Plasma levels of immune/inflammatory markers have been prospectively associated with lung cancer risk among never-smoking women in Shanghai. Here, we report associations between these markers and occupational exposure to diesel engine exhaust, using data from a cross-sectional molecular epidemiology study in China.
Abstract
The relationship between diesel engine exhaust (DEE), a known lung carcinogen, and immune/inflammatory markers that have been prospectively associated with lung cancer risk is not well understood. To provide insight into these associations, we conducted a cross-sectional molecular epidemiology study of 54 males highly occupationally exposed to DEE and 55 unexposed male controls from representative workplaces in China. We measured plasma levels of 64 immune/inflammatory markers in all subjects using Luminex bead-based assays, and compared our findings to those from a nested case–control study of these markers and lung cancer risk, which had been conducted among never-smoking women in Shanghai using the same multiplex panels. Levels of nine markers that were associated with lung cancer risk in the Shanghai study were altered in DEE-exposed workers in the same direction as the lung cancer associations. Among these, associations with the levels of CRP (β= −0.53; P = 0.01) and CCL15/MIP-1D (β = 0.20; P = 0.02) were observed in workers exposed to DEE and with increasing elemental carbon exposure levels (Ptrends <0.05) in multivariable linear regression models. Levels of a third marker positively associated with an increased lung cancer risk, CCL2/MCP-1, were higher among DEE-exposed workers compared with controls in never and former smokers, but not in current smokers (Pinteraction = 0.01). The immunological differences in these markers in DEE-exposed workers are consistent with associations observed for lung cancer risk in a prospective study of Chinese women and may provide some insight into the mechanistic processes by which DEE causes lung cancer.
Introduction
Diesel engine exhaust (DEE) consists of a complex mixture of gases and particulate matter (PM), including polycyclic aromatic hydrocarbons and nitro-polycyclic aromatic hydrocarbons, nitrogen and sulfur dioxides and other organic gases and vapors, with the relative fractions of the specific components varying according to differences in the engine characteristics (1). In 2012, a working group organized by the International Agency for Research on Cancer concluded that there is sufficient evidence in humans for the carcinogenicity of DEE, based on the weight of evidence for lung cancer and limited evidence for a positive association with bladder cancer (2). This conclusion for lung cancer was supported by ‘strong evidence’ suggesting that DEE can induce cancer through genotoxic mechanisms in humans (3).
The relationship between long-term occupational exposure to DEE and immune/inflammatory markers that have been directly and prospectively associated with lung cancer risk is not well understood. We recently conducted the first population-based, prospective evaluation of associations between plasma levels of immune/inflammatory markers measured using a multiplex panel and future risk of lung cancer in an Asian population. This nested case–control study within the Shanghai Women’s Health Study (SWHS), which included never-smoking women, identified 10 markers associated with future lung cancer risk (4). In particular, higher levels of three markers (SIL-6R, CCL2/MCP-1 and CCL15/MIP-1D) were each associated with increased risk of incident lung cancer, whereas seven markers (interleukin-21 [IL-21], CX3CL1/Fractalkine, SVEGFR2, SVEGFR3, STNFRI, IL-10 and CRP) were inversely associated with lung cancer risk.
We previously reported in a cross-sectional molecular epidemiology study, which included 54 workers exposed to DEE and 55 unexposed control workers in China, that DEE exposure was associated with an immune response as reflected by higher cell counts of total lymphocytes, CD4+ and CD8+ T cells and B cells in the exposed workers (5). To follow-up on these findings and to evaluate the mechanistic basis of the DEE-lung cancer association, we measured plasma levels of immune/inflammatory markers among subjects in the DEE cross-sectional study using the same panel of markers measured in the nested case–control study of lung cancer risk in the SWHS. Our primary aim was to identify specific markers associated with both lung cancer risk and DEE, as this may provide insight into the underlying biologic processes by which DEE causes lung cancer.
Materials and methods
Study population
Characteristics of the study population and design have been described elsewhere (5). Briefly, the study population consisted of 54 male workers exposed to DEE while employed in a diesel engine manufacturing facility and 55 unexposed male control workers from the same local area as the exposed workers who were employed in four separate facilities with no DEE exposure, as verified through extensive site visits. Selected control facilities included a bottling department of a brewery (n = 24), a water treatment plant (n = 18), a meat packing facility (n = 8) and an administrative facility (n = 5). The job duties in these control facilities did not involve exposure to particulates or chemicals either known or suspected to be associated with genotoxicity, hematotoxicity or immunotoxicity. Exposed workers were frequency matched to controls by age (±5 years) and smoking status (never, former and current). Demographic and lifestyle characteristics were obtained for each worker through a questionnaire, and peripheral blood samples were collected from all workers as part of a health examination conducted by the local Center for Disease Control. Biologic samples were collected from each of the subjects immediately following their work shift, and all plasma samples used for this study were processed and stored consistently in a −80°C freezer within 4 hours of collection. The same sample collection procedures and processing lab were used for all subjects in the study, including both the exposed and unexposed groups. Informed consent was obtained from all subjects and the study was approved by Institutional Review Boards at the US National Cancer Institute and the National Institute of Occupational Health and Poison Control, China CDC.
Exposure assessment
An extensive exposure assessment survey, as described in detail elsewhere (5), was conducted in a diesel manufacturing facility and included an assessment of several DEE constituents including fine particulate matter (PM2.5), elemental carbon (EC) and organic carbon (OC). Briefly, repeated full-shift personal air samples of EC, OC and PM2.5 were collected using a portable device attached to the lapel near the breathing zone with an aerodynamic cut-off of 2.5 µm (PM2.5) at a flow rate of 3.5 l/min using quartz or Teflon filters. PM2.5 was assessed by preweighing and postweighing the Teflon filters in an environmentally controlled weighing room using a microbalance at 1 µg accuracy. EC and OC were measured on the quartz filters using NIOSH Method 5040 (6). Weights of PM2.5, EC and OC were divided by the volume of air drawn through the filters to calculate exposure concentrations (µg/m3). Exposure assessments were also conducted in a subset of the controls from each factory except for one, a brewery, where no measurements could be obtained. Exposure levels were averaged (geometric mean) by factory and assigned to all controls in that factory. Minimal variation in exposure levels was observed among control factories. An average concentration in the three control factories with measurements was assigned to the 24 controls enrolled at the brewery.
As previously reported, EC levels were highly correlated with levels of OC (rsp = 0.86, P < 0.0001), but no correlation was observed between EC and PM2.5 (rsp = 0.09, P = 0.53) among DEE exposed workers (5). In this study, EC was used as the main exposure proxy for DEE in the statistical analyses given that EC is considered a specific marker for DEE in occupational settings and has been the focus of recent epidemiological studies of lung cancer (7).
Laboratory measurement of immune/inflammatory markers
Circulating levels of 64 immune/inflammatory markers were measured in plasma samples using Luminex bead-based assays (Millipore, Billerica, MA) using procedures that have been reported in prior studies (8). The assay consisted of six panels of immune/inflammatory markers including pro- and anti-inflammatory cytokines, chemokines, acute-phase proteins and growth and angiogenesis factors (Supplementary Table 1 is available at Carcinogenesis Online). These markers represent several components of the inflammation process and may contribute to lung carcinogenesis through a variety of molecular mechanisms involving sustained angiogenesis and replicative potential, immune surveillance and mediation of innate immune responses, and increased DNA damage from reactive species that are byproducts of the inflammatory response. The specific biologic mechanisms underlying these evaluated classes of immune/inflammatory markers and carcinogenesis have been extensively described elsewhere (9,10). Samples from DEE-exposed workers and controls were evenly dispersed in each batch and all laboratory personnel were blinded to the exposure status of the subjects. Each plate included the same quality control sample to assess interbatch variation and blinded duplicate quality control samples were included on each plate to assess within-batch variation. Intraclass correlation coefficients >0.70 were observed for 85% of the measured markers (markers with intraclass correlation coefficients <0.70 were as follows: G-CSF, TSLP, IL-33, SEGFR, TPO, CCL21/6CKINE, CCL13/MCP-4, IL-12(P70), IL-1RA and CTACK). Three of these markers (TPO, IL-33 and TSLP) and one additional marker (IL-4) were below the lower limit of quantification in >60% of the samples and were excluded from the statistical analyses (Supplementary Table 1 is available at Carcinogenesis Online). Measurements less than the lower limit of quantification were assigned a value of one-half the detection limit for each individual marker.
Statistical analysis
Differences in demographic and lifestyle characteristics between DEE-exposed and unexposed workers were evaluated using Wilcoxon tests for continuous variables and chi-square tests for categorical variables. The relationship between DEE exposure and levels of the immune/inflammatory markers was initially assessed using Wilcoxon tests to evaluate differences between DEE-exposed workers and controls overall. Multiple linear regression models were used to evaluate differences in natural log-transformed concentrations of immune/inflammatory markers between DEE-exposed workers and controls and to conduct trend tests for the levels of EC categorized using a four-level ordinal variable based on unexposed workers and EC exposure tertiles among the exposed workers. All linear regression models were adjusted for age, current smoking, current alcohol consumption, recent infection (flu or respiratory infection within the past month) and body mass index. In addition, statistically significant associations between DEE and immune/inflammatory markers that were observed in the primary analyses were further adjusted for lymphocyte subsets to evaluate if these associations were independent of previously reported effects of DEE on these cell counts (5). Multiple comparisons were accounted for by calculating the false discovery rate using the Benjamini–Hochberg method. As the markers associated with lung cancer risk in the SWHS were based on non-smokers, we further conducted separate analyses stratified by smoking status to evaluate if associations between DEE exposure and each immune/inflammatory marker differed in current, former and never smokers, and formally evaluated interactions by including a cross-product term (DEE exposure × smoking status) in the multiple linear regression models. All statistical analyses were conducted using SAS v. 9.3. Software (Cary, NC).
Results
Exposed and control workers had the same mean age (42 years), body mass index (25 kg/m2) and similar distributions of recent respiratory infections (50 and 51%, respectively) and smoking history (current, 63 and 64%; former, 20 and 22% and never smokers, 17 and 15%, respectively)] (Table 1). The proportion of current alcohol use was lower in exposed workers (72%) compared with controls (86%). Workers exposed to DEE had a mean employment duration of about 20 years (± 7 years). For DEE constituents, we present both unadjusted exposure levels and levels that are adjusted for exposure levels in the control factories, which were assumed to reflect background outdoor levels in this region given the absence of occupational sources of DEE or PM exposures (Table 1). Background adjusted mean levels of EC, OC and PM2.5 in the exposed workers were 48.5 ± 22.1 µg/m3, 70.2 ± 25.9 µg/m3 and 0.1 ± 0.07 mg/m3, respectively. There was no association between smoking status and EC levels among the DEE-exposed subjects (P = 0.39).
Table 1.
Demographic and lifestyle characteristics of workers exposed to DEE and control workers in a cross-sectional molecular epidemiology study in China
| Characteristic | Controls (n = 55) | Exposed (n = 54) | P-value |
|---|---|---|---|
| Age (years), mean (SD) | 42.1 (7.4) | 42.0 (6.8) | 0.99a |
| Body mass index (kg/m2), mean (SD) | 25.2 (3.8) | 24.7 (3.4) | 0.57a |
| Work years in diesel factory, mean (SD) | 19.6 (7.1) | ||
| Smoking status | |||
| Current, n (%) | 35 (63.6) | 34 (63.0) | 0.95b |
| Former, n (%) | 12 (21.8) | 11 (20.4) | |
| Never, n (%) | 8 (14.5) | 9 (16.7) | |
| Current alcohol use | |||
| No, n (%) | 8 (14.5) | 15 (27.8) | 0.09b |
| Yes, n (%) | 47 (85.5) | 39 (72.2) | |
| Recent infection | |||
| No, n (%) | 27 (49.1) | 27 (50.0) | 0.92b |
| Yes, n (%) | 28 (50.9) | 27 (50.0) | |
| Elemental carbon, µg/m3 (SD)c | |||
| Unadjusted, mean (SD) | 11.1 (1.3) | 59.6 (22.1) | |
| Background adjusted, mean (SD) | 0 (NA) | 48.5 (22.1) | |
| OC, µg/m3 (SD)c | |||
| Unadjusted, mean (SD) | 68.7 (4.1) | 138.9 (25.9) | |
| Background adjusted, mean (SD) | 0 (NA) | 70.2 (25.9) | |
| PM2.5, mg/m3 (SD)c | |||
| Unadjusted, mean (SD) | 0.2 (0.07) | 0.4 (0.07) | |
| Background adjusted, mean (SD) | 0 (NA) | 0.1 (0.07) | |
Background values are adjusted for exposure levels in the control factories, which had no occupational sources of DEE, to reflect background outdoor levels in this region. Unadjusted values are original measurements.
a P-value from Wilcoxon test.
b P-value from χ2 test.
cOn the basis of detailed walk-through surveys, no DEE sources were identified in any of the control factories.
Of the 10 markers associated with lung cancer risk in the SWHS, levels of 9 were altered in DEE-exposed workers in the same direction as the lung cancer association reported in the case–control study (Table 2). Unadjusted mean levels of CRP were ~43% lower in DEE-exposed workers (Pwilcoxon = 0.04) and unadjusted mean levels of CCL15/MIP-1D (Pwilcoxon = 0.02) were ~21% higher in exposed workers than the controls. Associations with CRP (β = −0.53, P = 0.01) and CCL15/MIP-1D (β = 0.20, P = 0.02) were observed and remained noteworthy (the false discovery rate = 0.10) after accounting for multiple comparisons among the 10 strong a priori evaluated markers associated with lung cancer in the SWHS. Furthermore, these associations were apparent after adjustment for lymphocyte subsets (Supplementary Table 2 is available at Carcinogenesis Online). The direction and magnitude of the associations for CRP and CCL15/MIP-1D comparing DEE exposed workers and controls were also similar in sensitivity analyses that removed one control group at a time from the models and that adjusted for employment duration; however, the largest declines in CRP were observed among workers exposed to DEE >17 years compared with controls (data not shown).
Table 2.
Associations between selecteda immune/inflammatory markers and DEE exposure in a cross-sectional molecular epidemiology study in China, and comparisons with findings from a nested case–control study of lung cancer in the prospective SWHS
| Mean concentrations pg/ml (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Marker | Controls (n = 55) | Exposed (n =54) | Difference in mean levels (%) | Wilcoxon P-value b | Adjusted β c | Adjusted P-value c | FDR d | Lung cancer OR (95% CI) Q4 versus Q1 e | Lung cancer P-trend e |
| Significant in SWHS | |||||||||
| SIL-6R | 31912.0 (7333.7) | 29905.0 (7859.9) | −6.3 | 0.08 | −0.08 | 0.07 | 0.23 | 2.37 (1.40–4.02) | 0.0003 |
| IL-21 | 3.4 (8.6) | 2.6 (4.9) | −23.5 | 0.21 | −0.18 | 0.36 | 0.42 | 0.53 (0.31–0.93) | 0.004 |
| CX3CL1/Fractalkine | 89.7 (46.1) | 85.4 (93.6) | −4.8 | 0.09 | −0.11 | 0.46 | 0.46 | 0.54 (0.30–0.96) | 0.008 |
| SVEGFR2 | 36862.0 (16255.0) | 31771.0 (9662.9) | −13.8 | 0.23 | −0.11 | 0.13 | 0.26 | 0.45 (0.26–0.76) | 0.02 |
| SVEGFR3 | 14475.0 (8189.1) | 12190.0 (5397.3) | −15.8 | 0.27 | −0.13 | 0.19 | 0.32 | 0.53 (0.32–0.90) | 0.03 |
| CCL2/MCP-1 | 285.3 (73.8) | 290.9 (54.9) | +2.0 | 0.26 | 0.04 | 0.33 | 0.42 | 1.62 (0.94–2.80) | 0.03 |
| STNFRI | 2459.0 (781.3) | 2236.2 (629.1) | −9.1 | 0.16 | −0.09 | 0.12 | 0.26 | 0.49 (0.29–0.83) | 0.03 |
| IL-10 | 6.9 (8.7) | 5.5 (7.4) | −20.3 | 0.06 | −0.16 | 0.38 | 0.42 | 0.60 (0.34–1.05) | 0.04 |
| CRP | 1.6x107 (2.3 × 107) | 9.2x106 (1.4 × 107) | −42.7 | 0.04 | −0.53 | 0.01 | 0.10 | 0.63 (0.37–1.06) | 0.05 |
| CCL15/MIP-1D | 2260.4 (997.2) | 2740.5 (1098.4) | +21.2 | 0.02 | 0.20 | 0.02 | 0.10 | 1.86 (1.09–3.17) | 0.05 |
| Not Significant in SWHS | |||||||||
| CXCL11/I-TAC | 30.0 (16.5) | 24.2 (10.0) | -19.3 | 0.08 | −0.20 | 0.03 | 0.75 | 1.34 (0.79–2.29) | 0.17 |
| IL-16 | 46.9 (31.8) | 54.7 (24.9) | +16.6 | 0.02 | 0.22 | 0.02 | 0.75 | 0.77 (0.46–1.28) | 0.46 |
aMarkers associated with the risk of lung cancer in non-smoking Chinese women (SWHS) and/or in the cross-sectional study of DEE exposure.
bWilcoxon test for differences in marker concentrations between DEE-exposed and control workers.
cParameter estimates and P-values for DEE exposure from linear regression models of natural log-transformed immune/inflammatory marker levels adjusted for age, current smoking, current alcohol use, current infection and body mass index.
dFDR values computed from adjusted P-values using Benjamini–Hochberg method. To account for the fact that 10 measured markers were strong a priori hypotheses based on their association with lung cancer in the SWHS, these 10 markers were evaluated separately for the purposes of the FDR adjustment. FDR adjustment for the remaining markers is based on the 50 evaluated markers that were not associated with lung cancer risk in the SWHS.
eAssociations between immune/inflammatory markers and lung cancer risk in a nested case–control study of never-smoking women from the SWHS ([Shiels et al. (4)].
CI, confidence interval; FDR, false discovery rate.
Associations between DEE exposure and other immune/inflammatory markers that were not associated with the risk of lung cancer in the SWHS are shown in Table 2 and Supplementary Table 1 is available at Carcinogenesis Online. Of these 50 additional markers, levels of CXCL11/I-TAC (β = −0.20, P = 0.03) and IL-16 (β = 0.22, P = 0.02) were altered in DEE-exposed workers compared with controls (Table 2). The association with IL-16, but not CXCL11/I-TAC, was attenuated after adjustment for lymphocyte subsets (Supplementary Table 2 is available at Carcinogenesis Online); however, neither association remained noteworthy after further accounting for multiple comparisons (the false discovery rate = 0.75). There was no correlation among CRP, CCL15/MIP-1D, CXCL11/I-TAC and IL-16 either overall (rsp ranging from −0.15 to 0.13) or in exposed workers (rsp ranging from −0.19 to 0.14).
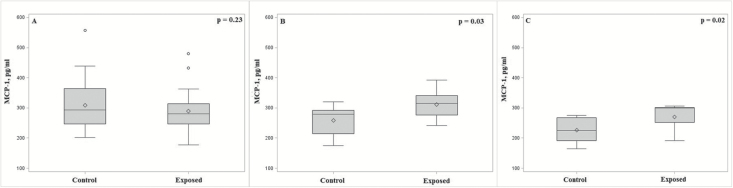
In analyses stratified by smoking status, we observed an interaction between DEE exposure and smoking on the levels of CCL2/MCP-1 (Pint = 0.01; Figure 1A–C). Among never and former smokers, unadjusted mean levels of CCL2/MCP-1 were higher to a similar degree (~20%) in DEE-exposed workers compared with controls (Figure 1B and C), which was consistent with the direction of the association between this marker and lung cancer risk in the SWHS. Conversely, CCL2/MCP-1 levels were ~3% lower in the DEE-exposed workers compared with controls among current smokers (Figure 1A). Levels of CCL2/MCP-1 were associated with DEE exposure in never (β = 0.24, P = 0.02) and former (β = 0.18, P = 0.03) smokers only. Several additional markers showed evidence of an association with DEE exposure in stratified analyses for current (CXCL11/I-TAC, CCL15/MIP-1D and SEGFR), former (IL-16) or never (CCL8/MCP2, CCL13/MCP4, CCL17/TARC and SILRII) smokers, but no evidence of a statistical interaction according to smoking status was apparent (Supplementary Table 3 is available at Carcinogenesis Online).
Figure 1.
(A–C) Box and whisker plot showing association between DEE exposure and CCL2/MCP-1, which showed a significant interaction with smoking status (Pinteraction = 0.01), stratified by current (A), former (B) and never smokers (C). Plots depict unadjusted mean (diamond) and median (line) marker levels with whiskers drawn to the most extreme values that lie within a fence (lower fence = first quartile minus 1.5 times the interquartile range; upper fence = third quartile plus 1.5 times the interquartile range). Observations outside each fence are shown as circles.
Of the four markers that showed an association in DEE-exposed workers compared with control workers overall, an exposure–response relationship with increasing levels of EC was observed for three (Table 3). Specifically, a monotonic exposure–response relationship with decreasing CXCL-11/I-TAC levels was observed with increasing levels of EC (Ptrend = 0.04). The levels of CRP were also consistently lower across tertiles of EC compared with controls and a trend was observed (Ptrend = 0.046), although a clear monotonic relationship was not observed as the lowest CRP levels were found among exposed workers with EC levels in the first tertile. Conversely, monotonic increasing concentrations of CCL15/MIP-1D were apparent across EC exposure groups (Ptrend= 0.005) (Table 3). Furthermore, two additional markers (SGP130 and SILRII) showed evidence of a trend (Ptrend < 0.05) with increasing EC levels, although a clear monotonic exposure-response relationship was not apparent and levels of these markers were not significantly different comparing DEE-exposed to control workers (Table 3; Supplementary Table 1 is available at Carcinogenesis Online). No exposure-response trends with EC exposure were observed for other evaluated markers (Supplementary Table 1 is available at Carcinogenesis Online). Increasing levels of PM2.5 exposure were associated with lower levels of CXCL11/I-TAC only (Ptrend <0.05), whereas five markers that showed evidence of an exposure-response relationship with EC were also associated with levels of OC in the same direction (Supplementary Table 4 is available at Carcinogenesis Online).
Table 3.
Associations between selecteda immune/inflammatory markers and EC exposure categories in a cross-sectional molecular epidemiology study in China
| EC exposure category b | |||||
|---|---|---|---|---|---|
| Marker | Control factory workers | Diesel engine manufacturing workers, EC | |||
| Significant in SWHS | Mean, pg/ml (SD), (n = 55) |
First tertile (n = 18) Mean, pg/ml (SD) |
Second tertile (n = 17) Mean, pg/ml (SD) |
Third tertile (n = 19) Mean, pg/ml (SD) |
P -trend adjusted, (all subjects)c |
| SIL-6R | 31912.0 (7333.7) | 29449.0 (7723.0) | 30605.0 (7470.7) | 29687.0 (8770.9) | 0.13 |
| IL-21 | 3.4 (8.6) | 3.2 (7.6) | 2.4 (4.2) | 2.2 (1.6) | 0.59 |
| CX3CL1/Fractalkine | 89.7 (46.1) | 100.6 (153.4) | 68.5 (41.0) | 85.9 (39.7) | 0.71 |
| SVEGFR2 | 36862.0 (16255.0) | 32013.0 (9884.0) | 33041.0 (11485.0) | 30244.0 (7593.7) | 0.11 |
| SVEGFR3 | 14475.0 (8189.0) | 12769.0 (5929.0) | 13133.0 (5866.0) | 10635.0 (4147.7) | 0.09 |
| CCL2/MCP-1 | 285.3 (73.8) | 286.9 (46.6) | 300.2 (68.2) | 286.4 (50.7) | 0.52 |
| STNFRI | 2459.0 (781.3) | 2267.0 (704.6) | 2283.0 (642.8) | 2156.7 (557.5) | 0.11 |
| IL-10 | 6.9 (8.7) | 6.5 (12.4) | 3.9 (1.8) | 5.9 (3.1) | 0.92 |
| CRP | 1.6 × 107 (2.3 × 107) | 6.2 × 106 (6.4 × 106) | 1.4 × 107 (2.2 × 107) | 7.2 × 106 (6.6 × 106) | 0.046 |
| CCL15/MIP-1D | 2260.4 (997) | 2366.6 (856.9) | 2914.9 (1175.9) | 2938.6 (1191.5) | 0.005 |
| Not Significant in SWHS | |||||
| CXCL11/I-TAC | 30.0 (16.5) | 25.0 (10.3) | 24.2 (10.6) | 23.4 (9.4) | 0.04 |
| IL-16 | 46.9 (31.8) | 51.9 (20.1) | 62.8 (33.5) | 50.2 (18.8) | 0.06 |
| SILRII | 9954.9 (2460.6) | 9683.2 (2554.6) | 11493.0 (2819.9) | 11154.0 (2600.8) | 0.03 |
| SGP130 | 232185.0 (42314.0) | 230348.0 (45553.0) | 262304.0 (51548.0) | 251148.0 (38594.0) | 0.046 |
aMarkers associated with the risk of lung cancer in the SWHS and/or that had a significant association in the cross-sectional study of DEE exposure.
bAdjusted concentrations are presented to characterize EC levels in tertiles of DEE-exposed workers. The median (range) EC (μg/m3) levels for the three categories: first tertile, 23.8 (6.1–39.0); second tertile, 49.7 (39.1–54.5) and third tertile, 69.4 (54.6–107.7).
c P-trend for EC exposure from linear regression models of natural log-transformed immune/inflammatory marker levels adjusted for age, current smoking, current alcohol use, current infection and body mass index. EC trend modeled using a four-level ordinal variable corresponding to controls not exposed to DEE and tertiles of EC among exposed workers.
Discussion
To further evaluate the immune-related effects of DEE exposure and to provide potential mechanistic insight into the DEE-lung cancer association, we conducted a cross-sectional molecular epidemiology study in China. Overall, we found that workers exposed to DEE had alterations in four markers compared with controls (CRP, CXCL11/I-TAC, IL-16 and CCL15/MIP-1D). Two of these (CRP and CCL15/MIP-1D) were considered strong a priori markers based on their associations with lung cancer risk in the SWHS. Interestingly, higher levels of another marker (CCL2/MCP-1), which were positively associated with increased lung cancer risk in the SWHS, were observed in DEE-exposed workers among never and former smokers, but not among current smokers. These findings extend our previous observations of immune-related effects in this study population, and may provide insight into the biologic effects of DEE exposure.
Whereas studies that have evaluated short-term exposure to DEE in humans have reported exposure-related alterations in inflammatory markers (11,12,13), few epidemiologic studies have examined immune markers in workers with long-term exposure to DEE. It is possible that individuals exposed to DEE on a chronic basis at relatively consistent exposure levels over a period of many years may have a distinct immunologic response involving expression of different immune markers or pathways, compared with the acute exposure setting. Another cross-sectional study in China, which included 137 workers occupationally exposed to DEE for an average of ~8 years and 108 unexposed controls (14), observed higher levels of CRP and reduced levels of four chemokines (IL-1β, IL-6, IL-8 and CCL4/MIP-1β) among exposed workers. Interestingly, levels of CCL4/MIP-1β and IL-1β were significantly lower among workers exposed to DEE for 5–10 years compared with control workers in that study, but no significant differences in levels of these markers were observed between workers exposed >10 years and controls. We also previously examined six immune markers among 41 exposed workers and 46 controls from our current study population, and also observed a strong decreasing trend in CCL4/MIP1-β levels with increasing PM2.5, but not EC, concentrations among DEE-exposed workers (Dai et al. submitted). In our current study, levels of CCL4/MIP-1β were inversely correlated with PM2.5 (rsp = −0.25; PM2.5Ptrend = 0.05), although there was no association with EC levels. Taken together, these data provide evidence for an association between DEE exposure and alterations in immune status, although results for specific markers, which were measured using different platforms, are not consistent across studies.
The strongest findings in our study were observed for CRP and CCL15/MIP-1D. CRP is an acute-phase protein that increases in plasma concomitantly with pro-inflammatory cytokines in response to acute inflammation and has been studied extensively in epidemiologic studies (15,16). Overall, epidemiologic evidence has suggested an increased risk of lung cancer in relation to higher circulating levels of CRP, but this association has differed by sex and smoking status. In particular, a meta-analysis of prospective studies reported a significant positive association between CRP and lung cancer in men but not in women (17), and a large study from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial observed a significant positive association for CRP and lung cancer in current and former smokers but not in never smokers (8). Recent preliminary evidence from a pooled analysis of twenty prospective cohorts also found that higher levels of CRP were associated with increased risk of lung cancer overall; however, a reduced risk of lung cancer (RR = 0.95, 95% confidence interval, 0.90–1.00) for higher levels of CRP was observed in never-smokers (18), which is consistent with the direction of the association observed among never-smoking women in the SWHS.
Our findings showing lower CRP levels among DEE-exposed workers are not consistent with the direction of our a priori hypothesis, but may be because of correlation patterns between CRP and other immune parameters or may signal a shift away from an acute inflammatory response in these workers who were exposed to DEE for an average of 20 years. For example, our previous study (5) showed a decrease in neutrophils and monocytes in DEE-exposed workers compared with controls, and each of these cell types were positively correlated with CRP in our current study. Although the CRP levels were also consistently lower in exposed workers relative to controls regardless of exposure duration in the current study, the largest differences were observed among those exposed for longer periods of time (i.e. >17 years). Interestingly, reduced levels of CRP have also been reported in a cross-sectional study of Chinese workers exposed to formaldehyde compared with an unexposed control population (19). Given that East Asians have lower background levels of CRP compared with Western populations (20), further evaluation of the relationship between levels of CRP in relation to environmental exposures and lung cancer risk would be informative in this population.
Higher levels of CCL15/MIP-1D were consistently associated with both higher risk of lung cancer in the SWHS and DEE exposure in our study. CCL15/MIP-1D is a member of the CC chemokine family and is particularly chemotactic for T cells and monocytes. It is expressed in human lung leukocytes and has been associated with asthma severity as well as angiogenesis and survival in relation to lung cancer (21,22). The increasing exposure–response relationship between levels of EC and this marker is consistent with our previous observations showing an exposure–response relationship between EC levels, CD4+ T cells and CD8+ T cells among DEE-exposed workers (5). There was no correlation between these T-cell subsets and levels of CCL15/MIP-1D, and further adjustment for lymphocyte counts had minimal effect on the CCL15/MIP-1D association, suggesting that this finding is independent of previously reported effects on blood cell counts.
CXCL-11/I-TAC and IL-16, which also showed differences in DEE-exposed workers, are also chemokines that function as attractants for specific types of white blood cells. Higher levels of CXCL11/I-TAC were previously observed to be associated with increased future risk of lung cancer in the PLCO Screening Trial, although the association was only observed in one of two phases of that study (8). These findings may provide insight into the immunologic effects of DEE exposure, although the absence of an association between these markers and lung cancer risk in the SWHS and the opposite direction of the CXCL-11/I-TAC finding in DEE-exposed workers compared with the lung cancer association in PLCO suggests that these markers are unlikely to be mediators of the DEE and lung cancer relationship. The observed associations for these two markers in our DEE cross-sectional study, but not in the study of lung cancer in the SWHS, could reflect differences by sex or exposure characteristics inherent to the two studies. For example, some immune markers or pathways involved in lung carcinogenesis may be distinct in the setting of high DEE exposure versus those involved in cases without identified high exposure to this known risk factor. Alternatively, these two markers may fundamentally not reflect changes that are pertinent to lung carcinogenesis in the Chinese population.
The risk of lung cancer in relation to DEE exposure was previously observed to be attenuated among heavy smokers (23). Therefore, we evaluated interactions between DEE exposure and levels of the evaluated markers by smoking status. Increasing levels of CCL2/MCP-1, which were positively associated with lung cancer risk in the SWHS, were higher in DEE-exposed workers among never and former smokers, but not current smokers. CCL2/MCP-1, a key chemokine that regulates migration of monocytes, has been observed to be associated with smoking (24) and was positively correlated with current smoking among controls in our study (rsp = +0.43, P = 0.001). Consequently, an effect of DEE on levels of this marker may be less apparent among current smokers compared with non-smokers given higher baseline levels among controls within the current smoking stratum. The plausibility for a relationship between DEE exposure and CCL2/MCP-1 levels is strengthened by animal studies showing increased expression levels of this marker following exposure to DEE (25,26).
A strength of our study was the availability of personal measurements of EC exposure, which enabled an evaluation of exposure-response relationships with levels of these markers. Furthermore, extensive site visits and exposure assessments in the control factories ensured that these workers were not exposed to occupational sources of DEE. Finally, a unique aspect of our study was the availability of immune marker data measured using the same multiplex panels from a nested case–control study of lung cancer in the SWHS, which provided an opportunity to link our findings in DEE-exposed workers to markers that are prospectively associated with risk of lung cancer in the same ethnic population. It would be informative in future studies to evaluate the relationship between these markers and the lower levels of DEE found in contemporary workplaces and urban areas such as Shanghai. These data would provide potential insight into the relevance of these markers with respect to lung cancer risk in the context of other exposure scenarios. The fact that we did identify some overlapping markers in our cross-sectional study of DEE exposure and the SWHS lung cancer study, despite differences in exposure characteristics, is intriguing and suggests that those specific markers may be on a common pathway involving both the immune response to DEE and lung carcinogenesis in the Chinese population.
The primary limitation of our study was the relatively small sample size, which resulted in an inability to conduct comprehensive analyses evaluating exposure-response relationships with EC by smoking status. In addition, all of the participants in our cross-sectional study were male and consequently the relationship between occupational DEE exposure and these markers may not be generalizable to women. Although the control factories were chosen from the same geographic area as the diesel engine testing facility, and exposed and control workers were very similar with respect to key demographic and lifestyle characteristics, we cannot definitively rule out that other unmeasured factors may have influenced the differences in immune marker levels that we observed. Therefore, larger studies will be needed to replicate and extend our findings in the Chinese population as well as to evaluate whether our observations are generalizable to other ethnic populations and women.
In summary, we measured plasma levels of 64 immune/inflammatory markers from a multiplex panel in a cross-sectional molecular epidemiology study of occupational DEE exposure in China. We observed associations between occupational DEE exposure and levels of three markers that were associated with lung cancer risk in the SWHS, including CRP and CCL15/MIP-1D overall as well as CCL2/MCP-1 in non-smokers only. Our observations may provide some insight into the underlying biologic mechanisms of the DEE-lung cancer association, but will require replication in larger studies of DEE exposure as well as in other population-based, prospective studies of Chinese individuals.
Funding
This work was supported by intramural funds from the National Cancer Institute and the National Institutes of Health, and the Key Program of National Natural Science Foundation of China [grant number NSFC 81130050].
Conflict of Interest Statement: None declared.
Supplementary Material
Abbreviations
- DEE
diesel engine exhaust
- EC
elemental carbon
- IL
interleukin
- OC
organic carbon
- PM
particulate matter
- SWHS
Shanghai Women’s Health Study
References
- 1. McDonald J.D., et al. (2011) Engine-operating load influences diesel exhaust composition and cardiopulmonary and immune responses. Environ. Health Perspect., 119, 1136–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International Agency for Research on Cancer (2012). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Diesel and Gasoline Engine Exhausts and some Nitroarenes http://monographs.iarc.fr/ENG/Monographs/vol105/mono105.pdf (15 October 2016, date last accessed).
- 3. Benbrahim-Tallaa L., et al. ; International Agency for Research on Cancer Monograph Working Group (2012) Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet. Oncol., 13, 663–664. [DOI] [PubMed] [Google Scholar]
- 4. Shiels M., et al. (2017) A prospective study of immune and inflammation markers and risk of lung cancer among female never smokers in Shanghai. Carcinogenesis, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lan Q., et al. (2015) Occupational exposure to diesel engine exhaust and alterations in lymphocyte subsets. Occup. Environ. Med., 72, 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The National Institute for Occupational Safety and Health (NIOSH). NIOSH Manual of Analytical Methods 4th Edition: Diesel Particulate Matter (as Elemental Carbon) http://www.cdc.gov/niosh/docs/2003–154/pdfs/5040.pdf (15 October 2016, date last accessed).
- 7. Vermeulen R., et al. (2014) Exposure-response estimates for diesel engine exhaust and lung cancer mortality based on data from three occupational cohorts. Environ. Health Perspect., 122, 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shiels M.S., et al. (2015) Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J. Natl. Cancer Inst., 107, djv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grivennikov S.I., et al. (2010) Immunity, inflammation, and cancer. Cell, 140, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho W.C., et al. (2011) The role of inflammation in the pathogenesis of lung cancer. Expert Opin. Ther. Targets, 15, 1127–1137. [DOI] [PubMed] [Google Scholar]
- 11. Pourazar J., et al. (2004) Diesel exhaust exposure enhances the expression of IL-13 in the bronchial epithelium of healthy subjects. Respir. Med., 98, 821–825. [DOI] [PubMed] [Google Scholar]
- 12. Xu Y., et al. (2013) Effects of diesel exposure on lung function and inflammation biomarkers from airway and peripheral blood of healthy volunteers in a chamber study. Part. Fibre Toxicol., 10, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stenfors N., et al. (2004) Different airway inflammatory responses in asthmatic and healthy humans exposed to diesel. Eur. Respir. J., 23, 82–86. [DOI] [PubMed] [Google Scholar]
- 14. Dai Y., et al. (2016) Long-term exposure to diesel engine exhaust affects cytokine expression among occupational population. Toxicol. Res., 5, 674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salazar J., et al. (2014) C-reactive protein: clinical and epidemiological perspectives. Cardiol. Res. Pract., 2014, 605810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo Y.Z., et al. (2013) Association between C-reactive protein and risk of cancer: a meta-analysis of prospective cohort studies. Asian Pac. J. Cancer Prev., 14, 243–248. [DOI] [PubMed] [Google Scholar]
- 17. Zhou B., et al. (2012) C-reactive protein, interleukin 6 and lung cancer risk: a meta-analysis. PLoS One, 7, e43075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muller D.C., et al. (2016) C-reactive protein and risk of lung cancer: a pooled analysis of 20 prospective cohorts. [abstract]. In Proceedings of the 107th Annual Meeting of the American Association for Cancer Research, 16–20 April2016; New Orleans, LA; Philadelphia, PA: AACR; Cancer Res 2016;76(suppl. 14):Abstract no. 4289.15. [Google Scholar]
- 19. Seow W.J., et al. (2015) Circulating immune/inflammation markers in Chinese workers occupationally exposed to formaldehyde. Carcinogenesis, 36, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saito I., et al. (2014) C-reactive protein and cardiovascular disease in East asians: a systematic review. Clin. Med. Insights. Cardiol., 8(suppl. 3), 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bodelon C., et al. (2013) Circulating levels of immune and inflammatory markers and long versus short survival in early-stage lung cancer. Ann. Oncol., 24, 2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shimizu Y., et al. (2012) CC-chemokine CCL15 expression and possible implications for the pathogenesis of IgE-related severe asthma. Mediators Inflamm., 2012, 475253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Silverman D.T., et al. (2012) The Diesel Exhaust in Miners study: a nested case-control study of lung cancer and diesel exhaust. J. Natl. Cancer Inst., 104, 855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McDermott D.H., et al. (2005) CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation, 112, 1113–1120. [DOI] [PubMed] [Google Scholar]
- 25. Saber A.T., et al. (2006) Cytokine expression in mice exposed to diesel exhaust particles by inhalation. Role of tumor necrosis factor. Part. Fibre Toxicol., 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rao K.M., et al. (2005) Time course of gene expression of inflammatory mediators in rat lung after diesel exhaust particle exposure. Environ. Health Perspect., 113, 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.