Abstract
Objective
To examine weight loss (WL) and excess weight loss (EWL) among newborns of caesarean delivery, comparing colloids plus crystalloids versus crystalloids only. Also, to examine different doses of intrapartum intravenous fluids on WL and EWL.
Design
Comparative safety retrospective cohort study.
Setting
University Teaching Hospital, Moncton, Canada.
Patients
Mothers exposed to intravenous fluids with caesarean delivery between 2008 and 2016.
Interventions
Exposure to colloids plus crystalloids was compared with crystalloids only, and dose-response analyses were performed for colloids, crystalloids and total intravenous fluids doses. Linear and logistic regression models were used, adjusting for potential confounders.
Main outcome measures
Infants’ WL was measured at days 1, 2 and 3 post partum, and EWL defined as loss of >7% of birth weight.
Results
From 801 mother-infant pairs, 176 were exposed to colloids plus crystalloids and 625 were exposed to crystalloids only (overall mean birth weight=3416 g, EWL=2%, 41.4% and 55.5% on days 1, 2 and 3, respectively). No significant difference in newborns’ WL was observed on any of the days assessed. Adjusted OR (95% CI) of EWL was 1.0 (0.3 to 3.3) at 24 hours, 1.0 (0.7 to 1.5) at 48 hours and 1.4 (0.9 to 2.2) at 72 hours. No dose-response relationship was detected with type-specific and total intravenous fluids exposures.
Conclusions
The risk of EWL was similar with colloids plus crystalloids and crystalloids only, suggesting that both therapeutic options can be considered during caesarean delivery. The absence of dose-response relationships adds confirmatory evidence to the intravenous fluids safety profiles.
Keywords: birth weight, weight loss, colloids, crystalloids, caesarean section
What is already known on this topic?
Use of intravenous fluids (ie, crystalloids and colloids) prior to and/or during anaesthesia is a common strategy to prevent maternal hypotension during caesarean sections.
Published literature provides conflicting evidence of association between intravenous fluids—specifically crystalloids—and excess weight loss.
What this study adds?
Weight loss difference and the risk of excess weight loss were similar to colloids plus crystalloids and crystalloids among women undergoing caesarean delivery.
No dose-response relationship observed between colloids, crystalloids or total intravenous fluids and an increased risk of excess weight loss.
Primary and sensitivity analyses suggest that both therapeutic options can be considered as safe during caesarean sections.
Introduction
Hypotension is the most significant adverse effect in women undergoing spinal anaesthesia for caesarean delivery, affecting on average 70% of pregnant women.1 2 The administration of intravenous fluids (ie, crystalloids and colloids) prior to and/or during anaesthesia represents one of the most common strategies to prevent maternal hypotension.1 Among the intravenous fluids, crystalloid solutions are the most frequently used, with normal saline (NS) and Ringer's lactate (RL) as the most common choices.3 Colloids are frequently used nowadays for several reasons. Preloading with crystalloids alone have shown poor effectiveness in decreasing hypotension.3 While crystalloids co-loading is considered superior, variable effectiveness were reported.1 3 Systematic reviews and meta-analyses indicate that preloading or co-loading with colloids—specifically hydroxyethyl starches (HES)—is superior to crystalloids alone, with volumes larger than 500 mL offering no significant additional benefits.1–5 Current practice advocate combining colloids and crystalloids solutions rather than colloids alone.2
While colloids are becoming an increasingly used choice as intravenous fluid in pregnant women undergoing caesarean section, no study has examined the impact of colloids—or colloids plus crystalloids combination—on excess weight loss (EWL) among newborns. Although normal physiological weight loss occurs after delivery,6 7 EWL (ie, loss of more than 7%–10% of birth weight) is considered a complication which increases the risk of hyperbilirubinaemia, hypernatremic dehydration, hospitalisations and long-term morbidities.8–10 Recent literature reports growing evidence of positive association between intravenous fluids—specifically crystalloids—and EWL (between 7% and 10%),11–14 and it remains unclear whether different intravenous fluid combinations present similar safety profiles. The primary objective of the current study was to examine amount of weight loss and EWL among newborns of caesarean delivery, comparing colloids plus crystalloids intrapartum intravenous fluids versus crystalloids only. The secondary objective was to examine the association between different doses of intrapartum intravenous fluids and newborn weight loss, as well as EWL.
Methods
Study design
A population-based retrospective cohort design was used. The cohort was selected from mothers hospitalised at the Dr George L. Dumont University Hospital Centre (New Brunswick, Canada) between April 2008 and June 2016. The average hospital stay for mothers after caesarean deliveries in New Brunswick is 72 hours. Trained medical staff extracted the information from the hospital medical records of mothers undergoing caesarean deliveries and their linked newborns’ files. Data collection was performed on two periods for logistical and sample size reasons: (1) records from all (n=320) caesarean deliveries between April 2008 and January 2010 and (2) a random sample of 500 caesarean deliveries between February 2010 and June 2016 were retained. Performing separate primary analysis for the two periods did not show major differences in results.
Cohort selection
The cohort inclusion criteria were: (1) a pregnancy with a recorded caesarean delivery between 1 April 2008 and 31 June 2016; (2) recorded singleton live birth; (3) maternal age at the beginning of pregnancy of 15–45 years; (4) gestational duration of 30–42 weeks and (5) exposure to colloids or crystalloids intravenous fluids before caesarean delivery. The exclusion criteria were: (1) missing data on intravenous fluids administration in addition to several other important variables and (2) infants with feeding difficulties leading to unsuccessful oral feeding (neither breast feeding nor formula) during the hospitalisation period (ie, nil per os).
Weight loss and EWL
From the infants’ medical records, we extracted information on birth weight and infants’ weight at 24, 48 and 72 hours. EWL was defined as loss of >7% of birth weight, calculated at 24, 48 and 72 hours post partum.6 7 15
Intravenous fluids exposure
Colloids (Voluven, Pentaspan and others/non-specified) plus crystalloids (RL, NS and others/non-specified) use was defined as the maternal intrapartum exposure to both colloids and crystalloids intravenous fluids for caesarean delivery. Crystalloids use (RL, NS and others/non-specified) was defined as the maternal intrapartum exposure to crystalloids only. Being ‘non-specified’ refers to cases where an indicator was present that a colloid or a crystalloid was administered, but no detail on the specific type was recorded. The doses of colloids were categorised as: 0, >0–500, >500 mL and doses of crystalloids were categorised as: 0, >0–1000, >1000–2000, >2000 mL. We calculated the total doses of intravenous fluids categorised into: 0–1000, >1000–2000, >2000–3000, >3000 mL.
Confounding variables
Two classes of potential risk factors were included in the analysis. First, gestational and maternal variables, including: maternal age in years at delivery, number of pregnancies, number of viable births, number of lost pregnancies, gestational diabetes (yes/no), hypertension during pregnancy (yes/no), pre-eclampsia (yes/no), cigarette smoking during pregnancy (yes/no), alcohol drinking during pregnancy (yes/no), drug use during pregnancy (yes/no), epidural before caesarean section (yes/no) and elective caesarean section (yes/no). Second, infant-related variables, including gestational age in weeks, Apgar score, fever during hospitalisation (yes/no), exclusive breast feeding during hospitalisation (ie, we excluded only formula and breastfeeding formula combined) (yes/no), phototherapy on days 1 (yes/no), 2 (yes/no) and 3 (yes/no), number of urine on each of day 1, 2 and 3 and number of stools on each of day 1, 2 and 3.
Statistical analysis
The descriptive statistics for the characteristics of the mother-infant pairs were calculated and compared between groups. Four regression forms were employed, two for the difference in weight according to types and doses of intravenous fluids, and two for EWL according to types and doses of intravenous fluids. We compared the amount of weight lost by infants in both groups (as absolute measure in grams) at 24, 48 and 72 hours post partum using crude and adjusted linear regressions, with crystalloids only users as reference group. Three regression models were constructed: model 1 (crude, ie, single univariate regression model), model 2 (adjusted for maternal age, gestational diabetes, hypertension during pregnancy, number of pregnancies, viable births, lost pregnancies, gestational age, pre-eclampsia, cigarette smoking, alcohol use, drug use, epidural before caesarean section and elective caesarean section) and model 3 (adjusted for the variables in model 2, in addition to Apgar score, exclusive breast feeding, number of stools, number of urine, presence of fever and phototherapy). The purpose of this methodology is that the additional variables in model 3 were measured during the postpartum period and not at baseline, and through them, the effect of intravenous fluids on weight loss could be mediated.
Additional regression models for colloids and crystalloids doses were used to examine trends and dose-response relationships with newborn weight loss, similarly with the total intravenous fluid doses. Logistic regression models were used to estimate crude and adjusted ORs and 95% CIs for EWL, with adjustment for potential confounders as listed above. Firth penalised maximum likelihood estimation method was used whenever quasi-complete separation in data was detected in logistic regression models.16
Sensitivity analysis
First, the maximum physiological limits of weight loss for newborns are controversial,17 18 thus, we reanalysed our data using EWL defined as (1) loss of >5% and (2) loss of >10% of birth weight. Second, suboptimal breast feeding and delayed onset of lactogenesis can lead to EWL.11 15 Therefore, we tested its potential effect using two different techniques: (1) performed all analyses among the subgroup of breastfed infants only and (2) excluded exclusive breast feeding as potential confounder from model 3. Using a type I error of 0.05% and 80% power, a sample size of 818 mother-infant pairs was estimated to be sufficient to detect a weight loss difference of 10% between the group exposed to colloids plus crystalloids versus the group exposed to crystalloids only. All statistical analyses were conducted using SAS software, V.9.3 (SAS Institute, Cary, North Carolina, USA). This study was approved by the Research Ethics Committee of the Vitalité Health Network.
Results
A total of 801 mother-infant pairs fulfilled our inclusion and exclusion criteria (see figure 1 for the selection process). From these, 176 mother-infant pairs were exposed to colloids plus crystalloids intrapartum and 625 were exposed to crystalloids only. Among the colloids plus crystalloids group, 103 (58.5%) were exposed to Voluven, 22 (12.5%) to Pentaspan and 51 (29%) to other colloids or non-specified. For crystalloids, 546 (87.3%) were exposed to RL only, 44 (7.1%) to RL and NS and 35 (5.6%) to other crystalloids or non-specified. The most frequently used doses of colloids and crystalloids were >500 mL and >1000–2000 mL, respectively. Overall, the mean birth weight for the newborns was 3416 g. During the hospitalisation period, EWL of >7% was detected in 15 infants (2%) at 24 hours, 318 infants (41.4%) at 48 hours, 351 infants (55.5%) at 72 hours and EWL of >10% was detected in 3 infants (0.4%) at 24 hours, 17 infants (2.2%) at 48 hours and 100 infants (15.8%) at 72 hours.
Figure 1.
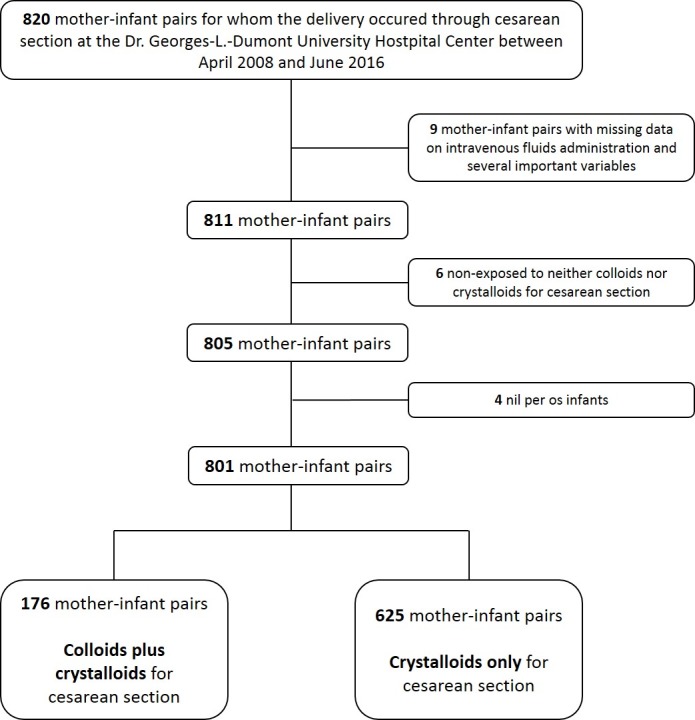
Cohort selection flow diagram.
In both groups, most women were aged ≤35 years, with approximately 1–4 gravidity, 0–1 parity and 0–1 abortus (Table 1). However, based on crude observations, women exposed to crystalloids only were more likely to have suffered from gestational diabetes, to have been exposed to epidural before caesarean section, to report cigarette smoking and alcohol drinking. The incidence of preterm deliveries and phototherapy on day 3 were higher among infants in the colloids plus crystalloids group, while exclusive breast feeding during hospitalisation was more successful in the crystalloids only group.
Table 1.
Characteristics of mother-infant pairs according to intrapartum colloids and crystalloids exposure for caesarean section
| Missing data | Colloids plus crystalloids | Crystalloids only | |
| No. (%) | No. of deliveries (%) | ||
| 176 (22.0) | 625 (78.0) | ||
| Gestational and maternal variables | |||
| Maternal age in years (mean±SD) | 0 (0) | 29.7 (±4.9) | 29.2 (±5.2) |
| ≤35 | 155 (88.1) | 547 (87.5) | |
| >35 | 21 (11.9) | 78 (12.5) | |
| Gravida | 2 (0.2) | ||
| 1 | 89 (50.9) | 285 (45.7) | |
| 2–4 | 79 (45.1) | 311 (49.8) | |
| ≥5 | 7 (4.0) | 28 (4.5) | |
| Para | 2 (0.2) | ||
| 0 | 104 (59.4) | 363 (58.2) | |
| 1 | 55 (31.4) | 200 (32.0) | |
| ≥2 | 16 (9.2) | 61 (9.8) | |
| Abortus | 2 (0.2) | ||
| 0 | 135 (77.1) | 439 (70.4) | |
| 1 | 33 (18.9) | 128 (20.5) | |
| ≥2 | 7 (4.0) | 57 (9.1) | |
| Cigarette smoking | 2 (0.2) | 21 (11.9) | 105 (16.9) |
| Alcohol drinking | 4 (0.5) | 14 (8.0) | 85 (13.7) |
| Drug use | 4 (0.5) | 6 (3.4) | 24 (3.9) |
| Gestational diabetes | 0 (0) | 5 (2.8) | 38 (6.1) |
| Hypertension during pregnancy | 0 (0) | 9 (5.1) | 41 (6.6) |
| Pre-eclampsia | 0 (0) | 11 (6.3) | 29 (4.6) |
| Epidural before caesarean section | 0 (0) | 53 (30.1) | 272 (43.5) |
| Elective caesarean section | 4 (0.5) | 73 (41.7) | 233 (37.5) |
| Infant-related variables | |||
| Birth weight in grams (mean±SD) | 12 (1.5) | 3383 (±572) | 3449 (±544) |
| Gestational age in weeks (mean±SD) | 12 (1.5) | 38.6 (±1.4) | 39.0 (±1.6) |
| Preterm delivery | 12 (1.5) | 19 (11.1) | 43 (7.0) |
| Low Apgar score (<7) at 1 min | 14 (1.7) | 8 (4.7) | 34 (5.5) |
| Fever during hospitalisation | 14 (1.7) | 4 (2.3) | 11 (1.8) |
| Phototherapy on day 1 | 13 (1.6) | 5 (2.9) | 9 (1.5) |
| Phototherapy on day 2 | 13 (1.6) | 9 (5.2) | 21 (3.4) |
| Phototherapy on day 3 | 14 (1.7) | 18 (10.5) | 36 (5.9) |
| Exclusive breast feeding during hospitalisation | 15 (1.9) | 46 (26.9) | 290 (47.2) |
| Urine during day 1 (mean±SD) | 15 (1.9) | 2.5 (±1.9) | 2.8 (±1.8) |
| Urine during day 2 (mean±SD) | 17 (2.1) | 4.0 (±1.7) | 3.8 (±1.7) |
| Urine during day 3 (mean±SD) | 73 (9.1) | 4.1 (±1.7) | 4.0 (±1.8) |
| Stools during day 1 (mean±SD) | 15 (1.9) | 2.5 (±1.6) | 2.6 (±1.6) |
| Stools during day 2 (mean±SD) | 17 (2.1) | 3.4 (±1.7) | 3.0 (±1.5) |
| Stools during day 3 (mean±SD) | 73 (9.1) | 3.0 (±1.6) | 3.0 (±1.6) |
| Colloids doses in mL* | 1 (0.1) | ||
| 0 | 0 (0) | 625 (78.1) | |
| >0–500 | 47 (5.9) | - | |
| >500 | 128 (16.0) | - | |
| Crystalloids doses in mL | 26 (3.2) | ||
| 0 | 16 (2.1)* | 0 (0) | |
| >0–1000 | 54 (7.0) | 168 (21.7) | |
| >1000–2000 | 84 (10.8) | 320 (41.3) | |
| >2000 | 18 (2.3) | 115 (14.8) | |
| Total intravenous fluid doses in mL | 26 (3.2) | ||
| >0–1000 | 22 (2.8) | 168 (21.7) | |
| >1000–2000 | 102 (13.2) | 320 (41.3) | |
| >2000–3000 | 42 (5.4) | 108 (13.9) | |
| >3000 | 6 (0.8) | 7 (0.9) | |
*Deliveries with missing data on crystalloids doses but available data on colloids doses (16 patients’ records) were included in the colloids plus crystalloids group in the statistical analysis.
Differences in weight
We observed no significant difference in newborns’ weight loss between women exposed to colloids plus crystalloids compared with crystalloids only intrapartum on any of the three days assessed (Table 2). Moreover, none of the doses of colloids, crystalloids or total intravenous fluids was found to be associated with a significant difference in weight loss, nor with a trend of increased or decreased risk over time. We obtained similar estimates for models 2 and 3 (only results from model 3 are presented, others are available on request).
Table 2.
Beta-coefficients and 95% CIs of the linear regression models for difference in weight at 24, 48 and >72 hours post partum according to maternal intrapartum colloids and crystalloids exposure
| Difference in weight in grams (β and 95% CI) | ||||||
| 24 hours post partum | 48 hours post partum | 72 hours post partum | ||||
| Model 1 (crude) | Model 3 | Model 1 (crude) | Model 3 | Model 1 (crude) | Model 3 | |
| Colloids plus crystalloids | −0.6 (-12.6 to 11.4) | −5.2 (-17.5 to 7.0) | −0.4 (-15.9 to 15.0) | −10.8 (-26.1 to 4.5) | −5.4 (-28.2 to 17.4) | −9.2 (-32.0 to 13.7) |
| Crystalloids only | Reference | Reference | Reference | Reference | Reference | Reference |
| Colloids doses in mL (vs 0) | ||||||
| >0–500 | −5.8 (-27.9 to 16.4) | −9.3 (-31.3 to 12.7) | −3.1 (-31.9 to 25.7) | −9.5 (-37.3 to 18.3) | −11.0 (-55.5 to 33.4) | −13.1 (-56.8 to 30.6) |
| >500 | −0.4 (-14.6 to 13.8) | −5.8 (-20.1 to 8.5) | 1.6 (-16.8 to 20.1) | −9.8 (-27.8 to 8.1) | −8.2 (-35.2 to 18.8) | −7.9 (-34.5 to 18.7) |
| Crystalloids doses in mL (vs 0) | ||||||
| >0–1000 | 4.2 (-33.0 to 41.4) | −2.6 (-40.9 to 35.7) | 5.0 (-43.3 to 53.3) | 13.9 (-34.3 to 62.1) | −0.03 (-73.6 to 73.5) | 39.8 (-35.6 to 115.1) |
| >1000–2000 | 2.9 (-33.9 to 39.6) | −1.5 (-39.4 to 36.4) | 9.5 (-38.2 to 57.2) | 21.9 (-25.8 to 69.6) | −7.6 (-80.1 to 64.9) | 39.5 (-35.1 to 114.1) |
| >2000 | −0.5 (-38.8 to 37.8) | −6.8 (-46.0 to 32.4) | 2.8 (-46.9 to 52.6) | 9.8 (-39.4 to 59.1) | −21.6 (-97.2 to 53.9) | 15.2 (-61.5 to 91.9) |
| Total intravenous fluid doses in mL: (vs >0–1000) | ||||||
| >1000–2000 | −4.5 (-16.6 to 7.6) | −2.9 (-15.0 to 9.2) | 4.3 (-11.5 to 20.1) | 8.1 (-7.1 to 23.3) | −7.4 (-31.7 to 17.0) | 3.5 (-20.2 to 27.3) |
| >2000–3000 | −1.6 (-16.9 to 13.7) | −2.7 (-17.8 to 12.3) | 0.8 (-19.1 to 20.7) | −0.3 (-19.2 to 18.6) | −24.8 (-54.6 to 4.9) | −22.1 (-50.9 to 6.6) |
| >3000 | −26.7 (-67.7 to 14.3) | −27.3 (-66.9 to 12.2) | −14.4 (-38.9 to 67.7) | −6.4 (-43.4 to 56.2) | −3.9 (-81.1 to 73.3) | −0.6 (-73.0 to 71.8) |
The regression coefficient represents the estimated difference in weight since birth.
Model 2 adjusted results were similar to the adjusted results of model 3 (only results from model 3 are presented in the table; results of model 2 are available on request).
Model 2 adjusted for maternal age, gestational diabetes, hypertension during pregnancy, number of pregnancies, viable births, lost pregnancies, gestational age, pre-eclampsia, cigarette smoking, alcohol use, drug use, epidural before caesarean section and election to have a caesarean section.
Model 3 adjusted for maternal age, gestational diabetes, hypertension during pregnancy, number of pregnancies, viable births, lost pregnancies, gestational age, pre-eclampsia, cigarette smoking, alcohol use, drug use, epidural before caesarean section, election to have a caesarean section, Apgar score, exclusive breast feeding, number of stools, number of urine, presence of fever and phototherapy.
Differences in EWL by group
We observed no significant difference in the risk of EWL (>7%) at 24 hours (adjusted OR: 1.0; 95% CI: 0.3 to 3.3), 48 hours (adjusted OR: 1.0; 95% CI: 0.7 to 1.5) or 72 hours (adjusted OR: 1.4; 95% CI: 0.9 to 2.2) (Table 3). Elective caesarean section was associated with a significant increased risk of EWL at 48 hours (adjusted OR: 1.7; 95% CI: 1.1 to 2.6) and at 72 hours (adjusted OR: 1.8; 95% CI: 1.1 to 2.9). Number of stools measured on each day was significantly associated with EWL (adjusted OR, 95% CI: 1.6, 1.1 to 2.3; 0.8, 0.7 to 0.9 and 0.7, 0.6 to 0.8 at 24, 48 and 72 hours, respectively). We obtained similar estimates for models 2 and 3.
Table 3.
Adjusted ORs and 95% CIs for excess weight loss (>7%) according to type of intrapartum intravenous fluid received by mothers for caesarean section
| 24 hours post partum | 48 hours post partum | 72 hours post partum | ||||
| Model 1 crude OR (95% CI) | Model 3 adjusted OR (95% CI)* | Model 1 crude OR (95% CI) | Model 3 adjusted OR (95% CI) | Model 1 crude OR (95% CI) | Model 3 adjusted OR (95% CI)* | |
| Colloids plus crystalloids | 0.9 (0.2 to 3.2) | 1.0 (0.3 to 3.3) | 0.9 (0.6 to 1.3) | 1.0 (0.7 to 1.5) | 1.3 (0.9 to 2.0) | 1.4 (0.9 to 2.2) |
| Crystalloids only | Reference | Reference | Reference | Reference | Reference | Reference |
| Maternal age in years | 0.9 (0.8 to 1.0) | 1.0 (0.9 to 1.1) | 1.0 (>0.9 to <1.1) | 1.0 (>0.9 to 1.0) | 1.0 (>0.9 to 1.1) | 1.0 (>0.9 to 1.1) |
| Gravida | 0.9 (0.6 to 1.4) | 0.3 (0.1 to >99) | 0.9 (0.8 to 1.0) | 0.7 (0.2 to 2.5) | 0.8 (0.7 to 0.9) | 8.8 (0.7 to >99) |
| Para | 0.9 (0.4 to 1.8) | 3.8 (<0.1 to 24.6) | 0.8 (0.7 to 1.0) | 1.2 (0.3 to 4.7) | 0.7 (0.5 to 0.8) | 0.1 (<0.1 to 0.8) |
| Abortus | 0.9 (0.4 to 1.8) | 3.3 (<0.1 to 19.7) | 0.9 (0.7 to 1.1) | 1.3 (0.4 to 5.0) | 0.8 (0.6 to 1.0) | 0.1 (<0.1 to 1.2) |
| Gestational age | 1.1 (0.8 to 1.6) | 0.9 (0.6 to 1.4) | 1.1 (1.0 to 1.3) | 1.0 (0.9 to 1.2) | 1.1 (1.0 to 1.2) | 0.9 (0.8 to 1.1) |
| Cigarette smoking | 2.8 (0.9 to 8.3) | 1.9 (0.5 to 5.9) | 0.7 (0.5 to 1.1) | 0.8 (0.5 to 1.3) | 0.5 (0.3 to 0.7) | 0.7 (0.4 to 1.2) |
| Drug use | 4.2 (0.9 to 19.7) | 2.8 (0.4 to 13.6) | 0.7 (0.3 to 1.5) | 0.6 (0.3 to 1.6) | 1.1 (0.5 to 2.6) | 1.3 (0.5 to 3.4) |
| Alcohol drinking | 1.1 (0.2 to 4.8) | 1.0 (0.2 to 3.7) | 1.1 (0.7 to 1.8) | 1.1 (0.7 to 1.7) | 1.2 (0.7 to 1.9) | 1.1 (0.7 to 2.0) |
| Hypertension during pregnancy | 0.5 (<0.1 to 3.7) | 0.2 (<0.1 to 2.6) | 0.5 (0.3 to 1.0) | 0.6 (0.3 to 1.3) | 0.7 (0.4 to 1.3) | 0.5 (0.2 to 1.1) |
| Gestational diabetes | 1.3 (0.2 to 10.1) | 1.6 (0.2 to 7.3) | 1.2 (0.6 to 2.2) | 1.4 (0.7 to 2.8) | 0.7 (0.4 to 1.5) | 1.2 (0.5 to 3.0) |
| Pre-eclampsia | 1.4 (0.2 to 10.7) | 3.8 (0.3 to 22.8) | 0.4 (0.2 to 0.8) | 0.6 (0.2 to 1.4) | 1.1 (0.5 to 2.0) | 1.5 (0.7 to 3.5) |
| Epidural before caesarean section | 1.3 (0.5 to 3.6) | 0.9 (0.2 to 3.6) | 1.3 (0.9 to 1.6) | 1.5 (1.0 to 2.4) | 0.9 (0.6 to 1.2) | 0.9 (0.5 to 1.4) |
| Elective caesarean section | 0.6 (0.2 to 1.8) | 0.6 (0.2 to 2.6) | 1.2 (0.9 to 1.6) | 1.7 (1.1 to 2.6) | 1.3 (1.0 to 1.9) | 1.8 (1.1 to 2.9) |
| Apgar score | 1.4 (0.6 to 3.2) | 1.2 (0.8 to 3.4) | 1.2 (1.0 to 1.3) | 1.2 (1.0 to 1.4) | 1.1 (1.0 to 1.2) | 1.1 (0.9 to 1.2) |
| Fever during hospitalisation | 1.6 (<0.1 to 13.2) | 1.5 (<0.1 to 13.6) | 2.6 (0.9 to 7.8) | 3.3 (1.1 to 10.4) | 0.8 (0.3 to 2.3) | 1.0 (0.3 to 3.1) |
| Exclusive breast feeding during hospitalisation | 0.7 (0.2 to 2.0) | 0.9 (0.3 to 2.7) | 1.3 (1.0 to 1.8) | 1.1 (0.8 to 1.5) | 1.6 (1.1 to 2.1) | 1.5 (1.0 to 2.2) |
| Phototherapy on day 1 | 4.0 (0.5 to 32.9) | 7.7 (0.7 to 46.1) | 1.1 (0.4 to 3.1) | 1.4 (0.4 to 4.8) | 0.3 (0.1 to 1.1) | 0.3 (0.1 to 1.2) |
| Phototherapy on day 2 | N/A | N/A | 1.1 (0.5 to 2.3) | 1.6 (0.7 to 3.9) | 0.9 (0.4 to 2.0) | 1.7 (0.7 to 4.4) |
| Phototherapy on day 3 | N/A | N/A | N/A | N/A | 0.7 (0.4 to 1.3) | 0.8 (0.4 to 1.6) |
| Urine during day 1 | 1.0 (0.8 to 1.4) | 0.8 (0.6 to 1.2) | 1.0 (0.9 to 1.1) | 1.0 (0.9 to 1.2) | 1.0 (0.9 to 1.1) | 1.1 (1.0 to 1.2) |
| Urine during day 2 | N/A | N/A | 0.8 (0.8 to 0.9) | 0.9 (0.8 to 1.0) | 0.8 (0.7 to 0.9) | 0.9 (0.8 to 1.0) |
| Urine during day 3 | N/A | N/A | N/A | N/A | 0.8 (0.7 to 0.9) | 0.9 (0.8 to 1.0) |
| Stools during day 1 | 1.5 (1.1 to 2.1) | 1.6 (1.1 to 2.3) | 1.1 (1.0 to 1.2) | 1.1 (1.0 to 1.2) | 1.0 (0.9 to 1.1) | 1.0 (0.8 to 1.1) |
| Stools during day 2 | N/A | N/A | 0.8 (0.7 to 0.9) | 0.8 (0.7 to 0.9) | 0.8 (0.7 to 0.9) | 0.9 (0.8 to 1.0) |
| Stools during day 3 | N/A | N/A | N/A | N/A | 0.7 (0.6 to 0.8) | 0.7 (0.6 to 0.8) |
Model 2 adjusted results were similar to the adjusted results of model 3 (only results from model 3 are presented in the table; results of model 2 are available on request).
Model 2 adjusted for maternal age, gestational diabetes, hypertension during pregnancy, number of pregnancies, viable births, lost pregnancies, gestational age, pre-eclampsia, cigarette smoking, alcohol use, drug use, epidural before caesarean section and election to have a caesarean section.
N/A, not applicable.
*Firth penalised maximum likelihood estimation method was used for quasi-complete separation.
Differences in EWL by dose
At 24 hours post partum, the number of cases of EWL was too small to examine specific crystalloids doses. However, neither colloids doses nor total intravenous fluids doses were associated significantly with EWL (Table 4). At 48 and 72 hours post partum, we observed no significant association between colloids, crystalloids or total intravenous fluids and EWL, with the exception of a marginal protective effect from EWL at 72 hours post partum for crystalloids at doses >1000–2000 (adjusted OR: 0.2; 95% CI: <0.1–0.9). No trend or dose-response relationship was observed with the increase in doses of intrapartum intravenous fluids (Table 4). Results from the three sensitivity analyses supported the primary analyses estimates (available in online supplementary table E1 to E5).
Table 4.
Crude and adjusted ORs and 95% CIs for excess weight loss (>7%) according to doses of intrapartum intravenous fluid received by mothers for caesarean section
| 24 hours post partum | 48 hours post partum | 72 hours post partum | |||||||
| No. of cases (%) | Model 1 (crude) OR (95% CI)* | Model 3 Adjusted OR (95% CI)* | No. of cases (%) | Model 1 (crude) OR (95% CI) | Model 3 Adjusted OR (95% CI) | No. of cases (%) | Model 1 (crude) OR (95% CI) | Model 3 Adjusted OR (95% CI)* | |
| Regression models for type-specific doses of intravenous fluid | |||||||||
| Colloids doses (in mL) | |||||||||
| 0 | 12 (2.0) | Reference | Reference | 252 (41.9) | Reference | Reference | 260 (53.8) | Reference | Reference |
| >0–500 | 2 (4.4) | 3.3 (0.6 to 11.8) | 2.7 (0.4 to 11.5) | 17 (37.0) | 0.9 (0.5 to 1.7) | 1.0 (0.5 to 2.1) | 20 (55.6) | 1.1 (0.6 to 2.3) | 1.3 (0.6 to 2.9) |
| >500 | 1 (0.8) | 0.7 (0.1 to 2.8) | 0.7 (0.1 to 3.1) | 48 (39.7) | 0.9 (0.6 to 1.4) | 1.0 (0.6 to 1.5) | 70 (62.5) | 1.4 (0.9 to 2.1) | 1.3 (0.8 to 2.1) |
| Crystalloids doses (in mL) | |||||||||
| 0 | 0 (0.0) | Reference | Reference | 6 (37.5) | Reference | Reference | 9 (75.0) | Reference | Reference |
| >0–1000 | 3 (1.4) | N/A | N/A | 92 (43.0) | 1.1 (0.4 to 3.4) | 0.8 (0.2 to 2.6) | 93 (54.1) | 0.5 (0.1 to 1.9) | 0.2 (<0.1 to 1.1) |
| >1000–2000 | 10 (2.6) | N/A | N/A | 156 (40.1) | 1.0 (0.3 to 3.0) | 0.6 (0.2 to 2.0) | 179 (56.7) | 0.5 (0.1 to 2.1) | 0.2 (<0.1 to 0.9) |
| >2000 | 1 (0.8) | N/A | N/A | 56 (44.8) | 1.2 (0.4 to 3.8) | 0.9 (0.2 to 3.0) | 61 (55.0) | 0.5 (0.1 to 2.1) | 0.3 (<0.1 to 1.2) |
| Regression models for total doses of intravenous fluid | |||||||||
| Total intravenous fluid doses (in mL) | |||||||||
| >0–1000 | 3 (1.7) | Reference | Reference | 82 (44.8) | Reference | Reference | 81 (55.5) | Reference | Reference |
| >1000–2000 | 8 (2.0) | 1.1 (0.3 to 4.4) | 1.1 (0.3 to 4.5) | 161 (39.3) | 0.8 (0.6 to 1.1) | 0.7 (0.5 to 1.0) | 183 (56.0) | 1.0 (0.7 to 1.5) | 0.8 (0.5 to 1.2) |
| >2000–3000 | 3 (2.2) | 1.3 (0.3 to 6.3) | 1.2 (0.2 to 6.1) | 64 (45.7) | 1.0 (0.7 to 1.6) | 1.0 (0.6 to 1.6) | 71 (55.9) | 1.0 (0.6 to 1.6) | 0.9 (0.5 to 1.6) |
| >3000 | 0 (0.0) | N/A | N/A | 3 (27.3) | 0.5 (0.1 to 1.8) | 0.5 (0.1 to 2.0) | 7 (63.6) | 1.4 (0.4 to 5.0) | 1.1 (0.3 to 4.6) |
Model 2 adjusted results were similar to the adjusted results of model 3 (only results from model 3 are presented in the table; results of model 2 are available on request).
Model 2 adjusted for maternal age, gestational diabetes, hypertension during pregnancy, number of pregnancies, viable births, lost pregnancies, gestational age, pre-eclampsia, cigarette smoking, alcohol use, drug use, epidural before caesarean section and election to have a caesarean section.
Model 3 adjusted for maternal age, gestational diabetes, hypertension during pregnancy, number of pregnancies, viable births, lost pregnancies, gestational age, pre-eclampsia, cigarette smoking, alcohol use, drug use, epidural before caesarean section, election to have a caesarean section, Apgar score, exclusive breast feeding, number of stools, number of urine, presence of fever and phototherapy.
N/A, not applicable.
*Firth penalised maximum likelihood estimation method was used for quasi-complete separation.
bmjpo-2017-000070supp001.pdf (197.1KB, pdf)
Discussion
This comparative safety study revealed two main findings. First, the newborn weight loss and the risk of EWL did not differ when colloids plus crystalloids or crystalloids only intravenous fluids were administered intrapartum for caesarean delivery. Second, there was no dose-response relationship between colloids, crystalloids or total intravenous fluids and an increased risk of EWL. The results were consistent in different models and sensitivity analyses on exclusively breastfed newborns and EWL of >5% and>10% showed similar results.
To the best of our knowledge, this study is the first to compare the risk of EWL for different types of intrapartum intravenous fluids for caesarean delivery. Previous studies have demonstrated comparable neonatal safety profiles of colloids versus crystalloids, specifically for neonatal acidosis (risk ratio (RR) 0.2; 95% CI: 0.01 to 4.1) and Apgar score (meta-analysis of 3 studies with 209 women: RR: 0.2; 95% CI: 0.03 to 2.1).1 Similarly, no significant difference in Apgar score was found among neonates exposed to colloids plus crystalloids versus crystalloids only (meta-analysis of 2 studies with 107 women: RR: 0.1; 95% CI: 0.01 to 1.2).1 Still, it is noteworthy that the number of events in those studies was low and medications used in these older trials (eg, dextrans and gelatins) no longer reflect the current practice of using HES as the preferred choice.2 4 5 Recently, the CAESAR trial compared two treatment regimens similar to the current study and reported no difference in Apgar score or umbilical pH.2
Of nine published studies, five reported significant positive associations between doses of intrapartum fluids and weight loss,11–14 19 whereas the other four reported no significant relationships.20–23 However, studies with significant findings are different from the current study since they were based on vaginal births and breastfed newborns11 12 19 or very small sample sizes.13 14 Our results corroborate the results of one case-control,23 two prospective cohort studies20 22 as well as the only RCT on intrapartum intravenous fluids and EWL to date.21
Previous studies hypothesised that since fluids move freely from the mother to her fetus, newborns may become overhydrated and a consequent correction for the newborns’ fluid balance is an increase in diuresis and weight loss.20 24 This hypothesis was partially supported by recent reports,11 12 but caution must be exercised as the observed weight loss could be attributed to insufficient feeding, especially among exclusively breastfed infants. Previous studies had suggested that the intrapartum administration of crystalloids could significantly affect the onset of lactogenesis12 15 25 through the development of breast engorgement, which in turn could negatively affect milk production25 and increase breast oedema.26 However, our results suggest that if this is the case, it will not lead to an increased risk of EWL. Future studies are warranted to fully examine the effect of intravenous fluids—and their specific types—on lactogenesis and EWL. In the current study, the rates of discharges (urine and stool) were comparable to similar studies published in the literature.20 23 27 A noteworthy finding is the significant association between elective caesarean delivery and EWL. It is possible that pregnant women opting for an elective caesarean delivery could have suffered from other obstetric conditions that leave their newborns more prone to EWL.
The present study has some important strengths. Our objective was to compare newborn weight loss and EWL after two widely used prevention and treatment options for hypotension during caesarean delivery. Through comparing similar intravenous fluid regimens, and accounting for numerous potentially confounding variables, we minimised the potential bias of confounding by indication, in this case hypotension itself. Indeed, maternal hypotension itself—and the associated reduction in uteroplacental blood supply—can lead to fetal acidosis, which cause weak rooting and sucking reflexes. The later factors can severely compromise lactogenesis, leading to significant newborn weight loss that can be erroneously attributed to intravenous fluids exposure.1 28–30 In addition, we used a large set of statistical models and sensitivity analyses to confirm the validity of our observed estimates. However, the results should be interpreted with consideration of the following limitations. The retrospective design has its limitations compared with a prospective study with efficient follow-up. We were unable to distinguish between preloading and co-loading of intravenous fluids administered. We did not have data on intrapartum oral fluids nor the exact onset of successful exclusive breast feeding (days 1, 2 or 3) and other related factors (eg, latching, dysfunctional sucking, etc). We did not have data on the degree of hypertension, the fluid regimens before the caesarean delivery decision nor the specific indications for caesarean delivery. The study was underpowered for some of the secondary analyses and future larger studies are warranted. Moreover, different tests were performed and no correction was applied for multiple testing. The study was conducted in one hospital centre in Canada, and results may not be directly generalisable to other hospital settings. Given that this is a comparative safety study with an active treatment regimen as a reference group, we cannot exclude the possibility that both intravenous fluid regimens have a deleterious effect on newborn weight loss. We used penalised maximum likelihood estimation, which produces the best estimates given the available information.31 However, given the very limited number of cases in some of the full models, effect estimates differed from crude models among some variables, with exceedingly inflated variance. The effect estimates of the primary and secondary exposures on the dependent variable (ie, EWL) are nonetheless valid since the full models were bias adjusted using penalisation.
In summary, in the current comparative safety study, the difference in weight loss and the risk of EWL were similar with colloids plus crystalloids and crystalloids intrapartum intravenous fluids among women undergoing caesarean delivery. The absence of dose-response relationships between both intravenous fluid types and EWL adds confirmatory evidence to their safety profiles. Future prospective studies are warranted to confirm the current study results. Combined with previous studies, these results suggest that both therapeutic options can be considered as safe during caesarean sections.
Acknowledgments
The authors would like to thank Mr Hamza Abdelalim for assistance with the data collection process.
Footnotes
Contributors: SE, ABa, BS, SD, AM, WH, ABl and MB have contributed to the concept and design of the study. SE and MB performed the analysis and drafted the first draft. SE, ABa, BS, SD, AM, WH, ABl and MB participated in the interpretation of the data. SE, ABa, BS, SD, AM, WH, ABl and MB contributed in drafting and revising of the full manuscript, and have approved the manuscript as submitted. SE, ABa, BS, SD, AM, WH, ABl and MB have met the criteria of authorship, and take public responsibility for the study contents.
Competing interests: None declared.
Ethics approval: Research Ethics Committee of the Vitalité Health Network.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data are available after discussion with the corresponding author in accordance with our data-sharing policy.
References
- 1.Cyna AM, Andrew M, Emmett RS, et al. Techniques for preventing hypotension during spinal anaesthesia for caesarean section Cochrane Database of Systematic Reviews: John Wiley & Sons, Ltd, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Mercier FJ, Diemunsch P, Ducloy-Bouthors AS, et al. 6% Hydroxyethyl starch (130/0.4) vs Ringer's lactate preloading before spinal anaesthesia for Caesarean delivery: the randomized, double-blind, multicentre CAESAR trial. Br J Anaesth 2014;113:459–67. doi:10.1093/bja/aeu103 [DOI] [PubMed] [Google Scholar]
- 3.Teoh WH, Westphal M, Kampmeier TG. Update on volume therapy in obstetrics. Best Pract Res Clin Anaesthesiol 2014;28:297–303. doi:10.1016/j.bpa.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 4.Mercier FJ. Cesarean delivery fluid management. Curr Opin Anaesthesiol 2012;25:286–91. doi:10.1097/ACO.0b013e3283530dab [DOI] [PubMed] [Google Scholar]
- 5.Ripollés Melchor J, Espinosa Á, Martínez Hurtado E, et al. Colloids versus crystalloids in the prevention of hypotension induced by spinal anesthesia in elective cesarean section. A systematic review and meta-analysis. Minerva Anestesiol 2015;81:1019–30. [PubMed] [Google Scholar]
- 6.Flaherman VJ, Schaefer EW, Kuzniewicz MW, et al. Early weight loss nomograms for exclusively breastfed newborns. Pediatrics 2015;135:e16–e23. doi:10.1542/peds.2014-1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller JR, Flaherman VJ, Schaefer EW, et al. Early weight loss nomograms for formula fed newborns. Hosp Pediatr 2015;5:263–8. doi:10.1542/hpeds.2014-0143 [DOI] [PubMed] [Google Scholar]
- 8.Moritz ML, Manole MD, Bogen DL, et al. Breastfeeding-associated hypernatremia: are we missing the diagnosis? Pediatrics 2005;116:e343–e347. doi:10.1542/peds.2004-2647 [DOI] [PubMed] [Google Scholar]
- 9.Escobar GJ, Gonzales VM, Armstrong MA, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med 2002;156:155–61. [DOI] [PubMed] [Google Scholar]
- 10.van Amerongen RH, Moretta AC, Gaeta TJ. Severe hypernatremic dehydration and death in a breast-fed infant. Pediatr Emerg Care 2001;17:175–80. doi:10.1097/00006565-200106000-00006 [DOI] [PubMed] [Google Scholar]
- 11.Chantry CJ, Nommsen-Rivers LA, Peerson JM, et al. Excess weight loss in first-born breastfed newborns relates to maternal intrapartum fluid balance. Pediatrics 2011;127:e171–e179. doi:10.1542/peds.2009-2663 [DOI] [PubMed] [Google Scholar]
- 12.Noel-Weiss J, Woodend AK, Peterson WE, et al. An observational study of associations among maternal fluids during parturition, neonatal output, and breastfed newborn weight loss. Int Breastfeed J 2011;6:9 doi:10.1186/1746-4358-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirth R, Weitkamp T, Dwivedi A. Maternal Intravenous Fluids and Infant Weight. Clin Lact 2012;3:59–63. doi:10.1891/215805312807009360 [Google Scholar]
- 14.Sheehan K FBE. The role of Intrapartum intravenous therapy and Newborn Weight loss: challenging the 7% Rule. Clinics in Mother and Child Health 2015;12:8–12. [Google Scholar]
- 15.Dewey KG, Nommsen-Rivers LA, Heinig MJ, et al. Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics 2003;112:607–19. doi:10.1542/peds.112.3.607 [DOI] [PubMed] [Google Scholar]
- 16.Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993;80:27–38. doi:10.1093/biomet/80.1.27 [Google Scholar]
- 17.Tawia S, McGuire L. Early weight loss and weight gain in healthy, full-term, exclusively-breastfed infants. Breastfeed Rev 2014;22:31–42. [PubMed] [Google Scholar]
- 18.Mezzacappa MA, Ferreira BG. Excessive weight loss in exclusively breastfed full-term newborns in a Baby-Friendly Hospital. Rev Paul Pediatr English Ed 2016;34:281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moen V, Brudin L, Rundgren M, et al. Hyponatremia complicating labour--rare or unrecognised? A prospective observational study. BJOG 2009;116:552–61. doi:10.1111/j.1471-0528.2008.02063.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamp JM, Macke JK. Relationships among intrapartum maternal fluid intake, birth type, neonatal output, and neonatal weight loss during the first 48 hours after birth. J Obstet Gynecol Neonatal Nurs 2010;39:169–77. doi:10.1111/j.1552-6909.2010.01106.x [DOI] [PubMed] [Google Scholar]
- 21.Watson J, Hodnett E, Armson BA, et al. A randomized controlled trial of the effect of intrapartum intravenous fluid management on breastfed newborn weight loss. J Obstet Gynecol Neonatal Nurs 2012;41:24–32. doi:10.1111/j.1552-6909.2011.01321.x [DOI] [PubMed] [Google Scholar]
- 22.Grossman X, Chaudhuri JH, Feldman-Winter L, et al. Neonatal weight loss at a US Baby-Friendly Hospital. J Acad Nutr Diet 2012;112:410–3. doi:10.1016/j.jada.2011.10.024 [DOI] [PubMed] [Google Scholar]
- 23.Thulier DJ. Maternal hydration and infant weight: a case-control study in breastfeeding infants delivered by cesarean section. ProQuest Diss Theses 2013;126 http://digitalcommons.uri.edu/oa_diss. [Google Scholar]
- 24.Zetterström R. Excessive fluid intake before and during delivery causing water intoxication in the mother and her offspring. Acta Paediatr 2007;91:867–8. doi:10.1111/j.1651-2227.2002.tb02846.x [DOI] [PubMed] [Google Scholar]
- 25.Cotterman KJ. Reverse pressure softening: a simple tool to prepare areola for easier latching during engorgement. J Hum Lact 2004;20:227–37. doi:10.1177/0890334404264224 [DOI] [PubMed] [Google Scholar]
- 26.Kujawa-Myles S, Noel-Weiss J, Dunn S, et al. Maternal intravenous fluids and postpartum breast changes: a pilot observational study. Int Breastfeed J 2015;10:18 doi:10.1186/s13006-015-0043-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulder PJ, Johnson TS, Baker LC. Excessive weight loss in breastfed infants during the postpartum hospitalization. J Obstet Gynecol Neonatal Nurs 2010;39:15–26. doi:10.1111/j.1552-6909.2009.01085.x [DOI] [PubMed] [Google Scholar]
- 28.Lyons G. Epidural is an outmoded form of regional anaesthesia for elective caesarean section. Proposer. Int J Obstet Anesth 1995;4:34–9. doi:10.1016/0959-289X(95)82288-L [DOI] [PubMed] [Google Scholar]
- 29.Robson SC, Boys RJ, Rodeck C, et al. Maternal and fetal haemodynamic effects of spinal and extradural anaesthesia for elective caesarean section. Br J Anaesth 1992;68:54–9. doi:10.1093/bja/68.1.54 [DOI] [PubMed] [Google Scholar]
- 30.Roberts SW, Leveno KJ, Sidawi JE, et al. Fetal acidemia associated with regional anesthesia for elective cesarean delivery. Obstet Gynecol 1995;85:79–83. doi:10.1016/0029-7844(94)P4401-9 [DOI] [PubMed] [Google Scholar]
- 31.Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. BMJ 2016;352:i1981 doi:10.1136/bmj.i1981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2017-000070supp001.pdf (197.1KB, pdf)


