Abstract
Objectives
Achieving endoscopic remission or decreasing the level of fecal biomarkers as an ideal therapeutic goal in ulcerative colitis has not been determined. This prospective study was to compare the clinical relevance of endoscopic score with fecal biomarkers for predicting relapse after clinical remission and mucosal healing (MH).
Methods
One hundred and sixty-four patients who achieved clinical remission and MH (Mayo endoscopic subscore (MES) 0 or 1) were included. At entry, fecal samples were collected for the measurement of calprotectin, lactoferrin, and hemoglobin. Thereafter patients received masalamine maintenance therapy, and were followed for 12 months.
Results
During 12-month study, 46 patients (28%) relapsed. The relapse rate was not significantly higher in 27/80 patients (34%) with MES 1 than in 19/84 patients (23%) with MES 0 (P = 0.16). The median fecal calprotectin, lactoferrin, and hemoglobin were significantly higher in patients with relapse than those in remission (calprotectin, 182 vs. 94 μg/g; lactoferrin, 185.5 vs. 111 μg/g; hemoglobin, 168 vs. 104 ng/mL; all P < 0.0001). A cutoff value of 115 µg/g calprotectin had 83% sensitivity and 81% specificity to predict relapse, whereas lactoferrin, 145 µg/g had 70% sensitivity and 79% specificity, and hemoglobin, 135 ng/mL showed 74% sensitivity and 73% specificity. The accuracy was significantly lower for hemoglobin as compared with calprotectin and lactoferrin.
Conclusions
Fecal calprotectin, lactoferrin, and to a lesser degree fecal hemoglobin appeared to be objective biomarkers for predicting future relapse after achieving clinical remission and MH. The predictive value of these biomarkers was higher than with MES.
Introduction
Endoscopic scoring systems have an essential role in the assessment of disease activity in patients with inflammatory bowel disease (IBD). There are numerous endoscopic indices, which have been used to assess disease activity in clinical trials of ulcerative colitis (UC) patients1,2. The Mayo endoscopic subscore (MES) is the most commonly used in both clinical trials and clinical practice settings3,4. MES divides the endoscopic findings into four levels of severity: 0, normal or inactive disease; 1, mild disease; 2, moderate disease; 3, severe disease3.
The paradigm for the management of IBD is shifting from alleviating symptoms towards more objective goals such as achieving mucosal healing (MH)5,6. The idea of using MH as an endpoint for the assessment of disease activity in patients with IBD started gaining popularity with the demonstration that medical therapy with azathioprine7 and then novel biologics8 could induce symptomatic improvement as well as endoscopic healing. A recent meta-analysis shows that MH achieved during medical therapy was associated with long-term clinical remission, colectomy avoidance, and corticosteroid-free clinical remission in patients with UC9. MH was defined as MES 0 or 1 in most relevant clinical trials. However, the MES has weaknesses including lack of a validated definition of MH and the inclusion of the subjective term “minimal” or “slight friability” (MES 1), which could affect the validity of assessing endoscopic disease activity10.
A number of studies11–14 have suggested that patients with the MES 1 are at a higher risk of future relapse than those with the MES of 0, indicating that MH should be limited to the MES 0. However, the MES is not an objective parameter, and it is difficult to discriminate between MES 0 and 1 in clinical practice. Therefore, MES is not the best parameter to identify patients at a high risk of future relapse.
Several fecal biomarkers have been evaluated for their utility to assess disease activity as alternative tests to endoscopy in patients with IBD15–19. Calprotectin and lactoferrin, both neutrophil-derived proteins, are frequently used fecal biomarkers in clinical practice settings. These biomarkers have been studied for their ability to identify patients with IBD, monitor disease activity, and assess response to therapy15–19. Likewise, fecal immunochemical test (FIT) for detecting hemoglobin in the stool has been widely used in colorectal cancer screening, and recently being adopted for assessing mucosal inflammation in patients with IBD20,21. These biomarkers can serve as low cost, simple, and convenient non-invasive tests to assess mucosal inflammation15–21.
In this prospective study, we were interested to compare the clinical significance of endoscopic score with fecal biomarkers for the prediction of future relapse in patients with UC after achieving clinical remission and MH with medical treatment. To our knowledge, this is the first large scale prospective study to compare the predictive value of the MES vs. fecal biomarkers for future relapse in UC patients with MH. We focused on three major fecal biomarkers, calprotectin, lactoferrin, and hemoglobin simultaneously using the same stool samples. This study will provide clinically relevant information for answering an important question: What should be the optimal treatment target in patients with quiescent UC, endoscopic remission or the level of fecal biomarkers below a given threshold.
Patients and methods
Study design and patient selection
This was a prospective, single center study undertaken at the Yokkaichi Hazu Medical Center, a referral center treating a large number of patients with IBD in the Mie Prefecture of Japan. Patient inclusion criteria were: (1) age between 20 and 75; (2) patients with endoscopic and histologic diagnosis of UC; (3) patients who achieved clinical remission (normal stool frequency and no rectal bleeding) with medical treatment; (4) patients who achieved MH defined as MES 0 or 1 at endoscopy performed when they went into clinical remission; (5) patients who were scheduled to receive mesalamine maintenance therapy after achieving clinical remission and MH; (6) patients who agreed to take and provide a stool sample for the assay of fecal biomarkers. Exclusion criteria were: (1) patients with proctitis alone; (2) patients who received non-steroidal anti-inflammatory drugs, anti-diarrheal (loperamide or codeine) or antispasmodic medications at entry; (3) patients who did not achieve MH (MES 2 or 3) at endoscopy performed when they went into clinical remission. A total of 164 patients who met the inclusion criteria were included. The baseline characteristics of the 164 eligible patients are presented in Table 1.
Table 1.
Baseline characteristics of 164 eligible patients of this study
| Median age at entry (IQR) | 35 (31–39) years |
| Male:female (n) | 101:63 |
| Median duration of UC before entry (IQR) | 48 (32–69) months |
| Severity of the current exacerbation before entry (n) | |
| Mild (Mayo score ≤ 4) | 73 (45%) |
| Moderate (5 ≤ Mayo score ≤ 8) | 71 (43%) |
| Severe (Mayo score ≥ 9) | 20 (12%) |
| Extent of UC (n) | |
| Left-sided colitis | 124 (76%) |
| Extensive colitisa | 40 (24%) |
| Extraintestinal manifestations (n) | |
| Arthritis | 18 (11%) |
| Erythema nodosum/pyoderma gangrenosum | 5 (3%) |
| Others | 6 (4%) |
| Induction therapy for the current exacerbation (n) | |
| Mesalamine (Pentasa 4 g/day) | 127 (77%) |
| Corticosteroids (prednisolone 30–60 mg/day) | 97 (59%) |
| Leucocytapheresis | 99 (60%) |
| Mayo endoscopic subscore (MES) at entry | |
| MES 0 | 84 (51%) |
| MES 1 | 80 (49%) |
IQR interquartile rage, UC ulcerative colitis
aInvolvement extends proximal to the splenic flexure
Medical treatment
At entry, all patients achieved clinical remission with medical treatment. As remission induction therapy, high-dose mesalamine (Pentasa 4 g/day) was used in 127 patients (77%), prednisolone (30–60 mg/day) in 97 patients (59%), and therapeutic leucocytapheresis (Adacolumn, JIMRO, Takasaki, Japan) in 99 patients (60%). Several patients received multiple therapies as induction therapy. For patients who achieved remission, the dose of prednisolone was to be tapered or discontinued. As remission maintenance therapy during the study period, orally administered mesalamine (Pentasa 1.5–3.0 g/day) was used in all patients. The mesalamine maintenance therapy was continued at the same dose unless there was a relapse. Twenty-two patients (13%) received concomitant azathioprine (25–100 mg/day).
Determination of clinical and endoscopic scores
All patients were reviewed in our clinic every 2 or 3 months up to 12 months after entry. Patients were advised to record their symptoms in a diary on a daily basis. At the clinic, patients’ compliance with medication, adverse effects, general well-being, stool frequency, stool consistency and presence or absence of abdominal pain, tenderness, tenesmus, rectal bleeding, and mucus in stool were recorded. Stool frequency and rectal bleeding were scored according to clinical sections in the Mayo score3. Clinical remission was defined as normal stool frequency (=score 0) and no rectal bleeding (=score 0). When a patient developed symptoms suggestive of a flare-up, endoscopic examination was immediately undertaken. Endoscopic score was according to the MES. The MES divides the endoscopic findings into four levels of severity: 0, normal or inactive disease; 1, mild disease (erythema, decreased vascular pattern, mild friability); 2, moderate disease (marked erythema, absent vascular pattern, friability, erosions); 3, severe disease (spontaneous bleeding, ulceration). Relapse was defined as worsening of stool frequency and/or rectal bleeding with the MES of 2 or 3. At entry, peripheral blood samples were collected for the measurement of white blood cell count (WBC), hemoglobin (Hb), platelet count, total protein, albumin, creatinine, urea, sodium, potassium, chloride, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, lactic dehydrogenase, total bilirubin, cholesterol, and C-reactive protein (CRP).
Measurements of fecal biomarkers
Patients were requested to collect a stool sample in the early morning within 5 days, and store at room temperature before their clinic visit. Fecal calprotectin was measured by a Human Calprotectin enzyme-linked immunosorbent assay Kit, (Cell Sciences, Canton, MA, USA). Lactoferrin was measured by a colloidal gold agglutination reagent (Auto Lf-Plus, Alfresa Pharma Corp., Osaka, Japan) by using a high-throughput discrete clinical chemistry analyzer (Hemo Techt NS-Plus C, Alfresa Pharma Corp.). Hemoglobin was measured by a colloidal gold agglutination reagent (i-FOBT Hemoglobin NS-Plus, Alfresa Pharma Corp.) by using the aforementioned clinical chemistry analyzer. Laboratory investigators were blinded to the clinical data.
Ethical considerations
Before initiating this study, our investigation protocol was reviewed and approved by the Institutional Review Board at the Yokkaichi Hazu Medical Center (the study site). All included patients agreed to participate in this study after being informed of the study purpose, and the nature of the procedures involved. Further, all investigations were conducted in accordance with the Good Clinical Practice Guidelines for investigations involving human subjects. Likewise, adherence was made to the Helsinki Declaration at all times.
Statistics
Comparisons of frequencies were done by applying the chi-square test with Yates’ correction. Differences between median values were compared by the Mann–Whitney U test or by the Kruskal–Wallis test if more than two groups were to be compared. Correlations were calculated by using the Spearman’s rank correlation coefficient (r). The cumulative relapse-free rate was determined by the Kaplan–Meier estimator graphs, and was compared between the groups by using the log-rank test. P < 0.05 was considered statistically significant. In addition, to find an optimal cutoff value to predict future relapse, a receiver operating characteristic (ROC) curve was constructed. An ROC curve was to be a plot of the true positive rate (sensitivity) against the false positive rate (1 – specificity) for different cutoff values of a diagnostic test. In general, the closer the curve follows the left hand-border and then the top border of the ROC space, the more accurate is the test. We defined the most optimal cutoff points by looking at the sensitivity and specificity for different cutoff values. The accuracy of the diagnostic test was determined by the area under the ROC curve (AUC).
Results
The overall clinical and laboratory findings
During the 12 months follow-up, 46 patients (28%) relapsed. The median time from entry to relapse was 8 months, interquartile range (IQR), 5–10 months. The median fecal calprotectin, lactoferrin and hemoglobin levels for all 164 patients were 100 μg/g (IQR: 84.5–171 μg/g), 118 μg/g (IQR: 92–177.5 μg/g), and 117 ng/mL (range: 83–189.5 ng/mL), respectively. There was significant correlation between the three fecal biomarkers: calprotectin vs. lactoferrin, r = 0.95, P < 0.0001; calprotectin vs. hemoglobin, r = 0.92, P < 0.0001; lactoferrin vs. hemoglobin, r = 0.95, P < 0.0001. The relationship between clinical parameters and fecal biomarkers was investigated. The following variables were included: age at entry (<35/≥35 years), gender, duration of UC before entry (<48/≥48 months), severity of the current exacerbation before entry (mild/moderate/severe), extent of UC (left-sided/extensive), and extraintestinal manifestations. None of these variables was significantly associated with the levels of fecal calprotectin, lactoferrin, or hemoglobin (Supplementary Table 1). Likewise, there was no significant correlation between the three fecal biomarkers and blood parameters such as WBC count, Hb, platelet count, albumin, or CRP (Supplementary Table 2).
Endoscopic findings
At the start of our follow-up, the MES was 0 in 84 patients (51%) and 1 in 80 patients (49%). The rate of subsequent relapse was higher in 27 of 80 patients (34%) with the MES 1 than in 19 of 84 patients (23%) with the MES 0. However, the difference was not statistically significant (P = 0.16). Similarly, the cumulative relapse-free rate was not significantly different between patients with the MES 0 and 1 (P = 0.11, Fig. 1). A post-hoc power analysis showed that this comparison was underpowered (power = 0.39). A larger sample size (226 for each group, with power = 0.80) was required to reach a significance level.
Fig. 1.
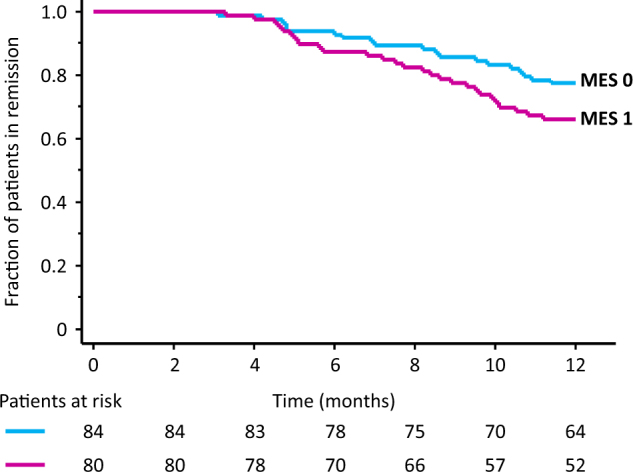
The cumulative relapse-free rate was not significantly different between patients with the Mayo endoscopic subscore (MES) 0 and 1 at entry ( P = 0.11)
There was a significant relationship between the MES and the fecal biomarkers. The median calprotectin, lactoferrin, and hemoglobin levels were significantly higher in patients with MES 1 than those with MES 0 (calprotectin, 112 (IQR: 86.5–244.5) vs. 96 (IQR: 81.5–112) μg/g, P = 0.01; lactoferrin, 130 (IQR: 93–204) vs. 113 (IQR: 89–138) μg/g, P = 0.02; hemoglobin, 128 (IQR: 90.5–208) vs. 112.5 (IQR: 82.5–144.5) ng/mL, P = 0.04).
Fecal biomarkers and subsequent relapse rate
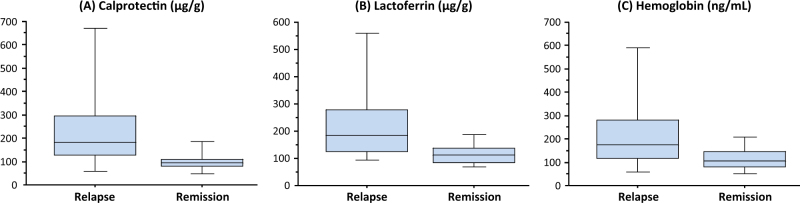
There was a significant relationship between the occurrence of relapse and the levels of fecal biomarkers. The median calprotectin, lactoferrin, and hemoglobin levels were significantly higher in patients (n = 46) with relapse than those (n = 118) in remission (calprotectin, 182 (IQR: 124.5–300) vs. 94 (IQR: 81.5–110.5) μg/g, P < 0.0001; lactoferrin, 185.5 (IQR: 125–284.5) vs. 111 (IQR: 85–138) μg/g, P < 0.0001; hemoglobin, 168 (IQR: 115–280.5) vs. 104 (IQR: 78.5–147) ng/mL, P < 0.0001; Fig. 2A–C). Subgroup analyses were conducted based on the concomitant use of azathioprine during remission maintenance therapy. In patients with azathioprine (n = 22), the median calprotectin, lactoferrin, and hemoglobin levels were significantly higher in patients (n = 6) with relapse than those (n = 16) in remission (calprotectin, 332 vs. 90 μg/g, P = 0.001; lactoferrin, 254.5 vs. 122 μg/g, P = 0.009; hemoglobin, 230.5 vs. 119 ng/mL, P = 0.02). Similarly, in patients without azathioprine (n = 142), the median calprotectin, lactoferrin and hemoglobin levels were significantly higher in patients (n = 40) with relapse than those (n = 102) in remission (calprotectin, 178 vs. 94.5 μg/g, P < 0.0001; lactoferrin, 180.5 vs. 103.5 μg/g, P < 0.0001; hemoglobin, 166 vs. 99.5 ng/mL, P = 0.0002).
Fig. 2.
The relationship between the levels of fecal biomarkers and subsequent clinical course. The median levels of calprotectin (A), lactoferrin (B), and hemoglobin (C) were significantly higher (all P < 0.0001) in patients who relapsed during the 12 months follow-up time (n = 46) compared with those who remained in remission (n = 118). The boxes represent interquartile ranges, whereas the horizontal lines indicate median values and whiskers indicate the upper and lower limits.
Fecal biomarkers for the prediction of subsequent relapse
ROC curves were constructed to determine an optimal cutoff value for fecal biomarkers to predict future relapse (Fig. 3). A cutoff value of 115 µg/g calprotectin had a sensitivity of 83% (95% confidence interval (CI): 72–94%), a specificity of 81% (95% CI: 74–88%), a positive predictive value (PPV) of 63% (95% CI: 51–76%), and a negative predictive value (NPV) of 92% (95% CI: 87–97%) with an AUC of 0.81 (95% CI: 0.70–0.88) to predict future relapse. Likewise, a cutoff value of 145 µg/g lactoferrin had a sensitivity of 70% (95% CI: 56–83%), a specificity of 79% (95% CI: 71–86%), a PPV of 56% (95% CI: 43–69%), and a NPV of 87% (95% CI: 81–93%) with an AUC of 0.78 (95% CI: 0.68–0.86). A cutoff value of 135 ng/mL fecal hemoglobin had a sensitivity of 74% (95% CI: 61–87%), a specificity of 73% (95% CI: 65–81%), a PPV of 52% (95% CI: 39–64%), and a NPV of 88% (95% CI: 81–94%) with an AUC of 0.72 (95% CI: 0.62–0.81). The AUC was significantly lower for hemoglobin compared with calprotectin or lactoferrin (hemoglobin vs. calprotectin, P = 0.0009; hemoglobin vs. lactoferrin, P = 0.02). The AUC was not significantly different between calprotectin and lactoferrin (P = 0.27).
Fig. 3. Receiver operating characteristic curves were constructed to determine an optimal cutoff value for fecal biomarkers to predict future relapse.
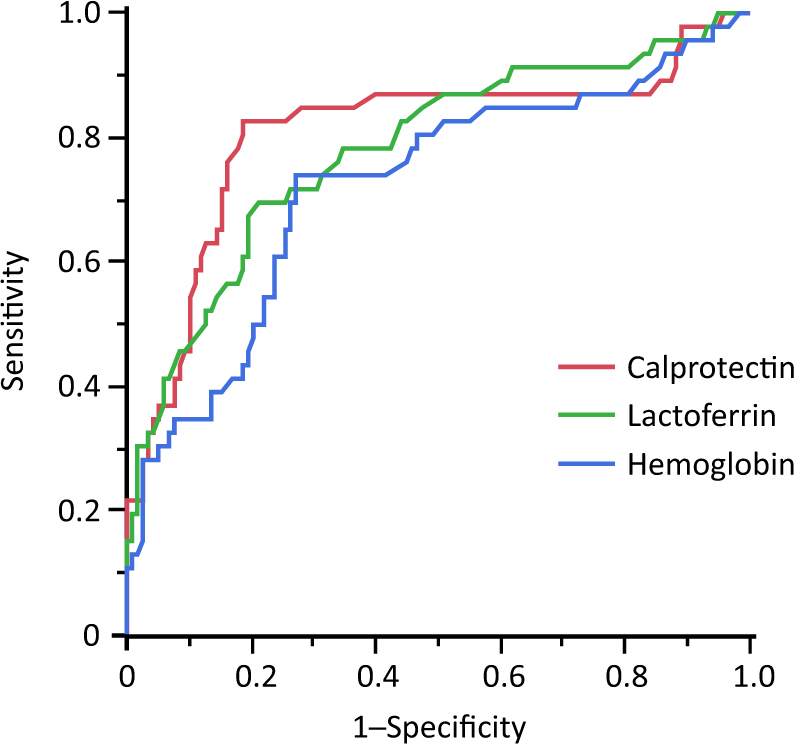
The optimal cutoff levels of fecal calprotectin, lactoferrin, and hemoglobin for the prediction of future relapse were 115 µg/g, 145 µg/g, and 135 ng/mL, respectively
The cumulative relapse-free rate was significantly higher in patients with low fecal calprotectin (<115 µg/g) compared with those with higher level (≥115 µg/g, P < 0.0001) shown in Fig. 4A. Likewise, the cumulative relapse-free rate was significantly higher in patients with low fecal lactoferrin (<145 µg/g) compared with those with higher level (≥145 µg/g, P < 0.0001) shown in Fig. 4B. A similar trend is shown in Fig. 4C for those with low fecal hemoglobin (<135 ng/mL) vs. higher level (≥135 ng/mL, P < 0.0001).
Fig. 4. The Kaplan–Meier estimator graphs showing the cumulative relapse-free rates for patients with high and low fecal biomarkers.
The relapse-free rate was significantly higher in patients with low fecal biomarkers compared with those who had elevated biomarkers (all P < 0.0001)
In patients with a baseline MES 0 only, the optimal cutoff value for the prediction of relapse was also determined. A cutoff value of 121 µg/g calprotectin had a sensitivity of 79% (95% CI: 61–97%), a specificity of 91% (95% CI: 84–98%), a PPV of 71% (95% CI: 52–91%), and a NPV of 94% (95% CI: 88–100%). A cutoff value of 127 µg/g lactoferrin had a sensitivity of 68% (95% CI: 48–89%), a specificity of 72% (95% CI: 61–83%), a PPV of 42% (95% CI: 25–59%), and a NPV of 89% (95% CI: 80–97%). A cutoff value of 138 ng/mL fecal hemoglobin had a sensitivity of 63% (95% CI: 41–85%), a specificity of 78% (95% CI: 68–88%), a PPV of 46% (95% CI: 27–65%), and a NPV of 88% (95% CI: 80–96%).
Clinical measures and medications vs. relapse rate
The following measures did not significantly affect the relapse rate. Age at entry (<35/≥35 years), gender, duration of UC before entry (<48/≥48 months), severity of the current exacerbation before entry (mild/moderate/severe), extent of UC (left-sided/extensive), extraintestinal manifestations, induction medication (mesalamine, prednisolone, leucocytapheresis), and maintenance medication (mesalamine with/without azathioprine) (Supplementary Table 3). Similarly, these parameters did not significantly affect the cumulative relapse-free rate: age (P = 0.26), gender (P = 0.60), duration of UC before entry (P = 0.53), severity of the current exacerbation before entry (P = 0.55), extent of UC (P = 0.23), extraintestinal manifestations (P = 0.62), induction medication (mesalamine P = 0.77, prednisolone P = 0.29, leucocytapheresis P = 0.75), and maintenance medication (P = 0.99).
Further, during the 12 months follow-up time, relapse was observed in 28 (27%) of the 103 patients on 3 g/day mesalamine, 15 (31%) of the 49 patients on 2.25 g/day mesalamine, and 3 (25%) of the 12 patients on 1.5 g/day mesalamine (P = 0.88). The cumulative relapse-free rate was not significantly different among the 3 subgroups (P = 0.90).
Discussion
This study was prospectively designed and rigorously conducted with a large number of homogeneous patients, aiming to compare the clinical relevance of the MES with 3 validated fecal biomarkers in patients with quiescent UC. To our knowledge, this is the first large scale study, which has evaluated the predictability of endoscopic score vs. fecal biomarkers for disease relapse in UC patients with MH. One hundred and sixty-four UC patients who achieved MH (MES 0 or 1) when going into clinical remission were included. At entry, fecal samples were collected for the measurement of these three major biomarkers, calprotectin, lactoferrin, and hemoglobin. The biomarkers were simultaneously measured at our institute in the same fecal samples without allowing the laboratory personnel knowing the donors. The novelty of this study was not to assess the relapse predictable value of fecal biomarkers, but rather to compare the predictive value of endoscopic assessment to one of these fecal biomarkers.
Following remission induction, the patients were treated with mesalamine as maintenance therapy, and were regularly observed during the following 12 months. The maintenance therapy was continued at the same dose unless a patient experienced relapse, which was defined as worsening of symptoms (stool frequency or rectal bleeding) with deterioration of endoscopic findings (MES 2 or 3). We found that the rate of future relapse tended to be higher in patients with the MES 1 compared with the MES 0 (34 vs. 23%), but statistically, the difference did not reach significance level. However, this analysis appeared to be underpowered, therefore, further work with a larger sample size is necessary. In contrast, all three fecal biomarkers were significantly elevated in patients with relapse as compared with those in remission. Accordingly, we could calculate relevant cutoff values with high sensitive and specific to predict future relapse. However, the sensitivity and specificity for the prediction of relapse appeared to be higher for fecal calprotectin (83 and 81%, respectively) as compared with lactoferrin (70 and 79%) or hemoglobin (74 and 73%). Also, for hemoglobin, the AUC in the ROC plot was significantly lower as compared with calprotectin or lactoferrin, suggesting that data from assay of fecal hemoglobin alone could be a poor predictor of UC relapse. In addition, we found that there was a significant positive relationship between the fecal biomarkers and the MES, but the predictive value of the biomarkers appeared to be higher than MES.
In a recent study22 involving 52 UC patients, the sensitivity of fecal calprotectin for the prediction of sustained clinical remission was evaluated in IBD patients with MH (MES 0). The relapse rate was only 8% after a median follow-up of 11 months (range: 5–15 months). Median fecal calprotectin level at entry was significantly higher in patients who relapsed compared with patients who maintained remission (284 vs. 37 mg/kg). Fecal calprotectin level <56 mg/kg was found to optimally predict the absence of relapse during the follow-up, with a sensitivity of 64% and a specificity of 100%. This study found that low calprotectin level identifies IBD patients who remain in remission. Other studies11–14 have suggested that MES was appropriate for prediction of future relapse. Nonetheless, the sample size in those studies was small. Further, the timing of endoscopic evaluation was not consistent among their patients, and multiple medications were used as maintenance therapy. Under these conditions, it is difficult to rigorously assess the value of endoscopic findings on the future relapse. Further, in our experience, the MES is not an objective parameter because, it was difficult to discriminate between the MES 0 and 1. In contrast, with the fecal biomarkers, use of predetermined, and known cutoff values reflect convenience and reliability.
In the present study, we found that clinical parameters such as age, gender, duration of UC, the severity of the current exacerbation, the extent of UC, and extraintestinal manifestation did not affect the levels of fecal biomarkers. Further, there was no significant correlation between the biomarkers and the conventional laboratory measurements including WBC count, Hb, platelet count, albumin, and CRP. Both calprotectin and lactoferrin are derived from polymorphonuclear neutrophils, primarily from those, which infiltrate the colonic mucosa15–19. When the concentration of neutrophils in the mucosa reaches a threshold level, the patient may experience a clinical relapse. Accordingly, an elevated amount of calprotectin or lactoferrin in the stool is secondary to mucosal inflammation, which may lead to relapse.
As mentioned in the “Patients and Methods” section, endoscopic evaluation was done in patients with worsening clinical symptoms, and the presence of both clinical symptoms and endoscopic activity was defined as relapse. Endoscopic examination was not performed for all patients during the follow-up, so the value of fecal biomarkers for the prediction of endoscopic relapse could not be adequately evaluated, but we found that the levels of fecal biomarkers were not elevated in symptomatic patients without endoscopic activity. Therefore, fecal biomarker testing may be more appropriate for monitoring endoscopic relapse than for clinical relapse.
FIT also can quantify the amount of hemoglobin in fecal samples, and was originally used for screening colorectal cancer. Several studies20,21 reported that hemoglobin detected by FIT is an appropriate biomarker for the assessment of mucosal inflammation in patients with UC. In one study21 comparing the value of FIT and fecal calprotectin, both FIT and calprotectin were suggested to efficiently predict MH in patients with UC. Calprotectin, lactoferrin, and hemoglobin are stable, convenient to measure at a low cost, and may be used as a non-invasive approach to monitor mucosal inflammation and predict subsequent relapse in patients with UC. This should spare the patients from complicated endoscopic procedures.
Our study has certain limitations. First, the different induction treatments used before entry and the change in therapy after remission induction, could have limited the accuracy of our findings. Second, histological evaluation was not done in our patients who achieved clinical and endoscopic remission. The relationship between histological inflammation and fecal biomarkers should have further strengthened our findings. Third, in this study measurement of fecal biomarkers was undertaken only at baseline, consecutive monitoring was not done in predicting future relapse.
In conclusion, fecal calprotectin, lactoferrin, and to a lesser degree hemoglobin appeared to be objective biomarkers for predicting relapse in UC patients after achieving clinical remission together with MH. The predictive value of these biomarkers is higher than MES as well as being available in fecal samples for assay. Likewise, use of these biomarkers should spare the patients from the unpleasant endoscopic procedures. On the basis of our experience, we believe that these biomarkers may serve as low cost, non-invasive, and clinically relevant parameters to predict UC relapse during maintenance therapy. It is hoped that the outcomes of this study might serve as the basis for establishing the optimal treatment target in patients with quiescent UC; endoscopic remission or decreasing fecal biomarkers below a given threshold. Additional large scale studies in this clinical setting should strengthen our findings. Further, a future study should investigate whether or not early medical intervention is beneficial for the prevention of relapse in patients with elevated fecal biomarkers.
Study Highlights
What is current knowledge
The paradigm for the management of IBD is shifting from alleviating symptoms towards achieving mucosal healing.
Simple and objective biomarkers for the prediction of relapse are expected to show greater clinical benefits.
Achieving endoscopic remission or decreasing the level of fecal biomarkers as an ideal therapeutic goal in UC has not been determined.
What is new here
Fecal calprotectin, lactoferrin, and to a lesser degree fecal hemoglobin appear to be objective biomarkers for predicting relapse after achieving clinical and endoscopic remission.
The predictive value of these fecal biomarkers is higher than with endoscopic score.
This study may be useful for establishing the optimal treatment target in patients with quiescent UC.
Electronic supplementary material
Conflict of interest
Guarantor of the article: Takayuki Yamamoto, MD, PhD, FACG.
Specific author contributions: Study design, planning and conducting the study, collection and interpretation of data, and drafting/editing the manuscript: T.Y.; planning the study, collection and interpretation of data, and drafting the manuscript: T.S. and S.U.; editing the manuscript: K.M. All the authors have approved the final draft submitted.
Financial support: None.
Potential competing interests: None.
Footnotes
Supplementary information
The online version of this article (10.1038/s41424-018-0006-7) contains supplementary material, which is available to authorized users.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rizzello F, et al. Review article: monitoring activity in ulcerative colitis. Aliment. Pharmacol. Ther. 2002;16:3–6. doi: 10.1046/j.1365-2036.16.s4.1.x. [DOI] [PubMed] [Google Scholar]
- 2.D’Haens G, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–786. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 4.Stange EF, et al. European Crohn’s and Colitis Organisation (ECCO). European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J. Crohns Colitis. 2008;2:1–23. doi: 10.1016/j.crohns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Walsh A, Palmer R, Travis S. Mucosal healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest. Endosc. Clin. N. Am. 2014;24:367–378. doi: 10.1016/j.giec.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Peyrin-Biroulet L, et al. Scientific Committee of the European Crohn’s and Colitis Organization. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J. Crohns Colitis. 2011;5:477–483. doi: 10.1016/j.crohns.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 7.D’Haens G, et al. Healing of severe recurrent ileitis with azathioprine therapy in patients with Crohn’s disease. Gastroenterology. 1997;112:1475–1481. doi: 10.1016/S0016-5085(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 8.Rutgeerts P, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Shah SC, et al. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2016;14:1245–1255. doi: 10.1016/j.cgh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Samaan MA, et al. A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm. Bowel. Dis. 2014;20:1465–1471. doi: 10.1097/MIB.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama K, et al. Clinical study of the relation between mucosal healing and long-term outcomes in ulcerative colitis. Gastroenterol. Res Pract. 2013;2013:192794. doi: 10.1155/2013/192794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barreiro-de Acosta M, et al. Evaluation of the risk of relapse in ulcerative colitis according to the degree of mucosal healing (Mayo 0 vs 1): a longitudinal cohort study. J. Crohns Colitis. 2016;10:13–19. doi: 10.1093/ecco-jcc/jjv158. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, et al. Effect of mucosal healing (Mayo 0) on clinical relapse in patients with ulcerative colitis in clinical remission. Scand. J. Gastroenterol. 2016;51:1069–1074. doi: 10.3109/00365521.2016.1150503. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino T, et al. The predictive variable regarding relapse in patients with ulcerative colitis after achieving endoscopic mucosal healing. Intest. Res. 2016;14:37–42. doi: 10.5217/ir.2016.14.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibble J, et al. A simple method for assessing intestinal inflammation in Crohn’s disease. Gut. 2000;47:506–513. doi: 10.1136/gut.47.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tibble JA, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 17.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin JF, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm. Bowel. Dis. 2014;20:1407–1415. doi: 10.1097/MIB.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 19.Mao R, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm. Bowel. Dis. 2012;18:1894–1899. doi: 10.1002/ibd.22861. [DOI] [PubMed] [Google Scholar]
- 20.Nakarai A, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am. J. Gastroenterol. 2013;108:83–89. doi: 10.1038/ajg.2012.315. [DOI] [PubMed] [Google Scholar]
- 21.Takashima S, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am. J. Gastroenterol. 2015;10:873–880. doi: 10.1038/ajg.2015.66. [DOI] [PubMed] [Google Scholar]
- 22.Mooiweer E, et al. Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: a plea for deep remission. J. Crohns Colitis. 2015;9:50–55. doi: 10.1093/ecco-jcc/jju003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.