Abstract
Background
Methicillin-resistant Staphylococcus aureus (MRSA), a bacterial pathogen, is a predominant cause of skin and soft tissue infections in the United States. Swine-production facilities have been recognized as potential environmental reservoirs of MRSA. Previous research has shown an increased prevalence of asymptomatic MRSA colonization of swine farm workers, however the relationship between MRSA infection and community-level swine exposure is not well understood.
Methods
Using the Farm Location and Agricultural Production Simulator, we estimated the number of farms with greater than 1000 swine per residential ZIP code and the overall residential ZIP code-level swine density (swine/km2). We evaluated the association between these swine exposure metrics and both MRSA infections and colonization among all state-wide inpatient hospitalizations for skin and soft tissue infection from January 2008 through July 2011. Logistic regression models were used to analyze continuous and categorical exposures in relation to different MRSA outcomes after adjusting for confounding variables.
Results
For every increase in 100 swine/km2 within a residential ZIP code, the adjusted OR (aOR) for MRSA infection was 1.36 (95% CI: 1.28–1.45) and was 1.39 (95% CI: 1.33–1.45) comparing the highest swine exposure category to the referent which was characterized by no residential ZIP code swine exposure. For every additional large farm (i.e., >1000 swine) per ZIP code, the aOR for MRSA infection was 1.06 (95% CI: 1.04–1.07). The aOR for ZIP codes with any large farms compared to those with no large farms was 1.24 (95% CI: 1.19–1.29).
Conclusions
These publicly-available, ecological exposure data demonstrated positive associations between swine exposure measures and individual-level MRSA infections among skin and soft tissue inpatients. Future research can address measurement error related to these data by improving exposure assessment precision, specification of MRSA outcomes, and better characterization of specific environmental exposure pathways.
Keywords: methicillin-resistant Staphylococcus aureus (MRSA), antimicrobial resistance, swine, zoonosis, concentrated animal feeding operations (CAFOs)
1. Introduction1
Over the last two decades, Methicillin-resistant Staphylococcus aureus (MRSA) has garnered increased attention, with a shift from a largely nosocomial etiology, affecting individuals with preexisting health conditions to that of community origin, affecting populations with limited healthcare exposures (Grundmann et al., 2006; Levy, 2010; Mediavilla et al., 2012). The first cases of community-acquired MRSA (CA-MRSA) were documented in the 1990s (Herold et al., 1998) and have since increased dramatically (Dukic et al., 2013). Because asymptomatic MRSA colonization is a risk factor for MRSA infection (Davis et al., 2004), active surveillance for MRSA carriers is a potential preventive practice in hospital settings (Diekema and Edmond, 2007). Likely due to improvements in hospital surveillance and biosecurity, hospital acquired MRSA (HA-MRSA) cases have decreased, leading to a higher relative proportion of CA-MRSA overall (Dantes et al., 2013; Lowy, 2013; David e al., 2014). This change in incidence and disease dynamics highlight the need to identify and characterize community-level risk factors for MRSA colonization and infection.
Swine production facilities have been identified as environmental reservoirs of MRSA (Silbergeld et al., 2008b; Gilchrist et al., 2007). Although the first livestock-associated (LA)-MRSA strain, ST398, was traced to swine farms and has been well researched (Voss et al., 2005; van Loo et al, 2007), other potential LA-MRSA strains have been identified more recently (Monecke et al., 2011; Molla et al., 2012; Frana et al., 2013). Previous studies showed an elevated prevalence of MRSA colonization in swine farmers and swine veterinarians (Smith et al., 2009; van Cleef et al., 2011; van Cleef et al, 2014; Frana et al., 2013; Huang et al., 2014; Nadimpalli et al., 2015; Rinsky et al., 2013; Smith et al., 2013). The spread of antibiotic resistance has been a major concern since the introduction of subtherapeutic antibiotics for growth promotion into conventional livestock production systems (Starr and Reynolds, 1951; Levy et al., 1976). Exposing microorganisms to consistent subtherapeutic levels of antibiotics may accelerate the formation of antibiotic resistant bacteria (Akwar et al., 2007; Silbergeld et al., 2008a). These bacteria can colonize and infect swine, contaminate their containment facilities and be excreted in waste. Further selective pressure by excreted and dispersed residual antibiotics (Hamscher et al., 2003; Dolliver and Gupta, 2008; Manzetti and Ghisi, 2014) and horizontal transfer of antibiotic resistant genes (Witte, 2000; Gilchrest et al., 2007; Marshall and Levy, 2011) can result in additional proliferation of both exogenous and endogenous antibiotic resistant bacteria in swine waste and the environment.
The shift of swine production to larger confinement operations has increased the volume of waste and may amplify the selection of antibiotic resistant bacteria and antibiotic resistant genes (Silbergeld et al., 2008b). Swine facility-level fate and transport studies have shown MRSA colonization of buildings and immediate surroundings (Leedom Larson et al., 2011) and the environmental dispersion of MRSA and their genetic markers via aerosolization and deposition (Chapin et al., 2005; Gibbs et al., 2006; Schulz et al., 2012), spills and discharges from waste storage, direct land application for fertilizing crops (Chee-Sanford et al., 2009; Heuer et al., 2011), runoff into surface waters (Pruden et al., 2012), and groundwater leaching (Chee-Sanford et al., 2001). While previous research shows that even indirect exposure may increase MRSA colonization risk among neighboring communities (Bisdorff et al., 2011; van Reijn et al., 2014; Feingold et al., 2012; Carrel et al., 2014), the relative importance of different environmental media and specific exposure pathways is incompletely understood (Appendix A, Fig. A.2).
To better understand how swine production may contribute to MRSA outcomes, we analyzed routinely-collected administrative hospitalization data and national swine inventory data to evaluate the association between community-level swine exposure and individual MRSA infection and colonization among skin and soft tissue infection (SSTI) inpatients from 2008 to 2011 in Illinois. We hypothesized that there would be increased risk of All MRSA infections and colonization for individuals living in ZIP codes with increasing numbers of swine/km2 and increasing numbers of large-scale facilities housing greater than 1000 swine. Additionally, we hypothesized that swine exposure would be more strongly associated with CA-MRSA compared to HA-MRSA.
2. Methods
2.1. Case ascertainment and outcome definitions
We utilized the Illinois Hospital Association’s (IHA) Hospital Discharge Database (IHD) to identify MRSA infection and colonization among all state-wide SSTI inpatient hospitalizations from January 2008 through July 2011. The IHA membership includes 97% of Illinois hospitals; the remaining nonmember hospitals treat 7% of all Cook County hospitalizations and are located in Cook County, a primarily urban area encompassing the city of Chicago, IL (Illinois Department of Public Health, 2015). Records stored in this database include all diagnoses identified and procedures reported using the 9th revision of the International Classification for Diseases coding (ICD-9) for up to 24 diagnostic positions. Although residential address is not available, the database included several relevant individual-level covariates, including ZIP code and county of residence, admission date, age, sex, type of health insurance and other comorbid risk factors (Appendix A, Table A.2).
Study inclusion criteria required records to contain ICD-9 codes 680–686, indicating an SSTI, and to have a residential ZIP code and Federal Information Processing Standard (FIPS) County code within Illinois. Presence of MRSA was determined primarily using specified codes for MRSA infection, 041.12, 038.12, or 482.42, which were introduced to ICD-9 in the 2008 update (CDC/NCHS, 2013). To account for any outdated coding, we also included records with the code assignment for MRSA infection used prior to 2008, which requires both one code for Staphylococcus aureus infection (038.11, 482.41 or 041.11) along with the supplementary V09.0 code indicating resistance to penicillins (CDC/NCHS, 2013). The primary supplementary code used to identify MRSA colonization was V02.54. This specified colonization code was added to ICD-9 in 2008; thus, we also included the pre-2008 unspecified colonization code V02.59. For the All MRSA analysis, MRSA-positive SSTI inpatients were compared to those without MRSA coding. For the MRSA-colonization analysis, MRSA-positive SSTI with MRSA colonization coding were compared to those without colonization coding.
2.1.1. HA- and CA-MRSA coding designation
To assess the difference in risk from swine exposure for HA- and CA-MRSA, we identified cases following a modified version of the Centers for Disease Control and Prevention’s (CDC) definition for HA-MRSA. CDC case definition defines a community-acquired (CA) MRSA to be lacking the elements of a classical hospital acquired (HA) infection that includes the following: the appearance of MRSA more than 48 h after hospital admission; a history of hospitalization, surgery, dialysis, or residence in a long-term care facility within 1 year of the MRSA infection; the presence of an indwelling catheter or a percutaneous device; or previous MRSA infection (Fridkin et al., 2005). Given that we did not have the ability to account for full patient history and specific time windows for diagnosis and clinical events, these components of the definition were not included in our analysis (Fridkin et al., 2005; Fig. A.1). Despite the incomplete patient history, inpatients were considered to have previous healthcare exposure if they were transferred or referred from another health care facility, or if they had a supplementary ICD-9 code indicating they were admitted for postoperative or other aftercare at any time during the study period (Table A.1). MRSA-positive SSTI inpatients with previous healthcare exposure were designated as HA-MRSA. If the MRSA infection was not present on admission, it was considered HA-MRSA if the record included procedural codes (excluding nonoperative and noninvasive therapeutic procedures) or indicated that the patient had undergone dialysis, an artificial opening, or an indwelling device during the hospitalization. If there was no evidence of previous healthcare exposure, inpatients with a MRSA infection that was present on admission were considered CA-MRSA. Following the CDC definition, any MRSA case that was not defined as HA-MRSA was assumed to be CA-MRSA. In our infection-specific analysis among All MRSA cases, HA-MRSA cases were included along with CA-MRSA cases.
2.2. Community-level demographic and land use data
Covariate data at the ZIP code-level was compiled from the American Community Survey 5-year estimates for 2007 to 2011 through the American FactFinder data repository (U.S. Census Bureau, 2015). To assess potential confounding by socioeconomic status, we used the socioeconomic deprivation index developed by Schwartz et al. (2011), which includes the following ZIP code-level covariates: percent of the population with less than high school education; percent of the civilian population not in the labor force; percent of the population below the federal poverty level; percent of the population receiving public assistance; percent of the civilian population who are unemployed; and percent of households with no vehicle access. Standardized Z-scores were calculated for each variable, which were summed to arrive at the deprivation index value. Positive numbers indicate increased deprivation and negative numbers indicate areas with high relative affluence. We also assessed additional covariates outside this index, including housing occupancy, median household income and individuals living in group quarters.
Rural/urban status was determined at the county level using USDA’s Economic Research Service’s 2013 Rural-Urban Continuum Code (RUCC) designations (USDA/ERS, 2013). RUCC values range between 1 and 9 and are based on county population characteristics and adjacency to a metropolitan area. Consistent with previous research (e.g., Messer et al, 2010), RUCC codes were combined into four categories. A value of 1 indicates “metropolitan urbanized” (RUCC 1, 2, 3), 2 indicates “nonmetropolitan urbanized” (RUCC 4, 5), 3 indicates “less urbanized” (RUCC 6, 7) and 4 indicates “thinly populated” (RUCC 8, 9).
2.3. Exposure assessment
Our primary analyses focused on ZIP code-level swine exposure, the finest residential resolution available for inpatients. Swine exposure was calculated as both a ZIP code area density (swine/km2) and as the number of facilities in a ZIP code housing >1000 swine (i.e., large farms). To derive the ZIP code-level swine density estimate, we utilized the web-based, publicly-available Farm Location and Agricultural Production Simulator (FLAPS) (Burdett et al., 2015) that disaggregates county and state-level U.S. Department of Agriculture Census of Agriculture data and simulates the locations and inventories of individual swine farms, using a probability surface that predicts the number and size of swine farms. We ran 100 FLAPS simulations for years 2007 and 2012, the most recent years for which Census of Agriculture data were available. Using ArcGIS 10.2 (ESRI, 2014) and the corresponding year’s U.S. Census Topologically Integrated Geographic Encoding and Referencing (TIGER) ZIP code shapefile (U.S. Census Bureau, 2014), we aggregated the total farms, total inventory, and number of large farms in each ZIP code. Each of the 100 simulation ZIP code aggregate values were averaged to obtain a single value for each ZIP code. Data were imputed across years between 2007 and 2012 using a smooth linear join interpolation, via the SAS Expand procedure, which fits a continuous curve to the data by connecting successive straight line segments (SAS/ETS® 13.2 User’s Guide, 2013). The final density estimate was calculated using the imputed values divided by the ZIP code area in km2. ZIP code swine estimates were aggregated to the county level and compared to Census of Agriculture estimates for evaluation of the method. County exposures were developed and analyzed separately and then compared to risks calculated for ZIP code exposures (Appendix A, Table A.2).
2.4 Statistical analysis
All data management and statistical analyses were completed using version 9.4 of the SAS system for Windows and ArcGIS desktop version 10.2.2 (SAS, 2013; ESRI, 2014). We used logistic regression to evaluate whether an increase in 100 swine/km2 within the residential ZIP code of an SSTI inpatient would increase their log likelihood for having a MRSA positive infection, or be colonized by MRSA, and assessed if there was a difference in these associations comparing CA-MRSA to HA-MRSA. We also examined whether an increase in one additional large swine farm per ZIP code increased the log likelihood for the same outcomes. We also evaluated these outcomes using binned ZIP code density and large operations into five exposure categories. The referent group for these categories included all individuals with no residential ZIP code-level swine exposure, while the other categories were created by evenly dividing the remaining study population into four exposure quartiles. In a separate analysis, the highest non-zero quartile was further divided into 50 swine/square km groupings up to > 150 swine/square km for a focused analysis of the highest exposures (Table B.3).
Potential confounding variables and effect measure modifiers were initially gleaned from the available literature and used to develop a conceptual diagram (Fig. A.2). Individual-level covariates examined as confounders included year and month of admission, chronic obstructive pulmonary disease (COPD), asthma, age (<1, ≥1 and <5, ≥5 and <10, ≥10 and <25, ≥25 and <50, ≥50 and <75, ≥75), sex, race (Black, White, Other), ethnicity (Hispanic, non-Hispanic), public insurance recipient status, and tobacco use. Community-level covariates included median household income and socioeconomic deprivation index (Schwartz et al., 2011). These variables were added individually to univariate logistic regression models as part of the confounding analysis. Variables that changed the unadjusted slope (i.e. beta coefficient) associated with swine exposure by greater than 10% were considered confounders. Confounders identified for at least one of the final models included sex, age, race, ethnicity, public insurance recipient status, asthma, COPD, tobacco use, and the socioeconomic deprivation index.
We undertook additional analyses stratified by the four Charlson comorbidity index scores (Charlson et al., 1987) ranging from “not-ill” to “moribund” and a MRSA-specific comorbidity index scoring system to assess the accuracy of our CA-HA designations and to examine the influence of comorbidity status on our study results (Appendices A.2, B.1 and B.4). We also stratified our analysis by RUCC to assess the potential for urbanicity and population density to modify the odds ratio for MRSA and hog exposure through population-driven transmission dynamics or land-use-related factors. Finally, sensitivity analyses were conducted to assess the impact of a different spatial scale of the continuous ZIP code exposure metric by analyzing county-level swine density (Appendix B.2), derived from published Agricultural Census data and National Agricultural Statistics Service survey data available through the Quickstats 2.0 data repository (USDA/NASS, 2015).
3. Results
3.1. Descriptive statistics for exposures and outcomes
The 2008–2011 hospitalization database included 219,200 records for SSTI inpatients in Illinois, of which 3982 (1.8%) were excluded due to missing ZIP codes. Among the 215,218 remaining SSTI inpatients, 25,644 (11.9%) were diagnosed with MRSA positive infections (i.e., All MRSA), as identified by ICD-9 code, and 3783 (1.7%) SSTI inpatients identified as colonized by MRSA by ICD-9 code. After interpolation of FLAPS model ZIP code-level averages between the Census of Agriculture years 2007 and 2012, 303 (21.9%) of the 1384 ZIP codes had no swine production, accounting for 46% of the study population. The average residential ZIP code-level exposure for non-MRSA SSTI inpatients was 8.9 (±20.7) swine/km2 and 0.3 (±1.0) large farms, compared to other MRSA outcome groups (mean density range: 10.1–10.7, mean number large farm range: 0.3–0.4) (Table 1). The All MRSA group resided in ZIP codes with statistically significantly higher average swine density (p-value <0.001).
Table 1.
Study characteristics for skin and soft tissue infection inpatients from 2008 to 2011 in Illinois.
| COVARIATE | No MRSA | All MRSA†ǂφ | HA-MRSA†ǂ | CA-MRSA†γφ | MRSA COL†φ |
|---|---|---|---|---|---|
| Individual-level n (%) | |||||
| SSTI inpatients | 189,574 | 25,644 | 5199 | 20,445 | 3783 |
| Sex | |||||
| Male | 95,973 (50.6) | 13,632 (53.2)* | 2729 (52.5) | 10,903 (53.3)* | 1867 (49.4) |
| Age | |||||
| <1 | 4162 (2.2) | 531 (2.1) | 23 (0.4) | 508 (2.5) | 32 (0.9) |
| ≥1 and <5 | 3051 (1.6) | 1132 (4.4) | 25 (0.5) | 1107 (5.4) | 48 (1.3) |
| ≥5 and <10 | 1732 (0.9) | 251 (1.0) | 5 (0.1) | 246 (1.2) | 16 (0.4) |
| ≥10 and <25 | 8397 (4.4) | 1940 (7.6) | 117 (2.3) | 1823 (8.9) | 140 (3.7) |
| ≥25 and <50 | 41,876 (22.1) | 7556 (29.5) | 1093 (21.0) | 6463 (31.6) | 819 (21.7) |
| ≥50 and <75 | 80,552 (42.5) | 9239 (36.0) | 2489 (47.9) | 6750 (33.0) | 1551 (41.0) |
| ≥75 | 49,804 (26.3) | 4995 (19.5) | 1447 (27.8) | 3548 (17.4) | 1177 (31.1) |
| Race | |||||
| Black | 31,163 (16.4) | 5102 (19.9) | 873 (16.8) | 4229 (20.7) | 559 (14.8) |
| White | 136,929 (72.2) | 17,676 (68.9) | 3783 (72.8) | 13,893 (68.0) | 2949 (78.0) |
| Other | 21,482 (11.3) | 2866 (11.2) | 543 (10.4) | 2323 (11.4) | 275 (7.3) |
| Ethnicity, Hispanic | 17,583 (9.3) | 2277 (8.8)* | 424 (8.2)* | 1853 (9.1) | 246 (6.5)* |
| Insurance Beneficiary | 120,664 (63.7) | 15,551 (60.6)* | 3766 (72.4)* | 11,785 (57.6)* | 2749 (72.8)* |
| Asthma | 14,252 (7.5) | 2126 (8.3)* | 438 (8.4)* | 1688 (8.3)* | 358 (9.5)* |
| COPD | 38,879 (20.5) | 4940 (19.3)* | 1276 (24.5)* | 3664 (17.9)* | 1015 (26.8)* |
| Tobacco use | 36,178 (19.1) | 5269 (20.6)* | 1145 (22.0)* | 4124 (20.2)* | 836 (22.1)* |
| County-level, n (%) | |||||
| RUCC | |||||
| 1, Metropolitan urbanized | 166,454 (87.8) | 21,662 (84.5) | 4408 (84.8) | 17,254 (84.4) | 3343 (88.4) |
| 2, Nonmetropolitan urbanized | 10,782 (5.7) | 1906 (7.4) | 396 (7.6) | 1510 (7.4) | 190 (5.0) |
| 3, Less urbanized | 11,609 (6.1) | 1966 (7.7) | 372 (7.2) | 1594 (7.8) | 241 (6.4) |
| 4, Thinly populated | 729 (0.4) | 110 (0.4) | 23 (0.4) | 87 (0.4) | 9 (0.2) |
| ZIP code-level, mean (Std Dev) | |||||
| Socioeconomic deprivation index†† | 1.7 (4.1) | 2.0 (4.1)* | 1.7 (4.0) | 2.1 (4.2)* | 1.2 (3.8)* |
| Median household Income ($1,000) | 56.8 (20.9) | 53.9 (19.7)* | 55.40 (20.1) | 53.5 (19.4)* | 58.9 (22.1)* |
| Density (swine/km2) | 8.9 (20.7) | 10.1 (21.4)* | 10.3 (21.7)* | 10.1 (21.3)* | 10.7 (23.2)* |
| Farms >1000 swine (N) | 0.3 (0.9) | 0.4 (1.0)* | 0.4 (1.0) | 0.3 (1.0)* | 0.4 (1.1)* |
| Aggregate ZIP code-level, n (%) | |||||
| Density Categories (swine/km2) | |||||
| 0 | 86,602 (45.8) | 10,613 (41.5) | 2174 (41.9) | 8439 (41.4) | 1595 (42.4) |
| 0.00004–1.48 | 26,197 (13.9) | 3155 (12.3) | 595 (11.5) | 2560 (12.6) | 529 (14.1) |
| 1.49–7.69 | 25,553 (13.5) | 3945 (15.4) | 776 (15.0) | 3169 (15.6) | 536 (14.2) |
| 7.70–19.90 | 25,353 (13.4) | 3956 (15.5) | 821 (15.8) | 3135 (15.4) | 506 (13.4) |
| 19.91–326.46 | 25,358 (13.4) | 3900 (15.3) | 820 (15.8) | 3080 (15.1) | 599 (15.9) |
| Farms >1000 swine | |||||
| 0 | 165,500 (87.5) | 21,879 (85.6) | 4428 (85.4) | 17,451 (85.6) | 3224 (85.6) |
| ≥1 | 23,563 (12.5) | 3690 (14.4) | 758 (14.6) | 2932 (14.4) | 541 (14.4) |
CA, Community-Acquired; COL, Colonization; COPD, Chronic Obstructive Pulmonary Disease; HA, Hospital Acquired; MRSA, Methicillin Resistant Staphylococcus Aureus; RUCC, Rural Urban Continuum Code; SSTI, Skin and Soft Tissue Infection; Std Dev, standard deviation.
Statistically significant difference compared to non-MRSA, at the α <0.05 level, using student’s T-test (means) and Chi-square test
Statistically significant difference in age distribution compared to non-MRSA, at the α <0.05 level, using Kolmogorov-Smirnov test
Statistically significant difference in race categories compared to non-MRSA, at the α <0.05level, using Chi-square test
Statistically significant difference in RUCC categories compared to non-MRSA at the α <0.05 level, using Chi-square test
Socioeconomic deprivation index was developed and described by Schwartz et al., 2011, the value reflects the sum of standardized z-scores of each component
3.2. Study characteristics for non-MRSA SSTI’s, MRSA infection and MRSA-colonization inpatients
CA-MRSA accounted for 80% of all MRSA infections (n=20,445). The All MRSA group and the CA-MRSA subgroup were also more often male, black, asthmatic, and tobacco users and were less often Hispanic, recipients of public insurance, had COPD, lower income, higher ZIP code socioeconomic deprivation index and higher swine exposures on average compared to non-MRSA SSTI inpatients (Table 1). In contrast, the age distribution of HA-MRSA and MRSA-colonization inpatients appeared similar to non-MRSA SSTI inpatients, with more inpatients in the two oldest age categories. HA-MRSA inpatients were similar to the MRSA-colonization group in every aspect described, except race and asthma, which were similar to distributions among the All MRSA infection population. A smaller proportion of the All MRSA group lived in RUCC 1 (most urban) areas, while more lived in RUCC 2 and 3 areas compared to the non-MRSA group (Table 2). While All MRSA and CA-MRSA inpatients were generally healthier (37% and 43% “not ill” Charlson Score) compared to the non-MRSA group (29% not ill), MRSA-colonized and HA-MRSA inpatients were less healthy (21% and 11%) (Appendix B.1).
Table 2.
Continuous Swine Exposures by Rural Urban Continuum Code.
| RUCC category | n | Density (swine/km2)
|
Large farms (n)
|
||
|---|---|---|---|---|---|
| Mean (Std Dev) | Range | Mean (Std Dev) | Range | ||
| 1, Metropolitan urbanized | 188,116 | 5.76 (17.29) | 0–247.26 | 0.12 (0.58) | 0–9 |
| 2, Nonmetropolitan urbanized | 12,688 | 32.48 (26.96) | 0–163.78 | 1.68 (1.70) | 0–8 |
| 3, Less urbanized | 13,575 | 33.13 (27.50) | 0–326.46 | 1.62 (1.78) | 0–11 |
| 4, Thinly populated | 839 | 21.77 (20.11) | 0–95.78 | 0.57 (0.73) | 0–2.6 |
RUCC, Rural Urban Continuum Code; Std Dev, standard deviation.
3.3. Swine exposure and MRSA infection and colonization among SSTI inpatients
Unadjusted odds ratios (ORs) were similar to adjusted ORs (aORs) irrespective of exposure metric, although the unadjusted OR for the swine density measure for the All MRSA SSTI group was slightly lower (1.27, data not shown) than the aOR (1.36). All MRSA group aORs compared to non-MRSA SSTI inpatients for every additional 100 swine/km2 (aOR=1.36, 95% CI: 1.28–1.45) were higher than for each additional large farm per ZIP code (aOR=1.06, 95% CI: 1.04–1.07) (Table 3). Associations similar in magnitude were detected for MRSA colonization and both the continuous swine density (aOR=1.22, 95% CI: 1.06–1.40) and large farm metrics (aOR=1.06, 95% CI: 1.03–1.09). We saw no evidence of an increased association for CA-MRSA compared to HA-MRSA with either continuous exposure metric (aORs=0.99), both CA- and HA-MRSA exhibited the same general relationship to continuous swine density exposures, represented by the All MRSA aORs.
Table 3.
Adjusted ORs (aORs) for All MRSA group compared to non-MRSA SSTI inpatients, MRSA-colonized compared to non-MRSA-colonized SSTI inpatients, and CA-MRSA compared to HA-MRSA for residential ZIP code swine exposures.
| Exposure | Outcome
|
|||||
|---|---|---|---|---|---|---|
| All MRSA†
|
MRSA Colonizationˠ
|
CA-MRSAǂ
|
||||
| Cases (n) | aOR (95% CI) | Cases (n) | aOR (95% CI) | Cases (n) | aOR (95% CI) | |
| Density, swine/km2 | ||||||
| 0 | 10,613 | 1.00 | 1595 | 1.00 | 8439 | 1.00 |
| 0.00004–1.48 | 3155 | 1.07 (1.02–1.11) | 529 | 0.98 (0.88–1.09) | 2560 | 1.14 (1.03–1.27) |
| 1.49–7.69 | 3945 | 1.33 (1.27–1.38) | 536 | 1.01 (0.91–1.11) | 3169 | 1.04 (0.94–1.15) |
| 7.70–19.90 | 3956 | 1.39 (1.34–1.45) | 506 | 0.93 (0.84–1.04) | 3135 | 0.99 (0.90–1.10) |
| 19.91–326.46 | 3900 | 1.39 (1.33–1.45) | 599 | 1.09 (0.99–1.21) | 3080 | 0.99 (0.90–1.09) |
| Continuous* | 25,569 | 1.36 (1.28–1.45) | 3765 | 1.22 (1.06–1.40) | 20,383 | 0.99 (0.85–1.14) |
| Farms >1000, n/ZIP | ||||||
| 0 | 165,500 | 1.00 | 3224 | 1.00 | 17,451 | 1.00 |
| ≥1 | 23,563 | 1.24 (1.19–1.29) | 541 | 1.08 (0.99–1.95) | 2932 | 0.99 (0.91–1.09) |
| Continuous** | 1.06 (1.04–1.07) | 1.06 (1.03–1.09) | 0.99 (0.96–1.02) | |||
aOR, adjusted odds ratio; CA, Community-Acquired; CI, confidence interval; HA, Hospital Acquired; MRSA, Methicillin Resistant Staphylococcus Aureus; SSTI, Skin and Soft Tissue Infection.
Per 100 swine/km2 increase
Per 1 large farm increase
Both Exposure Metrics: Adjusted for age, race, and socioeconomic deprivation index
Density: Adjusted for race, ethnicity, and socioeconomic deprivation index; Farms >1000: Adjusted for race, ethnicity, socioeconomic deprivation index, asthma, and COPD
Density: Adjusted for age, race, ethnicity, socioeconomic deprivation index, COPD, public insurance recipient status and tobacco use; Farms >1000: Adjusted for age, race, ethnicity, socioeconomic deprivation index, COPD, and public insurance recipient status
When the swine density exposure was evaluated categorically, increased aORs were detected for the All MRSA SSTI group compared to non-MRSA SSTI inpatients but were less consistent for the MRSA-colonization SSTI group compared to noncolonized SSTI inpatients (Table 3). Associations similar in magnitude were found for the All MRSA SSTI group for the 3rd through 5th swine density category exposures (aOR range=1.33–1.36). Subdivision of the 5th exposure category into four smaller categories (19.91–50, >50–100, >100–150, >150) revealed no dose-response pattern for the all MRSA SSTI group and inconsistent results for CA-MRSA compare to HA-MRSA. For the MRSA colonization group, increased aORs were detected at >50-100 swine/km2 (aOR=1.22, 95% CI: 1.03–1.43) and from >100-150 swine/km2 (aOR=1.53, 95%CI: 1.18–1.99). No association was detected at the highest exposure category (aOR=0.61, 95% CI: 0.32–1.23) but this category was limited by a small sample size (n=8 cases) (Appendix B.3).
3.4. Effect measure modification for continuous exposures
We saw no evidence of a gradient in estimated risk across levels of urbanicity and population density as indicated by RUCC levels. Associations were detected for the All MRSA SSTI group compared to non-MRSA SSTI inpatients within RUCC 1 for both swine density (aOR=1.25, 95% CI 1.16–1.36) and large farms (aOR=1.03, 95% CI: 1.01–1.06) (Table 4). The aORs for MRSA-colonized compared to noncolonized SSTI inpatients were similar in the metropolitan urbanized, nonmetropolitan urbanized and less urbanized categories for swine density and (aOR range: 1.31–1.72), but could not be estimated for the thinly populated regions due to too few colonization cases. The aORs for large farms and MRSA-colonization were similar to unstratified results for all RUCC categories (aOR range: 0.98–1.12).
Table 4.
Adjusted ORs (aORs) for All MRSA group compared to non-MRSA SSTI inpatients, MRSA-colonized compared to non-MRSA-colonized SSTI inpatients, and CA-MRSA compared to HA-MRSA for continuous residential ZIP code exposure metrics, stratified by RUCC.
| RUCC stratification group | Outcome
|
|||||
|---|---|---|---|---|---|---|
| All MRSA† | MRSA Colonizationˠ | CA-MRSAǂ | ||||
|
| ||||||
| Cases (n) | (aOR, 95% CI) | Cases (n) | (aOR, 95% CI) | Cases(n) | (aOR, 95% CI) | |
| Density, 100 swine/km2 | ||||||
| 1, Metropolitan urbanized | 21,588 | 1.25 (1.16–1.36) | 3323 | 1.31 (1.11–1.54) | 17,192 | 0.93 (0.77–1.24) |
| 2, Nonmetropolitan urbanized | 1896 | 0.65 (0.54–0.78) | 190 | 1.72 (1.04–2.84) | 1502 | 1.22 (0.77–1.93) |
| 3, Less urbanized | 1957 | 1.10 (0.92–1.31) | 240 | 1.49 (0.96–2.29) | 1587 | 0.90 (0.59–1.37) |
| 4, Thinly populated | 109 | 0.54 (0.13–2.25) | 9 | N/E | 86 | N/E |
| Farms >1000, n/ZIP | ||||||
| 1, Metropolitan urbanized | 21,588 | 1.03 (1.01–1.06) | 3323 | 1.12 (1.07–1.17) | 17,192 | 0.97 (0.92–1.03) |
| 2, Nonmetropolitan urbanized | 1896 | 0.93 (0.90–0.96) | 190 | 1.08 (1.00–1.72) | 1502 | 1.02 (0.95–1.10) |
| 3, Less urbanized | 1957 | 1.01 (0.98–1.03) | 240 | 1.09 (1.03–1.16) | 1587 | 0.98 (0.92–1.04) |
| 4, Thinly populated | 109 | 0.91 (0.66–1.25) | 9 | 0.99 (0.40–2.45) | 86 | 1.13 (0.56–2.28) |
aOR, adjusted odds ratio; CA, Community-Acquired; CI, confidence interval; HA, Hospital Acquired; MRSA, Methicillin Resistant Staphylococcus Aureus; N/E, not able to estimate; RUCC, Rural Urban Continuum Code; SSTI, Skin and Soft Tissue Infection.
We saw no evidence of a gradient in estimated risk by comorbidity as measured by the Charlson Index. Similar aORs were detected for MRSA infection when stratifying by Charlson Score for both exposure metrics, although a slightly larger aOR was found for the unhealthiest Charlson group. CA-MRSA compared to HA-MRSA showed larger aORs for healthiest and unhealthiest groups for both indices (Appendix B, Table B.4).
4. Discussion
We detected a consistent, statistically significant association between ZIP code-level swine exposure and both All MRSA and MRSA-colonization SSTI inpatient hospitalizations in Illinois from 2008 to 2011. The only other study identified on swine exposure and MRSA infection by Casey et al. (2013) may provide insight regarding potential environmental pathways, as the aOR for highest versus lowest quartile of residential distance to manure application site (aOR=1.38, 95% CI: 1.13–1.69) was larger than that of the inverse weighted distance to swine facilities (aOR=1.25, 95% CI: 0.99–1.58). The range of distance to application sites in their study was 2.6–7.5 km and most of the operations in their study applied manure off site. This suggests the potential importance of ambient, non-point source exposures and supports considering a density metric as we have included in this analysis. Despite using coarser, publicly-available data at the ZIP code-level, the magnitudes of our categorical exposure estimates are comparable to the estimates of Casey et al. (2013). We saw similar effect estimates for ZIP code-level and county-level swine density estimates (Appendix B.2). Unlike the increased ORs detected for large farms based on the ZIP code-level metric, we saw no associations at the county level. This may be due to the fact that the distribution of swine is not homogenous within a given county with variations in the number of swine at different locations, creating pockets of high and low exposures. Since conceptually, large farms act as a potential point-sources, the county scale may be less likely to capture their impact compared to the ZIP code.
Although our study suggests that the association between MRSA colonization and continuous swine exposure is similar to that of All MRSA and continuous swine exposure, we are aware that this may not be attributable to the same exposure pathway(s). Few existing studies have examined the relationship between swine exposure and non-occupational MRSA colonization; these studies generally focused on LA strains colonizing a small sample of individuals with and without direct contact with swine (Cuny et al., 2009; van Cleef et al. 2011; Bisdorff et al., 2011; van Rijen et al., 2014; Feingold et al., 2012). Using a swine density metric comparable to ours for their non-occupational colonization assessment, Feingold et al. (2012) reported that a log increase in municipal swine density resulted in an increased odds (1.37; 95% CI 1.01–1.87) for colonization with LA-MRSA compared with other MRSA strains. Because our data preclude an examination of specific MRSA strains in this analysis, it is not possible to determine whether the observed increase in risk of colonization is attributable to person-to-person transmission of LA-MRSA identified on many Illinois swine farms (Smith et al., 2009; Smith et al., 2013), or due to general increased exposure to environmental antibiotics, antibiotic-resistant bacteria, and antibiotic-resistant genes attributable to swine production, or due to other factors.
While the continuous swine exposure demonstrated similar associations for both All MRSA infection and MRSA-colonization, differences were noted between the two outcomes based on the categorical analysis. ZIP codes with the presence of one or more large farms resulted in an aOR for MRSA-colonization similar in magnitude to the highest density non-zero quartile (compared to their referents of 0); this suggests that large operations could be driving the overall colonization risk in this study. This is consistent with the only other study to our knowledge on non-LA-strain-specific, non-occupational MRSA colonization by Carrel at al. (2014), where the strongest associations were detected for veterans with exposures of greater than 2500 swine within a 1 mile (1.6 km) radius of their homes, compared to any swine within the same radius. We estimate that the local residential density in the Carrel et al. (2014) study was roughly 307 swine/km2. Our supplemental analysis of the upper non-zero exposure quartile precluded examination of values of this magnitude, as there were no colonization cases and only 81 hospitalization discharges in ZIP codes with such high densities. However, it appears that risk for MRSA colonization due to swine exposure appears to increase more substantially above 50 swine/km2, while All MRSA infection remains elevated in each category compared to the reference after 1.5 swine/km2 (Appendix B.3).
The exposure-response differences observed between infection and colonization may be due to chance. They may also result from inconsistent testing for colonization among hospitals, or could be due to differing exposure pathways or transmission and disease dynamics. Since MRSA colonization may not persist for more than a few days after contact with swine (van Cleef et al., 2011), the association with swine may be most readily detected in very high-density areas because of direct exposure to the MRSA organisms originating from swine facilities (Smith et al., 2009; Smith et al., 2013). Although it is an important risk factor for MRSA infection, positive test for MRSA colonization does not always precede onset of MRSA infection (Davis et al., 2004). The swine-MRSA infection association may be due to an increase in ambient antibiotic residues and specific genes that increase the potential for the transfer of antibiotic resistance to a pathologically relevant organism in a person’s immediate environment, rather than as a direct exposure to or colonization by MRSA organisms living on swine farms. For colonization, future research should consider the impact of differences in colonization testing across hospitals, as well as document the particular MRSA strain(s). Lastly, the exposure-response differences noted between infection and colonization could also be due to chance or exposure misclassification. Although the broad exposure ranges within the ZIP code swine density categories reduces the influence of exposure misclassification that may occur due to the error expected from the use of ZIP code-based exposure estimates, we would expect larger potential impact of this misclassification in categories with small cell sizes like the upper two MRSA colonization categories. Related to this, the study does not take into account the swine density metrics of surrounding residential ZIP codes, so if a person lives in a low density ZIP code that borders a very high density ZIP code, the assigned estimate may not adequately represent their true exposure.
We found that different swine exposure metrics did not increase the odds that a given MRSA hospitalization would have the CA-MRSA designation versus the HA-MRSA designation. Among both inpatient and outpatient MRSA cases, Casey et al. (2013) found similar associations between swine exposure and CA- (aOR=1.38, 1.13–1.69) and HA-MRSA (aOR=1.30, 1.05–1.61) compared to non-SSTI controls. These MRSA designations may be less etiologically relevant as environmental colonization by MRSA may occur prior to the development of the infection within the hospital. Likewise, exposure to healthcare settings may not always be predictive of CA or HA molecular characterization (David et al., 2008). Thus, there may be misclassification of the CA-MRSA outcome based on the “Present on Admission” data in our study, which could explain some of our inconsistent categorical results. Our study estimated a CA-MRSA prevalence of 80% in 2008 compared to 62% for the same year in a Chicago Medical Center study using molecular characterization techniques (David et al., 2014). Misclassification may also result from incomplete patient history, since the available records did not account for all previous visits to healthcare facilities, nor whether the inpatients normally reside in nursing homes. Many CA-MRSA cases could also be treated in outpatient settings, which may occur more frequently in rural settings with relatively higher hog exposure, due to hospital accessibility issues. In addition to the potential for outcome misclassification, demographic differences may explain the increased likelihood for CA-MRSA in the lowest swine density exposure category compared to the referent. If CA-MRSA cases were misclassified as HA-MRSA, our study results would be attenuated which may explain some of the null study findings.
Illinois is a useful study location to assess the potential role of swine exposures for MRSA infection, because there are areas within the state which represent combinations of both relatively low and high swine production with and without high human populations (Fig. 1). This variability allows for assessment of the impact of swine production, given different urban and rural land uses, which have been previously associated with MRSA (Naimi et al., 2003; Casey et al., 2013; Dantes et al., 2013). Additionally, we derived exposure estimates that had higher spatial resolution than county swine inventory, which is generally the lowest geographic resolution for reporting livestock inventory nationally (Appendix B, Table B.2). Although RUCC may not adequately capture the complex transmission dynamics across communities with different degrees of urbanicity, our analyses suggest that swine production may be a more important MRSA risk factor in more highly urbanized areas. It is important to note, however, that these findings are not consistent across categories and outcomes. When considering MRSA colonization via a person-to-person pathways, denser populations may expand the community reservoir for MRSA and increase the potential for persistent colonization of MRSA on fomites in the home environment (Desai et al., 2011; Davis et al., 2012). Future research could include a more refined urban-rural classification continuum to better understand the effect measure modification potential of population dynamics.
Fig. 1.
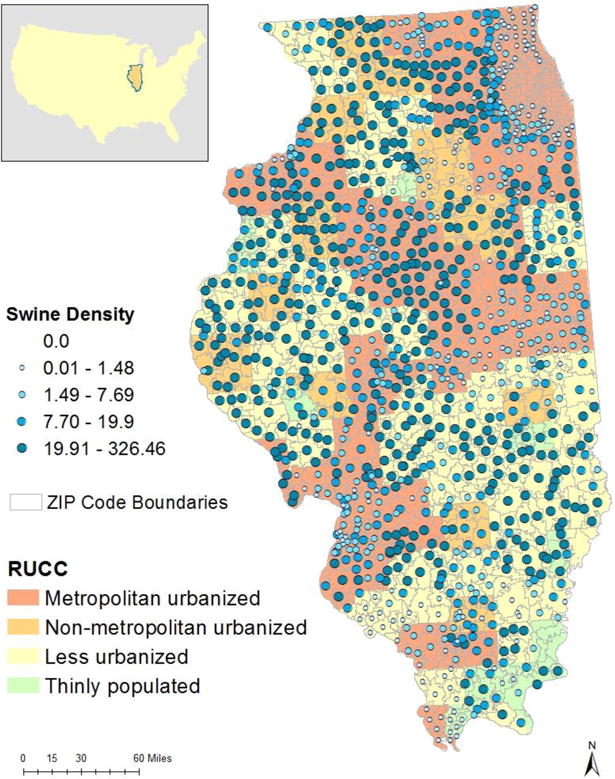
Map of the ZIP code aggregate swine density (swine/km2) categorical exposure groups across Rural and Urban Continuum Code (RUCC) urban-rural designations.
An additional study strength is that our records included both old and new MRSA ICD-9 coding, avoiding loss of cases attributable to administrative coding changes. This was especially important for 2008 where the majority (98%) of MRSA hospitalizations were identified using the older code assignment. The primary purpose of most administrative hospitalization databases is claims reimbursement and the Centers for Medicare and Medicaid Services reporting only requires nine diagnostic fields, and processing claims for reimbursement often causes truncation of records. This is concerning for characterizing MRSA cases, which requires a supplemental ICD-9 code (Shaefer et al., 2010; Jhung and Banerjee, 2009). Fortunately, IHA records are not truncated to the minimum Medicare and Medicaid claims requirements for the years included in our study, so the use of supplemental codes for resistant infection and comorbidity should increase case ascertainment compared to other less robust administrative databases. Lastly, our outcome data are relatively comprehensive since they comprise reported SSTI hospitalizations for 97% of the state of Illinois.
Also related to MRSA case ascertainment, there is some question in the literature as to the validity and completeness of administrative databases due to the possibility of detection differences from variable bacterial culture protocol, reporting, and coding variability across hospitals (Leal and Laupland, 2010; Schaefer et al., 2010; Casey et al., 2014). We are less confident, for example, that all secondary diagnoses and supplementary codes, indicating complications and comorbidities, are included consistently across hospitals. While administrative coders must undergo extensive certificate training, differences in ICD-9 administrative records at discharge could be influenced by clinician thoroughness, hospital throughput, and available resources, among other factors (Leal and Laupland, 2009). Additionally, the protocols for collecting bacterial cultures could differ across hospitals, potentially influencing our ability to accurately classify MRSA colonization cases. For example, some hospitals may swab all individuals with indication of infection, while others that do not routinely collect samples may only conduct a nasal swab upon admission for a pre-surgical patient or if an individual exhibits specific symptoms. In our data set, colonized inpatients appear to be older, less healthy individuals compared to all other SSTI inpatients. Given that colonization is almost certainly under-reported and because it may be differential based on hospital location, the direction of any potential bias is not known.
We acknowledge that hospital records alone may not be ideal for assessing environmental influences on all MRSA infections, as some MRSA cases could be diagnosed and treated in outpatient settings. Therefore, our study results may only be generalizable to hospitalized SSTI patients. In addition to case severity differences, there may be other fundamental differences between this population and those utilizing outpatient treatment, such as those using an emergency room in place of available primary care, or differential hospital accessibility based on urbanicity or residential proximity. Additionally, the use of SSTI hospitalizations as our study population may underestimate the underlying relationship between MRSA and swine exposures, as some research suggests that exposure may independently increase the risk of SSTIs (Casey et al., 2013). Though it would be preferable to have a comparison population that excludes records with conditions potentially associated with swine production, SSTI’s are an appropriate type of MRSA infection to explore, as they tend to have a higher proportion of CA-MRSA infection and may be more relevant for the exposure metrics examined here, compared to other syndromes like septicemia or respiratory MRSA infection.
The FLAPS model relies on the county and state reported number of farms identified in the 2007 and 2012 Censuses of Agriculture (Burdett et al., 2015) to allocate over 99% of the USDA census-reported swine population across the United States. Given uncertainties in the swine census data, the USDA/NASS (2012) has adjusted the estimated Illinois swine inventory total for errors due to nonresponse (15.7%), misclassification (1.4%), and coverage (0.8%). Because much of the uncertainty in swine inventory could result from nonresponse, there is potential for over or underestimating exposure if owners’ and operators’ propensity to respond is systematically related to farm size. Additional uncertainty introduced through our aggregation of farm locations predicted by FLAPS model to ZIP code is difficult to quantify, but use of 100 simulations for each census year provides a central tendency estimate of the modeled outcomes (Burdett, personal communication). Our exposure assessment at the ZIP code-level may be subject to the modifiable area unit problem (Openshaw, 1984). Because the farm locations predicted by FLAPS are based on a presence/absence probabilistic predicative model across a continuous grid, each simulation will have slightly different farm locations that can land indiscriminately inside or outside ZIP code boundaries. As a result, the final density measure variability tends to increase with ZIP codes of smaller areas that lie within counties of high swine production. In our data set, ZIP codes with the highest human population were within those with small areas. Though there was concern that smaller ZIP codes with higher human population would be assigned higher swine density due to MAUP and potentially bias results, evaluation of the FLAPS data showed no consistent relationship between ZIP code area and swine density estimates.
4.1. Conclusions and recommendations
Given the growing public health concern over antibiotic resistance, coupled with the predominance of large operations regularly administering subtherapeutic doses of antibiotics, additional studies are needed to increase the understanding of how swine production may contribute to environmental transmission of MRSA. Although we detect a consistent association for different ecological exposure metrics, routinely-collected national health and exposure data may not be sufficient for characterizing potential risk from specific exposure pathways. Therefore, more research is needed to delineate the contribution of person-to-person transmission from other environmental pathways of exposure via various media. In addition to assessing address-level information, a spatial analysis with integration of waste-movement modeling that incorporates additional sources of environmental exposures such as hospitals, slaughterhouses (van Cleef et al., 2011), and wastewater treatment plants (Pruden et al., 2012) and the inclusion of additional environmental covariates (e.g., soil type, slope, proximity to waterways, etc.) could improve delineation of the potential risk due to swine facilities. Finally, the addition of molecular characterization data for reported infections would not only improve case ascertainment, but would provide insight into strains related to swine production. This information, along with characterized strains from nearby facilities and within environmental media, would give further insight into the movement of resistance through the environment, important genetic mechanisms, and identification of strains with higher zoonotic potential.
Acknowledgments
We would like to thank Lee S. Friedman at the University of Illinois Chicago for assisting in data abstraction and acquisition.
Funding sources
This research was supported by Cooperative Agreement Number X3-83555301 from the U.S. Environmental Protection Agency (USEPA) and the Association of Schools and Programs of Public Health (ASPPH). The views expressed in this manuscript are those of the authors and do not necessarily reflect the views or policies of the USEPA or ASPPH.
APPENDICES
Appendix A. Methods
A.1. CA-HA case definition
CDC case definition defines a community-acquired (CA) MRSA to be lacking the elements of a classical hospital acquired (HA) infection that includes the following: the appearance of MRSA more than 48 h after hospital admission; a history of hospitalization, surgery, dialysis, or residence in a long-term care facility within 1 year of the MRSA infection; the presence of an indwelling catheter or a percutaneous device; or previous MRSA infection (Fridkin et al., 2005)
Table A.1.
IHA and ICD-9 codes for determination of CA and HA designations.
| HA Item (coding scheme) | Codes |
|---|---|
| PHE (IHA) | 02―Clinic referral |
| 04―Transfer from a different hospital | |
| 05―Transfer from a Skilled Nursing Facility or Intensive Care Facility | |
| 06―Transfer from another health care facility | |
| A―Transfer from a critical access hospital | |
| D―Transfer from the same facility | |
| E―Transfer from ambulatory service center | |
| F―Transfer from hospice | |
| PHE (ICD-9) | V45―Other post-procedural states |
| V51―Plastic surgery aftercare | |
| V50―Elective surgery aftercare | |
| V53.5–V53.6―Aftercare―Fitting and adjustment for prosthetic device | |
| V54―Other orthopedic aftercare | |
| V55―Attention to artificial openings | |
| V56―Encounter for dialysis or dialysis aftercare | |
| V58.3, V58.4, V58.7, V58.8, V58.9―Encounter for other and unspecified procedures and aftercare | |
| POA (IHA) | Y―Yes |
| Procedural codes (ICD-9) | 01–05―Operations on the nervous system |
| 06–07―Operations on the endocrine system | |
| 08–16―Operations on the eye | |
| 18―Operations on the ear | |
| 21–29―Operations on nose, mouth, and pharynx | |
| 30–34―Operations on the respiratory system | |
| 35–39―Operations on the cardiovascular system | |
| 40–41―Operations on the hemic and lymphatic system | |
| 42–54―Operations on the digestive system | |
| 55–59―Operations on the urinary system | |
| 60–64―Operations on the male genital organs | |
| 65–71―Operations on the female genital organs | |
| 72–75―Obstetrical procedures | |
| 76–84―Operations on the musculoskeletal system | |
| 85–86―Operations on the integumentary system | |
| Dialysis (ICD-9) | V56―Encounter for dialysis and dialysis catheter care, |
| V45.11―Renal dialysis status | |
| V56.2―Fitting and adjustment of peritoneal dialysis catheter | |
| Indwelling device (ICD-9) | V58.81―Fitting and adjustment of vascular catheter |
| V460-V461―Dependence on machine or device―aspirator and respirator | |
| 9961―Mechanical complication of other vascular device, implant, or graft | |
| 99931―Infection due to central venus catheter | |
| 9966―Infection and inflammatory reaction due to internal prosthetic device, implant, or graft | |
| Artificial opening (ICD-9) | V44―Artificial opening status |
CA, Community-Acquired; HA, Hospital Acquired; IHA, Illinois Hospital Association; ICD, International Statistical Classification of Diseases and Related Health Problems; PHE, Previous Healthcare Exposure; POA, Present on Admission
Fig. A.1.
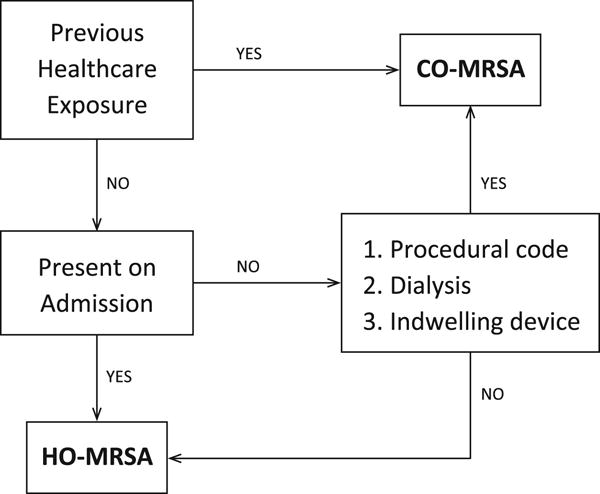
Decision flow chart depicting categorization as HA- and CA-MRSA
A.2. Comorbidity indices using additional diagnostic codes
To assess whether comorbid risk factors impact an individual’s susceptibility to MRSA via swine exposure, we utilized a modified Charlson Comorbidity Index (Charlson et al., 1987) as well as a simple index drawn from MRSA risk factors found within the existing literature (Table A.2). ICD-9 coding for the Charlson Index was assigned as previously described in the literature (Deyo et al., 1992). For the MRSA-specific index, we assigned a value of 1 to each behavioral and medical risk factor, which were then summed and subsequently stratified into 4 groups with ascending risk levels, as is done with Charlson Index. Group 1 indicates a score =0, group 2 indicates a score of 1 or 2, group 3, a score of 3 or 4 and group 4 includes all scores ≥5.
Table A.2.
Comorbid conditions included in the Charlson Index and MRSA-specific index items.
| Item | MRSA Lit | MRSA Score | Charlson Index | Charlson Score |
|---|---|---|---|---|
| Smoking | Y | 1 | N | |
| Alcohol Abuse | Y | 1 | N | |
| Intravenous Drug Use | Y | 1 | N | |
| Asthma | Y | 1 | N | |
| Chronic Noninfectious Skin Disease | Y | 1 | N | |
| Previous MRSA Infection | Y | 1 | N | |
| Diabetes | Y | 1 | Y | 1 |
| HIV | Y | 1 | Y | 6 |
| Myocardial Infarction | Y* | 1 | Y | 1 |
| Congestive Heart Failure | Y* | 1 | Y | 1 |
| Peripheral Vascular Disease | N | Y | 1 | |
| Cerebrovascular Disease | N | Y | 1 | |
| Dementia | N | Y | 1 | |
| COPD | Y | 1 | Y | 1 |
| Rheumatologic Disease | N | Y | 1 | |
| Peptic Ulcer Disease | N | Y | 1 | |
| Mild Liver Disease | N | Y | 1 | |
| Hemiplegia or Paraplegia | Y** | 1 | Y | 2 |
| Renal Disease | N | Y | 2 | |
| Malignancy | Y† | 1 | N | 2 |
| Moderate to Severe Liver disease | N | Y | 3 | |
| Metastatic Solid Tumor | N | Y | 6 |
COPD, Chronic Obstructive Pulmonary Disease; HIV, human immunodeficiency virus; MRSA, Methicillin Resistant Staphylococcus Aureus; N, no; Y, yes.
MRSA literature describes heart disease, as a risk factor for developing infection―these more specific items are from the CCI
MRSA associated with sedentary lifestyle
Immunodeficiency
A.3. County exposure calculation
County level data was accessed through the National Agricultural Statistical Service’s (NASS) Quickstats 2.0 online data repository and supplemented with NASS annual survey estimates (USDA/NASS, 2015). The Census of Agriculture is a complete census of all farms, taken every 5 years, while NASS survey inventory estimates are based on an annual survey administered in counties where production of the commodity in question is economically important. For Illinois, the majority of counties have annual swine inventory available for all years.
County Level swine densities were estimated from census data, and supplemented with NASS Annual Survey data in the case of matchable unpublished, nonzero entries. For years where the Census of Agriculture withheld a given county inventory to protect operator’s privacy, we reviewed the corresponding NASS inventory to assess the likelihood of more likely actual zero counts. All other missing data were imputed across years using a smooth linear join interpolation available within the SAS expand procedure (SAS/ETS® 13.2 User’s Guide, 2013). The imputed swine inventories were divided by the square kilometers (km2) to obtain a swine/km2 spatial density estimate.
Fig. A.2.
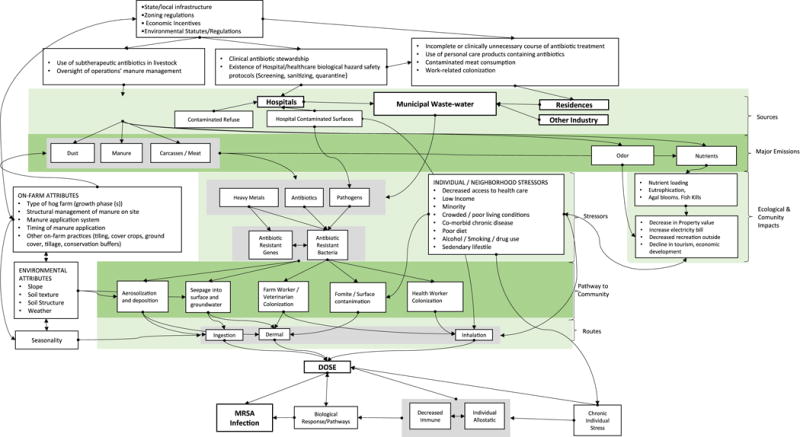
Conceptual model for CAFO swine operations and antibiotic resistance, MRSA colonization and infection
Appendix B. Results
Table B.1.
Comorbidity indices by outcome.
| COVARIATE | No MRSA | MRSAγ | MRSA COLγ | HA-MRSAγ | CA-MRSAγ |
|---|---|---|---|---|---|
| n (%) | |||||
| CI Score | |||||
| not ill | 55,890 (29.5) | 9419 (36.7) | 808 (21.4) | 596 (11.3) | 8838 (43.2) |
| mildly ill | 86,913 (45.9) | 10,791 (42.1) | 1834 (48.7) | 2768 (53.2) | 8030 (39.3) |
| moderately ill | 35,249 (18.6) | 4125 (16.1) | 905 (23.9) | 1467 (28.2) | 2662 (13.0) |
| severely ill | 10,886 (5.7) | 1215 (4.7) | 215 (5.7) | 378 (7.3) | 837 (4.1) |
| MRSA Score | |||||
| 0 | 58,082 (30.6) | 8639 (33.7) | 180 (4.8) | 749 (14.4) | 7892 (38.6) |
| 1 to 2 | 102,502 (54.1) | 13,457 (52.5) | 894 (23.6) | 3327 (64.0) | 10,142 (49.6) |
| 3 to 4 | 26,634 (14.1) | 3263 (12.7) | 2132 (56.4) | 1046 (20.1) | 2219 (10.9) |
| >5 | 1720 (0.9) | 191 (0.7) | 694 (18.4) | 77(1.5) | 114 (0.6) |
|
| |||||
| County-level Mean(Std Dev) | |||||
| Density (swine/km2) | 9.3 (19.8) | 10.3 (20.4)** | 11.3 (22.8)** | 10.7 (20.8)* | 10.2 (20.4) |
| Farms >1000 swine | 3.1 (6.1) | 3.4 (6.1)** | 3.7 (6.8) | 3.6 (6.4) | 3.4 (6.1) |
CA, Community-Acquired; CI, confidence interval; COL, Colonization; HA, Hospital Acquired; MRSA, Methicillin Resistant Staphylococcus Aureus; Std Dev, standard deviation.
Statistically significant difference in comorbidity index categories compared to non-MRSA, at the α <0.001 level, using chi-square test
Table B.2.
County-level ORs and aORs for All MRSA compared to non-MRSA SSTI inpatients, MRSA-colonized compared to noncolonized SSTI inpatients and CA-MRSA compared to HA-MRSA among SSTI inpatients, for continuous residential county swine exposure.
| Exposure | Outcome
|
|||||
|---|---|---|---|---|---|---|
| All MRSA† | MRSA Colonizationˠ | CA-MRSAǂ | ||||
|
|
|
|||||
| Cases (n) | aOR (95% CI) | Cases (n) | aOR (95% CI) | Cases (n) | aOR (95% CI) | |
| Density, swine/km2 | 25,569 | 1.32 (1.24–1.42) | 3765 | 1.24 (1.06–1.44) | 20,383 | 0.99 (0.85–1.17) |
| Farms >1000, n/ZIP | 25,569 | 1.01 (1.01–1.01) | 3765 | 1.01(1.00–1.01) | 20,383 | 1.00 (1.00–1.01) |
aOR; adjusted odds ratio; CA, Community-Acquired; CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; HA, Hospital Acquired; MRSA, Methicillin Resistant Staphylococcus Aureus; OR, odds ratio; SSTI, Skin and Soft Tissue Infection.
Both Metrics: Adjusted for age, race, and socioeconomic deprivation
Density: Adjusted for year, age, ethnicity, socioeconomic deprivation, and COPD; Farms >1000: Adjusted for race, ethnicity, socioeconomic deprivation, and COPD
Density: Adjusted for race, ethnicity, and socioeconomic deprivation; Farms >1000: Adjusted for race, socioeconomic deprivation, and COPD
Table B.3.
Adjusted ORs (aORs) for MRSA-positive compared to non-MRSA SSTI inpatients, MRSA-colonized compared to non-MRSA-colonized SSTI inpatients, and CA-MRSA compared to HA-MRSA for more finely parsed upper categorical residential ZIP code swine exposures.
| Exposure | Outcome
|
|||||
|---|---|---|---|---|---|---|
| All MRSA†
|
MRSA Colonizationˠ
|
CA-MRSAǂ
|
||||
| Cases (n) | aOR (95% CI) | Cases (n) | aOR (95% CI) | Cases (n) | aOR (95% CI) | |
| Density, swine/km2 | ||||||
| 0 | 10,613 | 1.00 | 1595 | 1.00 | 8439 | 1.00 |
| 0.00004–1.48 | 3155 | 1.07 (1.02–1.11) | 529 | 0.98 (0.88–1.08) | 2560 | 1.14 (1.03–1.27) |
| 1.49–7.69 | 3945 | 1.33 (1.27–1.38) | 536 | 1.01 (0.91–1.11) | 3169 | 1.04 (0.94–1.15) |
| 7.70–19.90 | 3956 | 1.39 (1.34–1.45) | 506 | 0.93 (0.84–1.04) | 3135 | 1.00 (0.90–1.10) |
| 19.91–50 | 2649 | 1.41 (1.35–1.48) | 376 | 1.02 (0.91–1.15) | 2093 | 0.98 (0.88–1.10) |
| 50.1–100 | 908 | 1.37 (1.27–1.47) | 155 | 1.22 (1.03–1.43) | 720 | 1.01 (0.84–1.19) |
| 100.1–150 | 266 | 1.32 (1.16–1.51) | 60 | 1.53 (1.18–1.99) | 204 | 0.90 (0.67–1.21) |
| >150 | 77 | 1.31 (1.03–1.67) | 8 | 0.61 (0.32–1.23) | 63 | 1.37 (0.76–2.48) |
aOR, adjusted odds ratio; CA, Community-Acquired; CI, confidence interval; HA, Hospital Acquired; MRSA, Methicillin Resistant Staphylococcus Aureus; SSTI, Skin and Soft Tissue Infection.
Per 100 swine/km2 increase**Per 1 large farm increase
Both Exposure Metrics: Adjusted for age, race, and socioeconomic deprivation index
Density: Adjusted for race, ethnicity, and socioeconomic deprivation index; Farms >1000: Adjusted for race, ethnicity, socioeconomic deprivation index, asthma, and COPD
Density: Adjusted for age, race, ethnicity, socioeconomic deprivation index, COPD, public insurance recipient status and tobacco use; Farms >1000: Adjusted for age, race, ethnicity, socioeconomic deprivation index, COPD, and public insurance recipient status
Table B.4.
Stratification by comorbidity indices: Continuous residential ZIP code swine exposure and All MRSA compared to non-MRSA SSTI inpatients, and non-MRSA Colonized inpatients compared to MRSA-colonized SSTI inpatients, and CA-MRSA compared to HA-MRSA.
| Comorbidity Score | Outcome
|
|||||
|---|---|---|---|---|---|---|
| All MRSA†
|
MRSA Colonizationˠ
|
CA-MRSAǂ
|
||||
| Cases (n) | aOR (95% CI) | Cases (n) | aOR (95% CI) | Cases (n) | aOR (95% CI) | |
| Charlson Score | ||||||
| Density, 100 swine/km2 | ||||||
| not ill | 9,419 | 1.42 (1.28–1.56) | 808 | 1.12 (0.82–1.52) | 8,838 | 1.58 (1.00–2.48) |
| mildly ill | 10,791 | 1.31 (1.20–1.44) | 1,834 | 1.29 (1.06–1.57) | 8,030 | 0.82 (0.67–1.01) |
| moderately ill | 4,125 | 1.24 (1.07–1.44) | 905 | 1.34 (1.02–1.78) | 2,662 | 0.93 (0.68–1.27) |
| severely ill | 1,215 | 1.73 (1.31–2.28) | 215 | 0.81 (0.37–1.77) | 837 | 1.10 (0.63–1.94) |
| Farms >1000, n/ZIP | ||||||
| not ill | 9,419 | 1.07 (1.05–1.09) | 808 | 1.03 (0.97–1.10) | 8,838 | 1.10 (1.00–1.21) |
| mildly ill | 10,791 | 1.05 (1.03–1.07) | 1,834 | 1.07 (1.03–1.12) | 8,030 | 0.95 (0.91–1.00) |
| moderately ill | 4,125 | 1.03 (0.99–1.06) | 905 | 1.07 (1.01–1.14) | 2,662 | 0.98 (0.91–1.05) |
| severely ill | 1,215 | 1.11 (1.05–1.18) | 215 | 1.00 (0.85–1.17) | 837 | 1.04 (0.92–1.17) |
| MRSA Score | ||||||
| Density, 100 swine/km2 | ||||||
| 0 | 8,639 | 1.44 (1.30–1.59) | 894 | 1.10 (0.82–1.47) | 7,892 | 1.33 (0.91–1.95) |
| 1 to 2 | 13,457 | 1.34 (1.23–1.46) | 2,132 | 1.23 (1.04–1.51) | 10,142 | 0.92 (0.76–1.12) |
| 3 to 4 | 3,263 | 1.25 (1.05–1.48) | 694 | 1.31 (0.95–1.82) | 2,219 | 0.80 (0.57–1.12) |
| >5 | 191 | 1.37 (0.66–2.84) | 42 | 1.32 (0.33–5.25) | 114 | 1.46 (0.37–5.74) |
| Farms >1000, n/ZIP | ||||||
| 0 | 8,639 | 1.08 (1.05–1.10) | 894 | 1.04 (0.97–1.10) | 7,892 | 1.10 (1.01–1.20) |
| 1 to 2 | 13,457 | 1.05 (1.03–1.07) | 2,132 | 1.06 (1.02–1.11) | 10,142 | 0.96 (0.92–1.00) |
| 3 to 4 | 3,263 | 1.02 (0.98–1.06) | 694 | 1.08 (1.00–1.15) | 2,219 | 0.97 (0.89–1.04) |
| >5 | 191 | 0.99 (0.84–1.18) | 42 | 0.93 (0.64–1.34) | 114 | 1.32 (0.88–1.98) |
CA, Community-Acquired; HA, Hospital Acquired; MRSA, Methicillin Resistant Staphylococcus Aureus; SSTI, Skin and Soft Tissue Infection.
Footnotes
Abbreviations: MRSA, Methicillin-resistant Staphylococcus aureus; IHA, Illinois Hospital Association; OR, odds ratios; aOR, adjusted odds ratios; CA‑MRSA, community-acquired MRSA; HA-MRSA, hospital acquired MRSA; LA‑MRSA, livestock-associated MRSA; SSTI, skin and soft tissue infection; IHD, Illinois Hospital Discharge Database; ICD-9, International Classification for Diseases coding 9th revision; FIPS, Federal information processing standard; CDC, Centers for Disease Control and Prevention; USDA, U.S. Department of Agriculture; RUCC, rural-urban continuum code; FLAPS, farm location and agricultural production simulator; ArcGIS, aeronautical reconnaissance coverage geographic information system; ESRI, Environmental Systems Research Institute; TIGER, Topologically Integrated Geographic Encoding and Referencing; SAS, Statistical Analysis System; ETS, Econometrics and Time Series; COPD, chronic obstructive pulmonary disease; NASS, National Agricultural Statistics Service; COL, colonization; Std Dev, standard deviation; CI, confidence interval; N/E, not able to estimate, PHE, previous healthcare exposure; POA, present on admission; HIV, human immunodeficiency virus; CAFO, concentrated animal feeding operation
Research Concerning Human Subjects
The use of these hospitalization records was approved by the University of Illinois, Chicago Internal Review Board.
Conflict of interest
There are no conflicts of interest to report.
References
- Akwar TH, Poppe C, Wilson J, Reid-Smith RJ, Dyck M, Waddington J, Shang N, Dassie SA, McEwen SA. Risk factors for antimicrobial resistance among fecal Escherichia coli from residents on forty-three swine farms. Microb Drug Resist. 2007;13(1):69–76. doi: 10.1089/mdr.2006.9999. http://doi.org/10.1089/mdr.2006.9999. [DOI] [PubMed] [Google Scholar]
- Bisdorff B, Scholhölter J, Claußen K, Pulz M, Nowak D, Radon K. MRSA-ST398 in livestock farmers and neighbouring residents in a rural area in Germany. BMC Proc. 2011;5(Suppl 6):P169. doi: 10.1017/S0950268811002378. http://doi.org/10.1186/1753-6561-5-S6-P169. [DOI] [PubMed] [Google Scholar]
- Burdett CL, Kraus BR, Garza SJ, Miller RS, Bjork KE. Simulating the distribution of individual livestock farms and their populations in the United States: An example using domestic swine (Sus scrofa domesticus) farms. PLoS One. 2015;10(11):e0140338. doi: 10.1371/journal.pone.0140338. Epub 2015 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel M, Schweizer ML, Sarrazin MV, Smith TC, Perencevich EN. Residential proximity to large numbers of swine in feeding operations is associated with increased risk of methicillin-resistant Staphylococcus aureus colonization at time of hospital admission in rural Iowa veterans. Infect Control Hosp Epidemiol. 2014;35(2):190–192. doi: 10.1086/674860. http://doi.org/10.1086/674860. [DOI] [PubMed] [Google Scholar]
- Casey JA, Cosgrove SE, Stewart WF, Pollak J, Schwartz BS. A population-based study of the epidemiology and clinical features of methicillin-resistant Staphylococcus aureus infection in Pennsylvania, 2001–2010. Epidemiol Infect. 2012;141(6):1166–1179. doi: 10.1017/S0950268812001872. http://doi.org/10.1017/S0950268812001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JA, Curriero FC, Cosgrove SE, Nachman KE, Schwartz BS. High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern Med. 2013;173(21):1980–1990. doi: 10.1001/jamainternmed.2013.10408. http://doi.org/10.1001/jamainternmed.2013.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JA, Shopsin B, Cosgrove SE, Nachman KE, Curriero FC, Rose HR, Schwartz BS. High-density livestock production and molecularly characterized MRSA infections in Pennsylvania. Environ Health Perspect. 2014;122(5):464–470. doi: 10.1289/ehp.1307370. http://doi.org/10.1289/ehp.1307370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC/NCHS (Centers for Disease Control and Prevention/National Center for Health Statistics) Conversion Table of New ICD-9-CM Codes. Office of Information Services; 2013. October 2013. Available http://www.cdc.gov/nchs/data/icd/ICD-9-CM_FY14_CNVTBL_Final.pdf. [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Chapin A, Rule A, Gibson K, Buckley T, Schwab K. Airborne multidrug-resistant bacteria isolated from a concentrated swine feeding operation. Environ Health Perspect. 2005;113(2):137–142. doi: 10.1289/ehp.7473. http://doi.org/10.1289/ehp.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee-Sanford JC, Amniov RI, Krapac IJ, Garrigues-Jeanjean N, Mackie RI. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl Environ Microbiol. 2001;67(4):1494–1502. doi: 10.1128/AEM.67.4.1494-1502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee-Sanford JC, Mackie RI, Koike S, Krapac IG, Lin YF, Yannarell AC, Maxwell S, Aminov RI. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J Environ Qual. 2009;38(3):1086–1108. doi: 10.2134/jeq2008.0128. http://doi.org/10.2134/jeq2008.0128. [DOI] [PubMed] [Google Scholar]
- Cuny C, Nathaus R, Layer F, Strommenger B, Altmann D, Witte W. Nasal colonization of humans with methicillin-resistant Staphylococcus aureus (MRSA) CC398 with and without exposure to pigs. PLoS One. 2009;4(8):1–6. doi: 10.1371/journal.pone.0006800. http://doi.org/10.1371/journal.pone.0006800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Fridkin S. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970–8. doi: 10.1001/jamainternmed.2013.10423. http://doi.org/10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David MZ, Glikman D, Crawford SE, Peng J, King KJ, Hostetler MA, Daum RS. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235–1243. doi: 10.1086/533502. http://doi.org/10.1086/533502. [DOI] [PubMed] [Google Scholar]
- David MZ, Cadilla A, Boyle-Vavra S, Daum RS. Replacement of HA-MRSA by CA-MRSA infections at an academic medical center in the midwestern United States, 2004-5 to 2008. PLoS One. 2014:e92760. doi: 10.1371/journal.pone.0092760. eCollection 2014. http://doi.org/10.1371/journal.pone.0092760. [DOI] [PMC free article] [PubMed]
- Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis. 2004;39(6):776–782. doi: 10.1086/422997. http://doi.org/10.1086/422997. [DOI] [PubMed] [Google Scholar]
- Davis MF, Iverson SA, Baron P, Vasse A, Silbergeld EK, Lautenbach E, Morris DO. Household transmission of meticillin-resistant Staphylococcus aureus and other staphylococci. Lancet Infect Dis. 2012;12(9):703–716. doi: 10.1016/S1473-3099(12)70156-1. http://doi.org/10.1016/S1473-3099(12)70156-1. [DOI] [PubMed] [Google Scholar]
- Desai R, Pannaraj PS, Agopian J, Sugar CA, Liu GY, Miller LG. Survival and transmission of community-associated methicillin-resistant Staphylococcus aureus from fomites. Am J Infect Control. 2011;39(3):219–225. doi: 10.1016/j.ajic.2010.07.005. http://doi.org/10.1016/j.ajic.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Diekema DJ, Edmond MB. Look before you leap: active surveillance for multidrug-resistant organisms. Clin Infect Dis. 2007;44(8):1101–1107. doi: 10.1086/512820. http://doi.org/10.1086/512820. [DOI] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- Dolliver H, Gupta S. Antibiotic losses in leaching and surface runoff from manure-amended agricultural land. J Environ Qual. 2008;37(3):1227–1237. doi: 10.2134/jeq2007.0392. http://doi.org/10.2134/jeq2007.0392. [DOI] [PubMed] [Google Scholar]
- Dukic VM, Lauderdale DS, Wilder J, Daum RS, David MZ. Epidemics of community-associated methicillin-resistant staphylococcus aureus in the United States: a meta-analysis. PLoS One. 2013;8(1):e52722. doi: 10.1371/journal.pone.0052722. Epub 2013 Jan 2. http://doi.org/10.1371/journal.pone.0052722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10.2.2. Redlands, CA: Environmental Systems Research Institute; 2014. Available http://resources.arcgis.com/en/help/ [Google Scholar]
- Feingold BJ, Silbergeld EK, Curriero FC, van Cleef BA, Heck ME, Kluytmans JA. Livestock density as risk factor for resistant staphylococcus aureus, the Netherlands. Emerg Infect Dis. 2012;18(11):1841–1849. doi: 10.3201/eid1811.111850. http://doi.org/10.3201/eid1811.111850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frana TS, Beahm AR, Hanson BM, Kinyon JM, Layman LL, Karriker LA, Ramirez A, Smith TC. Isolation and characterization of methicillin-resistant staphylococcus aureus from pork farms and visiting veterinary students. PLoS One. 2013;8(1):1841–1849. doi: 10.1371/journal.pone.0053738. http://doi.org/10.1371/journal.pone.0053738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, Harriman K, Harrison LH, Lynfield R, Farley MM. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352(14):1436–1444. doi: 10.1056/NEJMoa043252. http://doi.org/10.1097/01.AOG.0000170861.29348.64. [DOI] [PubMed] [Google Scholar]
- Gilchrist MJ, Greko C, Wallinga DB, Beran GW, Riley DG, Thorne PS. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ Health Perspect. 2007;115(2):313–316. doi: 10.1289/ehp.8837. http://doi.org/10.1289/ehp.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs SG, Green CF, Tarwater PM, Mota LC, Mena KD, Scarpino PV. Isolation of antibiotic-resistant bacteria from the air plume downwind of a swine confined or concentrated animal feeding operation. Environ Health Perspect. 2006;114(7):1032–1037. doi: 10.1289/ehp.8910. http://doi.org/10.1289/ehp.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368(9538):874–885. doi: 10.1016/S0140-6736(06)68853-3. http://doi.org/10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- Hamscher G, Pawelzick HT, Sczesny S, Nau H, Hartung J. Antibiotics in dust originating from a pig-fattening farm: A new source of health hazard for farmers? Environ Health Perspect. 2003;111(13):159–1594. doi: 10.1289/ehp.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279(8):593–598. doi: 10.1001/jama.279.8.593. http://doi.org/10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- Heuer H, Schmitt H, Smalla K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr Opin Microbiol. 2011;14(3):236–243. doi: 10.1016/j.mib.2011.04.009. http://doi.org/10.1016/j.mib.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Huang E, Gurzau AE, Hanson BM, Kates AE, Smith TC, Pettigrew MM, Spinu M, Rabinowitz PM. Detection of livestock-associated methicillin-resistant Staphylococcus aureus among swine workers in Romania. Journal of Infection and Public Health. 2014 doi: 10.1016/j.jiph.2014.03.008. http://doi.org/10.1016/j.jiph.2014.03.008. [DOI] [PubMed]
- Illinois Department of Public Health. Hospital Discharge Database: Database Description. 2015 Available http://app.idph.state.il.us/emsrpt/hospitalization.asp, accessed July, 2015.
- Jhung MA, Banerjee SN. Administrative coding data and health care-associated infections. Clin Infect Dis. 2009;49(6):949–955. doi: 10.1086/605086. http://doi.org/10.1086/605086. [DOI] [PubMed] [Google Scholar]
- Leal JR, Laupland KB. Validity of ascertainment of co-morbid illness using administrative databases: A systematic review. Clin Microbiol Infect. 2010;16(6):715–721. doi: 10.1111/j.1469-0691.2009.02867.x. http://doi.org/10.1111/j.1469-0691.2009.02867.x. [DOI] [PubMed] [Google Scholar]
- Leedom Larson KR, Harper AL, Hanson BM, Male MJ, Wardyn SE, Dressler AE, Wagstrom EA, Tendolkar S, Diekema DJ, Donham KJ, Smith TC. Methicillin-resistant staphylococcus aureus in pork production shower facilities. Appl Environ Microbiol. 2011;77(2):696–698. doi: 10.1128/AEM.01128-10. http://doi.org/10.1128/AEM.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SB, FitzGerald GB, Macone AB. Changes in intestinal flora of farm personnel after introduction of a tetracycline-supplemented feed on a farm. N Engl J Med. 1976;295(11):583–588. doi: 10.1056/NEJM197609092951103. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Commentary: methicillin-resistant staphylococcus aureus where is it coming from and where is it going? JAMA Intern Med. 2013;173(21):16–17. doi: 10.1001/jamainternmed.2013.8277. [DOI] [PubMed] [Google Scholar]
- Manzetti S, Ghisi R. The environmental release and fate of antibiotics. Mar Pollut Bul. 2014;79(1-2):7–15. doi: 10.1016/j.marpolbul.2014.01.005. http://doi.org/10.1016/j.marpolbul.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Marshall BM, Levy SB. Food animals and antimicrobials: Impacts on human health. Clin Microbiol Rev. 2011;24(4):718–733. doi: 10.1128/CMR.00002-11. http://doi.org/10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA) Curr Opin Microbiol. 2012;15(5):595–588. doi: 10.1016/j.mib.2012.08.003. http://doi.org/10.1016/j.mib.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Messer LC, Luben TJ, Mendola P, Carozza SE, Horel SA, Langlois PH. Urban-rural residence and the occurrence of cleft lip and cleft palate in Texas, 1999-2003. Ann Epidemiol. 2010;20(1):32–39. doi: 10.1016/j.annepidem.2009.09.006. http://doi.org/10.1016/j.annepidem.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Molla B, Byrne M, Abley M, Mathews J, Jackson CR, Fedorka-Cray P, Sreevatsan S, Wang P, Gebreyes WA. Epidemiology and genotypic characteristics of methicillin-resistant Staphylococcus aureus strains of porcine origin. J Clin Microbiol. 2012;50(11):3687–3693. doi: 10.1128/JCM.01971-12. http://doi.org/10.1128/JCM.01971-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, Chow H, Ip M, Jatzwauk L, Jonas D, Kadlec K, Keams A, Laurent F, O’Brien FG, Pearson J, Ruppelt A, Schwarz S, Scicluna E, Slickers P, Tan HL, Weber S, Ehricht R. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6(4):e17936. doi: 10.1371/journal.pone.0017936. http://doi.org/10.1371/journal.pone.0017936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadimpalli M, Rinsky JL, Wing S, Hall D, Stewart J, Larsen J, Nachman EE, Love DC, Pierce E, Pisanic N, Strelitz J, Harduar-Morano L, Heaney CD. Persistence of livestock-associated antibiotic-resistant Staphylococcus aureus among industrial hog operation workers in North Carolina over 14 days. Occup Environ Med. 2015;72:90–99. doi: 10.1136/oemed-2014-102095. http://doi.org/10.1136/oemed-2014-102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Johnson SK, Vandenesch F, Fridkin S, O’Boyle C, Danila RN, Lynfield R. Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290(22):2976–2984. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- Openshaw S. The modifiable areal unit problem Concepts and Techniques in Modern Geography No. 38. Geo Books; Norwich: 1984. [Google Scholar]
- Pruden A, Arabi M, Storteboom HN. Correlation between upstream human activities and riverine antibiotic resistance genes. Environ Sci Technol. 2012;46(21):11541–11549. doi: 10.1021/es302657r. [DOI] [PubMed] [Google Scholar]
- Rinsky JL, Nadimpalli M, Wing S, Hall D, Baron D, Price LB, Larsen J, Stegger M, Stewart J, Heaney CD. Livestock-associated methicillin and multidrug resistant staphylococcus aureus is present among industrial, not antibiotic-free livestock operation workers in North Carolina. PLoS One. 2013;9(7) doi: 10.1371/journal.pone.0067641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. The SAS system for Windows. Release 9.2. SAS Inst.; Cary, NC: 2013. Available http://support.sas.com/software/92/ [Google Scholar]
- Schaefer MK, Ellingson K, Conover C, Genisca AE, Currie D, Esposito T, Panttila L, Ruestow P, Martin K, Cronin D, Costello M, Sokalski S, Fridkin S, Srinivasan A. Evaluation of International classification of diseases, ninth revision, clinical modification codes for reporting methicillin-resistant staphylococcus aureus infections at a hospital in Illinois. Infect Control and Hosp Epidemiol. 2010;31(5):463–468. doi: 10.1086/651665. [DOI] [PubMed] [Google Scholar]
- Schulz J, Friese A, Klees S, Tenhagen BA, Fetsch A, Rösler U, Hartung J. Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock-associated methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol. 2012;78(16):5666–5671. doi: 10.1128/AEM.00550-12. http://doi.org/10.1128/AEM.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BS, Stewart WF, Godby S, Pollak J, DeWalle J, Larson S, Mercer DG, Glass TA. Body Mass Index and the built and social environments in children and adolescents using electronic health records. Am J Prevent Med. 2011;41(4):e17–e28. doi: 10.1016/j.amepre.2011.06.038. http://doi.org/10.1016/j.amepre.2011.06.038. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Ann Rev Pub Health. 2008a;29:51–169. doi: 10.1146/annurev.publhealth.29.020907.090904. http://doi.org/10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Davis M, Leibler JH, Peterson AE. One reservoir: redefining the community origins of antimicrobial-resistant infections. Med Clin N Am. 2008b;92(6):1391–1407. doi: 10.1016/j.mcna.2008.07.003. http://doi.org/10.1016/j.mcna.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Smith TC, Male MJ, Harper AL, Kroeger JS, Tinkler GP, Moritz ED, Capuano AW, Herwaldt LA, Diekema DJ. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS One. 2009;4(1):e4258. doi: 10.1371/journal.pone.0004258. Epub 2008 Jan 23. http://doi.org/10.1371/journal.pone.0004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TC, Gebreyes WA, Abley MJ, Harper AL, Forshey BM, Male MJ, Martin HW, Molla BZ, Sreevatsan S, Thakur S, Thiruvengadam M, Davies PR. Methicillin-resistant staphylococcus aureus in pigs and farm workers on conventional and antibiotic-free swine farms in the USA. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063704. http://doi.org/10.1371/journal.pone.0063704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr MP, Reynolds DM. Streptomycin resistance of coliform bacteria from turkeys fed streptomycin. Am J Public Health Nations Health. 1951;41(11 Pt 1):1375–1380. doi: 10.2105/ajph.41.11_pt_1.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA/ERS (U.S. Department of Agriculture Economic Research Service) Rural-Urban Continuum Codes. 2013 Available http://www.ers.usda.gov/briefing/rurality/RuralUrbCon/
- USDA/NASS (U.S. Department of Agriculture/National Agricultural Statistics Service) Census of Agriculture Illinois State and County Data. Washington, DC: USDA/NASS; 2012. (Geographic Area Series). Vol 1, part 13: AC-12-A-13. Available http://permanent.access.gpo.gov/gpo44362/ilv1.pdf. [Google Scholar]
- USDA/NASS (U.S. Department of Agriculture/National Agricultural Statistics Service) Quick Stats 2.0. Washington, DC: USDA?NASS; 2015. Available http://www.nass.usda.gov/Quick_Stats/ [Google Scholar]
- U.S. Census Bureau. 2014 TIGER/Line Shapefiles (machine readable data files) Washington, DC: U.S. Deparment of Commerce; 2014. Available https://www.census.gov/geo/maps-data/data/tiger-line.html. [Google Scholar]
- U.S. Census Bureau; American Community Survey. 2007–2011 American Community Survey 5-Year Estimates, American FactFinder. Washington, DC: U.S. Department of Commerce; 2015. < http://factfinder.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t>; (20 July 2015) [Google Scholar]
- van Cleef BAGL, Monnet DL, Voss A, Krziwanek K, Allerberger F, Struelens M, Zemlickova H, Skov RL, Vuopio-Varkila J, Cuny C, Friedrich AW, Spiliopoulou I, Pászti J, Hardardottir H, Rossney A, Pan A, Pantosti A, Borg M, Grundmann H, Mueler-Premru M, Olsson-Liljequist B-O, Widmer A, Harbarth S, Schweiger A, Unal S, Kluytmans JAJW. Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg Infect Dis. 2011;17(3):502–505. doi: 10.3201/eid1703.101036. http://doi.org/10.3201/eid1703.101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Cleef BAGL, van Benthem BHB, Verkade EJM, van Rijen M, Kluytmans-van Den Bergh MFQ, Schouls LM, Duim B, Wagenaar JA, Graveland H, Bos MEH, Heederik D, Kluytmans JAJW. Dynamics ofmethicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus carriage in pig farmers: a prospective cohort study. Clin Microbiol Infect. 2014;20:764–771. doi: 10.1111/1469-0691.12582. http://doi.org/10.1111/1469-0691.12582. [DOI] [PubMed] [Google Scholar]
- van Loo I, Huijsdens X, Tiemersma E, De Neeling AJ, van De Sande-Bruinsma N, Beaujean D, Voss A, Kluytmans J. Emergence of methicillin-resistant Staphylococcus aureus of animal origin in humans. Emerg Infect Dis. 2007;13(12):1834–1839. doi: 10.3201/eid1312.070384. http://doi.org/10.3201/eid1312.070384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijen MML, Bosch T, Verkade EJM, Schouls L, Kluytmans JAJW. Livestock-associated MRSA carriage in patients without direct contact with livestock. PLoS One. 2014;9(6):e100294. doi: 10.1371/journal.pone.0100294. http://doi.org/10.1371/journal.pone.0100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis. 2005;11(12):1965–1966. doi: 10.3201/eid1112.050428. http://doi.org/10.3201/eid1112.050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W. Ecological impact of antibiotic use in animals on different complex microflora: Environment. Int J Antimicrob Agents. 2000;14(4):321–325. doi: 10.1016/s0924-8579(00)00144-8. http://doi.org/10.1016/S0924-8579(00)00144-8. [DOI] [PubMed] [Google Scholar]


