Abstract
Introduction/background
Heart failure is a major cause of cardiovascular morbidity and mortality. This review covers current heart failure treatment guidelines, emerging therapies that are undergoing clinical trial, and potential new therapeutic targets arising from basic science advances.
Sources of data
A non-systematic search of MEDLINE was carried out. International guidelines and relevant reviews were searched for additional articles.
Areas of agreement
Angiotensin-converting enzyme inhibitors and beta-blockers are first line treatments for chronic heart failure with reduced left ventricular function.
Areas of controversy
Treatment strategies to improve mortality in heart failure with preserved left ventricular function are unclear.
Growing points
Many novel therapies are being tested for clinical efficacy in heart failure, including those that target natriuretic peptides and myosin activators. A large number of completely novel targets are also emerging from laboratory-based research. Better understanding of pathophysiological mechanisms driving heart failure in different settings (e.g. hypertension, post-myocardial infarction, metabolic dysfunction) may allow for targeted therapies.
Areas timely for developing research
Therapeutic targets directed towards modifying the extracellular environment, angiogenesis, cell viability, contractile function and microRNA-based therapies.
Keywords: heart failure, HFrEF, HFpEF, hypertrophy, fibrosis, contractile dysfunction, microRNA, clinical trials
Introduction
Heart failure is a major cause of cardiovascular morbidity and mortality, with global prevalence predicted to increase significantly over the next few decades.1 Important advances in the management of patients with heart failure and reduced left ventricular ejection fraction (HFrEF) have been made, with evidence-based use of angiotensin-converting enzyme (ACE) inhibitors, beta-adrenergic receptor blockers and devices such as biventricular pacemakers (cardiac resynchronization) and implantable defibrillators contributing to a significant reduction in mortality and hospitalization. However, mortality remains high even in optimally treated patients. Furthermore, 40% or more of patients have heart failure with preserved left ventricular ejection fraction (HFpEF), a condition where no therapies that reduce mortality have yet been identified in large randomized controlled trials.2 A greater understanding of the pathophysiological mechanisms responsible for cardiac remodelling and other changes in heart failure is required for the development of new therapeutic targets and treatments. Here, we provide an overview of current heart failure treatment guidelines, emerging treatments that are being trialled in humans, and novel therapeutic targets that may arise from advances in understanding the molecular and cellular mechanisms driving heart failure.
Current heart failure treatment guidelines
Current guidelines differ for patients with impaired or preserved left ventricular ejection fraction (LVEF), termed HFrEF and HFpEF respectively. HFpEF patients are commonly categorized by an LVEF > 45−50% with evidence of LV diastolic dysfunction.
Both the 2016 European Society of Cardiology (ESC) guidelines and the 2013 American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) guidelines recommend the use of an ACE inhibitor (or an angiotensin receptor blocker in those intolerant of ACE inhibitors) as well as a beta-blocker in all HFrEF patients. In patients with persistent symptoms, a mineralocorticoid receptor antagonist should be added. Loop or thiazide diuretics are used to improve symptoms of fluid retention and in acute decompensation. Additional therapies may be indicated for patients who remain symptomatic, e.g. ivabradine, cardiac resynchronization therapy, digoxin, hydralazine and isosorbide dinitrate, and most recently the angiotensin receptor-neprilysin inhibitor valsartan/sacubitril. Heart failure patients at high risk of ventricular arrhythmia benefit from implantable cardioverter-defibrillators.
For patients with HFpEF, the ESC and ACCF/AHA guidelines recommend symptomatic management with diuretics and appropriate treatment of co-morbidities (e.g. hypertension, ischaemic heart disease and atrial fibrillation). No treatments have been identified that reduce mortality in HFpEF; in practice, many patients receive similar treatment to those with HFrEF.
Emerging therapeutics and drugs in development
There are a large number of novel pharmacological agents that are currently undergoing clinical trial for the treatment of acute decompensated and chronic heart failure, both HFrEF and HFpEF3 (Table 1). The mechanism of action of these treatments includes vasodilatation and counteracting neurohormonal activation (valsartan/sacubitril, ularitide and cenderitide), enhancement of cardiac myocyte contractility (omecamtiv mecarbil) and growth factor therapy (neuregulin-1).
Table 1.
Ongoing clinical trials or studies that have recently completed phase 3 trials
| Therapy | Drug type | Hypothesized mechanism | Key trials to date | Key findings to date | Future or on-going studies |
|---|---|---|---|---|---|
| Ularitide (urodilatin) | Endogenous natriuretic peptide | Natriuresis, vasodilatation, antifibrotic and antihypertrophy effects | SIRIUS-1, SIRIUS-2 | Improved haemodynamics, improved dyspnoea, reduced plasma NT-proBNP | TRUE-AHF (NCT01661634) (Phase 3) – acute decompensated heart failure |
| Cenderitide | Recombinant human BNP | Natriuresis, vasodilatation, antifibrotic and antihypertrophy effects | Animal studies only | Increased cGMP and reduced fibrosis in rat model of cardiac fibrosis | Number of small phase 1 trials assessing pharmacokinetics, safety and tolerance |
| Omecamtiv mecarbil | Direct myosin activator | Prolonged systolic ejection time | ATOMIC-AHF | Reduced dyspnoea with highest dose Reduced HR and SBP | COSMIC-HF – stable HFrEF (NCT01786512) |
| Neuregulin-1 | Cardiac growth factor – acts via ErbB4 | Regulation of cardiac structure, homeostasis and physiology | Effectiveness and safety of rhNRG-1 in chronic heart failure | Increases in LVEF and reduced LV cavity dimensions | NRG-1β (GGF-2) in HFrEF (Phase 1) (NCT01258387), rhNRG-1 (Phase 2/3) (NCT01251406) |
| Serelaxin | Recombinant human relaxin-2 | Haemodynamic effects, reverse myocardial fibrosis | Pre-RELAX-AHF, RELAX-AHF | Improved dyspnoea, no effects on mortality/hospitalization | RELAX-AHF-EU (NCT02064868), RELAX-AHF-2 (NCT01870778), RELAX-AHF-ASIA (NCT02007720) (all Phase 3) |
| Perhexiline | Metabolism modulator | Improved myocardial energetics | Small trials in heart failure and hypertrophic cardiomyopathy | Improved energetics and symptom status | Perhexiline in patients with HFpEF (NCT00839228) |
| Sildenafil | cGMP phosphodiesterase V inhibitor | Improved haemodynamics and cardiac remodelling | RELAX | Possible reduction in LV systolic function, no effect on clinical or structural measurements | Sildenafil in HFpEF (EudraCT 2010-020153-14, 2011-001674-25, 2013-001659-10) |
| Riociguat, vericiguat | Soluble guanylate cyclase agonists | Pulmonary and systemic vasodilatation | DILATE-1, LEPHT (riociguat) | Increased stroke volume | SOCRATES-REDUCED – HFrEF (NCT01951625), SOCRATES-PRESERVED – HFpEF (NCT01951638) |
| Sitaxsentan | ETA antagonists | Systemic and pulmonary arterial vasodilatation, reduced hypertrophy | Effectiveness of sitaxsentan in HFpEF | Increased exercise tolerance, no change to echocardiographic indices of function | – |
| Valsartan/sacubitril | Angiotensin receptor blocker and neprilysin inhibitor | Inhibition of breakdown of natriuretic peptides and RAAS | PARADIGM-HF, PARAMOUNT-HF | 20% reduction in relative risk of CV mortality and heart failure hospitalization compared with ACE inhibitor | PARAGON-HF– HFpEF (NCT01920711) (Phase 3) |
| Ranolazine | Metabolism modulator, Na channel modulator | Improved function, reduced arrhythmia | RALI-DHF | Improved measures of haemodynamics but no improvement of relaxation parameters or NT-pro-BNP levels | RAZE (NCT01505179) |
| Bendavia | Mitochondrial enhancer | Improved metabolic gene expression and reduced hypertrophy | Animal studies only | Reduced fibrosis at MI border and cardiac hypertrophy post-infarction | Bendavia in stable HFrEF (NCT02388464) and HFpEF (Mito-HFpEF) (both phase 1) |
| Ivabradine | If inhibitor | Inhibition of aldosterone signalling, reduced heart rate | SHIFT | Improved clinical outcomes (HF hospitalization and CV mortality) | EDIFY (EudraCT 2012-002742-20) (Phase 2) |
| Finerenone | Non-steroidal MRA | Inhibition of aldosterone signalling | ARTS (phase IIa) | Decreased NT-proBNP, improved renal profile vs. spironolactone | ARTS-HF (NCT01807221) (Phase 2b) |
BNP – B-natriuretic peptide; MRA – mineralocorticoid receptor antagonist; rhNRG-1 – recombinant human neuregulin-1; GGF-2 – glial growth factor 2, ECM – extracellular matrix, ETA – endothelin-1 receptor A.
Natriuretic peptides such as brain-natriuretic peptide (BNP) are produced by cardiomyocytes in response to myocardial stretch and mediate physiological responses that counteract renin–angiotensin–aldosterone system (RAAS) activation in heart failure. They act on the kidneys to increase glomerular filtration through afferent arteriolar vasodilatation and efferent vasoconstriction, and inhibit sodium and water reabsorption in the collecting ducts. Through generation of cGMP, natriuretic peptides stimulate peripheral vasodilatation and a reduction in afterload, as well as having antifibrotic and antihypertrophic effects on the heart. Levels of BNP or its amino-terminal cleavage equivalent (NT-pro BNP) are raised in heart failure, with low levels having a high negative predictive value for exclusion of heart failure as a cause of symptoms. Higher levels are suggestive of severe heart failure or acute decompensation, although levels may also be raised in other conditions (e.g. arrhythmia, pulmonary embolism and renal failure). In addition to their role in diagnosis and as biomarkers, natriuretic peptides have become a target for heart failure. A promising new therapy for heart failure is the dual-acting angiotensin receptor and neprilysin inhibitor valsartan/sacubitril (formerly known as LCZ696). Neprilysin is an enzyme responsible for the breakdown of natriuretic peptides and several other vasoactive peptides including angiotensin II. In the phase 3 randomized, placebo-controlled PARADIGM-HF trial, LCZ696 treatment was compared to ACE inhibition with enalapril in 8436 patients with chronic heart failure with an ejection fraction of <40%.4 The benefit of treatment with LCZ696 in reducing cardiovascular mortality or hospitalization for heart failure led to a premature termination of the trial after a mean follow-up of 27 months—at which point there was a 20% risk reduction in the primary endpoint (HR 0.80, 0.73−0.87, P < 0.001), with a number needed to treat of 21. Valsartan/sacubitril received US FDA approval in July 2015,5 with introduction into the UK healthcare system this year.
Ularitide (or urodilatin) is an endogenous natriuretic peptide and a current phase 3 trial (TRUE-AHF) will evaluate the effects of 48 h continuous IV infusion of ularitide in patients with acute decompensated heart failure. Cenderitide is a recombinant natriuretic peptide that has been shown to exert antifibrotic effects in animal models.6 Various phase 1 and 2 clinical studies have been initiated to evaluate safety and tolerance of cenderitide in patients with acute and chronic heart failure, although little peer-reviewed data is available as yet.
Serelaxin is a novel human recombinant relaxin-2 molecule that has shown promise in phase 3 clinical trials in the treatment of acute heart failure. Relaxin-2 is an endogenous peptide that plays an important role in the adaptive haemodynamic changes that occur during pregnancy. In the largest randomized placebo-controlled study of Serelaxin, RELAX-AHF, improvements in dyspnoea were demonstrated with 48-h Serelaxin infusion in patients with acute heart failure, although no demonstrable effects on hospitalization or mortality were observed.7 Serelaxin mediates haemodynamic changes (lowers systemic vascular resistance, increases cardiac output and renal blood flow) through activation of G-protein coupled receptors (GPCR) on vascular smooth muscle cells and is also likely to act directly on cardiomyocytes to protect against oxidative-stress mediated injury and cardiac fibrosis.
Riociguat and vericiguat are novel activators of soluble guanylate cyclase that bypass natriuretic peptide or nitric oxide (NO) signalling to enhance cGMP signalling. They have been found to be efficacious in certain types of pulmonary hypertension and in animal models of heart failure, and are now being tested for benefit in chronic heart failure.
Omecamtiv mecarbil is the first direct myosin activator. It binds to cardiac myosin and increases the transition rate of myosin into actin-bound form, prolonging systolic ejection time and having a different mechanism of action from other positive inotropic agents that have been unsuccessful in previous heart failure trials. In the phase 2b placebo-controlled ATOMIC-AHF trial, the highest dose of omecamtiv mecarbil resulted in significant improvement in dyspnoea in patients with acute decompensated heart failure and reduced LVEF, although pooled analysis of all doses showed similar effects to placebo. Those with stable, chronic HFrEF have been enrolled into the phase 2 COSMIC-HF trial studying omecamtiv mecarbil with 544 patients.
Neuregulin, a cardiac growth factor that signals through ErbB receptors, has important roles in cardiac development, growth and maintenance of function and has emerged as a potential novel therapy in heart failure. Evidence for the importance of growth factor signalling comes from both the animal models and the clinical adverse effects of ErbB blockade in cancer patients. The monoclonal antibody trastuzumab improves survival in HER2-positive breast cancer, but its use is associated with the development of cardiac dysfunction and heart failure in many patients. A phase 2 double-blind placebo-controlled study in 44 patients with stable HFrEF who were given recombinant human neuregulin-1 for 10 h over 10 days showed greater increases in LVEF and improvements in echocardiographic measurements with neuregulin-1.
Novel therapeutic targets
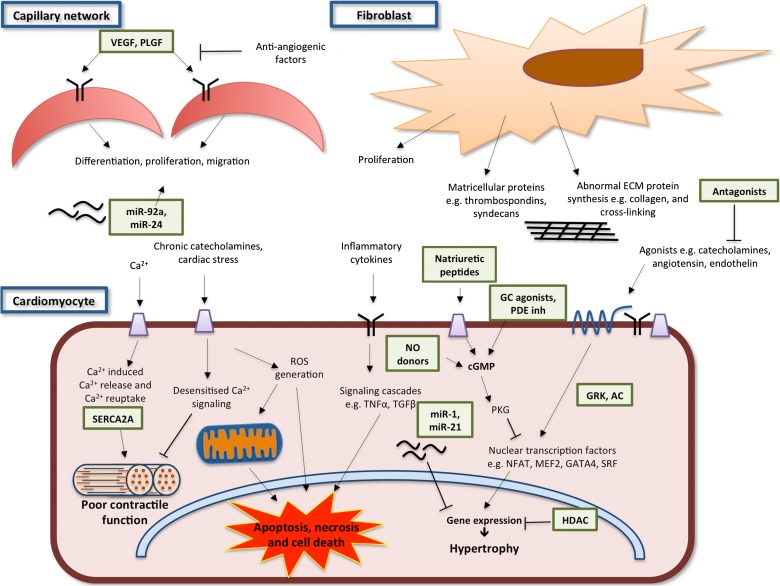
The heart failure phenotype includes several different components that may at least in part be subject to distinct regulation and are potentially amenable to therapeutic manipulation (see Fig. 1). These include cardiac hypertrophy, abnormalities of excitation–contraction (EC) coupling, impaired cardiomyocyte viability, abnormal protein homeostasis, oxidative stress, energetic dysfunction, arrhythmia, extracellular matrix remodelling and chamber dyssynchrony.
Fig.1.
Pathophysiological mechanisms and key therapeutic targets (green boxes) in heart failure. The heart failure phenotype involves cardiomyocyte hypertrophy, abnormalities of excitation–contraction coupling, impaired cardiomyocyte viability, abnormal protein homeostasis, oxidative stress, energetic dysfunction, arrhythmia, extracellular matrix remodelling and chamber dyssynchrony. These changes are mediated by intracellular signalling pathways and the interactions of multiple cell types within the heart including fibroblasts, endothelial cells and their associated capillary network and extracellular matrix. Key signalling pathways amenable to therapeutic targeting are shown in the figure and further described in the text. VEGF (vascular endothelial growth factor), PLGF (placental growth factor), SERCA2A (sarcoplasmic/endoplasmic reticulum Ca2+ ATPase), HDAC (histone deacetylase), GRK (G-protein coupled receptor kinase), AC (adenylate cyclase), NO (nitric oxide) and PDE inh(phosphodiesterase inhibitors).
Hypertrophy
A feature that is commonly present in heart failure, especially in the earlier stages, is cardiomyocyte hypertrophy. This is a complex pathological growth process that is a near-universal response to chronically increased cardiac workload but which eventually leads to impaired cardiac function and is associated with adverse cardiovascular outcomes.8 A wide range of agonists (e.g. catecholamines, angiotensin II, cytokine growth factors and endothelin), intracellular signalling cascades (e.g. mitogen-activated and other protein kinases) and nuclear transcription factors (e.g. nuclear factor of activated T cells [NFAT], myocyte enhancer factor 2 [MEF2], GATA binding protein 4 [GATA4] and serum response factor [SRF]) are involved in pro-hypertrophic gene expression. Additional points of regulation include counter-regulatory networks that inhibit hypertrophy (e.g. cGMP/protein kinase G [PKG]), epigenetic factors (e.g. histone deacetylases [HDAC]) that modulate gene expression and post-translational modification of mRNA by microRNAs (discussed later). These signalling networks provide a wide range of potential therapeutic targets, which may affect not only hypertrophy but also other components of the heart failure phenotype.
In principle, detrimental signalling pathways could be inhibited or beneficial pathways could be enhanced.9 Activation of c-GMP-dependent PKG by NO and natriuretic peptide receptor signalling inhibits hypertrophic signalling, and drugs to stimulate this pathway may hold promise. These include the cGMP phosphodiesterase inhibitors (e.g. sildenafil) and activators of guanylate cyclase, the enzyme that produces cGMP. Preliminary trials of the guanylate cyclase activator cinaciguat suggested that its use in acute decompensated heart failure improved afterload, filling pressures and dyspnoeic symptoms. However three randomized, placebo-controlled trials in the COMPOSE programme failed to demonstrate the improvement of dyspnoea symptoms, and together with an excess of hypotensive adverse events resulted in its early termination. Initial trials of riociguat in patients with pulmonary hypertension and heart failure (LEPHT and DILATE-1) showed improvements in haemodynamic and echocardiographic parameters. The recently published phase 2, double-blind, placebo-controlled, dose-finding SOCRATES-REDUCED trial of 456 patients with LVEF < 45% and recent deterioration in symptoms failed to demonstrate statistically significant change in NT-proBNP levels after 12 weeks of treatment compared with placebo, although exploratory analysis suggested a possible dose–response relationship. A similar trial is currently being completed in patients with HFpEF and results are awaited.10
Extracellular matrix and interstitium
Cardiac myocytes exist in a complex, dynamic fibrous extracellular matrix. Fibroblasts are the most numerous cell type and lay down many structural proteins including collagen, and interact with myocytes both physically and through paracrine signals. Pathophysiological changes to this environment in response to cardiac stress result in fibrosis, contractile dysfunction and increased propensity for re-entrant arrhythmia, while in the later stages of heart failure they contribute to ventricular dilatation and dyssynchrony. Indeed ACE inhibitors and angiotensin receptor blockers exert their effects in part through modulation of maladaptive cardiac remodelling and reduction in fibrosis. Collagen fibres are responsible for providing structural integrity and its production and deposition in response to cardiac stress may be a homeostatic response designed to maintain adequate tensile strength. Dysregulation of this process results in excess stiffness with associated cardiac dysfunction. In vivo experimental evidence has shown that a number of matricellular proteins (e.g. thrombospondins and syndecans) are important in preventing systolic dysfunction after infarction, but persistent expression increases collagen production, stiffness and diastolic dysfunction. Severe fibrosis is a significant predictor of mortality and cardiac events in HFrEF.11 Underlying mechanisms involved in the development of fibrosis include RAAS activation, transforming growth factor beta (TGFβ), tumour necrosis factor alpha (TNFα), oxidative stress and haemodynamic factors. Cross-linking of collagen is an important post-translational modification that increases tensile strength, and is regulated by lysyl oxidase. Collagen cross-linking is typically abnormal in the failing heart. The turnover of the extracellular matrix is regulated by a family of matrix metalloproteases and their inhibitors, and this is especially important in cardiac dilatation as well as post-myocardial infarction rupture. Targeting abnormal extracellular matrix remodelling and fibrosis in heart failure has long been considered a promising therapeutic target but no clinically applicable therapies have so far emerged.
MicroRNA
MicroRNAs (miRNA) are short, highly conserved, anti-sense RNA strands that target mRNA in a complementary manner and reduce protein expression by inhibiting mRNA translation and targeting their breakdown. Typically, a single miRNA has multiple mRNA targets, often in the same pathway. By regulating expression of multiple genes at the post-transcriptional level, miRNA are an exciting and novel therapeutic target for modulating networks of genes that may be involved in maladaptive remodelling. The role of miRNA in cardiac hypertrophy, fibrosis and remodelling is the focus of much research and their potential to be used as biomarkers as well as therapeutic targets is being increasingly recognized.12 Several miRNA have been shown to be associated with cardiac hypertrophy, among which miR-1 is one of the best understood. Animal models of heart failure have shown that administration of exogenous miR-1 improves hypertrophy, fibrosis and apoptosis.13 Another miRNA, miR-21, has been found experimentally to be an important mediator of cardiac fibroblast activation by promoting TGFβ signalling, and it induces fibrosis in animal models when over-expressed. Reducing transcription of miR-21 reduced fibrosis and attenuated cardiac dysfunction in response to pressure overload.14 Other miRNA that have been implicated as being important in maladaptive cardiac processes and have shown promise in animal models of heart failure include miR-30, miR-133, miR-208a, miR-199a, miR-199b, miR-132, miR-212/132, miR-29 and miR-101. While miRNA-based therapies (e.g. inhibition of miRNA effects using antagomirs) represent an attractive option in heart failure, several important factors relating to methods of delivery, pharmacokinetics and target organ and gene specificity still need to be addressed.12
Microvasculature and angiogenesis
Efficient blood supply to cardiomyocytes is necessary to meet their high metabolic demands and oxygen requirements. Unlike physiological settings such as pregnancy and chronic exercise, pathological cardiac stressors such as hypertension and chronic myocardial infarction are associated with an abnormal balance between the extent of hypertrophy and the level of myocardial blood supply. This may relate to abnormal angiogenic properties or altered capillary preservation and results in a relative impairment of local tissue oxygenation. Various factors have been implicated, including endothelial dysfunction, myocardial fibrosis and an abnormal balance between pro- and anti-angiogenic factors.15 Microvascular dysfunction may be seen early in heart failure, while inhibiting angiogenesis can lead to myocardial dysfunction.16 Promoting myocardial angiogenesis or the maintenance of appropriate local blood flow is therefore a target for the development of novel therapeutic agents.
Proliferation, differentiation and migration of endothelial cells require growth factors such as vascular endothelial growth factor (VEGF) and placental growth factor (PLGF).17,18 While phase I studies of recombinant human VEGF (rhVEGF) showed promise, the VIVA and RAVE trials did not demonstrate significant positive effects in patients with stable ischaemic heart disease and peripheral artery disease respectively. Both studies used systemically delivered rhVEGF, and possibly were unable to achieve high enough plasma concentrations to ensure adequate myocardial uptake.18 Targeting VEGF to the myocardium may be able to improve this, though this is not without its difficulties or limitations. Myocardial angiogenesis is also under the control of miRNAs, of which miR-92a and miR-24 have been best studied.19 Pre-clinical studies suggest that this may be a promising area for therapeutic intervention.12,20
In addition to pro-angiogenic growth factors, formation of new vessels and maintenance of existing ones is also regulated by anti-angiogenic factors. These usually act through blocking the activation and effects of VEGF signalling or by triggering apoptosis in endothelial cells. They are increasingly recognized to have important pathophysiological roles in specific causes of heart failure, including peripartum cardiomyopathy and chemotherapy-related cardiomyopathy.
Contractile function
A cardinal feature of heart failure is an impairment of the cardiomyocyte EC coupling machinery and calcium homeostasis.21Appropriate EC-coupling is essential for normal myocardial contraction and relaxation and the changes in contractile performance during exercise, mediating an efficient coupling between cyclical changes in calcium and the contractile machinery. Several steps are required, including the entry of calcium via sarcolemmal L-type channels, which stimulates Ca2+-induced Ca2+ release from the sarcoplasmic reticulum (SR) via ryanodine receptor (RyR) channels and the subsequent binding of Ca2+ to the myofibrillar apparatus to initiate contraction. Relaxation of the contractile machinery is a result of reuptake of Ca2+ into the SR by SERCA2a and efflux from the cardiomyocyte via the Na2+/Ca2+ exchanger. In the failing heart, these processes are typically dysregulated and are therefore an important therapeutic target.22 Indeed, agents such as the cardiac myosin activator omecamtiv mecarbil target elements of this machinery. A key modulator of efficient calcium signalling and EC-coupling is the beta-adrenergic signalling pathway through an increase in cAMP-dependant protein kinase activity (PKA). PKA enhances calcium entry via L-type channels, calcium release from the SR, calcium sensitivity of the myofilaments and calcium re-uptake into the SR through the phosphorylation of L-type channels, RyR, troponin I and myosin binding protein C and phospholamban (with impact on the SERCA pump) respectively. Beta-adrenergic pathways are desensitized in heart failure (probably due to chronic beta-adrenergic stimulation) and this contributes to contractile dysfunction, arrhythmia and exercise intolerance. Although augmenting this pathway might seem intuitively beneficial, previous studies of beta-adrenergic agonists or augmenting PKA signalling were found to be detrimental—potentially by causing energetic imbalance and increasing calcium overload. Indeed, the opposite strategy of chronic beta-blockade promotes some reverse remodelling and decreases mortality. Nevertheless, other approaches to target abnormal EC-coupling in heart failure remain under investigation. One idea is to target beta-adrenergic desensitization at the level of GPCR kinases (GRKs), which promote the degradation of beta-receptors and are found to be increased in heart failure. Inhibition of an isoform GRK2 may be beneficial to supporting contractile function23 and represents a promising target to boost beta-receptor function. However, the signalling is complex and further downstream of the G-protein are adenylate cyclases (ACs) that convert ATP to cAMP when GPCR are activated. AC5 and 6 are the major isoforms in the heart. Interestingly inhibition of AC5 may in fact be cardioprotective.24 It is likely that isoform-specificity of GRKs and ACs will be crucial in establishing successful therapies. The downstream signalling of PKA to specific targets within the cardiomyocyte is mediated by A-kinase anchoring proteins (AKAPs) which reside on myofilaments, RyR and phospholamban, therefore providing specificity of PKA action.25 Targeting specific PKA-AKAP interactions may be an interesting therapeutic approach. Further downstream in the signalling pathway, the activity of SERCA2a (which regulates calcium re-uptake into the SR) has long been a target of interest. SERCA2a is downregulated in heart failure and this is thought to be a major contributor to contractile impairment. Attempts at increasing SERCA2a levels by gene therapy have already progressed to human trials with encouraging initial results. However, a randomized, double-blind, placebo-controlled, phase 2b trial of AAV1/SERCA2a therapy in 250 patients with chronic heart failure recently reported negative results.26 Reducing Ca2+ leak from RyR channels (which in particular may increase arrhythmia) or inhibiting Na2+ accumulation are other potential targets that could be of therapeutic benefit.27 Another potential agent that directly modifies myofilament proteins to enhance their Ca2+ sensitivity is nitroxyl (HNO).28 HNO donors target the redox sensitive cysteines in EC-coupling machinery (RyR2 and SERCA2a effects) and myofilament proteins, therefore enhancing both contractile and relaxation properties.
Cell viability
Cardiomyocyte death is recognized to be a central feature of cardiac remodelling and progression to heart failure.29 Cell death in the failing heart occurs either by necrosis or by apoptosis. The underlying triggers of cell death in chronic heart failure are not fully understood but several mechanisms have been proposed including the gradual accumulation of damaged macromolecules from oxidative stress, persistent catecholamine and RAAS signalling, activation of TNFα signalling and chronic inflammatory signalling.30 Mitochondrial permeability transition pore (MPTP) opening is a major mechanism for necrotic cell death and various methods to inhibit MPTP have been shown to prevent cell death or reduce adverse remodelling in pre-clinical studies e.g. inhibition of CaMKII31 or cyclophilin D.32 Dysfunctional mitochondria arising from intracellular damage due to oxidative stress are also thought to be a central player in inducing a process termed sterile inflammation.33 Incomplete autophagocytosis of mitochondrial DNA can activate toll-like receptor 9 (TLR9) signalling cascades leading to the production of proinflammatory cytokines and further cellular damage in the failing heart. Similarly, the activation of inflammasomes—multiprotein complexes that identify harmful substances such as products of oxidative stress within the cell—are also implicated in ischaemic heart failure.34 Hence targeting inflammatory receptors and cytokine signalling may provide potential targets that can limit cell death in the failing heart. Autophagy is a key process in cellular renewal and either too much or too little autophagic activity also impacts on cardiac remodelling and the progression to heart failure.35 A key upstream regulator of autophagic signalling is through the mechanistic target of rapamycin (mTOR) complex and targeting this complex is seen as a potential therapeutic target.36 Cell viability is also determined by other cellular processes including the ubiquitin-proteasome system (UPS) and the endoplasmic reticulum (ER) stress response, with both systems serving to maintain intracellular protein homeostasis.37 Disruption of these mechanisms also leads to the activation of cell death pathways. Targeting the proteasome and ER stress signalling cascades and their associated molecular chaperones may be another potential target for heart failure therapeutics.38
Metabolism and energetic dysfunction
For the heart to perform its role as a pump it utilizes significant amounts of ATP and therefore relies on substrates such as carbohydrates and fatty acids for its energetic requirements.39 Heart failure is characterized by an impaired ability to generate sufficient ATP to support pump function. Defects in glycolysis, glucose and fatty acid oxidation and oxidative phosphorylation are observed in the failing heart. Perhexiline, which is currently undergoing trials (see Table 1), is a metabolic modulator whose primary action is thought to inhibit fatty acid oxidation and stimulate glucose utilization thereby improving the efficiency of ATP generation within the failing heart. As well as maintaining energetic functions the heart utilizes substrates that cross-talk with other remodelling processes including hypertrophy, fibrosis and cell viability.39 Therefore manipulating substrate metabolism and energetics offers another potential strategy to target the remodelling process in heart failure.
Conclusions
Existing drug therapies (beta-blockers and ACE inhibitors) that reduce mortality in chronic heart failure were introduced nearly two decades ago. A wide range of new therapies that are currently undergoing clinical trials have been reviewed in this article. In addition, increased knowledge about the molecular and cellular processes underlying the development of cardiac remodelling—recognized as a central process in the development of heart failure21—is providing diverse potential therapeutic targets. Targeting components of the heart failure phenotype, such as hypertrophy, fibrosis and cell viability, that have hitherto not been direct therapeutic targets may prove to be valuable. Despite this apparent progress, it should be noted that there is a striking lack of therapies that are proven to reduce mortality in HFpEF. In addition, more information is required on the differences in pathophysiology and response to therapy among diverse causes of heart failure, e.g. diabetes and metabolic dysfunction, as well as individual patient factors that determine response to therapy. As well as the current approaches to discovery, it is increasingly recognized that the use of systems biology approaches to understand the interactions that drive complex biology at several levels (e.g. alterations occurring at the genomic, transcriptomic and metabolomic levels) will be invaluable. Finally, more discovery science in large animal models and in human experimental medicine is also required.
Conflict of interest statement
The authors have no potential conflicts of interest.
Funding
The authors’ work is supported by the British Heart Foundation and the Department of Health via a National Institute for Health Research (NIHR) Biomedical Research Centre award to Guy's & St Thomas’ NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016; 133:e38–360. [DOI] [PubMed] [Google Scholar]
- 2. Ziaeian B, Fonarow GC.. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016; 13:368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. von Lueder TG, Krum H.. New medical therapies for heart failure. Nat Rev Cardiol 2015; 12:730–40. [DOI] [PubMed] [Google Scholar]
- 4. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004. [DOI] [PubMed] [Google Scholar]
- 5. Hubers SA, Brown NJ.. Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition. Circulation 2016; 133:1115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soeki T, Kishimoto I, Okumura H, et al. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J Am Coll Cardiol 2005; 45:608–16. [DOI] [PubMed] [Google Scholar]
- 7. Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet (London, England) 2013; 381:29–39. [DOI] [PubMed] [Google Scholar]
- 8. Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990; 322:1561–6. [DOI] [PubMed] [Google Scholar]
- 9. Schiattarella GG, Hill JA.. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation 2015; 131:1435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pieske B, Butler J, Filippatos G, et al. Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES). Eur J Heart Fail 2014; 16:1026–38. [DOI] [PubMed] [Google Scholar]
- 11. Aoki T, Fukumoto Y, Sugimura K, et al. Prognostic impact of myocardial interstitial fibrosis in non-ischemic heart failure. -Comparison between preserved and reduced ejection fraction heart failure. Circ J 2011; 75:2605–13. [DOI] [PubMed] [Google Scholar]
- 12. Vegter EL, van der Meer P, de Windt LJ, et al. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail 2016; 18:457–68. [DOI] [PubMed] [Google Scholar]
- 13. Karakikes I, Chaanine AH, Kang S, et al. Therapeutic cardiac-targeted delivery of miR-1 reverses pressure overload-induced cardiac hypertrophy and attenuates pathological remodeling. J Am Heart Assoc 2013; 2:e000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thum T, Gross C, Fiedler J, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008; 456:980–4. [DOI] [PubMed] [Google Scholar]
- 15. Oka T, Akazawa H, Naito AT, et al. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 2014; 114:565–71. [DOI] [PubMed] [Google Scholar]
- 16. Tocchetti CG, Gallucci G, Coppola C, et al. The emerging issue of cardiac dysfunction induced by antineoplastic angiogenesis inhibitors. Eur J Heart Fail 2013; 15:482–9. [DOI] [PubMed] [Google Scholar]
- 17. Accornero F, Molkentin JD.. Placental growth factor as a protective paracrine effector in the heart. Trends Cardiovasc Med 2011; 21:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taimeh Z, Loughran J, Birks EJ, et al. Vascular endothelial growth factor in heart failure. Nat Rev Cardiol 2013; 10:519–30. [DOI] [PubMed] [Google Scholar]
- 19. Icli B, Dorbala P, Feinberg MW.. An emerging role for the miR-26 family in cardiovascular disease. Trends Cardiovasc Med 2014; 24:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernardo BC, Gao XM, Winbanks CE, et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci USA 2012; 109:17615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tarone G, Balligand JL, Bauersachs J, et al. Targeting myocardial remodelling to develop novel therapies for heart failure: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur J Heart Fail 2014; 16:494–508. [DOI] [PubMed] [Google Scholar]
- 22. Shah AM, Mann DL.. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet 2011; 378:704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sato PY, Chuprun JK, Schwartz M, et al. The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol Rev 2015; 95:377–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okumura S, Takagi G, Kawabe J, et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci USA 2003; 100:9986–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Najafi A, Sequeira V, Kuster DW, et al. Beta-adrenergic receptor signalling and its functional consequences in the diseased heart. Eur J Clin Invest 2016; 46:362–74. [DOI] [PubMed] [Google Scholar]
- 26. Greenberg B, Butler J, Felker GM, et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016; 387:1178–86. [DOI] [PubMed] [Google Scholar]
- 27. Marks AR. Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest 2013; 123:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sabbah HN, Tocchetti CG, Wang M, et al. Nitroxyl (HNO): A novel approach for the acute treatment of heart failure. Circ Heart Fail 2013; 6:1250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moe GW, Marin-Garcia J.. Role of cell death in the progression of heart failure. Heart Fail Rev 2016; 21:157–67. [DOI] [PubMed] [Google Scholar]
- 30. Briasoulis A, Androulakis E, Christophides T, et al. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev 2016; 21:169–76. [DOI] [PubMed] [Google Scholar]
- 31. Joiner ML, Koval OM, Li J, et al. CaMKII determines mitochondrial stress responses in heart. Nature 2012; 491:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elrod JW, Wong R, Mishra S, et al. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest 2010; 120:3680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakayama H, Otsu K.. Translation of hemodynamic stress to sterile inflammation in the heart. Trends Endocrinol Metab 2013; 24:546–53. [DOI] [PubMed] [Google Scholar]
- 34. Mezzaroma E, Toldo S, Farkas D, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci USA 2011; 108:19725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lavandero S, Chiong M, Rothermel BA, et al. Autophagy in cardiovascular biology. J Clin Invest 2015; 125:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schiattarella GG, Hill JA.. Therapeutic targeting of autophagy in cardiovascular disease. J Mol Cell Cardiol 2015; 95:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nishida K, Yamaguchi O, Otsu K.. Degradation systems in heart failure. J Mol Cell Cardiol 2015; 84:212–22. [DOI] [PubMed] [Google Scholar]
- 38. Fu HY, Sanada S, Matsuzaki T, et al. Chemical Endoplasmic Reticulum Chaperone Alleviates Doxorubicin-Induced Cardiac Dysfunction. Circ Res 2016; 118:798–809. [DOI] [PubMed] [Google Scholar]
- 39. Taegtmeyer H, Young ME, Lopaschuk GD, et al. Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association. Circ Res 2016; 118:1659–701. [DOI] [PMC free article] [PubMed] [Google Scholar]