Summary
Epilepsy and psychogenic nonepileptic seizures (PNES) can coexist and may present in two forms: sequential and simultaneous. In sequential presentations, epileptic seizures (ES) are treated and PNES emerge later. Simultaneous recording of ES and PNES by video‐electroencephalogram (vEEG) is less well described. We retrospectively reviewed all patients diagnosed with PNES by vEEG following standard seizure induction practices over a 21‐month period. Within this cohort, we established the prevalence of coexisting epilepsy using clinical and electrographic data acquired from our epilepsy‐specific patient record. We identified patients with simultaneous PNES and ES recorded during a single vEEG admission, establishing the frequency and emergent timing of each type. Of our 262 monitored patients, 59 were diagnosed with PNES. Nineteen of the patients with PNES had coexisting epilepsy (prevalence rate of 7.3% or 32% of those with PNES). Sixteen patients had PNES and ES recorded during the same admission, and the remaining three patients had sequential PNES following successful treatment of ES. PNES occurred earlier (mean, within 1.21 days), with ES occurring later (mean, within 4.86 days). The simultaneous occurrence of PNES and ES recorded during a single admission is more common than previously reported. Identifying this group of patients may require a significantly longer period of vEEG monitoring and a detailed analysis of each individual's historical seizure events.
Keywords: Psychogenic nonepileptic seizures, Epileptic seizures, Simultaneous PNES/ES, Sequential ES/PNES, Video‐EEG
Psychogenic nonepileptic seizures (PNES) are common in epilepsy practice, with an estimated prevalence of two to 33 per 100,000.1 The initial clinical suspicion is on the basis of the history and examination. A number of semiological features strongly suggestive of PNES have been described and include maintained awareness, gradual onset or termination, discontinuous, irregular, or asynchronous motor activity including side‐to‐side head movement, pelvic thrusting, stuttering, and weeping, eye closure, and the ability of bystanders to modulate the intensity of the symptoms.2, 3 The gold standard for differentiating PNES from epileptic seizures (ES) continues to be continuous video‐electroencephalography (vEEG).4 Admission to an epilepsy monitoring unit (EMU) results in the definitive diagnosis of PNES in almost 90% of patients.3
PNES pose a diagnostic challenge, with an average delay to diagnosis of up to 7.2 years.5 Concurrent epilepsy is frequently seen in patients with PNES and may complicate the diagnostic process. The percentage of PNES patients with coexisting epilepsy has varied from 3.6% to 58% in different studies.6, 7, 8, 9 Both ES and PNES were recorded in one admission in between 4.6% and 21% of patients diagnosed with PNES by vEEG.5, 8, 9, 10
The coexistence of epilepsy and PNES can be said to take one of two forms, sequential or simultaneous.11 Sequential epilepsy and PNES are typically seen in patients with childhood or adolescent epilepsy. The epilepsy remits or is controlled by antiepileptic drugs (AEDs), and then PNES emerge later in life. In contrast, simultaneous epilepsy and PNES describes patients with both active epilepsy and PNES. It has been long thought that sequential epilepsy and PNES are more common than simultaneous epilepsy and PNES. The sequential presentation of PNES following resolution of ES suggests that in some patients, PNES may be a learned behavior or may result from a subconscious psychological or social dependence on being disabled by epilepsy.12
The aim of this study was to establish the prevalence of coexisting epilepsy and PNES and to determine the frequency of simultaneous PNES and ES recorded during the same admission to our EMU. We sought to define the differential clinical aspects of this subgroup. We also attempted to characterize the relationship between PNES and ES recorded by vEEG, establishing the frequency and emergent timing of each type.
Methods
Patient profiles
We performed a retrospective analysis of all patients monitored by vEEG between June 2013 and February 2015 at the National Epilepsy Programme at Beaumont Hospital, Dublin. Clinical and demographic data on patients were acquired from our epilepsy‐specific electronic patient record.13 Figure 1 demonstrates the profile of patients admitted to our EMU during this period. During this period, there were 20 nondiagnostic studies. Studies where no interictal epileptiform abnormalities were identified, studies where no events were captured, and studies where patients did not tolerate the investigation were defined as nondiagnostic.
Figure 1.
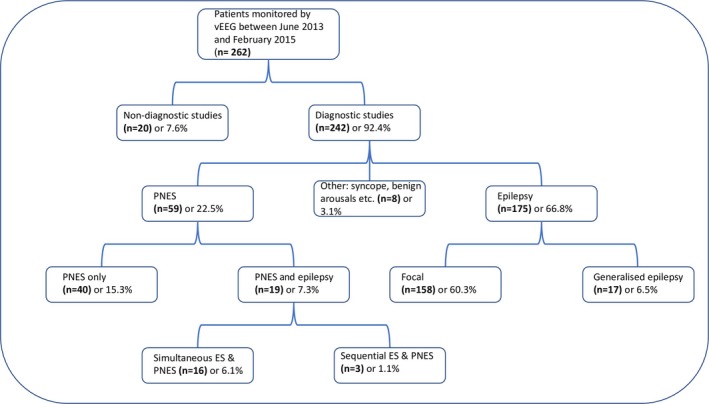
Profile of patients admitted to our epilepsy monitoring unit during the study period. ES, epileptic seizures; PNES, psychogenic nonepileptic seizures; vEEG, video‐electroencephalography.
We identified patients with recorded PNES and determined the prevalence of coexisting epilepsy within our EMU cohort. PNES patients were deemed to have coexisting epilepsy when ES were captured by vEEG, interictal epileptiform activity was recorded, or historical clinical and electrographic data confirmed epilepsy. Using Krumholz and Ting's classification,11 we subcategorized patients with PNES and epilepsy into two groups: (1) simultaneous PNES/ES (PNES and ES recorded during a single admission for vEEG monitoring) and (2) sequential ES/PNES (epilepsy successfully treated or in remission with PNES captured during vEEG monitoring). Within the simultaneous PNES/ES subgroup, we determined the frequency and emergent timing of PNES and ES.
Video‐EEG monitoring
Patients were generally admitted to the EMU for three indications: diagnostic classification, presurgical evaluation, or treatment adjustment. The standard international 10–20 system of electrode placement was used, including bilateral anterior temporal electrodes. Standard referential and bipolar montages were used for EEG review, as well as specifically reformatted arrays when necessary. Our monitoring unit runs a continuous 7‐day service, and thus monitoring continues over the weekend. A push button was available for the patient and/or staff to flag the occurrence of subjective or objective behavioral events. Continuous computerized spike and seizure detection was performed throughout the recording period. Behavior was monitored by continuous video recordings of patient activities and by maintenance of a log of activities by both the patient and the nursing staff. EEG was reviewed on a daily basis, and interim reports were generated as required. Electrocardiogram samples were also reviewed daily. Standard seizure induction practices were used, including careful drug reduction, sleep deprivation, photic stimulation, and hyperventilation.
Statistical analysis
We collected patient clinical and electrographic data such as sex, age, epilepsy type, number of AEDs, frequency of PNES and ES recorded, and emergent timing of PNES and ES and performed descriptive statistical analyses.
Results
From June 2013 to February 2015, 262 patients were monitored, and of those, 59 (32%) were diagnosed with PNES. In this cohort, 19 patients (23% of those with PNES) were found to have both epilepsy and PNES. The prevalence rate of coexisting PNES and epilepsy was 7.3% (19 of 262) of all EMU admissions, whereas the prevalence rate of PNES in patients with recorded ES or interictal epileptiform abnormalities was 9.8% (19 of 194). Simultaneous PNES and ES were captured during the same period of monitoring for 16 patients, whereas the remaining 3 patients had sequential ES and PNES, with a history of epilepsy in remission and PNES captured on current vEEG (see Table 1).
Table 1.
General characteristics of study population and patients with coexisting PNES and epilepsy
| Total number of patients monitored | 262 | |
| Female, n (%) | 162 (61.8) | |
| Age, yr (SD) | 37.58 (12.42) | |
| Total number of patients diagnosed with PNES (%) | 59 (22.5) | |
| Total number of patients with coexisting PNES and epilepsy (%) | 19 (7.3) | |
| Variables | Simultaneous PNES/ES, n = 16 | Sequential ES/PNES, n = 3 |
| Age, yr (SD) | 39.9 (11.3) | 33.7 (6.8) |
| Female, n (%) | 13 (81.25) | 3 (100) |
| Epilepsy type | 14 focal | 3 focal |
| 2 generalized | ||
| Age at 1st ES, yr (SD) | 12 (11.1) | 18.3 (5.9) |
| Family history of epilepsy, n (%) | 8 (50%) | 3 (100%) |
| Epilepsy surgery, n (%) | 2 (12.5%) | 2 (66.7%) |
| Comorbid psychiatric diagnosis, n (%) | 6 (37.5%) | 2 (66.7%) |
| Intellectual disability, n (%) | 4 (25%) | 0 |
| Number of AEDs at time of monitoring (SD) | 2.7 (1.6) | 1.7 (1.2) |
| Number of AEDs trialed (SD) | 7.5 (4) | 7 (3) |
AED, antiepileptic drug; ES, epileptic seizures; PNES, psychogenic nonepileptic seizures; SD, standard deviation.
Both subgroups were similar in terms of age, gender, and epilepsy type (see Table 1). Overall, 17 patients had focal epilepsy. Patients with coexisting epilepsy and PNES tended to have their first seizure in adolescence. All patients had drug‐resistant epilepsy, with a mean number of AEDs tried of approximately seven per patient. A family history of epilepsy, comorbid psychiatric illness, and intellectual disability were relatively common in patients with a dual diagnosis. Two of the patients with sequential ES and PNES had previous epilepsy surgery.
In patients with simultaneous PNES and ES, the number of nonepileptic events recorded largely outnumbered the number of ES. On average, 18.6 ± 12 PNES were recorded per patient. In comparison, we captured 3.1 ± 2.9 ES per patient. We recorded ES in 50% of the simultaneous PNES/ES patients prior to drug weaning (see Table 2). In the remaining seven simultaneous PNES/ES patients, ES emerged after AEDs were gradually withdrawn and in general, PNES were more likely to occur earlier in comparison to ES. The mean duration to first PNES was 1.2 ± 0.4 days, whereas the mean duration to first ES was 4.9 ± 3.6 days in the simultaneous PNES/ES group. Our data demonstrated a delayed emergence of ES in contrast to PNES necessitating extension of the monitoring period to >7 days in five cases (see Table 2).
Table 2.
Individual patient data for patients with simultaneous PNES and ES
| No. | Age, yr | Sex | Epilepsy type | Seizure type | AEDs, n | Drug ↓ prior to ES | Time to 1st ES, days | PNES, n |
|---|---|---|---|---|---|---|---|---|
| 1 | 28 | F | Focal (frontal) | GTC | 3 | No | 2 | 29 |
| 2 | 38 | F | Focal (temporal) | CPS | 3 | Yes | 3 | 18 |
| 3 | 44 | F | Focal (frontal) | CPS | 2 | No | 2 | 18 |
| 4 | 28 | M | Focal (temporal) | CPS | 4 | Yes | 10 | 18 |
| 5 | 17 | F | Generalized | GTC | 2 | No | 6 | 24 |
| 6 | 24 | F | Generalized | Myoclonus | 1 | No | 3 | 6 |
| 7 | 41 | F | Focal (temporal) | CPS | 1 | Yes | 7 | 40 |
| 8 | 38 | F | Focal (temporal) | CPS | 2 | Yes | 12 | 4 |
| 9 | 46 | F | Focal (frontal) | CPS | 2 | No | 1 | 20 |
| 10 | 54 | F | Focal (frontal) | 2° GTC | 2 | Yes | 9 | 36 |
| 11 | 60 | M | Focal (temporal) | CPS | 2 | No | 1 | 27 |
| 12 | 42 | F | Focal (frontal) | CPS | 2 | Yes | 5 | 2 |
| 13 | 48 | F | Focal (temporal) | CPS | 5 | Yes | 6 | 15 |
| 14 | 51 | F | Focal (temporal) | 2° GTC | 2 | No | 3 | 3 |
| 15 | 41 | M | Focal (temporal) | 2° GTC | 3 | Yes | 8 | 10 |
| 16 | 38 | F | Focal (frontal) | CPS | 7 | No | 1 | 3 |
AED, antiepileptic drug; CPS, complex partial seizure; ES, epileptic seizures; F, female; GTC, generalized tonic–clonic; M, male; PNES, psychogenic nonepileptic seizures.
Illustrative cases
Case 1 (simultaneous PNES/ES)
A 40‐year‐old woman with a history of childhood encephalitis was admitted for a diagnostic confirmation of suspected PNES. A collateral history revealed two separate distinct events. During her more frequent episodes, she would hyperventilate and asynchronously move her limbs. These were associated with apparent loss of awareness. Less frequently, she would have staring episodes with speech arrest and altered awareness. As a consequence of these spells, she was involved in two road traffic accidents. Prior to vEEG monitoring, she had a normal routine EEG and magnetic resonance imaging of the brain. She was monitored for 8 days and had 40 episodes with loss of awareness and asynchronous limb jerking without associated EEG changes consistent with PNES. By day 6 after drug reduction, theta slowing and sharp waves were seen over the right temporal region. On day 7, she had a complex partial seizure with loss of awareness, oral automatisms, speech arrest, and left head deviation with associated ictal EEG changes in the right temporal region.
Case 2 (sequential ES/PNES)
We conducted 6 days of continuous vEEG monitoring on a 38‐year‐old left‐handed woman with recurrent episodes of whole body shaking. She has a history of focal epilepsy secondary to cortical dysplasia. We recorded 22 of the patient's typical events over 6 days. These were associated with body jerking, hand tremor, unresponsiveness, and reported numbness of the right side. The patient had frequent “jumps” where she would have upper limb jerks. There were no changes seen in the EEG to support a diagnosis of active epileptic seizures as a cause or provoking factor in these episodes. The observed behavior was consistent with PNES. Collateral history and video review involving her next of kin confirmed PNES to be the sole current seizure burden with controlled ES. It is our standard practice in such cases to seek a next of kin unbiased review of events, following patient permission for this.
Discussion
The prevalence of coexisting epilepsy and PNES in our cohort (7.3%) was greater than reported in previous studies.8, 9, 10 Furthermore, our results contrast Krumholz and Ting's11 assertion that sequential ES/PNES are more common than simultaneous ES/PNES. It has been postulated that PNES may occur after ES in focal epilepsy, due to ictal disinhibition of emotion or impulse control facilitating the development of conversion symptoms.14 In our EMU, all 16 patients with simultaneous ES and PNES developed PNES during the first 2 days of monitoring prior to developing ES. Although the latency between first PNES and ES may be related to drug reduction, half the patients with simultaneous PNES and ES had no changes made to their AEDs during monitoring. Patients with a dual diagnosis tended to be on multiple AEDs and had a long history of epilepsy. In other series, patients with ES/PNES had drug‐resistant epilepsy15 or early onset of epilepsy predating the onset of PNES.6 These findings support the theory of model learning as a possible basis for their PNES.12
In those with PNES and ES recorded during a single EMU admission, the mean number of PNES captured outnumbered ES approximately 6:1. Furthermore, after recording a PNES we waited an additional 3.65 days per patient to record an ES where the clinical history was suspicious for epilepsy. We are unaware of previous reports characterizing this approach and temporal relationship. The two illustrative cases histories demonstrate the contrasting challenges and presentation of simultaneous and sequential PNES/ES. Our first case emphasizes the importance of persisting with monitoring when the history is suspicious for epilepsy. As interictal abnormalities did not manifest until after drug reduction at day 5 of monitoring, this patient could easily, but erroneously, have been diagnosed with PNES only. A period of monitoring off AEDs may be necessary in patients with recorded PNES and risk factors or clinical indicators for epilepsy to confidently rule out a dual diagnosis. Our second case was presented because the initial clinical assumption was that most of her events were epileptic in nature with an established diagnosis of epilepsy secondary to cortical dysplasia. However, the prolonged period of vEEG and collateral history revealed an exclusive burden of PNES, with controlled ES.
Outpatient EEG with video recording is often used as a diagnostic tool for PNES.16 However, this technique is not suitable for many patients, given the concern that an individual patient may be experiencing both ES and PNES in the community. Because of the complex comorbidity of PNES with ES, longer vEEG recordings and EMU admissions may be necessary to confidently detect the presence of both types of seizures, especially when historical indicators and risk factors for epilepsy are present. As the treatment of PNES alone involves tapering and stopping AEDs, failure to consider a diagnosis of coexisting epilepsy could have devastating consequences. As some patients required longer periods of monitoring to establish the diagnosis of simultaneous PNES and ES, our study emphasizes the advantage of being able to monitor patients 24 h per day, 7 days per week.
In conclusion, patients with simultaneous PNES and ES may be more common than previously reported. Patients may have treatment‐resistant epilepsy, and have early onset of epilepsy with PNES occurring later. When monitored by vEEG, PNES tend to occur earlier in the recording, whereas ES emerge later. To ensure maximal diagnostic accuracy, we recommend longer periods of vEEG monitoring, particularly when there are clinical indicators for epilepsy.
Disclosure
The authors declare no conflicts of interest. We read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Biography
Hany El‐Naggar is a consultant neurologist and researcher at the Royal College of Surgeons, Ireland.

References
- 1. Brown RJ, Syed TU, Benbadis S, et al. Psychogenic nonepileptic seizures. Epilepsy Behav 2011;22:85–93. [DOI] [PubMed] [Google Scholar]
- 2. Syed TU, LaFrance WC Jr, Kahriman ES, et al. Can semiology predict psychogenic nonepileptic seizures? A Prospective Study. Ann Neurol 2011;69:997–1004. [DOI] [PubMed] [Google Scholar]
- 3. Dickinson P, Looper KJ. Psychogenic nonepileptic seizures: a current overview. Epilepsia 2012;53:1679–1689. [DOI] [PubMed] [Google Scholar]
- 4. Gedzelman ER, LaRoche SM. Long‐term video EEG monitoring for diagnosis of psychogenic nonepileptic seizures. Neuropsychiatr Dis Treat 2014;10:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reuber M, Fernandez G, Bauer J, et al. Diagnostic delay in psychogenic nonepileptic seizures. Neurology 2002;58:493–495. [DOI] [PubMed] [Google Scholar]
- 6. Reuber M, Qurishi A, Bauer J, et al. Are there physical risk factors for psychogenic non‐epileptic seizures in patients with epilepsy? Seizure 2003;12:561–567. [DOI] [PubMed] [Google Scholar]
- 7. Benbadis SR, Agrawal V, Tatum WO. How many patients with psychogenic nonepileptic seizures also have epilepsy? Neurology 2001;57:915–917. [DOI] [PubMed] [Google Scholar]
- 8. Martin R, Burneo JG, Prasad A, et al. Frequency of epilepsy in patients with psychogenic seizures monitored by video EEG. Neurology 2003;61:1791–1792. [DOI] [PubMed] [Google Scholar]
- 9. Chen‐Block S, Abou‐Khalil BW, Arain A, et al. Video‐EEG results and clinical characteristics in patients with psychogenic nonepileptic spells: the effect of a coexistent epilepsy. Epilepsy Behav 2016;62:62–65. [DOI] [PubMed] [Google Scholar]
- 10. Krumholz A, Thomas D. Relevance of psychogenic and epileptic seizures coexisting in an epilepsy monitoring unit population. Neurology 2014;82(suppl 10):P4.257. [Google Scholar]
- 11. Krumholz A, Ting T. Co‐existing epileptic and nonepileptic seizures In Kaplan PW, Fisher RS. (Eds) Imitators of epilepsy. 2nd Ed New York: Demos Medical Publishing, 2005:261–276. [Google Scholar]
- 12. Reuber M. The etiology of psychogenic non‐epileptic seizures: toward a biopsychosocial model. Neurol Clin 2009;27:909–924. [DOI] [PubMed] [Google Scholar]
- 13. Fitzsimons M, Dunleavy B, O'Byrne P, et al. Assessing the quality of epilepsy care with an electronic patient record. Seizure 2013;22:604–610. [DOI] [PubMed] [Google Scholar]
- 14. Devinsky O, Gordon E. Epileptic seizures progressing into nonepileptic conversion seizure. Neurology 1998;51:1293–1296. [DOI] [PubMed] [Google Scholar]
- 15. Herskovitz M. Psychogenic nonepileptic seizure patterns in patients with epilepsy. Psychosomatics 2015;56:78–84. [DOI] [PubMed] [Google Scholar]
- 16. McGonigal A, Oto M, Russell AJ, et al. Outpatient video EEG recording in the diagnosis of non‐epileptic seizures: a randomised controlled trial of simple suggestion techniques. J Neurol Neurosurg Psychiatry 2002;72:549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]


