Abstract
Although increased awareness leading to early detection and prevention, as well as advancements in treatment strategies, have resulted in superior clinical outcomes, African American women with breast cancer continue to have greater mortality rates, compared to Caucasian American counterparts. Moreover, African American women are more likely to have breast cancer at a younger age and be diagnosed with aggressive tumor sub-types. Such racial disparities can be attributed to socioeconomic differences, but it is increasingly being recognized that these disparities may indeed be due to certain genetic and other non-genetic biological differences. Tumor microenvironment, which provides a favorable niche for the growth of tumor cells, is comprised of several types of stromal cells and the various proteins secreted as a consequence of bi-directional tumor-stromal cross-talk. Emerging evidence suggests inherent biological differences in the tumor microenvironment of breast cancer patients from different racial backgrounds. Tumor microenvironment components, affected by the genetic make-up of the tumor cells as well as other non-tumor-associated factors, may also render patients more susceptible to the development of aggressive tumors and faster progression of disease resulting in early onset, thus adversely affecting patients’ survival. This review provides an overview of breast cancer racial disparity and discusses the existence of race-associated differential tumor microenvironment and its underlying genetic and non-genetic causal factors. A better understanding of these aspects would help further research on effective cancer management and improved approaches for reducing the racial disparities gaps in breast cancer patients.
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer and second leading cause of cancer-related deaths in women in the United States (1). Moreover, the incidence, mortality and length of survival post-diagnosis are reported to vary among different ethnic and racial groups (2). Epidemiological data suggest that the women of African American (AA) racial background are disproportionately affected with BC, compared to their Caucasian American (CA) counterparts. Although the overall incidence of BC is higher in CA women compared to AA women, the latter are diagnosed at a younger age and more frequently with an aggressive type of breast cancer (2). AA women under 50 are diagnosed with BC with 30–40% greater frequency and have more aggressive disease than CA women (2). Further, the tumors in AA women rapidly progress to an advanced stage and are associated with poor survival for all ages, compared to those in CA women (2). Recent statistics suggest that from the year 2008 to 2012, BC incidence rates have increased tremendously in AA, as compared to CA, in seven southern states (3). According to the American Cancer Society (ACS), the 5 year survival rate is significantly lower for AA women than CA women (4). In addition, across all ages and tumor subtypes, the age-adjusted mortality rates for AA is the highest (32.4/100000) among all ethnic/racial groups studied (4). Despite these recognitions, the clear understanding of actual causes associated with such disparities remains elusive. It has been advocated that access to health care, cultural/behavioral, reproductive practices and socioeconomic factors contribute to BC racial disparities (5). Moreover, growing body of evidence suggests that biological factors such as differences at the genetic and molecular level are also crucial for observed BC disparity (6,7). It is also being recognized that the molecular differences within the tumor cells alone may not entirely be responsible for the observed BC racial disparities, and differential tumor-microenvironment (TME) can also play a significant role in the overall outcome (8). Changes in TME may not only occur due to tumor- or stromal cell-associated factors, but can also be influenced greatly by social, behavioral and life-style factors.
In this review article, we focus on differential TME in BC patients of different racial backgrounds, factors affecting TME and the role that TME might play in BC racial disparities. Understanding such basis of BC disparity will eventually help in developing a blueprint for targeted therapies to curtail the unfavorable outcomes of BC in AA population.
Disparity in breast cancer incidence and mortality
It is evident from the available literature that the incidence and survival for BC patients are considerably different in AA and CA racial groups. According to the ACS, the overall incidence of BC in the United States is higher in CA (125.4/100000) than AA women (116.4/100000) (4). DeSantis CE et al. reported that BC incidence rate increased among AA and Asian/Pacific Islander women between 2008 and 2012 (3); however, the incidence rate remained largely stable among CA. This is after decades of continuous increase in the incidence rate of BC women towards the end of last century. Interestingly, the data from 1989 to 2012 in seven southern states on the death rate associated with BC shows a reduction by 36%, but the disparity in mortality between CA and AA has continued to amplify and the death rate has elevated to 42% in AA women (3). This study is supported by a report from 50 largest cities of USA for the years 1990 through 2009 suggesting the prevalent higher mortality in AA women (9). The widening disparity in BC mortality appears to continue in view of the realization that while the incidence of BC has remained stable in CA women over the last decade, it has increased slightly in the AA women. Moreover, it has also been suggested that incidence of BC is more in young AA compared to CA women. This notion is supported by the fact that AA women under 50 years of age are diagnosed with BC more frequently than CA (2). These observations are consistent with other independent studies reporting more than 10% of BC cases in AA women diagnosed at < 40 years, compared to 5% in CA women (10). SEER data indicate that the age-specific incidence rate in AA women aged 30–39 years is 48.36 per 100000, compared to 40.79 for CA women, clearly suggesting that AA women are more susceptible to BC at a younger age (10).
Tumor microenvironment: modulator of tumor progression, metastasis and therapeutic outcome
Tumor cells do not live and thrive in isolation. They are surrounded by various other cells with which they constantly interact. This creates a unique environment, the ‘TME’, which comprises of tumor cells, immune cells, fibroblasts, lymphocytes, several signaling factors, tumor vasculature and the proteins secreted by these cells forming, extracellular matrix (ECM). It is believed that the tumor cells ‘corrupt’ the components of their TME so that these cells/factors in the TME start supporting the growth, angiogenesis and metastasis of tumor cells (11). In fact, the interaction is bidirectional wherein tumor cells remodel TME components and the cross-talk between tumor-stromal cells results in conditions conducive for mutual growth and sustenance. Cancer-associated fibroblasts (CAFs), present in TME actively participate in the growth and invasiveness of the tumor cells by secreting growth factors, cytokines and various inflammatory mediators (12). CAFs are also found to be activated by TME growth factors and cytokines such as TGF-β, monocyte chemotactic protein (MCP1), fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) (12). Activated CAFs are a major source of secreted growth factors such as VEGF that promote tumor growth, encourage vascular permeability and angiogenesis (13). Further, CAF produces MMP-2 and MMP-9, and urokinase-type plasminogen activator (uPA), which degrade ECM and support tumor invasion and metastasis (14). It was demonstrated that CAFs could modulate both premalignant and malignant mammary epithelial cells to acquire a mesenchymal-like phenotype that was associated with increased dissemination and metastasis, whereas, normal fibroblasts favored the maintenance of an epithelial-like phenotype and suppressed the tumor growth and metastasis (15). CXCL12/CXCR4 pathway, another exemplary tumor-stromal interaction, is very well known to promote growth, metastasis, and chemoresistance in various cancers (16–18). CXCL12 is a ligand for chemokine receptor, CXCR4, and is abundantly produced by tumor-associated stromal cells at the primary and metastatic sites. Earlier studies from our laboratory demonstrated that activation of CXCL12/CXCR4 signaling protected pancreatic cancer cells from gemcitabine cytotoxicity by potentiating intrinsic survival mechanisms (19). In another study, we identified a novel role of CXCL12/CXCR4 signaling axis in SHH upregulation in pancreatic cancer, where both CXCL12 and SHH predominantly acted in a paracrine manner (16).
The immune cells also hold a prominent place in TME. Immune suppressor cells such as macrophages and T regulatory cells (Treg) are known to accelerate the growth and aggressiveness of tumor (20). The bidirectional interaction between immune cells and other components of TME shapes their phenotype and responses towards tumors. Tumor-associated macrophages (TAMs) or M2 macrophage significantly contribute in creating immune suppressive microenvironment by secreting cytokine such as TGF-β and IL-10 (21). TAMs promote malignant cell migration, invasion, metastases, and are associated with poor prognosis (21). In TME, Tregs, that are found around tumor mass, exert immune suppressive function by secreting transforming growth factor-beta (TGF-β), IL-10, and cell-mediated contact through cytotoxic T-lymphocyte antigen 4 (CTLA4), thus preventing recognition and killing of tumor cells by the immune system (22). As a result, elevated numbers of Tregs in the TME has been associated with poor prognosis in many types of cancer (20).
Cells within the TME communicate with each other by different means and vesicular communication, especially exosome-mediated transport, is believed to be one of the important modes of inter-cellular communication (23,24). For last several years, there has been considerable interest in understanding the role of exosomes in BC tumorigenesis, metastasis and drug resistance (25,26). CAFs in the TME have been reported to affect BC progression by transferring stemness and EMT-promoting miRNAs to breast tumor cells through the exosomes (27). Similarly, immune cells in the TME, such as TAMs, promote aggressiveness of BC cells through exosome-mediated delivery of invasion-potentiating miRNAs to BC (28). Exosomes-mediated transfer of miR-9 (29) and miR-10b (30), the miRNAs involved in BC metastasis and drug resistance (31–33), is another example how the transport through exosomes influences BC progression. Further, exosomes secreted from TME stromal cells contribute to BC chemo- and radiation-resistance by targeting multiple signaling pathways (25). Exosomes from M1-polarized macrophages in the TME have been suggested to induce inflammatory microenvironment (34) and those from BC cells have been shown to play a role in their communication with TME, and to contribute to metastasis niche formation in an orthotopic BC mouse model (35). Exosomes from patient-derived CAFs have recently been reported to reprogram cancer cells’ metabolic machinery by delivering several intact metabolites, such as amino acids, lipids and TCA-cycle intermediates, for quick consumption and utilization by nutrient-deprived cancer cells, thus aiding growth under stressed conditions (36).
Tumor microenvironment in breast cancer racial disparity
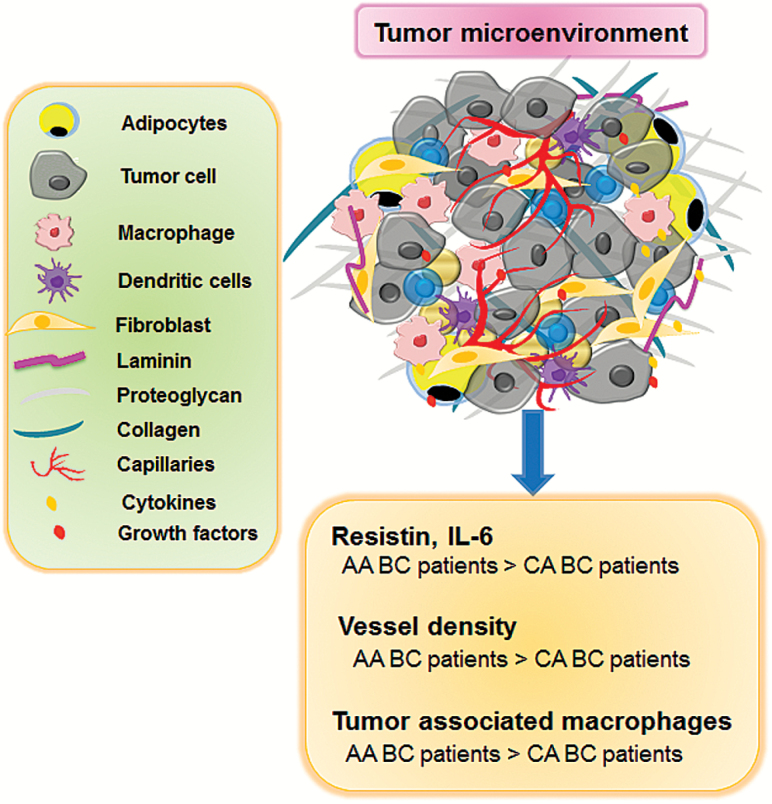
Since BC in AA women is multifaceted and complex, it is challenging to declare a single factor responsible for BC disparity. The TME, which offers fertile soil for BC progression and metastasis, is distinctly different in AA BC population, compared to CA population (Figure 1). As discussed below, growing body of evidence argues the presence of differential TME in AA and CA BC women. Infiltrated immune cells, adipocytes, tumor-associated fibroblast, and stroma contribute substantially to differential expression of genes, which further advocate the crucial role of TME in BC. The difference(s) in TME can be the decisive factor for BC clinical outcome. Therefore, an understanding of the differential TME is critical for novel therapeutic interventions to bridge the gap between clinical outcomes.
Figure 1.
Racially-disparate tumor microenvironment in breast tumors. The TME of BC contains tumor cells, macrophages, dendritic cells, adipocytes, fibroblasts, cytokines and growth factors. Higher vessel density, increased macrophage recruitment and elevated cytokines create differential TME in BC patients of AA racial group. The inflammatory microenvironment induced growth, angiogenesis, metastasis and therapy resistance ultimately leads to poor clinical outcome in AA BC patients.
Disparate expression of cytokines
Differential expression of inflammatory mediators in AA and CA BC patients has been noted by several studies (37). Cytokines in circulation are potentially important tools for prediction and prognosis of the disease. A differential expression of cytokines provides the clue about the fluctuations in TME, and can also be helpful for timely therapeutic interventions (37). Infiltrated and adipose tissue-resident macrophages are an important source of pro-inflammatory cytokines in TME (38). Patients with BC display dramatically high levels of resistin in circulation and these elevated levels correlate positively with tumor size and stage (39). Conversely, reduced expression of resistin results in improved disease-free and overall survival (39). Furthermore, we and others have reported relatively greater levels of pro-inflammatory cytokines, resistin and IL-6, in AA BC patients as compared to Caucasian patients (37,40,41). More importantly, we have also provided functional evidence for the elevated resistin and IL-6 in BC patients, which along with differential expression of CAP1 (resistin receptor) could explain racially-disparate outcomes (37,42).
The pivotal role of IL-6 has been implicated in several malignancies including BC, and IL-6 genotypes are identified to influence BC risk (43). Large amount of IL-6 is secreted by adipocytes in circulation for the maintenance of homeostasis (43). Higher expression of IL-6 is observed in adipocytes isolated from breast tumor and, moreover, the expression correlates positively with the tumor size (43). The levels of IL-6 vary with the level of obesity and, interestingly, weight loss dramatically reduces the IL-6 in circulation (44). Studies, including our own findings, suggest elevated expression of IL-6 in AA, compared to CA BC patients (37,40). IL-6 controls the regulatory activities of resistin, insulin and estrogen, which are all important for BC (37,45). In addition, when BC cells were exposed to resistin, elevated secretion of IL-6 was noted in a dose and time-dependent manner (37). Moreover, AA BC cells produced more IL-6 compared to CA BC cells after resistin treatment. These findings suggest that resistin and IL-6 coordinate to BC progression in AA patients.
Martin and coworkers have shown that vascular endothelial growth factor (VEGF) and syndecan-1, the known inducers of angiogenesis, are highly expressed in the tumor specimens of AA, compared to CA BC patients (46). Furthermore, adipose tissues mainly devoted to storing energy have emerged as active regulators of pathologic and physiologic processes, including immunity and inflammation. Adipocyte-secreted pro-inflammatory factors not only help in the maturation of cancer cells but also facilitate aggressiveness. Adipose tissues produce multiple proteins such as leptin, adipsin, visfatin, IL-6 and TNF-α, commonly termed as adipokines (47). These factors are vital for the balanced functioning of physiological processes. Inflated obesity in AA population dramatically modifies the functions of adipocytes and produces a differential clinical outcome in disease pathobiology. A study exploring ethnic differences in serum levels of adiponectin before and after adjusting BMI found a significantly higher level of C-reactive protein (CRP) and IL-6 in AA population. Unchecked growth of adipocytes leads to a release of monocyte chemoattractant protein 1 (MCP-1) in TME which activates resident macrophages and triggers macrophages infiltration. Moreover, these soluble factors alter the phenotype of macrophage to TAMs which assist tumor cells rampant growth (48). Thus, there seems to be ample evidence suggesting a disparate expression of TME cytokines in AA versus CA women.
Disparity in cellular components of tumor microenvironment
Time and again it has been shown that stromal components of TME contribute critically to cancer progression. Among immune components, TAMs are found in high number, playing a major role in unchecked cancer progression, thus contributing to poor prognosis (48). Macrophages are large white blood cells that are an integral part of immune system. They detect, engulf and destroy the invading pathogens and apoptotic cells (49). They also work as specialized antigen presenting cells, and alert T cells to act on foreign materials (49). However, in TME, instead of killing cancer cells, macrophages are polarized from M1 to M2 phenotype, resulting in TAMs. The polarized TAMs dynamically provide extra fuel for tumor cells growth and help in the immune escape, angiogenesis, tumor invasion and metastasis (49). TAMs-secreted factors stimulate tumor associated fibroblasts and manipulate T and B cells to harness pro-tumorigenic microenvironment.
It has been demonstrated that BCs from distinct racial groups display differential TAM populations (50). AA BC patients exhibit a higher number of TAMs, compared to CA BC patients (46,50). The cytokines secreted by TAMs promote the growth of cancer cells and help evade host immune system. Prevalent obesity in AA women makes the case even severe, as the organs with increased fat reserves harbor more macrophages (51). The high number of TAMs in AA tumors is a direct outcome of increased chemotactic cytokines in the TME of AA patients that attract macrophages (46). Some of the crucial chemotactic factors that attract macrophages are resistin, MCP-1, VEGF and CSF-1 (52). Macrophage-secreted resistin triggers further infiltration of macrophages and other immune cells to develop TME, and contributes to inflammatory process (53). TME captivates the infiltrated macrophages and converts their phenotype to TAMs. The tumor supporting macrophage phenotype not only stays in TME but also proliferates at a higher rate in AA BC patients, compared to CA patients (54). Biological processes associated with chemotaxis and angiogenesis are critical for tumor growth and progression. Martin et al. performed immunohistochemical analysis of microvessel density on tumor specimens from different race/ethnic background and demonstrated that the microvessel density, which boosts angiogenesis, is considerably high in breast tumors of AA patients, as compared to CA patients (46). Infiltration of macrophages and advanced microvessel density has also been shown to be associated with poor prognosis (46). Thus, the poor clinical outcome in AA BC patients can be attributed to higher angiogenesis and recruitment of macrophages.
Factors influencing tumor microenvironment
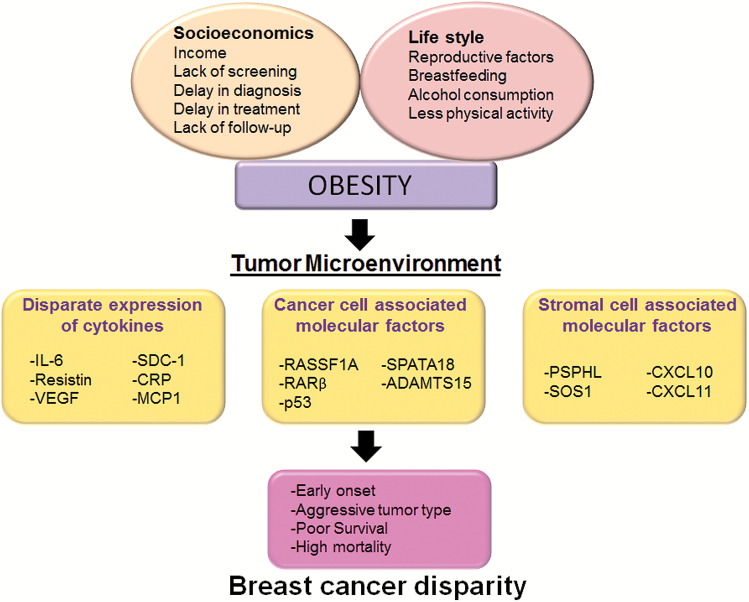
TME evolves with tumor and is continuously influenced by tumor- and/or stromal cell-associated factors and by bi-direction cross-talk between tumor and stromal compartments. Besides, several other behavioral and life-style factors may also impact TME (Figure 2). The comprehensive role of these factors in TME development is discussed below.
Figure 2.
Factors associated with breast cancer racial disparities. TME is a result of complex interplay of multiple factors such as socioeconomic, life style and obesity. Socioeconomics and lifestyle may give rise to the incidence of obesity further contributing to differential TME in AA population. Altered TME fuels BC progression and significantly contributes to early onset, aggressive tumor phenotype and poor survival leading to BC racial disparity.
Molecular factors associated with tumor cell
The perception of the involvement of genetic factors emerged from the realization that AA populations have adverse outcome even among those with a socioeconomic status similar to CA population (55). To understand this biological basis, efforts have been made to analyze differences in prominent gene mutations, chromosomal abnormalities (56) and gene expression patterns (41) with a hope to reveal intricate molecular mechanisms that influence TME and the overall outcome. Heterogeneity and genetic instability of tumors are typical characteristics of cancer, and BC is one of the most heterogeneous cancers (57). This heterogeneity poses a significant challenge in pinpointing specific factor(s) linked with aggressive tumor phenotypes and poorer clinical outcome. As discussed above, AA women have more aggressive BC at each clinical stage than their EA counterparts that affects prognosis and survival rate. Several abnormalities in cancer-associated genes p53 (7), RASSF1A (58) and RARβ (retinoic acid receptor beta) (58) have been predominantly identified in AA women. Also, tumor suppressor gene P53 has been reported to be mutated at a greater frequency in AA BC (2,59). p53 plays a role in tumor progression by altering host immune responses. It has been demonstrated that p53 dysfunction alters the tumor milieu towards pro-tumor inflammation (59), while p53 restoration or reactivation induces antitumor immunity (60), indicating the crucial role of p53 in shaping TME.
RASSF1A, a tumor suppressor involved in G1 cell cycle arrest and various apoptotic pathways, is methylated in 76% of AA compared to just 29% of CA BC patients in <50 years age group (58). It inhibits the accumulation of cyclin D1, and thus induces cell cycle arrest. Interestingly, RASSF1A is an important regulatory element that restricts NF-κB activity and protects against inflammation-induced injury (61). Enhanced activation of NF-κB and elevated production of NF-κB-regulated cytokines is commonly observed in many inflammatory diseases (62). Another gene that is methylated in AA tumors is RARβ (58). It binds the biologically active form of vitamin A and mediates signaling for cell growth and differentiation. Ablated expression of RARβ has been suggested to induce chronic inflammation and reduce apoptosis and may be involved in early stage cervical carcinogenesis (63). Furthermore, it has been linked with greater the incidence of lymph node metastasis (64). Thus, RASSF1A and RARβ are epigenetically regulated factors that differentially alter TME in racially distinct BC patients.
Polymorphisms in nucleotide excision repair genes have also been noted in AA women (65). For example, spermatogenesis associated 18 (SPATA18) was shown to be downregulated in age and stage-matched AA tumors (41). SPATA18 controls the mitochondrial quality by repairing or eliminating unhealthy mitochondria (66). Mitochondria produce reactive oxygen species (ROS) and initiate cellular apoptosis. Damage in mitochondria can alter apoptotic pathways and/or induce mutations in mitochondrial DNA which may promote cancer. Further, altered mitochondrial function plays a role in the process of inflammation, and promotes the activation NF-κB pathway and NLRP3 inflammasome. The NF-κB /NLRP3 inflammasome pathways are known to activate the production inflammatory cytokines (67).
ADAM metallopeptidase with thrombospondin type 1 motif, 15 (ADAMTS15) was another gene shown to be downregulated in age and stage-matched AA tumors (41). A large proportion of BC-associated deaths are due to metastases, and alteration of the TME is one essential determinant of metastasis. ADAMTSs (A disintegrin and metalloprotease domains with thrombospondins motifs) are multifaceted extracellular proteases that have been associated with both oncogenic and tumor-protective functions. ADAMTSs secreted by tumor or stromal cells can modify the primary TME by proteolytic-dependent or independent mechanisms and can deliver pro-tumor functions or anti-oncogenic properties (68). Downregulation of ADAMTS15 helps tumor cell migration and metastasis to distant organs (69). Its low expression is associated with a poor clinical outcome in BC (70). It is possible that the decreased expression of ADAMTS15 could have adverse consequences in AA BC progression.
Molecular factors associated with stromal cells
The progression of BC and its aggressiveness depends a lot on the interactions between various components within the TME. The crosstalk between tumor cells and their surrounding stromal components have attracted a lot of interest in this context. Several stromal components are implicated in architecting the TME (71) and the importance of stroma in BC therapy response and clinical outcomes have been recognized (71). In order to study the differentially expressed genes in stromal cells, Martin et al. performed a gene expression analysis on tumor stroma collected after microdissection from the breast tissue (46). The analysis revealed differential expression of PSPHL (phosphoserine phosphatase-like) and SOS1 (the sons of sevenless drosophila homolog 1). In addition, they identified a set of five tumor stromal genes PSPHL, CXCL10, CXCL11, ISG20 and GMDS associated with BC racial disparity by Gene Set Enrichment Analysis. The level of expression of this five-gene signature was observed to be higher in AA (94%), compared to CA women (82%) (46).
PSPHL, the stroma-associated gene found to be differentially expressed (46), is known to induce the expression of several cytokines and growth factors resulting in the activity of extracellular matrix remodeling (72). There seems to be a direct role of PSPHL in racially disparate tumor-stromal crosstalk because higher expression of PSPHL has been noted in the breast tumors, TME and non-malignant breast stroma of AA women, compared to CA women (72). In addition to BC, significantly higher expression of PSPHL has also been reported in prostate tumors, the surrounding microenvironment, and in primary cell cultures derived from AA men, compared to CA men (73). Furthermore, higher expression of PSPHL was also noted in in endometrial tumors from AA compared to CA women (74). It, thus, appears that PSPHL plays a role in racial disparity in multiple cancers and its levels are relatively higher in both men and women of AA racial background. The elevated expression of PSPHL in AA populations has been linked to less favorable outcomes (74). Considering the aggressive BC developed by AA population, PSPHL comes across as a promising stromal-associated therapeutic target to counter AA breast tumors (72,74). SOS1, a member of the RAS gene family, was the other stroma-associated gene found to be differentially expressed in AA BC tumors in the study (72) discussed above. It participates in cell growth, cell differentiation and triggers RAS/MAPK signaling pathway. The RAS/MAPK pathway helps in the progression of BC by multiple means including the promotion of growth, aggressiveness and immune surveillance (72), and, thus, higher expression of SOS1 in AA BC patients may also contribute to the most aggressive phenotype and poor prognosis. Altogether, differentially expressed stroma-associated genes can influence the tumor -stromal crosstalk within the TME, and such TME-influencing genes can form the molecular basis of racial disparity (Table 1). Thus, these genes can serve as potential therapeutic targets in AA BC patients.
Table 1.
Differentially expressed TME-related genes in African American (AA) versus Caucasian American breast cancer patients
| S. No. | Name | Title | Expression status in AA, relative to CA | Reference |
|---|---|---|---|---|
| 1 | RETN | Resistin | 2.25 folds higher | (41) |
| 2 | VEGF | Vascular endothelial growth factor | 1.59 folds higher | (46) |
| 3 | SDC1 | Syndecan1 | 1.56 folds higher | (46) |
| 4 | p53 | Tumor protein p53 | Mutated | (7) |
| 5 | RASSF1A | Ras association domain-containing protein 1 | Upregulated promoter methylation | (58) |
| 6 | RARβ | Retinoic acid receptor beta | Upregulated promoter methylation | (58) |
| 7 | SPATA18 | Spermatogenesis associated 18 | 1.28 folds lower | (41) |
| 8 | ADAMTS15 | ADAM metallopeptidase with thrombospondin type 1 motif, 15 | 1.29 folds lower | (41) |
| 9 | PSPHL | Phosphoserine phosphatase-like | Upregulated 5 folds in tumor epithelium/4.59 folds in tumor stroma | (46) |
| 10 | SOS1 | Sons of sevenless drosophila homolog 1 | 1.57 folds higher | (46) |
| 11 | CXCL10 | C-X-C motif chemokine 10 | 5.65 folds higher | (46) |
| 12 | CXCL11 | C-X-C motif chemokine 11 | 1.96 folds higher | (46) |
| 13 | IL-6 | Interleukin-6 | Upregulated | (37) |
Other factors influencing tumor microenvironment
The profound influence of TME on tumorigenesis is the net result of the complex and mutual relationship between tumor and stromal components which mutually influence the activity of each other through direct or paracrine signaling. The immediate proximity and bidirectional crosstalk between cancer cells and adipocytes are believed to expedite the aggressiveness of BC (75). Adipocytes are potential candidates that influence tumor behavior by producing growth factors, hormones, cytokines and adipokines (75). Adipocytes and BC cells synthesize leptin, a survival, pro-angiogenic and proliferation factor for cancer cells that also activates tumor stromal cells, mostly the endothelial cells and fibroblasts. Activated stromal cells further contribute to the production of a tumor-supportive extracellular matrix, thus, creating an ideal environment for the growth of cancer cells. Increasing incidence of BC has been precisely linked with the growing trend of women who adopt certain consumption habits, such as the high-fat diets, uptake of alcoholic beverages and tobacco all of which are risk factors for several malignancies (76). The overconsumption of high-fat foods increases both the hyperplasia and hypertrophy of adipose tissue which leads to the production of leptin that contributes to aggressive BC phenotype (77). Avery et al. compared 164 AA and 172 CA BC patients and revealed elevated serum level of leptin in AA BC patients after adjusting for BMI (78). Leptin acts in a paracrine as well as an autocrine manner to activate various signaling pathways involved in the BC pathogenesis (79). Leptin receptor (Ob-R) is expressed in adipose-derived stromal cells (ADSCs) as well as mammary gland epithelial cells. Moreover, ADSCs are known to synthesize and secrete 17-β estradiol (E2) whose exposure further stimulates neoplastic cell proliferation and promotes BC aggressiveness (80).
It is becoming increasingly apparent that obese BC patients have worse survival than BC patients with normal weight, with mortality as much as 30% higher (81). According to the Centers for Disease Control and Prevention, AA population has 51% higher obesity rates, compared to CA (82). Further, in comparison to CA, AA BC patients are more likely to consume a diet that is low in vegetables and fruits; are less frequently involved in the regular physical exercise, and, consequently, have more chances to be obese (81). Interestingly higher level of resistin is detected in serum obtained from obese subjects compared to lean subjects and has a positive correlation with BMI (83). Furthermore, a positive correlation has been observed between body fat and levels of serum resistin, which declines after weight loss or post-gastric bypass (83). Resistin possesses potent pro-inflammatory properties and is considered a potential mediator in several diseases including BC (37,45).
The incidence of triple-negative breast cancer (TNBC), a BC subtype that is marked by the lack of expression of estrogen and progesterone receptors and absence of ERBB2/ HER2 amplification, is particularly high in AA women (84). Moreover, AA women with TNBC have a worse clinical outcome compared to CA women with TNBC (85). Interestingly, Carolina breast cancer study have suggested a link of high BMI / high waist:hip ratio with increased risk of TNBC in premenopausal AA women (86). Obesity itself has been identified as a risk factor for TNBC in premenopausal women (85,87–89) and the observation that AA women have higher prevalence of overall obesity than other racial/ethnic groups (90) suggests a possible role of obesity in higher incidence of TNBC in AA women. There are several ways by which obesity may drive the aggressive TNBC phenotype in AA women. Obesity promotes inflammation by increasing circulating levels of inflammatory cytokines, resistin, IL6, IL-8, insulin and leptin. These cytokines in turn activate various oncogenic signaling pathways and transcription factors resulting in the poor prognosis in TNBC patients (91,92).
Alcohol consumption is another risk factor associated with BC. In a hospital-based study, McDonald PA et al. examined the influence of alcohol consumption on BC survival in women of AA racial background (93). Interestingly, their study indicated that alcohol consumption was associated with 2.7-fold increase in the risk of death. The increase of immune suppressor cells and alteration in macrophage function under the influence of alcohol exposure raised the possibility that alcohol consumption may promote tumorigenesis (94). Moreover, alcohol-associated failure of basic macrophage function may lead to loss of tissue integrity that allows invasion of tumor cells. Furthermore, a role of alcohol has been documented in the macrophage stimulation that might have tumor-supporting consequences (94). Activated macrophages promote tumorigenesis by secreting various proinflammatory cytokines such as resistin, which we have demonstrated to facilitate growth and aggressiveness (37). Thus, alcohol consumption can trigger a cascade of events in the BC TME, which can range from activation of macrophages to the release of stimulatory cytokines.
Poverty is a crucial factor that leads to BC risk-promoting lifestyle, contributing to increased morbidity and mortality (95). AA living in urban areas may experience safety issues leading to risk-promoting lifestyle. For instance, lack of safe open space and park reduces the ability to engage in regular exercise (81). Several observational studies have suggested that participation in moderate physical activities improves the survival in women with BC (96). In addition, regular participation in moderate-to-vigorous physical activity reduces the risk of postmenopausal BC and improves survival following a BC diagnosis (97). It is important to note that the immune system plays an instrumental role in TME in potential malignancies. Exercise modulates the biological function of human monocyte and increases their mobility (98). Acute exercise suppresses the sarcoma cell growth in vitro by peritoneal macrophages of NMRI mice (99). Moreover, short-term exercise increases the in vitro cytotoxic capacity of peritoneal macrophages against adenocarcinoma cells (100). The cytokine induced by exercise are generally associated with M1 phenotype macrophages. It is important to note the M2 phenotype macrophages, also known as TAMs, are mainly associated with tumor progression.
Taken together, it is evident that a number of other factors, such as diet, lifestyle etc. indirectly affect TME through their modulatory effects on various intermediate factors that affect inflammation, immune cells, and the adipocytes. These perturbed intermediate factors interact with the cellular components in the TME, often resulting in the release of cytokines.
Conclusion and perspective
It is quite evident that TME likely plays important roles in prevalent BC disparity. Differential expression of cytokines, adipokines and chemical messengers has gained prominence in recent years due to the multiple implications of these factors in cancer development leading to their recognition as attractive tools for the diagnosis. Moreover, their differential expression in AA BC patients makes them a lucrative target for therapy. To decrease the disparate burden of BC in AA women, the origin of this disparity must be defined. Differential TME significantly contributes to survival disparities. However, analysis of factors-associated with higher mortality in AA will be critical to preparing a blueprint of risk reduction strategies. Considering the unique TME and its role in AA BC pathobiology, decoding the diverse TME signature of BC in AA women is essential. The studies aimed at understanding the differential gene signature and the differentially expressed cytokines in TME of AA versus CA BC patients have just started emerging. Further, although a role of exosomes in TME has been recognized, there is, as yet, no published report suggesting differences in exosome numbers or contents among BC patients of different racial backgrounds. However, a study in prostate cancer that involved proteomic profiling of serum-derived exosomes from AA, CA and Hispanic prostate cancer patients, suggested differences in the exosome contents of patients representing these three distinct ethnicities (101). It is plausible that such studies evaluating exosome contents in racially disparate BC patients are underway, and the results would soon start appearing.
At this point, the available data is too little to make substantial progress in terms of exploiting TME differences for effective management of disparate clinical outcomes. However, it does provide a strong rationale for designing further studies to delineate the intricate cross talks between the tumor cells and the stromal cells in AA versus CA BC patients. Identification of wide-spread molecular changes would provide molecular signatures that can not only help explain the observed disparities in both incidence and the mortality, but also in the development of approaches for reducing these disparity gaps. Critical understanding of the significance of differential TME in BC will hopefully translate to therapeutic interventions in the future and improve the outcomes of BC in women of various racial and ethnic backgrounds.
Funding
This work is supported by National Institutes of Health/National Cancer Institute [CA204801 (to S.S.) and CA185490 (to A.P.S.)] and USAMCI. S.K.S. has an SBIR contract funding [HHSN261201600039C] from National Institutes of Health/National Cancer Institute.
Conflict of Interest Statement: A.P.S. and S.S. are co-founders and serve on executive management team of Tatva Biosciences LLC, which is involved in the development of tools and models for cancer health disparity research. S.K.S. serves as the Director of Cell Biology and Genetics at Tatva Biosciences LLC.
References
- 1. Siegel R.L., et al. (2015) Cancer statistics, 2015. CA. Cancer J. Clin., 65, 5–29. [DOI] [PubMed] [Google Scholar]
- 2. Danforth D.N., Jr (2013) Disparities in breast cancer outcomes between Caucasian and African American women: a model for describing the relationship of biological and nonbiological factors. Breast Cancer Res., 15, 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeSantis C.E., et al. (2016) Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA. Cancer J. Clin., 66, 31–42. [DOI] [PubMed] [Google Scholar]
- 4. American Cancer Society. Breast Cancer Facts and Figures 2011–2012 (2011). American Cancer Society, Atlanta, GA. [Google Scholar]
- 5. Vona-Davis L., et al. (2009) The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J. Womens. Health (Larchmt)., 18, 883–893. [DOI] [PubMed] [Google Scholar]
- 6. Deshmukh S.K., et al. (2017) Biological basis of cancer health disparities: resources and challenges for research. Am. J. Cancer Res., 7, 1–12. [PMC free article] [PubMed] [Google Scholar]
- 7. Dookeran K.A., et al. (2010) p53 as a marker of prognosis in African-American women with breast cancer. Ann. Surg. Oncol., 17, 1398–1405. [DOI] [PubMed] [Google Scholar]
- 8. Elledge R.M., et al. (1994) Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J. Natl. Cancer Inst., 86, 705–712. [DOI] [PubMed] [Google Scholar]
- 9. Hunt B.R., et al. (2014) Increasing Black:White disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol., 38, 118–123. [DOI] [PubMed] [Google Scholar]
- 10. Surveillance, Epidemiology, and End Results (SEER) Program Public-Use Data (2000) (1973–1998), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2001, based on the August 2000 submission. Report. [Google Scholar]
- 11. Balkwill F.R., et al. (2012) The tumor microenvironment at a glance. J. Cell Sci., 125 (Pt 23), 5591–5596. [DOI] [PubMed] [Google Scholar]
- 12. Xing F., et al. (2010) Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci. (Landmark. Ed)., 15, 166–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukumura D., et al. (1998) Tumor induction of VEGF promoter activity in stromal cells. Cell, 94, 715–725. [DOI] [PubMed] [Google Scholar]
- 14. Cirri P., et al. (2011) Cancer associated fibroblasts: the dark side of the coin. Am. J. Cancer Res., 1, 482–497. [PMC free article] [PubMed] [Google Scholar]
- 15. Dumont N., et al. (2013) Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia, 15, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh A.P., et al. (2012) CXCL12/CXCR4 protein signaling axis induces sonic hedgehog expression in pancreatic cancer cells via extracellular regulated kinase- and Akt kinase-mediated activation of nuclear factor κB: implications for bidirectional tumor-stromal interactions. J. Biol. Chem., 287, 39115–39124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neklyudova O., et al. (2016) Altered CXCL12 expression reveals a dual role of CXCR4 in osteosarcoma primary tumor growth and metastasis. J. Cancer Res. Clin. Oncol., 142, 1739–1750. [DOI] [PubMed] [Google Scholar]
- 18. Morimoto M., et al. (2016) Enhancement of the CXCL12/CXCR4 axis due to acquisition of gemcitabine resistance in pancreatic cancer: effect of CXCR4 antagonists. BMC Cancer, 16, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh S., et al. (2010) CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br. J. Cancer, 103, 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beyer M., et al. (2009) Regulatory T cells: major players in the tumor microenvironment. Curr. Pharm. Des., 15, 1879–1892. [DOI] [PubMed] [Google Scholar]
- 21. Allavena P., et al. (2008) The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol., 66, 1–9. [DOI] [PubMed] [Google Scholar]
- 22. Campbell D.J., et al. (2011) Treg cells: patrolling a dangerous neighborhood. Nat. Med., 17, 929–930. [DOI] [PubMed] [Google Scholar]
- 23. Staals R.H., et al. (2010) The human exosome and disease. Adv. Exp. Med. Biol., 702, 132–142. [PubMed] [Google Scholar]
- 24. Wendler F., et al. (2017) Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene, 36, 877–884. [DOI] [PubMed] [Google Scholar]
- 25. Boelens M.C., et al. (2014) Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell, 159, 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris D.A., et al. (2015) Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One, 10, e0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Donnarumma E., et al. (2017) Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget., doi: 10.18632/oncotarget.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang M., et al. (2011) Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol. Cancer, 10, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baroni S., et al. (2016) Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis., 7, e2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh R., et al. (2014) Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol. Cancer, 13, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmad A., et al. (2014) Up-regulation of microRNA-10b is associated with the development of breast cancer brain metastasis. Am. J. Transl. Res., 6, 384–390. [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmad A., et al. (2015) Functional role of miR-10b in tamoxifen resistance of ER-positive breast cancer cells through down-regulation of HDAC4. BMC Cancer, 15, 540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang J., et al. (2013) Overexpressions of microRNA-9 and microRNA-200c in human breast cancers are associated with lymph node metastasis. Cancer Biother. Radiopharm., 28, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng L., et al. (2017) Exosomes from M1-polarized macrophages potentiate the cancer vaccine by creating a pro-inflammatory microenvironment in the lymph node. Mol. Ther., 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suetsugu A., et al. (2013) Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv. Drug Deliv. Rev., 65, 383–390. [DOI] [PubMed] [Google Scholar]
- 36. Zhao H., et al. (2016) Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife, 5, e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Deshmukh S.K., et al. (2015) Resistin and interleukin-6 exhibit racially-disparate expression in breast cancer patients, display molecular association and promote growth and aggressiveness of tumor cells through STAT3 activation. Oncotarget, 6, 11231–11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jung H.S., et al. (2006) Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc. Res., 69, 76–85. [DOI] [PubMed] [Google Scholar]
- 39. Lee Y.C., et al. (2012) Resistin expression in breast cancer tissue as a marker of prognosis and hormone therapy stratification. Gynecol. Oncol., 125, 742–750. [DOI] [PubMed] [Google Scholar]
- 40. Park N.J., et al. (2013) Inflammatory cytokine levels and breast cancer risk factors: racial differences of healthy Caucasian and African American women. Oncol. Nurs. Forum, 40, 490–500. [DOI] [PubMed] [Google Scholar]
- 41. Stewart P.A., et al. (2013) Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS One, 8, e82460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deshmukh S.K., et al. (2017) Resistin potentiates chemoresistance and stemness of breast cancer cells: Implications for racially disparate therapeutic outcomes. Cancer Lett., 396, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knüpfer H., et al. (2007) Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res. Treat., 102, 129–135. [DOI] [PubMed] [Google Scholar]
- 44. Eder K., et al. (2009) The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res., 58, 727–736. [DOI] [PubMed] [Google Scholar]
- 45. Gonullu G., et al. (2005) Relation between insulin resistance and serum concentrations of IL-6 and TNF-alpha in overweight or obese women with early stage breast cancer. Cytokine, 31, 264–269. [DOI] [PubMed] [Google Scholar]
- 46. Martin D.N., et al. (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One, 4, e4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Derosa G., et al. (2013) Adipocytokine levels in obese and non-obese subjects: an observational study. Inflammation, 36, 914–920. [DOI] [PubMed] [Google Scholar]
- 48. Pollard J.W. (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer, 4, 71–78. [DOI] [PubMed] [Google Scholar]
- 49. Hao N.B., et al. (2012) Macrophages in tumor microenvironments and the progression of tumors. Clin. Dev. Immunol., 2012, 948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mukhtar R.A., et al. (2011) Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res. Treat., 130, 635–644. [DOI] [PubMed] [Google Scholar]
- 51. Amano S.U., et al. (2014) Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab., 19, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cursiefen C., et al. (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest., 113, 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Qatanani M., et al. (2009) Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J. Clin. Invest., 119, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lidia G., et al. (2015) Breast cancer-associated macrophages undergo proliferation at different rates across ethnicities: results of a pilot study. [abstract]. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research. American Association for Cancer Research, Philadelphia, PA, pp. 75. [Google Scholar]
- 55. Wojcik B.E., et al. (1998) Breast carcinoma survival analysis for African American and white women in an equal-access health care system. Cancer, 82, 1310–1318. [DOI] [PubMed] [Google Scholar]
- 56. Loo L.W., et al. (2011) Genome-wide copy number alterations in subtypes of invasive breast cancers in young white and African American women. Breast Cancer Res. Treat., 127, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Polyak K. (2011) Heterogeneity in breast cancer. J. Clin. Invest., 121, 3786–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mehrotra J., et al. (2004) Estrogen receptor/progesterone receptor-negative breast cancers of young African-American women have a higher frequency of methylation of multiple genes than those of Caucasian women. Clin. Cancer Res., 10, 2052–2057. [DOI] [PubMed] [Google Scholar]
- 59. Caleffi M., et al. (1994) p53 gene mutations and steroid receptor status in breast cancer. Clinicopathologic correlations and prognostic assessment. Cancer, 73, 2147–2156. [DOI] [PubMed] [Google Scholar]
- 60. Ventura A., et al. (2007) Restoration of p53 function leads to tumour regression in vivo. Nature, 445, 661–665. [DOI] [PubMed] [Google Scholar]
- 61. Gordon M., et al. (2013) The tumor suppressor gene, RASSF1A, is essential for protection against inflammation -induced injury. PLoS One, 8, e75483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoesel B., et al. (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer, 12, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Albino-Sanchez M.E., et al. (2016) Decreased RARβ expression induces abundant inflammation and cervical precancerous lesions. Exp. Cell Res., 346, 40–52. [DOI] [PubMed] [Google Scholar]
- 64. Shinozaki M., et al. (2005) Distinct hypermethylation profile of primary breast cancer is associated with sentinel lymph node metastasis. Clin. Cancer Res., 11, 2156–2162. [DOI] [PubMed] [Google Scholar]
- 65. Kato I., et al. (2009) African American-preponderant single nucleotide polymorphisms (SNPs) and risk of breast cancer. Cancer Epidemiol., 33, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kitamura N., et al. (2011) Mieap, a p53-inducible protein, controls mitochondrial quality by repairing or eliminating unhealthy mitochondria. PLoS One, 6, e16060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. López-Armada M.J., et al. (2013) Mitochondrial dysfunction and the inflammatory response. Mitochondrion, 13, 106–118. [DOI] [PubMed] [Google Scholar]
- 68. Cal S., et al. (2015) ADAMTS proteases and cancer. Matrix Biol., 44–46, 77–85. [DOI] [PubMed] [Google Scholar]
- 69. Kelwick R., et al. (2015) Metalloproteinase-dependent and -independent processes contribute to inhibition of breast cancer cell migration, angiogenesis and liver metastasis by a disintegrin and metalloproteinase with thrombospondin motifs-15. Int. J. Cancer, 136, E14–E26. [DOI] [PubMed] [Google Scholar]
- 70. Porter S., et al. (2006) ADAMTS8 and ADAMTS15 expression predicts survival in human breast carcinoma. Int. J. Cancer, 118, 1241–1247. [DOI] [PubMed] [Google Scholar]
- 71. Hanahan D., et al. (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21, 309–322. [DOI] [PubMed] [Google Scholar]
- 72. Field L.A., et al. (2012) Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer, 118, 1334–1344. [DOI] [PubMed] [Google Scholar]
- 73. Wallace T.A., et al. (2008) Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res., 68, 927–936. [DOI] [PubMed] [Google Scholar]
- 74. Allard J.E., et al. (2012) Analysis of PSPHL as a candidate gene influencing the racial disparity in endometrial cancer. Front. Oncol., 2, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dirat B., et al. (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res., 71, 2455–2465. [DOI] [PubMed] [Google Scholar]
- 76. Anand P., et al. (2008) Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res., 25, 2097–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rose D.P., et al. (2002) Adverse effects of obesity on breast cancer prognosis, and the biological actions of leptin (review). Int. J. Oncol., 21, 1285–1292. [PubMed] [Google Scholar]
- 78. Avery T.P., et al. (2011) Racial variation of leptin levels in women with breast cancer. 2011 ASCO Annual Meeting. J. Clin. Oncol., 29. [Google Scholar]
- 79. Saxena N.K., et al. (2013) Multifaceted leptin network: the molecular connection between obesity and breast cancer. J. Mammary Gland Biol. Neoplasia, 18, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lønning P.E., et al. (2009) Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J. Steroid Biochem. Mol. Biol., 117, 31–41. [DOI] [PubMed] [Google Scholar]
- 81. Patterson R.E., et al. (2010) Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas, 66, 5–15. [DOI] [PubMed] [Google Scholar]
- 82. Differences in prevalence of obesity among black, white, and Hispanic adults - United States, 2006–2008 (2009). Morb. Mortal. Wkly. Rep., 58, 740–744. [PubMed] [Google Scholar]
- 83. Azuma K., et al. (2003) Correlation between serum resistin level and adiposity in obese individuals. Obes. Res., 11, 997–1001. [DOI] [PubMed] [Google Scholar]
- 84. Sørlie T., et al. (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA, 98, 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dietze E.C., et al. (2015) Triple-negative breast cancer in African-American women: disparities versus biology. Nat. Rev. Cancer, 15, 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Carey L.A., et al. (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA, 295, 2492–2502. [DOI] [PubMed] [Google Scholar]
- 87. Kwan M.L., et al. (2009) Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res., 11, R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee E., et al. (2011) Characteristics of triple-negative breast cancer in patients with a BRCA1 mutation: results from a population-based study of young women. J. Clin. Oncol., 29, 4373–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pierobon M., et al. (2013) Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Res. Treat., 137, 307–314. [DOI] [PubMed] [Google Scholar]
- 90. Beydoun M.A., et al. (2009) Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity (Silver Spring)., 17, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Creighton C.J., et al. (2009) Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. USA, 106, 13820–13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hartman Z.C., et al. (2013) Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res., 73, 3470–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McDonald P.A., et al. (2002) Breast cancer survival in African American women: is alcohol consumption a prognostic indicator? Cancer Causes Control, 13, 543–549. [DOI] [PubMed] [Google Scholar]
- 94. Meadows G.G., et al. (2015) Effects of Alcohol on Tumor Growth, Metastasis, Immune Response, and Host Survival. Alcohol Res., 37, 311–322. [PMC free article] [PubMed] [Google Scholar]
- 95. Vona-Davis L., et al. (2009) The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J. Womens. Health (Larchmt)., 18, 883–893. [DOI] [PubMed] [Google Scholar]
- 96. Barbaric M., et al. (2010) Effects of physical activity on cancer survival: a systematic review. Physiother. Can., 62, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Friedenreich C.M. (2010) The role of physical activity in breast cancer etiology. Semin. Oncol., 37, 297–302. [DOI] [PubMed] [Google Scholar]
- 98. Gabriel H., et al. (1992) Mobilization of circulating leucocyte and lymphocyte subpopulations during and after short, anaerobic exercise. Eur. J. Appl. Physiol. Occup. Physiol., 65, 164–170. [DOI] [PubMed] [Google Scholar]
- 99. Lötzerich H., et al. (1990) Potentiation of cytostatic but not cytolytic activity of murine macrophages after running stress. Int. J. Sports Med., 11, 61–65. [DOI] [PubMed] [Google Scholar]
- 100. Woods J.A., et al. (1993) Exercise increases inflammatory macrophage antitumor cytotoxicity. J. Appl. Physiol. (1985)., 75, 879–886. [DOI] [PubMed] [Google Scholar]
- 101. Turay D., et al. (2016) Proteomic Profiling of Serum-Derived Exosomes from Ethnically Diverse Prostate Cancer Patients. Cancer Invest., 34, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]