Upregulation of p27 contributes to ISO inhibition of BC cell growth. ISO promotes p27 expression via increasing binding of FOXO1 to its promoter. p27 is induced by decreasing binding of miR-182 to its mRNA 3′-UTR upon ISO treatment.
Abstract
There are few approved drugs available for the treatment of muscle-invasive bladder cancer (MIBC). Recently, we have demonstrated that isorhapontigenin (ISO), a new derivative isolated from the Chinese herb Gnetum cleistostachyum, effectively induces cell-cycle arrest at the G0/G1 phase and inhibits anchorage-independent cell growth through the miR-137/Sp1/cyclin D1 axis in human MIBC cells. Herein, we found that treatment of bladder cancer (BC) cells with ISO resulted in a significant upregulation of p27, which was also observed in ISO-treated mouse BCs that were induced by N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN). Importantly, knockdown of p27 caused a decline in the ISO-induced G0–G1 growth arrest and reversed ISO suppression of anchorage-independent growth in BC cells. Mechanistic studies revealed that ISO promoted p27 expression at mRNA transcription level through increasing direct binding of forkhead box class O1 (FOXO1) to its promoter, while knockdown of FOXO1 attenuated ISO inhibition of BC cell growth. On the other hand, ISO upregulated the 3′-untranslated region (3′-UTR) activity of p27, which was accompanied by a reduction of miR-182 expression. In line with these observations, ectopic expression of miR-182 significantly blocked p27 3′-UTR activity, whereas mutation of the miR-182-binding site at p27 mRNA 3′-UTR effectively reversed this inhibition. Accordingly, ectopic expression of miR-182 also attenuated ISO upregulation of p27 expression and impaired ISO inhibition of BC cell growth. Our results not only provide novel insight into understanding of the underlying mechanism related to regulation of MIBC cell growth but also identify new roles and mechanisms underlying ISO inhibition of BC cell growth.
Introduction
Bladder cancer (BC) is the most common cause of death in patients with urinary system malignancies. Both the incidence and mortality of BC have risen steadily year by year in recent decades. It is estimated that about 79030 Americans will be diagnosed with BC and that 16870 will die of this disease in 2017 (1). Metastasis is the primary cause of death elicited by BC, with a 5-year survival rate of about 8.1% (2). Muscle-invasive bladder cancer (MIBC) represents 25–40% of all BC and one-third of patients with MIBC have already undetected metastases at the time of treatment (3). Current treatment strategies for BC range from transurethral resection for superficial tumors to systemic chemotherapy for locally advanced or metastatic BC (2). However, the effectiveness of chemotherapy for BC is still far from satisfactory (2). For these reasons, identification of the key molecules responsible for mediating human BC development and defining of more effective and less toxic alternate chemotherapeutic therapies are of tremendous importance for improving the clinical outcome of this disease.
p27 (also known as CDKN1B) is a member of the Cip/Kip family of cyclin-dependent kinase (CDK) inhibitor proteins and acts as a key suppressor of cell proliferation to induce cell-cycle arrest in G1 phase (4). In addition to its initial identification as a CDK inhibitor, p27 has also emerged as an intrinsically unstructured, multifunctional protein in CDK-independent manners (5). Our recent studies demonstrate that p27 suppresses Hsp27 and Hsp70 expressions at the transcriptional level through the JNK2/c-Jun- and HSF-1-dependent pathways (6) and inhibits cyclooxygenase-2 expression by inhibiting p38β- and p38δ-mediated CREB phosphorylation upon arsenite exposure (7). Furthermore, we have found that loss of p27 upregulates MnSOD in a STAT3-dependent manner and that it disrupts intracellular redox activity and enhances cell migration (8). These studies provide the molecular mechanisms for the tumor suppressive role of p27.
It has been reported that p27 knockout results in an increase of relative weights of urinary bladders and section areas of carcinomas in N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN)-treated mice, indicating that expression of p27 plays an important role in urinary bladder carcinogenesis (9). Importantly, p27 has been identified as a major player in cell-cycle control in poorly differentiated high-grade MIBC, and decreased p27 expression is associated with poor overall and post-relapse survival (10,11). Although induction of p27 is frequently found to be involved in inhibition of BC cell proliferation (12,13), nothing is known about the mechanisms underlying the p27 regulation. Therefore, the upstream regulators still need to be identified in human BC progression.
Isorhapontigenin (ISO) is a novel derivative of stilbene, which is isolated and purified from a Chinese herb Gnetum cleistostachyum (14–16). Our published work shows that ISO can induce cell-cycle arrest at G0/G1 phase and inhibit anchorage-independent cell growth through inhibiting cyclin D1 expression in both RT4 human non-invasive BC cells and UMUC3 human invasive BC cells (15). In addition, our recent work has demonstrated that ISO inhibition of cyclin D1 is mediated by the miR-137/Sp1 pathway in UMUC3 and T24T invasive BC cells both in vitro and in vivo (17). On the other hand, we have shown that relatively high doses of ISO exhibit anticancer activity accompanied by downregulating the X-linked inhibitor of apoptosis protein, thus inducing apoptosis in T24T BC cells (16). We have also found that ISO specifically suppresses invasion of UMUC3 and T24T BC cells through the targeting of the STAT1-FOXO1-MMP2 axis and that ISO treatment completely reverses the BBN-induced mouse invasive to non-invasive bladder tumors (18). Moreover, our most recent results show that ISO treatment induces autophagy and inhibits UMUC3 BC cells growth through MAPK8-JUN-dependent transcriptional induction of SESN2 (19). These studies reveal that ISO may act as a promising preventive and/or therapeutic drug against human BC. In the present study, we address the role of p27 upregulation in anchorage-independent growth inhibition of ISO on human BC cells. In addition, we have further discovered that p27 is upregulated at the transcriptional level through induction of forkhead box class O1 (FOXO1), as well as at the post-transcriptional level by repression of miR-182 upon ISO treatment.
Materials and methods
Plasmids, antibodies and other reagents
The shRNA for human p27 and FOXO1 was purchased from Open Biosystems (Thermo Fisher Scientific, Huntsville, AL). GFP–p27 was a gift from Dr Gustavo Baldassarre (Division of Experimental Oncology, Centro di Riferimento Oncologico, National Cancer Institute, Aviano, Italy) (20) and have been used in our previous studies (8). The human full-size p27 promoter-luciferase construct (P27 PF) and the deletion mutants (p27 KPN Ι and p27 Sac II) were subcloned into the luciferase reporter plasmid pGVB2 and were kindly provided by Dr Toshiyuki Sakai (Department of Molecular-Targeting Cancer Prevention, Kyoto Prefectural University of Medicine, Kyoto, Japan) (21). Human FOXO1 promoter-luciferase reporter was cloned into the pGL3 luciferase assay vector and was a gift from Dr Jean-Baptiste Demoulin (De Duve Institute, Catholic University of Louvain, BE-1200 Brussels, Belgium) (22). Three different lengths of human p27 3′-untranslated region (3′-UTR) fragments (0–1344 bp, 545–1344 bp, and 1166–1344 bp) were cloned and inserted into firefly luciferase reporter vector pmiR-RB-REPORT (RiboBio Co., Ltd, Guzhou, China), and the mutation of the miR-182-binding site in the p27 3′-UTR was created using site-directed mutagenesis by the overlap extension PCR method. The hsa-miR-182 mimics and negative control miRNA mimics were chemically synthesized by RiboBio Co., Ltd. The antibodies against FOXO1, FOXO3a, FOXO4, NF-κB p65, p-NF-κB p65 (Ser536), E2F1 and PARP were commercially purchased from Cell Signaling Technology (Boston, MA). The antibodies against p27, p21 and GAPDH were bought from Santa Cruz Biotechnology (Santa Cruz, CA). ISO with purity greater than 99.9% was purchased from Higher Biotech (Shanghai, China). ISO was dissolved in dimethyl sulfoxide (DMSO, Sigma, St Louis, MO) to make a 20 mM stock solution, and the same concentration of DMSO was also made and used as a vehicle control in all experiments.
Cell culture and transfection
Human invasive BC cell line T24 was described and used in our previous studies (18), and the invasive BC cell line TCCSUP and non-invasive BC cell line RT4 were obtained from American Type Culture Collection (ATCC, Manassas, VA). The cell lines were regularly authenticated on the basis of morphology, viability, growth, recovery and chemical response as well and were most recently confirmed 4–6 months before use by using a short tandem repeat method. T24 cells were maintained in Dulbecco’s modified Eagle medium/Ham’s F-12 (1:1 vol) mixed medium supplemented with 5% FBS, 1% penicillin/streptomycin and 2 mM l-glutamine. TCCSUP cells and mouse embryonic fibroblasts (MEFs) were cultured in Dulbecco’s modified Eagle medium supplemented with 10% FBS (HyClone, Logan, UT), 1% penicillin/streptomycin and 2 mM l-glutamine (Life Technologies, Rockville, MD). RT4 cells were maintained in 1640 medium supplemented with 10% FBS, 1% penicillin/streptomycin and 2 mM l-glutamine. Transfections were carried out using PolyJet™ DNA In Vitro Transfection Reagent (SignaGen Laboratories, Gaithersburg, MD) according to the manufacturer’s instructions. The transfected cells were then respectively selected with G418, hygromycin or puromycin (Life Technologies) for 4–6 weeks. Surviving cells were pooled as stable mass transfectants, as described in our previous studies (23,24).
Anchorage-independent growth assay
Anchorage-independent growth ability was evaluated in soft agar, as described in our previous studies (25). Briefly, 3 ml of 0.5% agar in basal modified Eagle’s medium supplemented with 10% FBS was layered onto each well of six-well cell culture plates. Cells (3 × 104) suspended in 1 ml of normal medium were mixed with 2 ml of 0.5% agar in basal modified Eagle’s medium supplemented with 10% FBS. One milliliter of the mixture was then added to each well on top of the 0.5% agar layer. After incubation at 37°C in 5% CO2 for 2 weeks, colonies with more than 32 cells were scored and presented as colonies per 104 cells.
Cell-cycle analysis
The cells were treated with ISO at 20 µM or control vehicle (0.1% DMSO) and were then harvested and fixed in 75% ethanol overnight. Next, the cells were suspended in the staining buffer containing 0.1% Triton X-100, 0.2 mg/ml RNase A and 50 mg/ml propidium iodide at 4°C. DNA content was then evaluated using a flow cytometry Epics XL flow cytometer (Beckman Coulter, Brea, CA). The results were analyzed with EXPO32 software.
Western blotting
Western blot assay was tested, as previously described (26). Briefly, cells were plated in six-well plates and cultured in normal FBS (5% for T24 cells and 10% for TCCSCP cells) medium at 70–80% confluence. The cells were then cultured in 0.1% FBS medium for 12 h and subsequently treated with different doses of ISO for the indicated time. The cells were washed once with ice-cold phosphate-buffered saline, and cell lysates were prepared with a lysis buffer [10 mM Tris–HCl (pH 7.4), 1% SDS and 1 mM Na3VO4]. An equal amount (80 μg) of total protein from each cell lysate was subjected to Western blot with the indicated antibody, as described in previous studies (8,27). Immunoreactive bands were detected by using the alkaline phosphatase-linked secondary antibody and the ECF Western blotting system (Amersham Biosciences, Piscataway, NJ). The images were acquired, and the protein levels were quantified by using the Typhoon FLA 7000 imager (GE Healthcare, Pittsburgh, PA).
Nuclear extract preparation
Nuclear extracts were prepared, as previously described (23). T24 cells were seeded into 10 cm culture dishes until 70–80% confluence, cultured in 0.1% FBS medium for 12 h and then treated either with vehicle (0.1% DMSO) or 20 μM ISO for 18 h. The nuclear proteins were extracted by a Nuclear/Cytosol Fractionation Kit (BioVison Technologies, Mountain View, CA) according to the manufacturer’s protocols. Equal protein concentrations were measured by a protein quantification assay kit (Bio-Rad, Hercules, CA), and nuclear extracts were stored at −80°C before use.
Actinomycin D chase experiment
Actinomycin D (Act D) chase experiment was performed for mRNA stability assay. T24 cells were plated in six-well plates and cultured in 5% FBS medium at 70–80% confluence. The cells were then cultured in 0.1% FBS medium for 12 h and subsequently treated with vehicle (0.1% DMSO) or 20 μM ISO in the presence or absence of 10 mg/ml Act D. The total RNA was isolated and subjected to reverse transcription-polymerase chain reaction (RT-PCR) for determination of p27 mRNA levels after treatment of ISO for 3 h and for detection of foxo1 mRNA levels after treatment of ISO for 6 h.
RT-PCR and quantitative RT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen Corp., Carlsbad, CA), and cDNAs were synthesized by using the SuperScript® III First-Strand Synthesis System for RT-PCR (Invitrogen Corp.). A pair of oligonucleotides (forward: 5′-GATG ATCTTGAGGCTGTTGTC-3′ and reverse: 5′-CAGGGCTGCTTTTAACTCTG-3′) were adopted to amplify human gapdh cDNA as loading control. The human foxo1 cDNA fragments were amplified by a pair of human p27 specific PCR primers (forward: 5′-ACGGGAGCCCTAGCCTGGAGC-3′; reverse: 5′-TGCCCTTCTCCACCTCTT GCC-3′). The PCR products were then separated on 2% agarose gels and stained with ethidium bromide. The images were visualized and scanned by using the UV lights with a FluorChem SP imaging system (Alpha Innotech, San Leandro, CA) as previously described (28). The Quantitative RT-PCR analysis was conducted for miRNA assay. Total RNA was extracted using the miRNeasy Mini Kit (Qiagen, Valencia, CA), and analysis of miRNAs expression was performed by the miScript PCR system (Qiagen) and the 7900HT Fast Real-time PCR system. The ΔΔCT value was used to calculate the relative expression of miRNAs, using U6 as endogenous control.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was performed using an EZ-ChIP kit (Millipore, Billerica, CA) following the manufacturer’s instructions. Briefly, T24 cells were treated with either 0.1% DMSO only or 20 μM ISO for 18 h. Genomic DNA and the proteins were then cross-linked with 1% formaldehyde. The cross-linked cells were pelleted, resuspended in lysis buffer and sonicated to generate 200–500 bp chromatin DNA fragments. After centrifugation, the supernatants were diluted 10-fold and then incubated with anti-FOXO1 antibody or the control rabbit IgG at 4°C overnight. Subsequently, the immune complex was captured by protein G agarose saturated with salmon sperm DNA, then eluted with the elution buffer. DNA–protein cross-linking was reversed by heating at 65°C for 4 h. After DNA was purified, PCR was performed with the following pair of primers: 5′-GCTCGCCAGTCCATTTGAT-3′ and 5′-CTCGCACGTTTGACATCTTTC-3′ to specifically amplify the region containing the FOXO1 responsive elements on the human p27 promoter. The PCR products were separated on 2% agarose gels and stained with ethidium bromide. Following this, the images were scanned by using a UV light.
Luciferase assay
As described in our previous studies (15,16), dual-luciferase reporter assay was performed by using the luciferase assay system (Promega Corp., Madison, WI). Each of the indicated luciferase reporters was transfected into human BC cells. After ISO treatment, cells were extracted with passive lysis buffer [25 mM Tris-phosphate (pH 7.8), 2 mM EDTA, 1% Triton X-100 and 10% glycerol]. The luciferase activity was measured using a microplate luminometer LB 96V (Berthold GmbH & Co. KG, Bad Wildbad, Germany). The firefly luciferase signal was normalized to the Renilla luciferase transfection control.
Immunohistochemistry paraffin of mouse bladder tissues
Mouse bladder tissues obtained from our previous study (29) were immunostained by using p27 antibody (Santa Cruz, CA) . The resultant immunostaining images were captured using the AxioVision Rel.4.6 computerized image analysis system (Carl Zeiss, Oberkochen, Germany), and protein expression levels were analyzed by calculating the integrated optical density per stained area using Image-Pro Plus version 6.0 (Media Cybernetics, MD), as described in our previous study (29).
Statistical methods
Statistical analyses were performed using SPSS 19.0 software (SPSS, Chicago, IL). Student’s t-test was utilized to compare continuous variables and summarized as means ± standard deviations (SD), between different groups. P < 0.05 was considered statistically.
Results
Upregulation of p27 contributed to ISO inhibition of anchorage-independent growth in human BC cells
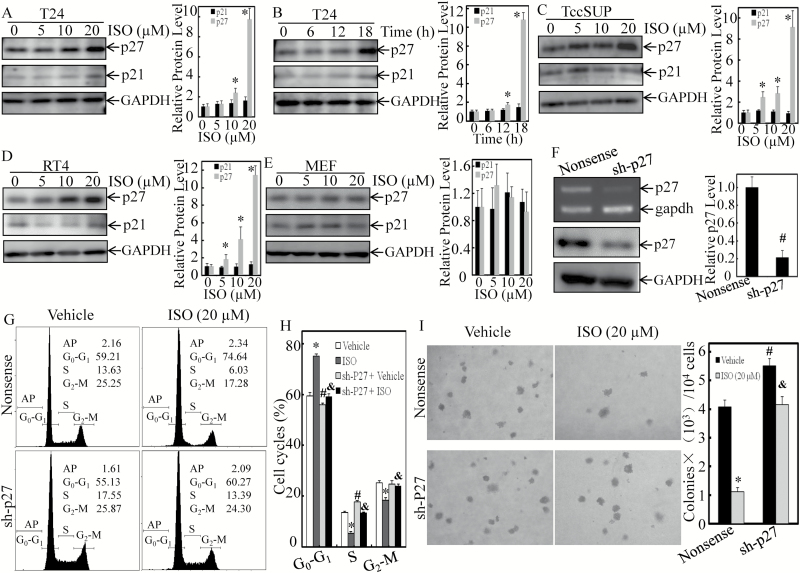
ISO has been shown to inhibit cell growth accompanied with G0/G1 growth arrest via inhibiting cyclin D1 expression in human invasive BC cells, as shown in our previous study (15,17). Two representative human high-grade invasive BC cell lines T24 (grade 3) with no metastatic ability and TCCSUP (grade 4) with metastatic ability, as well as non-invasion BC cell line RT4 were used in the present study. To test whether CDK inhibitors are involved in G0/G1 growth arrest induced in human BC cells, following ISO treatment, p21 and p27 expression was evaluated. As shown in Figure 1A, ISO induced p27 protein expression in a dose-dependent manner in T24 cells, while there was only a slight effect on the expression of p21 protein. Treatment of T24 cells with 20 µM ISO also resulted in a gradual induction in p27, but not p21 protein levels over various time points (Figure 1B). Similarly, p27 and p21 protein expression profiles were consistently obtained in TCCSUP and p53 proficient RT4 cells upon ISO treatment (Figure 1C and D). Meanwhile, protein expression of p27 and p21 was only slightly upregulated by ISO in p53-proficient MEFs (Figure 1E), suggesting that ISO might selectively upregulate p27 protein level in cancer cells. To address the contribution of p27 upregulation by ISO to the inhibition of BC cell growth, we transfected p27 shRNA into T24 cells, and the stable p27 knockdown transfectant was identified by RT-PCR and western blot (Figure 1F). The stable transfectant was then treated with ISO to determine how knockdown of p27 regulates cell-cycle and anchorage-independent growth. As shown in Figure 1G and H, knockdown of p27 caused a declined in the G0–G1 growth arrest of T24 cells by ISO. Accordingly, knockdown of p27 also reversed ISO suppression of anchorage-independent growth in T24 cells (Figure 1I). These results demonstrate that p27 upregulation contributes to anchorage-independent growth inhibition of ISO in human BC cells.
Figure 1.
Upregulation of p27-mediated ISO inhibition of anchorage-independent growth in human BC cells. (A) Human MIBC T24 cells were treated with ISO at the indicated doses for 18 h, and the cell extracts were subjected to western blotting to determine p21 and p27 protein expressions. (B) T24 cells were treated with 20 µM ISO for the indicated time points, and the cell extracts were subjected to western blotting to determine p21 and p27 protein expressions. (C and D) Human MIBC TCCSUP cells (C) and non-invasive BC RT4 cells (D) were treated with ISO at the indicated doses for 18 h, and the whole cell lysates were used for western blot to determine p21 and p27 protein expressions. (E) MEFs were treated with ISO at the indicated doses for 18 h, and the whole cell lysates were used for western blot to determine p21 and p27 protein expressions. GAPDH was used as a protein loading control. (F) T24 cells were then stably transfected with nonsense shRNA or p27 shRNA (sh-p27) constructs, respectively. The knockdown efficiency of p27 mRNA and protein was assessed by RT-PCR and western blotting. (G and H) p27 stable knockdown transfectant and nonsense transfectant were then used for determination of cell cycle by flow cytometry (G), and statistical significance of each cell cycle was analyzed (H) in the presence of either vehicle or the indicated concentration of ISO treatment for 18 h. (I) Anchorage-independent growth of p27 stable knockdown transfectant, as well as nonsense transfectant, was detected in the presence of either vehicle or the indicated concentration of ISO treatment for 18 h. Each of the experiment was replicated three times, and the error bars represent the SD. The symbol ‘*’ indicates a significant difference in comparison with the vehicle control group, the symbol ‘#’ indicates a significant difference between nonsense transfectant and shRNA transfectant and the symbol ‘&’ indicates a significant difference in comparison with nonsense transfectant upon ISO treatment (P < 0.05).
ISO promoted p27 expression at the mRNA transcription level in human BC cells
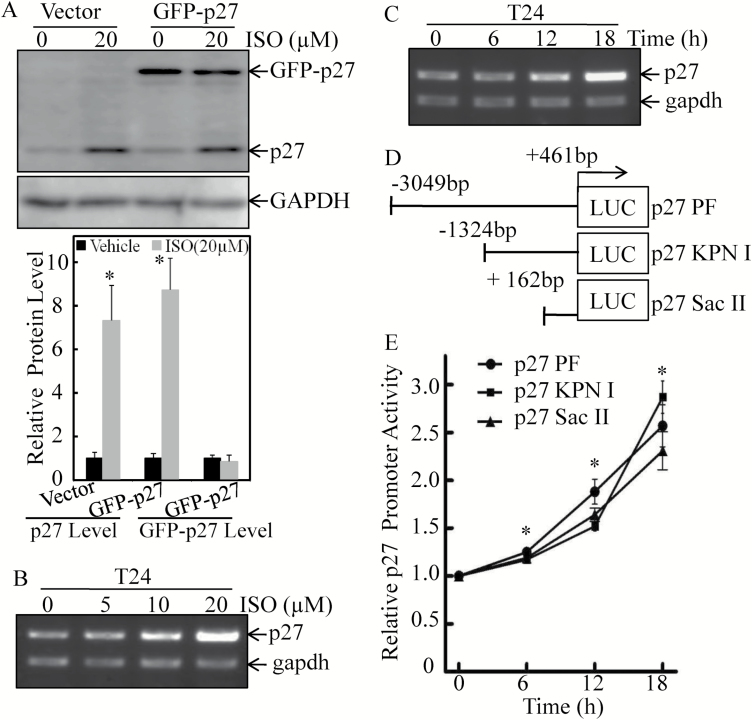
It has been reported that p27 protein downregulation is usually achieved by proteasomal degradation in several types of human cancers (30). To address whether ISO treatment regulates p27 protein degradation, both transfectants of T24 (vector) and T24 (GFP-p27) were employed. As shown in Figure 2A, ISO treatment increased endogenous p27 protein expression in both T24 (vector) and T24 (GFP-p27) cells, whereas it did not affect exogenous GFP-p27 expression in T24 (GFP-p27) cells, suggesting that protein degradation was not involved in ISO regulation of p27 expression. We subsequently evaluated the mRNA levels of p27 in T24 cells. Consistent with the results obtained from the protein levels, p27 mRNA expression was also markedly upregulated upon ISO treatment in a dose- and time-dependent manner (Figures 2B and C). These results suggest that ISO may upregulate p27 expression at the mRNA transcription level. To address this notion, the full-length and two 5′ truncated constructs of p27 promoter-driven luciferase reporters (Figure 2D) were transfected into T24 cells respectively, and the promoter activities were all significantly upregulated in a time-dependent manner upon ISO treatment (Figure 2E). These results reveal that ISO could promote p27 expression at mRNA transcription level in human BC cells.
Figure 2.
ISO-induced p27 expression at the mRNA transcription level in human BC cells. (A) GFP-p27 expression construct was used to stably transfect into T24 cells. The stable transfectants, T24 (vector) and T24 (GFP-p27), were identified by western blotting and were further for use in the determination of p27 protein expression. GAPDH was used as the protein loading control. (B) T24 cells were treated with ISO at the indicated doses for 18 h, and the cell extracts were subjected to RT-PCR for determination of p27 mRNA expression. (C) T24 cells were treated with 20 µM ISO for the indicated time points, and the cell extracts were subjected to RT-PCR for determination of p27 mRNA expression, using gapdh as the mRNA loading control. (D and E) T24 cells stably transfected with the indicated promoter-driven luciferase reporters (p27 PF, p27 KPN Ι and p27 Sac ΙΙ) (D) were treated with 20 μM ISO for the indicated times (E). Luciferase activity was evaluated by the dual-luciferase reporter assay system. Each of the experiment was replicated three times, and the error bars represent the SD. Symbol ‘*’ indicates a significant difference between vehicle and ISO-treated groups (P < 0.05).
The increased direct binding of FOXO1 to the p27 promoter mediated ISO-induced upregulation of p27 transcription in human BC cells
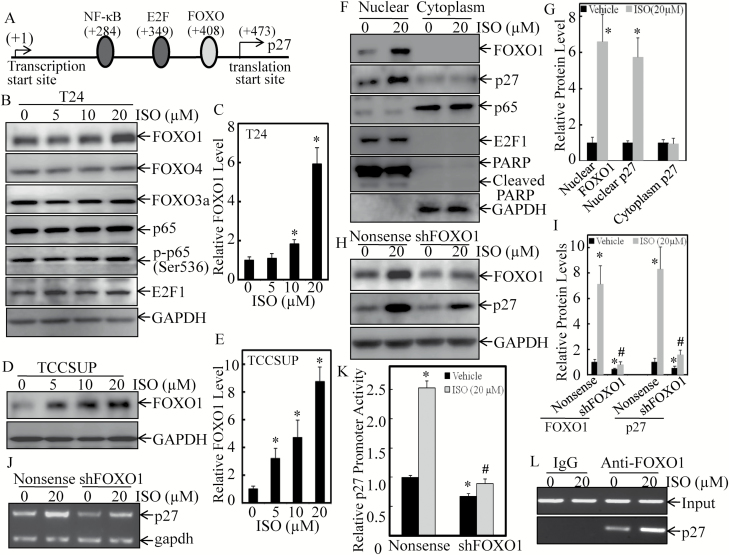
Although p27 proteasomal degradation and subcellular localization have been intensively investigated, the upstream regulators of its promoter transcription in cancer cells remain to be addressed (4). It was noted that ISO-induced activity of 5′ truncated p27 promoter (p27 Sac ΙΙ) was comparable with the full-length construct (p27 PF; Figure 2E), indicating the narrowed region (+162 bp to +461 bp relative to the transcription start site) is critical for promoter functionality. To explore the transcription factor that is responsible for ISO upregulation of p27 transcription, TFANSFAC® Transcription Factor Binding Sites Software (Biological Database, Wolfenbutel, Germany) was used for bioinformatics analysis, and it showed that this narrowed p27 promoter region contained the putative DNA-binding sites of various transcription factors, including the NF-κB, E2Fs and FOXOs (Figure 3A). We next evaluated the expression and the nuclear translocation changes of these factors upon ISO treatment for 18 h. As shown in Figure 3B and C, treatment of T24 cells with ISO resulted in the dramatic induction of FOXO1 in a dose-dependent manner, while there was no effect on expression of FOXO3a, FOXO4, NF-κB p65, p-NF-κB, p65 (Ser536) or E2F1. Similar upregulation of FOXO1 was observed in TCCSUP cells upon ISO treatment (Figure 3D and E). The results obtained from distributions of the transcription factors between nuclear and cytoplasm fractions further revealed that ISO treatment mainly targeted nuclear fraction of FOXO1 in T24 cells (Figure 3F and G), indicating that ISO upregulation of the FOXO1 transcription factor is potentially functional. Moreover, the increase of p27 was mainly located in nuclear rather than cytoplasm, which was consistent with its role in regulating cell-cycle progression.
Figure 3.
The increased direct binding of FOXO1 to p27, the promoter, contributed to ISO-induced upregulation of p27 transcription in human BC cells. (A) Schematic representation of transcription factor binding sites of the human p27 gene promoter. (B and C) T24 cells were treated with either vehicle or ISO with the indicated concentrations for 18 h. Expression of the related transcription factors in the whole cell lysates was determined by western blotting, and GAPDH was used as protein loading control. (D and E) TCCSUP cells were treated either with the vehicle or ISO with the indicated concentrations for 18 h. Expression of FOXO1 in the whole cell lysates was determined by western blotting, and GAPDH was used as protein loading control. (F and G) T24 cells were treated either with the vehicle or 20 μM ISO for 18 h. The cell extracts were used to isolate cell nuclear and cytoplasm fractions and were then subjected to western blotting, with the specific antibodies, as indicated. PARP and GAPDH were used as markers for nuclear and cytoplasm fractions, respectively. (H and I) T24 (nonsense) and T24 (sh-FOXO1) cells were treated either with the vehicle or 20 μM ISO for 18 h. Cell lysates were subjected to western blotting with the specific antibodies indicated. (J) T24 (nonsense) and T24 (sh-FOXO1) cells were treated either with the vehicle or 20 μM ISO for 18 h, and cell lysates were used for evaluation of p27 mRNA expression by RT-PCR. (K) T24 (nonsense) and T24 (sh-FOXO1) cells were transfected with p27 promoter-driven luciferase reporter, and the stable transfectants were then treated with 20 μM ISO for 18 h. Cells were then subjected to determination of luciferase activity using dual-luciferase reporter assay. (L) T24 cells were treated either with the vehicle or 20 μM ISO for 18 h. ChIP assay was carried out with anti-FOX1 antibody. The DNAs were extracted from protein G agarose with anti-FOXO1 antibody or with control IgG. Total sonicated nuclei were used as input control. The specific FOXO1 binding DNA regions of p27 promoter were then amplified by PCR. Each of the experiment was replicated three times, and the error bars represent the SD. The symbol ‘*’ indicates a significant increase in comparison with the vehicle control group (P < 0.05), and the symbol ‘#’ indicates a significant decrease in comparison with nonsense transfectant upon ISO treatment (P < 0.05).
To test the relation of FOXO1 induction to p27 upregulation following ISO treatment in BC cells, we next evaluated the effects of FOXO1 knockdown on p27 expression in T24 cells. As shown in Figure 3H–J, knockdown of FOXO1 decreased the basal levels of p27 protein and mRNA and attenuated ISO-induced upregulation of p27. Accordingly, the basal level of p27 promoter activity in T24 (sh-FOXO1) transfectant was also downregulated in comparison with T24 (nonsense) transfectant, and ISO induction of p27 promoter activity was impaired in T24 (sh-FOXO1) transfectant (Figure 3K). To verify whether upregulation of FOXO1 by ISO was associated with its specific binding to p27 promoter in intact cells, we performed ChIP assays followed by PCR with primers specifically targeting the FOXO1 binding region in the human p27 promoter in T24 cells. As shown in Figure 3L, FOXO1 did bind to the p27 promoter, and this binding was significantly increased upon ISO treatment. These results demonstrate that the increased direct binding of FOXO1 to the p27 promoter mediates ISO upregulation of p27 transcription in human BC cells.
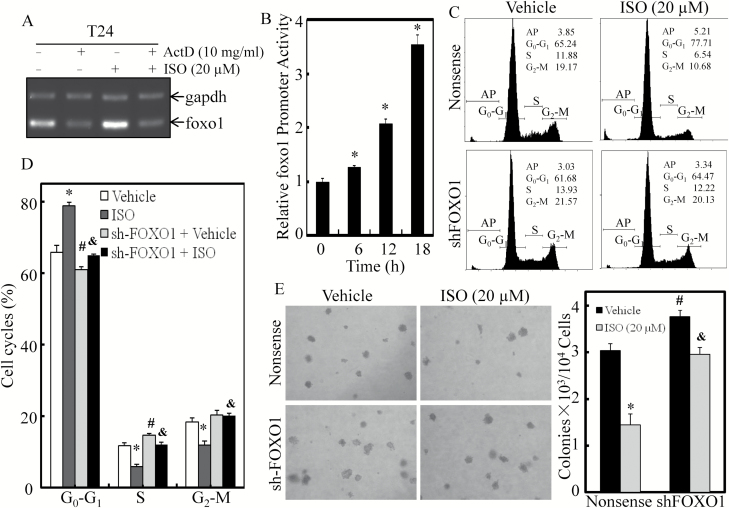
ISO upregulated FOXO1 expression at the mRNA transcription level without affecting mRNA stability, and knockdown of FOXO1 attenuated ISO inhibition of BC cell growth
Our recent study has discovered that ISO could upregulate foxo1 mRNA transcription, rather than affecting its protein degradation in T24T and UMUC3 BC cells (18). In the present study, we carried out additional experiments to exclude the involvement of ISO regulation of p27 mRNA stability using Act D. The results showed that ISO did not affect foxo1 mRNA level in Act D-treated T24 cells, whereas it increased foxo1 mRNA expression in the absence of Act D treatment (Figure 4A), suggesting that ISO did not regulate foxo1 mRNA stability. Consistently, foxo1 promoter activity was significantly enhanced in a time-dependent manner upon ISO treatment in T24 cells (Figure 4B). To determine the role of FOXO1 induction in ISO inhibition of BC cell growth, we subsequently evaluated cell-cycle and anchorage-independent growth in T24 (sh-FOXO1) stable transfectant. Knockdown of FOXO1 reduced the G0–G1 growth arrest of T24 cells by ISO (Figure 4C and D), as well as attenuated ISO inhibition of BC cell growth in soft agar assay (Figure 4E). Taken together, these data reveal that transcriptional induction and transactivation of FOXO1 mediates ISO upregulation of p27 transcription and protein expression, which leads to G0–G1 growth arrest and inhibits BC cell growth.
Figure 4.
ISO upregulated FOXO1 expression at the mRNA transcription level, and knockdown of FOXO1 reversed ISO inhibition of BC cell growth. (A) T24 cells were treated with 20 µM ISO for 6 h in the presence or absence of Act D. The total RNA was isolated and subjected to RT-PCR for determination of foxo1 mRNA levels. The gadph mRNA levels were used as loading control. (B) T24 cells stably transfected with the promoter-driven luciferase reporter were treated with 20 μM ISO for the indicated times. Luciferase activity was evaluated by the dual-luciferase reporter assay system. (C and D) FOXO1 stable knockdown transfectant and nonsense transfectant were used for determination of cell cycle by flow cytometry (C), and statistical significance of each cell cycle was analyzed (D) in the presence of either vehicle or the indicated concentration of ISO treatment for 18 h. (E) T24 (nonsense) and T24 (sh-FOXO1) cells were used for anchorage-independent growth assay in the presence of either the vehicle or the indicated concentration of ISO treatment. Each of the experiment was replicated three times, and the error bars represent the SD. The symbol ‘*’ indicates a significant difference in comparison with the vehicle control group, the symbol ‘#’ indicates a significant difference between nonsense transfectant and shRNA transfectant and the symbol ‘&’ indicates a significant difference in comparison with nonsense transfectant upon ISO treatment (P < 0.05).
Inhibition of miR-182 expression was responsible for ISO upregulation of p27 mRNA 3′-UTR activity
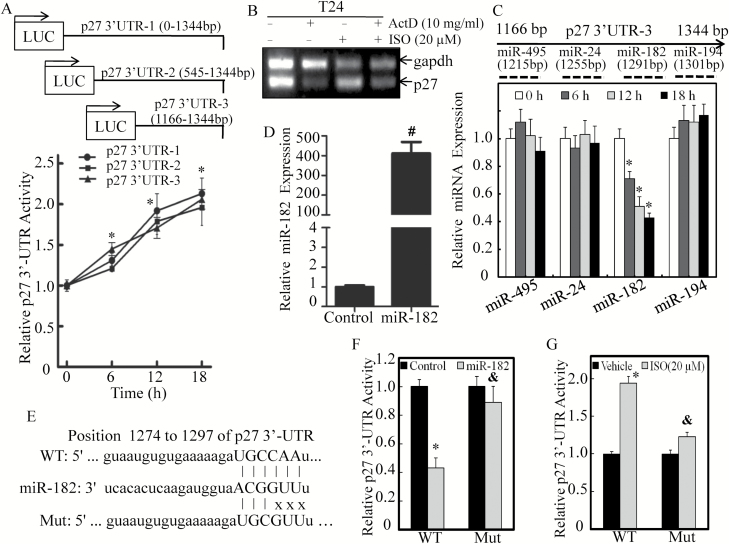
p27 may be regulated at multiple levels through a variety of mechanisms including transcriptional, mRNA stability, translational and protein degradation. The above results have confirmed the mRNA transcription role and excluded protein degradation involvement in ISO upregulation of p27 expression. It has been well recognized that 3′-UTR of the transcript can influence mRNA stabilization and protein translation regulation (31). To explore whether p27 is regulated at the level of either mRNA degradation or protein translation, we transfected the full-length and two 5′ truncated constructs of p27 mRNA 3′-UTR luciferase reporters into T24 cells and treated the transfectants with ISO. As shown in Figure 5A, ISO treatment also resulted in a significant upregulation of p27 3′-UTR activity in a time-dependent manner in T24 cells. Moreover, the shortest truncation form of p27 3′-UTR and the full-length p27 3′-UTR showed comparable inductions of luciferase activity after ISO treatment (Figure 5A), suggesting that the narrowed region (1166–1344 bp of p27 3′-UTR) contains the essential regulatory elements. Consistently, the p27 mRNA level was also increased upon ISO treatment in Act D-treated T24 cells (Figure 5B), indicating that ISO could stabilize p27 mRNA. Considering that miRNAs have been acknowledged as important regulators targeting mRNA 3′-UTR, we used the miRanda database to analyze potential miRNA-binding sites in this 1166–1344 bp region of p27 3′-UTR and four potential miRNA-binding sites, including miR-495, miR-24, miR-182 and miR-194, were identified (Figure 5C). We subsequently evaluated the expression levels of these potential miRNAs by qPCR in T24 cells upon ISO treatment. As shown in Figure 5C, ISO treatment led to a specific gradual inhibition of miR-182 expression without affecting miR-495, miR-24 and miR-194 levels. To test the role of miR-182 in the regulation of p27 3′-UTR activity, miR-182 mimics were transfected into T24 cells, and the overexpression of miR-182 was verified by real-time PCR (Figure 5D). Meanwhile, the miR-182 targeted site mutated p27 3′-UTR luciferase reporter was constructed (Figure 5E). Both wild-type and mutant of p27 3′-UTR luciferase reporter were then transfected into T24 (control) and T24 (miR-182) transfectants, respectively. The results showed that ectopic expression of miR-182 could significantly block p27 3′-UTR luciferase reporter activity, whereas mutation of the miR-182-binding site at p27 3′-UTR luciferase reporter effectively reversed such inhibition in T24 cells (Figure 5F), indicating that miR-182 is able to directly bind to p27 3′-UTR and repress its activity. In line with these observations, the point mutation of miR-182-binding site in p27 3′-UTR luciferase reporter also led to a significant loss of ISO induction effect on its activity (Figure 5G). Collectively, these findings reveal that inhibition of miR-182 expression and the decreased direct binding of miR-182 to p27 mRNA 3′-UTR are responsible for ISO upregulation of p27 3′-UTR activity.
Figure 5.
Inhibition of miR-182 expression contributed to ISO upregulation of p27 mRNA 3′-UTR activity. (A) T24 cells stably transfected with the indicated 3′-UTR-driven luciferase reporters were treated with 20 μM ISO for the indicated times. Luciferase activity was evaluated by the dual-luciferase reporter assay system. (B) T24 cells were treated with 20 µM ISO for 3 h in the presence or absence of Act D. The total RNA was isolated and subjected to RT-PCR for determination of p27 mRNA levels. (C) Schematic representation of potential miRNA-binding sites in p27 mRNA-3′-UTR was analyzed. T24 cells were treated with either vehicle or 20 μM ISO for 6 to 18 h. The expressions of the indicated miRNAs were evaluated by quantitative RT-PCR. (D) miR-182 mimics were transfected into T24 cells, and the overexpression of miR-182 was determined by quantitative RT-PCR. (E) Schematic sequence of the intact miR-182-binding site in WT (wild-type) p27 mRNA 3′-UTR and its mutation (Mut) of p27 3′-UTR luciferase reporter. WT p27 3′-UTR and its mutated luciferase reporters were transiently stably into T24 cells. Luciferase activities after miR-182 overexpression (F) or upon 20 µM ISO treatment for 18 h (G) were evaluated by the dual-luciferase reporter assay system. The results are presented as p27 3′-UTR luciferase activity relative to medium control (relative p27 3′-UTR activity). Each of the experiment was replicated three times, and the error bars represent the SD. The symbol ‘*’ indicates a significant difference in comparison with the vehicle control group, the symbol ‘#’ indicates a significant difference between scramble control and miR-182 transfectants and the symbol ‘&’ indicates a significant difference between WT p27 3′-UTR transfectant and Mut p27 3′-UTR transfectant upon ISO treatment (P < 0.05).
Ectopic expression of miR-182 attenuated ISO induction of p27 expression and impaired ISO inhibition of BC cell growth
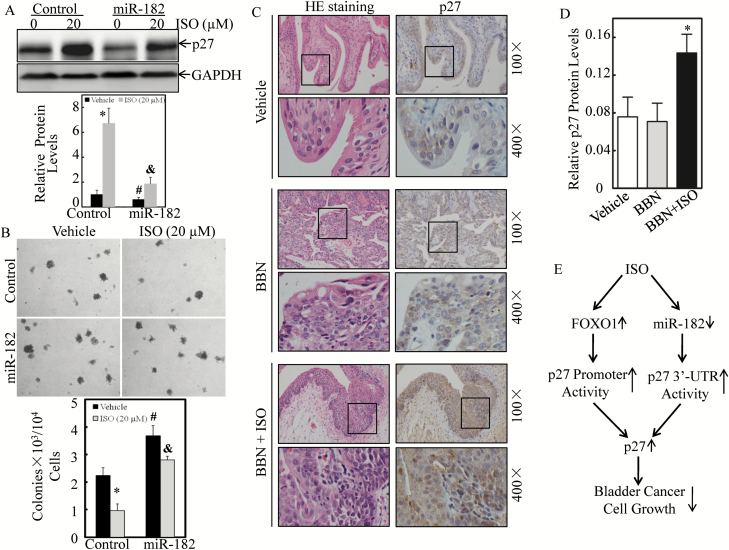
Next, the T24 (control) and T24 (miR-182) transfectants were treated with ISO to determine the contribution of miR-182 inhibition by ISO to p27 expression and BC cell growth. As shown in Figure 6A, ectopic expression of miR-182 downregulated the basal level of p27 protein and attenuated ISO-induced p27 expression. Consequently, BC cell growth was promoted, and the ISO inhibition of BC cell growth was impaired by overexpression of miR-182 in soft agar assay (Figure 6B). These data demonstrate that miR-182 is not only downregulated by ISO in BC cells but that this downregulation also contributes to ISO induction of p27 expression as well as inhibition of BC cell growth.
Figure 6.
Ectopic expression of miR-182 attenuated ISO induction of p27 expression and reversed ISO inhibition of BC cell growth, and ISO promoted p27 expression in BBN-treated mouse bladder tissues. (A) T24 (Control) and T24 (miR-182) cells were treated with either the vehicle or 20 μM ISO for 18 h. Cell lysates were subjected to western blotting to determine p27 protein expression, and GAPDH was used as protein loading control. (B) T24 (Control) and T24 (miR-182) cells were used for anchorage-independent growth assay in the presence of either the vehicle or the indicated concentration of ISO treatment. Each of the experiment was replicated three times, and the error bars represent the SD. (C) IHC-P staining by using an antibody specific against p27 was performed in BBN-induced mouse bladder tissues, and protein expression levels were analyzed by calculating the integrated optical density per stained area using Image-Pro Plus version 6.0 (D). Results are the means ± SD of 12 mice in each group. The symbol ‘*’ indicates a significant difference as compared with the vehicle control group, the symbol ‘#’ indicates a significant difference between scramble control and miR-182 transfectants and the symbol ‘&’ indicates a significant difference in comparison with scramble vector transfectant upon ISO treatment (P < 0.05). (E) The proposed schematic for the pathways underlying ISO inhibition of human BC cell growth through upregulation of p27 in both transcriptional and post-transcriptional levels.
ISO upregulated p27 expression in BBN-induced mouse bladder tissues
To address the inductive effect of ISO on p27 expression in vivo, we used the BBN-induced mouse invasive BC tissues that were obtained in our previous study (29). The results showed that p27 expression was markedly elevated in mouse bladder tissues that were co-treated with BBN and ISO (BBN + ISO) in comparison to with BCs that were treated with BBN alone (Figures 6C and D). Collectively, our results reveal that p27 expression is transcriptionally enhanced by increasing binding of FOXO1 to its promoter and post-transcriptionally upregulated through decreasing binding of miR-182 to its mRNA 3′-UTR, which mediates growth inhibition of ISO on human BC cells as diagramed in Figure 6E.
Discussion
The Chinese herb G. cleistostachyum has been used to treat BC for hundreds of years, and ISO is a new derivative isolated from this herb (14). Our recent in vivo studies have observed that ISO treatment results in a dramatic inhibition of BC cells xenograft tumor growth (17) and BBN-induced mouse invasive BC formation (18). The concentration of ISO in ISO-treated mouse serum could reach to 47.9 µM without any observable toxicity to experimental mice (15). In addition, we have demonstrated that ISO effectively induces cell-cycle arrest at G0/G1 phase and inhibits anchorage-independent cell growth through miR-137/Sp1/cyclin D1 axis in human BC cells (15,17). Herein, we found that upregulation of p27, a key CDK inhibitor, also mediated anchorage-independent growth inhibition of ISO on invasive human BC cells. Moreover, our study identified that p27 expression was transcriptionally upregulated by enhancing the binding of FOXO1 to its promoter and post-transcriptionally induced through decreasing binding of miR-182 to its mRNA 3′-UTR upon ISO treatment. These findings reveal that ISO can effectively inhibit growth of cancer cells via multiple pathways, indicating that ISO is a promising compound for the treatment of BC patients.
p27 binds to various cyclin/CDK complexes and acts as a negative regulator of cell-cycle progression to prevent G0/G1 to S-phase transition when localized in the nucleus (32). Although p27 is rarely mutated or deleted in human, its expression levels are frequently reduced in diverse human cancers, including carcinomas of the prostate, breast, colon, ovary, lung, brain, stomach, skin and bladder (10,33). p27 is subject to multiple levels of regulation via various signaling pathways, while regulation of its post-translational modifications and subcellular localization has been intensively investigated (4,34). Misregulation of p27 that results in increased protein degradation has been reported to be related with cancer development (4), and this fact makes p27 protein extremely attractive target for finding new proteasome inhibitors with selective antitumor activities due to stabilizing of p27 protein in tumor cells (34); nevertheless, the transcriptional regulation of p27 expression still needs to be elucidated in cancer cells. Previous studies suggest that Forkhead box O (FOXO) proteins, FOXO3a and FOXO1, could regulate the promoter activity of p27, which is involved in the inhibition of HEK293 cells and human umbilical vein endothelial cells proliferation (35,36), as well as anti-inflammatory drug-suppressed proliferation of human osteoblasts (37), and T-cell proliferation and survival (38). FOXO1 has been found to be induced by ISO at the transcriptional level in human T24T and UMUC3 invasive BC cells and mediated ISO inhibition of BC cells invasion through downregulation of MMP-2, in our most recent research (18). Distinctly different from the role of FOXO1 in suppression of BC cell invasion, our data presented here demonstrate that FOXO1 could directly bind to the p27 promoter and result in upregulation of p27 expression, which in turn mediates ISO inhibition of BC cell anchorage-independent growth in T24 cells. Therefore, we not only address the transcriptional regulation of p27 by FOXO1 in BC cancer cells but also explore the potential FOXO1/p27-related growth inhibitory effects of ISO in the present study.
miRNAs mainly form imperfect interactions with sequences in the 3′-UTR of their targeted mRNAs, causing translational suppression and sometimes promoting mRNA degradation as well (39). miR-182 was first identified and cloned from mouse eye (40) and has been reported to overexpress and play an oncogene role in melanoma, malignant glioma, endometrial cancer, breast cancer, lung cancer, medullary thyroid carcinoma and hepatocellular carcinoma (41–45). miR-182 has also been found to be related to MIBC tumor aggressiveness and is associated with both poor recurrence-free and overall survival in previous studies (46). However, nothing is known about the downstream effect of miR-182 in BC cells. Our current results show that ISO treatment leads to the inhibition of miR-182 expression in human T24 MIBC cells. Moreover, we have demonstrated that miR-182 is able to directly bind to p27 3′-UTR, and inhibition of miR-182 mediates ISO-induced post-transcriptional upregulation of p27 and ISO suppression of BC growth. Our findings are supported by the previous study, in which miR-182 was found to suppress the 3′-UTR activity of p27 in human breast cancer cells (47). Thus, our experimental evidences reveal the importance of both transcriptional and post-transcriptional upregulation of p27 expression in the anticancer activity of ISO. On the other hand, postphosphorylation and polyubiquitination of p27 could be another regulatory mechanism for its translocation, and the tumorigenic properties of p27 have also been proposed in recent years especially when located in cytoplasm (4). The exact function of cytoplasmic p27 is an active focus of some recent studies, but different and even contradictory findings have been reported (8,48). In previous studies, we have found that p27 plays a crucial negative role in cell migration by inhibiting MnSOD expression in a STAT3-dependent manner in MEFs (8). However, it is notable that cytoplasmic p27 has been reported to promote epithelial–mesenchymal transition and increase human breast and BC invasion and metastasis (49). Herein, the results obtained from distributions of the transcription factors between nuclear and cytoplasm fractions reveal that ISO treatment mainly upregulates nuclear fraction of p27 in T24 cells, indicating that ISO-induced p27 expression plays an important role in repressing cycle progression. Moreover, our recent studies demonstrate that FOXO1 is responsible for ISO-induced inhibition of BC cell invasion (18). Since p27 is a downstream effector of FOXO1 upon ISO treatment, the related investigation of identifying the exact function of p27 in ISO suppression of BC cell invasion is an ongoing project in our group.
In conclusion, we have demonstrated that expression of p27 can be transcriptionally enhanced by increasing binding of FOXO1 to its promoter and post-transcriptionally upregulated through decreasing the binding of miR-182 to its mRNA 3′-UTR in human BC cells. Moreover, our results reveal that upregulation of p27 expression contributes to anchorage-independent growth inhibition of ISO on invasive human BC cells. These findings not only provide novel insight into understanding of the underlying mechanisms related to regulation of invasive BC cell growth but they also identify new roles and mechanisms underlying the natural compound ISO that specifically inhibit such growth.
Funding
This work was supported by grants from National Institutes of Health (CA177665, CA165980, CA112557, ES000260 to C.S.H.) and Key Project of Science and Technology Innovation Team of Zhejiang Province (2013TD10 to C.S.H. and H.S.H.).
Conflict of Interest Statement: The authors disclose no potential conflicts of interest.
Acknowledgements
We thank Dr Toshiyuki Sakai from Kyoto Prefectural University of Medicine for providing human p27 promoter-luciferase constructs; Dr Jean-Baptiste Demoulin from Catholic University of Louvain for the generous gift of human FOXO1 promoter-luciferase reporter; Dr Gustavo Baldassarre from Centro di Riferimento Oncologico, National Cancer Institute for providing GFP-p27.
Abbreviations
- 3′-UTR
3′-untranslated region
- Act D
actinomycin D
- BBN
N-butyl-N-(4-hydroxybutyl) nitrosamine
- BC
bladder cancer
- CDK
cyclin-dependent kinase
- ChIP
chromatin immunoprecipitation
- FOXO1
forkhead box class O1
- ISO
isorhapontigenin
- MEFs
mouse embryonic fibroblasts
- MIBC
muscle-invasive bladder cancer
- RT-PCR
reverse transcription-polymerase chain reaction
- SD
standard deviations.
References
- 1. Siegel R.L., et al. (2017)Cancer statistics, 2017. CA. Cancer J. Clin., 67, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Alfred Witjes J., et al. (2017)Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur. Urol., 71, 462–475. [DOI] [PubMed] [Google Scholar]
- 3. Abdollah F., et al. (2013)Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol., 37, 219–225. [DOI] [PubMed] [Google Scholar]
- 4. Lee J., et al. (2009)The function of p27 KIP1 during tumor development. Exp. Mol. Med., 41, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sharma S.S., et al. (2016)The non-canonical functions of p27(Kip1) in normal and tumor biology. Cell Cycle, 15, 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu J., et al. (2010)p27 suppresses arsenite-induced Hsp27/Hsp70 expression through inhibiting JNK2/c-Jun- and HSF-1-dependent pathways. J. Biol. Chem., 285, 26058–26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Che X., et al. (2013)p27 suppresses cyclooxygenase-2 expression by inhibiting p38β and p38δ-mediated CREB phosphorylation upon arsenite exposure. Biochim. Biophys. Acta, 1833, 2083–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang D., et al. (2014)Loss of p27 upregulates MnSOD in a STAT3-dependent manner, disrupts intracellular redox activity and enhances cell migration. J. Cell Sci., 127(Pt 13), 2920–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hikosaka A., et al. (2008)Susceptibility of p27 kip1 knockout mice to urinary bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine may not simply be due to enhanced proliferation. Int. J. Cancer, 122, 1222–1228. [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Beltran A., et al. (2004)Prognostic factors in stage T1 grade 3 bladder cancer survival: the role of G1-S modulators (p53, p21Waf1, p27kip1, Cyclin D1, and Cyclin D3) and proliferation index (ki67-MIB1). Eur. Urol., 45, 606–612. [DOI] [PubMed] [Google Scholar]
- 11. Korkolopoulou P., et al. (2000)Cell cycle regulators in bladder cancer: a multivariate survival study with emphasis on p27Kip1. Hum. Pathol., 31, 751–760. [DOI] [PubMed] [Google Scholar]
- 12. Yu C., et al. (2012)The tumor-suppressor gene Nkx2.8 suppresses bladder cancer proliferation through upregulation of FOXO3a and inhibition of the MEK/ERK signaling pathway. Carcinogenesis, 33, 678–686. [DOI] [PubMed] [Google Scholar]
- 13. Miyake M., et al. (2010)1-Tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea (PD173074), a selective tyrosine kinase inhibitor of fibroblast growth factor receptor-3 (FGFR3), inhibits cell proliferation of bladder cancer carrying the FGFR3 gene mutation along with up-regulation of p27/Kip1 and G1/G0 arrest. J. Pharmacol. Exp. Ther., 332, 795–802. [DOI] [PubMed] [Google Scholar]
- 14. Huang K.S., et al. (2000)Stilbene dimers from the lianas of Gnetum hainanense. Phytochemistry, 54, 875–881. [DOI] [PubMed] [Google Scholar]
- 15. Fang Y., et al. (2013)Cyclin d1 downregulation contributes to anticancer effect of isorhapontigenin on human bladder cancer cells. Mol. Cancer Ther., 12, 1492–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fang Y., et al. (2012)The Chinese herb isolate isorhapontigenin induces apoptosis in human cancer cells by down-regulating overexpression of antiapoptotic protein XIAP. J. Biol. Chem., 287, 35234–35243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zeng X., et al. (2016)Induction of miR-137 by isorhapontigenin (ISO) directly targets Sp1 protein translation and mediates its anticancer activity both in vitro and in vivo. Mol. Cancer Ther., 15, 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang G., et al. (2016)Isorhapontigenin (ISO) Inhibits invasive bladder cancer formation in vivo and human bladder cancer invasion in vitro by targeting STAT1/FOXO1 axis. Cancer Prev. Res. (Phila)., 9, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang Y., et al. (2016)SESN2/sestrin 2 induction-mediated autophagy and inhibitory effect of isorhapontigenin (ISO) on human bladder cancers. Autophagy, 12, 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baldassarre G., et al. (2005)p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell, 7, 51–63. [DOI] [PubMed] [Google Scholar]
- 21. Minami S., et al. (1997)Molecular cloning and characterization of the human p27Kip1 gene promoter. FEBS Lett., 411, 1–6. [DOI] [PubMed] [Google Scholar]
- 22. Essaghir A., et al. (2009)The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J. Biol. Chem., 284, 10334–10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song L., et al. (2006)IKKbeta programs to turn on the GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50 NF-kappaB in arsenite response. J. Cell Biol., 175, 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Song L., et al. (2007)p85alpha acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol. Cell. Biol., 27, 2713–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo W., et al. (2008)Anti-cancer effects of JKA97 are associated with its induction of cell apoptosis via a Bax-dependent and p53-independent pathway. J. Biol. Chem., 283, 8624–8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ouyang W., et al. (2005)Cyclin D1 induction through IkappaB kinase beta/nuclear factor-kappaB pathway is responsible for arsenite-induced increased cell cycle G1-S phase transition in human keratinocytes. Cancer Res., 65, 9287–9293. [DOI] [PubMed] [Google Scholar]
- 27. Liu J., et al. (2011)X-linked inhibitor of apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP dissociation inhibitor (RhoGDI)-dependent regulation of the cytoskeleton. J. Biol. Chem., 286, 15630–15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang D., et al. (2010)JNK1 mediates degradation HIF-1alpha by a VHL-independent mechanism that involves the chaperones Hsp90/Hsp70. Cancer Res., 70, 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang G., et al. (2016)Isorhapontigenin (ISO) inhibits invasive bladder cancer formation in vivo and human bladder cancer invasion in vitro by targeting STAT1/FOXO1 axis. Cancer Prev. Res. (Phila)., 9, 567–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belletti B., et al. (2005)p27(kip1) functional regulation in human cancer: a potential target for therapeutic designs. Curr. Med. Chem., 12, 1589–1605. [DOI] [PubMed] [Google Scholar]
- 31. Matoulkova E., et al. (2012)The role of the 3′ untranslated region in post-transcriptional regulation of protein expression in mammalian cells. RNA Biol., 9, 563–576. [DOI] [PubMed] [Google Scholar]
- 32. Escobar-Hoyos L.F., et al. (2015)Keratin-17 promotes p27KIP1 nuclear export and degradation and offers potential prognostic utility. Cancer Res., 75, 3650–3662. [DOI] [PubMed] [Google Scholar]
- 33. Chu I.M., et al. (2008)The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat. Rev. Cancer, 8, 253–267. [DOI] [PubMed] [Google Scholar]
- 34. Nickeleit I., et al. (2008)Argyrin a reveals a critical role for the tumor suppressor protein p27(kip1) in mediating antitumor activities in response to proteasome inhibition. Cancer Cell, 14, 23–35. [DOI] [PubMed] [Google Scholar]
- 35. Sakamaki J., et al. (2009)Regulation of FOXO1-mediated transcription and cell proliferation by PARP-1. Biochem. Biophys. Res. Commun., 382, 497–502. [DOI] [PubMed] [Google Scholar]
- 36. Potente M., et al. (2003)11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J. Biol. Chem., 278, 29619–29625. [DOI] [PubMed] [Google Scholar]
- 37. Li C.J., et al. (2010)The PI3K/Akt/FOXO3a/p27Kip1 signaling contributes to anti-inflammatory drug-suppressed proliferation of human osteoblasts. Biochem. Pharmacol., 79, 926–937. [DOI] [PubMed] [Google Scholar]
- 38. Stahl M., et al. (2002)The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J. Immunol., 168, 5024–5031. [DOI] [PubMed] [Google Scholar]
- 39. Shenouda S.K., et al. (2009)MicroRNA function in cancer: oncogene or a tumor suppressor?Cancer Metastasis Rev., 28, 369–378. [DOI] [PubMed] [Google Scholar]
- 40. Lagos-Quintana M., et al. (2003)New microRNAs from mouse and human. RNA, 9, 175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang L., et al. (2010)miR-182 as a prognostic marker for glioma progression and patient survival. Am. J. Pathol., 177, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stenvold H., et al. (2014)Stage and tissue-specific prognostic impact of miR-182 in NSCLC. BMC Cancer, 14, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu J., et al. (2016)MicroRNA-182 targets SMAD7 to potentiate TGFβ-induced epithelial-mesenchymal transition and metastasis of cancer cells. Nat. Commun., 7, 13884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdelrahman M.M., et al. (2016)Enhancing NK cell cytotoxicity by miR-182 in hepatocellular carcinoma. Hum. Immunol., 77, 667–673. [DOI] [PubMed] [Google Scholar]
- 45. Spitschak A., et al. (2017)MiR-182 promotes cancer invasion by linking RET oncogene activated NF-κB to loss of the HES1/Notch1 regulatory circuit. Mol. Cancer, 16, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pignot G., et al. (2013)microRNA expression profile in a large series of bladder tumors: identification of a 3-miRNA signature associated with aggressiveness of muscle-invasive bladder cancer. Int. J. Cancer, 132, 2479–2491. [DOI] [PubMed] [Google Scholar]
- 47. Krishnan K., et al. (2013)MicroRNA-182-5p targets a network of genes involved in DNA repair. RNA, 19, 230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhatia B., et al. (2010)p27(Kip1), a double-edged sword in Shh-mediated medulloblastoma: tumor accelerator and suppressor. Cell Cycle, 9, 4307–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao D., et al. (2015)Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation. Oncogene, 34, 5447–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]