Summary
Objective
Automated seizure detection and alarming could improve quality of life and potentially prevent sudden, unexpected death in patients with severe epilepsy. As currently available systems focus on tonic–clonic seizures, we want to detect a broader range of seizure types, including tonic, hypermotor, and clusters of seizures.
Methods
In this multicenter, prospective cohort study, the nonelectroencephalographic (non‐EEG) signals heart rate and accelerometry were measured during the night in patients undergoing a diagnostic video‐EEG examination. Based on clinical video‐EEG data, seizures were classified and categorized as clinically urgent or not. Seizures included for analysis were tonic, tonic–clonic, hypermotor, and clusters of short myoclonic/tonic seizures. Features reflecting physiological changes in heart rate and movement were extracted. Detection algorithms were developed based on stepwise fulfillment of conditions during increases in either feature. A training set was used for development of algorithms, and an independent test set was used for assessing performance.
Results
Ninety‐five patients were included, but due to sensor failures, data from only 43 (of whom 23 patients had 86 seizures, representing 402 h of data) could be used for analysis. The algorithms yield acceptable sensitivities, especially for clinically urgent seizures (sensitivity = 71–87%), but produce high false alarm rates (2.3–5.7 per night, positive predictive value = 25–43%). There was a large variation in the number of false alarms per patient.
Significance
It seems feasible to develop a detector with high sensitivity, but false alarm rates are too high for use in clinical practice. For further optimization, personalization of algorithms may be necessary.
Keywords: Epilepsy, Seizure monitoring, Heart rate, Accelerometry, Sudden unexpected death in epilepsy
Key Points.
Nocturnal seizure detection devices are badly needed for intractable patients and their caregivers
Nocturnal seizure alarms should be limited to clinically urgent seizures
Combined sensors have high sensitivity
The number of false positive alarms in the current general system is still too high
To lower false positives, algorithm optimization will require some degree of personalization
Despite rapid expansion of pharmaceutical and surgical treatment options, approximately 30% of epilepsy patients continue to have seizures.1 In an unsupervised environment, seizures can be dangerous due to falls, harm from violent movements, confusional wandering, or status epilepticus. The situation is especially delicate during the night, when supervision is difficult. Seizures during sleep often go unnoticed, especially in patients who sleep alone. Sudden unexpected death in epilepsy (SUDEP) occurs most often during the night, and unsupervised patients are at risk.2
Automated seizure detection and alarming of a caregiver could improve quality of care of patients with severe epilepsy and potentially prevent SUDEP.3 Application in a home setting can also guide patient care through evaluation of therapy, and improve the quality of clinical trials where seizure frequency is used as an outcome. Patient diaries are generally unreliable.4
Whereas (video)‐electroencephalography (EEG) is used clinically to detect seizures, EEG is not suitable for long‐term use in institutions or at home. Non‐EEG signals such as heart rate (HR) and motion are more patient‐friendly and easier to measure and use for automatic detection.5 Several systems are available, but independent validation of detection algorithms is often lacking, and sensitivity and false alarm rates (FARs) are not sufficient for monitoring.6, 7 Audio systems that are often used at night detect only a minority of the seizures.7, 8 Many systems focus on specific seizure types, mainly generalized tonic–clonic (GTC) seizures. Detection of a broader range of seizure types could be more clinically relevant. When considering patient safety, the main seizure types where patients would need caregiver attendance are: (1) tonic–clonic seizures, as these are associated with SUDEP9; (2) generalized tonic (GT) seizures, where respiratory distress can occur; (3) hypermotor (HM) seizures, where there is a high risk of injury; and (4) clusters of myoclonic and/or short tonic seizures, where intervention medication and comforting is often provided.
In this article, we describe changes in non‐EEG signals during nocturnal seizures and nonseizure periods. We describe a universal, multimodal seizure detection algorithm, using real‐time data from on‐body sensors measuring HR and movement. We describe shortcomings, and discuss potential value in various clinical and home settings.
Methods
Study design and participants
This multicenter, prospective cohort study was approved by the Medical Ethics Committee of the University Medical Centre Utrecht and the institutional review boards of the Stichting Epilepsie Instellingen Nederland (SEIN), Heemstede and Zwolle, the Netherlands and Kempenhaeghe epilepsy centers in the Netherlands. All participants and/or legal guardian(s) provided written informed consent. The study population consisted of adults and children older than 2 years, with a history of nocturnal seizure frequency >1 seizure/week, admitted to one of the centers for long‐term (>24 h) video‐EEG monitoring between March 2012 and March 2014. HR and accelerometry were obtained separately. The clinical video‐EEG was used as a gold standard to assess occurrence of seizures. Measurements were performed during the night.
Data collection
Accelerometry and electrocardiography (ECG) signals were measured with a Shimmer sensor (Shimmer, Ireland) worn in an armband around the upper arm. The Shimmer combines three‐dimensional accelerometers with chest leads for ECG recording, and has a wireless transmitter to send signals to a personal computer. A trained EEG technician, blinded to the seizure detector data, retrospectively analyzed the video‐EEG and marked the occurrence of seizures. Ictal video‐EEG was then annotated by two independent clinical neurophysiologists blinded to the seizure detector data. They classified the seizures as GTC, GT, HM,10 or seizure cluster, defined as a series of at least five tonic or myoclonic spasms within 3 min. Seizures were also labeled “clinically urgent” when attendance or intervention was deemed necessary, based on seizure severity, postictal arousal state, breathing difficulties, and distress. A final classification was made through consensus discussion. Seizures shorter than 10 s and seizures classified as “other” were annotated as nonseizure data for the development of the seizure detector.
Signal preprocessing
HR was extracted from the ECG data using a MATLAB implementation by Afonso et al.11 The data were split into two equal parts to create a training and a test set (Fig. 1). A seizure was considered detected when, within 5 min before and 5 min after the seizure, a detection was recorded by the algorithm. Detections >5 min after a seizure were considered false positives. False positive detections <5 min apart were scored as one. Average sensitivity and FAR per patient and time from start of seizure until detection (detection delay) were calculated to assess performance (response time). FAR was calculated as FAR per standardized night (number of false alarms per 8 h).
Figure 1.
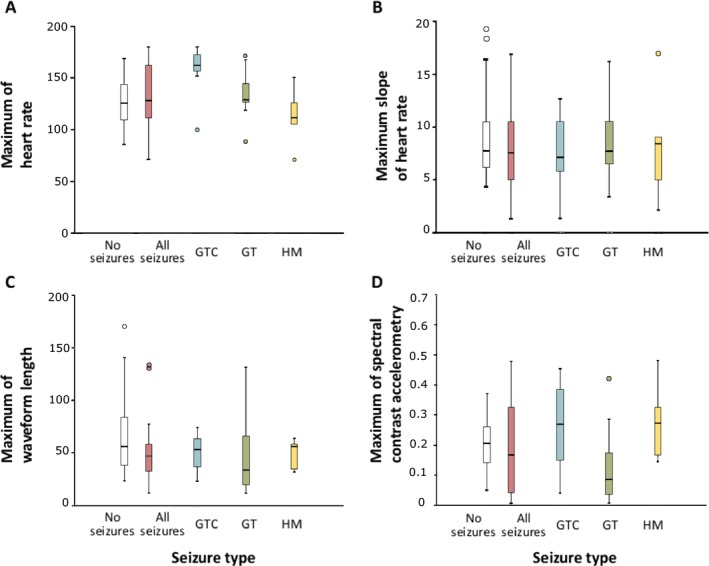
Boxplots showing median, 25th percentile, 75th percentile, and range of maximum heart rate (A), maximum slope of the heart rate (B), maximum summed waveform length (C), and maximum of spectral contrast (Kalitzin et al.25) in accelerometry (D) of included patients during seizure and nonseizure periods. Variation among patients is generally high, which can be seen in the wide range of maximum values found during seizures as well as during nonseizure periods. Maximum of the heart rate shows the most distinction for generalized tonic–clonic (GTC) seizures. GT, generalized tonic; HM, hypermotor.
Seizure detection: physiological characterization of ictal HR and movement
HR as beats per minute and summed waveform length of the accelerometry signal were used to detect seizures. The sum of the waveform lengths of acceleration in all three axes was taken as a measure for total amount of movement. HR and total movement were used to detect seizures. Both HR and summed waveform lengths were resampled at 1 Hz to allow easier interpretation of the results. Many authors have shown that HR increases can precede motor phenomena in several types of seizures.12, 13, 14 This relation is not fixed in time, and the delay between an HR increase and motion activity varies among patients and with seizure type. Three algorithms were explored: (1) one based on movement, (2) one based on HR, and (3) one combining both modalities. Algorithms were optimized in the training set and evaluated on the test set.
For algorithm 1, the number of seconds in which summed waveform length was higher than a fixed threshold within a fixed window was counted. A detection was recorded when this number was higher than the window length divided by four. For example, when using a window length of 30 s, eight or more counts of summed waveform lengths higher than the fixed threshold will generate a detection. Window length (range = 8–35 s) and threshold (range = 5–35) were optimized in the data of the patients with seizures from the training set. The combination of window length and threshold with the highest sensitivity and positive predictive value (PPV) was used to test the final performance of the algorithm in the test set.
For algorithm 2, HR at the beginning and at the end of a set window length (8–35 s) was calculated. If the HR had risen by a predefined factor, or if the HR exceeded a predefined “extreme” threshold, a detection was made. The factor and the extreme threshold depended on the baseline HR at the start of the window length. A low baseline HR required a lower factor and a lower extreme threshold. Window length, factors, and extreme thresholds were again optimized in the data of patients with seizures from the training set, whereas the sensitivity and PPV of these optimized combinations were assessed in the test set.
The last algorithm (3) combined the optimized algorithms for summed waveform length and HR. A detection was made when at least one of the algorithms fulfilled the set conditions. This “either HR or accelerometry” approach was chosen because seizures with a lot of motion may not trigger the algorithm based on HR because of the motion‐induced artifacts in the HR signal.
A more detailed description can be found in the Appendix S1.
The funding source had no role in the study design, data collection, data analysis, preparation of this article, or decision to submit this article for publication.
Results
Data were collected in 95 patients from three different centers. Due to failures in the wireless connection between sensors and recording software and internal sensor failures, data from 52 patients could not be used (median age = 14 years, range = 4–63, 48% male, six patients with seizures; Table 1). For eight patients, recorded data could only partly be used due to temporary sensor failure during the night. This resulted in a dataset of 43 patients with 402 h of recorded data. Major motor seizures were recorded in 23 patients. The other 20 patients were used to include extra seizure‐free data to train the algorithms and to test performance for occurrence of false alarms. Median age of included patients was 15 years (range = 2–65), 30 patients were male (70%), and 15 patients had intellectual disability (35%). In patients with seizures, the median number of seizures was two, ranging from one to 15 seizures per night (Table 2).
Table 1.
Number and type of seizures in patients not included in the dataset (six patients with seizures)
| Seizure type | Seizures, n (patients, n) | Clinically urgent seizures, n (%) |
|---|---|---|
| Tonic–clonic | 7 (3) | 7 (100%) |
| Tonic | 5 (2) | 3 (60%) |
| Hypermotor | 1 (1) | 1 (100%) |
| Cluster | ||
| Total | 13 (6) | 11 (85%) |
Table 2.
Number and type of seizures included in the dataset (23 patients with seizures)
| Seizure type | Seizures, n (patients, n) | Clinically urgent seizures, n (%) |
|---|---|---|
| Tonic–clonic | 18 (7) | 17 (94%) |
| Tonic | 41 (12) | 22 (54%) |
| Hypermotor | 18 (5) | 13 (72%) |
| Cluster | 9 (5) | 7 (78%) |
| Total | 86 (23) | 59 (69%) |
Seizures and nonseizure episodes
Fig. 1 shows maximum HR, slope of HR, movement, and spectral contrast of movement during seizure and nonseizure periods. When considering all seizure types together, there is large overlap between maximum values during seizures and nonseizure periods. Maximum HR is generally higher for GTC seizures, much less so for GT seizures, and unremarkable for HM seizures. Slope of the HR and summed waveform length show less distinct patterns. Spectral contrast is higher for GTC and HM seizures, although the range of values is wide for seizures as well as for nonseizure periods. Variation among patients is large for all signals. In Fig. 2 and Fig. S1–S3, examples are shown. GTC seizures show the most distinct patterns of HR and motion. In some cases, it is difficult to distinguish GT and HM seizures from nonseizure activity.
Figure 2.
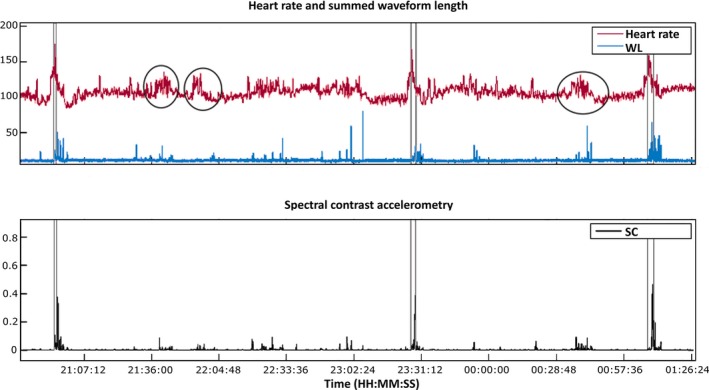
Heart rate and accelerometry data in a patient with three generalized tonic–clonic seizures. The clinical seizures occur between the gray vertical lines. The top panel shows heart rate (HR) and summed waveform length (WL), and the bottom panel shows spectral contrast (SC) of the accelerometry. The black circles highlight non–seizure‐related rises in HR. All seizures come with a high rise in HR, an increase in summed waveform length, and high spectral contrast in the range of 2–6 Hz. In all three seizures, the rise in HR is visible before seizure onset. Also, HR has reached high levels before movement is registered, which is due to the tonic phase, which starts the seizure and in which amplitude of movement is very low.
Performance of algorithms
Table 3 shows the performance of the three algorithms. Their average sensitivity and FAR based on stepwise fulfillment of conditions are shown in Fig. S4. Sensitivity is much higher when only clinically urgent seizures are considered, with a marginal increase in FAR. The highest sensitivity (87%) is reached in clinically urgent seizures based on both HR and movement. One GTC seizure and one HM seizure are missed with this algorithm; all other seizures (23) are detected. However, this comes with a high FAR of 6.3. Detection of clinically urgent seizures based only on motion has the lowest FAR of 2.3 false alarms/night. In this scenario, one extra GTC seizure, one GT seizure, two HM seizures, and three seizure clusters are missed (sensitivity = 71%). The algorithm based on only HR yields more false alarms (4.6), but only misses two extra GT seizures and one cluster of seizures and detects five of six GTC seizures (sensitivity for all seizures = 84%). Most seizures (90%) were detected within 30 s after or even before seizure onset. Only two seizures (5%) were detected >1 min after seizure onset. The average delay in detection was 13 s.
Table 3.
Overview of sensitivity and false alarm rate (number of false alarms per night) for evaluated algorithms
| All seizures | Clinically urgent seizures | |||
|---|---|---|---|---|
| Sensitivity | FAR | Sensitivity | FAR | |
| Stepwise algorithm, HR | 60% | 4.3 | 74% | 4.6 |
| Stepwise algorithm, Mvt | 56% | 2.3 | 71% | 2.3 |
| Stepwise algorithm, HR/Mvt | 71% | 5.9 | 87% | 6.3 |
FAR, false alarm rate; HR, heart rate; Mvt, movement.
The number of false alarms generated by the algorithms varied highly between patients. In eight of 22 patients in the test set, FAR was below two. Fig. S5 shows a patient with one GTC seizure. The seizure was detected early through motion detection and rise in HR. One false alarm was generated by the algorithm based on motion at the beginning of the night, when the patient was still awake and interacting with a caregiver. In some patients, HR or movement patterns were erratic, causing many false alarms (Fig. S6–S7). Fig. S6 shows a patient with three GT seizures. All were detected, but many false alarms were generated by restless movement during the night. In this patient, an HR‐based detector seems more suitable. Fig. S7 shows a patient with five single and five clusters of short GT seizures. In this case, not enough motion is generated to measure significant increases through accelerometry. HR‐based detection generates many false alarms, because signal quality is suboptimal and there are many arousals. Extra sensors or more sophisticated features may be necessary in this patient.
Discussion
Based on our findings, it does not seem feasible to develop a “one‐algorithm‐fits‐all” universal seizure detector. Sensitivities are good, especially for clinically urgent seizures (71–87%), but come with a high FAR (2.3–5.7). There was a large variation in number of false alarms, indicating that for some patients a one‐algorithm‐fits‐all system is suitable, whereas in others it is not. Particular weaknesses in our study are the reliability of HR detection in the face of movement, and the stability of wireless transmission of the Shimmer sensor.
Our findings are in line with other studies of automatic, non–EEG‐based detection of motor seizures.7 Most studies do not aim for a universal detector, but focus on, for exampe, GTC seizures for which new algorithms15 and validation of existing devices16, 17 show promising results, with sensitivities around 90% and FARs < 1/24 h. We also found the highest sensitivity of detection for GTC seizures and could, by raising detection thresholds, lower the number of false alarms. The focus on GTC seizures is understandable, as they are usually clinically urgent, with high rises in HR and distinct rhythmic movements. However, other seizure types can also be clinically urgent, or important for diagnostic purposes. Caregivers expect a detection system to alarm in any motor seizure that would require attendance, because it poses risks or major discomfort. This is different from using a system that would inform a neurologist about the actual number of seizures during the night. Restricting detection to GTC seizures would substantially improve performance of our system, but we considered this incompatible with what caregivers would want.18 Studies on detection of multiple seizure types until now show poor overall results. Evaluation of three types of mattress sensors did not yield sensitivities of >30%.19, 20 The use of HR to detect tonic, myoclonic, and GTC seizures yielded variable results, with a best performance (in one patient only) of 90% and PPV of around 50%. A more recent study in complex partial seizures before secondary generalization and GTC, using HR variability, also suggests that either individualization of algorithms or selection of patients based on previous video‐EEG and ECG findings is necessary.21
Methodological considerations
We need to consider the missing data in our study. Due to information transport issues and sensor failure, data from 52 of 95 patients were not usable for analysis. The main reasons were the sensor not being connected to the base station via Bluetooth, a bad connection between the ECG leads and the Shimmer sensor, and human error in handling hardware or data. Missing data seemed to occur in a random fashion, although sensor failure could sometimes be related to specific movements and therefore have led to a selection bias. In patients with seizures and failure of the ECG sensor, ECG data from the video‐EEG were used. This was not done in patients without seizures.
Even with less data than expected, we stuck to the principle of splitting data in an independent training and test set, instead of using double cross‐validation,15 which leads to less generalizable results. Our goal was a proof‐of‐concept for development of a universal seizure detector; therefore, we wanted to test performance of the algorithm in an independent test set.
We tried to build an alternative algorithm using HR and accelerometry parameters at the same time (switching from an OR to an AND paradigm). The problem is that both signals are not relevant at the same time during a seizure. During a convulsive seizure, the accelerometer signal shows good features, but the HR signal is too noisy due to motion artifacts. During a seizure with a minor motor component, the accelerometer signal has no variation, whereas the HR signal might show significant changes. If technology can provide a portable and noninvasive HR sensor that is not sensitive to motion artifacts, then we can use both signals for alarming. In this case, the false positives will be lower than in the current approach. We recently changed to the use of photoplethysmography as a sensor for HR, with much better results.
The assumption in the algorithms are all based on observations of physiological changes during nocturnal seizures, making them easily generalizable to other patients and leading to a low computational load. Adding other features with physiological rationale, such as electromyography (EMG) to measure the high‐frequency muscle activity during tonic seizures, may improve these algorithms.
A major strength of our study is the inclusion of children and adults with intellectual disability, who are most in need of automatic seizure detection.
Seizures versus arousals
We wonder whether physiological differences between short tonic and HM seizures and normal arousals during the night are large enough to allow automatic detection. For HM seizures, studies have shown that similar cortical pattern generators are involved as in arousals.22 Sympathetic activation is similar before clinical onset of HM seizures and of periodic limb movements.14, 23
Practical issues in seizure detection
Technical failures make the sensors used in this study unsuitable for long‐term, in‐home use. More robust sensor design and transmission need to be developed. The relatively high sensitivity of detection algorithms is promising, especially in clinically urgent seizures, and compares favorably with other devices.24 However, the FAR is currently prohibitive. Especially in the home‐based and institutional setting with alarm, tolerance for false alarms will be low. At home, this means unwanted interruption of much needed sleep. In institutions, where one caregiver often cares for many patients, if all these patients have a seizure detector yielding four to five erratic false alarms per night, the caregiver will be continuously attending to false alarms. In a diagnostic in‐hospital or research setting, the high number of false alarms is perhaps less bothersome, and results can be corrected for a known FAR.
Future directions
To bring automatic seizure detection another step further, we gain several suggestions from our study. First, in some patients distinguishing nocturnal HM and GT seizures from arousals is difficult when only accelerometer and HR data are used. Use of video/audio automated analysis is promising25 and has the advantage of not requiring something attached to the body. EMG‐based analysis shows promise for detection of the tonic phase of GTC seizures26 and to improve detection of GT seizures. Another interesting modality concerns autonomic imbalance before, during, and after seizures. Although cortical arousal mechanisms for HM seizures and nocturnal nonseizure events are probably very similar, differences in the timing and degree of sympathetic activation were found.14 HR variability is not a robust indicator of sympathetic activity,27 but electrodermal activity may be used instead.15
Second, performance of algorithms varies between patients. This is dependent on the dominant seizure type, but also on their HR and movement patterns during nocturnal arousals. As suggested by other researchers, personalized detection algorithms could improve performance of a detector in these patients.6, 21 To develop these, more data per patient are necessary to identify patterns in arousal‐like episodes and during several seizure types.
Lastly, further validation of algorithms in long‐term data is needed to assess their true performance. This requires measurements, preferably in a home setting, with very robust sensors. This may also give insight in the practical role of a seizure detector. This is essential, as a detector that is not accepted by patients or caregivers is of no value.18
Conclusion
It seems feasible to develop a detector with a high sensitivity for clinically relevant seizure types, including GT seizures, seizure clusters, and HM seizures besides tonic–clonic seizures. A physiological approach with stepwise fulfillment of conditions was used to develop these algorithms. Current FARs, however, are too high for use in clinical practice. Further optimization and personalization of algorithms in long‐term data from a home setting could solve this issue. Also, new generations of (lead‐independent) devices, new sensor modalities, and new methods of wireless transmission will lead to improvements. These next steps in development of automatic seizure detection at home will ultimately help improve the quality of care of patients with persistent seizures.
Disclosure
The authors declare no conflicts of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Study Funding
This study was funded with a Disease Management of Chronic Diseases grant from the Dutch National Science Foundation (300040003) and a supplementary grant from the Dutch Epilepsy Foundation (Epilepsiefonds).
Supporting information
Appendix S1. Specification of methods.
Figure S1. Heart rate and accelerometry data in a patient with two generalized tonic seizures. The seizures occur between the vertical gray lines. The top panel shows heart rate and summed waveform length of accelerometry, and the bottom panel shows spectral contrast of the accelerometry. Around the occurrence of the seizures, a small rise in heart rate (HR) is visible. Between seizures, shorter and longer rises in HR occur. The accelerometer is not sensitive enough to pick up the low‐amplitude and high‐frequency motion occurring during these tonic seizures.
Figure S2. Heart rate and accelerometry data in a patient with three hypermotor seizures. The seizures occur between the vertical gray lines. The top panel shows heart rate and summed waveform length of accelerometry, and the bottom panel shows spectral contrast of the accelerometry. Two sudden rises in heart rate occur in this patient during the night, unrelated to seizures (black circles). Distinct increases in summed waveform length and spectral contrast are visible only during seizure periods.
Figure S3. Heart rate (HR) and accelerometry data in a seizure‐free period. The top panel shows HR and summed waveform length, and the bottom panel shows spectral contrast in accelerometry. The black circles highlight non–seizure‐related rises in heart rate and movement. In this patient, three arousal‐like events are seen with a high increase in summed waveform length and an increase in HR. This pattern can be difficult to discern from, for instance, hypermotor seizures. The third arousal‐like event seems to give rise to a more permanent state of arousal, with a shift in baseline HR.
Figure S4. Sensitivity and false alarm (FA) rate (number of FAs per 8‐h night) of three tested algorithms in all seizures and in clinically urgent seizures. The algorithm based on heart rate and movement has the highest sensitivity but also generates many false alarms. HR, heart rate; Mvt, movement.
Figure S5. Performance of the stepwise fulfillment of condition‐based algorithms in a patient with one generalized tonic–clonic seizure (between blue bars in upper panel). One false alarm was generated by the algorithm based on motion at the beginning of the night. HR, heart rate; Mvt, movement.
Figure S6. Performance of the stepwise fulfillment of condition‐based algorithms in a patient with three tonic seizures. Seizures are indicated in the top panel with blue vertical lines. All three seizures are detected; however, many false alarms are generated by increases in summed waveform length due to restless movement during the night. HR, heart rate; Mvt, movement.
Figure S7. Performance of the stepwise fulfillment of condition‐based algorithms in a patient with five tonic seizures and five clusters of short tonic seizures (seizures indicated with blue bars in upper panel). Heart rate–based detection generates many false alarms, partly because of suboptimal quality of the signal and partly because of many arousal‐like events in nonseizure periods. HR, heart rate; Mvt, movement.
Biography
Judith van Andel has completed her doctorate at University Medical Center Utrecht, focusing on seizure detection.

References
- 1. Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 2011;52:657–678. [DOI] [PubMed] [Google Scholar]
- 2. Lamberts RJ, Thijs RD, Laffan A, et al. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia 2012;53:253–257. [DOI] [PubMed] [Google Scholar]
- 3. Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 4. Hoppe C, Poepel A, Elger CE. Accuracy of patient seizure counts. Arch Neurol 2007;64:1595–1599. [DOI] [PubMed] [Google Scholar]
- 5. Osorio I, Schachter S. Extracerebral detection of seizures: a new era in epileptology? Epilepsy Behav 2011;22(suppl 1):S82–S87. [DOI] [PubMed] [Google Scholar]
- 6. Van de Vel A, Cuppens K, Bonroy B, et al. Non‐EEG seizure‐detection systems and potential SUDEP prevention: state of the art. Seizure 2013;22:345–355. [DOI] [PubMed] [Google Scholar]
- 7. Van Andel J, Thijs RJ, De Weerd AW, et al. Non‐EEG based ambulatory seizure detection designed for home use: what is available and how will it influence epilepsy care? Epilepsy Behav 2016;57:82–89. [DOI] [PubMed] [Google Scholar]
- 8. Nijsen TM, Arends JB, Griep PA, et al. The potential value of three‐dimensional accelerometry for detection of motor seizures in severe epilepsy. Epilepsy Behav 2005;7:74–84. [DOI] [PubMed] [Google Scholar]
- 9. Hesdorffer DC, Tomson T, Benn E, et al. Do antiepileptic drugs or generalized tonic‐clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia 2012;53:249–252. [DOI] [PubMed] [Google Scholar]
- 10. Lüders H, Acharya J, Baumgartner C, et al. Semiological seizure classification. Epilepsia 1998;39:1006–1013. [DOI] [PubMed] [Google Scholar]
- 11. Afonso V, Tompkins W, Nguyen T, et al. ECG beat detection using filter banks. IEEE Trans Biomed Eng 1999;46:192–202. [DOI] [PubMed] [Google Scholar]
- 12. Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: prevalence and definition of an objective clinical sign. Epilepsia 2002;43:847–854. [DOI] [PubMed] [Google Scholar]
- 13. Mayer H, Benninger F, Urak L, et al. EKG abnormalities in children and adolescents with symptomatic temporal lobe epilepsy. Neurology 2004;63:324–328. [DOI] [PubMed] [Google Scholar]
- 14. Calandra‐Buonaura G, Toschi N, Provini F, et al. Physiologic autonomic arousal heralds motor manifestations of seizures in nocturnal frontal lobe epilepsy: implications for pathophysiology. Sleep Med 2012;13:252–262. [DOI] [PubMed] [Google Scholar]
- 15. Poh MZ, Loddenkemper T, Reinsberger C, et al. Convulsive seizure detection using a wrist‐worn electrodermal activity and accelerometry biosensor. Epilepsia 2012;53:e93–e97. [DOI] [PubMed] [Google Scholar]
- 16. Narechania AP, Garic II, Sen‐Gupta I, et al. Assessment of a quasi‐piezoelectric mattress monitor as a detection system for generalized convulsions. Epilepsy Behav 2013;28:172–176. [DOI] [PubMed] [Google Scholar]
- 17. Beniczky S, Polster T, Kjaer TW, et al. Detection of generalized tonic–clonic seizures by a wireless wrist accelerometer: a prospective, multicenter study. Epilepsia 2013;54:e58–e61. [DOI] [PubMed] [Google Scholar]
- 18. Van Andel JM, Leijten FSS, Van Delden JJM, et al. What makes a good home‐based nocturnal seizure detector? A value sensitive design. PLoS One 2015;10:e0121446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Poppel K, Fulton SP, McGregor A, et al. Prospective study of the Emfit movement monitor. J Child Neurol 2013;28:1434–1436. [DOI] [PubMed] [Google Scholar]
- 20. Fulton S, Van Poppel K, McGregor A, et al. Prospective study of 2 bed alarms for detection of nocturnal seizures. J Child Neurol 2013;28:1430–1433. [DOI] [PubMed] [Google Scholar]
- 21. Jeppesen J, Beniczky S, Johansen P, et al. Detection of epileptic seizures with a modified heart rate variability algorithm based on Lorenz plot. Seizure 2015;24:1–7. [DOI] [PubMed] [Google Scholar]
- 22. Tassinari CA, Cantalupo G, Högl B, et al. Neuroethological approach to frontolimbic epileptic seizures and parasomnias: the same central pattern generators for the same behaviours. Rev Neurol 2009;165:762–768. [DOI] [PubMed] [Google Scholar]
- 23. Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep 2007;30:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patterson AL, Mudigoudar B, Fulton S, et al. SmartWatch by SmartMonitor: assessment of seizure detection efficacy for various seizure types in children, a large prospective single‐center study. Pediatr Neurol 2015;53:309–311. [DOI] [PubMed] [Google Scholar]
- 25. Kalitzin S, Petkov G, Velis D, et al. Automatic segmentation of episodes containing epileptic clonic seizures in video sequences. IEEE Trans Biomed Eng 2012;59:3379–3385. [DOI] [PubMed] [Google Scholar]
- 26. Conradsen I, Beniczky S, Hoppe K, et al. Automated algorithm for generalized tonic–clonic epileptic seizure onset detection based on sEMG zero‐crossing rate. IEEE Trans Biomed Eng 2012;59:579–585. [DOI] [PubMed] [Google Scholar]
- 27. Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho‐vagal balance. Front Physiol 2013;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Specification of methods.
Figure S1. Heart rate and accelerometry data in a patient with two generalized tonic seizures. The seizures occur between the vertical gray lines. The top panel shows heart rate and summed waveform length of accelerometry, and the bottom panel shows spectral contrast of the accelerometry. Around the occurrence of the seizures, a small rise in heart rate (HR) is visible. Between seizures, shorter and longer rises in HR occur. The accelerometer is not sensitive enough to pick up the low‐amplitude and high‐frequency motion occurring during these tonic seizures.
Figure S2. Heart rate and accelerometry data in a patient with three hypermotor seizures. The seizures occur between the vertical gray lines. The top panel shows heart rate and summed waveform length of accelerometry, and the bottom panel shows spectral contrast of the accelerometry. Two sudden rises in heart rate occur in this patient during the night, unrelated to seizures (black circles). Distinct increases in summed waveform length and spectral contrast are visible only during seizure periods.
Figure S3. Heart rate (HR) and accelerometry data in a seizure‐free period. The top panel shows HR and summed waveform length, and the bottom panel shows spectral contrast in accelerometry. The black circles highlight non–seizure‐related rises in heart rate and movement. In this patient, three arousal‐like events are seen with a high increase in summed waveform length and an increase in HR. This pattern can be difficult to discern from, for instance, hypermotor seizures. The third arousal‐like event seems to give rise to a more permanent state of arousal, with a shift in baseline HR.
Figure S4. Sensitivity and false alarm (FA) rate (number of FAs per 8‐h night) of three tested algorithms in all seizures and in clinically urgent seizures. The algorithm based on heart rate and movement has the highest sensitivity but also generates many false alarms. HR, heart rate; Mvt, movement.
Figure S5. Performance of the stepwise fulfillment of condition‐based algorithms in a patient with one generalized tonic–clonic seizure (between blue bars in upper panel). One false alarm was generated by the algorithm based on motion at the beginning of the night. HR, heart rate; Mvt, movement.
Figure S6. Performance of the stepwise fulfillment of condition‐based algorithms in a patient with three tonic seizures. Seizures are indicated in the top panel with blue vertical lines. All three seizures are detected; however, many false alarms are generated by increases in summed waveform length due to restless movement during the night. HR, heart rate; Mvt, movement.
Figure S7. Performance of the stepwise fulfillment of condition‐based algorithms in a patient with five tonic seizures and five clusters of short tonic seizures (seizures indicated with blue bars in upper panel). Heart rate–based detection generates many false alarms, partly because of suboptimal quality of the signal and partly because of many arousal‐like events in nonseizure periods. HR, heart rate; Mvt, movement.


