Abstract
Introduction
Non-alcoholic fatty liver disease is the commonest cause of liver disease worldwide, and is rapidly becoming the leading indication for liver transplantation.
Sources of data
Original articles, reviews and meta-analyses, guidelines.
Areas of agreement
NAFLD strongly correlates with obesity and insulin resistance; currently, the best management strategy is weight loss and treatment of the metabolic syndrome.
Areas of controversy
Recent data suggest that the presence of fibrosis and not non-alcoholic steatohepatitis (NASH) is the predictor of clinical outcome.
Growing points
Many phase 2 and 3 trials are underway. Drugs hoped to be effective are obeticholic acid, elafibranor, glucagon-like peptide-1 analogues and CCR2/5 inhibitors.
Areas timely for developing research
Improved understanding of the pathophysiology of NAFLD should help us identify which patients progress to significant liver disease and to develop therapies to target this population.
Keywords: non-alcoholic fatty liver disease, cardiovascular disease, NASH, fibrosis, metabolic syndrome, obesity, assessment, treatment
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the commonest cause of liver disease in Western countries, with an overall prevalence of 25% in the general population1 increasing to 70% in the obese population2 and those who have Type 2 diabetes mellitus (T2DM).2,3 Moreover, the number of affected individuals is expected to increase over the forthcoming years,4 in line with increasing obesity due to the adoption of a high-fat diet and sedentary lifestyle. In the USA, it has become the second commonest cause for liver transplantation and is likely to become the leading cause in the next 10 years.5 This review will cover what is already known about the disease, current management strategies and discuss areas of contention requiring further research and development.
Pathophysiology
Free fatty acid (FFA) and hepatic triglyceride (TG) accumulation is a cardinal feature of NAFLD, and commonly occurs in the setting of insulin resistance and obesity. Liver injury usually occurs in the presence of these features, mediated by inflammatory cytokines, mitochondrial dysfunction secondary to nutrient excess and oxidative stress.6,7 The extent of hepatic inflammatory damage is also influenced by extrahepatic factors such as adipose tissue signalling,7 the effect of gut microbiota8 and polymorphisms such as PNPLA3 and TLF613 which are currently being explored.
In most patients, the only response to obesity/insulin resistance is simple steatosis, or non-alcoholic fatty liver (NAFL), which is defined as steatosis ≥5% and is believed to follow a relatively benign course. However, in a proportion of patients with steatosis1 a more profound inflammatory liver damage occurs, termed non-alcoholic steatohepatitis (NASH), which is characterized by the presence of lobular inflammation and hepatocellular damage (ballooning). This carries a worse prognosis, with 40% developing progressive fibrosis leading to cirrhosis in 10–27%, and hepatocellular carcinoma (HCC) in about 4–27% of those with cirrhosis.1,9,10
NAFLD is also an independent risk factor for cardiovascular disease (CVD) and diabetes mellitus,6 and indeed, ischaemic heart disease and stroke are the leading cause of morbidity and mortality in patients with NAFLD.9
Areas of controversy
How important is NASH?
NASH reflects hepatocellular damage and often the commencement of fibrosis progression and yet several long-term outcome studies have suggested that it is the fibrosis stage, rather than the presence of NASH or an elevated NAFLD activity score (NAS) that predicts patient outcomes (see Table 1).11,12 This may be a reflection of retrospective studies with insufficient power and/or it may be that NASH is a more dynamic entity which may spontaneously resolve as opposed to fibrosis, the presence of which is more intractable.
Table 1.
NASH CRN histological scoring system
| NAFLD activity score (NAS) (0–8) |
| Sum of scores for steatosis, lobular inflammation and hepatocellular ballooning |
Steatosis (0–3)
|
Lobular inflammation (0–3)
|
Hepatocyte ballooning (0–2)
|
Score
|
| Fibrosis stage |
1 Perisinusoidal or periportal
|
| 2 Perisinusoidal and portal/periportal fibrosis |
| 3 Bridging fibrosis |
| 4 Cirrhosis |
Growing points
It is likely that certain single nucleotide polymorphisms (SNPs) predispose some individuals to NAFLD. Genome-wide association studies have identified several potentially important genetic variants; the polymorphism seen in patatin-like phospholipase domain-containing 3 (PNPLA3) and farnesyl diphosphate farnesyl transferase-1 (FDFT-1) appears to be most significant. A non-synonymous SNP, rs738409 (c.444 C > G, I148M) in PNPLA3, encoding the adiponutrin protein, is linked to increased hepatic TG content and increased severity of NASH and fibrosis in NAFLD.13 Three other SNPs have been associated with the lobular inflammation phenotype: SNP rs1227756 on chromosome 10 in the COL13A1 (and collagen, type XIII, α 1) gene, rs6591182 on chromosome 11 and rs887304 on chromosome 12 in the EF-hand calcium-binding domain 4B(EFCAB4B) gene, and another SNP in transmembrane 6 superfamily member 2 (TM6SF2) (rs58542926 c.449 C > T, E167K) also has a strong association with NAFLD and disease progression to fibrosis and cirrhosis.13,14 It is therefore possible that in future we will be able to risk stratify patients according to the presence of genetic polymorphisms.
Recently, gut microbiota has been shown to have a potential role in the development of steatohepatitis and fibrosis in NAFLD. Lipopolysaccharides (LPSs) from Gram-negative gut microflora are absorbed into intestinal capillaries and enter the portal system, activating toll-like receptors (TLRs) on hepatocytes, Kupffer cells and hepatic stellate cells and exerting a pro-inflammatory effect. The clearance of LPS is believed to be impaired in NAFLD, leading to a cascade of bacterial overgrowth, increased intestinal permeability and stimulation of inflammatory cytokines and chemokines, resulting in hepatic injury and fibrosis.8,15 There is particular interest in Porphyromonas, a Gram-negative coccus that has been associated with several components of the metabolic syndrome, as well as complications of chronic liver disease, but more work is needed to establish its exact role in the pathogenesis of human NASH8.
Assessment
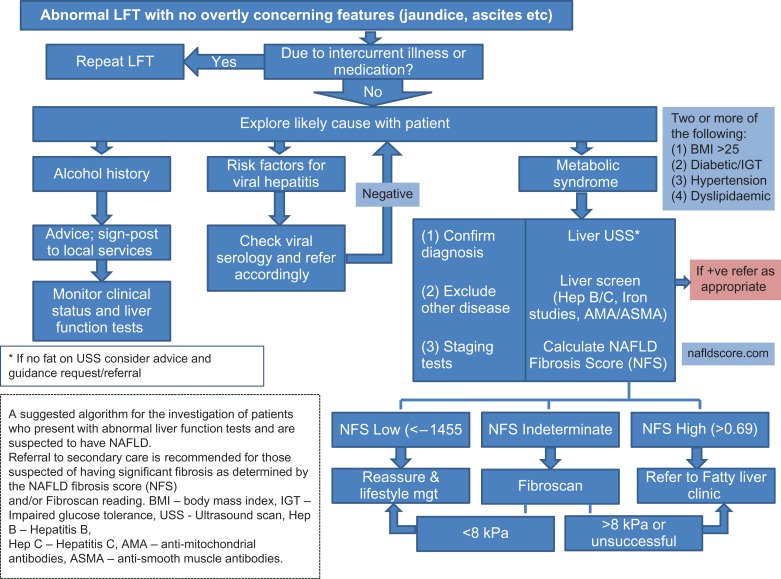
In a clinical setting, it is important to identify those patients that are at risk of progressive liver fibrosis, as these individuals will require regular monitoring, lifestyle interventions and management of their cardiovascular risk factors. Notably, most subjects with NAFLD are generally asymptomatic, with the diagnosis often made following an incidental finding of a fatty liver on ultrasound scan (USS) or abnormal LFTs.16 Figure 1 illustrates a suggested pathway for patients presenting with abnormal LFTs who are suspected to have NAFLD.
Fig. 1.
A suggested algorithm for the investigation of patients who present with abnormal liver function tests and are suspected to have NAFLD. Referral to secondary care is recommended for those suspected of having significant fibrosis as determined by the NAFLD fibrosis score (NFS) and/or fibroscan reading. BMI, body mass index; IGT, impaired glucose tolerance; USS, ultrasound scan; Hep B, hepatitis B; Hep C, hepatitis C; AMA, anti-mitochondrial antibodies; ASMA, anti-smooth muscle antibodies
Serum markers
Levels of serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) are usually increased up to 1.5- to 4-fold but rarely exceed five times the upper limit of normal in the setting of NAFLD. Gamma glutamyl transpeptidase (GGT) and alkaline phosphatase levels may also be elevated, but the serum prothrombin time, bilirubin level and serum albumin level are normal, except in patients with NAFLD-associated cirrhosis. About a quarter of NAFLD patients may have antinuclear antibodies (ANAs) in low titres (<1:320), and serum ferritin level may be raised in 20–50% of NAFLD patients, which is often associated with more advanced disease.9 Plasma cytokeratin-18 (CK-18) is a filament protein in the liver, with caspase-cleaved fragments released into blood stream following hepatocyte injury and apoptosis as seen in the setting of NASH. Levels of CK-18 fragments have been shown to correlate with histologically confirmed NASH in several groups (area under the receiver operated curve (AUROC) of 0.83 and sensitivity of 77%), although it is not clear whether they have the precision to have a diagnostic role or help monitor response to therapy.17,18
The enhanced liver fibrosis (ELF) test combines three candidate serum biomarkers for fibrosis: hyaluronic acid (HA), procollagen III amino-terminal peptide (PIIINP) and tissue inhibitor of metalloproteinase 1 (TIMP-1), which have been shown to correlate with the level of liver fibrosis seen histologically. A cut-off of 10.51 has been demonstrated to have a sensitivity of 100% and a specificity of 98% for detecting advanced fibrosis19; it is likely that ELF testing will be incorporated into upcoming UK guidelines to be used as a screening tool in the primary-care setting.
Where NAFLD is detected, a liver screen is generally performed to exclude autoimmune, viral and genetic causes followed by an assessment to determine the presence of NASH or fibrosis in order to risk stratify the patient for progression of liver disease.
Imaging for steatosis and inflammation
Ultrasound scan (USS) is the commonest modality for diagnosing liver steatosis, as defined by hyper-echogenicity of the liver parenchyma relative to the kidney or spleen,20 and is widely used due to its simplicity, non-invasive nature and low cost.21 It is, however, highly operator-dependent, non-reproducible and can be limited by abdominal gas or patient body habitus, but more importantly it is unable to distinguish simple steatosis from advanced fibrosis or cirrhosis.20
The use of the FibroScan® device with the controlled attenuation parameter (CAP) facility can also be used to assess hepatic steatosis. Ultrasound signals acquired by the FibroScan® are attenuated by liver fat which can be measured using a standard probe, giving a value between 100 and 400 dB/m.22 One prospective study in 153 patients compared the percentage of steatosis on liver biopsy with CAP readings found that using a cut-off of 283 dB/m, the CAP was 76% sensitive, 79% specific and had positive and negative predictive values of 87% and 64%, respectively. The AUROCs of the CAP for ≥5%, >;33% and >66% steatosis in this study were 0.79, 0.76 and 0.70, respectively.23 A larger study by de Ledinghen et al. compared CAP readings with histology in 440 patients and had similar finding grades of steatosis (>10%, >33% and >66%). AUROCs were 0.79 (95% CI, 0.74–0.84; P < 0.001), 0.84 (95% CI, 0.80–0.88; P < 0.001) and 0.84 (95% CI, 0.80–0.88; P < 0.001), respectively.24 In both studies only the M probe was used, and the failure rate for those with a BMI > 40 kg/m2 was 58.4%,23,24 although an XL probe is now available which has a lower failure rate and has similar accuracy in pilot studies.25
Magnetic resonance imaging (MRI) techniques and magnetic resonance spectroscopy (MRS) have been shown to detect lower levels of steatosis (<5) as well as identify changes in fat content accurately. Magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF) is a novel, image-based modality that permits quantification of the entire fat content of the liver, and which correlates strongly with MRS measured liver fat and histologically determined steatosis grade.26 Multi-parametric magnetic resonance (MR) imaging is another non-invasive technique under development and involves a three-stage process: T1 mapping for fibrosis/inflammation imaging, T2 mapping for liver iron quantification and proton magnetic resonance spectroscopy (1H-MRS) for liver fat quantification. The results allow quantification of hepatic fibrosis, iron and steatosis and in preliminary studies predict clinical outcomes in patients with chronic liver disease.27,28
Imaging for fibrosis
Transient elastography (TE), through assessment of liver stiffness measurement (LSM), is widely available in most secondary or tertiary centres for the assessment of liver fibrosis.29 Several studies have provided moderate quality evidence for the diagnostic accuracy of TE over a range of thresholds, and an XL probe is being validated for use in obese subjects. Wong et al. demonstrated a sensitivity of 91% and a specificity of 75% in the detection of significant (≥F3) fibrosis using a cut off of >7.9 kPa.30 The same group confirmed efficacy to detect ≥F3 fibrosis in those with a BMI ≥30 with a sensitivity and a specificity of 90% using a cut off of 7.2 kPa.31 Acoustic radiation force impulse (ARFI) imaging (ACUSON S2000™; Siemens Medical Solutions, Mountain View, CA, USA) is another ultrasound-based method for the assessment of liver stiffness based on the measurement of shear waves. Preliminary studies have shown that using a threshold of 4.24 kPa, advanced fibrosis (Stage 3 or 4) is detected with a sensitivity of 90% and a specificity of 90%. It is comparable to TE, and has the possible benefit that it can be undertaken during a routine US assessment.32,33
Magnetic resonance elastography (MRE) has also been shown to be useful for the detection of significant fibrosis (Stage 2 or above) and cirrhosis in all aetiologies of liver disease, including NAFLD.34,35 For detection of significant fibrosis, MRE showed 100% sensitivity, 96.5% specificity and 98.9% accuracy and 88.2% sensitivity, 91.1% specificity and 93.5% accuracy for cirrhosis.34 The ability to provide a summative assessment of fibrosis of the liver is a major advantage, although as with most elastography modalities the presence of significant inflammation can increase elastography readings.35
Liver biopsy
Liver biopsy remains the gold standard for both diagnosis and staging of disease, with NASH as defined by the presence of hepatocellular injury (ballooning, apoptosis/necrosis, presence of Mallory's hyaline and giant mitochondria), and inflammation (neutrophil and other inflammatory cell infiltrate),36 being detected solely on histology. Several scoring systems exist to help quantify these histological changes, the commonest being the NASH Clinical Research Network (CRN) classification which encompasses the NAS, which grades steatosis, lobular inflammation and hepatocellular ballooning, and a 0–4 score for liver fibrosis (see Table 1). More recently, the steatosis, activity, fibrosis (SAF) score was proposed37, which aims to accurately diagnose NASH and reduces inter-observer variability by further defining ballooning according to the size and shape of hepatocytes, and lobular inflammation according to the number of inflammatory foci per lobule. When used in the fatty liver inhibition of progression (FLIP) algorithm, patients can be further divided into those with NASH and those with simple steatosis.37 Liver histology remains the mainstay for outcomes in clinical trials and is required for seeking regulatory approval of new therapies.
Areas of controversy
Should we screen for NAFLD?
Many physicians advocate screening for NAFLD, and multiple methods have been proposed for this purpose, including imaging techniques such as USS, MRI and TE, or using blood tests such as the fatty liver index or AST/ALT ratio. Early identification of patients with or at risk of NAFLD may facilitate beneficial changes in lifestyle and prompt aggressive treatment of features of the metabolic syndrome, thereby reducing long-term morbidity and mortality from both liver disease and CVD. However, given the high prevalence of NAFLD (7–90% depending on the population and screening tool used),1 limited treatment options and the significant financial burden involved in screening, robust cost-effectiveness analyses are necessary to support this approach.38
Treatment
Lifestyle modification
Unhealthy diets such as those rich in fructose, trans-fatty acids and saturated fat are believed to be associated with the development of NAFLD.39 Dietary sugars such as fructose are used as a substrate for lipogenesis leading to hepatic fatty infiltration, inflammation and possibly fibrosis. Fat consumption, especially cholesterol, and trans or saturated fatty acids have also been shown to be steatogenic and seem to increase visceral adiposity.40 A recent review of dietary interventions in NAFLD suggested that restriction and modulation of simple and high glycaemic carbohydrates and total and saturated fats can improve metabolic parameters such as insulin resistance, decrease liver enzyme levels and reduce the grade of steatosis, independent of weight loss.41 However, few studies included liver biopsies, none were randomized control trials, and the authors were unable to conclude that benefits of dietary modification were truly independent of weight loss. Lifestyle modification, if successfully implemented, can result in weight loss with improvements in all histological aspects of NAFLD. A large prospective cohort study by Vilar-Gomez et al. investigated the effect of various degrees of weight loss on liver histology in 261 patients, and found that improvements in inflammation (resolution of NASH or reduction in NAS) correlated with the magnitude of weight loss.41 Notably, a greater degree of weight loss (≥10%) was required for improvement in inflammation in those patients deemed higher risk at baseline (female sex, fasting glucose > 5.5 mmol/L, many ballooned cells at baseline, BMI > 35). Furthermore, those achieving ≥10% weight reduction were also seen to have regression in fibrosis.41 One of the major challenges with lifestyle change once achieved is being able to sustain it for the longer term which is lacking in studies thus far.
It is likely that a reduction in calorific intake to bring about weight loss is the most beneficial dietary modification in NAFLD, and there is little evidence to favour one dietary intervention over another. In fact there are no RCTs, systematic reviews or comparative prospective cohort studies investigating diet alone, but several trials have shown that dietary intervention in addition to exercise appears to be the most effective.42
Exercise
Current obesity guidelines recommend 30 min of moderate exercise five times weekly43 to aid weight loss and improve cardiovascular health. However, there is no consensus as to what the ideal duration or intensity is for NAFLD, and both moderate-intensity aerobic and resistance training have been shown to reduce intrahepatic lipid (IHL) independent of weight loss and dietary modification.44,45 One study also showed evidence for histological improvements in patients with NASH following a 24-week moderate-intensity aerobic programme, although greater benefits were seen in those who also made dietary modifications.46 Most studies involve regimens of exercise for up to 60 min thrice weekly, much less than the guidelines for obesity. However, in most studies, the exercise was not monitored and so true level of participation is unknown.42
There is increasing interest in high-intensity interval training (HIIT), a modified form of sprint interval training using high-intensity bouts of exercise followed by recovery periods, which has been proposed as a less time-consuming alternative to continuous moderate-intensity alternatives.47 Studies have demonstrated at least equivalent if not greater improvements in cardiovascular fitness with HIIT compared to moderate-intensity exercise in a broad range of populations, including those with obesity and the metabolic syndrome.48 A meta-analysis of HIIT also showed significant improvements in fasting glucose and glycated haemoglobin A1c (HBA1c) in this subgroup of volunteers,49 suggesting potential improvements in insulin sensitivity. A recent study of HIIT in NAFLD showed a significant improvement in IHL, but no significant changes in measurements of insulin resistance (HBA1c, 2-h insulin, HOMA2-ß and HOMA2-S) following a thrice weekly 30 min HIIT intervention for 12 weeks.50
Diet supplements/probiotics
Consumption of omega-3 fatty acid has been found to be low in patients with NAFLD,51 and there have been several randomized control studies of the benefits of omega-3 polyunsaturated fatty acid (PUFA) supplementation. They are believed to alter hepatic gene expression, promote fatty acid oxidation, reduce inflammation and improve insulin sensitivity.52 A recent meta-analysis concluded that there were small but significant improvements in hepatic steatosis and a trend towards reduced ALT in adults with omega-3 fatty acid supplementation; however, the quality of evidence was low and there was heterogeneity in the type and dosage of supplements used.53 Notably, dietary and lifestyle interventions were made in some but not all of the studies.53 Similar results have been seen in children and young people; a double-blind, placebo-controlled randomized trial in 51 children found that liver fat measured by MRI was reduced by 53.4% (95% CI, 33.4–73.4) in the docosahexaenoic acid (DHA, an omega-3 fatty acid) group, as compared with 22.6% (6.2–39.0) in the placebo group (P = 0.040).54 Although reports of side effects and adverse events were low in both children and adults, long-term data are lacking, and more evidence is required before routine use can be recommended.
Many probiotic formulae have been studied in an attempt to target potential imbalance in gut microbiome described above, and have shown some success in improving hepatic steatosis, ALT levels and TE scores15 in adults. Larger studies are needed to confirm these findings, and describe their role and ideal dosage in NAFLD.
Alcohol—to drink or not to drink?
Advice on alcohol consumption in the setting of NAFLD is controversial. Whilst there are data suggesting that modest consumption (1 unit/day) is associated with a reduced prevalence of NAFLD55 and CVD,56 other studies refer to the harmful synergy between alcohol and obesity.57 Pragmatically, most recommend consumption within standard limits with the exception of those with advanced fibrosis in whom abstinence is advised.
Caffeine
For some time, caffeine has been believed to be hepatoprotective, although its potential role in NAFLD has been unclear. A recent meta-analysis of four cross-sectional and two case–control studies concluded that caffeine from coffee was associated with reduced prevalence of hepatic fibrosis in patients with NAFLD.58 More studies are needed before recommendations could be made regarding ideal daily consumption.
Pharmacotherapy
There are currently no approved pharmacotherapies for NAFLD, with the main focus being the management of components of the metabolic syndrome such as insulin resistance, hypertension and hyperlipidaemia. Hypertension and hyperlipidaemia should generally be managed according to local guidelines in the recognition that statins are not only safe in NAFLD but are associated with a reduced mortality.12,59 There are no particularly favoured agents for control of hypertension, although previous studies had suggested that angiotensinogen-receptor blockers may have additional anti-fibrotic effects.60
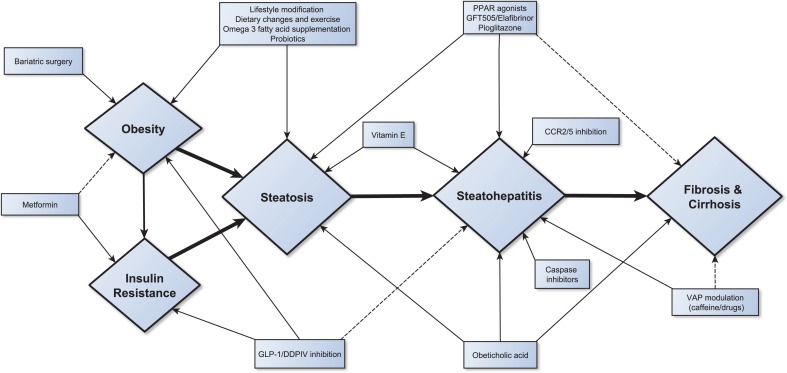
A range of medications have been studied specifically in NAFLD with some proceeding into late-phase trials. Figure 2 summarises potential therapies and indicates the proposed mechanism of action.
Fig. 2.
Schematic for NAFLD treatment
Metformin is the first-line agent for T2DM, and reduces the risk of all diabetes-related end-points including microvascular disease, myocardial infarction, large vessel disease and cardiovascular mortality, in addition to aiding weight loss.61 Although studies have not demonstrated any improvements in liver enzymes or liver histology, there is epidemiological evidence to suggest that it is associated with a reduced incidence of liver and non-liver malignancies including HCC in those with NASH cirrhosis by as much as 7%.62
Pioglitazone
Pioglitazone improves insulin sensitivity, reduces hepatic steatosis, inflammation and to a lesser degree fibrosis63 in patients with NASH, and has been shown to result in an 18% reduction in death, myocardial infarction and stroke in patients with T2DM.64 The PIVENS trial assigned 247 non-diabetic adults with NASH to receive pioglitazone, vitamin E or placebo, for 96 weeks. The primary outcome was a significant change in histological features of NASH, as assessed with the use of the NASH CRN classification. Whilst pioglitazone did not meet its primary end-point,65 serum alanine and aspartate aminotransferase levels were reduced (P < 0.001), and there was a reduction in hepatic steatosis (P < 0.001) and lobular inflammation (P = 0.004), but not in fibrosis scores (P = 0.12 for pioglitazone). Subsequent meta-analyses have also demonstrated efficacy in inducing resolution of NASH63. However, subjects in the PIVENS trial who received pioglitazone gained more weight than did those who received vitamin E or placebo,65 a side effect seen in several other studies. Furthermore, concerns regarding the long-term safety of pioglitazone have limited its use. Two meta-analyses have found an increased risk of congestive cardiac failure, despite reductions in other cardiovascular mortality. In the study by Lincoff et al., heart failure was reported in 200 (2.3%) of pioglitazone-treated patients compared with 139 (1.8%) control patients (HR, 1.41; 95% CI, 1.14–1.76; P = 0.002).64,66 Concerns have also been raised regarding the risk of bladder cancer, following a study demonstrating relative odds ratio of 4.30 (95% CI, 2.82–6.52) for pioglitazone compared with other antidiabetic medications, based on adverse event reporting to the US Food and Drug Administration (FDA) between 2004 and 2009.67 There is a possible reduction of bone density with pioglitazone; thiazolidinedione use causes peroxisome proliferator-activated receptor gamma (PPAR-γ) activation which increases bone resorption; while decreasing bone formation, a significant concern as those with diabetes are already at increased risk of osteoporosis.68
Liraglutide
Liraglutide is a GLP-1-receptor agonist approved for use in diabetes, which has been shown to induce improvements in peripheral, hepatic and adipose insulin resistance, alongside reductions in de novo lipogenesis.69 In a proof of concept RCT, it met its primary end-point and induced resolution of NASH in both diabetic and non-diabetic patients,70 although further studies are needed to corroborate this effect. The use of the higher 3 mg dose of liraglutide in an obese cohort without diabetes over 70 weeks demonstrated significant weight loss in those on liraglutide vs placebo (63.2% vs 27.1% for 5% weight loss and 33.1% vs 10.6% for 10% loss).71 Side effects were minimal and the higher dose appeared well tolerated.
GFT505
PPARs are nuclear receptors that play key roles in the regulation of metabolism and inflammation. GFT505 is a new dual agonist of the PPARα and δ receptors, and has been shown to improve lipid and glucose metabolism in T2DM, and steatosis, inflammation and fibrosis in mouse models of NAFLD.72 A small study (n = 22) in an obese population has shown that GFT505 improved peripheral and hepatic insulin sensitivity, and significantly reduced insulin-suppressed plasma FFA concentrations, fasting plasma TGs and LDL cholesterol.73Post hoc analysis of a recently published randomized phase IIb study showed patients clearing NASH (as defined by the disappearance of ballooning together with either the disappearance of lobular inflammation or the persistence of mild lobular inflammation (score of 0 or 1) without worsening of fibrosis) with 120 mg oral elafibranor (GFT505). When compared with placebo, improvement in NASH was more pronounced in those with NAS ≥4, (19% vs 9%; P = 0.013) compared those with NAS ≤ 4 (19% vs 12%; P = 0.045), and it is likely that PPAR agonism will have role in pharmacotherapy for NASH in the future.74,75
Vitamin E
Vitamin E is an antioxidant and has potential mechanism to reduce oxidative stress in NASH. It is the most widely investigated antioxidant, and has been shown to improve steatosis and inflammation in several RCTs in both diabetic and non-diabetic children and adults.76,77 However, the trials have been heterogeneous, comparing different doses of vitamin E against various agents as well as placebo, and in two studies the participants had lost weight, making it difficult to draw adequate conclusions. Despite meeting the primary end-point in the PIVENS trial, there are persisting concerns regarding the risk of prostate cancer and haemorrhagic stroke in higher doses.78,79 as well as reports of increased all-cause mortality80 The SELECT study compared selenium vs vitamin E vs placebo for a primary outcome of Gleason grade ≥7 prostate cancer, and showed a relative risk of 17% with vitamin E. However, absolute risk was lower at 1.6 per 1000 person-years was 1.6 for vitamin E, and it is possible that identifiable SNPs affecting vitamin E metabolism may be responsible for the increased risk.78 A meta-analysis investigating the effect of vitamin E on the incidence stroke reported an increase in the relative risk of haemorrhagic stroke by 22%, while the risk of ischaemic stroke was reduced by 10%. Given the severity of outcomes following haemorrhagic stroke, the authors could not recommend the use of vitamin E.79 Despite the potential benefits for NASH, the longest prospective trial is 2 years,77 and given the long-term concerns, the risks and benefits of therapy must be carefully discussed with patients in clinical practice.
Obeticholic acid
Obeticholic acid (OCA) is a synthetic variant of the natural bile acid (chenodeoxycholic acid), a potent activator of the farnesoid X nuclear receptor, which down-regulates lipogenesis. A randomized, placebo-controlled trial in NAFLD (the FLINT study) demonstrated improvement in histological features of NASH (steatosis, hepatocyte ballooning and inflammation) as well as fibrosis.81 Increased levels of low-density lipoprotein (LDL) and reduced high-density lipoprotein (HDL) were also seen in this group, which will need to be monitored in the ongoing phase III study. There was also a high incidence of pruritus (23%) which may be an important consideration for a condition with minimal symptoms.81
Bariatric surgery
Bariatric surgery offers an invasive but effective means of sustainable weight loss. There have been no RCTs investigating the benefits of bariatric surgery in NAFLD, but meta-analysis of cohort studies suggests an improvement in steatosis by 91.6%, steatohepatitis by 81.3% and fibrosis by 65.5%, following bariatric surgery.82 Furthermore, improvements in insulin resistance, dyslipidaemia and other obesity-related comorbidities have been demonstrated. No single technique is recommended for NAFLD but bypass procedures are believed to be the most effective for weight loss.83 RCTs and long-term follow-up studies are required to fully evaluate the risks and benefits of surgery over lifestyle modification and pharmacotherapy.
Growing points
LOXL2 antibody/inhibitors
LOXL2 is one of a family of enzymes involved in modifying the extracellular matrix, promoting cross-linking of cellular collagen and fibrosis.84 Serum LOXL2 levels have been shown to correlate with fibrosis in NAFLD, and both an antibody and an inhibitor have been developed, with phase 2b trials underway for the former (clinical trials.gov identifier: NCT01672866 and NCT01672879).
Vascular adhesion protein-1
The adhesion molecule vascular adhesion protein-1 (VAP-1) is a membrane-bound amine oxidase that promotes leukocyte recruitment to the liver, and the soluble form (sVAP-1) accounts for the most circulating monoamine oxidase activity, has insulin-like effects, and can initiate oxidative stress. An absence or blockade of functional VAP-1 in murine hepatic injury models has been shown to reduce inflammatory cell recruitment to the liver and attenuate fibrosis. Furthermore, serum sVAP-1 levels are elevated in patients with NAFLD compared with those in control individuals, and targeting VAP-1 is believed to have therapeutic potential for NAFLD and other chronic fibrotic liver diseases.85
CCR2/CCR5 antagonist
The C-C chemokine receptor types 2 and 5 (CCR2 and CCR5), and their respective ligands, C-C chemokine ligand types 2 (CCL2/monocyte chemoattractant protein-1 [MCP-1]) and 5 (CCL5/RANTES) are involved in recruitment of inflammatory cells to the liver and activation of hepatic stellate cells which promote fibrosis.86 Inhibition of CCR2 or CCR5 in murine models of liver injury demonstrated reduction in fibrosis; an oral dual CCR2/CCR5 antagonist (Cenicriviroc) has now been developed and a phase IIb trial is currently underway.87
Liver transplantation
Transplantation for NAFLD is rising, and, with it, expertise in the selection and management of both graft and patient peri-operatively.88 Patients often have significant comorbidities, yet a recent meta-analysis showed a tendency towards death from CVD or sepsis, but otherwise similar 5-year outcomes for NASH recipients compared with other aetiologies.89 Higher rates of renal dysfunction are observed in patients with NASH after transplantation, and therefore use of mycophenolate and lower serum levels of Tacrolimus is recommended.90
Conclusions
NAFLD is the fastest growing cause for liver disease worldwide, and in the light of the obesity epidemic, shows no sign of waning. Liver steatosis alone is relatively benign, but the presence of fibrosis has significant implications for cardiovascular- and liver-related morbidity and mortality. The factors determining development of steatohepatitis and fibrosis are poorly understood, and warrant further investigation. Nevertheless, identifying those with NASH and fibrosis is crucial, as these patients should usually be managed within a secondary care setting, and may benefit from pharmacological and non-pharmacological interventions, regular modification of risk factors and participation in clinical trials.
There are currently no non-invasive tests for steatohepatitis, but several for fibrosis. Currently, once patients at high-risk group have been identified, management is focussed on encouraging weight loss and managing features of the metabolic syndrome, in an attempt to halt progression of the disease and reduce cardiovascular mortality. Exercise and weight loss remain the most effective strategy for disease management, but is limited by the ability to sustain lifestyle changes in this population group. Identifying dietary and exercise regimens that are the easiest to adopt and lead to long-standing lifestyle reform will improve liver and cardiovascular outcomes. These would ideally be tailored to individual needs and abilities, but this is a resource-heavy approach, and may not be practicable in most healthcare systems.
Trials for pharmacological agents have historically been limited by small study cohort sizes, a dearth of high-quality studies, and concerns regarding efficacy and side effects. However, there is now multiple large phase II/III RCT in progress with both new and existing agents, with the FDA assigning breakthrough designation for several of them in light of the significant clinical unmet need in NASH. NAFLD is a highly complex condition with multiple parallel pathways and thus it is likely that therapy will be personalized and consist of multiple therapies.
Acknowledgements
Grant support: P.N.N. and S.A.T. are supported by the NIHR Birmingham Liver Biomedical Research Unit based at the University Hospitals Birmingham and the University of Birmingham. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of Interest statement
The authors have no potential conflicts of interest.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of non-alcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence and outcomes. Hepatology 2015; 10.1002/hep.28431. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2. Smits MM, Iannou GN, Boyko EJ, et al. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J Gastroenterol Hepatol 2013;28:664–70. [DOI] [PubMed] [Google Scholar]
- 3. Szczpaniak LS, Nurenburg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 2005;288:462–8. [DOI] [PubMed] [Google Scholar]
- 4. Visscher TLS, Heitmann BL, Rissanen A, et al. A break in the obesity epidemic? Explained by biases or misinterpretation of the data. Int J Obes 2005;39:189–198. [DOI] [PubMed] [Google Scholar]
- 5. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547–5. [DOI] [PubMed] [Google Scholar]
- 6. Haas JT, Francque S, Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annu Rev Physiol 2015;78:18.1–18.25. [DOI] [PubMed] [Google Scholar]
- 7. Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010;103:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999;30:1356–62. [DOI] [PubMed] [Google Scholar]
- 10. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820–32. [DOI] [PubMed] [Google Scholar]
- 11. Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- 12. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anstee QM, Seth D, Day CP. Genetic factors that affect risk of alcoholic and non-alcoholic fatty liver disease. Gastroenterology 2016;doi: 10.1053/j.gastro.2016.01.037.. [DOI] [PubMed] [Google Scholar]
- 14. Chalasani N, Guo X, Loomba R, et al. Genome-wide association study identifies variants associated with histologic features of nonalcoholic fatty liver disease. Gastroenterology 2010;139:1567–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelishadi R, Farajian S, Mirlohi M. Probiotics as a novel treatment for non-alcoholic fatty liver disease; a systematic review on the current evidences. Hepat Mon 2013;13:e7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Armstrong MJ, Houlihan DD, Bentham L, et al. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Heptol 2012;56:234–40. [DOI] [PubMed] [Google Scholar]
- 17. Feldstein AE, Wieckowska A, Lopez AR, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 2009;50:1072–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baršić N, Lerotić I, Smirčić-Duvnjak L, et al. Overview and developments in noninvasive diagnosis of nonalcoholic fatty liver disease. World J Gastroenterol 2012;18:3945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nobili V, Parkes J, Bottazzo G, et al. Performance of ELF serum 36 markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology 2009;136:160–7. [DOI] [PubMed] [Google Scholar]
- 20. Hamer OW, Aguirre DA, Casola G, et al. Fatty liver: imaging patterns and pitfalls. Radiographics 2006;26:1637–53. [DOI] [PubMed] [Google Scholar]
- 21. Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis 2007;11:37–54. [DOI] [PubMed] [Google Scholar]
- 22. Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010;36:1825–35. [DOI] [PubMed] [Google Scholar]
- 23. Myers RP, Pollett A, Kirsch R, et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int 2012;32:902–10. [DOI] [PubMed] [Google Scholar]
- 24. de Lédinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol 2014;60:1026–31. [DOI] [PubMed] [Google Scholar]
- 25. Sasso M, Audière S, Kemgang A, et al. Liver steatosis assessed by controlled attenuation parameter (CAP) measured with the XL probe of the FibroScan: a pilot study assessing diagnostic accuracy. Ultrasound Med Biol 2016;42:92–103. [DOI] [PubMed] [Google Scholar]
- 26. Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology 2013;58:1930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric magnetic resonance for the non-invasive diagnosis of liver disease. J Hepatol 2014;60:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pavlides M, Banerjee R, Sellwood J, et al. Multiparametric magnetic resonance imaging predicts clinical outcomes in patients with chronic liver disease. J Hepatol 2016;64:308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brener S. Transient elastography for assessment of liver fibrosis and steatosis: an evidence-based analysis. Ont Health Technol Assess Ser 2015;15:1–45. [PMC free article] [PubMed] [Google Scholar]
- 30. Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454–62. [DOI] [PubMed] [Google Scholar]
- 31. Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol 2012;107:1862–71. [DOI] [PubMed] [Google Scholar]
- 32. Palmeri ML, Wang MH, Rouze NC, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol 2011;55:666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoneda M, Suzuki K, Kato S, et al. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology 2010;256:640–7. [DOI] [PubMed] [Google Scholar]
- 34. Venkatesh SK, Yin M, Takahashi N, et al. Non-invasive detection of liver fibrosis: MR imaging features vs. MR elastography. Abdom Imaging 2015;40:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen J, Talwalkar JA, Yin M, et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 2011;259:749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hubscher SG. Review. Histological assessment of non-alcoholic fatty liver disease. Histopathology 2006;49:450–65. [DOI] [PubMed] [Google Scholar]
- 37. Bedossa P. FLIP Pathology Consortium Hepatology . Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014;60:565–75. [DOI] [PubMed] [Google Scholar]
- 38. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592–609. [DOI] [PubMed] [Google Scholar]
- 39. Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol 2011;17:3377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ferolla SM, Silva LC, Ferrari MLA, et al. Dietary approach in the treatment of nonalcoholic fatty liver disease. World J Hepatol 2015;28:2522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367–78. [DOI] [PubMed] [Google Scholar]
- 42. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;57:157–66. [DOI] [PubMed] [Google Scholar]
- 43. National Institute for Clinical Excellence (NICE) Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children (2006). [PubMed]
- 44. Johnson N, Sachinwalla T, Walton DW, et al. Aerobic exercise training reduces heptic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105–12. [DOI] [PubMed] [Google Scholar]
- 45. Hallsworth K, Fattakhova G, Hollingsworth KG. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eckard C, Cole R, Lockwood J, et al. Prospective histopathologic evaluation of lifestyle modification in non-alcoholic fatty liver disease: a randomised trial. Therap Adv Gastroenterol 2013;6:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gibala MJ. High-intensity interval training: a time-efficient strategy for health promotion. Curr Sports Med Rep 2007;6:211–3. [PubMed] [Google Scholar]
- 48. Gibala MJ, Little JP, Macdonald MJ, et al. Physiological adaptions to low-volume, high-Intensity interval training in health and disease. J. Physiol 2012;590:1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jelleyman C, Yates T, O'Donovan G, et al. The effects of high-intensity training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev 2015;16:942–61. [DOI] [PubMed] [Google Scholar]
- 50. Hallsworth K, Thoma C, Hollingsworth K, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomised controlled trial. Clin Sci 2015;129:1097–105. [DOI] [PubMed] [Google Scholar]
- 51. Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n–6/n–3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci 2004;106:635–43. [DOI] [PubMed] [Google Scholar]
- 52. Masterton GS, Pleveris JN, Hayes PC. Review article: omega-3 fatty acids—a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol 2010;31:679–92. [DOI] [PubMed] [Google Scholar]
- 53. Parker HM, Johnson A, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;56:944–51. [DOI] [PubMed] [Google Scholar]
- 54. Pacifico L, Bonci E, Di MM, et al. A double-blind, placebo-6 controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease. NMCD 2015;25:734–41. [DOI] [PubMed] [Google Scholar]
- 55. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ronksley PE, Brien SE, Turner BJ, et al. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hart CL, Morrison DS, Batty GD, et al. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ 2010;340:c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shen H, Rodrigue AC, Shiani A, et al. Association between caffeine consumption and nonalcoholic fatty liver disease: a systemic review and meta-analysis. Therap Adv Gastroenterol 2016;9:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tziomalos K, Athyros VG, Paschos P, et al. Nonalcoholic fatty liver disease and statins. Metabolism 2015;64:1215–23. [DOI] [PubMed] [Google Scholar]
- 60. Musso G, Gambino R, Cassader M, et al. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 2010;52:80–104. [DOI] [PubMed] [Google Scholar]
- 61. Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2016; 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 62. Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 2013;62:606–15. [DOI] [PubMed] [Google Scholar]
- 63. Boettcher E, Csako G, Pucino F, et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2012;35:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lincoff AM, Wolski K, Nicholls SJ, et al. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298:1180–8. [DOI] [PubMed] [Google Scholar]
- 65. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for non-alcoholic steatohepatitis. N Engl J Med 2010;362:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007;370:1129–36. [DOI] [PubMed] [Google Scholar]
- 67. Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care 2011;34:1369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lecka-Czernik B. Bone loss in diabetes: use of antidiabetic thiazolidinediones and secondary osteoporosis. Curr Osteoporos Rep 2010;8:178–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Armstrong MJ, Hull D, Guo K, et al. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol 2016;64: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679–90. [DOI] [PubMed] [Google Scholar]
- 71. Astrup A, Carraro R, Finer N. Safety, tolerability and sustained weight loss over 2 years with the once daily human GLP-1 analog, liraglutide. Int J Obes 2012;36:843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Staels B, Rubenstrunk A, Noel B, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology 2013;58:1941–52. [DOI] [PubMed] [Google Scholar]
- 73. Cariou B, Hanf R, Lambert-Porcheron S, et al. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care 2013;36:2923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ratziu V, Harrison S, Francque S, et al. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016; http://dx.doi.org/10.1053/j.gastro.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 75. Newsome PN. Entering the GOLDEN age for therapies in NASH. Gastroenterology 2016;150:1073–76. [DOI] [PubMed] [Google Scholar]
- 76. Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sumida Y, Naito Y, Tanaka S, et al. Long-term (> = 2 yr) efficacy of vitamin E for non-alcoholic steatohepatitis. Hepatogastroenterology 2013;60:1445–50. [DOI] [PubMed] [Google Scholar]
- 78. Chan JM, Darke AK, Penney KL, et al. Selenium or vitamin E-related gene variants, interaction with supplementation, and risk of high-grade prostate cancer in SELECT. Cancer Epidemiol Biomarkers Prev 2016; 10.1158/1055-9965.EPI-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 2010;341:c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37–46. [DOI] [PubMed] [Google Scholar]
- 81. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mummadi RR, Kasturi KS, Chennareddygari S, et al. Effects of bariatric surgery on non-alcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2008;6:1396–402. [DOI] [PubMed] [Google Scholar]
- 83. Hafeez S, Ahmed MH. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: a future treatment by choice or by chance. J Obes 2013; 10.1155/2013/839275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moon HJ, Finney J, Ronnebaum T, et al. Human lysyl oxidase-like 2. Biorgan Chem 2014;57:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Weston CJ, Shepherd EL, Claridge LC, et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest 2015;125:501–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Seki E, De Minicis S, Gwak GY, et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest 2009;119:1858–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Friedman S, Sanyal A, Goodman Z, et al. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials 2016;47:356–65. [DOI] [PubMed] [Google Scholar]
- 88. Newsome PN, Allison ME, Andrews PA. Guidelines for liver transplantation for patients with non-alcoholic steatohepatitis. Gut 2012;61:484–500. [DOI] [PubMed] [Google Scholar]
- 89. Wang X, Li J, Riaz DR, et al. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:394–402. [DOI] [PubMed] [Google Scholar]
- 90. Houlihan DD, Armstrong MJ, Davidov Y, et al. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: time to reconsider immunosuppression regimens. Liver Transpl 2011;17:1292–8. [DOI] [PubMed] [Google Scholar]