Abstract
Ductal carcinoma in situ (DCIS) can be a precursor of invasive breast cancer. Since the advent of screening mammography in the 1980's, the incidence of DCIS has increased dramatically. The value of screen detection and treatment of DCIS is a matter of controversy, since it is unclear to what extent detection and treatment of DCIS prevents invasive disease and reduces breast cancer mortality. The aim of this paper is to provide an overview of existing Cancer Intervention and Surveillance Modelling Network (CISNET) modeling approaches for the natural history of DCIS, and to compare these to other modeling approaches reported in the literature. Five of the six CISNET models currently include DCIS. Most models assume that some, but not all, lesions progress to invasive cancer. The natural history of DCIS cannot be directly observed and the CISNET models differ in their assumptions and in the data sources used to estimate the DCIS model parameters. These model differences translate into variation in outcomes such as the amount of overdiagnosis of DCIS with estimates ranging from 34%-72% for biennial screening from age 50-74 years. The other models described in the literature also report a large range in outcomes with progression rates varying from 20%-91%. In the future, DCIS data by grade from active surveillance trials, development of predictive markers of progression probability, and evidence from other screening modalities, such as tomosynthesis, may be utilized to inform and improve the models' representation of DCIS and might lead to convergence of the model estimates. Until then, the CISNET model results consistently show a considerable amount of overdiagnosis of DCIS, supporting the safety and value of observational trials for low-risk DCIS.
Keywords: Cancer simulation, breast cancer epidemiology, simulation models, ductal carcinoma in situ
Introduction
Ductal carcinoma in situ (DCIS) represents a spectrum of abnormal cells confined to the breast duct and is a risk factor for invasive breast cancer development [1]. Before the introduction of mammography screening, DCIS was not often diagnosed. Since the advent of screening mammography in the 1980s, the incidence of DCIS has increased dramatically. In the United States, the incidence of DCIS increased from 5.8 per 100,000 women in 1975 to 68.9 per 100,000 women in 2010 [2-4]. By the year 2020, more than one million US women are expected to be living with and have been treated for a DCIS diagnosis [1].
The etiology of DCIS is presumably heterogeneous and its natural history is poorly understood as onset, progression and regression rates are not directly observable. Some DCIS lesions likely represent a precursor to subsequent invasive breast cancer, but DCIS may also remain indolent for sufficiently long that a woman dies of other causes [5-7]. The proportion of untreated DCIS that will progress to invasive breast cancer is unknown [1], and therefore, the impact of detecting and treating DCIS, particularly for any given woman, is unclear. Treating some DCIS lesions will probably prevent invasive disease, and consequently might reduce breast cancer mortality, thus can be considered a benefit. Other lesions might remain indolent in the absence of treatment with only harms related to their treatment (representing overdiagnosis and overtreatment). Since we do not know which and how many DCIS lesions will progress, the value of screen detection and treatment of DCIS remains unknown and is a matter of considerable controversy.
Despite the uncertainty around the natural history of DCIS, some predictors for progression have been identified. For example, younger age at diagnosis and black ethnicity are associated with higher breast cancer-specific mortality among patients with DCIS [8, 9]. Other identified factors for progression include estrogen receptor (ER) negative status, larger DCIS tumor size, and comedonecrosis [9]. In addition, DCIS progression to invasive breast cancer can be predicted by cytologic grade [5, 7, 9]. Pathologists use three grading categories: corresponding to well (grade 1), moderately (grade 2), and poorly (grade 3) differentiated DCIS [10], also referred to as “low grade”, “intermediate grade”, and “high grade”, respectively. Grade has been found to be associated with recurrence [11, 12] and the survival benefit of surgical treatment has been found to be lower for low-grade DCIS than that for intermediate or high-grade DCIS [13]. Furthermore, the DCIS Score, based on Oncotype DX, has been found to be associated with recurrence of DCIS (either as DCIS or invasive breast cancer) [14].
These identified prognostic factors for recurrence may enable physicians to tailor treatment strategies. Specifically, recommending treatment that is less aggressive would be appropriate for DCIS that has a low risk for future recurrence, and predictors such as age, ER status, and/or grade might be used to identify low-risk lesions. Thus, understanding the natural history of DCIS and its recurrence and progression predictors to guide treatment strategies is important for both clinical and public health decisions. However, investigating the natural history of DCIS is difficult as ideal high-quality data is lacking, given that progression paths are not directly observable. In addition, data are also limited because survival for women diagnosed with DCIS is very high and a trial would need to enroll very large numbers of women and follow them for a lifetime to be adequately powered to detect an impact of screening and treatment on mortality or other endpoints. Moreover, the natural history of DCIS is difficult to study because the standard of care is immediate treatment following diagnosis. In these instances (comparative) modeling can be useful, for example to provide a range of plausible DCIS progression and regression rates by evaluating what set of assumptions about these rates best fit the existing observable data. In addition, in natural history models, the difference in risk of progression based on age, grade and ER status can be included by allowing varying transition rates for these factors, which has already been done in a well-established microsimulation model to include grade [15].
Furthermore, within the Cancer Intervention and Surveillance Modelling Network (CISNET) comparative modeling work has been done. Previously, three CISNET models estimated the amount of DCIS overdiagnosis in women age 74 and older. The results indicated that at older ages harms began to outweigh benefits, largely as a consequence of the increasing amount of overdiagnosis of DCIS at older ages [16], which is partly due to the higher death rate from competing causes with aging. Together, these modeling papers, on one hand highlight the uncertainty regarding the natural history of DCIS, but also show the potential value of modeling in providing information where results are consistent.
The aim of this paper is to provide an overview of the ways CISNET models simulate the natural history of DCIS, illustrate how different assumptions affect results, to compare the CISNET models to other models described in the literature, and to highlight developments that might lead to model improvements or refinements.
CISNET models
CISNET DCIS models – model overview
CISNET is a consortium of National Cancer Institute (NCI)-sponsored investigators who use statistical modeling to improve our understanding of cancer control interventions in prevention, screening, and treatment and their effects on population trends in incidence and mortality. The CISNET breast models have been described in detail previously and recently updated descriptions have been given [17-22]. Briefly, the models are designed to match breast cancer incidence and mortality rates observed in the US. Four models are micro-simulation models (models developed by Erasmus MC, University Medical Center Rotterdam, model E; Georgetown University Medical Center, and Albert Einstein College of Medicine, model G-E; MD Anderson Cancer Center, model M; and University of Wisconsin, Madison and Harvard Medical School, model W), one model uses an analytic approach (model developed by Dana-Farber Cancer Institute, model D), and the remaining model is a hybrid Monte Carlo simulation (model developed by Stanford University, model S). The micro-simulation models include natural history components that approximate tumor progression in size and stage (https://resources.cisnet.cancer.gov/registry/site-summary/breast/). Five of the six CISNET models currently include DCIS (all except model S). Most models assume that some, but not all, lesions progress to invasive cancer, for example by including three different types of preclinical DCIS: DCIS that progresses to invasive disease during the preclinical phase, progressive DCIS that is diagnosed clinically, and DCIS that does not progress (and might regress). However, the models differ in natural history of DCIS (Table 1) and model structure (see Figure 1), with different pathways for the progression and regression of DCIS and breast cancer. For example, invasive cancer can either develop through pre-clinical screen-detectable DCIS (Figure 1C), or also develop directly from pre-clinical DCIS that is not detectable at screening (Figure 1A and 1B). In the models, DCIS can regress from pre-clinical screen-detectable DCIS to pre-clinical undetectable DCIS (Figure 1A) or to an absorbing ‘no breast cancer’ state and disappear (“cease to exist”) (Figure 1B and 1C). One model (model W) allows regression of pre-clinical DCIS as well as invasive disease (Figure 1D). Although the regression of breast cancer, especially invasive disease, is controversial, there is some evidence supporting the possibility of regressing tumors, including epidemiologic evidence [23] and a case report on regression of breast on imaging [24].
Table 1.
Natural history of DCIS in the CISNET models.
| Model | in situ or DCIS?* | Do all tumors start as in situ? | Progression/regression | Model structure |
|---|---|---|---|---|
| D | DCIS only | Yes, but some DCIS is not screen detectable and assumed to progress to invasive directly | DCIS progress to clinical DCIS or invasive breast cancer at exponential rates with mean sojourn time of 1.5-3 years; DCIS may also go back to a state in which it is undetectable [19] | Figure 1A |
| E | All in situ | Yes | DCIS progress to clinical or invasive breast cancer at an exponential rate with age and calendar year dependent sojourn times; DCIS may also regress [22] | Figure 1B |
| GE | DCIS only | Yes | DCIS progress to clinical or invasive breast cancer at an exponential rate with mean sojourn time of 2.97 years; DCIS may also regress [21] | Figure 1C |
| M | Model M is not a natural history model. It does not specify how tumors grow. It is an empirical model to describe screening, incidence, treatment and mortality. Under different screening scenarios, different stage distribution tables obtained from observed data [28] are used to assign tumor stages: DCIS, stages I, II, III or IV. DCIS patients are assumed to have the same survival as normal population, given age and birth year, no matter what treatments they receive.[18] | |||
| W | All in situ. Model W also separated in situ into DCIS and non-DCIS in situ | Yes | All tumors, including DCIS, progress according to a Gompertz-type growth function, where the growth parameter is a random variable distributed with Gamma. Small size defines in situ. All tumors grow until they reach a maximum size. All tumors progress although a subset with “limited malignant potential” (LMP) stop at early invasive. LMPs comprise approximately 30-50% of all onset tumors [17] | Figure 1D |
Model D: Dana-Farber Cancer Institute, Boston, Massachusetts. Model E: Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands. Model GE: Georgetown University Medical Center, Washington, DC, and Albert Einstein College of Medicine, Bronx, New York. Model M: MD Anderson Cancer Center, Houston, Texas. Model W: University of Wisconsin, Madison, Wisconsin, and Harvard Medical School, Boston, Massachusetts.
in situ: DCIS and lobular carcinoma in situ (LCIS)
Figure 1.
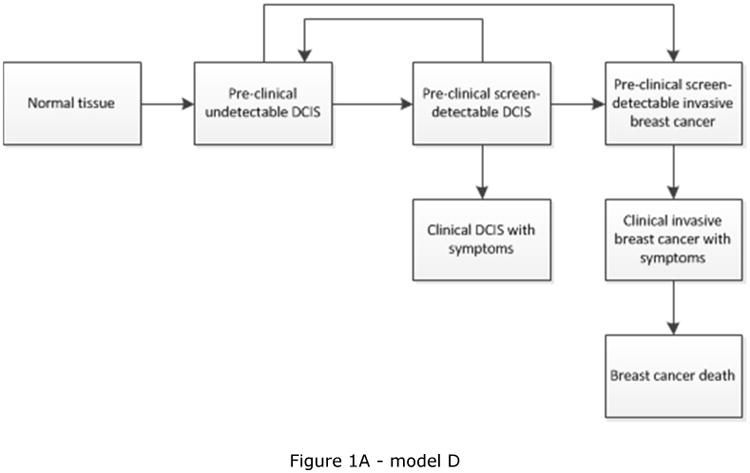
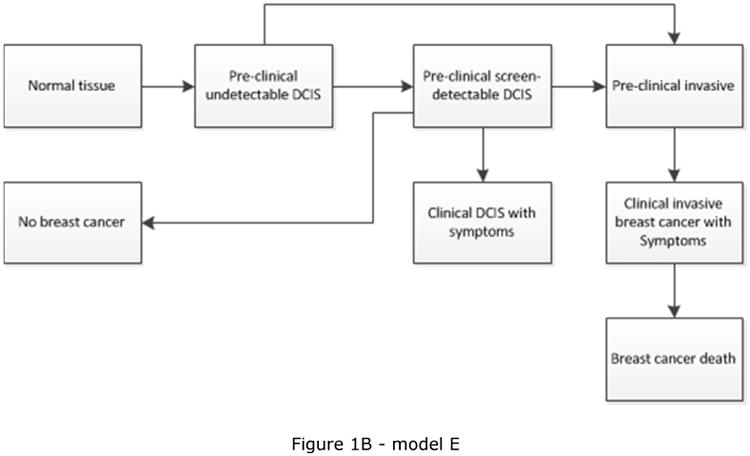
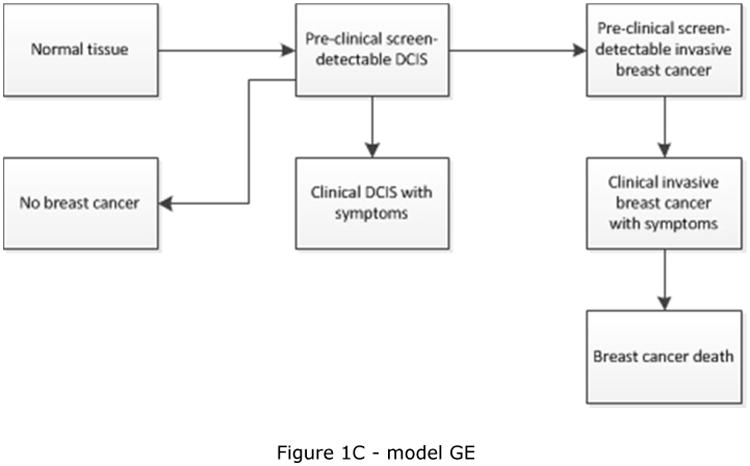
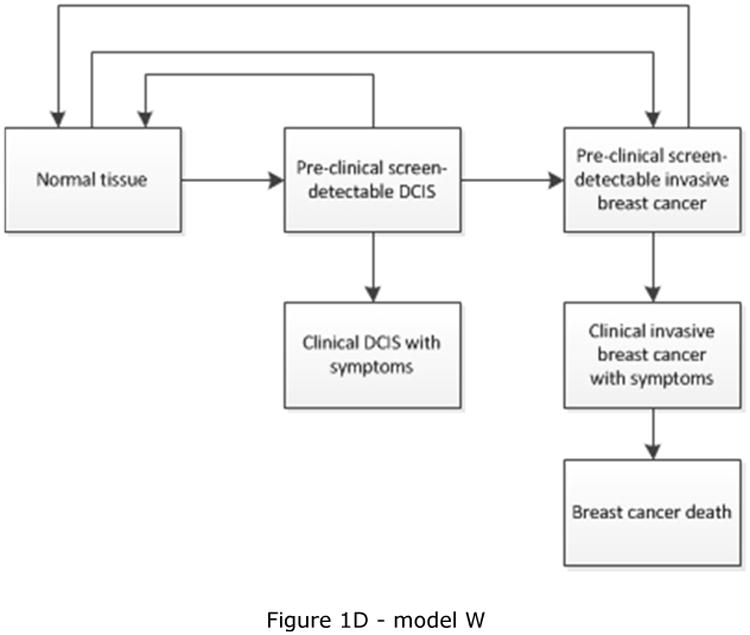
Schematic overview of models for the natural history of DCIS and invasive breast cancer. Invasive cancer can either develop through pre-clinical screening detectable DCIS (Figure 1C), or also develop directly from pre-clinical DCIS not detectable at screening (Figure 1A, 1B and 1D). Models include progression from preclinical screen-detectable DCIS to either clinical DCIS or preclinical invasive disease (Figure 1A, 1B, 1C, 1D), regression from preclinical DCIS to normal tissue (Figure 1D), to pre-clinical undetectable DCIS (Figure 1A), or to a ‘no breast cancer’ (absorbing) state in which women are no longer at risk for developing DCIS or invasive breast cancer (Figure 1B and 1C). Regression from invasive disease is also possible (Figure 1D).
Most of the CISNET models have used data from the Surveillance, Epidemiology, and End Results (SEER) Program [25], typically age-specific incidence over time, combined with data from other sources (Wisconsin cancer registry for model W, Dutch data for model E) to estimate DCIS parameters, although one model used data from another source to develop their model (Norwegian data for model D) [26]. All CISNET models include a certain probability for mammography to detect DCIS at screening (Table 2). Specifically models D and GE use the same detection mechanism for DCIS as for invasive disease by including a sensitivity of screening. Model W uses the detection probability as a function of tumor size and because in situ lesions are small the likelihood of detecting DCIS is lower than that for detecting invasive breast cancer. Model E includes two separate detection mechanisms; DCIS detection is modeled by including a sensitivity, whereas screen-detection of invasive disease is modeled by a threshold diameter. Thus, in some models the sensitivity of a screening test differs for DCIS and invasive cancer.
Table 2.
Detection mechanism of DCIS in the CISNET models.
| Model | Clinical detection mechanism | Screen detection mechanism | Detection mechanism DCIS vs. invasive cancer |
|---|---|---|---|
| D | Some DCIS progress to clinical DCIS with symptoms - this rate matches age-specific incidence rate of DCIS in pre-screening era | Sensitivity varying by screening modality, age, calendar year | Same mechanism for DCIS and invasive cancer by test sensitivity |
| E | Some DCIS progress to clinical DCIS with symptoms - this rate matches age-specific incidence rate of DCIS in pre-screening era | Sensitivity varying by calendar year | DCIS is detected by test sensitivity; invasive disease is detected using a threshold diameter |
| GE | Progressive DCIS are clinically detected the same as more advanced lesions. Non-progressive DCIS are NEVER clinically detected. | Sensitivity varying by screening modality, age, calendar year | Same mechanism for DCIS and invasive cancer by test sensitivity |
| M | Model M makes no explicit mechanism assumptions regarding DCIS detection. | ||
| W | Some DCIS are clinically diagnosed similarly as more advanced lesions. Clinical detection probability is an increasing function of tumor size and varies by age and calendar year. Clinical detection probabilities are in general smaller than screen detection probabilities; therefore a tumor is less likely to be detected via clinical surfacing than by screening. | Sensitivity varying by is tumor size, age, calendar year | Detection probability is an increasing function of tumor size, thus because in situ are small by definition, likelihood of detection of DCIS is less than that for invasive cancer |
Model D: Dana-Farber Cancer Institute, Boston, Massachusetts. Model E: Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands. Model GE: Georgetown University Medical Center, Washington, DC, and Albert Einstein College of Medicine, Bronx, New York. Model M: MD Anderson Cancer Center, Houston, Texas. Model W: University of Wisconsin-Madison, Madison, Wisconsin, and Harvard Medical School, Boston, Massachusetts.
CISNET models – analysis
The CISNET models were recently applied to evaluate screening outcomes of various screening strategies differing by age at which screening starts (40, 45, or 50 years) and screening interval (annual, biennial) for the US female population [27]. We assessed the results of those prior analyses by focusing on the (as yet unpublished) model-specific rates of DCIS detection and overdiagnosis of the five CISNET models that include DCIS [28]. Overdiagnosis was defined as the detection of tumors that would not have been detected in a woman's lifetime in the absence of screening. We estimated the detection and overdiagnosis rate per 1000 women screened followed from age 40 over their lifetimes. In addition, the percentage overdiagnosis was calculated by dividing the rate of overdiagnosed DCIS by the rate of detected DCIS. We focus on four screening scenarios: biennial screening from 50-74 years (base), more frequent screening (annual screening from age 50-74 years; A50-74), an earlier starting age (biennial screening from age 40-74 years; B40-74), and later stopping age (biennial screening from age 50-84 years; B50-84).
CISNET models – results and implications
For biennial screening between age 50 and 74 years, the five models that include DCIS predict that 154.4 women (median; range across five models 137.4 – 158.5; Table 3) are diagnosed with breast cancer per 1000 women followed from age 40 over their lifetimes. Of these women, 26.7 (25.8 – 32.3) are diagnosed with DCIS and 128.2 (110.7 – 131.8) with invasive disease. Of the women diagnosed with DCIS, 15.6 (9.0-18.8) are overdiagnosed, representing 51.3% (33.7%-71.8%) of the detected DCIS (Table 3). In contrast, for invasive disease, the models estimate that of the 128.2 (110.7-131.8) breast cancers detected, 3.3 (1.8-15.4) are overdiagnosed, corresponding to 2.6% (1.5%-12.0%; Table 3). This means that 2.6% (1.5-12.0%) of the invasive breast cancers that are detected would not have been detected in the absence of screening and are overdiagnosed. There is no direct connection between the amount of overdiagnosis of DCIS and overdiagnosis of invasive disease in the models. For example, one model predicts relatively low overdiagnosis percentages for DCIS as well as invasive breast cancer (model GE), whereas another model predicts relatively high percentages for both (model M). In contrast, there are also models that have modest estimates of DCIS overdiagnosis combined with relatively high estimates of invasive disease overdiagnosis (model W) or the other way around (model E).
Table 3.
Detection and overdiagnosis of DCIS and invasive disease across the CISNET models for biennial screening from age 50-74 years.
| Model | DCIS dx per 1000 | DCIS overdx per 1000 | %overdx DCIS | invasive dx per 1000 | invasive overdx per 1000 | %overdx invasive | total dx per 1000 | overdx per 1000 | %overdx (DCIS + invasive) |
|---|---|---|---|---|---|---|---|---|---|
| D | 30.2 | 15.5 | 51.3% | 128.3 | 3.3 | 2.6% | 158.5 | 18.8 | 11.9% |
| E | 25.8 | 16.1 | 62.4% | 131.8 | 2.0 | 1.5% | 157.6 | 18.1 | 11.5% |
| GE | 26.7 | 9.0 | 33.7% | 110.7 | 1.8 | 1.6% | 137.4 | 10.8 | 7.9% |
| M | 26.2 | 18.8 | 71.8% | 128.2 | 15.4 | 12.0% | 154.4 | 34.2 | 22.2% |
| W | 32.3 | 15.6 | 48.3% | 114.8 | 9.9 | 8.6% | 147.1 | 25.5 | 17.3% |
|
| |||||||||
| Median | 26.7 | 15.6 | 51.3% | 128.2 | 3.3 | 2.6% | 154.4 | 18.8 | 11.9% |
Model D: Dana-Farber Cancer Institute, Boston, Massachusetts. Model E: Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands. Model GE: Georgetown University Medical Center, Washington, DC, and Albert Einstein College of Medicine, Bronx, New York. Model M: MD Anderson Cancer Center, Houston, Texas. Model W: University of Wisconsin-Madison, Madison, Wisconsin, and Harvard Medical School, Boston, Massachusetts.
When annual screening from age 50-74 years is simulated, the models estimate 0.1-14.0 additional cases of DCIS being detected of which 0.1-13.7 are overdiagnosed (Table 4). Also, the models differ for the source for additional DCIS cases. For Models D, M, the increase in detection of DCIS is entirely overdiagnosis, whereas in models E, GE, W it is combination of overdiagnosis and earlier detection of lesions with progressive potential.
Table 4.
Changes in DCIS detection and overdiagnosis of DCIS when moving from biennial 50-74 years to other screening scenarios.
| change in DCIS detection | change in DCIS overdiagnosis |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | A50-74 | B40-74 | B50-84 | A50-74 | B40-74 | B50-84 | A50-74 | B40-74 | B50-84 | |
| D | 0.1 | 0.0 | 2.8 | 0.1 | 0.1 | 2.8 | 100% | N/A | 100% | |
| E | 8.5 | 4.8 | 5.6 | 6.7 | 3.3 | 5.2 | 79% | 69% | 93% | |
| GE | 3.2 | 3.6 | 6.3 | 0.4 | 1.2 | 3.0 | 13% | 33% | 48% | |
| M | 13.6 | 5.0 | 5.5 | 13.7 | 5.1 | 5.6 | 101% | 102% | 102% | |
| W | 14.0 | 2.4 | 9.7 | 7.1 | 1.5 | -1.1 | 51% | 63% | -11% | |
A50-74: annual screening from age 50-74 years.
B40-74: biennial screening from age 40-74 years.
B50-84: biennial screening from age 50-84 years.
Model D: Dana-Farber Cancer Institute, Boston, Massachusetts. Model E: Erasmus MC, University Medical Center Rotterdam, Rotterdam, the Netherlands. Model GE: Georgetown University Medical Center, Washington, DC, and Albert Einstein College of Medicine, Bronx, New York. Model M: MD Anderson Cancer Center, Houston, Texas. Model W: University of Wisconsin-Madison, Madison, Wisconsin, and Harvard Medical School, Boston, Massachusetts.
In addition, the order of scenarios that have the largest increase in overdiagnosis of DCIS varies across models, as well as the magnitude of the increase. For example, for annual screening the increase in overdiagnosis varies between 0.1 and 13.7 overdiagnosed DCIS cases across models. Some models estimate the largest change in detection and overdiagnosis when annual screening is considered (models E, M, W), whereas other models predict the largest increase when upper age of screening is extended to age 84 (models D and GE).
For the biennial screening scenario from age 50-74 years, the highest percentage of overdiagnosis of DCIS and invasive breast cancer was estimated by model M followed by W. This can be explained by the modeling choice of model M to assume a rather stable trend in breast cancer incidence (background trend) over time and, therefore, assign more of the increase to overdiagnosis than other CISNET models. Model W assumes that some invasive disease is non-progressive, and consequently, has a higher estimate for overdiagnosis than the other three models, especially for invasive disease.
For the other scenarios, annual screening from age 50-74 years, biennial screening from age 40-74 years, and biennial screening from age 50-84 years, there are two clusters of models: models D and M assign the increase in detection of DCIS when screening more intensively entirely to overdiagnosis. For model M that is again related to the stable background trend and for model D, the screen detectable period for DCIS is relatively short. The other three models (models E, GE, and W) only assign a proportion of the increase to overdiagnosis and a proportion to earlier diagnosis. Models E and GE assign most of the increase to overdiagnosis when moving to older ages and a smaller percentage when moving to younger ages.
Literature
Description of other DCIS models in the literature
To improve the understanding of the natural history of DCIS, we conducted a literature search to identify DCIS models that have been described in the literature. We searched PubMed and JSTOR for “DCIS natural history modeling” and “DCIS progression”, and selected the articles that focus on the estimation of key DCIS natural history parameters, such as mean sojourn time for screen-detectable pre-clinical DCIS, and percent of DCIS cases that progress to either invasive cancer, clinical DCIS, or potentially regress. We identified 10 relevant studies, of which nine include DCIS natural history modeling (Table 5). Among them, four studies use Markov models [29-32] and five use simulation models [15, 33-36], with parameters estimated with either maximum likelihood, Bayesian Gibbs sampling or least square methods, and varying assumptions about DCIS natural history pathways. Seven studies assumed that all invasive breast cancers progress through a pre-clinical in situ or DCIS state that can be detected at screening [15, 29, 32-34, 36], whereas the other two studies assumed that some DCIS or in situ lesions first become visible on mammograms as small invasive tumors [30, 35]. DCIS or in situ is assumed to have both progressive and non-progressive paths in eight studies [15, 29-34, 36], with one study also including non-progressive invasive cancers [36].
Table 5.
Overview of studies on modeling DCIS.
| 1st Author (Year), Journal |
Paper title | Approaches/Models for DCIS natural history |
Data sources | Natural History assumptions | All invasive cancers progress through screening detectable DCIS? |
Screening detectable DCIS might regress to a non-detectable stage |
Regression | Progression | Mean sojourn time | Mammography sensitivities to detect DCIS/in situ |
|---|---|---|---|---|---|---|---|---|---|---|
| Yen (2003), Eur J Cancer. [32] | Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening | Markov model | Swedish two county trial, service screening programs from UK, US Netherlands, and Australia | Healthy cases can progress to pre-clinical screen detectable progressive or non-progressive DCIS; progressive DCIS progress to invasive breast cancer; non-progressive DCIS regress to a separate state where no tumor is apparent. | Yes | Yes | 37% (19%-46%) at 1st screening; 4% (3%-21%) at 2nd screening | To invasive: 100-%non-progression | for non-progression: 30y (6y-37y), for progression to invasive: 3mo (2mo-5mo) | Not specified |
| Ozanne (2011), Breast Cancer Res Treat. [35] | Characterizing the impact of 25 years of DCIS treatment | Simulation model | US SEER (1975-2005) incidence | The percentage of the DCIS lesions that are assumed to progress to invasive breast cancer varies between 0% and 100%. The initial assumption that DCIS is a short-term obligate precursor of invasive cancer must be reevaluated based on the results. | No | Not specified | Not specified | To invasive: 20% of progression rate matches SEER data best | Not specified | Not specified |
| de Gelder (2011), Epi Rev. [33] | Interpreting overdiagnosis estimates in population-based mammography screening | Simulation model | Dutch population data from public screening program | Healthy cases can progress to pre-clinical screen detectable DCIS or invasive breast cancers; pre-clinical screen detectable DCIS can regress, progress to clinical DCIS, or progress to invasive breast cancer. | Yes | Yes | 11% of DCIS regress | To clinical DCIS: 28% ; To invasive: 61% | 2.6y | for DCIS: 72% |
| Gunsoy (2012), Breast Cancer Res. [29] | Modeling the overdiagnosis of breast cancer due to mammography screening in women aged 40-49 in the United Kingdom | Markov model | UK Age trial | Healthy cases can progress to pre-clinical screen detectable progressive in-situ or non-progressive in-situ; progressive in situ progress to invasive breast cancers | Yes | No | Not specified | To invasive: 45% (95%CI: 23%-75%) at 1st screen, 60% (95%CI: 40%-78%) at incidence screen | for pre-clinical non-progressive DCIS to clinical DCIS: 1.3y (95%CI: 0.4y-3.4y), for pre-clinical progressive DCIS: 0.11y (95%CI: 0.05y-0.19y). | for in situ: 82% (95%CI: 43%-99%) |
| Tan (2013), Br J Cancer. [31] | Quantifying the natural history of breast cancer | Markov model (Bayesian) | Swedish randomized trials | Healthy cases can progress to pre-clinical screen detectable progressive DCIS or non-progressive DCIS; progressive DCIS progress to invasive breast cancer. | Yes | Yes | Not specified | 91%(95%CI: 85%-97%) aggressive | for aggressive DCIS to invasive 0.5mo (95%CI: 0-1mo) | for DCIS: 88% (95%CI: 83%-92%) |
| Ryser (2016), J Natl Cancer Inst. [30] | Outcomes of Active Surveillance for DCIS: A Computational Risk Analysis | Markov model | US SEER (1999-2011) for cumulative mortality estimates and natural history model summarized from a variety of studies | Healthy cases can progress to the pre-clinical screen detectable progressive DCIS or non-progressive DCIS; progressive DCIS progress to localized invasive breast cancer. | No | Yes | Not specified | 24.4% (11.3%-67%) | for progressive DCIS to localized invasive (did not specify whether to pre-clinical or clinical invasive): 9.8y (6.4y-13.5y) | for MRI: 84% (77%-100%); for mammography: 40% (33%-50%) |
| Duffy (2016), Lancet Oncol. [37] | Screen detection of ductal carcinoma in situ and subsequent incidence of invasive interval breast cancers: a retrospective population-based study | Poisson regression | UK National Health Service Breast Screening Program (NHSBSP) | Not specified. | Not specified | Not specified | Not specified | 1 invasive interval cancer case is estimated to be avoided per 5 DCIS cases | Not quantified, but short | Not specified |
| de Koning (2006), Breast Cancer Res. [34] | Overdiagnosis and overtreatment of breast cancer: microsimulation modelling estimates based on observed screen and clinical data | Simulation model | Dutch pilot studies in Utrecht & Nijmegen; EORTC | Healthy cases can progress to pre-clinical screen detectable DCIS; pre-clinical screen detectable DCIS cases can progress to clinical DCIS or invasive breast cancer. | No | Yes | Not specified | To either invasive or clinical : 90% | Dutch pilot study suggests 2.8y with 99% sensitivity. Nijmegen data suggests 2.5y. EORTC trial suggests 5y with 40% sensitivity. | |
| Seigneurin (2011), BMJ. [36] | Overdiagnosis from non-progressive cancer detected by screening mammography: stochastic simulation study with calibration to population based registry data | Simulation model (Bayesian) | Isere, France incidence rates of breast cancer and DCIS (1991-2006) with some screening information | Healthy cases can progress to in situ; in situ cases can be non-progressive, progressive to clinical, and progressive to invasive; invasive cancer can also be non-progressive or progressive. | Yes | Yes | 6% non-progressive in situ (95%CI 0%--17%) | To invasive: 91% (95%CI: 84%-97%) | Not specified | Not specified |
| van Luijt (2016), Breast Cancer Res. [15] | The distribution of ductal carcinoma in situ (DCIS) grade in 4232 women and its impact on overdiagnosis in breast cancer screening | Simulation model | Nationwide network and registry of histopathology and cytopathology in the Netherlands (PALGA) data | Healthy cases can progress to different grades of DCIS; lower grade DCIS can progress to higher grade DCIS and vice versa; each grade of DCIS can progress to invasive cancer that are charaterized by tumor stage. | Yes | Yes | 4% low, 2% intermediate, and 1% for high grade DCIS | To invasive: 16% low, 31% intermediate, 53% for high grade DCIS | Not specified | Not specified |
Note: ranges present values estimated from different studies or data sources unless otherwise specified
These 10 studies used various data sources including different combinations of: i) data aggregated from population registries [15, 30, 35, 36], ii) observed national screening service program data [32, 33, 37], iii) detailed data from randomized screening trials [29, 31, 32, 34] and iv) estimates made from previously reported studies including studies of DCIS first overlooked at mammography [30, 36]. Generally, more detailed screening data makes it possible to deduce more realistic natural history models, fitting the model using data from different screening rounds and screening histories [29, 32]. In addition to the different data sources, three studies include all in situ lesions [29, 31, 36], while seven others only include DCIS [15, 30, 32-35, 37].
Parameters in the literature useful for DCIS modeling
The estimated proportion of DCIS progressing to invasive cancer varies widely in the literature (Table 5), mainly due to the available data, study-specific model assumptions, and different model structures. When all invasive breast cancer is assumed to go through a pre-clinical screen detectable DCIS state, the estimated progression rate of DCIS to invasive varies from 61% to 91% [15, 29, 31-34, 36]. When this assumption is not made, the estimated progression rate from DCIS to invasive varies from 20% to 24.4% [30, 35]. Some studies report a large proportion of progressive DCIS [31, 33, 34, 36], while other studies report that most DCIS cases do not progress to invasive cancer [30, 35]. When the proportion of progressive DCIS is reported by screening round, the subsequent screening rounds often reported smaller proportions of progressive DCIS [29, 32] compared to initial screening, as cases with a long sojourn time were diagnosed in earlier screening exams. High-grade DCIS cases have a larger proportion progressing to invasive than low-grade DCIS cases [15].
As for the mean sojourn time, when all invasive cancer are assumed to be screen detectable at a pre-clinical DCIS stage, the estimated mean sojourn time for progressive DCIS cases in the pre-clinical screen-detectable DCIS state are usually short varying from 1 month to 5 years [29, 31, 32, 34, 35]. On the other hand, the sojourn time estimates are much longer if it is assumed that only a small fraction of invasive cancers comes from pre-clinical screen-detectable DCIS [30]. The estimated mean sojourn time in pre-clinical screen-detectable DCIS state for DCIS cases that progress to clinical DCIS or regress is in typically longer than the mean sojourn time of DCIS cases that progress to invasive cancer [29, 32].
The mammography sensitivity for DCIS varies from 40% to 99% [29, 31, 33, 34]. The mean sojourn time for progressive DCIS in the pre-clinical screen detectable DCIS state tends to be smaller when mammography sensitivity is high. These variations reveal the uncertainty regarding the natural history of DCIS, highlighting the need and potential directions of CISNET modeling.
Discussion
While the CISNET models have generated relatively similar results and conclusions in most other respects, DCIS detection rates and overdiagnosis reveal more variation in results, with predicted DCIS incidence ranging from 25.8 – 32.3 per 1000 women age 40 followed over their lifetimes, and estimates of DCIS overdiagnosis ranging from 34%-72% for biennial screening from age 50 to 74 years. The large difference in the predicted amount of overdiagnosis of DCIS between models likely reflects the continued uncertainty about DCIS natural history, in particular the progression rates, which is also reflected in the results from other models described in the literature with reported progression rates varying from 20% to 91%.
In the literature outside of CISNET, several approaches have been proposed to model DCIS. The variations in model structure, assumptions and results make it challenging to deduce good overall estimates of key natural history parameters. Given the uncertainties in the DCIS models, a realistic approach to DCIS modeling is to adopt several plausible sets of model parameters and to evaluate a range of outcomes generated from the models. The CISNET models are well-suited for this type of analysis. CISNET models have the ability to project long-term implications for DCIS assumptions in terms of breast cancer outcomes such as life expectancy and overdiagnosis, and can thus assess how much early detection impacts breast cancer mortality. Also, moving forward, CISNET models are capable of utilizing multiple models and vary model parameters, to explore the impact of different DCIS assumptions on outcomes more systematically. In addition, both the impact of screening and treatment on DCIS-related outcomes can be systematically reviewed and compared. Although it remains to be seen to what extent these analyses will provide sufficiently accurate and consistent findings to inform clinical practice, the comparative modeling effort of the CISNET models will likely contribute to a greater understanding of DCIS.
Despite the large difference in the predicted amount of overdiagnosis of DCIS between models, all models indicated that the amount of overdiagnosis of DCIS is substantial (i.e., 34%-72% for biennial screening from age 50-74 years), indicating that per 1000 women followed over their lifetimes 9-19 are overdiagnosed with DCIS and the majority of those women will undergo treatment for their non-invasive disease. Almost all women (98%) diagnosed with DCIS undergo a surgical procedure [13, 38] and recent work found an increase in the utilization of mastectomy with reconstruction and contralateral risk-reducing mastectomy over time [39]. There was also an increase in the proportion of women undergoing adjuvant radiation therapy after surgery from 58.5% in 1998-1999 to 70% during 2006-2011 [39].
Modeling estimates might improve and results might converge when new data becomes available. A unique opportunity to improve DCIS natural history modeling comes from trials on active surveillance. Several trials are currently underway to evaluate active surveillance approaches for DCIS. In the UK, the Low Risk DCIS Trial (LORIS), is comparing surgical excision to active surveillance without excision [40, 41]. Similarly, the European Organisation for Research and Treatment of Cancer (EORTC) has started a trial on the management of low-risk DCIS (LORD), which is a randomized, multicenter, non-inferiority trial, between standard therapy approach versus active surveillance [42]. In the US a prospective, randomized trial, Comparing Operative to Medical Endocrine Therapy for low-risk DCIS (COMET), has recently been funded. Women diagnosed with low-risk DCIS will be randomized to receive either guideline-concordant care of surgical intervention, with or without radiation, or active surveillance of a mammogram every 6 months for 5 years. Patients in both trial arms are free to choose endocrine therapy. Also, in the US, several research networks, called cooperative groups, that conduct cancer clinical research primarily under the sponsorship of the NCI, are presently testing the use of neo-adjuvant hormonal therapy in postmenopausal women with ER-positive DCIS prior to surgery; those with a complete response based on magnetic resonance imaging (MRI) will not receive additional therapy. However, it will take a long time before results are available, e.g., for LORIS initial results are expected in 2020 and for LORD the results are not expected before 2029. When they do become available these data present a unique opportunity to validate models by comparing the model projections to the final trial data.
In the meantime, thus, before final results from these trials become available, the models can be used to evaluate which assumptions affect outcomes most. Also, data from several different sources might be used and combined to compare model outcomes and see what model structure and progression rates fit the data best. For example, data from different screening modalities can inform models, as the ability to detect DCIS varies across modalities. Screening ultrasound is less likely to detect DCIS compared to mammography in the small number of controlled experiments available that make this comparison, because ultrasound is unlikely to detect micro-calcifications. MRI may be more sensitive than mammography [43, 44] by detecting the pathophysiologic properties like basement membrane permeability in DCIS [45] perhaps explaining the tendency of MRI to detect intermediate and high grade DCIS more readily than mammography. By using a particular set of parameters and modelling different screening modalities, it might become possible to narrow down the range of plausible progression parameters. Furthermore, data by ER and grade might be used to refine the models. Subsequently, the updated and refined models can be used to simulate active surveillance strategies and quantify the predicted outcomes for subgroups of women varying by age and with DCIS varying by grade and ER status.
Until then, the model results consistently show a considerable amount of overdiagnosis of DCIS, which increases with more frequent screening. This indicates that women undergoing regular screening with a screen-detected DCIS are quite likely to be overdiagnosed. Thus, given the substantial amount of overdiagnosis estimated by the CISNET models for DCIS in general, the model results support the safety and value of observational trials for low-risk DCIS.
Acknowledgments
Support: This work was supported by the National Institutes of Health under National Cancer Institute Grants U01CA152958 and U01CA199218. ESB was funded in part by National Institutes of Health grant R01CA165229.
Contributor Information
Nicolien T. van Ravesteyn, Department of Public Health, Erasmus Medical Center, Rotterdam, the Netherlands
Jeroen J. van den Broek, Department of Public Health, Erasmus Medical Center, Rotterdam, the Netherlands
Xiaoxue Li, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute and in the Department of Biostatistics, Harvard TH Chan School of Public Health, Massachusetts, USA.
Harald Weedon-Fekjær, Center for Biostatistics and Epidemiology, Research Support Services, Oslo University Hospital, Oslo, Norway.
Clyde B. Schechter, Departments of Family and Social Medicine and Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA
Oguzhan Alagoz, Department of Industrial and Systems Engineering, University of Wisconsin-Madison, Madison, Wisconsin, USA.
Xuelin Huang, Department of Biostatistics, The University of Texas MD Anderson Cancer Center, Houston, USA.
Donald L. Weaver, Department of Pathology and Laboratory Medicine, University of Vermont, Burlington, Vermont, USA
Elizabeth S. Burnside, Department of Radiology, University of Wisconsin-Madison School of Medicine and Public Health, Madison, Wisconsin
Rinaa S. Punglia, Department of Radiation Oncology, Dana-Farber Cancer Institute and Harvard Medical School, Boston, Massachusetts, USA
Harry J. de Koning, Department of Public Health, Erasmus Medical Center, Rotterdam, the Netherlands
Sandra J. Lee, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute and Harvard Medical School, and in the Department of Biostatistics, Harvard TH Chan School of Public Health, Massachusetts, USA
References
- 1.Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, et al. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22-24, 2009. J Natl Cancer Inst. 2010;102(3):161–9. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 2.Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010(41):139–41. doi: 10.1093/jncimonographs/lgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102(3):170–8. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 4.Gangnon RE, Sprague BL, Stout NK, Alagoz O, Weedon-Fekjaer H, Holford TR, et al. The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer Epidemiol Biomarkers Prev. 2015;24(6):905–12. doi: 10.1158/1055-9965.EPI-14-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins LC, Tamimi RM, Baer HJ, Connolly JL, Colditz GA, Schnitt SJ. Outcome of patients with ductal carcinoma in situ untreated after diagnostic biopsy: results from the Nurses' Health Study. Cancer. 2005;103(9):1778–84. doi: 10.1002/cncr.20979. [DOI] [PubMed] [Google Scholar]
- 6.Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103(12):2481–4. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 7.Mokbel K, Cutuli B. Heterogeneity of ductal carcinoma in situ and its effects on management. Lancet Oncol. 2006;7(9):756–65. doi: 10.1016/S1470-2045(06)70861-0. [DOI] [PubMed] [Google Scholar]
- 8.Esserman L, Yau C. Rethinking the Standard for Ductal Carcinoma In Situ Treatment. JAMA Oncol. 2015;1(7):881–3. doi: 10.1001/jamaoncol.2015.2607. [DOI] [PubMed] [Google Scholar]
- 9.Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast Cancer Mortality After a Diagnosis of Ductal Carcinoma In Situ. JAMA Oncol. 2015;1(7):888–96. doi: 10.1001/jamaoncol.2015.2510. [DOI] [PubMed] [Google Scholar]
- 10.Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. J Natl Cancer Inst Monogr. 2010;2010(41):134–8. doi: 10.1093/jncimonographs/lgq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverstein MJ, Lagios MD. Choosing Treatment for Patients With Ductal Carcinoma In Situ: Fine Tuning the University of Southern California/Van Nuys Prognostic Index. JNCI Monographs. 2010;2010(41):193–6. doi: 10.1093/jncimonographs/lgq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerlikowske K, Molinaro A, Cha I, Ljung BM, Ernster VL, Stewart K, et al. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. Journal of the National Cancer Institute. 2003;95(22):1692–702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]
- 13.Sagara Y, Mallory MA, Wong S, Aydogan F, DeSantis S, Barry WT, et al. Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA surgery. 2015;150(8):739–45. doi: 10.1001/jamasurg.2015.0876. [DOI] [PubMed] [Google Scholar]
- 14.Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, et al. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105(10):701–10. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Luijt PA, Heijnsdijk EA, Fracheboud J, Overbeek LI, Broeders MJ, Wesseling J, et al. The distribution of ductal carcinoma in situ (DCIS) grade in 4232 women and its impact on overdiagnosis in breast cancer screening. Breast Cancer Res. 2016;18(1):47. doi: 10.1186/s13058-016-0705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Ravesteyn NT, Stout NK, Schechter CB, Heijnsdijk EA, Alagoz O, Trentham-Dietz A, et al. Benefits and harms of mammography screening after age 74 years: model estimates of overdiagnosis. J Natl Cancer Inst. 2015;107(7) doi: 10.1093/jnci/djv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alagoz O, Ergun MA, Cevik M, Sprague BL, Fryback DG, Gangnon R, et al. The University Of Wisconsin Breast Cancer Epidemiology Simulation Model: An Update (Medical Decision Making. 2016. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Li Y, Song J, Berry DA. Medical Decision Making. 2016. The MD Anderson CISNET Model for Estimating Benefits of Adjuvant Therapy and Screening Mammography for Breast Cancer: An Update. submitted. [Google Scholar]
- 19.Lee SJ, Li X, Huang H. Models for Breast Cancer Screening Strategies Updated for Ductal Carcinoma In Situ and Subgroups Medical Decision Making. 2016 submitted. [Google Scholar]
- 20.Mandelblatt JS, Near AM, Miglioretti DL, Munoz D, Sprague BL, Trentham-Dietz A, et al. Common Model Inputs in Collaborative Breast Cancer Medical Decision Making. 2016 doi: 10.1177/0272989X17700624. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schechter CB, Near AM, Jayasekera J, Chang Y, Mandelblatt JS. Structure, Function, and Applications of the Georgetown-Einstein (GE) Breast Cancer Simulation Model Medical Decision Making. 2016 doi: 10.1177/0272989X17698685. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Broek JJ, van Ravesteyn NT, Heijnsdijk EA, de Koning HJ. Estimating the effects of risk-based screening and adjuvant treatment using the MISCAN-Fadia continuous tumor growth model for breast cancer. Medical Decision Making. 2016 submitted. [Google Scholar]
- 23.Segnan N, Minozzi S, Armaroli P, Cinquini M, Bellisario C, González-Lorenzo M, et al. Epidemiologic evidence of slow growing, nonprogressive or regressive breast cancer: A systematic review. International journal of cancer. 2016;139(3):554–73. doi: 10.1002/ijc.30105. [DOI] [PubMed] [Google Scholar]
- 24.Burnside ES, Trentham-Dietz A, Kelcz F, Collins J. An example of breast cancer regression on imaging. Radiology case reports. 2006;1(2):27–37. doi: 10.2484/rcr.v1i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases. Linked To County Attributes - Total U.S., 1969-2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012. based on the November 2011 submission.
- 26.Larsen IK, Småstuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, et al. Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. European journal of cancer. 2009;45(7):1218–31. doi: 10.1016/j.ejca.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 27.Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, et al. Collaborative Modeling of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Ann Intern Med. 2016;164(4):215–25. doi: 10.7326/M15-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandelblatt JS, Cronin K, de Koning H, Miglioretti DL, Schechter CS, Stout N. Collaborative modeling of US breast cancer screening strategies. 2015 [Google Scholar]
- 29.Gunsoy NB, Garcia-Closas M, Moss SM. Modelling the overdiagnosis of breast cancer due to mammography screening in women aged 40 to 49 in the United Kingdom. Breast Cancer Res. 2012;14(6):R152. doi: 10.1186/bcr3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryser MD, Worni M, Turner EL, Marks JR, Durrett R, Hwang ES. Outcomes of Active Surveillance for Ductal Carcinoma in Situ: A Computational Risk Analysis. J Natl Cancer Inst. 2016;108(5) doi: 10.1093/jnci/djv372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan KH, Simonella L, Wee HL, Roellin A, Lim YW, Lim WY, et al. Quantifying the natural history of breast cancer. Br J Cancer. 2013;109(8):2035–43. doi: 10.1038/bjc.2013.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen MF, Tabar L, Vitak B, Smith RA, Chen HH, Duffy SW. Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. Eur J Cancer. 2003;39(12):1746–54. doi: 10.1016/s0959-8049(03)00260-0. [DOI] [PubMed] [Google Scholar]
- 33.de Gelder R, Heijnsdijk EA, van Ravesteyn NT, Fracheboud J, Draisma G, de Koning HJ. Interpreting overdiagnosis estimates in population-based mammography screening. Epidemiol Rev. 2011;33(1):111–21. doi: 10.1093/epirev/mxr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Koning HJ, Draisma G, Fracheboud J, de Bruijn A. Overdiagnosis and overtreatment of breast cancer: microsimulation modelling estimates based on observed screen and clinical data. Breast Cancer Res. 2006;8(1):202. doi: 10.1186/bcr1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozanne EM, Shieh Y, Barnes J, Bouzan C, Hwang ES, Esserman LJ. Characterizing the impact of 25 years of DCIS treatment. Breast Cancer Res Treat. 2011;129(1):165–73. doi: 10.1007/s10549-011-1430-5. [DOI] [PubMed] [Google Scholar]
- 36.Seigneurin A, Francois O, Labarere J, Oudeville P, Monlong J, Colonna M. Overdiagnosis from non-progressive cancer detected by screening mammography: stochastic simulation study with calibration to population based registry data. BMJ. 2011;343:d7017. doi: 10.1136/bmj.d7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffy SW, Dibden A, Michalopoulos D, Offman J, Parmar D, Jenkins J, et al. Screen detection of ductal carcinoma in situ and subsequent incidence of invasive interval breast cancers: a retrospective population-based study. Lancet Oncol. 2016;17(1):109–14. doi: 10.1016/S1470-2045(15)00446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baxter NN, Virnig BA, Durham SB, Tuttle TM. Trends in the treatment of ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2004;96(6):443–8. doi: 10.1093/jnci/djh069. [DOI] [PubMed] [Google Scholar]
- 39.Shiyanbola OO, Sprague BL, Hampton JM, Dittus K, James TA, Herschorn S, et al. Emerging trends in surgical and adjuvant radiation therapies among women diagnosed with ductal carcinoma in situ. Cancer. 2016;122(18):2810–8. doi: 10.1002/cncr.30105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis A, Fallowfield L, Rea D. The LORIS Trial: Addressing overtreatment of ductal carcinoma in situ. Clin Oncol (R Coll Radiol) 2015;27(1):6–8. doi: 10.1016/j.clon.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Francis A, Thomas J, Fallowfield L, Wallis M, Bartlett JM, Brookes C, et al. Addressing overtreatment of screen detected DCIS; the LORIS trial. Eur J Cancer. 2015;51(16):2296–303. doi: 10.1016/j.ejca.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Elshof LE, Tryfonidis K, Slaets L, van Leeuwen-Stok AE, Skinner VP, Dif N, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study. Eur J Cancer. 2015;51(12):1497–510. doi: 10.1016/j.ejca.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Warner E, Causer PA, Wong JW, Wright FC, Jong RA, Hill KA, et al. Improvement in DCIS detection rates by MRI over time in a high-risk breast screening study. The breast journal. 2011;17(1):9–17. doi: 10.1111/j.1524-4741.2010.01018.x. [DOI] [PubMed] [Google Scholar]
- 44.Lehman CD. Magnetic Resonance Imaging in the Evaluation of Ductal Carcinoma In Situ. JNCI Monographs. 2010;2010(41):150–1. doi: 10.1093/jncimonographs/lgq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhl CK. Why do purely intraductal cancers enhance on breast MR images? Radiology. 2009;253(2):281–3. doi: 10.1148/radiol.2532091401. [DOI] [PubMed] [Google Scholar]


