Abstract
Objective
UK guidelines recommend that diagnosis of autism in children requires assessment by a multidisciplinary team. With growing numbers of referrals for assessment, diagnostic services have been under increasing pressure to meet the level of need. This study aimed to explore the number of hours of professional time required to complete such an assessment based on current practice in secondary care child development centres across the UK, and from this we calculate the cost of assessment.
Design
An online questionnaire, using SurveyMonkey.com, was sent to 20 child development centres asking them to retrospectively record team members involved at each stage of assessment and time taken, including report writing and administration for a typical assessment. Costs were estimated based on the hourly rate for each team member, including salary, on-costs and trust overheads.
Results
12 questionnaires (60%) were returned. 10 centres adopted a two-stage approach to assessment with an initial ‘screening’ clinic determining whether the child needed to proceed to full multidisciplinary assessment. Median professional time involved was 13 hours (IQR 9.6–15.5 hours). This resulted in a median cost of £809 ($1213, based on conversion rate £1 equal to US$1.5 (November 2015)), (IQR £684–£925) ($1026–$1388)).
Implications
This study confirms that multidisciplinary diagnostic assessment of a child with possible autism requires significant professional time, with staff costs of approximately £800 ($1200) per child. This does not include costs of intervention, parent psychological education, investigation and assessment and management of comorbidities. If growing waiting times for diagnostic assessment are to be avoided, funding for diagnostic services needs to reflect the human resources required and the resulting costs of that assessment.
Keywords: autism, costing, neurodevelopment, paediatric staffing
What is already known on this topic?
UK (National Institute for Health and Care Excellence) guidelines recommend that diagnostic assessment of a child for possible autistic spectrum disorder should be carried out by a multidisciplinary team.
With increasing incidence of autistic spectrum disorder, there has been increasing demand on diagnostic services in the UK, resulting in long waiting times for assessment.
Traditional funding of child development services by block contract has struggled to respond in a timely manner to increasing demand on these services.
What this study adds?
Most secondary care child development centres taking in part in this study adopted a two-stage process for assessment of a child with possible autistic spectrum disorder.
The full process typically requires around 13 hours of professional time to complete, although initial ‘screening’ assessment only takes 1–2 hours.
This costs around £800 (US$1200) per child for a completed diagnostic assessment.
Introduction
Autism spectrum disorder (ASD) is redefined in the Diagnostic and Statistical Manual of Mental Disorders (DSM), Fifth Edition,1 by the presence of ‘persistent deficits in social communication and social interaction across multiple contexts’ and ‘restricted, repetitive patterns of behavior [sic], interests, or activities’. The International Classification for Diseases (ICD) XI β2 similarly suggests ‘ASD is characterized by persistent deficits in the ability to initiate and sustain reciprocal social interaction and social communication, and by a range of restricted, repetitive, inflexible patterns of behaviour and interests’. Presentation varies significantly, for example, the relative severity of deficits in social communication and repetitive behaviours1 or associated intellectual levels or verbal abilities of the child.1 2 With no confirmatory laboratory test, diagnosis requires building an accurate picture of the child across settings. The combination of complexity and increasing demand for diagnostic assessment for possible ASD means services are coming under increasing pressure, resulting in long waiting times warranting a better understanding of professional time involved alongside resulting financial costs. Additionally, a clearer picture of diagnostic pathways is vital in informing appropriate future provision of services.
UK National Health Service (NHS) recommends diagnostic practice based on National Institute for Health and Clinical Excellence (NICE) guidelines3 developed following rigorous systematic review of the evidence base. Assessment by a multidisciplinary team including a core team of a paediatrician, speech therapist and psychologist3 is recommended as good clinical practice to determine whether a child meets diagnostic criteria in line with DSM IV,4 ICD 105 (as in this study) or now DSM V.1 Teams may be based in secondary care child development centres (CDC), whether in a local hospital or community setting, of which there are 179 in the UK,6 or Child and Adolescent Mental Health Services (CAMHS). Complex cases may also be referred to specialist tertiary centres, responsible for supporting secondary care services.
Alternative explanations of a child’s social communication, and associated comorbidities, may need to be identified, so NICE recommends3 that assessment of neurodevelopmental disorders (eg, developmental coordination disorder), mental and behavioural disorders (eg, ADHD and mood disorder), developmental regression (eg, Rett’s syndrome), ‘maltreatment’ and visual or hearing impairment should be considered. In addition to a core team, NICE advises access to other disciplines, for example, occupational therapy, to ‘construct a profile for each child or young person, for example (their) intellectual ability…speech, language and communication, fine and gross motor skills, and mental and emotional health including self-esteem’.3 Younger children are mostly assessed within CDCs in UK practice, presenting with concerns about neurodevelopment or developmental regression. As children approach adolescence, they often develop secondary mental health difficulties such as depression, and therefore may access child psychiatry and clinical psychology, mainly based in CAMHS services.
Use of validated formal structured history, such as the Autism Diagnostic Interview-Revised,7 and observation for autistic behaviours, for example, Autism Diagnostic Observation Scale (ADOS),8 is encouraged. However, NICE recognises that ‘no single tool alone [has] adequate sensitivity and specificity for diagnosis of autism’.9While some countries have developed their own guidelines,10 11 others, for example, Australia,12 recognise NICE as ‘gold standard’. Most aspire to a multidisciplinary approach, which is not always achievable, such as when performed by a single practitioner or ‘office paediatrician’.10–12
With changes in the way health services are financed in the UK, it was hoped a move to funding specific pathways with a tariff per patient would improve patient care. As Monitor comments:
The design of the payment system influences…quality of NHS care for patients in lots of ways.[T]he payment system…can make sure commissioning groups pay providers enough money to cover the costs of caring for patients…If commissioners pay providers of NHS services too little, they won’t be able to afford to give the high standards of care that patients need and have the right to expect.13
However, community services within the UK NHS, including for ASD, continue to be resourced through block contracts. Budgets are allocated to providers to deliver a service such as a CDC but are less responsive to changing caseloads.
In the absence of national benchmarks for autism costs or agreed tariffs, the primary study aim was to calculate financial costs to the NHS of a typical multidisciplinary diagnostic assessment for a child with possible ASD. To achieve this, an additional main aim was to establish professional time involved in an ‘average’ pathway at secondary care CDCs across the UK. Secondary aims included determining typical numbers of children being seen in each centre, perceived likelihood of receiving a diagnosis of ASD and whether teams felt happy with the pathway offered. Multiple assessments and national numbers for other diagnoses were not considered.
Owing to the questions asked, no hypotheses were made.
Method
An online questionnaire, using SurveyMonkey.com, was sent to 20 CDCs identified through the British Association of Community Child Health Informatics Group (BACCH IG), approximately 11% of the UK total. Consultant paediatricians were approached through BACCH IG where there was an interest in addressing the question of costs via pathways to care. The questionnaire (see box and online supplementary appendix 1) was designed to allow teams to report the amount of time spent by members of a multidisciplinary team in completing full diagnostic assessment. This included space to describe stages of assessment, for example, for teams who run an initial general developmental clinic or for observation in educational settings. Teams also reported on their satisfaction with their pathway. Respondents were all senior community or neurodisability paediatricians within a team. Teams were contacted after initial interest through email and were contacted once more by email if they did not respond. The study took place between January and May 2013.
Box. Main questions in questionnaire (please see online supplementary appendix 1 for full questionnaire).
Name and centre.
What age children do you see for possible autism spectrum disorder (ASD)?
Do you run an initial/triage/screening/stage one clinic to determine whether a child warrants a formal diagnostic assessment for ASD? If so, please quantify professional time involved.
Diagnostic pathway. Do you gather information from home and or educational settings? Please quantify time involved. Please allow for typical travel involved.
Diagnostic clinic. Do you run a specific diagnostic clinic for children with possible ASD? If so please quantify professional time involved. If pathway is same regardless of age please answer as ‘standard pathway’.
Do you run a feedback/support clinic following diagnosis? Please quantify professional time involved.
Do you routinely take a number of clinic visits to reach a diagnosis, over and above initial, diagnostic and feedback clinics?
Roughly how many children referred with possible ASD do you see each year, and how many receive a diagnosis of ASD?
Are you happy with the pathway you offer?
bmjpo-2017-000052supp001.pdf (694.7KB, pdf)
Pathway costs were calculated by multiplying hourly unit costing of the different staff involved, based on ‘Unit Costs of Health and Social Care 2013’,14 by the amount of time each staff member contributed to a typical assessment (see online supplementary appendix 2 for example of calculation for one school age pathway). The unit costing is calculated from salary+salary on costs (14% of salary)+trust overheads+management (20%)+non-staff costs (50%)+capital overheads+travel+training costs.14 The study was approved by Brighton and Sussex Medical School Research Governance and Ethics Committee. Non-parametric statistical analysis was used, with simple descriptive data, including medians and IQRs, as results were not normally distributed and in anticipation of small sample size. Regression analysis was used to examine the relationships between variables.
bmjpo-2017-000052supp002.pdf (179.4KB, pdf)
Results
An encouraging 12 out of 20 (60%) questionnaires were returned (see figure 1 for distribution of centres). One CDC only assessed preschool children, with older children referred to the CAMHS or tertiary centre. Two delivered a service for 0–11 year olds, six 0–16 years and three 0–19 years. Ten out of 12 CDCs provided initial assessment before progressing to full diagnostic assessment, if required. A median of 140 children (IQR 90–177.5) was assessed in each centre annually. In the 10 centres running an initial clinic, a median of 70 (IQR 60–130) from 130 (54%) children progressed from this to full diagnostic assessment. A median of 60 (43%) (IQR 50–125) children entering the pathway received an autistic spectrum diagnosis. Related disorders, for example, language disorder, were diagnosed in approximately 27% of children.
Figure 1.
Distribution of participating centres across the UK.
The median length of time for a full assessment was 13 hours (IQR 9.6–15.5 hours), with similar times for preschool (12.5 hours) and school-age children (13.2 hours). This includes time for initial assessment (median 2 (IQR 1.75–2.5) hours). These figures exclude data from one centre that found it difficult to reflect their pathway using the questionnaire. Between three and seven staff (median of 4 staff in both preschool and school-age pathways with no statistical difference between pathways, P0.74) contributed time to assessment, most frequently a paediatrician and speech therapist and/or clinical psychologist (figure 2).
Figure 2.
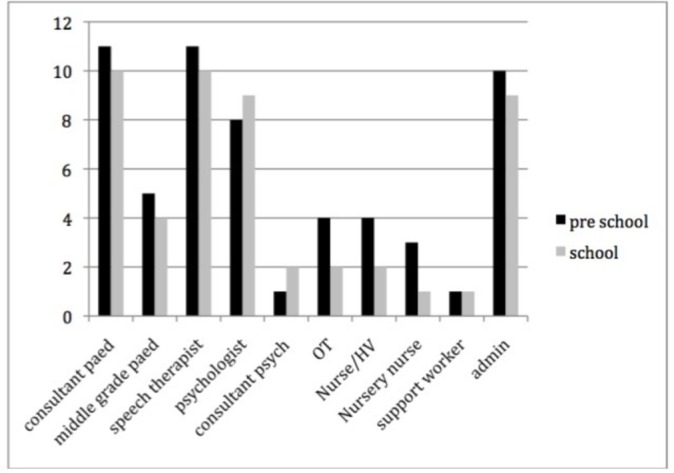
The number of staff from each discipline contributing to diagnostic assessment across the participating centres (OT short for occupational therapists, HV for health visitors).
In 10 centres carrying out an initial assessment, median cost was £147 ($220) (IQR £116–£148). The median cost of a full assessment, including initial appointment, was £809 ($1213), (IQR £684–£925) (see figure 3). Cost was directly related to length of time taken in the assessment (r 0.82, P<0.002, see figure 4) but not to the number of professionals involved (r 0.24, P=0.45). Cost was also closely related to number of doctor hours contributing to the assessment (r 0.84, P=0.001, see figure 5) but not statistically significantly to hours of speech therapy (r 0.02, P=0.9) or clinical psychology (r 0.54, P=0.07). The proportion of children receiving an ASD diagnosis was negatively related to the number of hours taken in assessment (r −0.52, P=0.19), cost of assessment (r −0.16, P=0.71) and number of professionals involved (r −0.43, P=0.25), but none of these reached statistical significance.
Figure 3.
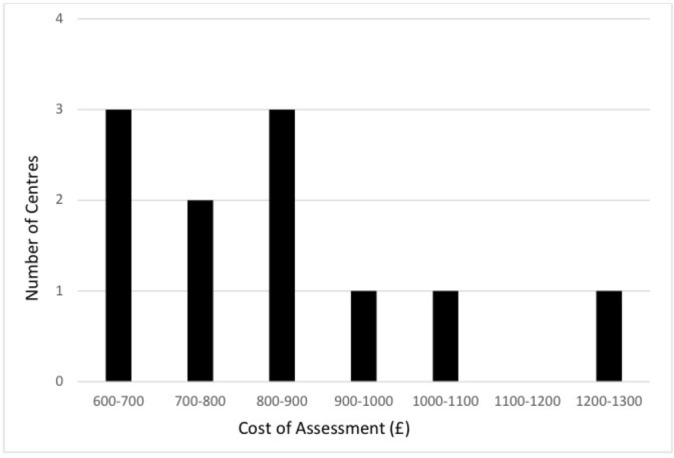
Distribution of average cost across preschool and school-age pathways for each centre. This excludes data from the centre that found it difficult to record their pathways accurately using the questionnaire.
Figure 4.
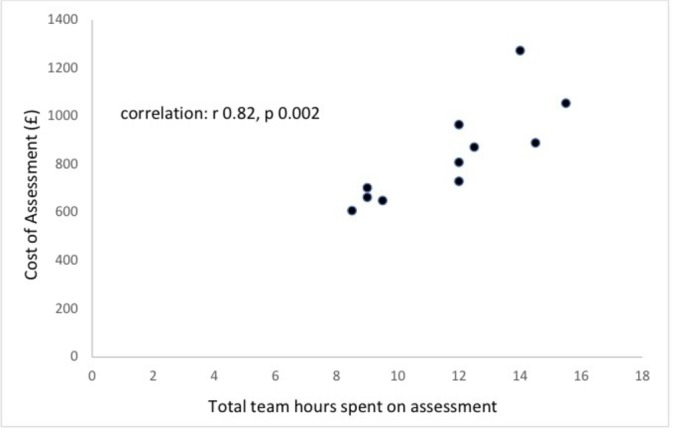
Relationship between cost and total length of time spent completing multidisciplinary assessment.
Figure 5.
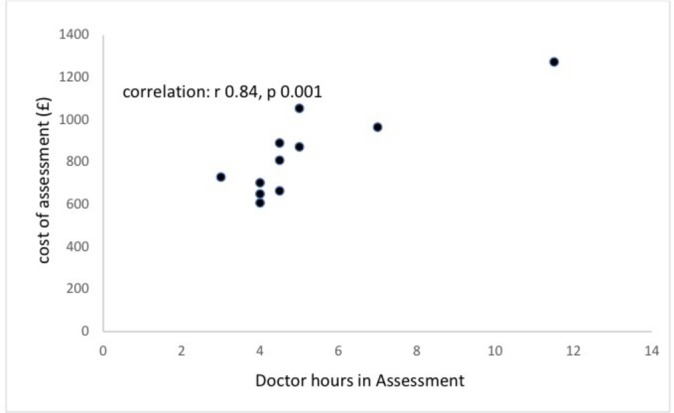
Relationship between cost and total length of time spent by doctors in completing a diagnostic assessment.
Nine respondents commented that available resources governed diagnostic pathways often expressing a need to increase capacity. Two units expressed satisfaction with their current pathways. One unit commented on difficulties in meeting NICE guidelines, while another commented that an increase in referrals has led to shorter assessments.
Three CDCs regularly provided long-term follow-up care for families with a new diagnosis. Two reported continued involvement only for specific issues such as need for medication. Another unit commented on its provision of short-term follow-up but experiencing increasing pressure to halt longer term follow-up. All centres could access other agencies for postdiagnosis input, for example, Early Bird programme.
Discussion
This study reported the amount of time that goes into an assessment of a child with possible autism and suggests most UK centres spend around 13 hours per child, costing between £650 and £1000 ($975–$1500). The most expensive outlier, a school-age pathway, costing £1446 ($2169), was delivered by two consultants, a paediatrician and child psychiatrist, working together. This reflects doctors’ hourly rates being generally twice that of other members of a multidisciplinary team. Consequently, the length of time spent by doctors in diagnostic assessment also appeared to directly influence cost compared with other disciplines, with a similar impact to that of the total length of time spent by the whole team. This would suggest that carrying out a multidisciplinary assessment is a good practice3 and allowing allied health professionals to carry out parts of the assessment not requiring doctor’s skills, for example, observational assessment using ADOS, could save costs. While this study reflects current practice across the NHS, it did not set out to determine relative merits of individual pathways nor scrutinise whether variation in expense gave a more reliable outcome.
This study was based on what teams believed their typical pathway looks like rather than actual patient journeys. There is potential for recall bias, with participating centres potentially over, or under, estimating the length of time taken in a typical assessment. Some centres reported difficulty completing the questionnaire, as it did not fit their pathway. While the method for calculating costings is recommended by health economists in the UK and includes recognition of trust on-costs, overheads and training, it may underestimate additional costs such as report writing (although centres were encouraged to include this), material costs (eg, Autism Diagnostic Interview paperwork) and ongoing service development. The number of responding centres was small, representing 7% of CDCs in the UK. Nevertheless, most centres showed consistency in time taken and cost, with a couple of outliers, with resulting figures of approximately 13 hours professional time, costing £650–£1000 giving an important starting point to inform future funding of diagnostic services for children with possible ASD at secondary care level. Findings are relevant to other health economies planning to adopt a similar evidence-based multidisciplinary approach to diagnostic assessment. Although costing of individual staff involved in this will vary internationally, the amount of professional time involved should be similar.
This study reports the costs of diagnostic assessment but does not include costs of ongoing support and follow-up, for example, genetic screening, completing advice for educational support (eg, education, health and care plan) and assessment and management of comorbidities. For example, an hour-long feedback discussion with a consultant paediatrician might cost £99 (+£45 allowing for half hour in report writing) or £52 (+£26) if this was with a band 7 nurse specialist. With the expansion of potential genetic screening for conditions associated with ASD such as Fragile X syndrome, following the introduction of microarray testing, a growing number of abnormalities, such as 16p11.2 microdeletion,15 have been identified. While this is not yet recommended for all children with ASD, this may become a routine, adding significantly to the overall costs of diagnostic assessment.
Interestingly, the five exemplar pathways in NICE guidelines3 have combined professional times ranging from 16 to 49 hours. While these might reflect ideal practice, respondents appear to reflect a more realistic length of assessment and personnel across the country. Most respondents stated that multidisciplinary pathways depended on resources available to them. Adopting a funding model that reflects realistic costs for conducting a multidisciplinary assessment and that is responsive to rates of referral could allow services to develop their ideal team, rather than make do with what they can afford. With growing capacity issues being identified internationally, this might also enable teams to respond to growing demand while achieving the timeliness and comprehensive assessment required by NICE,3 16 commissioners and families. This might also help to address concerns identified in a recent survey of over 1000 parents around their negative experiences of the diagnostic process and indeed post-diagnostic support.17
While additional funding required to adequately address diagnostic costs may be significant, it is evident that longer term costs to society are more challenging. Early diagnosis and intervention can help, enabling those caring for children to understand and better manage difficulties.18–20 Investing in high-quality initial assessment and support are likely to be offset by long-term savings that could include a reduction in widespread financial impact on families, including reduced employment, and bankruptcy.21–23
A number of studies modelled potential savings made by effective early interventions,24–26 giving projected lifetime individual savings of €1.1 million26 or $187 000 to $203 000 per child between the ages of 3 and 22 years old.24 Annual US costs have been estimated at $126 billion,25while in the UK, annual costs of supporting childhood autism is estimated to be approximately £2.7 billion27 and estimated £25 billion for adults.27 28 NICE suggests the mean annual total cost per child or young person with autism in the UK is £25 400.29 In childhood, the greatest financial burden falls on families and education, whereas in adults the main contributors are supportive living accommodation and individual productivity loss.30
Conclusions
The assessment required to explore possible diagnosis of ASD is complex, ideally demanding the involvement of a multidisciplinary team. This study suggests this typically takes 13 hours of professional time. Based on UK costings, this costs around £650–£1000 ($975–$1500) per child. As this reflects practice based in part on available resources, consideration should be given to basing costs on the NICE exemplars3 rather than who is available locally. Additional resources should be included for intervention, investigation, management of comorbid conditions and ongoing support that would need multiagency approaches including education and social care.
Acknowledgments
We would like to thank Dr A Johnston (Bishop Auckland), Dr Yvonne Parks (East Kent), Dr Sue Zeitlin (Norfolk), Dr Kim Pugh (Durham), Dr S Dipti (Mid Yorkshire), Dr Sian Hull (Ronnie Mackeith CDC), Dr Jo Crane (Chichester), Dr Ben Ko (Newham), Dr Nirmala Sellathurai (Hounslow and Richmond), Dr Karen Horridge (Sunderland) and Dr Sameena Shakoor (Tunbridge Wells) for completing questionnaires for their units. We would particularly like to recognise the support and advice from Drs Fawzia Rahman, Ben Ko and Gabriel Whitlingum from the British Association of Community Child Health informatics group in developing and proceeding with this study. We are also grateful for the advice given by Professor Heather Gage, health economist at University of Surrey, for advice over calculating costings.
Footnotes
Contributors: All five authors contributed to the writing of the paper. IM and WF conceived and lead the study and supervised MG and EG in completing their Independent Research Projects conducted as part of their medical degrees at Brighton and Sussex Medical School and led the writing up of the final paper. MG collected and analysed the initial data and presented this to the peer-reviewed BACCH national conference winning the presentation prize (2013), under supervision as above. This paper was based on his resulting dissertation, along with contributions from EG’s dissertation that continued the research, as well as winning the Brighton and Sussex Medical School silver award for medical student research projects. MG also contributed his prior experience, having worked as an accountant before studying medicine. AG brought the perspective of a commissioner, including a review of the relevant commissioning and costings literature, in writing the paper. IM also brought his knowledge from almost 20 years of clinical experience in diagnostic assessment of children with possible autistic spectrum disorder and his involvement in the BACCH Informatics Group to the study and paper.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The original data for individual centres are held by Dr Ian Male and Dr William Farr. Analysis of all data is contained in this paper. No additional data are available.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fifth Edition Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 2.World Health Organization. International classification of diseases XI beta draft: 6A02 Autism spectrum disorder. http://apps.who.int/classifications/icd11/browse/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f437815624 (accessed 5 Sep 2017).
- 3.National Institute for Health and Care Excellence. Autism: recognition, referral and diagnosis of children and young people on the autism spectrum (CG128. London, UK: National Institute for Health and Care Excellence, 2011. [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Fourth Edition Washington DC: American Psychiatric Association, 1994. [Google Scholar]
- 5.WHO. The ICD-10 classification of mental and behavioural disorders Clinical descriptions and diagnostic guidelines. Tenth edition Geneva: World Health Organization, 1992. [Google Scholar]
- 6.British Association of Community Child Health and Royal College of Paediatrics and Child Health. Covering all bases. community child health: a paediatric workforce guide. London, UK: RCPCH, 2017. [Google Scholar]
- 7.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994;24:659–85. doi:10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- 8.Lord C, Rutter M, Goode S, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord 1989;19:185–212. doi:10.1007/BF02211841 [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence. Evidence update 40; autism diagnosis in children and young people. Manchester, UK: National Institute for Health and Care Excellence, 2013. [Google Scholar]
- 10.American Academy of Neurology. Practice parameter: screening and diagnosis of autism. St Paul, MN: AAN Quality Standards Subcommittee, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Johnson CP, Myers SM. American Academy of Pediatrics Council on Children With Disabilities. Identification and evaluation of children with autism spectrum disorders. Pediatrics 2007;120:1183–215. doi:10.1542/peds.2007-2361 [DOI] [PubMed] [Google Scholar]
- 12.Taylor L, Brown P, Eapen V, et al. Autism spectrum disorder diagnosis in Australia: are we meeting best practice standards? Brisbane, Aus: Autism Co-operative Research Centre, 2016. [Google Scholar]
- 13.Monitor and NHS England. Towards an NHS payment system that does more for patient. http://www.monitor.gov.uk/regulating-health-care-providers-commissioners/regulating-prices-nhs-funded-care/towards-nhs-payment-system-does-more-patients (accessed 7 Jan 2014).
- 14.Curtis L, Unit costs of health and social care 2013. Canterbury, UK: Personal Social Services Research Unit, University of Kent, 2013. [Google Scholar]
- 15.Weiss LA, Shen Y, Korn JM, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med 2008;358:667–75. doi:10.1056/NEJMoa075974 [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence. QS51 Autism quality standard. Manchester, UK: National Institute for Health and Care Excellence, 2014. [Google Scholar]
- 17.Crane L, Chester JW, Goddard L, et al. Experiences of autism diagnosis: a survey of over 1000 parents in the United Kingdom. Autism 2016;20:153–62. doi:10.1177/1362361315573636 [DOI] [PubMed] [Google Scholar]
- 18.Diggle T, McConachie HR, Randle VR. Parent-mediated early intervention for young children with autism spectrum disorder. Cochrane Database Syst Rev 2003;1:CD003496 doi:10.1002/14651858.CD003496 [DOI] [PubMed] [Google Scholar]
- 19.Farmer J, Reupert A. Understanding autism and understanding my child with autism: an evaluation of a group parent education program in rural Australia. Aust J Rural Health 2013;21:20–7. doi:10.1111/ajr.12004 [DOI] [PubMed] [Google Scholar]
- 20.Gura GF, Champagne MT, Blood-Siegfried JE. Autism spectrum disorder screening in primary care. J Dev Behav Pediatr 2011;32:48–51. doi:10.1097/DBP.0b013e3182040aea [DOI] [PubMed] [Google Scholar]
- 21.Sharpe DL, Baker DL. Financial issues associated with having a child with Autism. J Fam Econ Issues 2007;28:247–64. doi:10.1007/s10834-007-9059-6 [Google Scholar]
- 22.Cidav Z, Marcus SC, Mandell DS. Implications of childhood autism for parental employment and earnings. Pediatrics 2012;129:617–23. doi:10.1542/peds.2011-2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavelle TA, Weinstein MC, Newhouse JP, et al. Economic burden of childhood autism spectrum disorders. Pediatrics 2014;133:e520–e529. doi:10.1542/peds.2013-0763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobson JW, Mulick JA, Green G. Cost-benefit estimates for early intensive behavioral intervention for young children with autism—general model and single state case. Behav Interv 1998;13:201–26. doi:10.1002/(SICI)1099-078X(199811)13:4<201::AID-BIN17>3.0.CO;2-R [Google Scholar]
- 25.Mandell DS. Understanding and addressing the impact of autism on the family. LDI Issue Brief 2012;17:1–4. [PubMed] [Google Scholar]
- 26.Peters-Scheffer N, Didden R, Korzilius H, et al. Cost comparison of early intensive behavioral intervention and treatment as usual for children with autism spectrum disorder in The Netherlands. Res Dev Disabil 2012;33:1763–72. doi:10.1016/j.ridd.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 27.Knapp M, Romeo R, Beecham J. Economic cost of autism in the UK. Autism 2009;13:317–36. doi:10.1177/1362361309104246 [DOI] [PubMed] [Google Scholar]
- 28.Reed P, Osborne LA. Diagnostic practice and its impacts on parental health and child behaviour problems in autism spectrum disorders. Arch Dis Child 2012;97:927–31. doi:10.1136/archdischild-2012-301761 [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence. CG170 Autism - management of autism in children and young people: costing statement. Manchester, UK: National Institute for Health and Care Excellence, 2013. [Google Scholar]
- 30.Buescher AV, Cidav Z, Knapp M, et al. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr 2014;168:721–8. doi:10.1001/jamapediatrics.2014.210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2017-000052supp001.pdf (694.7KB, pdf)
bmjpo-2017-000052supp002.pdf (179.4KB, pdf)


