Abstract
Background
Endoplasmic reticulum (ER) stress creates abnormalities in the insulin action, inflammatory responses, lipoprotein B100 degradation, and hepatic lipogenesis. Hepatic steatosis leads to a broad spectrum of hepatic disorders such as nonalcoholic fatty liver disease (NAFLD) and NASH. Amygdalin has beneficial effects on asthma, bronchitis, diabetes, and atherosclerosis. We designed this study to evaluate the effect of amygdalin on the ER stress induced hepatic steatosis.
Methods
Inbred mice received saline, DMSO and amygdalin, as control groups. ER stress was induced by tunicamycin (TM) injection. Amygdalin was administered 1 h before the TM challenge (Amy + TM group). Mice body and liver weights were measured. Hematoxylin and eosin (H&E) and oil red O staining from liver tissue, were performed. Alanin aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride and cholesterol levels were measured.
Results
Histological evaluation revealed that amygdalin was unable to decrease the TM induced liver steatosis; however, ALT and AST levels decreased [ALT: 35.33(2.15) U/L versus 92.33(6.66) U/L; (57.000, (50.63, 63.36), P < 0.001) and AST: 93(5.09) U/L versus 345(97.3) U/L, (252, (163.37, 340.62), P < 0.001)]. Amygdalin also decreased triglyceride and cholesterol plasma levels in the Amy + TM group [TG: 42.66(2.15) versus 53.33(7.24) mg/dL; (10.67, (3.80, 17.54), P = 0.006) and TC: 9.33(3.55) versus 112.66(4.31) mg/dL, (103.33, (98.25, 108.40) P < 0.001)].
Conclusion
Amygdalin improved the ALT, AST, and lipid serum levels after the TM challenge; however, it could not attenuate hepatic steatosis.
Keywords: amygdalin, ER stress, liver, tunicamycin, steatosis
Introduction
In certain circumstances, such as pharmacological stimuli, dietary demands, viral infections, and oxidative stress, the endoplasmic reticulum (ER) homeostasis may disrupt and induce ER stress phenomenon; this may lead to unfolded or misfolded protein accumulation (1). ER stress and unfolded or misfolded protein accumulation create abnormalities in the insulin action, inflammatory responses, and hepatic steatosis (2–4). An excess accumulation of triglycerides (steatosis) in the hepatocytes is closely associated with several hepatic disorders such as nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), fibrosis, and possibly cirrhosis (5). It has been known that disruption in the ER homeostasis pathway decreases the very low-density lipoprotein (VLDL) level, increases the degradation of lipoprotein B100, and alters the lipid related transcription factors (6, 7). Numerous documents demonstrated that tunicamycin (TM) challenge, an ER stress inducer agent, could induce pharmacological ER stress and hepatic steatosis in the in vitro and in vivo models (8). It has also been reported that naltrexone, melatonin, and taurine attenuate the ER stress induced hepatic steatosis and liver injury (9–11).
Amygdalin, a cyanide-containing substance, is abundant in the seeds of apricots, almonds, peaches, and other rosaceous plants. Previously, amygdalin was used for angiogenesis inhibition, asthma, bronchitis, emphysema, renal fibrosis, diabetes treatment, and pain relief (12–17). Several studies have also demonstrated the apoptotic and anticancer effects of amygdalin in various cells lines and tissues (18–20). Furthermore, amygdalin reveals attenuating effects on atherosclerosis in mice (21, 22).
Considering the aforementioned studies, in this study, we aimed to evaluate the effect of amygdalin on ER stress induced hepatic steatosis.
Materials and Methods
Reagents
Tunicamycin and amygdalin were purchased from Sigma-Aldrich. Amygdalin was dissolved in saline and tunicamycin was dissolved in dimethyl sulfoxide (DMSO).
Animals
Inbred C57/BL6 male mice weighing 23–25 g [Academic Center for Education, Culture & Research (ACECR), Tehran, Iran] were used in this study. Animals were kept in a temperature-controlled room and were exposed to 12:12 h light: dark cycle with free access to standard laboratory chow and water. All procedures were in accordance with the Guidelines for Animal Care and Use at the Qom University of Medical Sciences (IR.MUQ.REC.1396.33).
Animal Procedures
The animals were randomly divided into five equal groups (N = 6 per group). Control group: received normal saline (0.2 mL i.p.), vehicle group: received DMSO (0.2 mL i.p.), amygdalin group: received amygdalin (3 g/kg i.p.) (23), TM group: received single dose of TM (2 mg/kg body weight) to induce ER stress (8), and Amy + TM group: received single dose of amygdalin (3 g/kg i.p.) 1 h prior to TM administration (23). Thirty hours post-TM injection (8), the animals were anesthetised with sodium pentobarbital (24). Animals were weighed before and after the procedure. The abdomen was excised via a midline incision and the liver was removed and weighed. Liver index was calculated by the formula: liver weight/bodyweight × 100.
Hematoxylin and Eosin Staining
A part of the median lobe sections was further dissected, fixed in 10% buffered formaldehyde solution, and stained with hematoxylin and eosin (H&E) stain.
Oil-red O Staining
Another part of frozen tissue sections was stained with oil-red O to reveal the lipid droplet contents.
Serum Analysis
Blood was directly collected from the heart by a 2 mL syringe insertion. Serum was obtained from the centrifuged blood samples (3500 rpm for 20 min) and total cholesterol (TC), triglyceride (TG), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were measured by an autoanalyser (Sunrise, Austria).
Statistical Analysis
We justified our sample size. The sample size was calculated based on a pilot study. The serum cholesterol level in the two study groups was calculated as 112.1(4.3) and 102(4.2), respectively. Furthermore, based on the mean difference of the two groups (MF = 10), type one error (α = 0.05), and power (1-β = 0.90), the minimum sample size was calculated for each group as 4 animals; however, considering the attrition rate, we recruited 6 animals for each group. The sample size formula was:
Data were expressed as mean (SD). Data normality was checked by the Kolmogorov–Smirnov test. Statistical analysis was performed by one-way analysis of variance and Tukey’s posthoc test using SPSS for windows version 11.5. P < 0.05 was considered as statistically significant.
Results
Effect of Amygdalin on the Liver Index Measurement
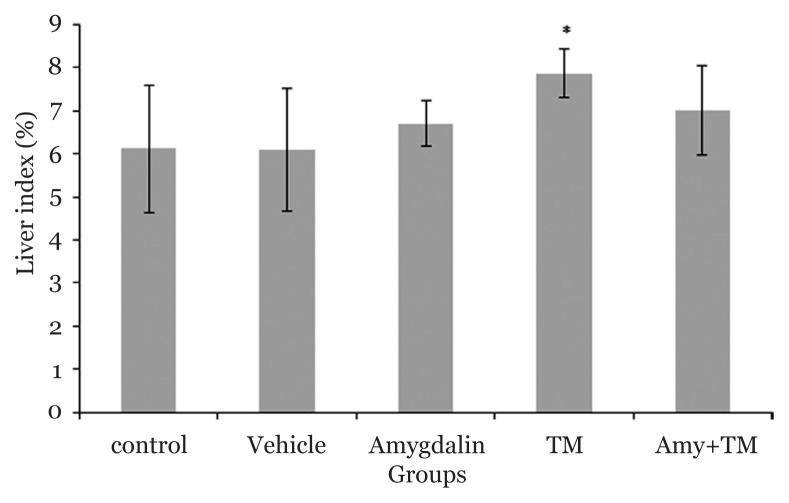
Mice body weights were measured before and at the end of the protocol. Data analysis revealed that the liver index (liver weight/bodyweight × 100) increased significantly in the ER stress group when compared with the control groups (7.85(0.56) versus 6.11(1.46), P = 0.021; 6.08(1.42), P = 0.017; and 6.7(0.51), P = 0.004, respectively); however, it did not decrease in the Amy + TM group when compared with TM group (Figure 1). Moreover, our finding did not reveal any significant difference between the amygdalin and Amy + TM groups (Figure 1).
Figure 1.
Liver index in different experimental groups. N = 6 per group, error bars represent standard deviation, *P < 0.05 when compared with control, vehicle, amygdalin groups. TM (Tunicamycin), Amy (Amygdalin)
Effect of Amygdalin on the Histological Changes
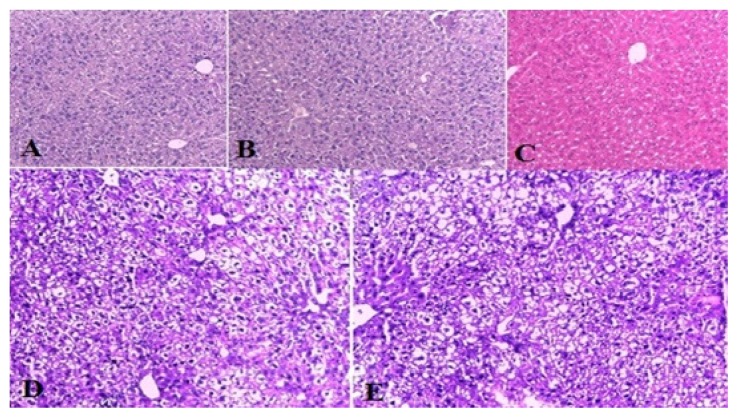
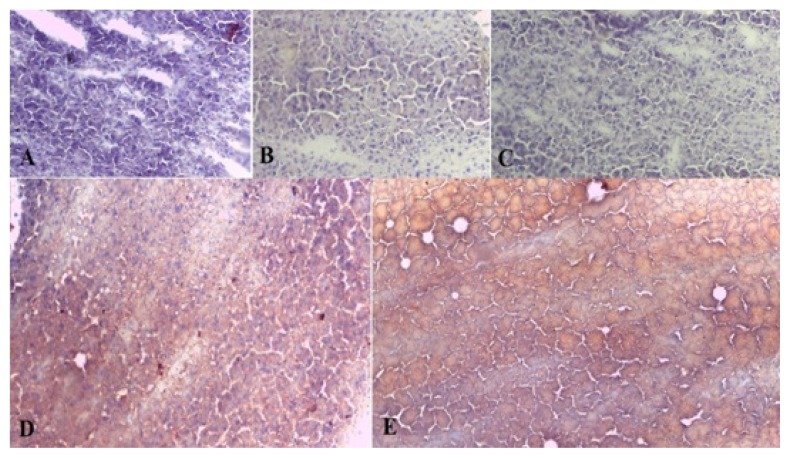
In this experiment, we used both H&E and oil red O staining. An expert pathologist, blinded to the experiment, performed the histopathological evaluations to determine the hepatic injuries. In H&E staining, the histological findings were scored based on the Kleiner et al. scoring system, as follows: steatosis (0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2) (25). As noted in Figure 2, no sign of steatosis or inflammation was seen in the saline, vehicle, and amygdalin groups; however, severe steatosis, cellular ballooning, and lobular inflammation was observed in the TM group when compared with the control animals; in contrast to our initial hypothesis, amygdalin did not decrease steatosis in the Amy + TM group when compared with the ER stress group (Figure 2, Table 1). Oil red O staining evaluations also confirmed the H&E staining results. No significant difference was observed between the TM and Amy + TM groups (Figure 3).
Figure 2.
Histological findings of liver tissues after H&E staining (magnification ×200) in different experimental groups. A; control: normal liver histology, B; vehicle: normal liver architecture, C; amygdalin: normal liver histology, D; TM (Tunicamycin): showing steatosis, ballooning degradation and lobular inflammation, E; amygdalin plus TM: showing steatosis and ballooning
Figure 3.
Histological findings of liver tissues after oil red O staining (magnification ×200) in different experimental groups. A; control: normal liver histology, B; vehicle: normal liver architecture, C; amygdalin: normal liver histology, D; TM (Tunicamycin): showing steatosis (red spots), E; amygdalin plus TM: showing steatosis (red spots)
Effect of Amygdalin on the Serum Lipids Levels
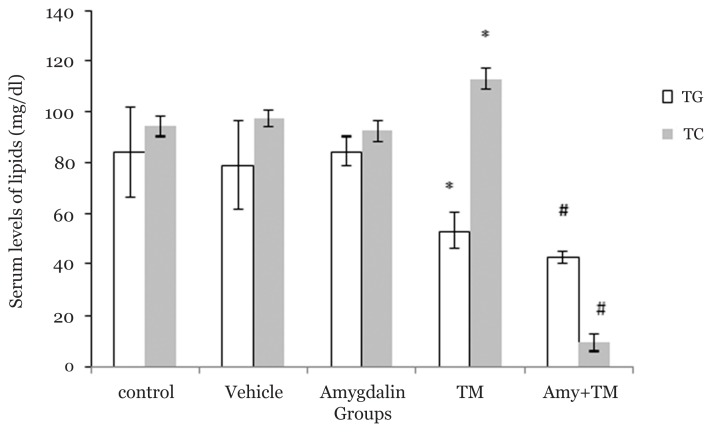
Our findings revealed that the TG level in the TM group significantly decreased when compared with the control, DMSO, and amygdalin groups (53.33(7.24) mg/dL versus 84(17.65) mg/dL, P = 0.002; 79(17.53) mg/dL, P = 0.007; and 84.33(5.7) mg/dL, P < 0.001, respectively). TC level significantly increased in the TM group when compared with the control, vehicle, and amygdalin groups (112.66(4.31) mg/dL versus 94.31(4.06) mg/dL, P < 0.001; 97.15(3.47) mg/dL, P = 0.000; and 92.35(3.77) mg/dL, P < 0.001, respectively). Pre-treatment of amygdalin also significantly decreased the TG and TC levels when compared with the TM group (TG: 42.66(2.15) versus 53.33(7.24) mg/dL, P = 0.006 and TC: 9.33(3.55) versus 112.66(4.31) mg/dL, P < 0.001; Figure 4).
Figure 4.
Lipid plasma levels in different experimental groups. N = 6 per group, error bars represent standard deviation, *P < 0.05 when compared to control, vehicle and amygdalin groups, respectively. # P < 0.05 when compared to TM group. TM (Tunicamycin), Amy (Amygdalin)
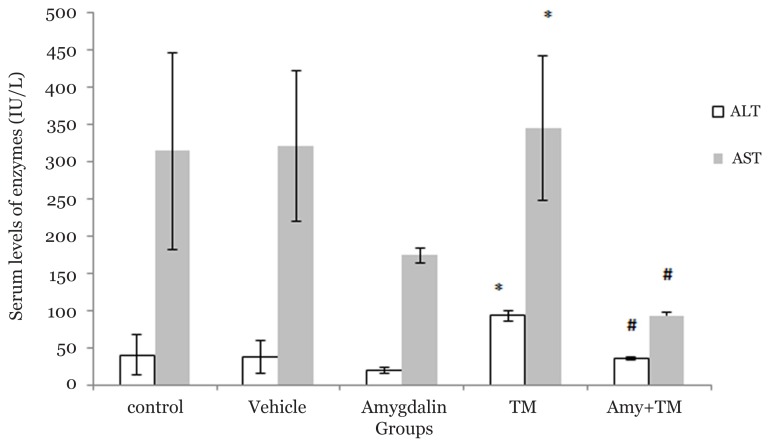
Effect of Amygdalin on the Liver Enzymes Levels
Blood sample analysis revealed that ALT serum levels significantly increased after the TM challenge when compared with the control, vehicle, and amygdalin groups (92.33(6.66) U/L versus 40 ± 26.69, P < 0.001; 37.2(21.55) U/L, P < 0.001; and 19.33(3.52) U/L, P < 0.001, respectively). Blood sample analysis also indicated that AST serum levels significantly increased after the TM challenge when compared with the amygdalin group (345(97.37) U/L versus 173.6(9.91) U/L, P < 0.001, respectively). Serum levels of ALT and AST significantly decreased in the Amy + TM group when compared with the TM group (ALT: 35.33(2.15) U/L versus 92.33(6.66) U/L, P < 0.001 and AST: 93(5.09) U/L versus 345(97.37) U/L, P < 0.001). Interestingly, our results revealed a considerable decrease in the AST level in the amygdalin group when compared with the saline and vehicle groups (173.66(9.91) U/L versus 314(132.24) U/L, P = 0.026 and 321(100.4) U/L, P = 0.005, respectively (Figure 5).
Figure 5.
Liver enzymes levels in different experimental groups. N = 6 per group, Error bars represent standard deviation, *P < 0.05 when compared to control, vehicle and amygdalin groups, respectively. # P < 0.05 when compared to TM group. (TM (Tunicamycin), Amy (Amygdalin)
Discussion
In this study, we reported that amygdalin reduced the plasma TG and TC as well as ALT and AST levels, alleviating liver inflammation, but unexpectedly it could not attenuate liver steatosis. Amygdalin is a cyanogenic glycoside with antidiabetic, antiatherosclerosis, and anticancer characteristics (12–16).
Liver index and our histological data (H&E and oil red O staining) showed that ER stress induction by TM challenge leads to severe steatosis and hepatocytes ballooning. These results were consistent with several previously published studies (26, 27); however, amygdalin administration before ER stress induction was unable to decrease lipid accumulation in the hepatic cells. Our pervious study reported that naltrexone attenuated ER stress induced liver steatosis (28). Heikkila, Cabbat (23) have previously reported that amygdalin pretreatment before alloxan-induced diabetes decreases the blood glucose by reactive hydroxyl radical scavenging. In another study, the potential effects of amygdalin in prevention of aortic atherosclerotic plaques and other vessels have been reported (21, 22). Nevertheless, our results showed that, at least in the liver, amygdalin did not improve ER stress induced steatosis. This may be due to the inadequate dose or time for the amygdalin pretreatment. Since the liver is an essential organ in lipid homeostasis, presumably the cyanogenic effects of amygdalin lead to loss of expression of the transcriptional factors and lipid metabolism related enzymes or hepatic cell destruction, as ER is destroyed after severe ER stress and is unable to activate the unfolded protein response in order to restore ER homeostasis and recovery mechanisms. Therefore, more studies are required to support these hypotheses.
Our other findings reported that amygdalin decreased the plasma TG and TC levels after the TM challenge. It has been revealed that amygdalin decreased TG, TC, and low-density lipoprotein in ApoE (−/−) mice (22). Zhao, Yang (21) also reported the protective effect of amygdalin in atherosclerosis development after one-month high fat diet. Our results are in accordance with these studies and confirm the amygdalin positive effect on plasma lipids reduction.
In the present study, amygdalin ameliorated the ALT and AST levels after ER stress induction. ALT and AST levels also considerably decreased in the amygdalin group. These enzymes could be interpreted as inflammatory indexes in the liver. It has been demonstrated that amygdalin reduced the proinflammatory cytokines such TNF-α, IL1, monocyte chemo-attractant protein-1 (MCP1), and inhibited toll-like receptor2 (TLR2) and TLR4 (27, 29). In another study, Jiagang et al. (22) have been shown that the antiatherosclerotic effect of amygdalin is mediated through the induction of regulatory T cells. Collectively, it is presumed that these findings delineate the amygdalin inhibitory effects on the inflammation processes in the liver; however, ample evidence is required to clarify the precise mechanism.
Conclusion
Our study revealed that amygdalin reduced the lipid plasma levels and liver inflammation, thus confirming the antilipid and anti-inflammatory effects of amygdalin; however, amygdalin could not attenuate the ER stress induced liver steatosis.
Acknowledgements
We appreciate the Qom University of Medical Sciences for supporting this study.
Footnotes
Authors’ Contributions
Conception and design: AM
Analysis and Interpretation of the data: AM
Provision of study materials or patients: AM, SAC, AB
Statistical expertise: AMB
Obtaining of funding: MF
Administrative, technical, or logistic support: MF, SAC, AB, SA
Collection and assembly data: SA
References
- 1.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. https://doi.org/10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyerovich K, Ortis F, Allagnat F, Cardozo AK. Endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. J Mol Endocrinol. 2016;57(1):1–17. doi: 10.1530/JME-15-0306. https://doi.org/10.1530/JME-15-0306. [DOI] [PubMed] [Google Scholar]
- 3.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. https://doi.org/10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Takatani T, Shirakawa J, Roe MW, Leech CA, Maier BF, Mirmira RG, et al. IRS1 deficiency protects β-cells against ER stress-induced apoptosis by modulating sXBP-1 stability and protein translation. Sci Rep. 2016;6:28177. doi: 10.1038/srep28177. https://doi.org/10.1038/srep28177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs CD, Claudel T, Kumari P, Haemmerle G, Pollheimer MJ, Stojakovic T, et al. Absence of adipose triglyceride lipase protects from hepatic endoplasmic reticulum stress in mice. Hepatology. 2012;56:270–280. doi: 10.1002/hep.25601. https://doi.org/10.1002/hep.25601. [DOI] [PubMed] [Google Scholar]
- 6.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. https://doi.org/10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. https://doi.org/10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JS, Zheng Z, Mendez R, Ha SW, Xie Y, Zhang K. Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett. 2012;211:29–38. doi: 10.1016/j.toxlet.2012.02.017. https://doi.org/10.1016/j.toxlet.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christopher L, Gentile CL, Nivala AM, Gonzales JC, Pfaffenbach KT, Wang D, et al. Experimental evidence for therapeutic potential of taurine in the treatment of nonalcoholic fatty liver disease. Am J Physiol Regul Integr Comp Physiol. 2011;301:1710–1722. doi: 10.1152/ajpregu.00677.2010. https://doi.org/10.1152/ajpregu.00677.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SJ, Kang HS, Lee JH, Park JH, Jung CH, Bae JH, et al. Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver. Biochem Biophys Res Commun. 2015;458(3):462–469. doi: 10.1016/j.bbrc.2015.01.117. https://doi.org/10.1016/j.bbrc.2015.01.117. [DOI] [PubMed] [Google Scholar]
- 11.Moslehi A, Nabavizadeh F, Zekri A, Amiri F. Naltrexone changes the expression of lipid metabolism-related proteins in the endoplasmic reticulum stress induced hepatic steatosis in mice. Clin Exp Pharmacol Physiol. 2017;44(2):207–212. doi: 10.1111/1440-1681.12695. https://doi.org/10.1111/1440-1681.12695. [DOI] [PubMed] [Google Scholar]
- 12.Chang LW, Zhu HP, Li WB, Liu HC, Zhang QS, Chen HB. Protective effects of amygdalin on hyperoxia-exposed type II alveolar epithelial cells isolated from premature rat lungs in vitro. ZhonghuaErKeZaZhi. 2005;43(2):118–123. [PubMed] [Google Scholar]
- 13.Guo J, Wu W, Sheng M, Yang S, Tan J. Amygdalin inhibits renal fibrosis in chronic kidney disease. Mol Med Rep. 2013;7(5):1453–1457. doi: 10.3892/mmr.2013.1391. https://doi.org/10.3892/mmr.2013.1391. [DOI] [PubMed] [Google Scholar]
- 14.Hwang HJ, Kim P, Kim CJ, Lee HJ, Shim I, Yin CS, et al. Antinociceptive effect of amygdalin isolated from Prunusarmeniaca on formalin-induced pain in rats. Biol Pharm Bull. 2008;31(8):1559–1564. doi: 10.1248/bpb.31.1559. https://doi.org/10.1248/bpb.31.1559. [DOI] [PubMed] [Google Scholar]
- 15.Mirmiranpour H, Khaghani S, Zandieh A, Khalilzadeh OO, Gerayesh-Nejad S, Morteza A, et al. Amygdalin inhibits angiogenesis in the cultured endothelial cells of diabetic rats. Indian J Pathol Microbiol. 2012;55(2):211–214. doi: 10.4103/0377-4929.97874. https://doi.org/10.4103/0377-4929.97874. [DOI] [PubMed] [Google Scholar]
- 16.Murnane JM, Ritter S. Alloxan-induced glucoprivic feeding deficits is blocked by D-glucose and amygdalin. Pharmacol Biochem Behav. 1985;22(3):407–413. doi: 10.1016/0091-3057(85)90041-3. https://doi.org/10.1016/0091-3057(85)90041-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhu H1, Chang L, Li W, Liu H. Effect of amygdalin on the proliferation of hyperoxia-exposed type II alveolar epithelial cells isolated from premature rat. J Huazhong Univ Sci Technology Med Sci. 2003;24(3):223–225. doi: 10.1007/BF02831995. [DOI] [PubMed] [Google Scholar]
- 18.Chang HK, Shin MS, Yang HY, Lee JW, Kim YS, Lee MH, et al. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol Pharm Bull. 2006;29(8):1597–1602. doi: 10.1248/bpb.29.1597. https://doi.org/10.1248/bpb.29.1597. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Ma J, Wang F, Hu J, Cui A, Wei C, et al. Amygdalin induces apoptosis in human cervical cancer cell line HeLa cells. Immunopharmacol Immunotoxicol. 2013;35(1):43–51. doi: 10.3109/08923973.2012.738688. https://doi.org/10.3109/08923973.2012.738688. [DOI] [PubMed] [Google Scholar]
- 20.Makarević J, Rutz J, Juengel E, Kaulfuss S, Reiter M, Tsaur I, et al. Amygdalin blocks bladder cancer cell growth in vitro by diminishing cyclin A and cdk2. PLoS One. 2014;9(8):105590. doi: 10.1371/journal.pone.0105590. https://doi.org/10.1371/journal.pone.0105590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao F, Yang Z. Amygdalin attenuates atherosclerosis progress through inhibiting of toll-like receptors expression and activity. J Animal Veterinary Advances. 2012;11:1613–1621. https://doi.org/10.3923/javaa.2012.1613.1621. [Google Scholar]
- 22.Jiagang D, Li C, Wang H, Hao E, Du Z, Bao C, et al. Amygdalin mediates relieved atherosclerosis in apolipoprotein E deficient mice through the induction of regulatory T cells. Biochem Biophys Res Commun. 2011;411(3):523–529. doi: 10.1016/j.bbrc.2011.06.162. https://doi.org/10.1016/j.bbrc.2011.06.162. [DOI] [PubMed] [Google Scholar]
- 23.Heikkila RE, Cabbat FS. The prevention of alloxan-induced diabetes by amygdalin. Life Sci. 1980;27(8):659–662. doi: 10.1016/0024-3205(80)90006-5. https://doi.org/10.1016/0024-3205(80)90006-5. [DOI] [PubMed] [Google Scholar]
- 24.Nikoukar LR, Nabavizadeh F, Mohamadi SM, Moslehi A, Hassanzadeh G, Nahrevanian H, et al. Protective effect of ghrelin in a rat model of celiac disease. Acta Physiol Hung. 2014;101(4):438–447. doi: 10.1556/APhysiol.101.2014.4.5. https://doi.org/10.1556/APhysiol.101.2014.4.5. [DOI] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. https://doi.org/10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Fang DL, Wan Y, Shen W, Cao J, Sun ZX, Yu HH, et al. Endoplasmic reticulum stress leads to lipid accumulation through upregulation of SREBP-1c in normal hepatic and hepatom cells. Mol Cell Biochem. 2013;381:127–137. doi: 10.1007/s11010-013-1694-7. https://doi.org/10.1007/s11010-013-1694-7. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto K, Takahara K, Oyadomari S, Okada T, Sato T, Harada A, et al. Induction of liver steatosis andlipid droplet formation in ATF6alpha-knockout mice burdened with pharmacological endoplasmic reticulum stress. Mol Biol Cell. 2010;21:2975–2986. doi: 10.1091/mbc.E09-02-0133. https://doi.org/10.1091/mbc.E09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moslehi A, Nabavizadeh F, Dehpour AR, Tavanga SM, Hassanzadeh G, Zekri A, et al. Naltrexone attenuates endoplasmic reticulum stress induced hepatic injury in mice. Acta Physiol Hung. 2014;101(3):341–352. doi: 10.1556/APhysiol.101.2014.3.9. https://doi.org/10.1556/APhysiol.101.2014.3.9. [DOI] [PubMed] [Google Scholar]
- 29.Hwang HJ, Lee HJ, Kim CJ, Shim I, Hahm DH. Inhibitory effect of amygdalin on lipopolysaccharide-inducible TNF-alpha and IL-1beta mRNA expression and carrageenan-induced rat arthritis. J Microbiol Biotechnol. 2008;18(10):1641–1647. [PubMed] [Google Scholar]