Abstract
Background
Infants born very preterm (VPT) and moderate-to-late preterm (MLPT) are at increased risk of long-term neurodevelopmental deficits, but how these deficits relate to early neurobehaviour in MLPT children is unclear. The aims of this study were to compare the neurobehavioural performance of infants born across three different gestational age groups: preterm <30 weeks’ gestational age (PT<30); MLPT (32–36 weeks’ gestational age) and term age (≥37 weeks’ gestational age), and explore the relationships between MRI brain abnormalities and neurobehaviour at term-equivalent age.
Methods
Neurobehaviour was assessed at term-equivalent age in 149 PT<30, 200 MLPT and 200 term-born infants using the Neonatal Intensive Care UnitNetwork Neurobehavioral Scale (NNNS), the Hammersmith Neonatal Neurological Examination (HNNE) and Prechtl’s Qualitative Assessment of General Movements (GMA). A subset of 110 PT<30 and 198 MLPT infants had concurrent brain MRI.
Results
Proportions with abnormal neurobehaviour on the NNNS and the HNNE, and abnormal GMA all increased with decreasing gestational age. Higher brain MRI abnormality scores in some regions were associated with suboptimal neurobehaviour on the NNNS and HNNE. The relationships between brain MRI abnormality scores and suboptimal neurobehaviour were similar in both PT<30 and MLPT infants. The relationship between brain MRI abnormality scores and abnormal GMA was stronger in PT<30 infants.
Conclusions
There was a continuum of neurobehaviour across gestational ages. The relationships between brain abnormality scores and suboptimal neurobehaviour provide evidence that neurobehavioural assessments offer insight into the integrity of the developing brain, and may be useful in earlier identification of the highest-risk infants.
Keywords: Neurodevelopment
What is already known on this topic?
Infants born very preterm (VPT) are at increased risk of long-term neurodevelopmental deficits.
Early interventions can improve developmental outcomes and thus early identification of infants at greatest need for these services is paramount.
Brain MRI abnormalities have been associated with neurobehaviour functioning in VPT infants, however, this relationship is yet to be explored in moderate-to-late preterm infants.
What this study hopes to add?
This study highlights a clear continuum of neurobehaviour with increased suboptimal functioning on three neurobehavioural assessments with decreasing gestational age.
The relationships between brain abnormality scores and suboptimal neurobehaviour provide evidence that neurobehavioural assessments may be useful in earlier identification of the highest-risk infants.
Background and rationale
Infants born preterm are at increased risk of long-term neurodevelopmental deficits in cognitive, neurosensory, physical and social-emotional development, as well as impairments in academic functioning compared with their term-born peers.1–4 Early interventions to mitigate some of these adverse neurodevelopmental deficits are promising,5 6 and thus, clinicians working with preterm infants and their families aim to identify those infants at greatest need for early intervention services. While major preterm brain injuries, such as high-grade intraventricular haemorrhage and cystic periventricular leukomalacia, are highly prognostic for adverse neurodevelopmental outcomes,6 7 other clinical predictors of long-term development have only modest prognostic utility. Magnetic resonance imaging (MRI) can detect more subtle preterm brain injury associated with adverse neurodevelopment, however, its use in routine clinical care is limited by availability and cost.8 Clinicians are increasingly using neonatal neurobehavioural assessments to identify high-risk infants and to help guide referrals for early intervention. Neurobehavioural assessments are valid and reliable tools which offer insights into the neurological integrity and behavioural functioning of an infant.9 10
Compared with their term-born peers, infants born very preterm (VPT: <32 weeks’ gestation) and moderate-to-late preterm (MLPT: 32–36 weeks’ gestation) present with increased rates of atypical neurobehaviour at term-equivalent age.11 12 Importantly, these neurobehavioural difficulties have been associated with later developmental deficits,13 14 providing support for the neurobehavioural assessment as a predictive tool for later neurodevelopmental outcome. While there is some evidence that MRI brain abnormalities are associated with neurobehaviour in VPT infants at term-equivalent age,15–17 this brain–neurobehaviour relationship has yet to be explored in MLPT infants.
The primary aim of this study was to compare the neurobehavioural performance of infants born across three different gestational age groups: preterm <30 weeks’ gestational age (PT<30); MLPT (32–36 weeks’ gestational age) and term age (≥37 weeks’ gestational age) using the Neonatal Intensive Care Unit Network Neurobehavioral Scale (NNNS), the Hammersmith Neonatal Neurological Examination (HNNE) and Prechtl’s Qualitative Assessment of General Movements (GMA).18 Secondary aims were to explore the relationships between MRI brain abnormalities (development and injury) and neurobehaviour at term-equivalent age in infants born preterm (PT<30 and MLPT).
Methods
Participants
Participants were derived from two longitudinal cohorts of infants recruited from the Royal Women’s Hospital in Melbourne, Australia, between November 2009 and December 2013. The first cohort comprised 149 PT<30 infants19 and the second cohort included 201 MLPT infants.20 In addition, a cohort of 201 term controls were recruited across the two cohorts. Infants with congenital abnormalities and/or infants with non-English speaking parents were excluded due to limited funding for interpreters. In the term control group, infants requiring admission to the special care or intensive care nurseries were excluded. Informed parental consent was obtained for all participants, and both studies were approved by the Royal Women’s Hospital’s and Royal Children’s Hospital’s Human Research Ethics Committees.
Perinatal data were recorded by research nurses, including gestation at birth, sex, birth weight Z-score (calculated according to gestational age and sex using the British Growth Reference norms),21 multiple birth, use of antenatal corticosteroids and respiratory support.
Neurobehavioural measures
At term-equivalent age (38–44 weeks’ postmenstrual age), neurobehaviour was assessed by one of five trained and certified assessors using NNNS,22 HNNE23 and GMA. All assessors had advanced GMA certification and were masked to the participants’ clinical history. All assessments were administered according to their standardised procedures, as previously published in the study protocol.19
Neonatal Intensive Care Unit Network Neurobehavioral Scale
The NNNS is a neurobehavioural assessment that examines the neurological integrity, behavioural functioning and responses to stress in high-risk infants using 45 items which correspond to 13 summary scales including habituation, attention, arousal, regulation, handling, quality of movement, excitability, lethargy, non-optimal reflexes, asymmetrical reflexes, hypertonicity, hypotonicity and stress.24 25 The habituation scale was not included in this study as infants were not consistently in an appropriate state (sleep) to administer the scale at the start of the assessment. The scoring and classification of infants’ performance on the NNNS summary scales has been described previously by our group.26
Hammersmith Neonatal Neurological Examination
The HNNE is primarily a neurological examination developed for term and preterm infants. It consists of 34 individual items with six subtotals including tone, tone patterns, reflexes, spontaneous movements, abnormal neurological signs and behaviour, which are added for a total score. Suboptimal performance on the HNNE was categorised as previously published.27
Prechtl’s Qualitative Assessment of General Movements
The GMA is an observational assessment of the infant’s spontaneous movements (or general movements (GMs)) with good predictive validity for neurodevelopmental outcomes, including cerebral palsy, motor impairment and cognitive outcomes.28 GMs were scored from video recordings according to Prechtl’s method of qualitative assessment.18 GMs were categorised as normal or abnormal, with abnormal GMs further categorised as poor repertoire, cramped synchronised or chaotic. GMs were classified unscorable if the infant was crying or hypokinetic.
Magnetic Resonance Imaging
Brain MRI was performed using the Siemens 3T Magnetom Trio MRI system (Siemens, Erlangen, Germany) during natural sleep, on the same day as neurobehavioural assessments. The details of the imaging protocol have previously been published, and the T1-weighted and T2-weighted structural brain images were used for the current study.20
A validated neonatal brain MRI scoring system was used to assess brain maturation, injury and size, using two-dimensional brain metrics29 and conventional methods of assessing brain injury.30 Four regional abnormality scores were calculated based on assessment of injury, growth and maturation: cerebral white matter, cortical grey matter, deep grey matter (basal ganglia and thalamus) and cerebellar abnormality.31 A global abnormality score was computed using the sum of the four regional abnormality scores; higher scores indicate greater abnormality. Brain MRI were scored independently by one of four experienced neuroradiologists and neonatologists who had received training in this scoring system with excellent inter-rater and intrarater reliability.20
Statistical analysis
Data were analysed using Stata V.14 (StataCorp). To explore the relationships between gestational age group and neurobehaviour at term-equivalent age, proportions with suboptimal neurobehaviour within gestational age groups were compared by χ2 test for trend. Relationships between brain MRI abnormalities and suboptimal neurobehaviour in the preterm infants (<37 weeks’ gestational age) were explored using logistic regression, fitted using generalised estimating equations to allow for multiple births, and adjusted for sex and age at brain MRI. For any significant relationships between neonatal brain abnormality and suboptimal neurobehaviour, an interaction term was included in the model to explore whether the relationship differed according to the preterm infants’ gestational age group (PT<30 or MLPT). We have previously demonstrated a strong relationship between brain abnormality scores and abnormal GMA in infants born <30 weeks’ gestational age,32 thus, an interaction term was included in this model, irrespective of the initial findings.
Results
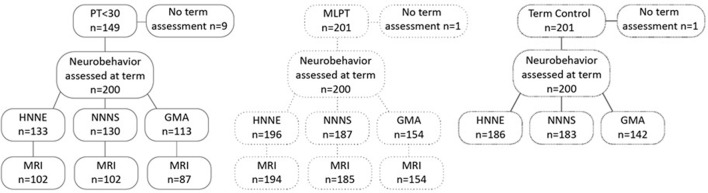
Neurobehavioural assessments were performed at term-equivalent age in 140 of 149 PT<30 infants, 200/201 MLPT infants and 200/201 term controls recruited for this study. Participant characteristics are summarised in table 1. A subset of PT<30 (n=110) and MLPT (n=198) infants had concurrent brain MRI. Infants ≥45 weeks’ postmenstrual age at the time of neurobehavioural assessment and brain MRI were excluded, as were infants who were not in an appropriate state to allow their GMA to be reliably scored (see figure 1). Differences between infants with and without neurobehavioural data are shown in online supplementary tables 1a–c. In the PT<30 group, six infants died prior to term-equivalent age and were not included in this baseline analysis.
Table 1.
Participant characteristics of infants assessed at term-equivalent age (based on HNNE sample size)
| PT<30 (n=133) | MLPT (n=196) | Term control (n=186) | |
| Postnatal corticosteroids, n (%) | 12 (9) | 0 | 0 |
| Multiple birth, n (%) | 59 (44) | 73 (37) | 2 (1) |
| Caesarean delivery, n (%) | 75 (72) | 134 (68) | 72 (39) |
| Gestational age at birth (weeks), mean (SD) | 27.9 (1.4) | 34.4 (1.3) | 39.7 (1.2) |
| Birth weight Z-score, mean (SD) | −0.33 (1) | −0.33 (1.2) | 0.23 (0.82) |
| Male, n (%) | 54 (52) | 93 (47) | 99 (53) |
| Respiratory distress at birth, n (%) | 102 (98) | 44 (22) | 0 |
| Postmenstrual age at neurobehavioural assessment (weeks), mean (SD) | 41.5 (1.9) | 41.4 (1.1) | 41.9 (1.5) |
| Postmenstrual age at MRI (weeks), mean (SD) | 42.4 (1.5) | 41.4 (1.1) | N/A |
| Global brain abnormality score, n participants, mean score (SD) | 101, 4.30 (2.02) | 187, 1.82 (1.87) | N/A |
| Cerebral WM brain abnormality score, n participants, mean score (SD) | 101, 2.29 (1.35) | 187, 0.98 (1.17) | N/A |
| Cortical grey matter abnormality score, n participants, mean score (SD) | 102, 1.39 (1.28) | 193, 0.62 (0.78) | N/A |
| Deep nuclear grey matter abnormality score, n participants, mean score (SD) | 102, 0.11 (0.34) | 194, 0.04 (0.19) | N/A |
| Cerebellar abnormality score, n participants, mean score (SD) | 102, 0.51 (0.73) | 192, 0.19 (0.58) | N/A |
HNNE, Hammersmith Neonatal Neurological Examination; MLPT, moderate-to-late preterm; N/A. not applicable (term infants did not have an MRI for this study); PT<30, preterm born <30 weeks’ gestational age; WM, white matter.
Figure 1.
Participant recruitment and neurobehavioural assessment and MRI follow-up. GMA, General Movements Assessment; HNNE, Hammersmith Neonatal Neurological Examination; MLPT, moderate-to-late preterm; NNNS, Neonatal Intensive Care Unit Network Neurobehavioral Scale; PT<30, preterm born <30 weeks’ gestational age.
bmjpo-2017-000136supp001.pdf (51.2KB, pdf)
Suboptimal neurobehaviour
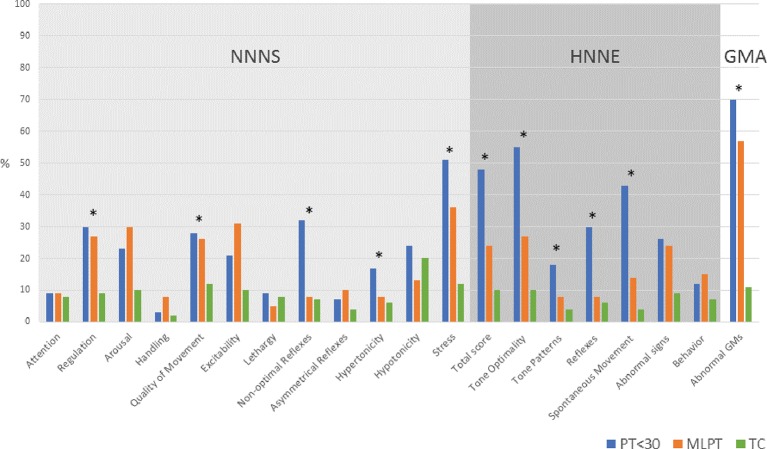
Scores on several neurobehaviour subscales were not independent of gestational age (p<0.05) (figure 2). On the NNNS, the proportions with suboptimal neurobehaviour decreased with increasing gestational age group for the following subscales: regulation, quality of movement, non-optimal reflexes, hypertonicity and stress. Excluding abnormal signs and behaviour, suboptimal neurobehaviour on the remaining HNNE subscales decreased with increasing age. Rates of abnormal GMA were higher with decreasing gestational age.
Figure 2.
Suboptimal neurobehaviour and abnormal GMs across gestational age groups. Single asterisk (*) denotes neurobehavioural subscale score not independent of gestational age (p<0.05). GMs, general movements; GMA, General Movements Assessment; HNNE, Hammersmith Neonatal Neurological Examination; MLPT, moderate-to-late preterm; NNNS, Neonatal Intensive Care Unit Network Neurobehavioral Scale; PT<30, preterm born <30 weeks’ gestational age; TC, term control.
Brain MRI abnormality and suboptimal neurobehaviour
Higher brain MRI abnormality scores were associated with suboptimal neurobehaviour on the NNNS and HNNE, with the exception of the deep grey matter abnormality score (table 2). A higher global brain abnormality score was associated with greater odds of being suboptimal on the lethargy domain and non-optimal reflexes on the NNNS, and greater odds of suboptimal reflexes, abnormal signs and total score on the HNNE. Similarly, a higher cerebral white matter abnormality score was associated with greater odds of being suboptimal on the lethargy domain and non-optimal reflexes on the NNNS, and greater odds of suboptimal reflexes, suboptimal spontaneous movements and total score on the HNNE. A higher cortical grey matter abnormality score was associated with non-optimal reflexes on the NNNS. A higher cerebellar abnormality score was associated with greater odds of being suboptimal on the lethargy domain on the NNNS and total score on the HNNE. The cerebellar abnormality score was the only brain MRI abnormality score associated with suboptimal tone on the HNNE. Brain abnormality scores were not associated with increased odds of abnormal GMA.
Table 2.
Relationship between brain abnormality scores and suboptimal neurobehaviour
| Suboptimal neurobehaviour | Global brain abnormality score OR (95% CI), p Value |
Cerebral WM brain abnormality score OR (95% CI), p Value |
Cortical grey matter abnormality score OR (95% CI), p Value |
Deep nuclear grey matter abnormality score OR (95% CI), p Value |
Cerebellar abnormality score OR (95% CI), p Value |
| NNNS attention | 1.03 (0.87 to 1.21), 0.67 | 1.18 (0.91 to 1.53), 0.20 | 0.75 (0.50 to 1.13), 0.16 | 1.59 (0.42 to 5.95), 0.50 | 1.08 (0.61 to 1.90), 0.80 |
| NNNS arousal | 0.93 (0.82 to 1.04), 0.21 | 0.89 (0.72 to 1.09), 0.26 | 1 (0.77 to 1.28), 0.98 | 0.74 (0.29 to 1.92), 0.54 | 0.77 (0.48 to 1.25), 0.30 |
| NNNS regulation | 0.99 (0.89 to 1.11), 0.88 | 1.01 (0.84 to 1.21), 0.94 | 0.98 (0.76 to 1.27), 0.90 | 0.21 (0.03 to 1.30), 0.09 | 1.01 (0.69 to 1.47), 0.97 |
| NNNS handling | 0.85 (0.68 to 1.07), 0.16 | 0.72 (0.42 to 1.23), 0.23 | 1.08 (0.78 to 1.51), 0.65 | Omitted* | 0.51 (0.18 to 1.44), 0.20 |
| NNNS quality of movement | 1.07 (0.94 to 1.21), 0.31 | 1.07 (0.87 to 1.32), 0.51 | 1.02 (0.78 to 1.34), 0.88 | 0.44 (0.11 to 1.70), 0.23 | 1.45 (0.97 to 2.16), 0.07 |
| NNNS excitability | 0.93 (0.83 to 1.05), 0.25 | 0.87 (0.71 to 1.06), 0.17 | 1.05 (0.83 to 1.35), 0.67 | 0.20 (0.03 to 1.50), 0.12 | 0.85 (0.57 to 1.27), 0.43 |
| NNNS lethargy | 1.20 (1.01 to 1.43), 0.04 | 1.44 (1.09 to 1.89), 0.01 | 0.78 (0.47 to 1.27), 0.32 | Omitted* | 2.22 (1.38 to 3.59),<0.01 |
| NNNS non-optimal reflexes | 1.23 (1.07 to 1.41),<0.01 | 1.30 (1.06 to 1.59), 0.01 | 1.33 (1.03 to 1.72), 0.03 | 1.12 (0.36 to 3.53), 0.85 | 1.47 (0.97 to 2.23), 0.07 |
| NNNS asymmetrical reflexes | 0.86 (0.69 to 1.08), 0.20 | 0.89 (0.63 to 1.26), 0.52 | 0.83 (0.50 to 1.36), 0.45 | Omitted* | 0.43 (0.18 to 1), 0.05 |
| NNNS hypertonicity | 1.07 (0.92 to 1.24), 0.40 | 1.17 (0.91 to 1.52), 0.23 | 1.07 (0.78 to 1.47), 0.68 | Omitted* | 0.99 (0.58 to 1.68), 0.97 |
| NNNS hypotonicity | 1.05 (0.93 to 1.19), 0.40 | 1.21 (1 to 1.46), 0.05 | 0.92 (0.67 to 1.27), 0.62 | Omitted* | 1.08 (0.67 to 1.73), 0.75 |
| NNNS stress | 1.10 (0.98 to 1.22), 0.11 | 1.15 (0.96 to 1.38), 0.12 | 1.05 (0.84 to 1.32), 0.66 | 0.90 (0.37 to 2.16), 0.81 | 1.35 (0.91 to 1.99), 0.13 |
| HNNE total score | 1.19 (1.05 to 1.33),<0.01 | 1.29 (1.06 to 1.57), 0.01 | 1.16 (0.91 to 1.49), 0.23 | 0.68 (0.23 to 2.02), 0.49 | 1.61 (1.09 to 2.38), 0.02 |
| HNNE tone optimality | 1.10 (0.97 to 1.23), 0.14 | 1.14 (0.94 to 1.38), 0.17 | 1 (0.77 to 1.29), 0.97 | 1.38 (0.58 to 3.29), 0.46 | 1.55 (1.02 to 2.34), 0.04 |
| HNNE tone patterns | 1.13 (0.98 to 1.31), 0.10 | 1.09 (0.86 to 1.37), 0.48 | 1.22 (0.87 to 1.70), 0.25 | 0.54 (0.09 to 3.06) 0.48 | 1.50 (0.91 to 2.46), 0.11 |
| HNNE reflexes | 1.17 (1.03 to 1.33), 0.01 | 1.37 (1.09 to 1.71), 0.01 | 1.05 (0.80 to 1.39), 0.72 | 0.38 (0.05 to 2.76), 0.34 | 1.41 (0.94 to 2.12), 0.10 |
| HNNE spontaneous movement | 1.11 (0.98 to 1.25), 0.09 | 1.26 (1.04 to 1.52), 0.02 | 1.04 (0.81 to 1.35), 0.74 | 0.42 (0.10 to 1.88), 0.26 | 1.15 (0.77 to 1.72), 0.50 |
| HNNE abnormal signs | 1.14 (1.01 to 1.28), 0.03 | 1.12 (0.93 to 1.34), 0.24 | 1.23 (0.94 to 1.61), 0.13 | 1.09 (0.44 to 2.69), 0.86 | 1.34 (0.88 to 2.06), 0.18 |
| HNNE behaviour | 1.11 (0.90 to 1.36), 0.32 | 1.20 (0.89 to 1.63), 0.23 | 1.02 (0.66 to 1.58), 0.94 | Omitted* | 1.52 (0.84 to 2.74), 0.16 |
| Abnormal GMs | 1.12 (0.99 to 1.26) 0.07 | 1.21 (1.00 to 1.46) 0.05 | 1.15 (0.91 to 1.45) 0.24 | 0.92 (0.41 to 2.05) 0.84 | 1.04 (0.71 to 1.53) 0.83 |
*Result omitted as too few numbers with suboptimal neurobehaviour and deep nuclear grey matter abnormality to analyse.
GMs, general movements; HNNE, Hammersmith Neonatal Neurological Examination; NNNS, Neonatal Intensive Care Unit Network Neurobehavioral Scale; WM, white matter.
When an interaction term was included in the model to explore whether the relationship differed according to the preterm infants’ gestational age group, statistically significant relationships between brain MRI abnormality scores and suboptimal neurobehaviour on the NNNS and HNNE were similar in both the PT<30 and MLPT groups (all p values for interaction >0.05). The relationships between brain MRI abnormality scores and abnormal GMA were stronger in the PT<30 group for global brain (OR 1.39; 95% CI 1.04 to 1.86; p=0.03) and cortical grey matter (OR 1.75; 95% CI 1.05 to 2.93; p=0.03) abnormality scores.
Discussion
This study demonstrates the continuum of neurobehaviour across gestational age groups, demonstrating more infants with suboptimal neurobehaviour for many items on the HNNE, NNNS and GMA with decreasing gestational age. This study extends previous research that has shown differences in neurobehaviour between VPT and term-born infants at term-equivalent age by exploring brain–behaviour relationships on concurrent neurobehavioural assessment and brain MRI.
Regulation and behavioural domains of the NNNS and HNNE
The current study demonstrated that suboptimal regulation on the NNNS (which assesses the infant’s capacity to organise motor activity, physiology and state during the examination)24 was more common with decreasing gestational age, and is consistent with other reports describing VPT infants as more irritable with poorer self-regulation at term-equivalent age than their term-born peers.33 Compromises to the development of preterm infants’ self-regulation may influenced by the ‘chaotic context’ of the neonatal intensive care unit environment,34 which includes perinatal care experiences associated with pain and stress to a system undergoing rapid growth and organisation.35 Previous studies have demonstrated a relationship between lower gestational age and greater exposure to stress in the neonatal period,36 which may explain the current study’s finding of increasing rates of suboptimal regulation at term-equivalent age in infants born at lower gestations.
Poor regulation is often exhibited through infant behaviours known as stress cues.37 Accordingly, it is not surprising that the current study also demonstrated greater suboptimal levels of stress-related behaviours on the NNNS with decreasing gestational age. The NNNS stress scale provides information about the infant’s capacity to regulate and organise multiple systems (eg, visual, state, physiological, motor) in response to handling and interaction demands of their environment. Interestingly, a significant proportion of the MLPT infants showed suboptimal stress responses on the NNNS (36%), suggesting that despite their greater gestational age, MLPT infants, like their PT<30 infant peers, are vulnerable to environmental stressors and may require external support to regulate their developing regulatory systems.38
Influenced in part by an infant’s regulatory capacity, it is not surprising that compared with their term-born peers, both PT <30 and MLPT infants had higher rates of suboptimal arousal and excitability on the NNNS, however, the relationship between these subscale scores and their dependence on gestational age did not reach statistical significance (p<0.05). This may be explained by the bidirectional nature of these scales, with higher scores representing overarousal or overexcitability and lower scores reflecting underarousal or underexcitability. The suboptimal score does not differentiate the direction of the pattern.
The lack of dependence on gestational age for some variables in the current study may be explained by the age of term infants at assessment. Compared with the MLPT infants, term infants had higher proportions of suboptimal lethargy and hypotonicity on the NNNS. These infants were often assessed before hospital discharge, when the physiological changes that occur in the first days after birth may have influenced the presentation of lethargy and apparent hypotonicity.39 The NNNS handling scale includes the strategies used to obtain focused attention and the non-significant relationship across groups is somewhat surprising. Term infants did not require as much supportive handling, as expected, but MLPT infants required more handling than the PT<30 infants, which may reflect their different responses to stress. On the HNNE, the proportions of suboptimality in the abnormal signs domain were similar between PT<30 and MLPT infants and the behavioural domain had low levels of suboptimality in all three groups.
Despite the effects of gestational age at birth on regulation at term-equivalent age, all three groups demonstrated similar orientation and attentional responses to visual and auditory stimuli, although perhaps at the cost of the PT<30 and MLPT infants’ stress and regulation performance.
Neurological domains of the NNNS and HNNE, and the GMA
Having spent longer in physiological flexion in utero, term-born infants often present with greater flexion and smoother movement patterns compared with preterm peers.16 40 41 Higher rates of tone abnormalities, both hypertonicity and hypotonicity, are reported in preterm infants at term-equivalent age.16 Consistent with this pattern, the current study demonstrated higher rates of suboptimal hypertonicity on the NNNS, suboptimal tone optimality and suboptimal tone patterns on the HNNE with decreasing gestational age. Similarly, there was poorer quality of movement with decreasing gestational age on all three neurobehavioural assessments. On the HNNE, PT<30 infants demonstrated substantially higher rates of suboptimal spontaneous movement (41%) compared with MLPT and term infants (14% and 4%, respectively), and there were higher rates of abnormal GMA with decreasing gestational age. Reduced quality of movement at lower gestational ages may reflect differences in central nervous system integrity and the extrauterine environment, including limited opportunities for movement.
Similarly, the reflex scales of the NNNS and HNNE also reflect maturation of the infants’ central nervous system with increasing suboptimal reflexes on the NNNS and HNNE with decreasing gestational age. Infants born PT<30 had a substantially higher rate of suboptimal reflexes (32%) compared with MLPT and term-born infants (9% and 7%, respectively). Furthermore, the rates of suboptimal total scores on the HNNE, a reflection of an infant’s overall performance on the more neurologically based assessment, increased significantly with decreasing gestational age, highlighting the impact of preterm birth on the maturation and integrity of the central nervous system. This finding contrasts with other studies having reported that motor reflexes do not seem to be differentially affected by a short gestation, severity of illness or brain injury.42
Brain–behaviour relationships
A number of relationships between brain MRI abnormality scores and suboptimal neurobehaviour were identified in the current study. These brain–behaviour relationships were more common on the HNNE than the NNNS, which may reflect its greater neurological focus.9
On the NNNS, various brain abnormality scores were associated with greater odds of non-optimal reflexes and suboptimal lethargy. In particular, the global, cerebral white matter and cortical grey matter abnormality scores were associated with suboptimal reflexes and the global, cerebral white matter and cerebellar with suboptimal lethargy on the NNNS. This is consistent with the study by Brown et al,17 who also reported both white matter signal abnormality and delayed gyral maturation to be associated with worse performance on the NNNS non-optimal reflex scale.
Previous studies have linked poor quality of movement with cerebral injury, in particular, white matter abnormality and poor outcome.10 Similarly, the current study demonstrated a significant relationship between white matter abnormality and suboptimal performance on the HNNE spontaneous movement scale. This is consistent with the study by Brown et al who reported an association between a worse grade of white matter abnormality and suboptimal HNNE spontaneous movement scores. In keeping with the current study’s findings, previous studies found no relationship between brain abnormality scores and the NNNS quality of movement scale.16 17 No other regional abnormality scores were associated with suboptimal movement in contrast with two earlier studies that reported delayed gyral maturation and higher grey matter abnormality were associated with lower HNNE spontaneous movement scores.17 43 On the GMA, however, global brain abnormality and cortical grey abnormality, a composite of signal abnormality, delayed gyration and dilated extracerebral space, was associated with abnormal GMA.
Limitations of the current study are important to consider in the interpretation of the findings. In particular, the low rates of brain abnormality scores may have influenced the power to detect relationships between brain abnormality and suboptimal neurobehavioural outcomes. Also, not all infants were able to have all assessments.
Conclusion
The current study demonstrated a clear continuum of neurobehaviour across three gestational age groups, with poorer behavioural regulation, increased stress response, greater tone abnormality, abnormal reflexes and poorer quality of movement with decreasing gestational age. A number of relationships between brain abnormality scores and suboptimal neurobehaviour were found, particularly the global, white matter and cerebellar abnormality scores with the more neurologically focused domains of the NNNS and HNNE evidence that the neurobehavioural assessment can provide insight into the integrity of the developing brain and can both supplement imaging findings when available or in the absence of MRI, provide guidance to clinicians who are identifying high-risk infants for early intervention services.
Footnotes
Contributors: ALE collected data, carried out the analyses, drafted the initial manuscript and reviewed and revised the manuscript. JMW collected data, drafted the initial manuscript and reviewed and revised the manuscript. JEO collected data and reviewed and revised the manuscript. RC critically reviewed and revised the manuscript. DKT, PJA, LWD, JLYC and AJS conceptualised and designed the study and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding: This work was supported in part by the Australian National Health and Medical Research Council (NHMRC) (Project Grant ID 1028822); Centre of Clinical Research Excellence Grant ID 546519; Centre of Research Excellence Grant ID 1060733; Senior Research Fellowship ID 1081288 to PJA; Early Career Fellowship ID 1053787 to JLYC, ID 1053767 to AJS; Career Development Fellowship ID 1108714 to AJS; Australian Postgraduate Scholarship to JEO, Murdoch Children’s Research Institute, Clinical Sciences Theme Grant, the Victorian Government Operational Infrastructure Support Program.
Competing interests: None declared.
Ethics approval: The Royal Women’s Hospital Human Research Ethics Board and the Royal Children’s Hospital Human Research Ethics board.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Anderson P, Doyle LW. Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 2003;289:3264–72. doi:10.1001/jama.289.24.3264 [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res 2011;69:11R–18. doi:10.1203/PDR.0b013e318212faa0 [DOI] [PubMed] [Google Scholar]
- 3.Cheong JL, Doyle LW. Increasing rates of prematurity and epidemiology of late preterm birth. J Paediatr Child Health 2012;48:784–8. doi:10.1111/j.1440-1754.2012.02536.x [DOI] [PubMed] [Google Scholar]
- 4.Spittle AJ, Treyvaud K, Doyle LW, et al. . Early emergence of behavior and social-emotional problems in very preterm infants. J Am Acad Child Adolesc Psychiatry 2009;48:909–18. doi:10.1097/CHI.0b013e3181af8235 [DOI] [PubMed] [Google Scholar]
- 5.Spittle AJ, Orton J, Doyle LW, et al. . Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev 2007;2:CD005495 doi:10.1002/14651858.CD005495.pub2 [DOI] [PubMed] [Google Scholar]
- 6.De Vries LS, Van Haastert IL, Rademaker KJ, et al. . Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr 2004;144:815–20. doi:10.1016/j.jpeds.2004.03.034 [DOI] [PubMed] [Google Scholar]
- 7.Vermeulen GM, Bruinse HW, de Vries LS. Perinatal risk factors for adverse neurodevelopmental outcome after spontaneous preterm birth. Eur J Obstet Gynecol Reprod Biol 2001;99:207–12. doi:10.1016/S0301-2115(01)00383-9 [DOI] [PubMed] [Google Scholar]
- 8.Plaisier A, Raets MM, Ecury-Goossen GM, et al. . Serial cranial ultrasonography or early MRI for detecting preterm brain injury? Arch Dis Child Fetal Neonatal Ed 2015;100:F293–F300. doi:10.1136/archdischild-2014-306129 [DOI] [PubMed] [Google Scholar]
- 9.Brown N, Spittle A. Neurobehavioral evaluation in the preterm and term infant. Curr Pediatr Rev 2014;10:65–72. doi:10.2174/157339631001140408121310 [DOI] [PubMed] [Google Scholar]
- 10.Spittle AJ, Brown NC, Doyle LW, et al. . Quality of general movements is related to white matter pathology in very preterm infants. Pediatrics 2008;121:e1184–e1189. doi:10.1542/peds.2007-1924 [DOI] [PubMed] [Google Scholar]
- 11.Duffy FH, Als H, McAnulty GB. Behavioral and electrophysiological evidence for gestational age effects in healthy preterm and fullterm infants studied two weeks after expected due date. Child Dev 1990;61:1271–86. doi:10.2307/1130893 [PubMed] [Google Scholar]
- 12.Jeng SF, Yau KI, Teng RJ. Neurobehavioral development at term in very low-birthweight infants and normal term infants in Taiwan. Early Hum Dev 1998;51:235–45. doi:10.1016/S0378-3782(98)00035-8 [DOI] [PubMed] [Google Scholar]
- 13.Wallace IF, Rose SA, McCarton CM, et al. . Relations between infant neurobehavioral performance and cognitive outcome in very low birth weight preterm infants. J Dev Behav Pediatr 1995;16:309–17. doi:10.1097/00004703-199510000-00001 [PubMed] [Google Scholar]
- 14.Spittle AJ, Walsh J, Potter C, et al. . Neurobehaviour at term is predictive of neurodevelopmental outcomes at two years of age for children born moderate-late preterm. Dev Med Child Neurol 2015;57:23. [DOI] [PubMed] [Google Scholar]
- 15.Spittle AJ, Doyle LW, Anderson PJ, et al. . Reduced cerebellar diameter in very preterm infants with abnormal general movements. Early Hum Dev 2010;86:1–5. doi:10.1016/j.earlhumdev.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 16.Pineda RG, Tjoeng TH, Vavasseur C, et al. . Patterns of altered neurobehavior in preterm infants within the neonatal intensive care unit. J Pediatr 2013;162:470–6. e471 doi:10.1016/j.jpeds.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown NC, Inder TE, Bear MJ, et al. . Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J Pediatr 2009;155:32–8. doi:10.1016/j.jpeds.2009.01.038 [DOI] [PubMed] [Google Scholar]
- 18.Einspieler C, Prechtl HFR, Bos AF, et al. ; Prechtl’s method on the qualitative assessment of general movements in preterm, term and young infants. Cambridge: Mac Keith Press, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Spittle AJ, Thompson DK, Brown NC, et al. . Neurobehaviour between birth and 40 weeks' gestation in infants born <30 weeks' gestation and parental psychological wellbeing: predictors of brain development and child outcomes. BMC Pediatr 2014;14:111 doi:10.1186/1471-2431-14-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh JM, Doyle LW, Anderson PJ, et al. . Moderate and late preterm birth: effect on brain size and maturation at term-equivalent age. Radiology 2014;273:232–40. doi:10.1148/radiol.14132410 [DOI] [PubMed] [Google Scholar]
- 21.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998;17:407–29. doi:10.1002/(SICI)1097-0258(19980228)17:4<407::AID-SIM742>3.0.CO;2-L [PubMed] [Google Scholar]
- 22.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures; appendix 3: summary score calculations. Pediatrics 2004;113:695–9. [PubMed] [Google Scholar]
- 23.Dubowitz L, Mercuri E, Dubowitz V. An optimality score for the neurologic examination of the term newborn. J Pediatr 1998;133:406–16. doi:10.1016/S0022-3476(98)70279-3 [DOI] [PubMed] [Google Scholar]
- 24.Tronick EZ, Olson K, Rosenberg R, et al. . Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics 2004;113(3 Pt 2):676–8. [PubMed] [Google Scholar]
- 25.Fink NS, Tronick E, Olson K, et al. . Healthy newborns' neurobehavior: norms and relations to medical and demographic factors. J Pediatr 2012;161:1073–9. doi:10.1016/j.jpeds.2012.05.036 [DOI] [PubMed] [Google Scholar]
- 26.Spittle AJ, Jennifer W, Olsen JE, et al. . Neurobehaviour in the first weeks after birth for infants born between 32-42 weeks’ gestation. Early Hum Dev 2015. [DOI] [PubMed] [Google Scholar]
- 27.Spittle AJ, Walsh J, Olsen JE, et al. . Neurobehaviour and neurological development in the first month after birth for infants born between 32-42 weeks' gestation. Early Hum Dev 2016;96:7–14. doi:10.1016/j.earlhumdev.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 28.Bosanquet M, Copeland L, Ware R, et al. . A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol 2013;55:418–26. doi:10.1111/dmcn.12140 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen The Tich S, Anderson PJ, Shimony JS, et al. . A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol 2009;30:125–31. doi:10.3174/ajnr.A1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013;34:2208–14. doi:10.3174/ajnr.A3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidokoro H, Anderson PJ, Doyle LW, et al. . Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 2014;134:444:e444–e453. doi:10.1542/peds.2013-2336 [DOI] [PubMed] [Google Scholar]
- 32.Olsen JE. Neurobehavioural trajectories of infants born <30 weeks' gestation from birth to term equivalent age: are preterm general movements related to neurobehavioural outcome at term equivalent age? (PhD thesis) 2014. [Google Scholar]
- 33.Brown NC, Doyle LW, Bear MJ, et al. . Alterations in neurobehavior at term reflect differing perinatal exposures in very preterm infants. Pediatrics 2006;118:2461–71. doi:10.1542/peds.2006-0880 [DOI] [PubMed] [Google Scholar]
- 34.Gorzilio DM, Garrido E, Gaspardo CM, et al. . Neurobehavioral development prior to term-age of preterm infants and acute stressful events during neonatal hospitalization. Early Hum Dev 2015;91:769–75. doi:10.1016/j.earlhumdev.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 35.Anand KJ, Scalzo FM. Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate 2000;77:69–82. doi:10.1159/000014197 [DOI] [PubMed] [Google Scholar]
- 36.Smith GC, Gutovich J, Smyser C, et al. . Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol 2011;70:541–9. doi:10.1002/ana.22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Als H. The newborn communicates. J Commun 1977;27:66–73. doi:10.1111/j.1460-2466.1977.tb01828.x [DOI] [PubMed] [Google Scholar]
- 38.Als H, Duffy FH, McAnulty GB, et al. . Early experience alters brain function and structure. Pediatrics 2004;113:846–57. doi:10.1542/peds.113.4.846 [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Yolton K, Khoury J. Earliest appropriate time for administering neurobehavioral assessment in newborn infants. Pediatrics 2011;127:e69–e75. doi:10.1542/peds.2010-1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubowitz L, Dubowitz V, Mercuri E. The neurological assessment of the preterm and full-term newborn infant. 2 edn London: Mac Keith Press, 1999. [Google Scholar]
- 41.Allen MC, Capute AJ. Tone and reflex development before term. Pediatrics 1990;85:393. [PubMed] [Google Scholar]
- 42.Constantinou JC, Adamson-Macedo EN, Mirmiran M, et al. . Neurobehavioral assessment predicts differential outcome between VLBW and ELBW preterm infants. J Perinatol 2005;25:788–93. doi:10.1038/sj.jp.7211403 [DOI] [PubMed] [Google Scholar]
- 43.Woodward LJ, Mogridge N, Wells SW, et al. . Can neurobehavioral examination predict the presence of cerebral injury in the very low birth weight infant? J Dev Behav Pediatr 2004;25:326–34. doi:10.1097/00004703-200410000-00004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2017-000136supp001.pdf (51.2KB, pdf)