging Systems, FOTODYNE, Hartland, WI).
2.7. Laser-induced CNV
Laser-induced rupture of Bruch’s membrane is a well-accepted and frequently utilized murine model of choroidal neovascularization (Lambert et al., 2013; Tobe et al., 1998) which our laboratory has successfully adopted for animal studies on AMD. Each experimental group underwent laser treatment one month after subretinal injection. The beam from a diode laser (532 nm; OcuLight GLx, Iridex, Mountain View, CA) was directed onto the retina via slit lamp, using a 22-mm coverslip as a contact lens. The treatment parameters were optimized for the formation of a cavitation bubble in the choroid without hemorrhage (spot diameter, 100 μm; intensity, 120 mW; duration, 100 ms). All laser burns were produced symmetrically and circumferentially 2–3 disc diameters from the optic nerve. Four laser spots (3, 6, 9, and 12 o’clock axes) were generated for CNV volume analysis, while eight laser spots (adding in the 1.5, 4.5, 7.5, and 10.5 clock–hour axes) were generated for the VEGF and HIF1-α experiments. The creation of a break in Bruch’s membrane at each spot was verified by visualization of a bubble at the time of photocoagulation.
2.8. Quantitation of VEGF
Multiple studies have demonstrated that VEGF levels in the RPE/ choroid climax at 3 days post laser photocoagulation (Ashikari et al., 2010; Mizutani et al., 2013; Nozaki et al., 2006; Sakurai et al., 2003). On this basis, globes from the AAV2.COMP-Ang1, AAV2.AcGFP, and PBS groups were harvested three days after laser-induced CNV. After isolation of RPE/choroid tissue, an enzyme-linked immunosorbent assay (ELISA) was performed per manufacturer’s instructions using the Quantikine Mouse VEGF Kit (R&D Systems, Minneapolis, MN). VEGF-A concentrations were calculated from the standard curve and corrected by total protein.
2.9. Quantitation and qualitation of HIF-1α
In addition to VEGF, hypoxia-inducible factor 1-alpha (HIF-1-alpha, HIF-1α), a transcription factor which is activated by decreased oxygen availability in the cellular environment, becomes elevated after laser CNV. In response to hypoxic conditions caused by the insult, HIF-1α reaches maximum concentrations at day 3 after laser-induced CNV and can be assayed by Western blot or ELISA analysis (Yang et al., 2009).
To investigate the possibility of a hypoxia-based association between AAV2.COMP-Ang1 and VEGF, we evaluated HIF-1α expression in the RPE/choroid with immunoblotting and ELISA. For immunoblotting, RPE/choroid lysates were detected usingWestern blot with anti–HIF–1α antibody (ab51608, Abcam, Cambridge, MA). For ELISA, the same antibody was used with the methods described above for VEGF quantification (Section 2.8).
2.10. Quantitation of CNV
Peak CNV volume has previously been shown to occur 7 days after laser-induced CNV (Mizutani et al., 2013). Accordingly, one week after laser injury, globes from the AAV2.COMP-Ang1, AAV2.AcGFP, and PBS groups were harvested. After removing the cornea and lens, the eye cup was fixed in 4% paraformaldehyde for 2 h at 4 °C. The retina was then dissected out. The sclera/choroid/ RPE complex was washed three times in PBS, permeated for 30 min in 1% triton X-100, blocked in 5% BSA with 0.2% tritonX-100 and 2 mmol/L MgCl2, and stained with 5ug/mL Alexa Fluor (AF) 568-conjugated isolectin GS-IB4 (1:200, Invitrogen) in blocking buffer overnight (Gaddipati et al., 2015). After three additional washings, samples were flat mounted on glass slides. Isolectin-568 and AAV2.AcGFP signal were analyzed by scanning laser confocal microscopy (Olympus America, Center Valley, USA). Four fields, comprising each of the laser spots, were imaged for each retina using the 40X oil objective, and CNV volume measurements were calculated (Sakurai et al., 2003; Uehara et al., 2013).
2.11. Statistical analysis
Data was collected and analyzed in Excel (Microsoft, Redmond, WA). Mean values and standard deviations were computed. The Student’s two-tailed t-test was performed to compare differences between two samples, with significance set at p < 0.05. Analysis of variance (ANOVA) with significance set at p < 0.05, followed by a post-hoc Bonferroni correction, was used for multiple pairwise comparisons involving three or more samples.
3. Results
3.1. The retinal detachment created by subretinal injection resolves spontaneously
Although there is a theoretical risk of permanent damage from the iatrogenically formed rhegmatogenous retinal detachment during subretinal injection, prior studies have demonstrated that this side effect is temporary and self-resolving (Lai et al., 2012). When tracked by OCT, the retina typically reattaches as the injected subretinal fluid dissipates, without any concomitant impairment to visual function on electroretinography (ERG) or optokinetic tracking (OKT) (Zhang et al., 2015), likely helped by the fact that the penetration of the needle is in the peripheral retina distant from the macula.
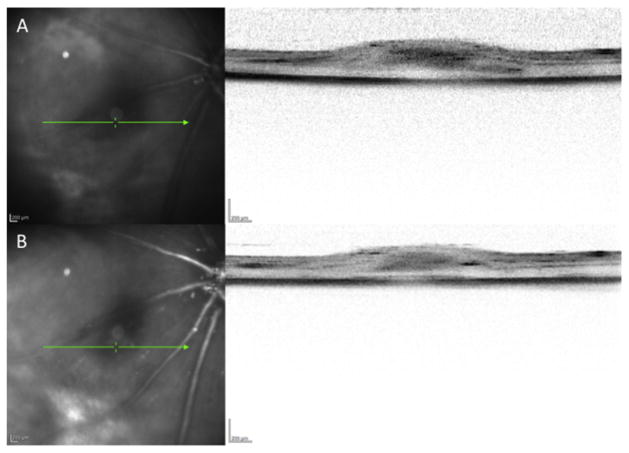
Corroborating the existing data on the safety of subretinal injections, we found that by 3 and 4 weeks post-injection, the retina had successfully reattached, with mild edema which gradually dissipated from one week to the next (Fig. 2, A and B). No other adverse injection-related sequelae were observed.
Fig. 2.
The retina reattaches after subretinal injection. A. Cross-sectional OCT image of the retina at the site of penetration 3 weeks post-injection demonstrating reapposition of the retina to the RPE/choroid with mild residual subretinal fluid. B. Sequential cross-sectional OCT image of the retina in the same location at 4 weeks post-injection showing continued attachment of the retina with progressive improvement in subretinal edema.
3.2. Subretinal AAV2 localizes and propagates within the RPE/ choroid
Subretinal AAV2 localization was first tested with well-established reporter genes: AcGFP, which has a fluorescent product (Cereso et al., 2014; Cronin et al., 2012; Li et al., 2008; Zhang et al., 2012, 2015), and LacZ, which has a chromogenic product (Hojo et al., 2004), to validate successful inoculation of the targeted tissue.
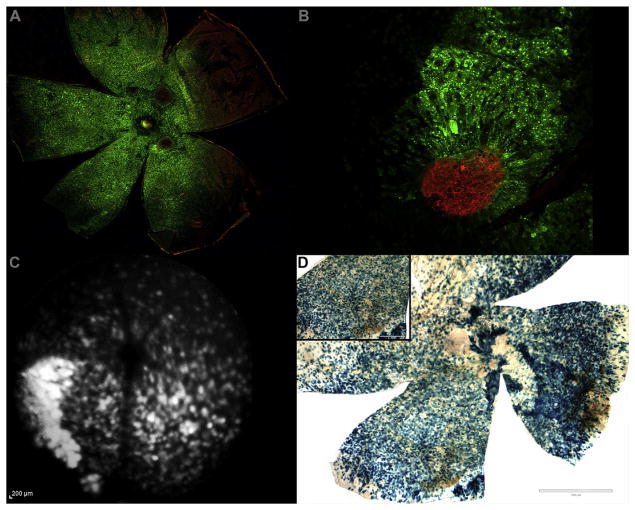
The structural integrity of flat mounted tissue from both AAV2.AcGFP and AAV2.COMP-Ang1 preparations appeared grossly intact, with no obvious signs of damage. A diffuse GFP signal was seen in the AAV2.AcGFP group (Campochiaro, 2011) using ex vivo confocal microscopy (Fig. 3A and B) and in vivo funduscopy (Fig. 3C). AAV2 transfection was verified in the AAV2.LacZ group along by blue X-gal signal (Jomary et al., 1994) (Fig. 3D).
Fig. 3.
AAV2 successfully transduces subretinal and outer retinal tissues. A. RPE/choroid from an eye treated with AAV2.AcGFP (green) and stained with isolectin (red). Red neovascular tufts (at 2 and 5 o’clock) surrounded by diffuse GFP signal validate CNV formation within the AAV2 treated tissue. B. High power magnification of laser-induced CNV (isolectin, red) within AAV2.AcGFP treated RPE/choroid (green). C. Funduscopic FA modality image showing diffuse in vivo expression of GFP autofluorescence in an eye AAV2.AcGFP. D. RPE/choroid from an eye treated with AAV2.LacZ and stained with X-gal. Blue signal indicates successful AAV transduction following subretinal administration. Scale bars = 1000 μm main, 400 μm inset. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
These viral control results confirmed efficient delivery, integration, and activity of AAV2 batches prepared per our protocol.
3.3. Subretinal AAV2.COMP-Ang1 expresses COMP-Ang1 protein in the RPE/choroid
Intravitreal administration of AAV2.COMP-Ang1 was previously shown to successfully transducer and treat retinas of diabetic mice (Cahoon et al., 2015). As this is the first paper to test the same AAV2 payload using a subretinal approach, we first confirmed expression of COMP-Ang1 protein in the RPE/choroid prior to experimental trials.
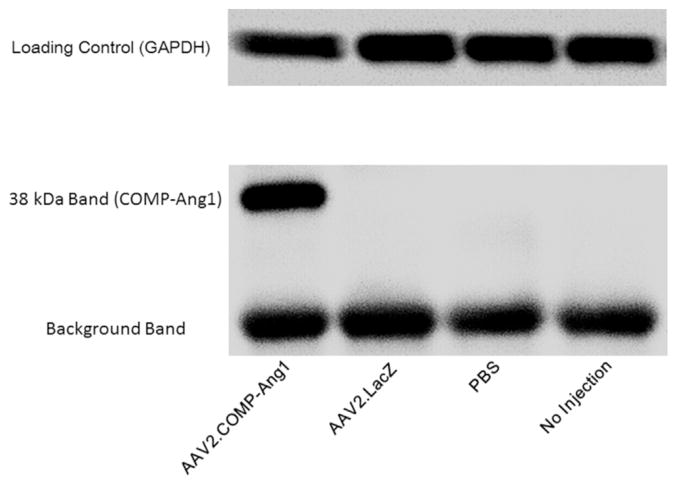
An anti-FLAG monoclonal antibody (Cho et al., 2004; Hwang et al., 2008) was used to detect the FLAG sequence incorporated in our AAV2.COMP-Ang1 gene construct. This antibody labeled a band of the expected size (38 kDa) in RPE/choroids of mice treated with AAV2.COMP-Ang1 that was not apparent in controls (Fig. 4).
Fig. 4.
AAV2 expresses COMP-Ang1 protein in RPE/choroid. Western blot for anti-FLAG antibody in the RPE/choroid at 2 months after subretinal injection. Detection of a 38 kDa band signifies successful expression of COMP-Ang1 protein in the RPE/choroid. GAPDH and background bands indicate similar total protein in all samples.
This data indicated that the incorporation of COMP-Ang1 plasmid DNA into an AAV2 vector is an effective technique for the delivery of COMP-Ang1 protein to the subretinal tissue.
3.4. Subretinal AAV2.COMP-Ang1 does not suppress HIF-1α in the RPE/choroid
CNV formation and VEGF production in AMD has been linked to hypoxia (Zhao et al., 2008). HIF-1α may be involved in CNV formation by controlling VEGF secretion (Zhang et al., 2007). As a vascular stabilizing agent, which restores normal perfusion, we surmised that AAV2.COMP-Ang1 would be able to prevent hypoxia and the resultant upsurge in HIF-1α levels in the RPE/choroid.
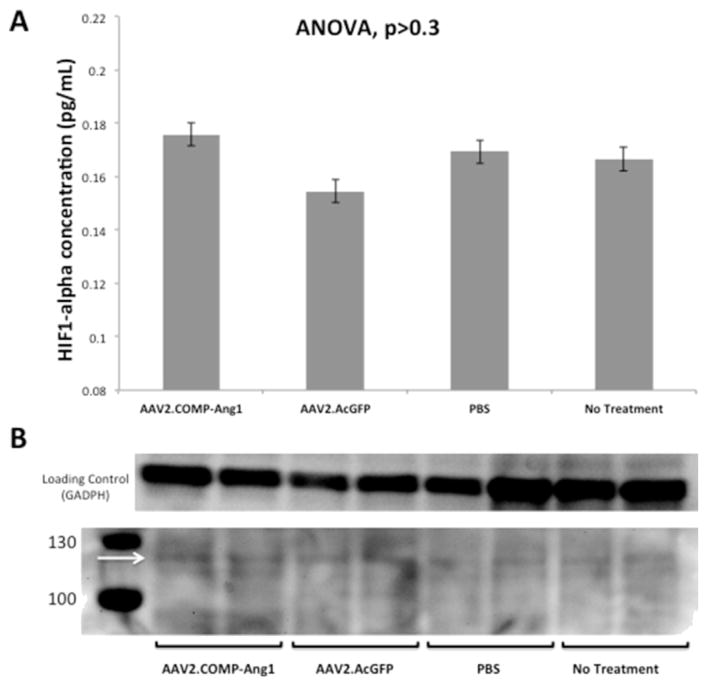
Our western blot for HIF-1α did not show any observable difference between bands from either our AAV2.COMP-Ang1 or control groups (Fig. 5B). Band intensities were calculated using densitometry analysis but were not significant (data not shown). HIF-1α concentrations from ELISA (Fig. 5A) were as follows (mean ratio ± SD): AAV2.COMP-Ang1, 0.18 ± 0.02 pg/mL; AAV2.GFP 0.16 ± 0.02 pg/mL; PBS 0.17 ± 0.02 pg/mL; no subretinal injection 0.17 ± 0.02 pg/mL. There was no difference between groups (p = 0.4).
Fig. 5.
AAV2.COMP-Ang1 does not suppress HIF-1α in RPE/choroid. A. Bar graph displaying mean HIF-1α levels in RPE/choroid per ELISA. There was no difference among HIF-1α levels among all groups (ANOVA p > 0.3). B. Western blot for anti–HIF–1α in RPE/choroid at 1 month after subretinal injection. There was no observable difference between bands from either our AAV2.COMP-Ang1 or control groups. Band intensities were calculated using densitometry analysis but were not significant (data not shown). The arrow denotes level of expected banding (~116 kDa).
These outcomes suggest that AAV2.COMP-Ang1 does not create a more normoxic environment in the RPE/choroid, contrary to our initial theory.
3.5. Subretinal AAV2.COMP-Ang1 suppresses VEGF levels in the RPE/ choroid
Since VEGF plays a central role in CNV pathogenesis, we explored the relationship between VEGF and AAV2.COMP-Ang1. ELISA is a common way to examine VEGF levels in the RPE/ choroid (Itaya et al., 2007; Kim et al., 2008; Zhang et al., 2015).
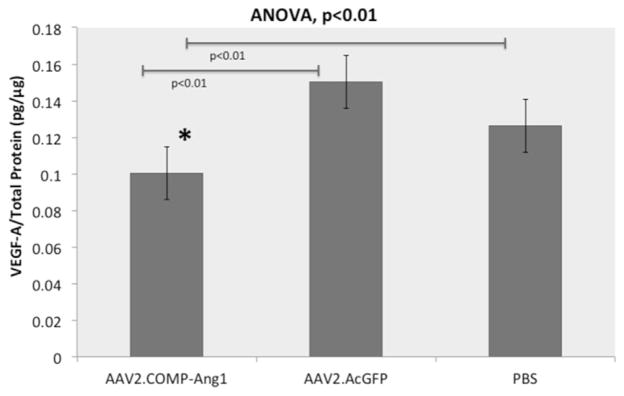
Our output VEGF-A/total protein ratios from ELISA, with n representing the number of mice, were as follows (mean ratio ± SD): AAV2.COMP-Ang1 0.10 ± 0.01 pg/μg (n = 6); AAV2.AcGFP 0.15 ± 0.03 pg/μg (n = 5); PBS 0.14 ± 0.03 pg/μg (n = 5) (Fig. 6). The VEGF concentration in the AAV2.COMP-Ang1 group was significantly decreased from both the AAV2.AcGFP (33%) and PBS (29%) (p < 0.01).
Fig. 6.
AAV2.COMP-Ang1 suppresses VEGF in the RPE/choroid. Bar graph displaying mean VEGF levels in the RPE/choroid. AAV2.COMP-Ang1 significantly reduces VEGF levels compared to AAV2.AcGFP and PBS (p < 0.01) as per ANOVA with Bonferroni correction. The asterisk designates a significant difference in the AAV2.COMP-Ang1 group compared to both controls with the Student’s t-test.
Consistent with earlier articles describing local suppression of VEGF levels at the site of COMP-Ang1 administration (Cahoon et al., 2015; Kim et al., 2008), these outcomes suggest that the subretinal injection of AAV2.COMP-Ang1 may downregulate VEGF expression in the RPE/choroid.
3.6. Subretinal AAV2.COMP-Ang1 suppresses CNV volume
Quantifying isolectin IB4 staining by confocal image analysis is an accepted method for appraising CNV, the dominant feature of neovascular AMD and the primary outcome of interest for this study (Gaddipati et al., 2015; Zhang et al., 2015).
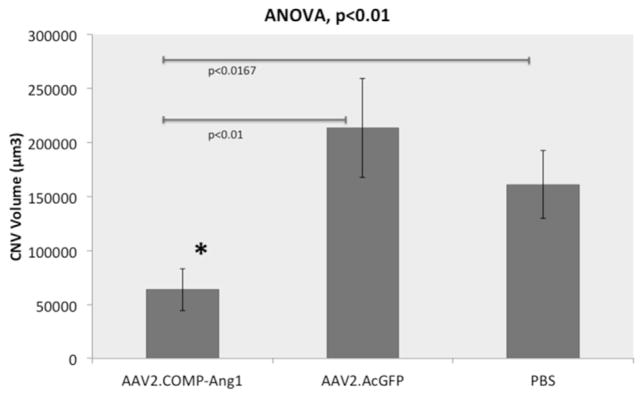
Our CNV volume measurements (Fig. 7), with n representing the number of laser spots assayed, were as follows (mean volume ± SD): AAV2.COMP-Ang1 6.36 ± 1.94 × 104 μm3 (n = 31); AAV2.AcGFP 21.32 ± 4.57 × 104 μm3 (n = 30); PBS 16.12 ± 3.16 × 104 μm3 (n = 40) (Fig. 8). The AAV2.COMP-Ang1 group had significantly less CNV than the AAV2.AcGFP group (70%, p < 0.01) and the PBS group (61%, p < 0.0167).
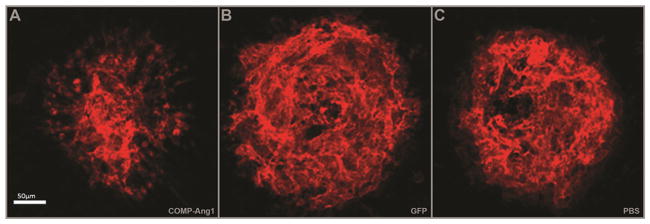
Fig. 7.
AAV2.COMP-Ang1 inhibits laser-induced CNV. Immunofluorescent images of RPE/choroid flat mounts representative of average laser-induced CNV spot size (A–C). Magnitude of CNV is visibly lower in the AAV2.COMP-Ang1 group compared to controls. Scale bar = 50 μm.
Fig. 8.
AAV2.COMP-Ang1 inhibition of laser induced CNV is statistically significant. Bar graph displaying mean CNV volume. AAV2.COMP-Ang1 significantly reduces CNV volume compared to AAV2.AcGFP (p < 0.01) and PBS (p < 0.0167) as per ANOVA with Bonferroni correction. The asterisk designates a significant difference in the AAV2.COMP-Ang1 group compared to controls with the Student’s t-test.
These results, revealing that AAV2.COMP-Ang1 has an anti-CNV effect, correlate well with existing data on CNV diminution by recombinant COMP-Ang1 protein in an identical model of AMD (Lee et al., 2014).
Taken together, our data posits the suppression of VEGF-mediated CNV formation as one of the mechanisms of action for AAV2.COMP-Ang1 in neovascular AMD, but does not provide a full picture of AAV2.COMP-Ang1’s therapeutic effect. The absence of a link with HIF-1α and the disproportionate difference in VEGF versus CNV suppression invites inquiry into other mechanisms which may be at play.
4. Discussion
This study expands our laboratory’s earlier findings on the efficacy and safety of using an AAV2 vector for delivery of COMP-Ang1 (Cahoon et al., 2015) and a subretinal modality for AAV2-mediated gene therapy in AMD (Zhang et al., 2015). To our knowledge, it is the first report of subretinal administration of AAV2.COMP-Ang1.
The broad transduction by AAV2.LacZ at 1 month post-injection, followed by sustained expression of COMP-Ang1 protein by AAV2.COMP-Ang1 at 3 months post-injection, together serve to validate subretinal AAV2 as a feasible vehicle delivering COMP-Ang1 to the RPE/choroid.
Our data builds upon previous reports, demonstrating that AAV2.COMP-Ang1 suppresses laser-CNV by up to 70%. As such, we sought to determine the mechanism for this anti-angiogenic activity. We initially speculated that AAV2.COMP-Ang1 counteracts dysfunctional angiogenesis in AMD through vascular stabilization and subsequent modulation of hypoxia-induced VEGF secretion (Nakajima et al., 2013; Xiao et al., 2008).
Although we found significant VEGF-A reduction by AAV2.COMP-Ang1 compared to controls, we were unable to find significant differences in HIF-1α expression by either ELISA or immunoblotting. This could be due to the fact that VEGF-A is regulated my other inflammatory and factors besides HIF-1α (Lee et al., 2014; Nagineni et al., 2012, 2003). Additionally, although we saw decreases in VEGF levels in mice treated with AAV2.COMP-Ang1 compared to controls, the VEGF suppression was minimal (29–33%) compared to a rather large decrease in CNV volume suppression (61–70%). As such, although our results indicate that laser-induced CNV suppression by AAV2.COMP-Ang1 may be partially due to VEGF-A reduction, hypoxia may not be the key mechanistic link. Additionally, there may be alternative, non-VEGF-A mediated mechanisms at play.
For example, Gavard et al. demonstrated that angiopoietin 1 treated endothelial cells inhibit VEGF-A pathways through sequestering the tyrosine kinase Src (Gavard et al., 2008). Angiopoietin 1 is also known to inhibit several inflammatory pathways (He et al., 2014; Ismail et al., 2012). Both Tie2 receptor expression and VEGF secretion can be found in the RPE cells (Oh et al., 1999; Otani et al., 1999; Zhang et al., 2015), vascular endothelial cells, choroidal fibroblasts (Kvanta, 1995), and macrophages (Gu et al., 2010; Jones et al., 2001; Lee et al., 2014; Nozaki et al., 2006; Sakurai et al., 2003) that constitute CNV membranes. Moreover, COMP-Ang1 administration, Tie2 activation, and VEGF inhibition all interfere with vascular endothelial-cadherin (VE-cadherin) and zonula occludens (ZO) sequestration after an inflammatory insult (Giannotta et al., 2013; Koh, 2013; Lee et al., 2014; Murakami et al., 2009), thereby strengthening the endothelial tight junctions responsible for maintaining the barrier function of vessels vital for preventing vision-threatening fluid leakage.
Current treatments for neovascular AMD target angiogenesis through direct inhibition of VEGF-A. Although effective in most cases, there are many patients that are insensitive to anti-VEGF-A therapy. There are other factors (PDGF-BB and PDGF-CC) that contribute to CNV formation, growth, and persistence (Hou et al., 2010). Additionally, TGF- β, IFN-γ, TNF-α, and IL-1β are known to be potent inducers of VEGF secretion (Nagineni et al., 2012, 2003), and macrophage infiltration is also responsible for increased VEGF levels (Lee et al., 2014), leading to CNV. In our current study, we demonstrated that AAV2.COMP-Ang1 suppresses CNV growth and may serve as an alternative therapy to direct anti-VEGF-A inhibition in combating neovascular AMD. Although in our present study we were unable to fully elucidate the exact mechanism for CNV and VEGF suppression, future investigation will focus on understanding this etiology and may provide a new candidate factor to target for anti-angiogenesis.
Subretinal inoculation is a common practice and has been used on mice, rats, dogs, primates (Jacobson et al., 2006a, 2006b). Currently in humans, there are several clinical trials investigating the subretinal delivery of RPE stem cells (Schwartz et al., 2012, 2015). Previous studies have commented on the safety of subretinal inoculation of recombinant adeno-associated viruses (Jacobson et al., 2006a; Zhang et al., 2015). Jacobson et al., investigating use of an AAV2 vector for gene delivery in a model of Leber’s congenital amaurosis noted that there was no optic nerve or brain viral inoculation, retinal thinning was rare and post-injection inflammation resolved by 3 months (Jacobson et al., 2006a). This study also demonstrated that retinal function (via ERG) was no different compared to controls or pre-injection testing (Jacobson et al., 2006a, 2006b).
In our lab, we have also previously demonstrated the safety of 1) subretinal inoculation of recombinant intracellular-VEGF modulating AAV2 vectors and 2) intravitreal inoculation of AAV2.COMP-Ang1. Zhang et al. (2015) investigated the use of subretinal AAV2-Flt23k delivery, which decreased intracellular-VEGF levels in RPE/ Choroid tissue. In assessing retinal health, mice treated with subretinal injection of AAV2.Flt23k showed no decrease in ERG response, no retinal thinning, and no apoptosis of RPE or choroid cells compared to controls. Recently, Cahoon et al. (2015) also noted the safety of AAV2.COMP-Ang1 administered via intravitreal route. Corroborating the results from Zhang et al. (2015), Cahoon et al. (2015) also noted no scotopic or photopic difference in b-wave amplitudes on ERG in mice treated with AAV2.COMP-Ang1 compared to AAV2.AcGFP or PBS treated mice. Our results add to these previous findings that subretinal administration of AAV2.COMP-Ang1 is both a safe and efficacious modality for CNV suppression. Although Zhang et al. (2015) and Cahoon et al. (2015) differed slightly from our experiment in either recombinant protein or location of injection (subretinal vs. intravitreal), their data support that sub-retinal injection of AAV2.COMP-Ang1 likely shares a similar safety profile.
To date, subretinal injections in humans have been limited. There is theoretical risk for this type of treatment route including rhegmetogenous retinal detachment, retinal hemorrhage, retinal degeneration and atrophy, as well as local and systemic inflammatory response. Despite these theoretical risks, several human trials assert that subretinal injections are both safe and efficacious (Bennett et al., 2012; Hauswirth et al., 2008; Maguire et al., 2008; Schwartz et al., 2015). Subretinal injections have been used as gene therapy for Leber congenital amaurosis type 2 (LCA2) (Testa et al., 2013), stem cell transplantation for age-related macular degeneration and Stargarts disease (Schwartz et al., 2012, 2015) during pars plana vitrectomy to reduce hemorrhage (Ehlers et al., 2015; Moisseiev et al., 2014). Several of these studies have also utilized AAV2 as a viral vector (Bennett et al., 2012; Hauswirth et al., 2008; Maguire et al., 2008). In these studies, safety assessment has revealed only minimal systemic and immunological response (Maguire et al., 2008). Early data appears to support safety of subretinal AAV2 administration in humans (Bennett et al., 2012; Hauswirth et al., 2008; Maguire et al., 2008). As such, the translation of AAV2 mediated therapies, delivered via a subretinal approach, into human models, is both relevant and vital.
Future directions for research include a focus on whether COMP-Ang1 antagonizes the pro-permeability activities of VEGF via CNV leakage assays, followed by an evaluation of combination therapy to characterize whether COMP-Ang1 can work in concert with and/ or reduce the need for VEGF blockers. Additionally, given prior data demonstrating that AAV2 expression peaks 4–8 weeks post-delivery (Wang et al., 2004), further study to identify the optimal concentrations and dosing intervals needed to maximize therapeutic outcomes is warranted.
5. Conclusions
Our results indicate that a) subretinally administered AAV2 is a suitable vector for long-lasting COMP-Ang1 expression in the RPE/ choroid tissue, b) AAV2.COMP-Ang1 suppresses CNV, and c) although VEGF suppression in the RPE/choroid may be one of the mechanisms by which AAV2.COMP-Ang1 exerts its therapeutic effect, this effect is not driven by hypoxia. Moreover, there may be additional non-VEGF mediated mechanisms responsible for the anti-angiogenic activity of COMP-Ang1. Taken together, we believe these findings suggest that AAV2.COMP-Ang1 has the potential to serve as an alternative or complementary option to anti-VEGF agents for the long-term amelioration of neovascular AMD.
Acknowledgments
Supported in part by an Unrestricted Grant from Research to Prevent Blindness, a Diabetes Metabolism Consortium Training Grant, and a National Institutes of Health Diabetes T32 Training Grant. Funding from Fight for Sight is also gratefully acknowledged.
Footnotes
6. Conflicts of interest
HU and BKA have filed a provisional patent application for AAV2.COMP-Ang1. The remaining authors have no proprietary interests or conflicts.
References
- Amado D, Mingozzi F, Hui D, Bennicelli JL, Wei Z, Chen Y, Bote E, Grant RL, Golden JA, Narfstrom K, Syed NA, Orlin SE, High KA, Maguire AM, Bennett J. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med. 2010;2:21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikari M, Tokoro M, Itaya M, Nozaki M, Ogura Y. Suppression of laser-induced choroidal neovascularization by nontargeted siRNA. Invest Ophthalmol Vis Sci. 2010;51:3820–3824. doi: 10.1167/iovs.09-5121. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol. 2006;290:H107–H118. doi: 10.1152/ajpheart.00542.2005. [DOI] [PubMed] [Google Scholar]
- Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, McCague S, Pierce EA, Chen Y, Bennicelli JL, Zhu X, Ying GS, Sun J, Wright JF, Auricchio A, Simonelli F, Shindler KS, Mingozzi F, High KA, Maguire AM. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra115. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- Broadhead GK, Hong T, Chang AA. Treating the untreatable patient: current options for the management of treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2014;92:713–723. doi: 10.1111/aos.12463. [DOI] [PubMed] [Google Scholar]
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, Group AS. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65. e55. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Cahoon JM, Rai RR, Carroll LS, Uehara H, Zhang X, O’Neil CL, Medina RJ, Das SK, Muddana SK, Olson PR, Nielson S, Walker K, Flood MM, Messenger WB, Archer BJ, Barabas P, Krizaj D, Gibson CC, Li DY, Koh GY, Gao G, Stitt AW, Ambati BK. Intravitreal AAV2. COMP-Ang1 prevents neurovascular degeneration in a murine model of diabetic retinopathy. Diabetes. 2015;64:4247–4259. doi: 10.2337/db14-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P, Ferreras A, Al Adel F, Wang Y, Brent MH. Dexamethasone intravitreal implant as adjunct therapy for patients with wet age-related macular degeneration with incomplete response to ranibizumab. Br J Ophthalmol. 2015;99:723–726. doi: 10.1136/bjophthalmol-2014-305684. [DOI] [PubMed] [Google Scholar]
- Campbell JP, Bressler SB, Bressler NM. Impact of availability of antivascular endothelial growth factor therapy on visual impairment and blindness due to neovascular age-related macular degeneration. Arch Ophthalmol. 2012a;130:794–795. doi: 10.1001/archophthalmol.2011.2480. [DOI] [PubMed] [Google Scholar]
- Campbell RJ, Gill SS, Bronskill SE, Paterson JM, Whitehead M, Bell CM. Adverse events with intravitreal injection of vascular endothelial growth factor inhibitors: nested case-control study. BMJ. 2012b;345:e4203. doi: 10.1136/bmj.e4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA. Gene transfer for neovascular age-related macular degeneration. Hum Gene Ther. 2011;22:523–529. doi: 10.1089/hum.2011.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereso N, Pequignot MO, Robert L, Becker F, De Luca V, Nabholz N, Rigau V, De Vos J, Hamel CP, Kalatzis V. Proof of concept for AAV2/5-mediated gene therapy in iPSC-derived retinal pigment epithelium of a choroideremia patient. Mol Ther Methods Clin Dev. 2014;1:14011. doi: 10.1038/mtm.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Huang L, Li X, Qi H, Zhou P, Yu W, Jiang YA, Wadsworth S, Scaria A. Prevalence of neutralizing factors against adeno-associated virus types 2 in age-related macular degeneration and polypoidal choroidal vasculopathy. Curr Gene Ther. 2013;13:182–188. doi: 10.2174/1566523211313030003. [DOI] [PubMed] [Google Scholar]
- Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella P, Auricchio A. AAV-mediated gene supply for treatment of degenerative and neovascular retinal diseases. Curr Gene Ther. 2010;10:371–380. doi: 10.2174/156652310793180670. [DOI] [PubMed] [Google Scholar]
- Comparison of Age-related Macular Degeneration Treatments Trials Research G. Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL., 3rd Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. [Google Scholar]
- Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P Eye Diseases Prevalence Research G. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- Cronin T, Chung DC, Yang Y, Nandrot EF, Bennett J. The signalling role of the avbeta5-Integrin can impact the efficacy of AAV in retinal gene therapy. Pharm (Basel) 2012;5:447–459. doi: 10.3390/ph5050447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, McGuire PG. Retinal and choroidal angiogenesis: pathophysiology and strategies for inhibition. Prog Retin Eye Res. 2003;22:721–748. doi: 10.1016/j.preteyeres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismuke DJ, Tenenbaum L, Samulski RJ. Biosafety of recombinant adeno-associated virus vectors. Curr Gene Ther. 2013;13:434–452. doi: 10.2174/15665232113136660007. [DOI] [PubMed] [Google Scholar]
- Ehlers JP, Petkovsek DS, Yuan A, Singh RP, Srivastava SK. Intrasurgical assessment of subretinal tPA injection for submacular hemorrhage in the PIONEER study utilizing intraoperative OCT. Ophthalmic Surg Lasers Imaging Retina. 2015;46:327–332. doi: 10.3928/23258160-20150323-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J Eye Diseases Prevalence Research G. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Angiopoietin- 1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol Histopathol. 2010;25:387–396. doi: 10.14670/HH-25.387. [DOI] [PubMed] [Google Scholar]
- Funk M, Karl D, Georgopoulos M, Benesch T, Sacu S, Polak K, Zlabinger GJ, Schmidt-Erfurth U. Neovascular age-related macular degeneration: intraocular cytokines and growth factors and the influence of therapy with ranibizumab. Ophthalmology. 2009;116:2393–2399. doi: 10.1016/j.ophtha.2009.05.039. [DOI] [PubMed] [Google Scholar]
- Fuxe J, Tabruyn S, Colton K, Zaid H, Adams A, Baluk P, Lashnits E, Morisada T, Le T, O’Brien S, Epstein DM, Koh GY, McDonald DM. Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation. Am J Pathol. 2011;178:2897–2909. doi: 10.1016/j.ajpath.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddipati S, Lu Q, Kasetti RB, Miller MC, Lu Q, Trent JO, Kaplan HJ, Li Q. IKK2 inhibition using TPCA-1-loaded PLGA microparticles attenuates laser-induced choroidal neovascularization and macrophage recruitment. PLoS One. 2015;10:e0121185. doi: 10.1371/journal.pone.0121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–454. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR Group V.I.S.i.O.N.C.T. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MG, Toth CA, Jaffe GJ, Fine SL, Blodi B, Klein ML, Martin AA, Hagstrom SA, Martin DF, Group CR. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121:150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Cui M, Bai Y, Chen F, Ma K, Zhou C, Guo L. Angiopoietin-1/Tie2 signaling pathway inhibits lipopolysaccharide-induced activation of RAW264.7 macrophage cells. Biochem Biophys Res Commun. 2010;392:178–182. doi: 10.1016/j.bbrc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DK, Shao YR, Zhang L, Shen J, Zhong ZY, Wang J, Xu G. Adenovirus-delivered angiopoietin-1 suppresses NF-kappaB and p38 MAPK and attenuates inflammatory responses in phosgene-induced acute lung injury. Inhal Toxicol. 2014;26:185–192. doi: 10.3109/08958378.2013.872213. [DOI] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U View Groups V.S. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Hojo M, Abe T, Sugano E, Yoshioka Y, Saigo Y, Tomita H, Wakusawa R, Tamai M. Photoreceptor protection by iris pigment epithelial transplantation transduced with AAV-mediated brain-derived neurotrophic factor gene. Invest Ophthalmol Vis Sci. 2004;45:3721–3726. doi: 10.1167/iovs.04-0059. [DOI] [PubMed] [Google Scholar]
- Hou X, Kumar A, Lee C, Wang B, Arjunan P, Dong L, Maminishkis A, Tang Z, Li Y, Zhang F, Zhang SZ, Wardega P, Chakrabarty S, Liu B, Wu Z, Colosi P, Fariss RN, Lennartsson J, Nussenblatt R, Gutkind JS, Cao Y, Li X. PDGF-CC blockade inhibits pathological angiogenesis by acting on multiple cellular and molecular targets. Proc Natl Acad Sci U S A. 2010;107:12216–12221. doi: 10.1073/pnas.1004143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Kim SH, Kim HZ, Steinmetz MO, Koh GY, Lee GM. High-level expression and purification of a designed angiopoietin-1 chimeric protein, COMP-Ang1, produced in Chinese hamster ovary cells. Protein J. 2008;27:319–326. doi: 10.1007/s10930-008-9140-5. [DOI] [PubMed] [Google Scholar]
- Ismail H, Mofarrahi M, Echavarria R, Harel S, Verdin E, Lim HW, Jin ZG, Sun J, Zeng H, Hussain SN. Angiopoietin-1 and vascular endothelial growth factor regulation of leukocyte adhesion to endothelial cells: role of nuclear receptor-77. Arterioscler Thromb Vasc Biol. 2012;32:1707–1716. doi: 10.1161/ATVBAHA.112.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya M, Sakurai E, Nozaki M, Yamada K, Yamasaki S, Asai K, Ogura Y. Upregulation of VEGF in murine retina via monocyte recruitment after retinal scatter laser photocoagulation. Invest Ophthalmol Vis Sci. 2007;48:5677–5683. doi: 10.1167/iovs.07-0156. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, Zeiss CJ, Komaromy AM, Kaushal S, Roman AJ, Windsor EA, Sumaroka A, Pearce-Kelling SE, Conlon TJ, Chiodo VA, Boye SL, Flotte TR, Maguire AM, Bennett J, Hauswirth WW. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006a;13:1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Boye SL, Aleman TS, Conlon TJ, Zeiss CJ, Roman AJ, Cideciyan AV, Schwartz SB, Komaromy AM, Doobrajh M, Cheung AY, Sumaroka A, Pearce-Kelling SE, Aguirre GD, Kaushal S, Maguire AM, Flotte TR, Hauswirth WW. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther. 2006b;17:845–858. doi: 10.1089/hum.2006.17.845. [DOI] [PubMed] [Google Scholar]
- Jomary C, Piper TA, Dickson G, Couture LA, Smith AE, Neal MJ, Jones SE. Adenovirus-mediated gene transfer to murine retinal cells in vitro and in vivo. FEBS Lett. 1994;347:117–122. doi: 10.1016/0014-5793(94)00512-5. [DOI] [PubMed] [Google Scholar]
- Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001;2:257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- Kim SR, Lee KS, Park SJ, Min KH, Lee KY, Choe YH, Hong SH, Koh GY, Lee YC. Angiopoietin-1 variant, COMP-Ang1 attenuates hydrogen peroxide-induced acute lung injury. Exp Mol Med. 2008;40:320–331. doi: 10.3858/emm.2008.40.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med. 2013;19:31–39. doi: 10.1016/j.molmed.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Kvanta A. Expression and regulation of vascular endothelial growth factor in choroidal fibroblasts. Curr Eye Res. 1995;14:1015–1020. doi: 10.3109/02713689508998523. [DOI] [PubMed] [Google Scholar]
- Lai CM, Estcourt MJ, Himbeck RP, Lee SY, Yew-San Yeo I, Luu C, Loh BK, Lee MW, Barathi A, Villano J, Ang CL, van der Most RG, Constable IJ, Dismuke D, Samulski RJ, Degli-Esposti MA, Rakoczy EP. Preclinical safety evaluation of subretinal AAV2. sFlt-1 in non-human primates. Gene Ther. 2012;19:999–1009. doi: 10.1038/gt.2011.169. [DOI] [PubMed] [Google Scholar]
- Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM, Rakic JM, Noel A. Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc. 2013;8:2197–2211. doi: 10.1038/nprot.2013.135. [DOI] [PubMed] [Google Scholar]
- Lazarovici P, Marcinkiewicz C, Lelkes PI. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr Pharm Des. 2006;12:2609–2622. doi: 10.2174/138161206777698738. [DOI] [PubMed] [Google Scholar]
- Lee J, Park DY, Park do Y, Park I, Chang W, Nakaoka Y, Komuro I, Yoo OJ, Koh GY. Angiopoietin-1 suppresses choroidal neovascularization and vascular leakage. Invest Ophthalmol Vis Sci. 2014;55:2191–2199. doi: 10.1167/iovs.14-13897. [DOI] [PubMed] [Google Scholar]
- Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, Hauswirth WW. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T, Ashikari M, Tokoro M, Nozaki M, Ogura Y. Suppression of laser-induced choroidal neovascularization by a CCR3 antagonist. Invest Ophthalmol Vis Sci. 2013;54:1564–1572. doi: 10.1167/iovs.11-9095. [DOI] [PubMed] [Google Scholar]
- Moisseiev E, Ben Ami T, Barak A. Vitrectomy and subretinal injection of tissue plasminogen activator for large submacular hemorrhage secondary to AMD. Eur J Ophthalmol. 2014;24:925–931. doi: 10.5301/ejo.5000500. [DOI] [PubMed] [Google Scholar]
- Muhlfriedel R, Michalakis S, Garcia Garrido M, Biel M, Seeliger MW. Optimized technique for subretinal injections in mice. Methods Mol Biol. 2013;935:343–349. doi: 10.1007/978-1-62703-080-9_24. [DOI] [PubMed] [Google Scholar]
- Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J Biol Chem. 2009;284:21036–21046. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni CN, Kommineni VK, William A, Detrick B, Hooks JJ. Regulation of VEGF expression in human retinal cells by cytokines: implications for the role of inflammation in age-related macular degeneration. J Cell Physiol. 2012;227:116–126. doi: 10.1002/jcp.22708. [DOI] [PubMed] [Google Scholar]
- Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, Hooks JJ. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: involvement of mitogen-activated protein kinases. J Cell Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Nakajima E, Shearer TR, Azuma M. Concerted inhibition of HIF-1alpha and -2alpha expression markedly suppresses angiogenesis in cultured RPE cells. Mol Cell Biochem. 2013;383:113–122. doi: 10.1007/s11010-013-1760-1. [DOI] [PubMed] [Google Scholar]
- Nambu H, Nambu R, Oshima Y, Hackett SF, Okoye G, Wiegand S, Yancopoulos G, Zack DJ, Campochiaro PA. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004;11:865–873. doi: 10.1038/sj.gt.3302230. [DOI] [PubMed] [Google Scholar]
- Nambu H, Umeda N, Kachi S, Oshima Y, Akiyama H, Nambu R, Campochiaro PA. Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J Cell Physiol. 2005;204:227–235. doi: 10.1002/jcp.20292. [DOI] [PubMed] [Google Scholar]
- Nishijima K, Ng YS, Zhong L, Bradley J, Schubert W, Jo N, Akita J, Samuelsson SJ, Robinson GS, Adamis AP, Shima DT. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58:353–363. [PubMed] [Google Scholar]
- Nozaki M, Sakurai E, Raisler BJ, Baffi JZ, Witta J, Ogura Y, Brekken RA, Sage EH, Ambati BK, Ambati J. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Investig. 2006;116:422–429. doi: 10.1172/JCI26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Takagi H, Takagi C, Suzuma K, Otani A, Ishida K, Matsumura M, Ogura Y, Honda Y. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1891–1898. [PubMed] [Google Scholar]
- Otani A, Takagi H, Oh H, Koyama S, Matsumura M, Honda Y. Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1999;40:1912–1920. [PubMed] [Google Scholar]
- Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya’ale D, Negrel AD, Resnikoff S. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11:67–115. doi: 10.1076/opep.11.2.67.28158. [DOI] [PubMed] [Google Scholar]
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group SUS. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Romano MR, Biagioni F, Besozzi G, Carrizzo A, Vecchione C, Fornai F, Lograno MD. Effects of bevacizumab on neuronal viability of retinal ganglion cells in rats. Brain Res. 2012;1478:55–63. doi: 10.1016/j.brainres.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- Roy K, Stein L, Kaushal S. Ocular gene therapy: an evaluation of recombinant adeno-associated virus-mediated gene therapy interventions for the treatment of ocular disease. Hum Gene Ther. 2010;21:915–927. doi: 10.1089/hum.2010.041. [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai E, Taguchi H, Anand A, Ambati BK, Gragoudas ES, Miller JW, Adamis AP, Ambati J. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:2743–2749. doi: 10.1167/iovs.02-1246. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM, Mickunas E, Gay R, Klimanskaya I, Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- Shin JY, Woo SJ, Ahn J, Park KH. Anti-VEGF-refractory exudative age-related macular degeneration: differential response according to features on optical coherence tomography. Korean J Ophthalmol. 2013;27:425–432. doi: 10.3341/kjo.2013.27.6.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaprasad S, Hykin P. What is new in the management of wet age-related macular degeneration? Br Med Bull. 2013;105:201–211. doi: 10.1093/bmb/ldt004. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Antivascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;8:CD005139. doi: 10.1002/14651858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MW. Clinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on aflibercept. Clin Ophthalmol. 2012;6:1175–1186. doi: 10.2147/OPTH.S33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, Wright JF, Wellman J, High KA, Auricchio A, Bennett J, Simonelli F. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology. 2013;120:1283–1291. doi: 10.1016/j.ophtha.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–1646. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozer K, Roller AB, Chong LP, Sadda S, Folk JC, Mahajan VB, Russell SR, Boldt HC, Sohn EH. Combination therapy for neovascular age-related macular degeneration refractory to anti-vascular endothelial growth factor agents. Ophthalmology. 2013;120:2029–2034. doi: 10.1016/j.ophtha.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Tranos P, Vacalis A, Asteriadis S, Koukoula S, Vachtsevanos A, Perganta G, Georgalas I. Resistance to antivascular endothelial growth factor treatment in age-related macular degeneration. Drug Des Devel Ther. 2013;7:485–490. doi: 10.2147/DDDT.S43470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara H, Cho Y, Simonis J, Cahoon J, Archer B, Luo L, Das SK, Singh N, Ambati J, Ambati BK. Dual suppression of hemangiogenesis and lymphangiogenesis by splice-shifting morpholinos targeting vascular endothelial growth factor receptor 2 (KDR) FASEB J. 2013;27:76–85. doi: 10.1096/fj.12-213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AY, Peng PD, Ehrhardt A, Storm TA, Kay MA. Comparison of adenoviral and adeno-associated viral vectors for pancreatic gene delivery in vivo. Hum Gene Ther. 2004;15:405–413. doi: 10.1089/104303404322959551. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bi H, Teng D, Zou Y, Pan X, Guo D, Cui Y. Potential protective effect of angiopoietin-1 on the leakage of rat choroidal neovascularization. Saudi Med J. 2013;34:584–590. [PubMed] [Google Scholar]
- Xiao Q, Zeng S, Lv M, Ling S. Small hairpin loop RNA targeting HIF-1alpha down-regulates VEGF and up-regulates PEDF in human retinal pigment epithelial cells under hypoxic condition. J Huazhong Univ Sci Technol Med Sci = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2008;28:460–464. doi: 10.1007/s11596-008-0419-8. [DOI] [PubMed] [Google Scholar]
- Yang XM, Wang YS, Zhang J, Li Y, Xu JF, Zhu J, Zhao W, Chu DK, Wiedemann P. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1alpha and VEGF in laser-induced rat choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:1873–1879. doi: 10.1167/iovs.08-2591. [DOI] [PubMed] [Google Scholar]
- Yodoi Y, Tsujikawa A, Nakanishi H, Otani A, Tamura H, Ojima Y, Hayashi H, Yoshimura N. Central retinal sensitivity after intravitreal injection of bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol. 2009;147:816–824. 824 e811. doi: 10.1016/j.ajo.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wang Y, Hui Y, Hu D, Wang H, Zhou J, Du H. Inhibition of VEGF expression by targeting HIF-1 alpha with small interference RNA in human RPE cells. Ophthalmologica. 2007;221:411–417. doi: 10.1159/000107502. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wu J, Wu X, Xu P, Tian Y, Yi M, Liu X, Dong X, Wolf F, Li C, Huang Q. Enhancement of rAAV2-mediated transgene expression in retina cells in vitro and in vivo by coadministration of low-dose chemotherapeutic drugs. Invest Ophthalmol Vis Sci. 2012;53:2675–2684. doi: 10.1167/iovs.11-8856. [DOI] [PubMed] [Google Scholar]
- Zhang X, Das SK, Passi SF, Uehara H, Bohner A, Chen M, Tiem M, Archer B, Ambati BK. AAV2 delivery of Flt23k intraceptors inhibits murine choroidal neovascularization. Mol Ther. 2015;23:226–234. doi: 10.1038/mt.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Wang YS, Hui YN, Zhu J, Zhang P, Li X, Dou GR. Inhibition of proliferation, migration and tube formation of choroidal microvascular endothelial cells by targeting HIF-1alpha with short hairpin RNA-expressing plasmid DNA in human RPE cells in a coculture system. Graefes Arch Clin Exp Ophthalmol. 2008;246:1413–1422. doi: 10.1007/s00417-008-0858-8. [DOI] [PubMed] [Google Scholar]
- Zhu YG, Qu JM, Zhang J, Jiang HN, Xu JF. Novel interventional approaches for ALI/ARDS: cell-based gene therapy. Mediat Inflamm. 2011;2011:560194. doi: 10.1155/2011/560194. [DOI] [PMC free article] [PubMed] [Google Scholar]