In this study, we investigated the association of glycemic index and RCC risk. Our findings suggest that a high-GI diet is associated with an increased risk of RCC, especially for individuals with hypertension or high body mass index.
Abstract
Carbohydrate intake affects postprandial glucose levels and insulin response, which plays a role in carcinogenesis. The relationship between carbohydrate intake, dietary glycemic index (GI) and glycemic load (GL), and risk of renal cell carcinoma (RCC) remains unclear. We conducted a case–control study including 854 patients with newly diagnosed RCC (cases) and 1255 healthy participants (controls) recruited since 2002. GI, GL and carbohydrate intake were obtained via a validated food frequency questionnaire. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multivariable logistic regression, adjusting for potential confounders. We found that higher GI was significantly associated with RCC risk with an OR of 1.32 (95% CI, 0.99–1.74; Ptrend = 0.026) (the highest versus the lowest quartiles). We also observed an inverse association between fiber intake and RCC risk with OR of 0.70 (95% CI = 0.50–0.99) as well as between starch intake and risk of RCC with OR of 0.65 (95% CI = 0.49–0.87). Individuals with a high-GI diet and hypertension or high body mass index (BMI) had a 2.7 times (OR = 2.67, 95% CI = 1.96–3.64) and two times (OR = 1.95, 95% CI = 1.29–2.92) higher RCC risk, respectively, than those without these factors. Our findings suggest that a high-GI diet is associated with an increased risk of RCC, whereas increased fiber and starch intakes appear to be associated with a decreased risk of RCC. We found that reducing GI levels and increasing fiber intake could be a dietary strategy to decrease RCC risk, especially for individuals with hypertension or high BMI.
Introduction
Renal cell carcinoma (RCC), the most common histologic type of kidney cancer, is now the eighth most frequent cancer in the USA (1). Established risk factors for RCC include hypertension, obesity, tobacco use, family history of kidney cancer and certain dietary factors (2). One such proposed dietary factor is carbohydrate intake, which has been implicated in the etiology of several other cancers (3).
Carbohydrate-rich foods affect postprandial glucose response and insulin levels at different rates, depending on the types of carbohydrates ingested, the amount of fiber the food contains and the food-processing method (4). The glycemic effect from any food is expressed as the glycemic index (GI), a ranking system of how quickly the food raises blood glucose levels, assuming the food contains a standardized amount of digestible carbohydrates (5). Glycemic load (GL) estimates the blood glucose response using the GI and the quantity of carbohydrates consumed. Compared with a low-GI diet, a high-GI diet will result in dramatic fluctuations of postprandial blood glucose levels, leading to high circulating levels of insulin and insulin-like growth factor I (IGF1), which play critical roles in regulating cell proliferation and differentiation and thus may affect cancer risk (6).
Observational studies have shown that a diet high in GI and/or GL is related to increased risk of multiple cancers including colorectal, breast and endometrial cancer (7,8), but there are few studies on kidney cancer and findings from available studies are inconsistent. A case–control study in Italy indicated that high-GI and high-GL diets were associated with an increased risk of RCC (9). In contrast, a cohort study in the United States (10) and a case–control study in Canada (11) that investigated GI, GL and the risk of several types of cancer did not find a statistically significant association between GI, GL and kidney cancer risk. The three studies were from three different countries with different study designs. In addition, although the three studies presented the multivariable-adjusted associations, some important potential confounders (e.g. sodium intake and overall diet quality) were not considered or adjusted. The few studies that have investigated associations between total carbohydrate intake, intake of different types of carbohydrates (starch, sugar and fiber), and RCC risk have also yielded conflicting results (12–14).
We therefore investigated the associations between GI, GL and carbohydrate intake (total carbohydrates, fiber, starch and sugar) and risk of RCC and whether these associations varied by known or potential risk factors using data from a large case–control study.
Materials and methods
Study population
Our study used data from an ongoing case–control study of kidney cancer initiated in 2002 and was approved by The University of Texas MD Anderson Cancer Center Institutional Review Board. Study design and subject recruitment criteria have been described previously (15). Briefly, all cases were patients with a recent diagnosis (diagnosis within 1 year of enrollment) of RCC that was histologically confirmed. Controls were healthy participants without a history of cancer (except non-melanoma skin cancer) who were identified and recruited using the random digit dialing method at the time cases were recruited. The overall response rate for RDD screening was 51% and, among those who agreed to participate, the response rate was 88%. The response rate for the eligible cases was 87%. All cases and controls gave written informed consent before participation in the study.
Data collection
An in-person interview was conducted by MD Anderson staff to obtain epidemiological data, including but not limited to, demographics, education, smoking, family history of cancer/kidney cancer, history of hypertension/diabetes prior to diagnosis (cases) or recruitment (controls) and physical activity. Body mass index (BMI; kg/m2) was calculated through self-reported usual height and weight and categorized into three groups (normal and underweight <25 kg/m2; overweight 25–29.9 kg/m2; obese ≥30 kg/m2) according to the standard classifications of the World Health Organization (WHO). Participants also reported the average frequency they spent on five broad groups of activities in the year before interview. The grouping of activities was based on strenuousness, and the activities included in each group have similar strenuousness. A metabolic equivalent value was assigned on the basis of the energy cost of each activity group (16,17). Energy expenditure from physical activity was calculated as the metabolic equivalent value of each activity multiplied by the frequency of each activity and then summed across all activities. The weekly metabolic equivalent score (MET) of activity was estimated, and participants were categorized into tertiles based on the distribution in the control group. A never smoker was an individual who had never smoked or had smoked less than 100 cigarettes in his or her lifetime. An individual who had smoked at least 100 cigarettes in his or her lifetime but had quit at least 12 months before diagnosis (for cases) or before the interview (for controls) was defined as a former smoker. Current smokers were those who were currently smoking or had quit <12 months before diagnosis (cases) or before enrollment (controls). Ever smokers included former and current smokers.
A previously validated food frequency questionnaire (a modified version of the National Cancer Institute’s Health Habits and History Questionnaire (18) was used to assess diet during the year before diagnosis (cases) or before enrollment (controls). The questionnaire includes a semi-quantitative food frequency list made up of food and beverage items and an open-ended section regarding dietary behaviors such as dining in restaurants and food preparation methods. For each type of food, a commonly used unit or portion size was specified and frequency was collected. The amount consumed (g/day) of each food or beverage item and total intake of energy, carbohydrates, fiber, starch, sugar and alcohol were calculated using the USA Department of Agriculture National Nutrient Database for Standard Reference and Dietary Studies (19). All nutrient and food variables of interest were energy adjusted for total caloric intake using the residual method (20).
GI and GL calculations
GI values (using glucose as the reference food) were obtained from published international tables (4) and were assigned to individual foods in the food frequency questionnaire with the best match possible, as previously described (21). The GL for each food was calculated by multiplying the available carbohydrate content of the food consumed by its GI value, and total daily GL was the sum of the GL of all individual food items divided by 100 (4). The overall GI, which reflects the average quality of carbohydrates consumed, was estimated by dividing the total GL by the total daily available carbohydrate intake. To avoid overestimating the glycemic effect from any food containing fiber, especially high-fiber foods, we removed fiber from the total carbohydrates to calculate available carbohydrates (4,10).
Statistical analysis
We excluded 95 individuals with outlying total energy intake (i.e., outside the interval delimited by the 25th percentile minus 1.5 times the interquartile range and the 75th percentile plus 1.5 times the interquartile range), and our final analysis included 854 cases and 1255 controls. Baseline characteristics were compared between cases and controls using the Student t-test for continuous variables and the Pearson chi-squared test for categorical variables. Participants were categorized into sex-specific quartiles by GI, GL, total carbohydrate, fiber, starch and sugar intake based on the distribution in the control group. GI and GL were also treated as continuous variables by dividing the original GI or GL by its interquartile range of the sex-specific distribution among the controls. Unconditional multivariable logistic regression was used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the association of GI, GL and carbohydrate intake with RCC risk after adjusting for potential confounders identified on the basis of a priori knowledge. We included age (continuous), sex and energy intake (kcal/day; continuous) in the minimally adjusted model (model 1). A second model (model 2) also adjusted for education, smoking status, history of hypertension, physical activity, usual BMI and family history of kidney cancer. The fully multivariable model (model 3) further included Healthy Eating Index (HEI)-2015, which was calculated according to (https://epi.grants.cancer.gov/hei/developing.html). Other potential confounders considered but not included in the multivariable model were race/ethnic, history of diabetes, alcohol consumption and sodium intake; these variables were not included because they did not improve the fit and predictive power of the model by the likelihood ratio test. Exposure variables were treated as ordinal variables to test for trends across quartiles in the model.
We also conducted stratified analyses to explore potential effect modifications on continuous increments of GI and GL values and RCC risk by known or potential risk factors. Multiplicative interaction was determined using the Wald statistic. Joint effects of GI and GL with hypertension, BMI were also assessed using the multivariable model by adjusting the appropriate covariates.
Analyses were conducted using Stata 13.0 (StataCorp LP, College Station, TX). Two-sided P < 0.05 were considered statistically significant.
Results
Table 1 shows the distribution of selected characteristics and dietary factors for cases and controls. Cases and controls differed in age, sex, education, ethnicity, family history of kidney cancer and hypertension. Cases were more likely to be obese and have a lower level of physical activity than controls (P < 0.001). Also, cases had significantly lower HEI-2015 (P < 0.001). GI and GL exposures and total energy intake were higher while fiber and alcohol intake were lower in cases than in controls. The top 30 foods that contributed most to the total GL were presented in Supplementary Table 1, available at Carcinogenesis Online.
Table 1.
Selected characteristics of cases (patients with renal cell carcinoma) and controls (healthy participants)a
| Variable | Case (N = 854) | Control (N = 1255) | P value |
|---|---|---|---|
| Mean age (SD), years | 58.72 (10.58) | 61.57 (10.31) | <0.001 |
| Sex | |||
| Male | 567 (66.4) | 959 (76.4) | |
| Female | 287 (33.6) | 296 (23.6) | <0.001 |
| Educationb | |||
| High school and below | 234 (27.7) | 230 (18.3) | |
| Some college | 242 (28.6) | 299 (23.8) | |
| College and above | 369 (43.7) | 725 (57.8) | <0.001 |
| Race/ethnicity | |||
| Non-Hispanic White | 765 (89.6) | 1187 (94.6) | |
| Other | 89 (10.4) | 68 (5.4) | <0.001 |
| Smoking status | |||
| Never | 449 (52.6) | 607 (48.4) | |
| Former | 313 (36.7) | 497 (39.6) | |
| Current | 92 (10.8) | 151 (12.0) | 0.161 |
| Family history of kidney cancerb | |||
| No | 812 (95.2) | 1224 (97.7) | |
| Yes | 41 (4.8) | 29 (2.3) | 0.001 |
| Hypertension | |||
| No | 334 (39.1) | 682 (54.3) | |
| Yes | 520 (60.9) | 573 (45.7) | <0.001 |
| Diabetes mellitusb | |||
| No | 702 (82.7) | 1046 (83.5) | |
| Yes | 147 (17.3) | 207 (16.5) | 0.629 |
| Usual BMI (kg/m2) b | |||
| Normal or underweight (<25) | 129 (15.3) | 312 (24.9) | |
| Overweight (25–29.99) | 341 (39.6) | 524 (41.8) | |
| Obese (≥30) | 374 (44.3) | 419 (33.4) | <0.001 |
| Physical activity (METs/week)b,c | |||
| Low | 399 (51.3) | 395 (32.0) | |
| Medium | 195 (25.1) | 416 (33.7) | |
| Intensive | 184 (23.7) | 424 (34.3) | <0.001 |
| Healthy Eating Index-2015, mean (SD) | 64.15 (9.40) | 67.33 (9.55) | <0.001 |
| Dietary factors, mean (SD)d | |||
| GI | 49.04 (5.85) | 47.75 (7.22) | <0.001 |
| GL | 119.20 (29.69) | 115.96 (25.71) | 0.007 |
| Total energy, kcal/day | 2351.02 (886.00) | 2016.06 (789.04) | <0.001 |
| Carbohydrate, g/day | 241.82 (81.76) | 241.17 (45.11) | 0.765 |
| Fiber, g/day | 20.07 (7.28) | 21.78 (7.62) | <0.001 |
| Starch, g/day | 36.72 (17.00) | 37.59 (15.10) | 0.222 |
| Sugar, g/day | 185.02 (48.68) | 181.80 (39.87) | 0.109 |
| Sodium, mg/day | 2892.89 (678.83) | 2882.85 (600.62) | 0.721 |
| Alcohol, g/day | 7.72 (23.47) | 11.16 (18.34) | <0.001 |
aData are number of individuals (%) unless otherwise indicated.
bParticipants with missing data: years of education, n = 10; family history of kidney cancer, n = 3; diabetes mellitus, n = 7; unknown usual BMI, n = 10; and unknown physical activity, n = 96.
cTertiles based on the METs/week distribution in the control group.
dVariables were energy adjusted using the residual method.
Table 2 presents adjusted ORs and corresponding 95% CIs in three models for the association between RCC risk and increasing quartile of GI and GL exposures and carbohydrate, fiber, starch and sugar intake. Higher GI diets were positively associated with RCC risk (Ptrend = 0.026), and the OR for the highest GI quartile was 1.32 (95% CI = 0.99–1.74) compared with the lowest quartile in the fully adjusted model. The OR was 1.12 (95% CI = 1.03–1.23) when GI was treated as a continuous variable. GL was not associated with RCC risk in any model. Participants in the highest quartile for intake of fiber and starch had a significantly lower risk of RCC than those in the lowest quartile (fiber: OR = 0.70, 95% CI = 0.50–0.99; starch: OR = 0.65, 95% CI = 0.49–0.87). As shown in Supplementary Table 2, available at Carcinogenesis Online, starches from high GI (>55) foods were more closely associated with RCC risk compared to starches from low GI (≤55) foods. Total carbohydrate intake was not significantly associated with RCC risk. The highest quartile for sugar intake was associated with a significantly higher risk of RCC compared with the lowest quartile (OR = 1.33, 95% CI = 1.01–1.74). Also, we analyzed the association between different types of sugar (glucose, fructose, sucrose, galactose, lactose and maltose) and RCC risk. As shown in Supplementary Table 3, available at Carcinogenesis Online, there were differences in relationship with RCC depending on the type of sugar, and higher intakes of glucose, fructose and sucrose were significantly associated with RCC risk while other sugars were not.
Table 2.
Associations of glycemic index, glycemic load and carbohydrate intake by quartilea with renal cell carcinoma risk
| Variable | OR (95% CI) | P value fortrend | Continuous OR(95% CI)e | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Glycemic index | ||||||
| Cases/controls, n | 192/314 | 158/314 | 195/314 | 309/313 | ||
| Model 1b | 1 (ref) | 1.04 (0.79–1.37) | 1.34 (1.02–1.75) | 1.76 (1.38–2.26) | <0.001 | 1.23 (1.12–1.36) |
| Model 2c | 1 (ref) | 0.94 (0.70–1.26) | 1.13 (0.84–1.51) | 1.45 (1.11-–1.90) | 0.003 | 1.15 (1.05–1.27) |
| Model 3d | 1 (ref) | 0.90 (0.67–1.22) | 1.06 (0.79–1.42) | 1.32 (0.99–1.74) | 0.026 | 1.12 (1.03–1.23) |
| Glycemic load | ||||||
| Cases/controls, n | 229/313 | 178/315 | 187/313 | 260/314 | ||
| Model 1b | 1 (ref) | 0.98 (0.75–1.27) | 1.04 (0.80–1.35) | 1.19 (0.93–1.52) | 0.146 | 1.13 (1.02–1.25) |
| Model 2c | 1 (ref) | 0.91 (0.69–1.21) | 1.00 (0.75–1.33) | 1.11 (0.84–1.45) | 0.381 | 1.10 (0.99–1.23) |
| Model 3d | 1 (ref) | 0.94 (0.71–1.25) | 1.05 (0.79–1.40) | 1.15 (0.88–1.51) | 0.232 | 1.12 (1.00–1.25) |
| Total carbohydrate | ||||||
| Cases/controls, n | 249/314 | 188/314 | 172/313 | 245/314 | ||
| Model 1b | 1 (ref) | 0.90 (0.70–1.17) | 0.82 (0.64–1.07) | 0.96 (0.75–1.23) | 0.612 | 1.00 (0.91–1.09) |
| Model 2c | 1 (ref) | 0.89 (0.67–1.17) | 0.86 (0.65–1.15) | 1.00 (0.77–1.31) | 0.967 | 1.01 (0.91–1.11) |
| Model 3d | 1 (ref) | 0.94 (0.71–1.24) | 0.93 (0.70–1.25) | 1.15 (0.87–1.52) | 0.362 | 1.06 (0.95–1.18) |
| Fiber | ||||||
| Cases/controls, n | 302/313 | 190/315 | 189/314 | 173/313 | ||
| Model 1b | 1 (ref) | 0.79 (0.61–1.01) | 0.74 (0.58–0.95) | 0.55 (0.43–0.71) | <0.001 | 0.77 (0.69–0.85) |
| Model 2c | 1 (ref) | 0.79 (0.60–1.04) | 0.73 (0.55–0.96) | 0.60 (0.45–0.79) | <0.001 | 0.81 (0.72–0.90) |
| Model 3d | 1 (ref) | 0.85 (0.64–1.14) | 0.82 (0.60–1.11) | 0.70 (0.50–0.99) | 0.049 | 0.85 (0.75–0.98) |
| Starch | ||||||
| Cases/controls, n | 229/314 | 239/313 | 200/315 | 186/313 | ||
| Model 1b | 1 (ref) | 1.24 (0.96–1.59) | 1.01 (0.78–1.30) | 0.75 (0.58–0.98) | 0.014 | 0.93 (0.84–1.02) |
| Model 2c | 1 (ref) | 1.14 (0.87–1.50) | 0.93 (0.71–1.23) | 0.64 (0.48–0.85) | 0.001 | 0.89 (0.80–0.99) |
| Model 3d | 1 (ref) | 1.16 (0.88–1.53) | 0.94 (0.71–1.24) | 0.65 (0.49–0.87) | 0.002 | 0.89 (0.80–0.99) |
| Sugar | ||||||
| Cases/controls, n | 251/314 | 161/314 | 164/313 | 278/314 | ||
| Model 1b | 1 (ref) | 0.80 (0.61–1.04) | 0.83 (0.64–1.09) | 1.18 (0.93–1.49) | 0.152 | 1.06 (0.97–1.15) |
| Model 2c | 1 (ref) | 0.78 (0.58–1.03) | 0.82 (0.62–1.10) | 1.21 (0.93–1.57) | 0.124 | 1.08 (0.98–1.19) |
| Model 3d | 1 (ref) | 0.82 (0.61–1.09) | 0.90 (0.67–1.21) | 1.33 (1.01–1.74) | 0.025 | 1.12 (1.02–1.23) |
aQuartiles are based on the distribution in the controls group by sex and are energy-adjusted.
bAdjusted for age, sex and total energy intake.
cAdjusted for age, sex, total energy intake, smoking status, hypertension, education, usual BMI, physical activity and family history of kidney cancer.
dAdjusted for age, sex, total energy intake, smoking status, hypertension, education, usual BMI, physical activity, family history of kidney cancer and Healthy Eating Index-2015.
eContinuous values were calculated by dividing intake by the difference between the 75th and 25th percentiles.
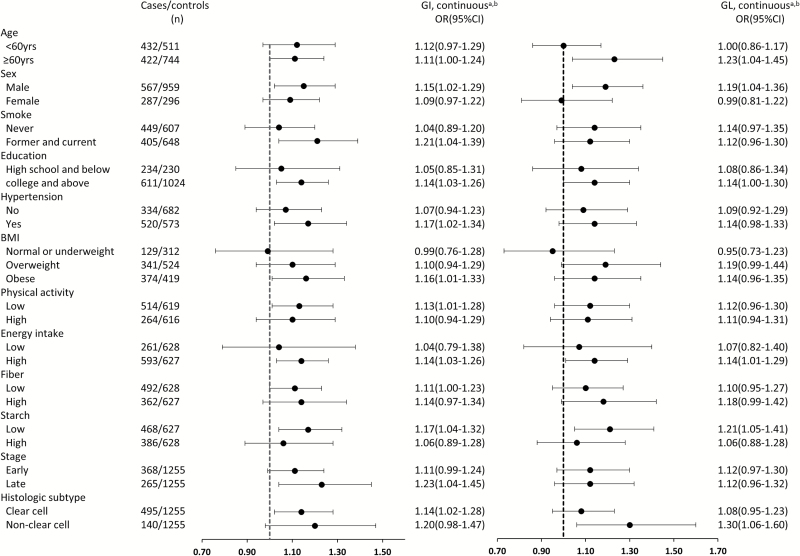
Figure 1 shows the association of GI and GL with RCC risk stratified by age, sex, smoking status, education, hypertension, BMI, physical activity, total energy intake, fiber intake, starch intake, stage and histological subtype. These results were consistent with our overall findings: the risk associated with RCC increased with the increase of GI as a continuous factor within each stratum, although no evidence of interaction was observed across these strata (Pinteraction > 0.05). The ORs of increasing GI for RCC appeared to be greater in in men, in former and current smokers, in individuals with hypertension, in individuals with low physical activity, in individuals who were obese or overweight, in individuals with low starch intake and for late state RCC. Also, we conducted a stratified analysis between GI/GL and RCC risk by four groups when BMI (<25 kg/m2 versus ≥25 kg/m2) and hypertension (yes or no) were modeled together (Supplementary Table 4, available at Carcinogenesis Online).
Figure 1.
Associations of glycemic index (GI) and glycemic load (GL) with renal cell carcinoma risk stratified by selected covariates. aContinuous values were calculated by dividing intake by the difference between the 75th and 25th percentiles; bFully adjusted model where appropriate and No P values for interaction across strata were statistically significant; cPhysical activity (Mets/week) was divided by median in control group; dEnergy intake (kcal/day) was divided by median in control group, by sex.
In stratified analyses between fiber intake and RCC risk by BMI and hypertension (Supplementary Table 5, available at Carcinogenesis Online), higher fiber intake was associated with lower RCC risk only among those who were hypertensive or those who were overweight/obese; the protective effect was the most pronounced among those who were hypertensive and overweight/obese.
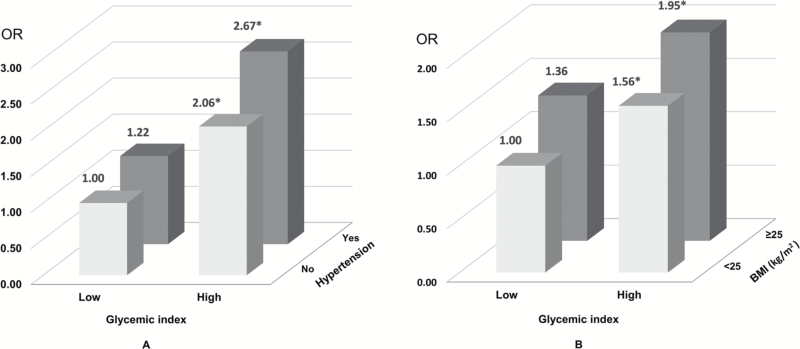
The joint effects of GI with hypertension (yes or no) or BMI (<25 kg/m2 or ≥25 kg/m2) on RCC risk are shown in Figure 2. The ORs for the combination of high GI with hypertension (yes), high BMI were 2.67 (95% CI = 1.96–3.64), 1.95 (95% CI = 1.29–2.92) respectively, compared with the opposite extremes.
Figure 2.
Joint effect of glycemic index with hypertension (A) or body mass index (BMI) (B) on renal cell carcinoma risk (*P values for ORs <0.05). The cutoff for glycemic index (low or high) was defined by the median values of the control group by sex. We used the fully adjusted model where appropriate.
Discussion
To our knowledge, this is the first U.S. study to report an independent positive association between high GI exposures and RCC risk. Our results indicate a 32% increased risk of RCC with the highest GI exposure, whereas the highest dietary fiber and starch intakes were associated with a 30 and 35% decreased risk of RCC, respectively. Our study also is the first to suggest that high GI levels combined with other factors (e.g. hypertension and being overweight or obese) are associated with greater RCC risk. We observed a 2.7 times higher risk of RCC in individuals with a high-GI diet and hypertension and a two times higher risk in overweight or obese individuals with a high-GI diet than in those without these factors.
Our observation that a high-GI diet was associated with a 32% increased risk of RCC is consistent with the Italian case–control study findings of an OR of 1.43 (95% CI = 1.05–1.95) for the highest quintile of GI compared with the lowest quintile (9). The Italian study also found an OR of 2.56 (95% CI = 1.78–3.70) for the highest quintile of GL, but our study did not find a significant association between GL and RCC risk. Meanwhile, the Canadian case–control study showed non-significantly increased ORs for RCC with high GI (OR = 1.18, 95%CI = 0.98–1.43) and high GL (OR = 1.09, 95% CI = 0.69–1.22) (11). In addition, the U.S. cohort study among a population older than 50 years found a non-significant reduction of RCC risk with high GI and GL (10). Different populations and different methods in these studies might account for the variations in findings and further research is needed essentially to clarify the potential role for high GI and GL exposures in RCC etiology.
Our results suggested that GI, but not GL which considers both the quality and quantity of carbohydrate, was associated with RCC risk, suggesting that the quality of carbohydrates may play a more important role than the quantity in renal cell carcinogenesis. Consistent with this notion, our results also showed that the total amount of carbohydrates was not associated with RCC risk. In addition, high dietary GI but not GL has been consistently and significantly associated with increased risk of colorectal cancer in both cohort and case–control studies (7). One study further analyzed carbohydrate intake from high GI and that from low medium GI foods separately (22). The results showed that only high carbohydrate intake from high GI foods significantly increased the risk of colorectal cancer, but the high carbohydrate intake from low GI foods significantly decreased the risk.
It has been suggested that a high-GI diet could increase cancer risk by elevating circulating glucose and insulin concentrations, which can promote glucose intolerance, insulin resistance and hyperinsulinemia (23). High levels of circulating insulin may promote cancer development by affecting insulin-like growth factor-binding proteins and by increasing the bioactivity of IGF1, which has proliferative, angiogenic, antiapoptotic and estrogen-stimulating properties (24). Additionally, data from a rat model of diabetes showed that sustained hyperglycemia resulted in an adaptive metabolic response, growth factor signaling alterations and subsequent transformation of tubular epithelial cells (the cells from which RCC develops) and nephron carcinogenesis (25). Another study showed that long-term exposure of tubular cells to hyperglycemia may cause disturbances in DNA repair mechanisms and thus may lead to RCC development (26). However, the evidence for the association between insulin and RCC is still debated, as controversial data have been reported a null or inverse association (27,28). Further research is needed to understand the underlying mechanisms linking GI/GL and RCC risk in human.
The few previous studies that have examined the relationship between RCC and intake of different types of carbohydrates have also yielded conflicting results. Similar to our findings for fiber intake, results from a cohort study in the U.S. showed that total fiber intake was associated with a significantly decreased risk of RCC (15–20%) (14), although two other prospective studies found null associations (29,30). Dietary fiber may lower RCC risk by slowing digestion and lowering the postprandial glucose response, which improves insulin sensitivity, reduces the generation and absorption of potential toxins, and lowers plasma levels of systemic inflammation biomarkers (14). A meta-analysis suggests that fiber from different sources may have different effects and fiber from vegetables and legume may play a more important role in RCC prevention compared with fiber from other sources (31). Although no association between starch intake and RCC risk was observed in the European Prospective Investigation into Cancer and Nutrition (EPIC) study (13), an increased risk of RCC with higher starch intake was found in a case–control study in Italy (12). Conversely, in our study, starch intake, especially when from high GI foods, was inversely associated with RCC risk. The source and nature of dietary starch may be a major determinant of glucose and insulin response, and major food sources for dietary starch likely differ among the Italian population (one of the populations with the highest starch intake in Europe), other European populations and the U.S. population, and this may partially explain the discrepant findings in studies of different populations.
In our study, total carbohydrate was not significantly associated with RCC risk and the finding is consistent with the results from the EPIC cohort (13). Furthermore, our results suggest that the highest level of total sugar intake may increase the risk of RCC and different types of sugar may have differential effects. Diets rich in sugar, rather than starch, have been shown to have adverse effects on health including diabetes, obesity and possibly some cancers by raising levels of triglycerides and insulin (32). As different components of carbohydrates have diverse effects on the postprandial glucose response and insulin levels, one scientific consensus suggested that reducing starch gelatinization and increasing viscous fiber or fructose content could reduce the GI of common carbohydrate-rich foods (5) and thus potentially reduce the risk of RCC.
In our study, the stratified analysis results indicated that the risk of increasing GI for RCC appeared to be greater in individuals with RCC risk factors, such as hypertension, obesity or overweight than in individuals without these risk factors, although lacking of statistically significant effect modification between the strata. According to our joint effect analysis results, individuals with both a high-GI diet and hypertension or high BMI had at least two times higher risk of RCC than individuals without these risk factors. Hypertension is a confirmed risk factor for RCC and is hypothesized to play a role in RCC pathogenesis through oxidative damage and lipid peroxidation (33). Furthermore, obesity-related insulin resistance and compensatory hyperinsulinemia are important mechanisms in RCC pathogenesis (34). Individuals with a high BMI tend to have a high-energy diet, with high intake of simple sugars, low intake of fiber and high GI exposures. This finding suggests that reducing GI exposures could reduce RCC risk for individuals with established risk factors, such as hypertension and high BMI. Additionally, our findings suggest that high GI levels combined with other risk factors may help identify high-risk individuals for targeted screening and intervention.
Our study has several strengths. To our knowledge, this is the first study to examine the association of GI, GL and intake of total carbohydrates, fiber, starch and sugar with RCC risk in a large group of patients with newly diagnosed, histologically confirmed RCC in the USA. We also evaluated the quantity and quality of carbohydrate intake through a validated food frequency questionnaire. Furthermore, we comprehensively controlled for many potential confounding factors and explored the independent and joint effects of GI and GL with these factors. Notably, given the potential influence of poor overall diet quality on RCC risk and the correlation between poor overall diet quality and high GI/GL diet, our full model also adjusted for overall diet quality (as indicated by the HEI-2015 score), which has never been done in previous studies. Our results did show that overall diet quality was a significant confounder for the associations of GI and fiber with RCC risk; after further adjustment of HEI-2015 score, the associations were attenuated but remained significant, suggesting them as independent risk factors for RCC risk.
However, our study has several potential limitations. It is possible that cases reported their dietary intake such as unhealthy dietary habits differently from healthy controls, leading to biased effect estimates. However, it is unlikely that cases and controls differentially reported dietary consumption based on GI values. In addition, measurement errors may occur when assessing dietary intake with the food frequency questionnaire, but these errors are expected to be non-differential between cases and controls. Third, residual confounding due to unmeasured or unknown confounders may be present, although comprehensive adjustment for known risk factors of RCC minimized this possibility. Finally, GI may not be able to capture all the inter-individual variations in responses to ‘high-GI foods’. For example, inter-individual differences in microbiota and the way they utilize different carbohydrate sources may also contribute to the inter-individual responses to ‘high-GI foods’.
In conclusion, our findings suggest that a high-GI diet is associated with an increased risk of RCC and that individuals with a high-GI diet and hypertension or high BMI may have a higher RCC risk than those without these factors. Also, increased total fiber and starch intake were associated with decreased RCC risk. Given the limited number of epidemiological studies of carbohydrates and related indexes and risk of RCC, further studies are warranted to establish causality and to explore underlying mechanisms.
Supplementary material
Supplementary data are available at Carcinogenesis online.
Funding
Supported by grants from the National Institutes of Health (NIH) (grant number R01 CA170298) and the Center for Translational and Public Health Genomics, Duncan Family Institute for Cancer Prevention, The University of Texas MD Anderson Cancer.
Conflict of Interest Statement: None declared.
Supplementary Material
Abbreviations
- BMI
body mass index
- CIs
confidence intervals
- GI
glycemic index
- GL
glycemic load
- HEI
healthy eating index
- ORs
odds ratios
- METs
metabolic equivalent score
- RCC
renal cell carcinoma
References
- 1. Siegel R.L., et al. (2016) Cancer statistics, 2016. CA. Cancer J. Clin., 66, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Scelo G., et al. (2016) International cancer seminars: a focus on kidney cancer. Ann. Oncol., 27, 1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gnagnarella P., et al. (2008) Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am. J. Clin. Nutr., 87, 1793–1801. [DOI] [PubMed] [Google Scholar]
- 4. Foster-Powell K., et al. (2002) International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr., 76, 5–56. [DOI] [PubMed] [Google Scholar]
- 5. Augustin L.S., et al. (2015) Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis., 25, 795–815. [DOI] [PubMed] [Google Scholar]
- 6. Pollak M. (2012) The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat. Rev. Cancer, 12, 159–169. [DOI] [PubMed] [Google Scholar]
- 7. Sieri S., et al. (2017) Dietary glycemic index, glycemic load and cancer: An overview of the literature. Nutr. Metab. Cardiovasc. Dis., 27, 18–31. [DOI] [PubMed] [Google Scholar]
- 8. Turati F., et al. (2015) High glycemic index and glycemic load are associated with moderately increased cancer risk. Mol. Nutr. Food Res., 59, 1384–1394. [DOI] [PubMed] [Google Scholar]
- 9. Galeone C., et al. (2009) Glycemic index, glycemic load and renal cell carcinoma risk. Ann. Oncol., 20, 1881–1885. [DOI] [PubMed] [Google Scholar]
- 10. George S.M., et al. (2009) Dietary glycemic index, glycemic load, and risk of cancer: a prospective cohort study. Am. J. Epidemiol., 169, 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu J., et al. ; Canadian Cancer Registries Epidemiology Research Group. (2013) Glycemic index, glycemic load and cancer risk. Ann. Oncol., 24, 245–251.22831983 [Google Scholar]
- 12. Bidoli E., et al. (2008) Macronutrients, fatty acids, cholesterol and renal cell cancer risk. Int. J. Cancer, 122, 2586–2589. [DOI] [PubMed] [Google Scholar]
- 13. Allen N.E., et al. (2009) A prospective analysis of the association between macronutrient intake and renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer, 125, 982–987. [DOI] [PubMed] [Google Scholar]
- 14. Daniel C.R., et al. (2013) Intake of fiber and fiber-rich plant foods is associated with a lower risk of renal cell carcinoma in a large US cohort. Am. J. Clin. Nutr., 97, 1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clague J., et al. (2009) Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol. Biomarkers Prev., 18, 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ainsworth B.E., et al. (1993) Compendium of physical activities: classification of energy costs of human physical activities. Med. Sci. Sports Exerc., 25, 71–80. [DOI] [PubMed] [Google Scholar]
- 17. Ainsworth B.E., et al. (2011) 2011 Compendium of Physical Activities: a second update of codes and MET values. Med. Sci. Sports Exerc., 43, 1575–1581. [DOI] [PubMed] [Google Scholar]
- 18. Block G., et al. (1994) Revision of dietary analysis software for the Health Habits and History Questionnaire. Am. J. Epidemiol., 139, 1190–1196. [DOI] [PubMed] [Google Scholar]
- 19. US. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 27 https://www.ars.usda.gov/ARSUserFiles/80400525/Data/SR27/sr27_doc.pdf. (28 November 2016, date last accessed).
- 20. Willett W.C., et al. (1997) Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr., 65(4 Suppl), 1220S–1228S; discussion 1229S. [DOI] [PubMed] [Google Scholar]
- 21. Flood A., et al. (2006) Methodology for adding glycemic load values to the National Cancer Institute Diet History Questionnaire database. J. Am. Diet. Assoc., 106, 393–402. [DOI] [PubMed] [Google Scholar]
- 22. Sieri S., et al. (2015) Dietary glycemic index and glycemic load and risk of colorectal cancer: results from the EPIC-Italy study. Int. J. Cancer, 136, 2923–2931. [DOI] [PubMed] [Google Scholar]
- 23. Djiogue S., et al. (2013) Insulin resistance and cancer: the role of insulin and IGFs. Endocr. Relat. Cancer, 20, R1–R17. [DOI] [PubMed] [Google Scholar]
- 24. Solarek W., et al. (2015) Insulin and IGFs in renal cancer risk and progression. Endocr. Relat. Cancer, 22, R253–R264. [DOI] [PubMed] [Google Scholar]
- 25. Dombrowski F., et al. (2007) Renal carcinogenesis in models of diabetes in rats: metabolic changes are closely related to neoplastic development. Diabetologia, 50, 2580–2590. [DOI] [PubMed] [Google Scholar]
- 26. Habib S.L., et al. (2014) Hyperactivation of Akt/mTOR and deficiency in tuberin increased the oxidative DNA damage in kidney cancer patients with diabetes. Oncotarget, 5, 2542–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Major J.M., et al. (2010) Insulin-like growth factors and risk of kidney cancer in men. Br. J. Cancer, 103, 132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spyridopoulos T.N., et al. ; Obesity and Cancer Oncology Group. (2012) Insulin resistance and risk of renal cell cancer: a case-control study. Hormones (Athens)., 11, 308–315. [DOI] [PubMed] [Google Scholar]
- 29. Bertoia M., et al. (2010) No association between fruit, vegetables, antioxidant nutrients and risk of renal cell carcinoma. Int. J. Cancer, 126, 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weikert S., et al. (2006) Fruits and vegetables and renal cell carcinoma: findings from the European prospective investigation into cancer and nutrition (EPIC). Int. J. Cancer, 118, 3133–3139. [DOI] [PubMed] [Google Scholar]
- 31. Huang T.B., et al. (2014) Dietary fiber intake and risk of renal cell carcinoma: evidence from a meta-analysis. Med. Oncol., 31, 125. [DOI] [PubMed] [Google Scholar]
- 32. Cust A.E., et al. (2009) Total dietary carbohydrate, sugar, starch and fibre intakes in the European Prospective Investigation into Cancer and Nutrition. Eur. J. Clin. Nutr., 63 Suppl 4, S37–S60. [DOI] [PubMed] [Google Scholar]
- 33. Sanfilippo K.M., et al. (2014) Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension, 63, 934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McGuire B.B., et al. (2011) BMI and the risk of renal cell carcinoma. Curr. Opin. Urol., 21, 356–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.