Abstract
Objective
Patients with cirrhosis are at increased risk for venous thromboembolism (VTE) and portal vein thrombosis (PVT). Cirrhosis due to non-alcoholic steatohepatitis (NASH) appears to be particularly prothrombotic. We investigated hospitalized patients with NASH cirrhosis to determine if they are at increased risk for VTE.
Methods
Data on adult hospitalized patients with cirrhosis and VTE (deep vein thrombosis and/or pulmonary embolism) between November 1, 2010 and December 31, 2015 were obtained. Cases with VTE were matched by age, gender, and model for end stage liver disease (MELD) score to corresponding controls without VTE.
Results
Two hundred and ninety subjects (145 matched pairs) with mean age of 58.4 ± 11.8 years and MELD score of 16.0 ± 7.2 were included. Baseline characteristics were similar between cases and controls. Independent adjusted risk factors for VTE included NASH (OR: 2.46, 95% CI: 1.07–5.65, p = 0.034), prior VTE (OR: 7.12, 95% CI: 1.99–25.5, p = 0.003), and presence of PVT (OR: 2.18, 95% CI: 1.03–4.58, p = 0.041). Thrombocytopenia was associated with decreased risk (OR: 0.49, 95% CI: 0.26–0.95, p = 0.035).
Conclusions
NASH is an independent risk factor for VTE among cirrhosis patients and provides further evidence that NASH is a hypercoagulable state. While all hospitalized patients with cirrhosis at risk for VTE should be considered for medical thromboprophylaxis, those with NASH cirrhosis are at particularly increased risk and therefore a high index of suspicion for VTE should be maintained even in the presence of thromboprophylaxis.
Introduction
Historically, patients with cirrhosis were thought to have an inherent “coagulopathy” that predisposed them to bleeding and, hence, protected against thrombosis. There is emerging evidence that shows a precariously rebalanced hemostatic system with simultaneous change in pro- and anti-hemostatic pathways1. This balance can be disrupted by hepatic decompensation, renal failure, active infection, or invasive procedures1. Hospitalized patients with cirrhosis are at increased risk for venous thromboembolism (VTE)2–4. Incidence rates of VTE in hospitalized patients with cirrhosis are between 0.5 and 8.2%5–11. Furthermore, a recent meta-analysis of eleven studies comprised of 695,012 subjects with cirrhosis documented a pooled adjusted odds ratio of 1.49 (95% CI: 1.22–1.76, p < 0.0001) when compared to subjects without cirrhosis4. While risk factors for VTE in hospitalized patients with cirrhosis are similar to other medical patients, disease-specific risk factors including hypoalbuminemia5,6, age8, male gender4, severity of liver disease9,10, and malnutrition11 have all been described. Despite an increased risk of VTE, medical thromboprophylaxis is often withheld in hospitalized patients with cirrhosis10 even with similar bleeding risks12.
The etiology of underlying liver disease may play a role in thrombotic risk in patients with cirrhosis. In particular, non-alcoholic steatohepatitis (NASH) may predispose to prothrombotic events through longstanding chronic inflammation leading to activation of the coagulation system13–20. Others have argued that obesity is more strongly associated with a hypercoagulable environment than NAFLD21. Despite the lack of a definitive mechanism, it is clear that patients with NASH develop clinically relevant thrombotic events. Multiple reports have shown an association between NASH cirrhosis and portal vein thrombosis (PVT) prior to liver transplantation when adjusting for comorbid metabolic risk factors22–24. Hospitalized patients with non-alcoholic fatty liver disease (NAFLD) may also be at increased risk for VTE25. For these reasons, we investigated the relationship between NASH and thrombosis further and hypothesize that hospitalized patients with NASH cirrhosis are at increased risk for VTE when adjusting for comorbid metabolic risk factors.
Methods
Case and control selection
Data on all hospitalized adult patients with cirrhosis and VTE between 2010 and 2015 were obtained from the University of Virginia Clinical Data Repository using billing and administrative codes. Cirrhosis of the liver was confirmed by direct review of the medical record by study personnel including histology (if available) or by biochemical, imaging, and endoscopic findings, suggesting advanced liver disease with portal hypertension. Deep vein thrombosis (DVT) was diagnosed by imaging with Doppler ultrasound formally interpreted by radiology. Pulmonary embolus (PE) was diagnosed by either computerized tomography (CT) of the chest or ventilation–perfusion scan formally interpreted by radiology. NASH was defined by review of the medical record for liver histology showing features of steatohepatitis or cryptogenic cirrhosis in the presence of metabolic risk factors (e.g., diabetes, hypertension, and obesity), both in the absence of significant alcohol intake (<100 g of alcohol intake per week). Cases with VTE were matched 1:1 by age (within 5 years), gender and model for end stage liver disease (MELD) score (within 2 points) to the corresponding controls with cirrhosis but no VTE. Cases with VTE were time matched within 90 days of VTE diagnosis to the corresponding controls to ensure reproducibility in medical assessment for and treatment of VTE. Hospitalized controls were presumed not to have VTE based on the absence of ICD codes for VTE. The diagnosis of VTE was confirmed by trained study personnel by review of available imaging and/or imaging reports that described a definitive thrombosis. Baseline patient characteristics were reviewed, including demographics (age, gender, and body mass index), etiology of liver disease, portal hypertensive complications (gastroesophageal varices, ascites, hepatic encephalopathy, portal vein thrombosis, and hepatocellular carcinoma), comorbidities (coronary artery disease, cardiomyopathy, diabetes, and hypertension), substance use (alcohol and tobacco smoking), medications, laboratory values, and imaging. VTE risk factors (prior admission within 90 days, presence of inherited or acquired thrombophilia, active cancer, respiratory failure, acute myocardial infarction or cerebrovascular accident, acute infection of any physiologic system, hormone therapy, recent trauma or surgery, prior VTE) were also collected. The Padua Prediction Score was calculated retrospectively and compared between groups (Table 1 shows a list of included variables)26. Immobility was defined according to the original definition put forth by Barbar et al.26 as bed rest >3 days (with bathroom privileges) due to physician order or patient limitations in functional status as was active cancer (local or metastatic cancer that had been treated with chemotherapy or radiation the previous 6 months).
Table 1.
Padua Prediction Score predicts risk of venous thromboembolism in acutely ill hospitalized medical patients (including those with cirrhosis)26
| Risk factor | Score |
|---|---|
| Active cancer ≤180 days | 3 |
| Previous VTE (excluding superficial thrombosis) | 3 |
| Reduced mobility | 3 |
| Inherited or acquired thrombophilic condition | 3 |
| Trauma/surgery ≤30 days | 2 |
| Age ≥70 years | 1 |
| CHF and/or respiratory failure | 1 |
| Acute MI or ischemic CVA | 1 |
| Acute infection and/or rheumatologic condition | 1 |
| Obesity (BMI >30 kg/m2) | 1 |
| Hormonal treatment | 1 |
A score ≥4 indicates increased risk of VTE
BMI body mass index, CHF congestive heart failure, CVA cerebrovascular accident, MI myocardial infarction, VTE venous thromboembolic disease
Statistical analysis
Univariate analysis was performed using paired ttest and McNemar’s test for continuous and categorical variables as appropriate. Conditional logistic regression models were constructed to assess risk factors for the development of new VTE utilizing the c-statistic as a standard measure of the predictive accuracy of the model. Patient characteristics were included as adjustment variables in the conditional logistic regression analysis if they had meaningful unadjusted levels of association in the paired univariate analysis (significant at p < 0.20), were clinically important, or had been shown to be significant in prior studies27,28. Categorical variables were analyzed as continuous variables where appropriate (albumin, BMI, and platelet count) prior to final model selection. Due to the inability to accurately capture grade of ascites based on data available for extraction in the medical record, we opted not to correct BMI for ascites. Covariates included in the final model were thrombocytopenia (platelet count <150 k/µL based on standard accepted definitions), hypoalbuminemia (serum albumin <2.8 g/dL as this has previously been shown to be the cutoff, where hypoalbuminemia is predictive of DVT risk5), proton pump inhibitor use, prior VTE, diabetes, coronary artery disease, PVT prior to VTE diagnosis, NASH, acute infection, and obesity (BMI >30 kg/m2 based on standard accepted criteria). BMI, thrombocytopenia, and hypoalbuminemia were included as categorical variables rather than continuous variables to improve clinical decision-making based on the findings of the model. All statistical tests for significance were two sided and a significance level p ≤ 0.05 was considered statistically significant. Data analysis and graph generation were performed using SAS Version 9.4 (Cary, NC, USA) and GraphPad Prism version 7.03 for Windows, GraphPad Software (La Jolla, CA, USA). Institutional review board approval was obtained from the University of Virginia Health Sciences Research Institutional Review Board. No data from prisoners or compromised individuals were included in this study.
Results
Two hundred and ninety subjects (145 matched pairs) with mean age of 58.4 ± 11.8 years and mean MELD score of 16.0 ± 7.2 (standard deviation) met inclusion criteria. Mean body mass index was 29.8 ± 0.6 kg/m2 (standard deviation). Sixty-two percent of the cohort was male and 77% had advanced cirrhosis with Child-Turcotte-Pugh Class B or C disease. Sixty-nine percent had ascites, 52% gastroesophageal varices, and 49% hepatic encephalopathy. The most common etiologies of cirrhosis were NASH (38%), chronic hepatitis C (26%), and alcoholic liver disease (24%). One hundred two cases had isolated DVT while 25 had PE without evidence of DVT. Eighteen cases were diagnosed with both PE and DVT simultaneously. Thirty-one (21%) cases with VTE had been admitted to the hospital within 90 days preceding their diagnosis of PE or DVT.
When comparing cases with VTE to controls without VTE, the two groups were similar with several notable exceptions (Table 2). While mean MELD scores were similar (15.7, 95% CI: 14.5–16.9 VTE vs. 16.3, 95% CI: 15.1–17.4, p = 0.505), cases with VTE had lower total bilirubin levels at the time of clot diagnosis (2.3 g/dL, 95% CI: 1.7–2.9 vs. 4.1 g/dL, 95% CI: 3.1–5.2, p = 0.003). Plasma creatinine and INR values were similar. Platelet counts were higher in the VTE group (154 k/μL, 95% CI: 136–171 VTE vs. 115 k/μL, 95% CI: 103–127 no VTE, p < 0.001) but albumin levels were similar. Rates of inherited or acquired hypercoaguability were similar between the two groups. Cases with VTE also had a greater incidence of acute infection (47 vs. 32%, p = 0.007). Prior VTE was also more prevalent in the VTE group (22 vs. 4%, p < 0.001) as was a diagnosis of PVT prior to VTE (23 vs. 14%, p = 0.049). There was a trend toward significance with higher rates of congestive heart failure (16 vs. 9%, p = 0.075) and respiratory failure (20 vs. 12%, p = 0.080) in the VTE group compared to the non-VTE group. Otherwise, other risk factors for clotting were similar between cases and controls, including hormone therapy, active cancer (including hepatocellular carcinoma), history of cerebrovascular accident, immobility and recent surgery or trauma. Metabolic risk factors coronary artery disease, diabetes mellitus, and hypertension were also similar between cases and controls. Body mass index was also similar (29.6 kg/m2, 95% CI: 28.4–30.8 vs. 30.0 kg/m2, 95% CI: 28.7–31.2, p = 0.667).
Table 2.
Unadjusted univariate analysis of baseline characteristics of 290 subjects with cirrhosis both in the presence and absence of venous thromboembolism
| VTE (n = 145)a | No VTE (n = 145) | pvalue | |
|---|---|---|---|
| Age | 58.6 (56.6–60.5) | 58.2 (56.3–60.2) | 0.820 |
| Male gender | 90 (62.1) | 90 (62.1) | 1.000 |
| Body mass index (kg/m2) | 29.6 (28.4–30.8) | 30.0 (28.7–31.2) | 0.667 |
| Disease etiology | |||
| NASH/Crypto | 62 (42.8) | 49 (34.3) | 0.175 |
| Autoimmune | 5 (3.5) | 3 (2.1) | |
| Cholestatic | 10 (7.0) | 10 (7.0) | |
| Alcohol | 30 (20.7) | 39 (27.3) | |
| Hepatitis C | 35 (24.4) | 40 (28.0) | |
| Hepatitis B | 2 (1.4) | 1 (0.7) | |
| Hemochromatosis | 2 (0.7) | 17 (11.9) | |
| Alcohol use | |||
| Active | 25 (17.5) | 28 (19.6) | 0.231 |
| Former | 36 (25.2) | 47 (32.9) | |
| Never | 82 (57.3) | 68 (47.6) | |
| Smoking history | |||
| Active | 36 (25.0) | 38 (27.0) | 0.394 |
| Former | 50 (34.7) | 57 (40.4) | |
| Never | 58 (40.3) | 36 (32.6) | |
| Child-Turcotte-Pugh | |||
| A | 30 (22.9) | 36 (35.5) | 0.745 |
| B | 58 (44.3) | 59 (41.8) | |
| C | 43 (48.9) | 45 (32.8) | |
| Laboratory values | |||
| MELD | 15.7 (14.5–16.9) | 16.3 (15.1–17.4) | 0.505 |
| Total bilirubin (g/dL) | 2.3 (1.7–2.9) | 4.1 (3.1–5.2) | 0.003 |
| Creatinine (g/dL) | 1.70 (1.35–2.05) | 1.38 (1.19–1.58) | 0.114 |
| INR | 1.50 (1.39–1.61) | 1.57 (1.47–1.61) | 0.340 |
| Sodium (mEq/L) | 135.5 (134.6–136.4) | 134.7 (133.8–135.7) | 0.248 |
| Albumin (g/dL) | 2.90 (2.79–3.01) | 2.87 (2.77–2.98) | 0.762 |
| Platelet count | 153.5 (135.7–171.3) | 115.0 (103.1–126.9) | <0.001 |
| Comorbidities | |||
| Stroke history | 20 (13.8) | 19 (13.1) | 0.863 |
| Active cancer | 18 (12.4) | 20 (13.8) | 0.729 |
| Acute infection | 68 (46.9) | 45 (31.5) | 0.007 |
| Congestive heart failure | 23 (15.9) | 13 (9.0) | 0.075 |
| Respiratory failure | 29 (20.0) | 18 (12.4) | 0.080 |
| Coronary artery disease | 27 (18.6) | 19 (13.3) | 0.217 |
| Diabetes | 73 (50.3) | 60 (41.7) | 0.139 |
| Hypertension | 88 (61.0) | 79 (54.9) | 0.316 |
| Medications | |||
| VTE prophylaxis | 53 (36.5) | 62 (42.8) | 0.285 |
| Therapeutic AC | 13 (9.5) | 9 (6.5) | 0.355 |
| Hormone therapy | 4 (2.8) | 2 (1.4) | 0.434 |
| Aspirin | 23 (16.8) | 20 (14.5) | 0.600 |
| Nonselective BB | 79 (57.6) | 68 (48.9) | 0.146 |
| Diuretics | 95 (69.3) | 91 (65.5) | 0.492 |
| Lactulose | 61 (44.5) | 61 (45.3) | 0.894 |
| Proton pump inhibitor | 94 (68.6) | 80 (57.6) | 0.057 |
| Rifaximin | 20 (14.6) | 27 (19.6) | 0.274 |
| Hypercoagulability | |||
| MTHFR homozygous | 4 (57.1) | 1 (12.5) | 0.307 |
| MTHFR heterozygous | 2 (28.6) | 0 (0.0) | 0.710 |
| ATIII deficiency | 18 (66.6) | - | - |
| Anticardiolipan IgG | 2 (12.0) | 2 (28.6) | 0.286 |
| Anticardiolipan IgM | 2 (8.7) | 1 (14.3) | 0.666 |
| Factor V Leiden hetero | 14 (93.3) | 6 (85.7) | 0.562 |
| Lupus anticoagulant | 0 (0.0) | 1 (16.7) | 0.057 |
| Protein C deficiency | 8 (57.1) | 3 (60.0) | 0.912 |
| Protein S deficiency | 2 (15.4) | 1 (25.0) | 0.659 |
| Prothrombin mutation | 1 (4.6) | 1 (16.7) | 0.307 |
| VTE risk factors | |||
| Prior VTE | 32 (22.1) | 5 (3.5) | <0.001 |
| Immobility | 31 (21.5) | 21 (14.5) | 0.119 |
| Trauma/surgery | 45 (31.0) | 38 (26.2) | 0.414 |
| Prior admission <90 days | 31 (21.4) | – | – |
| Padua Prediction Score | 4.50 (4.02–4.98) | 3.01 (2.64–3.37) | <0.001 |
| Portal hypertension decompensations | |||
| Ascites | 100 (69.4) | 100 (69.4) | 0.923 |
| Gastroesophageal varices | 76 (52.8) | 74 (51.0) | 0.767 |
| Hepatocellular carcinoma | 16 (11.1) | 18 (12.4) | 0.731 |
| Hepatic encephalopathy | 73 (50.3) | 70 (48.3) | 0.725 |
| Portal vein thrombosis | 28 (23.0) | 16 (13.8) | 0.049 |
| TIPS | 13 (9.2) | 16 (11.0) | 0.597 |
In general, the cases with venous thromboembolism and the control group were similar with the exception of platelet count, active infection, prior venous thromboembolism, and the Padua Prediction Score
Subjects were matched on age ±5 years, MELD score ±2 and gender
AC anticoagulation, AT antithrombin, BB beta blocker, MELD model for end stage liver disease, MTHFR methyltetrahydrofolate reductase, NASH non-alcoholic steatohepatitis, TIPS transjugular intrahepatic portosystemic shunt, VTE venous thromboembolism
aDVT = 102, PE = 25, DVT + PE = 18
Overall, cohort rate of chemical VTE prophylaxis was low at 42.4%. When comparing the two groups, rates of VTE prophylaxis were similar with 36.5% (n = 53) for cases with VTE vs. 42.8% (n = 62) for controls without VTE (p = 0.285). The type of medications prescribed for VTE prophylaxis were similar between the two groups as well: VTE-apixiban (n = 1), bivalirudin (n = 1), dalteparin (n = 1), heparin (n = 40), LMWH (n = 10), and no VTE-dalteparin (n = 1), heparin (n = 43), LMWH (n = 18), p = 0.456. Similar rates of therapeutic anticoagulation (9.5 vs. 6.5%, p = 0.355) and aspirin use (16.8 vs. 14.5%, p = 0.600) were seen when comparing cases and controls. Indications for anticoagulation were different, however, as the VTE group had eight subjects with previous VTE who were on therapeutic anticoagulation as the indication, three for atrial fibrillation, one for a mechanical heart valve, and one for coronary artery disease compared to the non-VTE group, where there were zero subjects with previous VTE, two with previous mesenteric vein thrombosis, three with atrial fibrillation, two with coronary artery disease, and one with an artificial heart valve (p = 0.007). The Padua Prediction Score was significantly higher in cases with VTE compared to controls without VTE (4.50, 95% CI: 4.02–4.98 vs. 3.01, 95% CI: 2.64–3.37, p < 0.001).
On adjusted multivariable analysis, independent risk factors for VTE included NASH (OR: 2.46, 95% CI: 1.07–5.65, p = 0.034), prior VTE (OR: 7.12, 95% CI: 1.99–25.5, p = 0.003), and the presence of PVT prior to the diagnosis of VTE (OR: 2.18, 95% CI: 1.03–4.58, p = 0.041) (Fig. 1). Thrombocytopenia was associated with decreased risk of VTE (OR: 0.49, 95% CI: 0.26–0.95, p = 0.035). While significant on unadjusted univariate analysis, acute infection was not predictive on adjusted multivariable analysis (OR: 1.52, 95% CI: 0.82–2.79, 95% CI: 0.182). Importantly, comorbid metabolic risk factors, such as diabetes, coronary artery disease, and obesity, were not significantly associated with VTE risk.
Fig. 1. Risk factors for venous thromboembolism in hospitalized patients with cirrhosis (adjusted multivariable analysis).
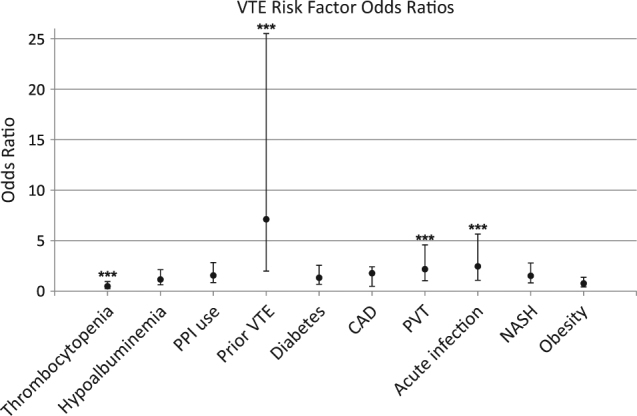
NASH cirrhosis patients were found to be at nearly 2.5-fold greater risk for VTE. CAD coronary artery disease; NASH non-alcoholic steatohepatitis; PPI proton pump inhibitor; PVT portal vein thrombosis; VTE venous thromboembolism. Adjusted for age, gender, and MELD. c-statistic 0.74. ***P ≤ 0.05
Discussion
This study confirms that NASH is a hypercoagulable state. NASH was found to be a strong independent risk factor for VTE in hospitalized patients with cirrhosis when adjusted for comorbid metabolic risk factors, including diabetes and obesity. This report highlights that the coagulation derangement is not just limited to local portal circulation with PVT risk23,29, but rather extends systemically as well. NASH cirrhosis patients were 2.46 times more likely to be diagnosed with a PE and/or DVT compared to a composite of all other etiologies of liver disease. NASH patients with cirrhosis should be considered among the highest risk patients to develop future VTE while hospitalized and medical thromboprophylaxis should be administered in the absence of contraindications.
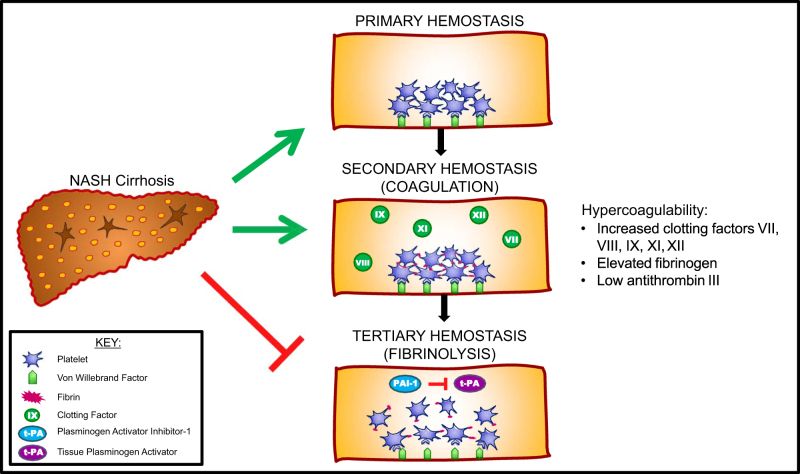
The mechanism leading to increased thrombotic risk in patients with NASH or NASH cirrhosis remains unknown. It has been postulated that repetitive injury from chronic inflammation from hepatic steatosis and lipid deposition over time leads to endothelial cell activation, oxidative injury, and necroapoptosis13–16. This proinflammatory process may lead to imbalance in hemostasis, thereby disrupting the delicate balance of hemostasis to favor hypercoagulability17,18. Abnormalities in elevated levels of vonWillebrand factor, mean platelet volume (surrogate of platelet activation), Factor VIII, fibrinogen and plasminogen activator inhibitor-1 have been reported in both NAFLD and NASH17,18. Levels of protein C and antithrombin are decreased in both NASH and NASH cirrhosis17,18. Plasminogen activator inhibitor-1 levels correlate with increasingly severe liver histopathology with greater levels of lobular inflammation, hepatocyte ballooning, steatosis, and fibrosis20. Figure 2 depicts a proposed mechanism for hypercoagulability in NASH.
Fig. 2. Proposed mechanism for hypercoagulability in NASH cirrhosis.
Prohemostatic changes are seen across all three phases of hemostasis in patients with NASH
Plasminogen activator inhibitor-1 inhibits breakdown of fibrin-based clots promoting thrombotic risk both in the macrovascular system as well as in local circulatory systems. Resultant intrahepatic thrombi induce tissue ischemia, which has the potential to accelerate liver disease progression through stellate cell activation and fibrogenesis30. A series by Papatheodoris et al.31 found that the presence of at least one thrombotic risk factor was associated with a nearly two-fold fibrosis stage increase in NASH patients, confirming earlier observational reports correlating thrombotic risk factors to the extent of hepatic fibrosis32.
Despite evidence suggesting a hypercoagulable state in patients with NASH and NASH cirrhosis, a recent report by Potze et al.21 challenges this notion. The authors found that hemostatic profiles were similar when comparing non-cirrhotic biopsy-proven NAFLD to controls without NAFLD with several exceptions. NAFLD patients had increased PAI-1 levels, less fibrinolysis and a greater degree of prothrombotic structure to the fibrin clot. However, these prohemostatic features were also found in obese controls leading the authors to conclude that prothrombotic risk was perhaps related to the presence of obesity rather than NAFLD per se21. This distinction is important given multiple reports and a recent meta-analysis confirming that metabolic syndrome predisposes patients to VTE in the absence of cirrhosis33–36. In fact, when distilling the metabolic syndrome into individual components, abdominal obesity was the strongest independent predictor of VTE and may be a better predictor than BMI33,34. However, these studies did not examine comorbid NAFLD or NASH. To date, only one other study has investigated NAFLD specifically and shown an association between NAFLD and VTE risk25. While the study controlled for obesity by matching cases and controls on BMI, it excluded patients with cirrhosis. We did not match for obesity in our study design. Rather, we included obesity as a predictor in the multivariable logistic regression model and we did not find obesity predictive of VTE risk in patients with cirrhosis.
Our cohort of patients was largely comprised of decompensated liver disease. In total, 77.3% had CTP Class B or C disease. By controlling for disease severity with matching by MELD score, we postulate that the coagulation balance in patients with NASH cirrhosis tips toward thrombosis as the patient becomes more decompensated. This could explain why we did not find NASH cirrhosis patients to be predisposed to DVT in our previous work as our prior cohort of patients was a much healthier population with significantly lower MELD scores and near-normal platelet counts5. Additionally, we did not analyze portal hypertensive complications in our previous work5. Future study confirming this from a mechanistic and translational standpoint would be interesting to undertake and may validate our findings.
Our study has several limitations. Namely, data on patient-centered long-term outcomes could not be captured due to a significant amount of missing longitudinal data. Because the study was retrospective, we also were unable to capture previously described abnormal biomarkers of coagulation. Thrombophilia testing was also not performed in the majority of patients. A matched case–control study design was chosen to provide greater statistical precision given the relatively low even rate of VTE and to allow for direct matching on confounding variables.
Other findings in this study beyond the primary objective deserve attention. Pre-existing PVT was significantly associated with future development of VTE, adding to the speculation that cirrhosis can be a hypercoagulable state. We have previously described pre-transplantation PVT to be predictive of post-transplantation hepatic artery thrombosis37,38. Whether or not the post-transplantation hypercoagulable milieu extends to post-operative PE or DVT has yet to be explored. Thrombocytopenia was associated with a lower risk of VTE, which is intriguing in that multiple reports have found thrombocytopenia was not predictive of bleeding risk. Whether or not thrombocytopenia is protective against clotting remains to be determined and should be validated with further prospective study. Recent reports have surfaced that reactive thrombocytosis following treatment of chronic hepatitis C with direct acting antiviral medications may be implicated in the development of PVT further implicating the role of the platelet in prohemostatic risk39. While hypoalbuminemia has previously been shown to be predictive of DVT development, our analysis did not confirm this finding5. As both thrombocytopenia and hypoalbuminemia can be considered markers of advanced liver disease, our matching by MELD score controlled for liver disease severity in order to avoid confounding with the primary outcome and may offer an explanation to the lack of confirmation of these previous findings. Similar to previous findings26,40, the Padua Prediction Score was predictive of VTE risk in patients with cirrhosis.
In conclusion, we have shown that NASH is an independent risk factor for VTE among cirrhosis patients, providing further evidence that NASH is a hypercoagulable state. While all hospitalized patients with cirrhosis should be considered for medical thromboprophylaxis, those with NASH cirrhosis are at particularly increased risk and a high index of suspicion for VTE should be reserved in these patients.
Study Highlights
What is current knowledge
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of liver disease worldwide.
NAFLD is associated with derangements in all three phases of hemostasis.
Patients with NASH cirrhosis are at increased risk for portal vein thrombosis independent of other comorbid metabolic conditions.
What is new here
The prothombotic state of NASH cirrhosis extends beyond the portal venous system and into the systemic circulatory system.
NASH is an independent risk factor for venous thromboembolism (VTE) among cirrhosis patients with nearly 2.5-fold greater risk when compared to all other etiologies of cirrhosis.
While all hospitalized patients with cirrhosis should be considered for medical thromboprophylaxis, those with NASH cirrhosis are at particularly increased risk and a high index of suspicion for VTE should be reserved in these patients.
Electronic supplementary material
Acknowledgements
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Competing interests
Guarantors of the article: Jonathan Stine
Specific authors’ contributions: All authors participated in the listed roles as follows: J.S.—planning/conducting study, collecting and/or interpreting data, drafting manuscript, and final approval. B.N.—collecting data, drafting manuscript, and final approval. A.Z.—collecting data, drafting manuscript, and final approval. N.I.—drafting manuscript and final approval. S.C.—drafting manuscript and final approval. C.A.—drafting manuscript and final approval. P.N.—planning/conducting study, collecting and/or interpreting data, drafting manuscript, and final approval.
Financial support:. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. This work was supported by a Transplant Hepatology Fellowship Award from the American Association for the Study of Liver Diseases (AASLD).
Potential competing interests: None.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41424-018-0002-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878–885. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 2.Sogaard KK, et al. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am. J. Gastroenterol. 2009;104:96–101. doi: 10.1038/ajg.2008.34. [DOI] [PubMed] [Google Scholar]
- 3.Sogaard KK, et al. Cirrhosis is associated with an increased 30-day mortality after venous thromboembolism. Clin. Transl. Gastroenterol. 2015;6:e97. doi: 10.1038/ctg.2015.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrosino P, et al. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta-analysis. Thromb. Haemost. 2017;117:139–148. doi: 10.1160/TH16-06-0450. [DOI] [PubMed] [Google Scholar]
- 5.Northup PG, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am. J. Gastroenterol. 2006;101:1524–1528. doi: 10.1111/j.1572-0241.2006.00588.x. [DOI] [PubMed] [Google Scholar]
- 6.Gulley D, et al. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig. Dis. Sci. 2008;53:3012–3017. doi: 10.1007/s10620-008-0265-3. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Fuster MJ, et al. Venous thromboembolism and liver cirrhosis. Rev. Esp. Enferm. Dig. 2008;100:259–262. doi: 10.4321/S1130-01082008000500002. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin. Gastroenterol. Hepatol. 2010;8:800–805. doi: 10.1016/j.cgh.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Dabbagh O, et al. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137:1145–1149. doi: 10.1378/chest.09-2177. [DOI] [PubMed] [Google Scholar]
- 10.Aldawood A, et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb. J. 2011;9:1. doi: 10.1186/1477-9560-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali M, et al. Deep vein thrombosis and pulmonary embolism in hospitalized patients with cirrhosis: a nationwide analysis. Dig. Dis. Sci. 2011;56:2152–2159. doi: 10.1007/s10620-011-1582-5. [DOI] [PubMed] [Google Scholar]
- 12.Intagliata NM, et al. Prophylactic anticoagulation for venous thromboembolism in hospitalized cirrhosis patients is not associated with high rates of gastrointestinal bleeding. Liver Int. 2014;34:26–32. doi: 10.1111/liv.12211. [DOI] [PubMed] [Google Scholar]
- 13.Targher G, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity. 2008;16:1394–1399. doi: 10.1038/oby.2008.64. [DOI] [PubMed] [Google Scholar]
- 14.Targher G, et al. Disorders of coagulation and hemostasis in abdominal obesity: emerging role of fatty liver. Semin. Thromb. Hemost. 2010;36:41–48. doi: 10.1055/s-0030-1248723. [DOI] [PubMed] [Google Scholar]
- 15.Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin. Liver Dis. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Cigolini M, et al. Liver steatosis and its relation to plasma haemostatic factors in apparently healthy men–role of the metabolic syndrome. Thromb. Haemost. 1996;76:69–73. [PubMed] [Google Scholar]
- 17.Tripodi A, et al. Procoagulant imbalance in patients with non-alcoholic fatty liver disease. J. Hepatol. 2014;61:148–154. doi: 10.1016/j.jhep.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Potze W, Siddiqui MS, Sanyal AJ. Vascular disease in patients with nonalcoholic fatty liver disease. Semin. Thromb. Hemost. 2015;41:488–493. doi: 10.1055/s-0035-1550433. [DOI] [PubMed] [Google Scholar]
- 19.Alkhouri N, et al. Mean platelet volume as a marker of increased cardiovascular risk in patients with nonalcoholic steatohepatitis. Hepatology. 2012;55:331. doi: 10.1002/hep.24721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verrijken A, et al. Prothrombotic factors in histologically proven NAFLD and NASH. Hepatology. 2013;59:121–129. doi: 10.1002/hep.26510. [DOI] [PubMed] [Google Scholar]
- 21.Potze W, et al. Preserved hemostatic status in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016;65:980–987. doi: 10.1016/j.jhep.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Stine JG, et al. Increased risk of portal vein thrombosis in patients with cirrhosis due to non-alcoholic steatohepatitis (NASH) Liver Transpl. 2015;21:1016–1021. doi: 10.1002/lt.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stine JG, et al. Advanced non-alcoholic steatohepatitis cirrhosis: a high-risk population for pre-liver transplant portal vein thrombosis. World J. Hepatol. 2017;9:139–146. doi: 10.4254/wjh.v9.i3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghabril, M. et al. Portal vein thrombosis is a risk factor for poor early outcomes after liver transplantation: analysis of risk factors and outcomes for portal vein thrombosis in waitlisted patients. Transplantation100, 126–133 (2016). [DOI] [PubMed]
- 25.Di Minno MN, et al. High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J. Gastroenterol. 2010;16:6119–6122. doi: 10.3748/wjg.v16.i48.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbar S, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J. Thromb. Haemost. 2010;8:2450–2457. doi: 10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]
- 27.Bursac Z, et al. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun GW, Shook TL, Kay GL. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J. Clin. Epidemiol. 1996;49:907–916. doi: 10.1016/0895-4356(96)00025-X. [DOI] [PubMed] [Google Scholar]
- 29.Stine JG, et al. Hepatic decompensation likely attributable to simeprevir in patients with advanced cirrhosis. Dig. Dis. Sci. 2015;60:1031–1035. doi: 10.1007/s10620-014-3422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verrijken A, et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2014;59:121–129. doi: 10.1002/hep.26510. [DOI] [PubMed] [Google Scholar]
- 31.Papatheodoridis GV, et al. Thrombotic risk factors and liver histologic lesions in non-alcoholic fatty liver disease. J. Hepatol. 2009;51:931–938. doi: 10.1016/j.jhep.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Assy N, et al. Association between thrombotic risk factors and extent of fibrosis in patients with non-alcoholic fatty liver diseases. World J. Gastroenterol. 2005;11:5834–5839. doi: 10.3748/wjg.v11.i37.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaya A, et al. The metabolic syndrome and its individual components: its association with venous thromboembolism in a Mediterranean population. Metab. Syndr. Relat. Disord. 2011;9:197–201. doi: 10.1089/met.2010.0117. [DOI] [PubMed] [Google Scholar]
- 34.Ageno W, et al. Association between the metabolic syndrome, its individual components, and unprovoked venous thromboembolism: results of a patient-level meta-analysis. Arterioscler. Thromb. Vasc. Biol. 2014;34:2478–2485. doi: 10.1161/ATVBAHA.114.304085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Minno MN, et al. Abnormally high prevalence of major components of the metabolic syndrome in subjects with early-onset idiopathic venous thromboembolism. Thromb. Res. 2011;127:193–197. doi: 10.1016/j.thromres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Ageno W, et al. The metabolic syndrome and the risk of venous thrombosis: a case-control study. J. Thromb. Haemost. 2006;4:1914–1918. doi: 10.1111/j.1538-7836.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 37.Stine JG, et al. Pre-transplant portal vein thrombosis is an independent risk factor for graft loss due to hepatic artery thrombosis in liver transplant recipients. HPB. 2016;18:279–286. doi: 10.1016/j.hpb.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stine JG, et al. Liver transplant recipients with portal vein thrombosis receiving an organ from a high-risk donor are at an increased risk for graft loss due to hepatic artery thrombosis. Transpl. Int. 2016;29:1286–1295. doi: 10.1111/tri.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stine JG, Prakash S, Northup PG. Portal vein thrombosis after hepatitis C eradication with direct acting antiviral therapy. Liver Int. 2017;38:185–186. doi: 10.1111/liv.13537. [DOI] [PubMed] [Google Scholar]
- 40.Bogari H, et al. Risk-assessment and pharmacological prophylaxis of venous thromboembolism in hospitalized patients with chronic liver disease. Thromb. Res. 2014;134:1220–1223. doi: 10.1016/j.thromres.2014.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.