Abstract
Objective
To determine the impact of one probiotics combination on the neurodevelopment of very preterm children at 2–5 years corrected gestational age (CA).
Design
Follow-up study of survivors of a double-blinded, placebo-controlled, randomised trial of probiotic effects on late-onset sepsis in very preterm infants that found reduced necrotising enterocolitis.
Setting
10 tertiary perinatal centres in Australia and New Zealand.
Patients
1099 very preterm infants born <32 weeks’ gestation and weighing <1500 g.
Intervention
Probiotics (Bifidobacterium infantis, Streptococcus thermophilus and Bifidobacterium lactis) or placebo administered from birth until discharge home or term CA, whichever came sooner.
Main outcome measures
Major neurodevelopmental impairment comprised any of moderate/severe cerebral palsy (Gross Motor Function Classification System score 2–5), motor impairment (Bayley-III Motor Composite Scale <–2SD or Movement Assessment Battery for Children <15th centile if >42 months’ CA), cognitive impairment (Bayley-III Composite Cognitive or Language Scales <–2SD or Wechsler Preschool and Primary Scale of Intelligence Full Scale Intelligence Quotient <–2SD if >42 months’ CA), blindness or deafness.
Results
Outcome data were available for 735 (67%) participants, with 71 deaths and 664/1028 survivors assessed at a mean age of 30 months. Survival free of major neurodevelopmental impairment was comparable between groups (probiotics 281 (75.3%) vs placebo 271 (74.9%); relative risk 1.01 (95% CI 0.93 to 1.09)). Rates of deafness were lower in probiotic-treated children (0.6% vs 3.4%).
Conclusion
Administration of the probiotics combination Bifidobacterium infantis, Streptococcus thermophilus and Bifidobacterium lactis to very preterm babies from soon after birth until discharge home or term CA did not adversely affect neurodevelopment or behaviour in early childhood.
Trial registration number
Australia and New Zealand Clinical Trials Register (ANZCTR): ACTRN012607000144415.
Keywords: Neurodevelopment
What is already known on this topic?
In very preterm infants, some prophylactic probiotics reduce necrotising enterocolitis (NEC) ≥ Bell Stage 2, mortality and late-onset sepsis.
Probiotics are increasingly considered standard care for very preterm infants in NICUs around the world.
Knowledge regarding the effects of probiotics on longer-term neurodevelopmental outcomes in early childhood is limited.
What this study adds?
The probiotics combination used in this trial reduced NEC ≥ Bell Stage 2 without adverse effects on neurodevelopment or behaviour in early childhood.
This adds to the safety of prophylactic probiotics administration to very preterm infants.
Introduction
Probiotics significantly reduce mortality, necrotising enterocolitis (NEC) ≥Bell Stage 2 and late-onset sepsis in preterm infants.1–3 However, despite improved survival of infants born very preterm, significant neurological sequelae, including cerebral palsy (CP) (10%) and major neurosensory impairment (50%), remain static.4
There is biological plausibility that probiotics could improve neurodevelopment in very preterm infants either by reducing NEC and the associated negative impact on neurodevelopment5 or by a primary effect on the brain–gut–microbiome axis. The intestinal microbiome performs essential functions, including digestion and metabolism of nutrients, protection against pathogen translocation and modulation of local and distal immune responses.6–8 The latter occurs via the brain–gut–microbiome axis through immune, endocrine and neural tissue cross-communication.9 10 Intestinal microbiome dysbiosis affects the brain–gut–microbiome axis and has been linked to physical and psychological disorders, including anxiety and depression.11–14 Immunomodulatory effects of probiotics, ‘live microorganisms that when administered in adequate amounts confer health benefits on the host’,15 include a reduction in proinflammatory substances, such as brain-derived neurotrophic factor and an increase in anti-inflammatory substances, such as Interleukin-6, both implicated in brain injury.16 17
Knowledge regarding the effects of probiotics on neurodevelopment and behaviour in early childhood is limited.18–20 The ProPrems randomised controlled trial (RCT) of 1099 infants born <32 weeks’ gestation and weighing <1500 g found no significant effect of prophylactic administration of the probiotics combination Bifidobacterium infantis, Streptococcus thermophilus and Bifidobacterium lactis on the primary outcome, definite late-onset sepsis, but did report a 54% reduction in the secondary outcome NEC ≥Bell Stage 2.2 This current study aimed to investigate the effects of probiotics on neurodevelopment and behaviour of a large cohort of very preterm participants in the ProPrems trial at 2–5 years of age, corrected for prematurity (corrected gestational age (CA)). We hypothesised that the administered probiotics would not adversely affect neurodevelopment in early childhood.
Methods
Design and participants
This was a neurodevelopmental follow-up study of participants of the ProPrems trial: a multicentre, double-blind, placebo-controlled randomised trial of 1099 very preterm infants recruited from October 2007 to November 2011 from tertiary perinatal centres in Australia (n=8) and New Zealand (n=2). Participants in the ProPrems trial were randomised to receive probiotics (n=548) or placebo (n=551), with the primary outcome being late-onset sepsis.2 21
Intervention
The details of the ProPrems trial have previously been described.2 To summarise, eligible infants born <32 completed weeks’ gestation and weighing <1500 g were randomised within 72 hours of birth. The intervention started when an infant was receiving at least 1 mL of milk 4 hourly, administered daily until discharge from hospital or term CA. The intervention was the probiotic combination Bifidobacterium infantis (BB–02 96579 300×106), Streptococcus thermophilus (S. thermophilus TH–4 15957 350×106) and Bifidobacterium lactis (BB-12 15954 350×106) (ABC Dophilus Probiotic Powder for Infants, Solgar, USA) with 1×109 total organisms per 1.5 g in a maltodextrin base powder. The placebo was maltodextrin powder.
Assessments
Our intention was for neurodevelopment and behavioural assessments to be performed at 2 years CA. However, as neurodevelopment was not an outcome of the original ProPrems trial, funding and ethical approval were obtained after some participants were older than 2 years CA. Therefore, assessments were performed as close to 2 years CA as possible and before 5 years CA. Families of surviving ProPrems participants were made aware of the follow-up study in regular study newsletters and were then approached by mail and then by telephone 3 weeks later about participation in follow-up assessments. Where assessments had already been performed at 2 years CA as part of routine local follow-up, retrospective consent was requested to include the data.
At 2–5 years CA, participants underwent a standardised neurological examination.22 The Bayley Scales of Infant and Toddler Development III (Bayley-III) assessed cognitive, language and motor development (n=597).23 Participants were assessed in a single session by a trained examiner, who was unaware of group assignment. As the Bayley-III overestimates development, likely due to a mixed sampling procedure used for the Bayley-III standardisation,24 25 the impairment cut-off was adjusted by 7.5 points (half a SD) for cognitive, language and motor scales, that is, major impairment cut-off was 77.5 (instead of 70) and mild impairment on the Bayley-III indices was 92.5 (instead of 85).23 CP was diagnosed using standard criteria, including severity according to the Gross Motor Function Classification System (GMFCS).22 Blindness was diagnosed when vision was worse than 6/60 in the better eye and deafness if amplification or cochlear implant was required. For children over 42 months’ CA (n=62) unable to be assessed by the Bayley-III because of the ceiling effect, cognition was assessed with the Wechsler Preschool and Primary Scale of Intelligence—Third Edition Australian Language Adaptation (WPPSI-III) and motor function was assessed with the Movement Assessment Battery for Children—2 (MABC).26 27
Caregivers completed questionnaires about their child’s socioemotional development using the Infant Toddler Social Emotional Assessment,28 including the four composite scales of externalising, internalising, dysregulation and social competence behaviour, and their family’s social risk.29 The Social Risk Index29 was developed and is used extensively within the Australian context to provide a cumulative score accounting for family structure, education of primary caregiver, employment status and occupation of primary income earner, language spoken at home and maternal age at birth of child. A composite score was calculated and dichotomised to higher and lower social risks.
Outcomes
The prespecified primary outcome was survival free of major neurosensory impairment. Major impairment was a composite outcome, defined as having one or more of the following: motor impairment, cognitive impairment, blindness and/or deafness. Motor impairment comprised any CP on neurological examination or GMFCS score of 2–5 or Bayley-III Motor Composite Scale <77.5 or Movement ABC <15th centile.22 23 27 Cognitive impairment comprised a score on the Bayley-III Cognitive or Language Scales of <77.5 or WPPSI-III Full Scale Intelligence Quotient of more than 2 SD below the normative mean (<70). If English was not the family’s first language, then only the Bayley-III cognitive score was included (n=23). Children for whom disability status could not be determined (ie, those with missing data for one or more primary outcome component and no disability on the non-missing components) were excluded from the primary outcome analysis (n=43).
Secondary outcomes included the individual components of the primary outcome and rates of disability. Mild disability comprised mild developmental delay (Bayley-III cognitive or language composite scores 77.5–92.5 or WPPSI-III 70–85) or mild CP (walking at 2 years, GMFCS 1). Moderate disability comprised moderate CP (not walking at 2 years but expected to walk eventually, GMFCS level 2 or 3) or deafness or moderate developmental delay (Bayley-III 62.5–77.5 or WPPSI-III 55–70). Severe disability comprised severe CP (unlikely ever to walk, GMFCS level 4 or 5), blindness or severe developmental delay (Bayley-III <62.5 or WPPSI-III <55).
This early childhood follow-up of ProPrems participants at 2–5 years CA was not part of the original protocol developed in 2006.21 Both the ProPrems RCT and this neurodevelopmental follow-up study were approved by the human research ethics committees at each participating centre. Written informed consent was obtained from parents or guardians both for the original study and the neurodevelopmental follow-up. Investigators and parents remained blinded to treatment allocation group during the follow-up period. The trial is registered with the Australia and New Zealand Clinical Trials Register (ANZCTR), number ACTRN012607000144415.
Statistical analysis
The ProPrems randomised trial sample size of 1100 participants had 80% power to detect a difference of 7% (16% probiotics vs 23% placebo) in the incidence of culture-proven sepsis. Assuming a similar follow-up rate of 90% to that seen in other Victorian cohorts at 2 years CA, this neurodevelopmental follow-up study would have more than 80% power to detect a difference of 8% (82% probiotics, 74% placebo) in the primary outcome (survival free of major neurosensory impairment at 2 years CA).
All analyses were performed on an intention-to-treat basis. (i) Unadjusted results: for the primary outcome and dichotomous secondary outcomes, we estimated the difference between probiotic and placebo using risk ratios (relative risks (RRs)) with 95% CIs.30 For continuous secondary outcomes, differences in means, with 95% CIs, were estimated. All continuous variables reported in this analysis had unskewed distributions. P values were estimated using Χ2 tests and t-tests. (ii) Adjusted results: we estimated risk ratios and differences in means adjusted for possible imbalance in prognostic factors between the two groups (intraventricular haemorrhage grade 3 or 4 or periventricular leukomalacia, retinopathy of prematurity ≥stage 3, bronchopulmonary dysplasia at 36 weeks CA, male sex, definite late-onset sepsis, birth gestation and birthweight z-score). For dichotomous outcomes, we used a generalised linear model with Gaussian distribution, log link and robust variance estimation.31 For continuous outcomes, we used multivariable linear regression. All analyses were performed using Stata/IC software, V.14.0 (StataCorp, Texas, USA).
Subgroup analyses were undertaken within the prespecified gestational age strata (<28 weeks) and birth weight (<1000 g) for the primary outcome, with binary regression used to assess evidence for interaction between treatment and subgroup. Analyses included only subjects with sufficiently complete follow-up data to determine outcomes. Multiple imputation of outcome variables was not considered appropriate since the only other information available was the trial data from the perinatal period.
Results
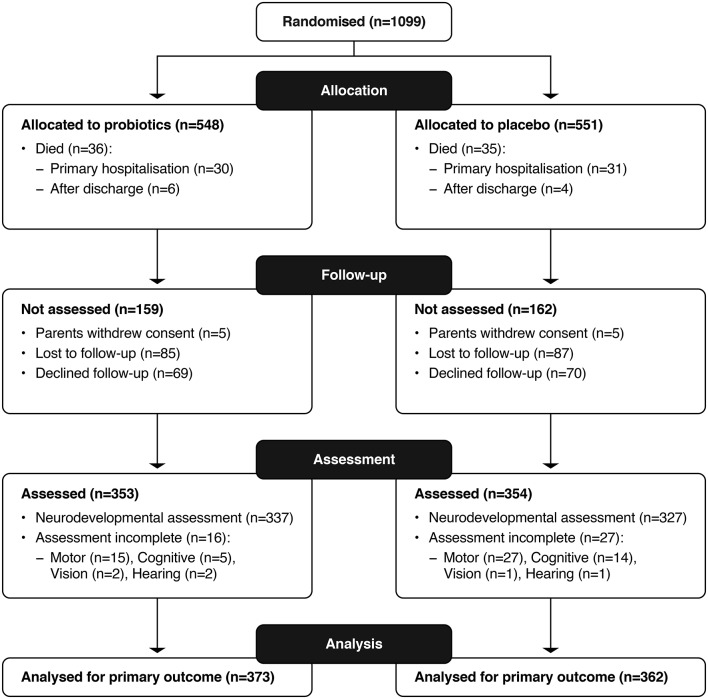
The primary outcome was available for 735 (67%) of the ProPrems trial participants, with 71 deaths and 664 survivors assessed (figure 1) at a mean age of 30 months CA. Assessed survivors were lighter at birth (birth weight mean (SD) in grams 1059 (257) vs 1098 (246); and <1000 g 289 (43.5%) vs 127 (34.9%)) and less mature at birth (gestation mean (SD) 27.8 (1.9) vs 28.4 (1.9) and <28 weeks 276 (41.6%) vs 118 (32.4%)) when compared with non-assessed surviving infants (see online supplementary table 1). Infants randomised to probiotics had lower rates of NEC ≥Bell Stage 2, compared with placebo (9 (2.4%) vs 20 (5.5%)) (table 1). Baseline characteristics between probiotics and placebo groups were comparable in the 664 survivors assessed (see online supplementary table 2). More primary caregivers were in full-time employment in the probiotics compared with placebo group (table 2); other characteristics were similar.
Figure 1.
Trial follow-up profile.
Table 1.
Baseline characteristics of 735 participants
| Probiotic group (n=373) |
Placebo group (n=362) |
|
| Perinatal characteristics | ||
| Multiple births, N (%) | 140 (37.5) | 127 (35.1) |
| Antenatal steroids (any), N (%) | 342 (91.7) | 334 (92.3) |
| Maternal antibiotics, N (%) | 183 (49.1) | 173 (47.8) |
| Chorioamnionitis, N (%) | 28 (7.5) | 34 (9.4) |
| Caesarean section, N (%) | 242 (64.9) | 253 (69.9) |
| Gestational age, mean (SD) weeks | 27.6 (2.0) | 27.6 (1.9) |
| <28 weeks, N (%) | 165 (44.2) | 171 (47.2) |
| Birth weight, mean (SD) g | 1042 (267) | 1027 (261) |
| <1000 g, N (%) | 177 (47.5) | 170 (47.0) |
| Birthweight z-score*, mean (SD) | −0.3 (1.1) | −0.4 (1.1) |
| Male, N (%) | 193 (51.7) | 207 (57.2) |
| 5 min Apgar score, median (IQR) | 8 (7–9) n=371 |
8 (7–9) n=358 |
| Age at enrolment into original RCT, mean (SD) days | 2.0 (0.9) | 2.1 (0.9) |
| Age commenced study powder, median (IQR) days† | 5.0 (4.0–7.0) n=371 |
5.0 (4.0–7.0) n=352 |
| Neonatal characteristics | ||
| Any EBM, n (%) | 356 (95.4) | 355 (98.1) |
| NEC (Bell Stage 2 or greater), n (%) | 9 (2.4) | 20 (5.5) |
| IVH Grade 3 or 4 or cystic PVL, n (%) | 17 (4.6) | 10 (2.8) |
| At least one episode of definite late-onset sepsis, n (%) | 57 (15.3) | 55 (15.2) |
| BPD at 36 weeks, n (%) | 117 (33.7) n=347 |
110 (32.7) n=336 |
| ROP ≥grade 3, n (%) | 18 (4.8) | 17 (4.7) |
*Z-score was calculated using the LMS British preterm growth data.
†Some babies died prior to commencing study powder (13 probiotics, 15 placebo).
BPD, bronchpulmonary dysplasia; EBM, expressed breast milk; IVH, intraventricular haemorrhage; NEC, necrotising enterocolitis; PVL, periventricular leukomalacia; RCT, randomised controlled trial; ROP, retinopathy of prematurity.
Table 2.
Characteristics of children with neurodevelopmental assessments
| Probiotic group (n=337) |
Placebo group (n=327) |
|
| Age at assessment, months corrected age, mean (SD) | 30.7 (8.9) n=336 |
30.1 (7.8) n=325 |
| Weight at assessment, kg mean (SD) | 12.8 (2.6) n=328 |
12.7 (2.1) n=322 |
| Height at assessment, cm mean (SD) | 90.4 (7.6) n=320 |
89.9 (6.4) n=311 |
| Head circumference at assessment, cm mean (SD) | 48.8 (1.9) n=319 |
48.9 (2.0) n=312 |
| Growth: z-scores, mean (SD) | n=329 | n |
| Mean (SD) Weight Height Head circumference |
n=329 −0.6(1.3) −0.2(1.3) −1.2(1.3) |
n=321 −0.6(1.3) −0.2(1.2) −1.1(1.4) |
| Sociodemographic variables | ||
| English only spoken at home, n (%) | 231 (82.5) n=280 |
207 (77.2) n=268 |
| Two caregivers (nuclear family structure), n (%) | 236 (87.1) n=271 |
220 (87.0) n=253 |
| Primary caregiver education, n (%) | ||
| <11 years of schooling 11–12 years of schooling Tertiary |
25 (9.3) 111 (41.1) 134 (49.6) n=270 |
31 (12.3) 94 (37.2) 128 (50.6) n=253 |
| Primary caregiver employment, n (%) | ||
| Full time Part time Unemployed, pension |
183 (67.5) 41 (15.1) 47 (17.3) n=271 |
144 (56.9) 60 (23.7) 49 (19.4) n=253 |
| Primary income earner, n (%) | ||
| Skilled/professional Semiskilled Unskilled |
141 (52.2) 85 (31.5) 44 (16.3) n=270 |
151 (59.7) 63 (24.9) 39 (15.4) n=253 |
| Maternal age, n (%) | ||
| >21 years 18–21 years <18 years |
264 (97.4) 7 (2.6) 0 n=271 |
244 (96.4) 8 (3.2) 1 (0.4) n=253 |
| SRI score, median (range) | 2.0 (1.0–3.0) n=269 |
2.0 (1.0–4.0) n=252 |
| Higher social risk (SRI≥2), n (%) | 152 (56.5) n=269 |
152 (60.3) n=252 |
SRI, Social Risk Index.38
bmjpo-2017-000176supp001.pdf (136.8KB, pdf)
Survival free of major neurosensory impairment was comparable between children who received probiotics and those who received placebo (281 (75.3%) vs 271 (74.9%); RR 0.98 (95% CI 0.76, 1.26); P=0.88), (table 3), even after adjusting for confounding variables (see online supplementary table 3). This relationship was similar in both gestational age and birthweight subgroups (P value for interaction >0.05). Given the high rates of loss to follow-up, we performed a sensitivity analysis to possible differential rates of survival free of major neurodevelopmental impairment in the study participants who did not attend follow-up. In order to achieve a P value <0.05 for a difference in survival free of major neurodevelopmental impairment rates between probiotics and placebo groups, we would have needed a difference of at least 14% between the groups who did not attend follow-up.
Table 3.
Primary composite outcome of survival free of major neurosensory impairment at 2–5 years corrected gestational age
| Probiotic group n=373 |
Placebo group n=362 |
Relative risk (95% CI) |
P value | |
| Survival without major neurosensory impairment* N (%) | 281 (75.3) | 271 (74.9) | 1.01 (0.93 to 1.09) | 0.88 |
| Subgroup analyses: | ||||
| Gestation | 0.08† | |||
| <28 weeks, n (%) ≥28 weeks, n (%) |
104 (63.0) 177 (85.1) |
118 (69.0) 153 (80.1) |
0.91 (0.78 to 1.07) 1.06 (0.97 to 1.16) |
0.25 0.19 |
| Birth weight | 0.62† | |||
| <1000 g, n (%) ≥1000 g, n (%) |
116 (65.5) 165 (84.2) |
113 (66.5) 158 (82.3) |
0.99 (0.85 to 1.15) 1.02 (0.94 to 1.12) |
0.85 0.62 |
*Major neurosensory impairment=motor impairment AND/OR cognitive impairment AND/OR blindness AND/OR deafness.
†Interaction P value.
Individual components of the primary outcome are summarised in table 4. Mortality rates, as well as neurodevelopmental disability and socioemotional development were similar between groups. However, fewer children treated with probiotics required amplification or cochlear implants for sensorineural deafness compared with placebo (2 (0.6%) vs 11 (3.4%); RR 0.18 (95% CI 0.04, 0.80); P=0.01). This result was not explained by differences in numbers of courses of antibiotics or in the total days of vancomycin or gentamicin (data not shown).
Table 4.
Secondary outcomes at 2–5 years corrected gestational age (CA)
| Probiotic group n=337 |
Placebo group n=327 |
Relative risk (95% CI) |
P value | |
| Components of primary outcome: | ||||
| Death before 24 months CA N (%) | 36/548 (6.6) | 35/551 (6.4) | 1.03 (0.66, 1.62) | 0.88 |
| Major neurosensory impairment N (%)* | 56 (16.6) | 56 (17.1) | 0.97 (0.69 to 1.36) | 0.86 |
| Disability, N (%) | 0.72 | |||
| None Mild Moderate Severe |
206 (61.1) 90 (26.7) 27 (8.0) 14 (4.2) |
203 (62.1) 77 (23.5) 30 (9.2) 17 (5.2) |
||
| Cerebral palsy (CP) N (%) | 19 (5.7) | 14 (4.3) | 1.33 (0.68 to 2.61) | 0.41 |
| CP severity | 0.55 | |||
| No CP Mild CP Moderate CP Severe CP |
301 (90.4) 23 (6.9) 3 (0.9) 1 (0.3) |
294 (90.2) 19 (5.8) 3 (0.9) 1 (1.5) |
||
| Motor impairment† N (%) | 31 (9.3) | 24 (7.4) | 1.25 (0.75 to 2.07) | 0.40 |
| Cognitive impairment† N (%) | 39 (11.6) | 40 (12.4) | 0.93 (0.62 to 1.41) | 0.74 |
| Deafness N (%) | 2 (0.6) | 11 (3.4) | 0.18 (0.04 to 0.80) | 0.01 |
| Blindness N (%) | 1 (0.3) | 0 (0.0) | 0.32 | |
| Bayley-III composite scores mean (SD) | ||||
| Mean difference (95% CI) | P value | |||
| Cognitive | 100.4 (17.1) n=299 |
99.2 (15.1) n=298 |
1.2 (−1.4, 3.8) | 0.36 |
| Language | 98.3 (16.8) n=289 |
98.5 (18.1) n=281 |
−0.3 (−3.1, 2.6) | 0.86 |
| Motor | 102.3 (11.6) n=299 |
100.7 (16.8) n=296 |
1.6 (−1.1, 4.3) | 0.24 |
| WPPSI | n=37 | n=25 | Mean difference (95% CI) | |
| FSIQ | 106.0 (21.6) | 1.3 (−8.3, 14.1) | 0.79 | |
| FSIQ<70 | 2 (5.4) | 1 (4.0) | 1.35 (0.1 to 14.1) | 0.80 |
| MABC | n=37 | n=23 | Mean difference (95% CI) | P value |
| <15th centile N (%) | 8 (21.6) | 5 (21.7) | 1.0 (0.4 to 2.7) | 0.99 |
| <5th centile N (%) | 3 (8.1) | 2 (8.7) | 0.9 (0.2 to 5.2) | 0.94 |
| ITSEA | n=165 | n=163 | Mean difference (95% CI) | P value |
| Months corrected age at testing, mean (SD) | 26.7 (3.3) | 27.5 (4.4) | −0.1 (−1.6, 0.1) | 0.09 |
| T scores, mean (SD) | n=165 | n=163 | ||
| Externalising | 48.6 (9.8) | 50.2 (11.4) | −1.7 (−4. 0.6) | 0.16 |
| Internalising | 48.1 (10.6) | 48.6 (11.2) | −0.5 (−2.9, 1.8) | 0.66 |
| Dysregulation | 48.1 (14.1) | 46.5 (12.9) | 1.6 (−1.4, 4.5) | 0.29 |
| Competence | 47.3 (12.0) | 46.4 (12.4) | 1 (−1.7, 3.6) | 0.48 |
| Of concern, n (%) | n=165 | n=163 | Relative risk (95% CI) | |
| Externalising | 15 (9.1) | 16 (9.8) | 0.93 (0.5 to 1.8) | 0.82 |
| Internalising | 16 (9.7) | 15 (9.2) | 1.05 (0.5, 2.1) | 0.88 |
| Dysregulation | 21 (12.7) | 14 (8.5) | 1.49 (0.8 to 2.8) | 0.22 |
| Competence | 21 (12.8) | 27 (16.7) | 0.77 (0.5 to 1.3) | 0.33 |
*Major neurosensory impairment=motor impairment AND/OR cognitive impairment AND/OR blindness AND/OR deafness.
†(Corrected by 0.5SD=7.5 points).
FSIQ, Full Scale Intelligence Quotient; ITSEA, Infant Toddler Social Emotional Assessment; MABC, Movement Assessment Battery for Children; WPPSI, Wechsler Preschool and Primary Scale of Intelligence.
Disability severity: mild disability=GMFCS1and/or Bayley-III cognitive or language composite scores <–1–2SD (77.5–92.5)and/or WPPSI <–1–2SD (70-85); moderate disability=GMFCS2–3and/or Bayley-III cognitive or language composite scores <–2–3SD (62.5–77.5)or WPPSI <–2–3SD (55-70) and/or deafness; severe disability=GMFCS4–5and/or Bayley-III cognitive or language composite scores <–3SD (62.5) or WPPSI <–3SD (55) and/or blindness.
CPseverity: mild CP=GMFCS1; moderate CP=GMFCS2 or 3; severe CP=GMFCS4 or 5.
Discussion
This follow-up study of a RCT found that probiotics administered to very preterm infants from soon after birth until discharge home or term CA did not affect neurodevelopmental outcomes or behaviour in early childhood. We previously reported that this probiotic combination administered in the perinatal period halved the rate of NEC ≥Bell Stage 2 in a population with a high breast milk feeding rate.2 This important benefit in very preterm infants without longer-term adverse consequences adds to the safety profile of prophylactic probiotics administration in this vulnerable population.
The three follow-up studies of probiotics administration to preterm infants have smaller sample sizes and were performed in different settings and preterm populations.18–20 Two cohorts from Taiwan (n=301)18 and Turkey (n=221)20 assessed children born weighing <1500 g at 3 years CA and <33 weeks or <1500 g at 18–22 months CA, respectively, using the Bayley Scales of Infant and Toddler Development II; these also found no differences in the incidence of survival without major neurodevelopmental impairment between probiotics and control groups (135/180 (75.0%) vs 138/187 (73.8%), P=0.94)18 and (100/121 (82.6%) vs 98/121 (81.0%), P=0.92).20 Reported rates of CP, visual loss, as well as cognitive and motor impairment were similar to ProPrems.18–20 Another trial enrolled 249 infants weighing <2500 g and <37 weeks’ gestation at birth and reported similar proportions with ‘suboptimal scores’ at 12 months’ CA in probiotic and control groups using the Hammersmith Infant Neurological Assessment (23/166 (13.8%) vs 24/83 (29%), P>0.05).19
In this study, the incidence of sensorineural hearing loss was lower in the probiotics group compared with placebo, although the overall rate for both groups was low. This finding could not be explained by post hoc analysis of ototoxic antibiotic therapy during the primary hospitalisation and has not been reported in other studies. It is unclear if lower rates of hearing loss in probiotics-treated children are real effects or type I errors. One possible explanation for this finding is that probiotics may reduce cochlear injury and therefore sensorineural hearing loss. Although only described until now in adult mice with Lactococcus lactis reducing age-related cochlear degeneration and hearing loss,32 sensorineural hearing loss in preterm infants is also predominantly mediated by cochlear and outer hair cell mediated injury.33 As this is only speculative, until this finding is replicated in other studies, we recommend that it must be interpreted with caution.
While the probiotics combination used in the ProPrems study does not negatively impact on neurodevelopment in early childhood, the rate of NEC ≥Bell stage 2 was low (2.0% vs 4.4%). Emerging evidence suggests that colonisation of the gut with exogenous probiotics can reverse the abnormal psychological effects associated with a dysbiotic intestinal microbiome and malfunctioning brain–gut–microbiome axis. For example, adult human studies have reported beneficial effects of probiotics on depression,34 35 as well as cognitive and neurobehavioural function.36 In these studies, benefits were experienced during probiotics administration. So, although there is a theoretical basis for a primary effect of probiotics on neurodevelopment in very preterm infants, neurobehavioural effects of probiotics may not be seen 2–5 years after probiotics were ceased.
Another possible reason for the lack of effect of this probiotic combination on neurobehaviour is the probiotic strains used. Probiotic effects are known to be strain, dose, condition and site specific37; so it is possible that we did not detect a difference in outcomes between the groups because the most appropriate probiotic strain, or strains, or dose for modulation of the brain–gut–microbiome axis was not used. Further evaluation based on experimental paradigms may be warranted.
The current study, with a sample size of 735 very preterm born children, is the largest follow-up study of probiotic effects in very preterm infants published until now. Assessors and parents were blinded to group allocation throughout the follow-up. We assessed neurodevelopment and socioemotional development.
We acknowledge limitations of this study, particularly that neurodevelopment was not a planned outcome of the ProPrems trial. Neither parents nor participating centres expected further evaluation in early childhood, which may explain the 172 surviving participants lost to follow-up and the 139 families who declined neurodevelopmental assessment (figure 1). The primary outcome was therefore available in only 67% of participants, increasing the risk of type 2 error. However, with 373 intervention and 362 control participants, there was 75% power to detect a difference of 82% versus 74% in survival free of major neurosensory impairment. Also, those who were assessed were at higher risk of adverse neurodevelopmental outcome, being of lower birth weight and gestational age compared with those who were not assessed. Following the sensitivity analyses, it is unlikely that a difference of disability-free survival between probiotics and placebo participants who attended follow-up would be ≥14%. Therefore. we do not believe our conclusions would be altered.
In addition, participants had a wide age range necessitating assessment with different measures, which assess slightly different concepts and which may have impacted on the power of the study to find differences between groups. However, based on the sensitivity analysis, the age range is unlikely to have influenced the study conclusions because numbers were small (n=62) and also because dichotomous outcomes based on SD (2SD below the mean) were used to standardise the different tests. Future trials should plan to assess long-term neurodevelopmental outcome within a narrow age window allowing for a standardised assessment battery.
In conclusion, administration of the probiotics combination Bifidobacterium infantis, Streptococcus thermophilus and Bifidobacterium lactis to very preterm infants from soon after birth until term CA did not negatively impact on survival free of neurodevelopmental impairment in early childhood. This probiotics mixture reduces NEC in very preterm infants, with apparent safety with respect to early childhood development.
Acknowledgments
We acknowledge the assistance of the physicians, psychologists, and research coordinators, and other staff who made this study possible. Most importantly, we sincerely thank the children and their families who participated in the ProPrems trial and follow-up study.
Footnotes
Contributors: SJ, LH and JC were involved in study design, literature review and planning data analysis. SJ prepared the first version of the manuscript assisted by LH. SD performed all data analyses. SJ, LH and GO conducted and coordinated the study. GO and PA assisted with planning assessments and with interpretation of results. SMG designed the original ProPrems randomised controlled trial. All authors reviewed and approved the final draft.
Funding: This work was supported by the National Health and Medical Research Council of Australia grant numbers 454629 and 1028050; SJ is also supported by Early Career Fellowship number 1073103, PA by Senior Research Fellowship number 1081288 and JC by Early Career Fellowship number 1053787).
Disclaimer: The probiotic combination ’ABC Dophilus Powder for Infants' was supplied at cost by Solgar, USA, which had no role in the trial design, conduct, data management or analysis or writing the manuscript. The authors have no financial disclosures relevant to this article to disclose.
Competing interests: None decalred.
Patient consent: Written informed consent was obtained from parents or guardians both for the original study and the neurodevelopmental follow-up. However, they were not BMJ patient consent forms.
Ethics approval: Human research ethics committees at participating 10 centres.
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: Group Information: Members of The ProPrems Study Groups are as follows. ProPrems Neuro Steering Group: SE Jacobs (principal investigator), L Hickey (chief investigator), GF Opie (chief investigator), SM Garland (chief investigator), JLY Cheong (chief investigator), S Donath (associate investigator), PJ Anderson (associate investigator), L Gold (associate investigator), KL Sia (associate investigator). ProPrems Steering Group: SM Garland (principal investigator), SE Jacobs (chief investigator), JM Tobin (chief investigator), SN Tabrizi (chief investigator), M Pirotta (chief investigator), S Donath (associate investigator), GF Opie (associate investigator), MLK Tang (associate investigator), CJ Morley (associate investigator). Trial Statistician: S Donath, Clinical Epidemiology and Biostatistics Unit, Murdoch Childrens Research Institute, Melbourne, Australia. Participating Hospitals and Investigators: SM Garland, SE Jacobs, CJ Morley, SN Tabrizi, L Hickey, JLY Cheong, The Royal Women’s Hospital; GF Opie, Mercy Hospital for Women; K Tan, A Lewis, A Veldman, E Carse, Monash Health; L Hickey, PJ Anderson, MLK Tang, Royal Children’s Hospital: all in Melbourne, Australia; J Travadi, IMR Wright, John Hunter Children’s Hospital; DA Osborn, Royal Prince Alfred Hospital; J Sinn, J Bowen, Royal North Shore Hospital; J Levison, JA Stack, Liverpool Hospital; – all in New South Wales, Australia; AG DePaoli, Royal Hobart Hospital, Tasmania, Australia; NC Austin, BA Darlow, Christchurch Women’s Hospital, University of Otago, Christchurch, New Zealand; JM Alsweiler, MJ Buksh, Auckland City Hospital and The Liggins Institute, University of Auckland, Auckland, New Zealand.
Contributor Information
ProPremsStudy Groups:
SE Jacobs, L Hickey, GF Opie, SM Garland, JLY Cheong, S Donath, PJ Anderson, L Gold, KL Sia, JM Tobin, SN Tabrizi, M Pirotta, MLK Tang, CJ Morley, K Tan, A Lewis, A Veldman, E Carse, J Travadi, IMR Wright, DA Osborn, J Sinn, J Bowen, J Levison, JA Stack, AG Depaoli, NC Austin, BA Darlow, JM Alsweiler, and MJ Buksh
References
- 1.AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014;4. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SE, Tobin JM, Opie GF, et al. . Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 2013;132:1055–62. doi:10.1542/peds.2013-1339 [DOI] [PubMed] [Google Scholar]
- 3.Rao SC, Athalye-Jape GK, Deshpande GC, et al. . Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-analysis. Pediatrics 2016;137:e20153684–1-16. doi:10.1542/peds.2015-3684 [DOI] [PubMed] [Google Scholar]
- 4.Doyle LW, Roberts G, Anderson PJ, et al. . Outcomes at age 2 years of infants < 28 weeks' gestational age born in Victoria in 2005. J Pediatr 2010;156:49–53. doi:10.1016/j.jpeds.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 5.Hintz SR, Kendrick DE, Stoll BJ, et al. . Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005;115:696–703. doi:10.1542/peds.2004-0569 [DOI] [PubMed] [Google Scholar]
- 6.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr 2002;22:283–307. doi:10.1146/annurev.nutr.22.011602.092259 [DOI] [PubMed] [Google Scholar]
- 7.Ley RE, Bäckhed F, Turnbaugh P, et al. . Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005;102:11070–5. doi:10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Ley RE, Mahowald MA, et al. . An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–131. doi:10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 9.Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 2011;23:187–92. doi:10.1111/j.1365-2982.2010.01664.x [DOI] [PubMed] [Google Scholar]
- 10.Kelly JR, Kennedy PJ, Cryan JF, et al. . Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392 doi:10.3389/fncel.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 2010;7:503–14. doi:10.1038/nrgastro.2010.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol 2015;6:110–9. doi:10.4291/wjgp.v6.i4.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. . Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut 2006;55:205–11. doi:10.1136/gut.2005.073817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Ling Z, Zhang Y, et al. . Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 2015;48:186–94. doi:10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 15.Hill C, Guarner F, Reid G, et al. . Expert consensus document. The International scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. doi:10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 16.Bercik P, Verdu EF, Foster JA, et al. . Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 2010;139:2102–12. doi:10.1053/j.gastro.2010.06.063 [DOI] [PubMed] [Google Scholar]
- 17.Desbonnet L, Garrett L, Clarke G, et al. . Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010;170:1179–88. doi:10.1016/j.neuroscience.2010.08.005 [DOI] [PubMed] [Google Scholar]
- 18.Chou IC, Kuo HT, Chang JS, et al. . Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. J Pediatr 2010;156:393–6. doi:10.1016/j.jpeds.2009.09.051 [DOI] [PubMed] [Google Scholar]
- 19.Romeo MG, Romeo DM, Trovato L, et al. . Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J Perinatol 2011;31:63–9. doi:10.1038/jp.2010.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sari FN, Eras Z, Dizdar EA, et al. . Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? Am J Perinatol 2012;29:579–86. doi:10.1055/s-0032-1311981 [DOI] [PubMed] [Google Scholar]
- 21.Garland SM, Tobin JM, Pirotta M, et al. . The ProPrems trial: investigating the effects of probiotics on late onset sepsis in very preterm infants. BMC Infect Dis 2011;11:210 doi:10.1186/1471-2334-11-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palisano R, Rosenbaum P, Walter S, et al. . Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 1997;39:214–23. doi:10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 23.Bayley N. Bayley Scales of Infant and Toddler Development, The Psycholgical Corporation. 3rd ed Harcourt Assessment, Inc, 2006. [Google Scholar]
- 24.Anderson PJ, De Luca CR, Hutchinson E, et al. . Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med 2010;164:352–6. doi:10.1001/archpediatrics.2010.20 [DOI] [PubMed] [Google Scholar]
- 25.Anderson PJ, Burnett A. Assessing developmental delay in early childhood - concerns with the Bayley-III scales. Clin Neuropsychol 2017;31:371–81. doi:10.1080/13854046.2016.1216518 [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Primary and Preschool Scale of Intelligence. 3rd ed Psychological Corporation, 2002. [Google Scholar]
- 27.Henderson SE, Sugden DA. Movement Assessment Battery for Children. 2nd ed: (Movement ABC-2): Pearson Assessment, 2007. [Google Scholar]
- 28.Carter AS, Briggs-Gowan MJ, Jones SM, et al. . The Infant-Toddler Social and Emotional Assessment (ITSEA): factor structure, reliability, and validity. J Abnorm Child Psychol 2003;31:495–514. doi:10.1023/A:1025449031360 [DOI] [PubMed] [Google Scholar]
- 29.Treyvaud K, Anderson VA, Howard K, et al. . Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics 2009;123:555–61. doi:10.1542/peds.2008-0477 [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ. Epidemiology M. Boston: Little, Brown, 1986. [Google Scholar]
- 31.Lumley T, Kronmal R, Ma S. Relative risk regression in medical research: models, contrasts, estimators, and algorithms. UW Biostatistics Working Paper Series. 2006. [Google Scholar]
- 32.Oike H, Aoki-Yoshida A, Kimoto-Nira H, et al. . Dietary intake of heat-killed Lactococcus lactis H61 delays age-related hearing loss in C57BL/6J mice. Sci Rep 2016;6:23556 doi:10.1038/srep23556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cristobal R, Oghalai JS. Hearing loss in children with very low birth weight: current review of epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed 2008;93:F462–F468. doi:10.1136/adc.2007.124214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Messaoudi M, Lalonde R, Violle N, et al. . Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 2011;105:755–64. doi:10.1017/S0007114510004319 [DOI] [PubMed] [Google Scholar]
- 35.Steenbergen L, Sellaro R, van Hemert S, et al. . A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun 2015;48:258–64. doi:10.1016/j.bbi.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 36.Davari S, Talaei SA, Alaei H, et al. . Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience 2013;240:287–96. doi:10.1016/j.neuroscience.2013.02.055 [DOI] [PubMed] [Google Scholar]
- 37.Shanahan F. Molecular mechanisms of probiotic action: it’s all in the strains!. Gut 2011;60:1026–7. doi:10.1136/gut.2011.241026 [DOI] [PubMed] [Google Scholar]
- 38.Roberts G, Howard K, Spittle AJ, et al. . Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health 2008;44:276–80. doi:10.1111/j.1440-1754.2007.01251.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2017-000176supp001.pdf (136.8KB, pdf)