Abstract
Objective
Non-Hodgkin’s lymphoma (NHL) is the most common childhood malignancy in sub-Saharan Africa. Survival rates for NHL are higher than 80% in high-income countries.
This study explores treatment outcomes of children with NHL in Kenya, a sub-Saharan low-income country, and the association between health insurance status at diagnosis and treatment outcomes.
Design
This was a retrospective medical records study. All children diagnosed with NHL in 2010, 2011 and 2012 were included. Data on treatment outcomes and health insurance status at diagnosis were collected.
Results
Of all 63 patients with NHL, 35% abandoned treatment, 22% had progressive or relapsed disease, 14% died and 29% had event-free survival. Most patients (73%) had no health insurance at diagnosis. Treatment outcomes in children with or without health insurance at diagnosis differed significantly (p=0.005). The most likely treatment outcome in children with health insurance at diagnosis was event-free survival (53%), whereas in children without health insurance at diagnosis it was abandonment of treatment (44%). Crude HR for treatment failure was 3.1 (95% CI 1.41 to 6.60, p=0.005) for uninsured versus insured children. The event-free survival estimate was significantly higher in children with health insurance at diagnosis than in patients without health insurance at diagnosis (p=0.003). Stage of disease at diagnosis was identified as a confounder of this association (adjusted HR=2.4, 95% CI 0.95 to 6.12, p=0.063).
Conclusions
Survival of children with NHL in Kenya is much lower compared with high-income countries. Abandonment of treatment is the most common cause of treatment failure. Health insurance at diagnosis was associated with better treatment outcomes and survival.
Keywords: childhood cancer, non-Hodgkin's lymphoma, health insurance status, low-income country
What is already known on this topic?
Poor childhood cancer survival in low and middle-income countries is mainly due to treatment abandonment.
Non-Hodgkin's lymphoma (NHL) is the most common childhood cancer in sub-Saharan Africa.
No study has investigated the association between health insurance status at diagnosis and treatment outcomes of children with NHL.
What this study hopes to add?
In this sub-Saharan African study, most children with NHL had no health insurance at diagnosis.
Health insurance at diagnosis was associated with reduced treatment abandonment and better survival of children with NHL.
This study illustrates the need for universal health coverage in low and middle-income countries to improve access to healthcare services and health outcomes of its populations.
Introduction
Non-Hodgkin’s lymphoma (NHL) is the most commonly diagnosed paediatric malignancy in sub-Saharan African countries. This increased incidence of NHL, compared with the rest of the world, is related to endemic malaria in many parts of this region. The development of Burkitt’s lymphoma, a subtype of NHL, is associated with Epstein-Barr virus and chronic malaria infection.1 2
Childhood cancer survival rates have increased substantially in the last decades, especially for patients with NHL in high-income countries. In 1975, the 5-year survival rate for patients with NHL younger than 15 years was 45%, which improved to 87% in 2010.3 Reported NHL survival rates in low and middle-income countries vary considerably and are particularly poor in Africa compared with high-income countries.4–10 In Malawi, the reported survival of children with Burkitt’s lymphoma was 33%.11 In North Africa, a survival rate of 61% was reported in children with Burkitt’s lymphoma from Algeria, Cameroon, Madagascar, Morocco, Tunisia and Senegal.12
The gap in NHL survival between high versus low and middle-income countries can be related to many factors including higher prevalence of malnutrition and low socioeconomic status in the latter countries.4–10 When families are poor, their access to healthcare services may be limited as required treatment at specialised cancer centres may be too expensive. Access to health insurance, which could help cover medical expenses, may be limited for the poor majority in low and middle-income countries. Lacking health insurance may cause delays in health-seeking behaviour, resulting in advanced disease stages at diagnosis, and it may force poor parents to abandon potentially curative cancer treatment for their children.4–10
To the best of our knowledge, no studies have been conducted that focus on associations between health insurance status at diagnosis and treatment outcomes in patients with NHL in high, middle or low-income countries. Our study explores treatment outcomes of children with NHL in Kenya, and its association with health insurance status at diagnosis.
Methods
Setting
Kenya is an equatorial country in East Africa with a population of 45 million people. Nearly half of the citizens are living below the poverty line. Kenya has a relatively young population as 42% of its inhabitants are between 0 and 14 years of age.13
Since 1966, the National Hospital Insurance Fund (NHIF) is Kenya’s government-owned health insurance. Every Kenyan above 18 years is eligible to enrol in NHIF. Contribution for self-employed or unemployed Kenyans is around US$2 per month which covers inpatient healthcare in government hospitals of the contributor and nuclear family members. Contribution for Kenyans working in formal sector is calculated based on their income. Despite its affordability, less than 10% of Kenyan population is insured.14 15
Our study was conducted at Moi Teaching and Referral Hospital (MTRH) in Eldoret. MTRH has an estimated service area of 16–18 million people, and subsequently 700 children under 15 years are expected to be diagnosed with a malignancy each year.2 However, in reality only 110 children are annually diagnosed with cancer.16 MTRH has a bed capacity for 700 patients. Paediatric ward consists of 72 beds of which 16 are allocated for oncology patients supervised by one paediatrician.2 17
At MTRH, NHL diagnosis is confirmed by lymph node biopsy and/or fine needle aspiration. To stage the tumour, an additional chest X-ray, ultrasound, bone marrow aspiration and cerebrospinal fluid cytology are performed. Tumour staging is done using the St Jude’s modification of Ann Arbor classification. After diagnosis is confirmed a chemotherapy regimen of COP reduction (cyclophosphamide, vincristine and prednisone) followed by eight courses of multimodal therapy is given every 3 weeks. Remission is determined with clinical and radiological assessment. At MTRH, lower intensity treatment is given compared with high-income settings, because access to expensive drugs (rituximab, high-dose treatments) and high-level supportive care are precluded. Nevertheless, various studies suggest that moderately intensive protocols are effective in underdeveloped areas.18–21
Study design
This was a retrospective medical records study. Inclusion criteria were: all children between 0 and 16 years of age, who were newly diagnosed with NHL at MTRH between January 2010 and December 2012. Further selection of patients did not take place.
Sociodemographic and clinical data were extracted from patients’ medical records using a structured data collection form. Sociodemographic characteristics included age at diagnosis, gender, ethnicity, patients’ residence and NHIF enrolment. Clinical characteristics included date of diagnosis, duration of symptoms before first hospital admission at MTRH (<1 month, 1–3 months, >3 months), staging of disease (stages I–IV), time to events and treatment outcomes.
Treatment outcomes were classified as treatment abandonment, progressive or relapsed disease, death and event-free survival. According to international consensus, treatment abandonment entails failure to start or continue scheduled curative treatment during four or more consecutive weeks.22
Our study was approved by MTRH’s Research Ethical Committee.
Data analysis
Data management and analysis were performed by SPSS V.20. Frequency distributions, means and medians were calculated. Relationships between treatment outcomes and patients’ sociodemographic or clinical characteristics were evaluated by χ2test and Fisher’s exact test. Cox regression survival analysis was applied. Event-free survival curves were estimated by Kaplan-Meier method. Log-rank test compared survival between groups. Event-free survival was measured from date of NHL diagnosis until first treatment failure (treatment abandonment, progressive or relapsed disease and death) or last follow-up date. Two-sided p value less than 0.05 was considered statistically significant.
Results
In total, 280 children were diagnosed with cancer between 2010 and 2012, of which NHL (23%) was the most common malignancy. From January 2010 to December 2012, a total of 63 patients were diagnosed with NHL. Table 1 illustrates patients’ sociodemographic and clinical characteristics. Most children (93%) were referred to MTRH by other healthcare facilities at primary care level (75%) and secondary care level (25%). Before patients were admitted to MTRH for the first time, 19% had received potential diagnosis of NHL, while 2% had already received some treatment destined for NHL. Of 44 children (70%), disease stage at diagnosis was known. Most patients presented late: stage I (7%), stage II (11%), stage III (66%), stage IV (16%).
Table 1.
Sociodemographic and clinical characteristics of children with non-Hodgkin’s lymphoma (N=63)
| Characteristics | n (%) |
| Age in years | |
| Mean ± SD | 7.7±3.3 |
| Median (range) | 7.8 (2–16) |
| Gender | |
| Male | 45 (71%) |
| Female | 18 (29%) |
| Tribe | |
| Luhya | 27 (43%) |
| Kalenjin | 20 (32%) |
| Luo | 8 (13%) |
| Kikuyu | 2 (3%) |
| Kisii | 2 (3%) |
| Maasai | 2 (3%) |
| Turkana | 1 (2%) |
| Teso | 1 (2%) |
| Distance to MTRH (n=62) | |
| <50 km | 2 (3%) |
| 50–100 km | 16 (26%) |
| >100 km | 44 (71%) |
| Comorbidities | |
| Malaria | 10 (26%) |
| HIV | 6 (14%) |
| Tuberculosis | 1 (2%) |
| Duration of symptoms before first hospital admission at MTRH (n=59) | |
| <1 month | 12 (20%) |
| 1–3 months | 24 (41%) |
| >3 months | 23 (39%) |
| Stage of disease at diagnosis (n=44) | |
| Stage I | 3 (7%) |
| Stage II | 5 (11%) |
| Stage III | 29 (66%) |
| Stage IV | 7 (16%) |
| Health insurance (NHIF) status at diagnosis (n=62) | |
| NHIF | 17 (27%) |
| No NHIF | 45 (73%) |
MTRH, Moi Teaching and Referral Hospital; NHIF, National Hospital Insurance Fund.
Treatment outcomes are summarised in figure 1. Treatment abandonment was the most common treatment failure, which occurred in 22 patients (35%): 9% abandoned prior to treatment and 91% during treatment. The second most common treatment failure was progressive or relapsed disease which affected 14 patients (22%): progressive disease (64%) and relapse (36%). The least common treatment failure was death. Of all nine patients (14%) who died, cause of death was classified as malignancy related (67%) and treatment related (33%). Figure 2 shows the event-free survival curve of all 63 children with NHL.
Figure 1.
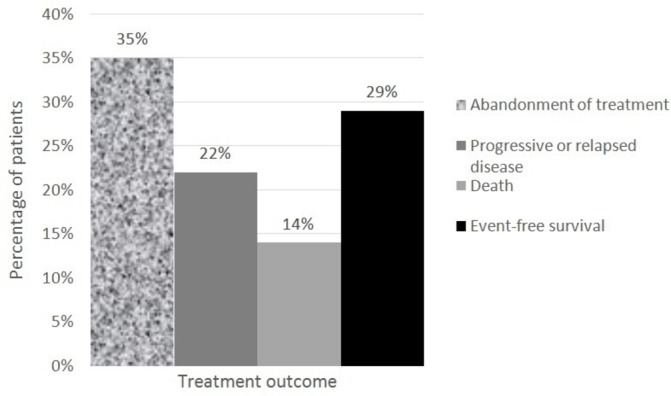
Treatment outcome in children with non-Hodgkin lymphoma (n=63).
Figure 2.
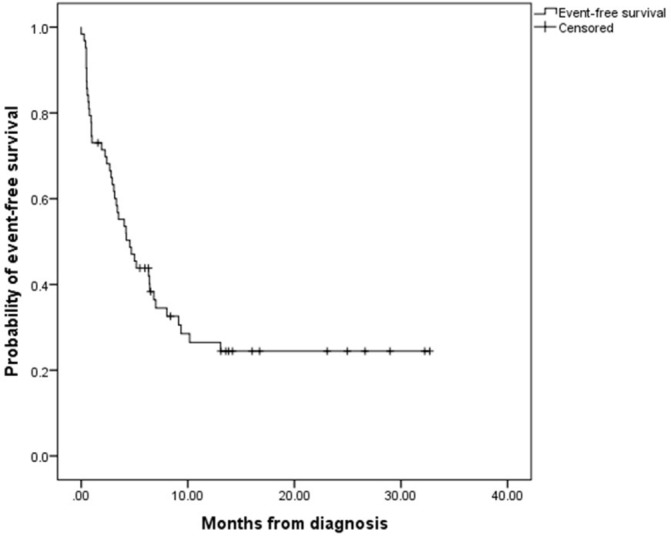
Kaplan-Meier estimates of event-free survival in children with non-Hodgkin lymphoma (n=63). Events included abandonment of treatment, death, and progressive or relapsed disease.
NHIF status at diagnosis was known for 62 children (98%). Of these 62 children, 17 (27%) had NHIF and 45 (73%) had no NHIF at diagnosis. Among those 44 for which disease stage was registered, disease stages III and IV were more often diagnosed in uninsured (n=28/31, 90%) than in insured (n=8/13, 62%) children (p=0.037). Most likely treatment outcome in children with NHIF at diagnosis was event-free survival (n=9, 53%), whereas in children without NHIF at diagnosis it was abandonment of treatment (n=20, 44%). Figure 3 illustrates that treatment outcomes in children with or without NHIF at diagnosis differed significantly (p=0.005). Crude HR for treatment failure was 3.1 (95% CI 1.41 to 6.60, p=0.005) for uninsured versus insured children. Figure 4 shows that event-free survival estimate was significantly higher in children with NHIF at diagnosis than in patients without (p=0.003). Of those children without NHIF at diagnosis, many (80%) enrolled during cancer treatment at MTRH leading the total NHIF registration level to 85%.
Figure 3.
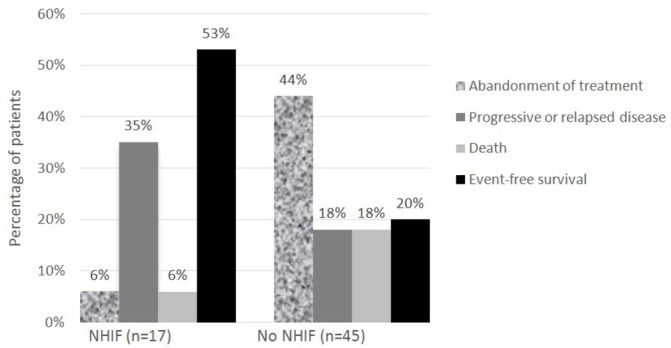
Treatment outcome in children with non-Hodgkin lymphoma per health-insurance (NHIF) status at diagnosis (n=62, P=0.005)
Figure 4.
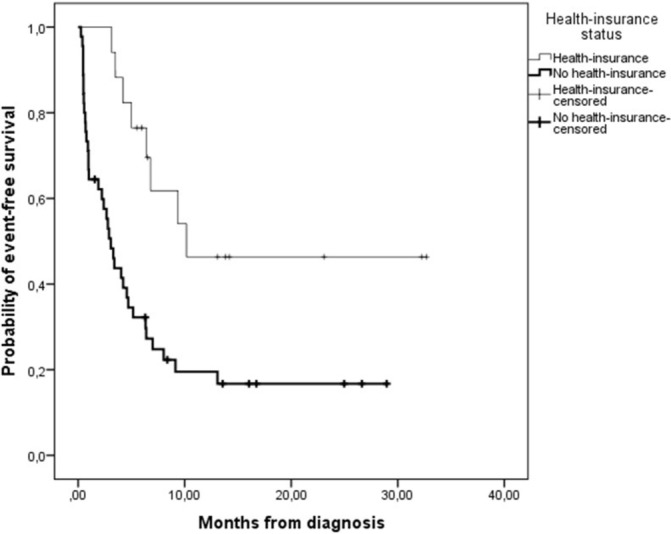
Kaplan-Meier estimates of event-free survival in children with non-Hodgkin lymphoma per health-insurance (NHIF) status at diagnosis (P=0.003). Events included abandonment of treatment, death, and progressive or relapsed disease. Heavy solid line, no NHIF at diagnosis (n=45); solid line, NHIF at diagnosis (n=17); heavy plus, no NHIF at diagnosis censored; plus, NHIF at diagnosis censored.
Following patients’ sociodemographic and clinical characteristics, age, gender, distance to MTRH, duration of symptoms and disease stage at diagnosis did not statistically significantly impact treatment outcomes and event-free survival estimates. Of all these characteristics, only disease stage at diagnosis was a confounder of association between NHIF and survival (adjusted HR=2.4, 95% CI 0.95 to 6.12, p=0.063).
Discussion
This study demonstrates a survival rate of 29% in children with NHL in Kenya which is lower compared with high-income countries who routinely achieve survival rates greater than 80%. Reasons underlying poor cancer survival in low-income settings include delays in health-seeking behaviour, poor nutritional status, scarcity of health facilities, healthcare system delays, treatment costs, inconsistent medication availability, lack of supportive care, lack of government prioritisation of cancer compared with other health issues and foremost treatment abandonment.22–27
Also at MTRH, the main reason for treatment failure was treatment abandonment with 35% of children prematurely stopping conventional medicine. By contrast, in high-income settings toxicity-related death and relapse are most common causes of treatment failure.23 Treatment abandonment seldom occurs in high-income countries, and if it does happen state support and intervention will assure that the child gets cancer treatment.22 23 Unfortunately, treatment abandonment is a critical problem in the rest of the world. Limited financial resources play a crucial role in families’ decision to abandon treatment. In low and middle-income countries social and economic assistance from the state and access to health insurance are often absent or lacking.16 22 23
We found that the vast majority of patients with NHL (71%) had no health insurance at diagnosis, despite the existence of an affordable health insurance in Kenya. At MTRH, treatment outcomes of childhood patients with NHL with or without health insurance at diagnosis differed significantly. The most likely treatment outcome in children with health insurance at diagnosis was event-free survival, whereas in children without health insurance at diagnosis it was treatment abandonment. Event-free survival estimate was significantly higher in children with health insurance at diagnosis than in patients without. With health insurance status making such a significant difference in treatment outcome and survival, there is a strong call for governmental campaigns to emphasise benefits of health insurance so that every Kenyan citizen gets insured.
A previous Kenyan study conducted among children with cancer at MTRH showed that having health insurance leads to shorter delays in health-seeking behaviour.24 Parents are more likely to seek treatment at a conventional healthcare facility if they have health insurance than if they do not have health insurance. In the latter case, families may resort to complementary medicine first. Earlier and adequate health-seeking behaviour leads to early-stage disease at diagnosis which has better prognosis, treatment options and improved survival. Major improvements would be feasible if health insurance was mandatory such that parents would visit conventional healthcare facilities first when their child is sick.15 24–26 Also this study showed that more uninsured than insured children come to the hospital with a substantial delay manifesting in stage III or IV of disease. Disease stage at diagnosis was recognised as a confounder of the association between NHIF and survival. A likely explanation is that families without health insurance report to conventional healthcare at a later stage of disease which limits chances of a positive treatment outcome. Further investigation on a larger group of patients with NHL to explore and consolidate the relationship between NHIF status and disease stage at diagnosis is required.
Worldwide health coverage would contribute to breaking the vicious cycle of ill health and impoverishment of already marginalised populations. For example, poor health of a family member frequently reduces the caregivers’ ability to work and earn daily wages as the patient needs to be accompanied to hospital. A child with cancer can thus drive a family without health insurance into long-term poverty because land and livestock need to be sold, savings used and siblings’ tuition payments stopped to pay for cancer treatment. Consequently, a family loses their means of earning a living and their chance to escape poverty through educational achievements of its young generation. By contrast, insured families are better protected from the financial abyss caused by the disease.27 28 In most low and middle-income countries, the vast majority of the population is poor and uninsured. Improving their access to the health sector through health insurance coverage will importantly prevent a loss of income and positively impact the nation’s economic growth.29
The importance of health coverage is also emphasised by the United Nations General Assembly’s unanimous acceptance of a ground-breaking resolution in which universal health coverage (UHC) is recommended for sustainable development. The General Assembly addresses governments to ‘urgently and significantly scale up efforts to accelerate the transition towards universal access to affordable and high quality healthcare services.’30 31 The aim of UHC is to ensure that all people receive medical care without suffering financially for it. By covering safe, effective, quality and affordable essential healthcare services, medicines and vaccines, UHC importantly reduces the financial risks which are associated with ill health and improves health standards and life expectancy, and protects household incomes.27 30 31 The World Bank supports low and middle-income countries by offering loans, advice and resources in their pursuit of UHC.27
Among our patients with NHL who abandoned treatment, only 9% refused treatment, while 91% dropped out after treatment initiation. Timing of abandonment, either prior or during treatment, depends importantly on accessibility of healthcare services.28 32 If families are denied access when their financial means are limited, more children will abandon treatment immediately after diagnosis without starting treatment. For instance, in India, 67% of childhood patients with cancer refused treatment and 33% departed after initially starting therapy.28 The low number of children refusing treatment at MTRH may be explained by hospital detention practices, which are defined as ‘refusal to release living patients after medical discharge is clinically indicated, or bodies of deceased patients, when families are unable to pay hospital bills.29 33 Children are thus initially admitted and treated. Families are subsequently not allowed to take their children home before medical invoices are covered. This can lead to painful situations, where families are desperately trying to find money while their children are left alone inside hospital. After bills are paid, families will hesitate to bring their child back to hospital for treatment continuation or follow-up with the risk of high medical expenses and detention. Thus, hospital detention practices can further exacerbate treatment abandonment.15 24 29
Male gender accounts for 71% of patients in our study. Globally, NHL is two to three times more common in boys than in girls. Therefore, we cannot relate our findings to gender-biased seeking of healthcare, as has been documented for other malignancies.23 34 In the latter cases, cultural aspects may explain the male predominance. Kenyan culture, for instance, is a paternalistic culture where boys are generally more appreciated than girls. Girls, once married, are not considered part of the family anymore. The father will leave his wealth and shamba (farmland) to his son. Therefore, to continue the family line and tradition, it is important that the boy survives.35 Yet, our NHL male/female ratio of 2.4:1 is in line with results from previous studies.36 37
The main limitations of our investigation were its small sample size and retrospective nature. Some restrictions of retrospective chart reviews need to be taken into account: only pre-existing data can be analysed, it cannot determine causation and some important data may be missing. We learnt that recording of clinical characteristics, such as disease stage at diagnosis, needs to be rigorously improved. Additional research on a larger group of patients with NHL at MTRH is required before definitive conclusions can be drawn.
In conclusion, survival of children with NHL in Kenya is much lower compared with high-income countries. The main reason for treatment failure was abandonment of treatment. Health insurance at diagnosis significantly improved treatment outcome and survival. Based on our study, we recommend that government implements obligatory health insurance for every Kenyan citizen. This can be achieved by making it mandatory to register with NHIF when obtaining birth certificates or national identity cards at the age of 18 years old. This will help reduce the risk of treatment failure, increase NHL survival and protect families from financial suffering.
Acknowledgments
We wish to acknowledge the Doctor to Doctor Program for their logistical support. We also acknowledge all the staff who work in the paediatric oncology unit of MTRH.
Footnotes
Contributors: HAM: data collection, data analysis and interpretation, drafting the article, critical revision of the article, final approval of the version to be published. FN: conception or design of the work, data analysis and interpretation, critical revision of the article, final approval of the version to be published. GO, JS, SM, TV, GJLK: conception or design of the work, critical revision of the article, final approval of the version to be published. SL: data collection, data analysis and interpretation, critical revision of the article, final approval of the version to be published. PMvdV: data analysis and interpretation, critical revision of the article, final approval of the version to be published. SM: conception or design of the work, data analysis and interpretation, drafting the article, critical revision of the article, final approval of the version to be published.
Funding: The study was conducted through a grant from the International Society of Paediatric Oncology.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Our study was approved by the Research Ethical Committee at MTRH.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Aka P, Kawira E, Masalu N, et al. Incidence and trends in burkitt lymphoma in Northern Tanzania from 2000 to 2009. Pediatr Blood Cancer 2012;59:1234–8. doi:10.1002/pbc.24194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mostert S, Njuguna F, Kemps L, et al. Epidemiology of diagnosed childhood cancer in Western Kenya. Arch Dis Child 2012;97:508–12. doi:10.1136/archdischild-2011-300829 [DOI] [PubMed] [Google Scholar]
- 3.Smith MA, Altekruse SF, Adamson PC, et al. Declining childhood and adolescent cancer mortality. Cancer 2014;120:2497–506. doi:10.1002/cncr.28748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown BJ, Bamgboye EA, Sodeinde O. Causes of death in childhood Cancer at the department of paediatrics, University College Hospital, Ibadan, Nigeria. Afr J Med Sci 2008;37:7–13. [PubMed] [Google Scholar]
- 5.Viana MB, Murao M, Ramos G, et al. Malnutrition as a prognostic factor in lymphoblastic leukaemia: a multivariate analysis. Arch Dis Child 1994;71:304–10. doi:10.1136/adc.71.4.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oakhill A, Mann JR. Poor prognosis of acute lymphoblastic leukaemia in Asian children living in the United Kingdom. Br Med J 1983;286:839–41. doi:10.1136/bmj.286.6368.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicsönmez G, Ozsoylu S, Yetgin S, et al. Poor prognosis of childhood acute lymphoblastic leukaemia. BMJ 1983;286:1437 doi:10.1136/bmj.286.6375.1437-c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moleti ML, Al-Hadad SA, Al-Jadiry MF, et al. Treatment of children with B-cell non-Hodgkin lymphoma in a low-income country. Pediatr Blood Cancer 2011;56:560–7. doi:10.1002/pbc.22905 [DOI] [PubMed] [Google Scholar]
- 9.Acquatella G, Insausti CL, García R, et al. Outcome of children with B cell lymphoma in Venezuela with the LMB-89 protocol. Pediatr Blood Cancer 2004;43:580–6. doi:10.1002/pbc.20116 [DOI] [PubMed] [Google Scholar]
- 10.Klumb CE, Schramm MT, De Resende LM, et al. Treatment of children with B-cell non-Hodgkin's lymphoma in developing countries: the experience of a single center in Brazil. J Pediatr Hematol Oncol 2004;26:462–8. doi:10.1097/00043426-200407000-00014 [DOI] [PubMed] [Google Scholar]
- 11.Hesseling P, Broadhead R, Mansvelt E, et al. The 2000 burkitt lymphoma trial in Malawi. Pediatr Blood Cancer 2005;44:245–50. doi:10.1002/pbc.20254 [DOI] [PubMed] [Google Scholar]
- 12.Harif M, Barsaoui S, Benchekroun S, et al. Treatment of B-cell lymphoma with LMB modified protocols in Africa--report of the French-African Pediatric Oncology Group (GFAOP). Pediatr Blood Cancer 2008;50:1138–42. doi:10.1002/pbc.21452 [DOI] [PubMed] [Google Scholar]
- 13.Central Intelligence Agency. The World Factbook. Kenya, 2016. https://www.cia.gov/library/publications/the-world-factbook/geos/ke.html. [Google Scholar]
- 14.NHIF. National Hospital Insurance Fund. Kenya: http://www.nhif.or.ke/healthinsurance/. [Google Scholar]
- 15.Mostert S, Njuguna F, van de Ven PM, et al. Influence of health-insurance access and hospital retention policies on childhood cancer treatment in Kenya. Pediatr Blood Cancer 2014;61:913–8. doi:10.1002/pbc.24896 [DOI] [PubMed] [Google Scholar]
- 16.Njuguna F, Mostert S, Slot A, et al. Abandonment of childhood cancer treatment in Western Kenya. Arch Dis Child 2014;99:609–14. doi:10.1136/archdischild-2013-305052 [DOI] [PubMed] [Google Scholar]
- 17.AMPATH: the Academic Model providing access to Healthcare. http://www.nhif.or.ke/healthinsurance/.
- 18.Dozzo M, Carobolante F, Donisi PM, et al. Burkitt lymphoma in adolescents and young adults: management challenges. Adolesc Health Med Ther 2017;8:11–29. doi:10.2147/AHMT.S94170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngoma T, Adde M, Durosinmi M, et al. Treatment of burkitt lymphoma in equatorial Africa using a Simple three-drug combination followed by a salvage regimen for patients with persistent or recurrent disease. Br J Haematol 2012;158:749–62. doi:10.1111/j.1365-2141.2012.09236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magrath I. Towards curative therapy in burkitt lymphoma: the role of early african studies in demonstrating the value of combination therapy and CNS prophylaxis. Adv Hematol 2012;2012:1–7. doi:10.1155/2012/130680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt's lymphoma. N Engl J Med 2013;369:1915–25. doi:10.1056/NEJMoa1308392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mostert S, Arora RS, Arreola M, et al. Abandonment of treatment for childhood cancer: position statement of a SIOP PODC Working Group. Lancet Oncol 2011;12:719–20. doi:10.1016/S1470-2045(11)70128-0 [DOI] [PubMed] [Google Scholar]
- 23.Arora RS, Pizer B, Eden T. Understanding refusal and abandonment in the treatment of childhood cancer. Indian Pediatr 2010;47:1005–10. doi:10.1007/s13312-010-0172-5 [DOI] [PubMed] [Google Scholar]
- 24.Njuguna F, Martijn H, Langat S, et al. Factors influencing time to diagnosis and treatment among pediatric oncology patients in Kenya. Pediatr Hematol Oncol 2016;33:186–99. doi:10.3109/08880018.2016.1169566 [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues KES, Latorre R, Camargo B. Delayed diagnosis in retinoblastoma. J Pediatr 2004;80:511–6. doi:10.2223/1266 [PubMed] [Google Scholar]
- 26.Chantada G, Fandiño A, Manzitti J, et al. Late diagnosis of retinoblastoma in a developing country. Arch Dis Child 1999;80:171–4. doi:10.1136/adc.80.2.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worldbank. Universal Health Coverage. http://www.worldbank.org/en/topic/universalhealthcoverage.
- 28.Kulkarni KP, Marwaha RK, Trehan A, et al. Survival outcome in childhood ALL: experience from a tertiary care centre in North India. Pediatr Blood Cancer 2009;53:168–73. doi:10.1002/pbc.21897 [DOI] [PubMed] [Google Scholar]
- 29.Mostert S, Njuguna F, Olbara G, et al. Corruption in health-care systems and its effect on cancer care in Africa. Lancet Oncol 2015;16:e394–e404. doi:10.1016/S1470-2045(15)00163-1 [DOI] [PubMed] [Google Scholar]
- 30.UN General Assembly Resolution adopted by the General Assembly on 12 December 2012. Global health and foreign policy A/RES/67/81. http://www.un.org/en/ga/search/view_doc.asp?symbol=A/RES/67/81.
- 31.WHO. Questions and answers on Universal Health Coverage and the post-2015 framework. http://www.who.int/contracting/documents/QandA_UHC_post-2015.pdf.
- 32.Mostert S, Sitaresmi MN, Gundy CM, et al. Comparing childhood leukaemia treatment before and after the introduction of a parental education programme in Indonesia. Arch Dis Child 2010;95:20–5. doi:10.1136/adc.2008.154138 [DOI] [PubMed] [Google Scholar]
- 33.Mostert S, Lam CG, Njuguna F, et al. Hospital detention practices: statement of a global taskforce. Lancet 2015;386:649 doi:10.1016/S0140-6736(15)61495-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce MS, Parker L. Childhood cancer registrations in the developing world: still more boys than girls. Int J Cancer 2001;91:402–6. doi:10.1002/1097-0215(200002)9999:9999<::AID-IJC1048>3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- 35.Moormann AM, Snider CJ, Chelimo K. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic burkitt lymphoma. Curr Opin Infect Dis 2011;24:435–41. doi:10.1097/QCO.0b013e328349ac4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mwanda OW, Rochford R, Moormann AM, et al. Burkitt's lymphoma in Kenya: geographical, age, gender and ethnic distribution. East Afr Med J 2004:S68–77. [DOI] [PubMed] [Google Scholar]
- 37.Hesseling PB, Broadhead R, Molyneux E, et al. Malawi pilot study of burkitt lymphoma treatment. Med Pediatr Oncol 2003;41:532–54. doi:10.1002/mpo.10322 [DOI] [PubMed] [Google Scholar]


