Summary
Objective
Safety in epilepsy monitoring units (EMUs) has become an increasing concern because adverse events occur in up to 10% of patients undergoing long‐term video EEG in EMUs. The aim of this study was to assess the effectiveness of a specific safety protocol in an EMU.
Methods
We retrospectively assessed the adverse event rates in a group without (group 1, 84‐month period, Innsbruck, Austria) and a group with (group 2, 33‐month period, Salzburg, Austria) personalized safety measures utilizing a standardized protocol for long‐term epilepsy monitoring in high‐risk patients. Differences in adverse event rates during and after long‐term video EEG between the two groups were calculated and compared.
Results
In group 1, 44/507 (9%, 95% confidence interval [CI] 6.5–11.5%) patients experienced 53 adverse events: 20/507 (4%, 95% CI 2.6–6.0%) patients had psychiatric events, 15/507 (3%, 95% CI 1.8–4.8%) patients sustained a total of 19 injuries during seizures, and 10/507 (2%, 95% CI 1.1–3.6%) patients had 13 episodes of status epilepticus; one adverse event was treatment‐related (valproic acid–induced encephalopathy; 1/507, 0.2%, 95% CI 0.0–1.1%). By using the new safety protocol in group 2, the adverse event rate was only 5% (95% CI 3.4–7.6%; 30 adverse events in 26/491; 45% reduction; p = 0.036), in contrast. These events included 13 psychiatric complications in 13/491 (2%, 95% CI 1.6–4.5%, p = 0.252) patients, 12 seizure‐related injuries in 9/491 (2%, 95% CI 1.0–3.4%, p = 0.250) patients, and 5 episodes of status epilepticus in 4/491 (1%, 95% CI 0.3–2.1%, p = 0.120) patients.
Significance
Implementation of personalized safety measures in high‐risk patients resulted in a clinically relevant reduction of adverse events in the EMU. Safety protocols are a valid tool to reduce the occurrence of adverse events in EMUs.
Keywords: Safety, Adverse events, Long‐term video EEG, Epilepsy monitoring unit
Key Points.
Adverse events occur in up to 10% of patients undergoing long‐term video EEG in epilepsy monitoring units
With a personalized safety protocol, the adverse event rate could be reduced from 9% (44/507 patients) to 5% (26/491 patients)
Implementation of safety strategies in defined high‐risk patients resulted in a clinically relevant reduction of adverse events
Safety protocols are a valid tool to reduce the occurrence of adverse events in epilepsy monitoring units
Patients’ safety has become a major concern in the organization of epilepsy monitoring units (EMUs) because it is an iatrogenic exposure of patients to seizures and their potentially harmful consequences.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Despite growing awareness for safety issues in the EMU, many aspects remain to be addressed. The International League Against Epilepsy, the American Clinical Neurophysiology Society, and the National Association of Epilepsy Centers (NAEC) introduced a series of guidelines and recommendations, which mainly address technical aspects for long‐term monitoring with a specific emphasis on the equipment, personnel, and technical procedures, whereas recommendations on patient safety are less specific.16, 17, 18 This might mirror the current culture of patients’ safety, which lacks evidence‐based safety standards and data‐driven guidelines for identifying and addressing the potential safety risks for patients. Furthermore, several surveys about safety issues in EMUs revealed wide variation in practice patterns.19, 20, 21 The results raise safety concerns and underline the necessity for standardized approaches and definition of protective measures. In 2012, a workgroup of the American Epilepsy Society provided expert consensus‐based recommendations for EMU safety practices regarding seizure observation, antiepileptic drug withdrawal, management of acute seizures/status epilepticus, and safe environments/activities for EMU patients.22 The consensus was based on expert opinions and on literature, but the effectiveness has not yet been investigated in clinical practice.
In a recent survey of the European Epilepsy Monitoring Unit Association among 48 EMUs in 18 European countries, 79% (38/48) reported on status epilepticus in the past 15 years, 73% (35/48) reported injuries, 35% (17/48) reported bone fractures, 67% (32/48) reported psychoses, and 4% (2/48) reported sudden unexpected death in epilepsy.19 In our own series of 507 consecutive patients undergoing video‐EEG monitoring, 44 (9%) patients experienced 53 adverse events (4% psychiatric events, 3% injuries, 2% status epilepticus).8 The most common adverse events were predicted by previous medical history as independent risk factors: any psychiatric comorbidity was associated with a 16‐fold increased risk of a psychiatric adverse event during or after video‐EEG monitoring. A history of seizure‐related injuries or status epilepticus resulted in a more than 3‐fold increased risk in each case to sustain these adverse events also during video‐EEG monitoring. On the basis of these findings, we implemented personalized measures and identified patients with a higher risk for adverse events. So far, only two retrospective studies evaluated the impact of specific trainings and protocols in the EMU on patients’ safety.23, 24 In one study, safety efforts including continuous 24‐h observation by EEG technologists, and enhanced staff education resulted in a significant 77% decrease in missed seizures and a 15% reduction in the fall rate per 1,000 patient days, but the adverse event rate apart from falls was not reported.23 In a second study, a ceiling lift system extending into the bathroom of the EMU as a single measure was investigated, which helped to prevent any fall over a 15‐month period, but other effects of the introduced measure have not been reported, nor other types of adverse events reported.24
The aim of this study was to assess the effectiveness of personalized safety measures on the frequency of the most common adverse events—psychiatric complications, seizure‐related injuries, and status epilepticus—in patients undergoing prolonged video‐EEG monitoring in an EMU.
Methods
Patients and data collection
We evaluated adverse events in 1,241 video‐EEG sessions of 998 patients in two groups: group 1 includes 507 consecutive patients with 596 video‐EEG sessions. This group was examined retrospectively from the EMU of the Department of Neurology, Medical University, Innsbruck, Austria, between January 1999 and December 2005. The video‐EEG investigation was performed without a specific safety protocol (“preimplementation”). The detailed procedures and results have been reported previously.8 Group 2 includes 491 consecutive patients with 645 video‐EEG sessions. These patients were evaluated retrospectively at the Department of Neurology, Paracelsus Medical University, Salzburg, Austria, between January 2013 and September 2015 (“postimplementation”). Based on the results of our previous work, we implemented specific safety measures in group 2 (described in detail below). The incidence of adverse events (psychiatric adverse events, seizure‐related injuries, status epilepticus, and treatment‐related side effects) was assessed and compared between the two groups. Convulsive status epilepticus was defined as a generalized or focal convulsive seizure lasting longer than 5 min or when consciousness was not regained between two consecutive convulsive seizures.25 For nonconvulsive status epilepticus, we used the older definition of continuous seizure activity without major motor signs lasting longer than 30 min to be comparable over both periods.26
Moreover, reason for referral, etiology of the epilepsy syndrome, duration of monitoring, as well as number and type of recorded seizures or events were analyzed.
Video EEG, technical equipment, and staff
Both EMUs at the Departments of Neurology in Salzburg and Innsbruck serve four inpatient beds, three of them with a 64‐channel video‐EEG system and one bed with 128‐channel video‐EEG system (Salzburg: MicromedTM, Mogliano Veneto, Italy; Innsbruck: NeurofileTM, IT‐medTM, Bad Homburg, Germany). Each EMU is integrated in a general neurological ward with 24 (Innsbruck) and 29 (Salzburg; Fig. 1) beds each and share the same architectural structure and adapted furnishing. The EMUs were staffed by four EEG‐monitoring technicians with epilepsy‐specific training and competencies who worked in overlapping 8‐h shifts during the week from 7 a.m. to 7 p.m. They are located in the unit and view video and EEG in real‐time mode. In addition, during the week one nurse cares for the patients with regular nurse rounds (7 a.m. to 7 p.m. every hour, 7 p.m. to 7 a.m. every 2 h) and on the weekend every 2 h (maximum nurse‐patient ratio of 1:7.5 during daytime, 1:18 during nighttime and on the weekend). During nighttime and weekends, two specially trained medical, psychology, or biology students provide full 24‐h observation in the EMU. Specialized epileptologists and board‐certified electroencephalographers were present during official working time (40 h per week) and on call for the rest of the time (E.T., J.D., I.U., G.W., J.H., M.L., and G.K.).
Figure 1.

Epilepsy Monitoring Unit, Salzburg, Austria.
General safety strategies and specific safety protocol
Written informed consent was obtained from all patients at admission after they were counseled by the consulting epileptologist. The information included the aim of the procedure, its potential risks, and the EMU safety protocol (for publication purposes we translated the informed consent sheet from German to English; see Fig. 2). In group 2, risk factors for adverse events were assessed at time of admission to the EMU as well as occurrence of adverse events at time of discharge with a checklist (for publication purposes, we translated it from German to English; see Fig. 3).
Figure 2.
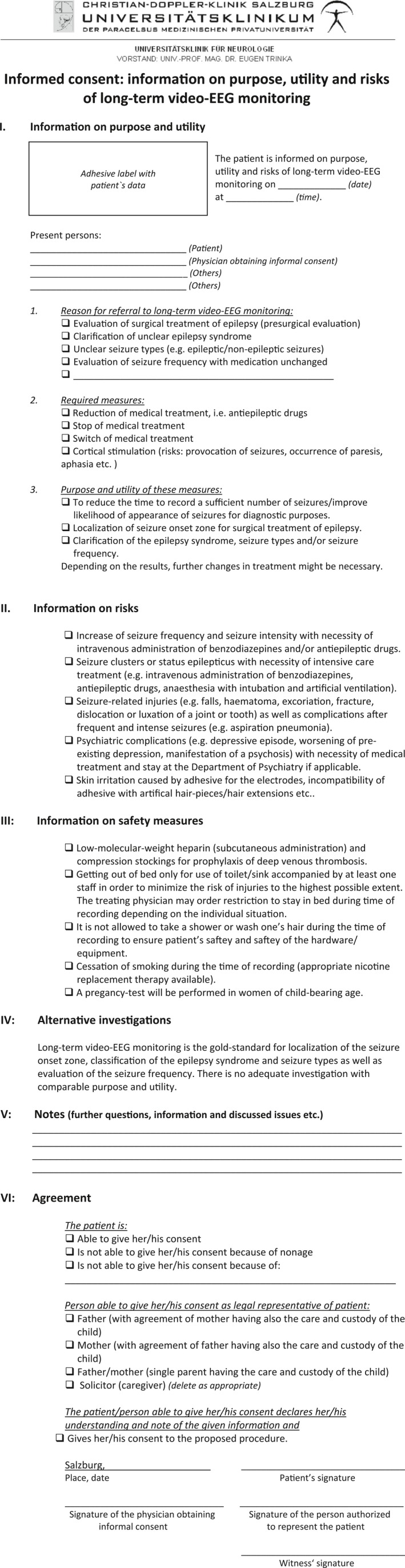
Informed Consent of the Epilepsy Monitoring Unit, Salzburg, Austria
Figure 3.
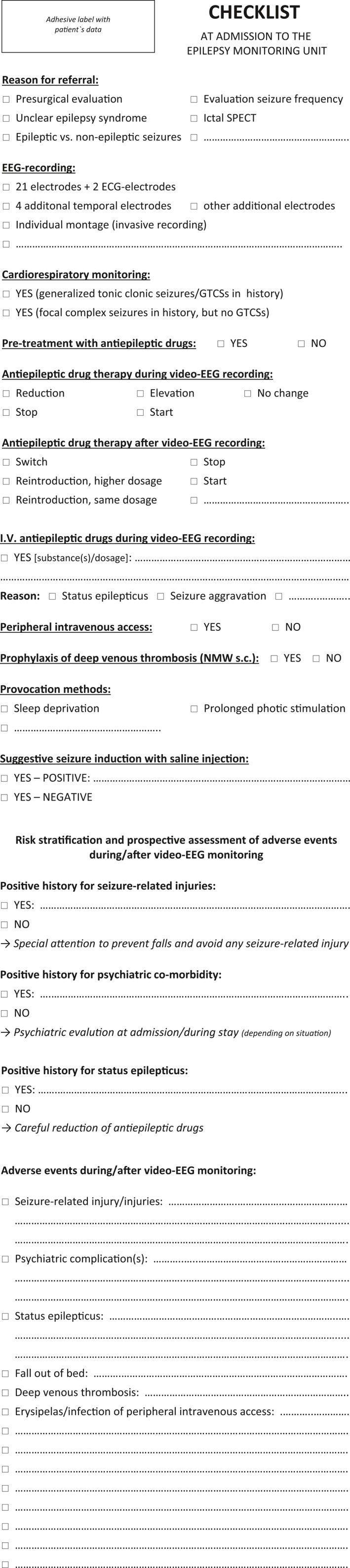
Check‐list of the Epilepsy Monitoring Unit, Salzburg, Austria, assessing Risk Factors for Adverse Events and Occurrence of Adverse Events during/after Long‐Term Video‐EEG
Several general safety precautions were applied to all patients of groups 1 and 2:
Continuous 24‐h surveillance by specially trained personnel for high patient safety and diagnostic accuracy, including testing of patients during and immediately after the seizures.
Vital signs are monitored continuously with cardiorespiratory telemetry (heart rate, oxygen saturation) in all patients.
Limited ambulation: all patients, especially those undergoing antiepileptic drug withdrawal and patients with implanted electrodes, are asked to leave their monitoring beds only temporarily for use of the toilet/bathroom. If they have to stand up, they are accompanied by a nurse. Moreover, all patients are instructed to take their meals in bed (sitting upright in bed).
Compression stockings and low‐molecular‐weight heparin subcutaneously are applied as well as physiotherapy performed daily to prevent deep vein thrombosis.
Adapted layout/furnishing to minimize the risk of seizure‐related injuries: sideboards with soft upholstery in up position (Fig. 1).
Withdrawal of the antiepileptic drugs is performed on an individual basis by the treating physician with respect to seizure frequency in the 3 months preceding the monitoring session, number and dosage of antiepileptic drugs, serum levels, age of the patient, and duration of the epilepsy. Therefore, antiepileptic drugs were tapered more slowly in patients with one or more seizures a week and more rapidly in patients with fewer than one seizure per month. The withdrawal of antiepileptic drugs was adjusted as necessary during monitoring.
Peripheral intravenous access during recording process (changed every 72 h at the latest) for intravenous administration of medical treatment in emergency situations such as seizure clusters and status epilepticus.
Written protocols for the treatment of seizure clusters and status epilepticus.
When an attack occurs, one staff member provides immediate bedside care to ensure patient's safety, performs peri‐ictal testing, including assessment of neurologic status according to a standardized protocol, and activates an audible alarm for additional nurse support.
Full access on a 24/7 basis to the medical staff of the Department of Neurology, including neurological intensive care unit, Department of Neurosurgery with neurosurgical intermediate care and intensive care unit, Department of Anesthesiology, and Department of Psychiatry.
In group 2, we implemented the following individualized measures for safety management in high‐risk patients for psychiatric complications, seizure‐related injuries, and status epilepticus:
In patients with psychiatric comorbidity and when antidepressive as well as antipsychotic treatment was considered, a psychiatrist was involved from the beginning of the video‐EEG monitoring.
In patients with a history of seizure‐related injuries, special attention was given to prevent falls and to avoid any seizure‐related injury: when out of bed the patient was always assisted by at least one staff member who stayed within arm's reach of the patient. For use of the bathroom, the accompanying person stayed outside and the door was left open; for use of the toilet, the person stayed outside the unlocked door or within arm's reach on patient's demand. Patients undergoing invasive recording were restricted to the bed at all times.
In patients with a history of status epilepticus, antiepileptic drugs, especially those possibly facilitating seizure clusters and status epilepticus, were tapered very carefully without complete withdrawal.27 According to our earlier published study, we defined “history of status epilepticus” as any type of status epilepticus occurring at any time before the monitoring session.8 Tapering of antiepileptic drugs was done on an individual basis. Basically, we followed general rules preassigned to our epilepsy center in patients with a history of status epilepticus: In case of monotherapy, a quarter to a third of the antiepileptic drug dosage was reduced every day or every 2 days, on average. In case of polytherapy, only one antiepileptic drug was tapered with half of the drug dosage every day or every 2 days. Further tapering of antiepileptic drugs was stopped after the first seizure occurred.
Statistical analysis
To summarize the clinical and demographic variables, counts were used for qualitative variables, and the median as well as the range were reported for quantitative variables. Ninety‐five percent confidence intervals for the group‐ and adverse‐event‐specific rates were calculated using Wilson's method.28 For comparisons of adverse event rates between group 1 and group 2, chi‐square tests were used if the number of counts was at least 1 in each cell. Otherwise, we used the two‐sided version of Fisher's exact test.29
According to the Austrian law on retrospective research, this retrospective study did not require the approval of the ethics committee.
Results
Demographic as well as clinical details of groups 1 and 2 are presented in Table 1. Groups differed slightly in the number of monitoring sessions (596 group 1 vs. 645 group 2) and etiology of epilepsy syndrome, with more symptomatic/structural/metabolic epilepsies in group 1 (52% group 1 vs. 40% group 2) but less cryptogenic/unknown epilepsy syndromes (21% group 1 vs. 30% group 2) compared to group 2. Patients of group 1 had a longer median duration of epilepsy than patients of group 2 (12 vs. 5 years). Fewer patients were referred for presurgical evaluation in group 2 (145/491; 30%) compared to group 1 (279/507; 55%), whereas the percentages of patients referred for an unclear epilepsy syndrome (32% Salzburg vs. 22% Innsbruck) as well as differentiation between epileptic versus nonepileptic seizures (30% Salzburg vs. 22% Innsbruck) were higher in group 2. Possibly related to that, a further noticeable difference is the number of recorded attacks (5,090 group 1 vs. 2,917 group 2) resulting from recorded epileptic seizures (4,594 group 1 vs. 2,471 group 2). In group 1, events occurred in 413/507 (81%) patients, and in 345/507 (68%) patients, an ictal EEG was recorded. In 94/507 (19%) patients, no events were recorded, but interictal epileptiform activity supported a positive diagnosis of epilepsy in 74/94. Therefore, accuracy of video‐EEG monitoring with establishing a clear diagnosis (epilepsy vs. nonepileptic attack disorders) was 96% (487/507) in group 1. In group 2, the accuracy was lower because of the lower number of recorded seizures but was still 85% (417/491): events were recorded in 238/491 (48%) patients, and an ictal EEG could be recorded in 38% (187/491). In 253/491 (52%) patients, no events were recorded, but history and interictal epileptiform activity supported the referral diagnosis of epilepsy or different types of nonepileptic attack disorders in 179 of them.
Table 1.
Demographic and clinical details of group 1 and group 2
| Group 1 | Group 2 | |
|---|---|---|
| Consecutive patients | 507 | 491 |
| City | Innsbruck | Salzburg |
| Men | 233 | 237 |
| Women | 274 | 254 |
| Median age, range (years) | 35, 9–80 | 40, 7–97 |
| Number of monitoring sessions | 596 | 645 |
| Distribution of number of stays | ||
| Repeated sessions (%) | 69/507 (14%) | 93/491 (19%) |
| Two times | 56 | 56 |
| Three times | 8 | 27 |
| Four times | 4 | 4 |
| Five times | 0 | 1 |
| Six times | 1 | 3 |
| Seven times | 0 | 1 |
| Eight times | 0 | 1 |
| Median monitoring duration per patient, range (days) | 4, 1–37 | 4, 1–32 |
| Invasive recordings | 27/507 (5%) | 7/491 (1%) |
| Consecutive epilepsy surgery | 19/27 | 4/7 |
| Reason for referral | ||
| Presurgical evaluation (%) | 279/507 (55%) | 145/491 (30%) |
| Unclear epilepsy syndrome (%) | 110/507 (22%) | 156/491 (32%) |
| Epileptic/nonepileptic seizures (%) | 113/507 (22%) | 147/491 (30%) |
| Evaluation of seizure frequency (%) | 5/507 (1%) | 43/491 (8%) |
| Etiology of epilepsy syndrome | ||
| Symptomatic/structural/metabolic (%) | 263/507 (52%) | 196/491 (40%) |
| Cryptogenic/unknown (%) | 109/507 (21%) | 147/491 (30%) |
| Idiopathic/genetic (%) | 26/507 (5%) | 39/491 (8%) |
| No epilepsy (%) | 108/507 (21%) | 105/491 (21%) |
| Median duration of epilepsy, range (years) | 12, 0–56 | 5, 0–79 |
| Number of recorded attacks | 5,090 | 2,917 |
| Epileptic seizures | 4,594 | 2,471 |
| Nonepileptic events | 496 | 448 |
| Median number of seizures per patient, range | 4, 0–889 | 0, 0–187 |
| Diagnosis of epilepsy based on ictal EEG (%) | 345/507 (68%) | 189/491 (38%) |
| Temporal seizure onset (%) | 194/507 (38%) | 79/491 (16%) |
| Frontal seizure onset (%) | 52/507 (10%) | 39/491 (8%) |
| Extratemporal or multifocal onset (%) | 26/507 (5%) | 15/491 (3%) |
| Generalized seizure onset (%) | 54/507 (11%) | 32/491 (7%) |
| Nonlocalizing seizure onset (%) | 19/507 (4%) | 24/491 (5%) |
Adverse events in both groups during and after long‐term video EEG are described in detail in Table 2. In group 1, 5 patients experienced two adverse events (3 patients with two seizure‐related injuries and 2 patients with two episodes of status epilepticus), and 2 patients had three adverse events (both with psychiatric complications and two seizure‐related injuries in the one patient and two episodes of status epilepticus in the other). In group 2, 2 patients experienced two adverse events (two falls during an epileptic seizure and two episodes of status epilepticus), and 1 patient had three adverse events (two falls during nonepileptic seizures and one fall during syncope). Before implementation of the new safety protocol, 53 adverse events occurred in 9% (44/507, 95% CI 6.5–11.5%) of the patients of group 1. Postimplementation, the overall adverse event rate was reduced to 26/491 (5%, p = 0.036; 45% reduction, 95% CI 3.4–7.6%) patients of group 2. No treatment‐related side effects occurred in group 2 (0/491, 95% CI 0.0–0.8%, p = 1.000). Further details of adverse event rates of group 1 and group 2, overall and for all four groups of adverse events, are given in Table 3. Table 4 shows the number of recorded attacks in patients with and without adverse events as well as the number of recorded attacks in presurgical and non‐pre‐surgical patients of group 1 compared to group 2. In both groups, patients with adverse events as well as presurgical patients had on average more recorded attacks than patients without adverse events and non‐pre‐surgical patients, respectively.
Table 2.
Adverse events in group 1 and group 2
| Adverse event | Group 1 | Group 2 |
|---|---|---|
| Psychiatric complications | 20 episodes in 20/507 (4%) pts.
|
13 episodes in 13/491 (2%) pts.
|
| Evaluation by psychiatrist | 20/20 pts. | 13/13 pts. |
| Hospitalization in Dept. of Psychiatry | 10/20 pts. | 1/13 pts. |
| Seizure‐related injuries | 19 episodes in 15/507 (3%) pts.
|
12 episodes in 9/491 (2%) pts.
|
| Status epilepticus | 13 episodes in 10/507 (2%) pts.
|
5 episodes in 4/491 (1%) pts.
|
| Treatment‐related side effects |
1/507 (0.2%) Acute encephalopathy after IV VPA under video‐EEG control for absence status |
– |
Dept., Department; GTCSs, generalized tonic‐clonic seizures; IV, intravenous; pt., patient; pts., patients; sGTCS, secondary generalized tonic‐clonic seizure; szs., seizures; VPA, valproic acid.
Table 3.
Adverse event rates of group 1 and group 2, overall and for every group of adverse events
| Adverse events | Group 1 (n = 507) | Group 2 (n = 491) | p Valuea |
|---|---|---|---|
| Overall number (%, 95% CI) | 44 (9, 6.5–11.5) | 26 (5, 3.4–7.6) | 0.036 |
| Psychiatric complications | 20 (4, 2.6–6.0) | 13 (2, 1.6–4.5) | 0.252 |
| Seizure‐related injuries | 15 (3, 1.8–4.8) | 9 (2, 1.0–3.4) | 0.250 |
| Status epilepticus | 10 (2, 1.1–3.6) | 4 (1, 0.3–2.1) | 0.120 |
| Treatment‐related side effect | 1 (0.2, 0.0–1.1) | 0 (0, 0.0–0.8) | 1.000 |
Chi‐square or Fisher's exact tests.
Table 4.
Number of recorded attacks in patients with and without adverse events and number of recorded attacks in presurgical and non‐pre‐surgical patients of group 1 compared to group 2
| Minimum | 1st quartile | Median | 3rd quartile | Maximum | |
|---|---|---|---|---|---|
| Group 1: Adverse events | 0 | 3 | 6 | 16 | 83 |
| Group 1: No adverse events | 0 | 1 | 4 | 9 | 889 |
| Group 2: Adverse events | 0 | 4 | 10 | 21 | 170 |
| Group 2: No adverse events | 0 | 0 | 0 | 4 | 187 |
| Group 1: Presurgical | 0 | 3 | 6 | 12 | 889 |
| Group 1: Non‐pre‐surgical | 0 | 0 | 2 | 5 | 119 |
| Group 2: Presurgical | 0 | 1 | 4 | 10 | 120 |
| Group 2: Non‐pre‐surgical | 0 | 0 | 0 | 1 | 187 |
In group 1, the rates of patients with adverse events were 11.5% (95% CI: 8.2–15.7%) in patients who were referred to the EMU for presurgical examinations (n = 279) and 5.3% (95% CI: 3.0–9.0%) in those who were referred to the EMU for other reasons (n = 228). In group 2, the percentages in those subgroups were 11% (n = 145; 95% CI: 6.0–13.4%) and 2.9% (n = 346; 95% CI: 1.0–4.8%), respectively. So, although the adverse event rates in presurgical patients of groups 1 and 2 were comparable, the adverse event rate in non‐pre‐surgical patients of group 2 was lower.
There was no mortality or permanent morbidity in patients with adverse events in both groups. The overall fall rate in group 1 was 6.6/1,000 inpatient‐days preimplementation, which could be reduced to 3.4/1,000 inpatient‐days in group 2 (48% reduction) postimplementation of the new safety protocol. Overall, falls due to seizures and syncope could be reduced from 17 falls in group 1 to 10 falls in group 2. In group 1, 12/17 falls occurred outside of bed (elsewhere than toilet/bathroom) and 5/17 falls on the toilet/at the bathroom. In group 2 only 3/10 falls were reported outside of bed (other than in the toilet/bathroom) but 7/10 falls on the toilet/at the bathroom.
Discussion
This study provides a systematic assessment of adverse events and complications in the EMU without and with implementation of personalized safety strategies in high‐risk patients for psychiatric complications, seizure‐related injuries, and status epilepticus. The group with personalized safety measures showed a clinically relevant lower rate of adverse events.
Optimizing patients’ safety management during prolonged video EEG has progressively become one of the key elements in the organization of EMUs. Previous work concerning patient safety mainly focused on reporting occurrence of adverse events in the EMU in selected patient groups or in consecutive patients.1, 2, 3, 4, 5, 6, 7, 9, 10, 11, 12, 13, 15 There is a need to establish standardized safety protocols to minimize adverse events and improve patients’ safety outcomes.3, 5, 7, 9, 11, 15 This was also emphasized by the NAEC in its revised guidelines for specialized epilepsy centers in 2010.18 In a review of 2014 summarizing the literature on patients’ safety in the EMU, the authors concluded that it is “time for revising practices”; they identified the lack of high‐level evidence as the main limiting factor to the development and release of appropriate standards and guidelines.14
In our previous study, we analyzed psychiatric comorbidity (16‐fold increased risk) and a positive history of seizure‐related injuries or status epilepticus (each more than 3‐fold increased risk) as independent risk factors in a large group of consecutive patients.8 On the basis of these findings, we employed individual safety measures in high‐risk patients undergoing prolonged video EEG in the EMU. The early involvement of a psychiatrist in case of any psychiatric comorbidity resulted in a reduction of psychiatric complications by half (from 4% to 2%). This measurement requires 24‐h availability of a psychiatrist, which was already strongly recommended by the German Austrian Swiss Quality Guidelines for presurgical evaluation.30, 31
Seizure‐related injuries could be diminished from 3% to 2% (19 episodes in 15/507 patients of group 1 vs. 12 episodes in 9/491 patients of group 2) after applying stricter safety rules. In comparing the location of falls (outside of bed other than in the toilet/bathroom vs. in the toilet/bathroom), the greatest reduction of falls was observed in the former group, from 66% in group 1 to 33% in group 2, most likely a consequence of closer observation and accompanied stand‐up. In contrast, falls on the toilet/at the bathroom haven't decreased at all, possibly because this area is nonaccessible to the accompanying person. Accompanied stand‐up with close supervision in patients with a history of seizure‐related injuries to avoid falls can be challenging with regard to patient privacy and autonomy despite appropriate counselling of the patients. In a recent study from the Mayo Clinic, both epileptic seizures as well as psychogenic nonepileptic seizures occurring in the bathroom were more likely to result in falls compared with events occurring elsewhere in the room.24 After implementation of a special ceiling lift system in the EMU that extended into the bathroom, zero falls occurred in the subsequent 15 months. This method seems to prevent seizure‐related falls very effectively, but acceptance by the patients as well as the high costs of the lift system might impede realization in many centers. Finally, the occurrence of status epilepticus as an adverse event in about 2–3% of the patients5, 8 underscores the necessity of 24‐h access to an intensive care unit in‐house.26 Management of antiepileptic drug withdrawal with standardized protocols of tapering is an unmet need in the organization of EMUs due to a lack of available guidelines. With the protocol established in our EMU in Salzburg, we could reduce the rate of status epilepticus from 2% to 1% postimplementation.
A study from the United States suggests that stricter safety rules help to improve patient safety in the EMU.23 The authors compared the incidence of missed seizures and only falls as one possible seizure‐related injury before and after implementation of new safety measures, whereas we additionally assessed the impact of personalized safety measures on psychiatric complications, status epilepticus, and any kind of seizure‐related injuries. In their study, stricter safety processes included dedicated staff education, improvement of patients’ supervision by nurses and EEG technicians, and immediate review of adverse events. This resulted in a significant 77% reduction of missed seizures, which was most likely the result of a continuous 24‐h observation by specially trained personnel. We didn't assess missed seizures in the two centers because 24‐h continuous observation of video‐EEG monitors involved EEG technologists and specially trained personnel in both EMUs. The reduction of the fall rate per 1,000 patient days (2.7 to 2.3, 15%) in this study was lower than in our patient group (6.6 to 3.4, 48%) with an already low fall rate in their study cohort.
In summary, we could observe a 45% reduction of the overall rate of adverse events postimplementation and clinically relevant reductions of the most common adverse events—psychiatric complications, seizure‐related injuries, and status epilepticus.
Our study provides further evidence that implementation of a personalized safety protocol can help prevent adverse events and complications in an EMU. We could identify individualized strategies in high‐risk patients that resulted in a demonstrable reduction of the adverse event rate, which may serve as evidence for the generation of guidelines and consensus statements regarding essential protocols in establishing EMU safety practices.
Limitations of our study are the retrospective assessment of the adverse event rates and the serial group design (evaluation of group 1 in 1999–2005; evaluation of group 2 in 2013–2015). The different locations (Innsbruck vs. Salzburg) as well as different staff who performed the video‐EEG investigations might also limit the interpretation of the results, although, essentially, the same treating physicians cared for the patients and trained the staff members in both centers. Despite the different time frames (84 vs. 33 months), the group size and number of monitoring admissions of both groups are comparable, whereas the different number of recorded epileptic seizures is noticeable. Taking into consideration the longer median duration of epilepsy in group 1, we cannot exclude a bias with more severe epilepsies and therefore higher seizure frequencies and so more recorded epileptic seizures in patients of group 1. This might also be supported by the fact that fewer patients were referred for presurgical evaluation in group 2 compared to in group 1 (30% vs. 55%). The number of recorded seizures in both groups depending on the occurrence of adverse events as well as on the referral reason of “presurgical/non‐pre‐surgical” may indicate that the adverse event group of group 1 contains the “more severe” cases, too. The number of recorded attacks tends to be lower in patients with adverse events in group 1 compared to in group 2, thus indicating that the safety protocol has a beneficial effect despite a larger average number of recorded attacks, and the contrary is true for the patients without adverse events. However, further examination of the potential confounding effect of the number of recorded attacks is difficult, because in both groups there are a few patients with very large numbers of attacks. The lower number of recorded epileptic seizures might also be a consequence of a more careful tapering of antiepileptic drugs, for example, in patients with a positive history of status epilepticus. Hence, the effect of the safety protocol on the reduction of adverse events might be overestimated and a lower adverse event rate might also be the consequence of fewer recorded epileptic seizures. Our study can only provide limited information on the management of antiepileptic drug withdrawal because this was done on an individual basis. Specifically, differences in the management of antiepileptic drug withdrawal in patients with status epilepticus between groups 1 and 2 are difficult to assess because withdrawal in all patients of group 1 was done on an individual basis as determined by the treating physician, whereas a more specific protocol was applied to patients in group 2. Thus, the available data do not allow provision of a detailed analysis of antiepileptic drug management withdrawal in both patient groups.
Psychiatric complications, seizure‐related injuries, and status epilepticus can be related to the occurrence and number of seizures during video‐EEG monitoring. Nevertheless, adverse events, for example, psychiatric complications, can also occur independently of the occurrence of seizures, which is the case in 2/44 patients of group 1 and 3/26 patients of group 2 as well as in the treatment‐related adverse event of group 1.
Further prospective multicenter studies are needed to evaluate the impact of the specific procedures on patients’ safety. Moreover, comparative research is still needed to determine safety strategies in reducing adverse events in the EMU setting.
Disclosure of Conflicts of Interest
J.D. has received honoraria and travel support from UCB Pharma, Gerot‐Lannach, Eisai, GlaxoSmithKline, and Neurodata GmbH/Micromed Austria. M.L. has received a travel grant from Medtronic and UCB Pharma. G. Kuchukhidze has received travel support from Eisai and UCB Pharma. J.H. has received speakers honoraria from UCB Pharma and travel support from Eisai and GL Pharma. I.U. has received advisory board/speakers honoraria from UCB Pharma, Eisai, GlaxoSmithKline, and GL Lannacher. G. Kalss has received travel support by UCB Pharma, Eisai, OEGN, Cyberonics, ILAE‐CEA, ILAE, and the Asian Oceanian Association of Neurology. A.R. has received travel support and speakers honoraria from Eisai. E.T. has acted as a paid consultant to Eisai, Ever Pharma, Biogen Idec, Medtronics, Bial, and UCB and has received speakers honoraria from Bial, Eisai, GL Pharma, GlaxoSmithKline, Boehringer, Viropharma, Actavis, Newbridge, Novartis, and UCB Pharma in the past 3 years. E.T. has received research funding from UCB Pharma, Biogen‐Idec, Red Bull, Merck, Bayer, the European Union, FWF Österreichischer Fond zur Wissenschaftsförderung, and Bundesministerium für Wissenschaft und Forschung. E.T. is also part of the European Reference Centre Pilot Project E‐PILEPSY (http://www.e-pilepsy.eu/). The remaining authors (G.Z., A.T., G.W., C.N., T.K., and Y.H.) have no conflicts of interest to declare. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guideline.
Acknowledgments
There are no acknowledged sources of support (grants from government agencies, private foundations, etc.), including funds obtained from private industry.
Biography
PD Dr. Judith Dobesberger is an epileptologist at the Department of Neurology in Salzburg, Austria.

References
- 1. Savard G, Andermann F, Olivier A, et al. Postictal psychosis after partial complex seizures: a multiple case study. Epilepsia 1991;32:225–231. [DOI] [PubMed] [Google Scholar]
- 2. Devinsky O, Abramson H, Alper K, et al. Postictal psychosis: a case control series of 20 patients and 150 controls. Epilepsy Res 1995;20:247–253. [DOI] [PubMed] [Google Scholar]
- 3. Sanders PT, Cysyk BJ, Bare MA. Safety in long‐term EEG/video monitoring. J Neurosci Nurs 1996;28:305–313. [DOI] [PubMed] [Google Scholar]
- 4. Haut SR, Swick C, Freeman K, et al. Seizure clustering during epilepsy monitoring. Epilepsia 2002;43:711–715. [DOI] [PubMed] [Google Scholar]
- 5. Rose AB, McCabe PH, Gilliam FG, et al. Occurrence of seizure clusters and status epilepticus during inpatient video‐EEG monitoring. Neurology 2003;60:975–978. [DOI] [PubMed] [Google Scholar]
- 6. Hui AC, Kwan P, Leung TW, et al. Diagnostic value and safety of long‐term video‐EEG monitoring. Hong Kong Med J 2007;13:228–230. [PubMed] [Google Scholar]
- 7. Noe KH, Drazkowski JF. Safety of long‐term video‐electroencephalographic monitoring for evaluation of epilepsy. Mayo Clin Proc 2009;84:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dobesberger J, Walser G, Unterberger I, et al. Video‐EEG monitoring: safety and adverse events in 507 consecutive patients. Epilepsia 2011;52:443–452. [DOI] [PubMed] [Google Scholar]
- 9. Atkinson M, Hari K, Schaefer K, et al. Improving safety outcomes in the epilepsy monitoring unit. Seizure 2012;21:124–127. [DOI] [PubMed] [Google Scholar]
- 10. Pati S, Kumaraswamy VM, Deep A, et al. Characteristics of falls in the epilepsy monitoring unit: a retrospective study. Epilepsy Behav 2013;29:1–3. [DOI] [PubMed] [Google Scholar]
- 11. Kandler R, Lai M, Ponnusamy A, et al. The safety of UK video telemetry units: results of a national service evaluation. Seizure 2013;22:872–876. [DOI] [PubMed] [Google Scholar]
- 12. Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 13. Ley M, Vivanco R, Massot A, et al. Safety study of long‐term video‐electroencephalogram monitoring. Neurologia 2014;29:21–26. [DOI] [PubMed] [Google Scholar]
- 14. Rheims S, Ryvlin P. Patients’ safety in the epilepsy monitoring unit: time for revising practices. Curr Opin Neurol 2014;27:213–218. [DOI] [PubMed] [Google Scholar]
- 15. Fahoum F, Omer N, Kipervasser S, et al. Safety in the epilepsy monitoring unit: a retrospective study of 524 consecutive admissions. Epilepsy Behav 2016;61:162–167. [DOI] [PubMed] [Google Scholar]
- 16. Velis D, Plouin P, Gotman J, et al. Recommendations regarding the requirements and applications for long‐term recordings in epilepsy. Epilepsia 2007;48:379–384. [DOI] [PubMed] [Google Scholar]
- 17. American Clinical Neurophysiology Society . Guideline twelve: guidelines for long‐term monitoring for epilepsy. J Clin Neurophysiol 2008;25:170–180. [DOI] [PubMed] [Google Scholar]
- 18. Labiner DM, Bagic AI, Herman ST, et al. Essential services, personnel, and facilities in specialized epilepsy centers—revised 2010 guidelines. Epilepsia 2010;51:2322–2333. [DOI] [PubMed] [Google Scholar]
- 19. Buelow JM, Privitera M, Levisohn P, et al. A description of current practice in epilepsy monitoring units. Epilepsy Behav 2009;15:308–313. [DOI] [PubMed] [Google Scholar]
- 20. Rubboli G, Beniczky S, Claus S, et al. A European survey on current practices in epilepsy monitoring units and implications for patients’ safety. Epilepsy Behav 2015;44:179–184. [DOI] [PubMed] [Google Scholar]
- 21. Kobulashvili T, Höfler J, Dobesberger J, et al. Current practices in long‐term video‐EEG monitoring services: a survey among partners of the E‐PILEPSY pilot network of reference for refractory epilepsy and epilepsy surgery. Seizure 2016;38:38–45. [DOI] [PubMed] [Google Scholar]
- 22. Shafer PO, Buelow JM, Noe K, et al. A consensus‐based approach to patient safety in epilepsy monitoring units: recommendations for preferred practices. Epilepsy Behav 2012;25:449–456. [DOI] [PubMed] [Google Scholar]
- 23. Spanaki MV, McCloskey C, Remedio V, et al. Developing a culture of safety in the epilepsy monitoring unit: a retrospective study of safety outcomes. Epilepsy Behav 2012;25:185–188. [DOI] [PubMed] [Google Scholar]
- 24. Spritzer SD, Riordan KC, Berry J, et al. Fall prevention and bathroom safety in the epilepsy monitoring unit. Epilepsy Behav 2015;48:75–78. [DOI] [PubMed] [Google Scholar]
- 25. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56:1515–1523. [DOI] [PubMed] [Google Scholar]
- 26. Recommendations of the Epilepsy Foundation of America's Working Group on Status Epilepticus . Treatment of convulsive status epilepticus. JAMA 1993;270:854–859. [PubMed] [Google Scholar]
- 27. Dobesberger J, Ristić AJ, Walser G, et al. Duration of focal complex, secondarily generalized tonic‐clonic, and primarily generalized tonic‐clonic seizures—a video‐EEG analysis. Epilepsy Behav 2015;49:111–117. [DOI] [PubMed] [Google Scholar]
- 28. Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927;22:209–212. [Google Scholar]
- 29. Irwin JO. Tests of significance for differences between percentages based on small numbers. Metron 1935;12:83–94. [Google Scholar]
- 30. Rosenow F, Bast T, Czech T, et al. Qualitätsleitlinien für prächirurgische Epilepsiediagnostik und operative Epilepsietherapie. Nervenarzt 2014;85:753–756. [DOI] [PubMed] [Google Scholar]
- 31. Rosenow F, Bast T, Czech T, et al. Revised version of quality guidelines for presurgical epilepsy evaluation and surgical epilepsy therapy issued by the Austrian, German and Swiss working group on presurgical epilepsy diagnosis and operative epilepsy treatment. Epilepsia 2016;57:1215–1220. [DOI] [PubMed] [Google Scholar]


