Abstract
Introduction
Friedreich’s ataxia is the most common inherited ataxia.
Sources of data
Literature search using PubMed with keywords Friedreich’s ataxia together with published papers known to the authors.
Areas of agreement
The last decade has seen important advances in our understanding of the pathogenesis of disease. In particular, the genetic and epigenetic mechanisms underlying the disease now offer promising novel therapeutic targets.
Areas of controversy
The search for effective disease-modifying agents continues. It remains to be determined whether the most effective approach to treatment lies with increasing frataxin protein levels or addressing the metabolic consequences of the disease, for example with antioxidants.
Areas timely for developing research
Management of Freidreich’s ataxia is currently focussed on symptomatic management, delivered by the multidisciplinary team. Phase II clinical trials in agents that address the abberrant silencing of the frataxin gene need to be translated into large placebo-controlled Phase III trials to help establish their therapeutic potential.
Keywords: Friedreich’s ataxia, epigenetics, antioxidants
Introduction
Friedreich’s ataxia (FRDA) is an autosomal recessive spinocerebellar ataxia. It is the most common inherited ataxia in Europe with prevalence showing large regional differences; between 1 in 20 000 in south-west Europe and 1 in 250 000 in the north and east of Europe.1 In the majority of cases the disease is caused by a homozygous GAA triplet repeat expansion in the frataxin (FXN) gene, and the shorter repeat expansion length correlates with age at onset and disease severity.2 The clinical phenotype is broad, but consistently involves gait and limb ataxia, dysarthria and loss of lower limb reflexes. Since the original description by Nicholaus Friedreich in 1863, our understanding of the genetic aetiology, pathophysiology and clinical phenotype has progressed significantly.3 Harding’s seminal case series provided an important description of FRDA in 115 patient, and the discovery of the causative mutation in 1996 has allowed neurologists to further extend and refine our understanding of the FRDA phenotype.4,5
Clinical features
FRDA is a multisystem disorder, affecting both the central and peripheral nervous systems, the musculoskeletal system, the myocardium and the endocrine pancreas. Whilst the ‘classical’ FRDA phenotype varies substantially, gait and limb ataxia, dysarthria and loss of lower limb reflexes with deep sensory loss are always detectable. Symptoms tend to present between the ages of 10 and 16, and the mixed ataxia is the result of peripheral sensory neuropathy, spinocerebellar tract degeneration and cerebellar pathology. Gait ataxia develops early and gait is characteristically unsteady, but not overtly broad-based. Loss of balance and trunk ataxia necessitate progressive degrees of support, with most patients being wheelchair-bound by the third decade. Limb ataxia affects dexterity and coordination such that basic daily activities become increasingly difficult, and nose–finger ataxia, upper limb dysdiadochokinesia and impaired heel–shin slide are all common early signs. Dysarthria consists of slow, slurred speech which progresses from early in the disease towards unintelligibility in the advanced stages. Lower limb reflexes are absent, reflecting the underlying peripheral neuropathy, and early loss of distal vibration sense reflects dorsal root ganglion and dorsal column atrophy.
The later stages of disease are associated with pyramidal weakness, particularly of the lower limbs, and distal wasting, which further exacerbates disability. Spasticity has typically been described in the more advanced stages of the disease, however one study using biomechanical techniques detected lower limb spasticity in ambulant patients, and in those with disease durations of less than 10 years.6 Indeed the high prevalence of extensor plantars in the classical phenotype betrays pyramidal involvement early in the disease course.4 Contractures and painful muscles spasms can develop secondary to spasticity. Dysphagia is common and progresses with the disease, and in advanced cases patients require modified diets, and eventually nasogastric feeding or gastrostomy.7 Common oculomotor abnormalities in FRDA include fixation instability with frequent square-wave jerks, and gaze-evoked nystagmus is less common. Around two-thirds of patients exhibit clinical or subclinical optic neuropathy, and acuity tends to decline slowly with the disease, to blindness in a small number.8 A rare but notable exception is apparent in a subpopulation of patients who present with rapidly progressive visual loss, similar to that seen in Leber’s Hereditary Optic Neuropathy.9 Aberrant central auditory processing, detectable as abnormal evoked potentials, is common in FRDA, and the associated temporal distortion of stimuli can exacerbate verbal communication difficulties.10 In some cases, sensorineural deafness develops. Urinary urgency and frequency are common complaints, affecting between 23% and 41%, and bladder overactivity is likely to reflect pyramidal involvement.7,11 Bowel symptoms, in the form of constipation or incontinence, are common, and are likely related to reduced mobility and corticospinal pathology. Sleep-related breathing problems, primarily in the form of obstructive sleep apnoea (OSA) are reported with higher frequency than in the normal population, and seems to be associated with increased disease duration and severity.12
Common musculoskeletal abnormalities include scoliosis, pes cavus and talipes equinovarus. Scoliosis is often seen early in the disease, and a signification proportion of patients will require surgical correction.13 Subtle cognitive deficits have been described involving various domains, including executive function, speed and attention, working memory and visuospatial reasoning.14 A number of small cohort studies have described significant rates of depression and anxiety amongst FRDA patients, and whilst these symptoms are considered by some to reflect a reactive condition in the context of a neurodegenerative disease, there is evidence to support a correlation between depression and frontal grey matter volume loss.15,16
The neurological phenotype of FRDA is the result of diffuse pathological processes affecting various components of the central and peripheral nervous systems. Cerebellar atrophy is prominent in the dentate nucleus and its efferent pathways in the superior cerebellar peduncles.16 The dorsal root ganglia are smaller than normal, and the large primary sensory neurons atrophy early in the disease. These changes lead to an axonal peripheral sensory neuropathy and posterior column loss.17 Spinal cord diameter is reduced at all levels, but atrophy is particularly prominent at the thoracic levels, and loss of corticospinal and spinocerebellar tracts is also evident. In line with these macroscopic findings, MRI studies have revealed significant grey and white matter loss in the deep cerebellar nuclei and brainstem, with the superior cerebellar peduncles showing significant atrophy.18 More recent volumetric and tractography techniques have extended these findings to include supratentorial structures, demonstrating grey matter loss in bilateral precentral gyri and progressive white matter changes in the corpus callosum and pyramidal tracts.19
FRDA is strongly associated with a cardiomyopathy, and it is thought that cardiac wall abnormalities are present in the majority of patients, though often these will be asymptomatic. Indeed the ECG reveals repolarisation abnormalities in most cases; typically T-wave inversion or flattening in lateral or inferior leads, and ST-segment depression or elevation. Common echocardiographic findings include increased end-diastolic septal and posterior wall thicknesses, and left ventricular hypertrophy (LVH) which is primarily concentric. Longitudinal data suggests that ~20% of patients exhibit reduced ejection fraction (EF), which tends to decline with age.20 One study described two distinct cardiac trajectories amongst FRDA patients over a 10-year follow-up period; a large low-risk group with normal EF at baseline, which declined slightly over time but remained within normal range, and a smaller high-risk group associated with a more pronounced and progressive decline in EF.21 Interestingly, evolution of EF was shown to be independent of hypertrophy, but correlated with the size of the shorter GAA repeat. The overall pattern of pathology seems to show a slow regression of LVH over time, and a progressive increase in left ventricular dilatation. The advanced stages of disease are associated with supraventricular tachyarrythmias, most commonly atrial fibrillation (AF) which, if sustained, can manifest as palpitations. Furthermore these arrythmias can contribute to worsening systolic function and eventually clinical heart failure, which accounts for >50% of deaths in FRDA.22 Diabetes mellitus (DM) is more prevalent in patients with FRDA when compared to age-matched control populations, with estimates varying between 1% and 32%.2,7 Younger age-at-onset and longer disease duration increase the risk of DM, and it can present acutely with ketoacidosis. Evidence suggests that both insulin deficiency, secondary to beta-cell apoptosis and insulin resistance contribute to glucose intolerance and eventually DM.
Whilst the ‘classical’ phenotype is by far the most prevalent and subsequently most commonly studied and described, a significant proportion of cases are described as ‘atypical’, most of which are delayed-onset.23 Whereas mean age of onset in classical FRDA is between 10 and 16 years,4,7,11 late-onset (LOFA) and very late-onset (VLOFA) Friedreich’s ataxia develop after the ages of 25 and 40 years, respectively. Whereas taken together these two forms of the disease might represent part of the same phenotypic spectrum, important distinctions have been described when they are compared to classical FRDA.24 Delayed-onset cases are characterised by a milder phenotype, slower progression of disease and a more variable collection of signs and symptoms. Gait and limb ataxia remain the most common presenting features, but dysarthria presents late, and spasticity and retained reflexes are encountered more frequently.25 Of particular note, non-neurological features including scoliosis, pes cavus, cardiomyopathy and diabetes are less frequent amongst the delayed-onset cases. As such, morbidity and mortality are less commonly attributable to cardiac complications, although of note the ECG is typically abnormal in most cases. A small number of patients will develop symptoms before the age of 5, and such cases are classified as early-onset FRDA. These cases are associated with larger GAA repeats, a more severe phenotype, faster disease progression and higher incidence of cardiac complications.23
FRDA is a progressive disorder, and increases in ataxia rating scales over time have been consistently reported. Evidence suggests that those with earlier onset disease suffer a more severe and progressive phenotype, although ceiling effects in the more advanced stages mean that scale score progression is of limited use over longer disease durations (around 20 years). The most common cause of death in FRDA is cardiac dysfunction, namely congestive heart failure or arryhthmia, and average age at death was reported as 36.5 years (range 12–87) in a large retrospective study.22 Other causes of death include stroke, ischaemic heart disease and pneumonia.
Genetics
The majority of cases of FRDA are associated with a pathological expansion in the non-coding first intron of the FXN gene. The remaining cases (1–3%) are associated with a compound heterozygous expansion with a point mutation or deletion. Expanded trinucleotide tracts containing less than ~40 repeats are found in normal chromosomes, and the pathological threshold seems to be 70. Triplet numbers in FRDA are most commonly between 600 and 900. Heterozygous carriers are healthy, and carrier rate amongst Europeans is ~1 in 85.26
The intronic GAA expansion silences the FXN gene, resulting in pathologically suppressed levels of the frataxin protein. The mechanisms underlying this silencing effect have been extensively investigated, in the hope that targeting the initial gene insult might offer a viable strategy for disease modification. Some evidence supports a physical blockade on transcription by the GAA repeat, for example in the form of ‘sticky DNA’ triplexes and R-loops.27 These structures could impede the progress of RNA polymerase II through the repeats and lead to stalled or aborted transcription. Silencing of the FXN gene might be the result of position effect variegation-like effects whereby GAA repeats induce abnormal heterochromatisation of nearby genes, rendering them transcriptionally inactive in certain cell populations. This effect is thought to arise when the transcriptional balance of euchromatic (transcriptionally active) and heterochromatic (transcriptionally inactive) factors is in equilibrium. Aberrant epigenetic mechanisms including DNA methylation and histone modification are thought to tip this balance towards heterochromatin and gene silencing in FRDA. DNA methylation is an important mediator of gene expression which is thought to regulate the majority of the human genome, and quantitative analyses in peripheral blood mononuclear cells from FRDA patients have confirmed abnormally extensive DNA methylation upstream of the repeat expansion on the silenced FXN locus.28 This methylation is inversely correlated with FXN expression and age of onset of disease, suggesting an important role in pathogenesis of the disease.29 Similar biochemical modifications of histones have been shown to modulate the chromatin structure and thus the transcriptional availability of genes. High levels of heterochromatic ‘marks’ have been identified in the regions flanking the GAA repeat expansion at the FXN locus, and suggest a bidirectionally spreading transcriptional suppression.30 Whilst important clarifications still need to be made, particularly with regards to the specific chromatin modifiers responsible for silencing the FXN gene and the extent of this heterochromatisation, the epigenetic basis of FRDA is providing a promising therapeutic approach to promoting frataxin expression.
Frataxin
Frataxin is a small, highly conserved protein, which is encoded in the nucleus, expressed in the cytoplasm as a precursor polypeptide, and then imported into the mitochondria. Early studies in yeast established a strong association between frataxin and mitochondrial iron, describing iron overload and susceptibility to oxidative stress when the frataxin homologue (Yfh1p) is deficient.31 In FRDA patients, endomyocardial biopsy analyses have revealed reduced activity of iron-sulphur cluster (ISC) containing subunits of the mitochondrial respiratory chain complexes I, II and II, and increased iron deposition.32 The ISCs are inorganic redox-active protein cofactors that are involved in a variety of cardinal functions within the cell, including oxidative phosphorylation, enzyme catalysis and gene regulation.
Bioinformatic studies have provided an important insight into the frataxin interactome, and two phylogenetically linked co-occurring genes were originally identified.33 The products of these two genes in a yeast model, SSQ1 and JAC1, are required for ISC assembly in mitochondria, and it has thus been postulated that frataxin plays an integral role in ISC biogenesis. Immunoprecipitant and recombinant protein studies have demonstrated a direct interaction between human frataxin and a preformed ISC core assembly complex.34 The exact nature of this interaction however, remains to be established. From a kinetics perspective, eukaryotic frataxin increases the rate of ISC formation, and furthermore it facilitates the formation of ISCs that are subsequently transferred to cytosolic aconitase to induce a molecular switch between enzymatic and RNA-binding functions.35 Recent evidence suggests that human frataxin activates ISC formation by accelerating sulphur transfer on the ISC assembly protein ISCU2, supporting a role for frataxin as an allosteric modulator.36
Whilst the function of frataxin is yet to be characterised completely, its role in the formation of ISCs is well supported. Other functional links have been made, in particular with haem biosynthesis and ferrochelatase, respiratory chain complex II, and mitochondrial chaperones.37 The role of frataxin in these pathways will require further investigation, particularly in in vivo models where the metabolic consequences of frataxin deficiency can be elucidated.
Mitochondria
It is essential for us to understand how mitochondrial frataxin deficiency precipitates the cellular, tissue and clinical phenotype of FRDA. A strong and consistent link has been made between frataxin deficiency, reduced activity of ISC-containing enzymes including respiratory chain complexes, and abnormal mitochondrial iron homoeostasis. Pathological intramitochondrial iron deposition has been reported in yeast knockout models, and cardiomyocytes from both conditional knockout mouse models and FRDA patients.38 The role of intracellular iron accumulation in the neuronal pathology of FRDA is less clear. Evidence from X-ray fluorescence and immunohistochemical studies has highlighted an important role for glial cells; intracellular ferritin expression is abnormally high in the hyperplastic microglial populations supporting the dentate nucleus and dorsal root ganglia, and in the latter, forms part of a constellation of maladaptive changes that might result in impaired trophic support of neurons.39,40
The iron-responsive element binding protein (IRP1) is an ISC-containing protein which, in conjunction with the structurally similar IRP2, exerts a regulatory effect by binding to iron-responsive elements (IRE) on the mRNA of proteins involved in iron metabolism. It is possible that the impaired ISC biosynthesis associated with frataxin deficiency activates IRP1-mediated cellular iron uptake via IRE-binding and the consequent expression of proteins such as transferrin receptor 1. This iron is then translocated to the mitochondria in an attempt to increase ISC synthesis. The lack of frataxin however impedes the ISC-assembly complex and so the iron is not utilised, instead accumulates and is oxidised.41 It is thought that high levels of free iron increases production of reactive oxygen species, resulting in a greater oxidative stress burden. Evidence from transgenic FRDA mice-derived neurons describes a functional imbalance between respiratory chain complexes I and II, which drives the formation of free radicals, resulting in glutathione depletion, lipid peroxidation and cell death.42 These toxic processes can be rescued by preventing lipid peroxidation and activating antioxidant pathways within the mitochondria,43 providing strong evidence for the therapeutic targeting of these processes in FRDA. These oxidative consequences of frataxin deficiency have also been linked to increased mitophagy, impaired cytoskeletal dynamics and mitochondrial transport, abnormal calcium homoeostasis, and altered lipid metabolism.44
Management
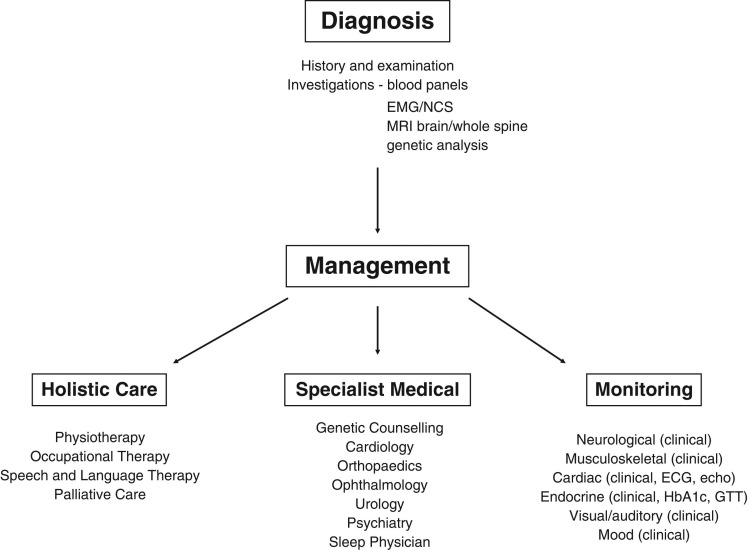
The complex and variable clinical phenotype of FRDA requires a broad multidisciplinary approach to management. Whilst significant progress has been made in the search for disease-modifying agents, we are yet to see any therapeutic options that actually halt the progression of the disease. As such, management currently focuses on symptoms. A comprehensive set of consensus clinical management guidelines have recently been published, which draw on published evidence and expert opinion to ensure best practice in the delivery of health services to individuals with FRDA.45,46 Of note, early referral to a specialist Ataxia Centre will facilitate access to the multidisciplinary team and ensure an approach that is tailored to the individual needs of each patient, with the aim of prolonging independence and maintaining quality of life. It is essential that newly diagnosed individuals are also referred to genetic counselling for discussions on inheritance, implications for family members, and the possibility of pre-symptomatic testing. Patient groups offer an invaluable source of information and support for patients and their families, e.g. Ataxia UK (www.ataxia.org.uk). An overview of the multidisciplinary service pathway is shown in Figure 1.
Fig. 1.
Flow chart for the diagnosis and management of Friedreich's ataxia.
Physiotherapy provides an important means of maintaining balance, flexibility, strength and accuracy of limb movements, all of which can help to ameliorate the functional consequences of gait and limb ataxia. Furthermore, aerobic exercise training may help to improve weakness and fatigue, which are prevalent and progressive.47 Passive stretching by the physiotherapist can provide temporary improvement of spasticity, however more prolonged muscle lengthening might require splinting, casting and orthoses.48 Spasticity and spasm may require pharmacological intervention, including oral options such as baclofen, tizanadine, low-dose gabapentin and benzodiazepines, or more advanced techniques including botulinum injections, intrathecal baclofen pumps.49 Rehabilitation may help counteract the effects of ataxia, weakness and spasticity on function in FRDA patients, by modifying physiological processes and thereby delaying, maintaining and even improving functional decline. In particular, intensive inpatient rehabilitation programmes have been shown to improve function in patients with a variety of neurodegenerative disorders, including FRDA.50 Occupational therapy allows for the assessment and optimisation of functional status, thereby reducing the impediments to activities of daily living. Specifically, prescription and provision of equipment to maximise independence, home/work modifications, retraining of functional skills, and management of educational and vocational issues, are all important components of the holistic management approach needed in FRDA. Musculoskeletal complications are common in FRDA and scoliosis affects most patients, often with onset early in the disease. Indications for surgical correction of scoliosis include a curve approaching 50%, and deformity causing functional problems such as poor sitting balance and poor head control.51,52 Whilst bracing has not been shown to affect prognosis, it may help delay surgical correction in young children.53
Problems with speech and swallowing are prevalent in FRDA, and require specialist input for assessment, monitoring and treatment. Dysarthria is a primary feature of FRDA, which progressively limits communication, and so early and comprehensive assessments are fundamental.54 Behavioural management strategies encompass a range of measures to help facilitate specific communication deficits, and constitute the main approach to speech problems.55 Measures can include physiotherapy to improve the physical aspect of speech generation, compensatory speaking strategies, developing alternative or augmentative modes of communication and managing the communication environment. Patients reporting symptoms or showing signs of dysphagia should have a swallowing assessment by a speech and language therapist.56 The multidisciplinary team can offer environmental modification and compensatory posture training in order to facilitate safe swallowing.57 Dietary modification can also be beneficial, and in severe cases nasogastric or gastrostomy feeding may be required for weight maintenance.
Patients should undergo visual screening, in line with national standards, and ophthalmological input should be sought in the case of visual symptoms. Memantine, acetazolamide or clonazepam may be of benefit for treating square-wave jerks or ocular flutter.58 A comprehensive auditory assessment should be completed at time of diagnosis, and should be followed-up with annual screening. Tactics involving optimisation of the listening environment might be beneficial in FRDA, and whereas conventional hearing aids are of little use, FM-listening devices may improve hearing and communication.59 Bladder symptoms should be carefully assessed, involving dipstick analysis for infection, and post-void residual (PVR) measurement. In cases of overactive bladder, antimuscarinics, e.g. oxybutynin, tolterodine and solifenacin, can provide symptomatic relief. Alternative options include intradetrusor botulinim toxin A injection, supra-pubic catheterisation, and in the cases of persistently elevated PVR volumes, intermittent self-catheterisation.60 Annual reviews should also include evaluation of sleep-disordered breathing using the Epworth Sleepiness Scale, and patients should be referred for polysomnography if OSA is suspected.61
The heart requires special attention in FRDA, as cardiac complications are a common cause of morbidity and mortality. An electrocardiogram and echocardiography should be performed at diagnosis, and specialist cardiological input should be sought if results are abnormal or if cardiac symptoms are present. There are no randomised controlled trials in FRDA assessing rhythm- or rate-control in supraventricular tachyarrythmias, and as such it is reasonable for clinicians to consider the recommendations included in the 2011 ACCF/AHA/HRS Focused Updates Incorporated Into the ACC/AHA/ESC 2006
Guidelines for the Management of Patients with Atrial Fibrillation.62 It is important to note that agents with negative inotropic or pro-arrhythmic effects are generally avoided in FRDA, especially in the presence of structural heart disease or heart failure. The decision on anticoagulation should include thromboembolic risk as defined by the CHA2DS2VASc score, and either warfarin or one of the novel oral anticoagulants is recommended. There is currently no evidence to support the treatment of patients with normal EF and no symptoms or signs of heart failure. A number of studies have investigated the effects of idebenone on LV thickness or LV mass index, but findings have been inconsistent.63,64 Recommendations from the 2009 Focused Update of the 2005 AHA/ACC Guidelines for the Diagnosis and Management of Heart Failure in Adults should be considered when managing FRDA patients with heart failure and reduced EF, again in the absence of specific RCT data.65 Patients should be started on either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and a beta blocker, and there is a strong evidence base demonstrating a reduction in mortality and hospitalisations with these agents.65 In cases of symptomatic heart failure with reduced ejection fraction, loop diuretics should be prescribed for fluid overload, and mineralocorticoid receptor antagonists can be used in patients with an EF <35%, and functional status defined as New York Heart Association Stage III or IV. In addition to the above options, digoxin can also be considered for symptomatic relief in HF with reduced EF, and it may also be considered a relevant option in patients with heart failure and AF. Patients with LVEF <35%, QRS duration of >0.12 s, and sinus rhythm should be considered for device therapy, including cardiac resynchronisation therapy, in line with the ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities.66 FRDA patients undergoing major surgery should have a thorough assessment of cardiac function as part of their pre-operative review, and peri- and post-operative care should be coordinated by a multidisciplinary team.
Annual oral glucose tolerance testing is recommended, and this can be augmented with serum HBA1c levels. Patients with impaired glucose tolerance or diabetes should be counselled on the importance of lifestyle changes, in particular dietary changes and physical exercise. Metformin should be avoided due to its inhibitory effect on complex I of the electron transport chain. Despite their antioxidant effects and beneficial effects on frataxin levels in FRDA patient fibroblasts, PPAR-γ agonists, specifically thiazolidinediones, should be used with caution due to the risk of water retention and congestive hear failure.67 The use of incretin analogues, for example exenatide, is endorsed by UK National Guidelines as part of specific triple therapy, and interestingly these agents have been shown to prevent apoptosis in both pancreatic beta cells and neurons.68,69 Their utility in FRDA however remains to be determined. Ultimately, it is typical to require insulin in these patients as sensitisation and lifestyle changes will eventually fail to control serum glucose levels.
Disease modification therapies
Whilst we have yet to see an effective disease modification strategy for FRDA, a number of molecules aimed at specific pathological processes have been evaluated in clinical trials (Table 1; for individual references see45). Progression of disease is typically measured using clinical scales, including the Friedreich’s Ataxia Rating Scale (FARS), the International Cooperative Ataxia Rating Scale (ICARS) and the Scale for the Assessment and Rating of Ataxia (SARA) which all broadly overlap but do include important distinctions, for example the inclusion of functional measures in the FARS. Scores have been shown to correlate well with one another, and the SARA exhibits the greatest sensitivity to longitudinal change in FRDA, a high construct validity, and requires the smallest sample size for an equivalently powered trial.70 Earlier clinical trials have focussed on the downstream consequences of frataxin deficieny, specifically the roles of oxidative stress and iron accumulation in FRDA, with antioxidant and iron chelation agents being evaluated both as monotherapy and in combination, yielding inconsistent results.71,72 Other approaches have attempted to treat FRDA by restoring frataxin levels. For example, the glycoprotein erythropoietin has been shown to increase frataxin levels in FRDA models, but clinical trials have as yet failed to consistently demonstrate clinical benefit.73,74 More recent approaches have focussed on the more upstream processes that yield frataxin deficiency. For example, histone deacetylase (HDAC) inhibition has emerged as a promising therapeutic strategy that builds on the developing evidence that FRDA is a gene silencing disease with an epigenetic basis. A first clinical trial in nicotinamide, a Class III HDAC inhibitor, has demonstrated sustained rises in frataxin levels in FRDA patients.75
Table 1.
Clinical trials in Friedreich's ataxia
| Agent | Therapeutic mechanism | Current level of investigation |
|---|---|---|
| Idebenone | Antioxidant | Phase III randomised placebo-controlled trial |
| CoQ10/Vitamin E | Antioxidant | Phase III randomised two-dose-arm trial |
| Carnitine/Creatine | Antioxidant | Phase II randomised placebo-controlled trial |
| Deferiprone | Iron chelator | Phase II randomised placebo-controlled trial |
| Deferiprone/Idebenone | Iron chelator/antioxidant | Phase II open-label trial |
| Deferiprone/Idebenone/Riboflavin | Iron chelator/antioxidants | Phase II open-label trial |
| EPO | Increase frataxin | Phase II randomised placebo-controlled trial |
| Carbamylated EPO | Increase frataxin | Phase II randomised placebo-controlled trial |
| A0001 | Antioxidant | Phase II placebo-controlled trial |
| Nicotinamide | Increase frataxin | Phase II open-label dose-escalation trial |
| RG2833 | Increase frataxin | Phase I crossover dose-escalation trial |
| Interferon gamma | Increase frataxin | Phase II dose-escalation trial |
| Resveratrol | Antioxidant | Phase II open-label non-randomised trial |
Conclusions
FRDA is a complex multisystem disorder caused by a GAA repeat expansion in the first intron of the FXN gene. The resulting frataxin deficiency impairs ISC biogenesis, disrupts mitochondrial iron homeostasis, and profoundly impacts sensitivity to oxidative stress. These processes yield a progressive picture of cell toxicity and death in the heart, the nervous systems and the pancreatic beta cells. The resultant phenotype is distinct yet highly variable, associated with progressive loss of mobility, cardiac dysfunction and early death. A disease-modifying treatment is yet to be approved, however various therapeutic strategies based on our advancing knowledge of the pathogenesis of FRDA are being evaluated. Our understanding of the genetic and epigenetic mechanisms of FXN gene silencing has preceded the use of HDAC inhibitors, and the identification of new pathways that upregulate frataxin remain the most promising approach to, and indeed the ultimate goal of, treatment of this disease. This fascinating and tragic condition is providing unique insights into molecular genetics, bioenergetics and cellular iron dynamics, and it is with great anticipation that we hope these insights can be translated into effective treatments to halt or even prevent this disease in the future.
Conflict of interest statement
None declared.
Acknowledgements
We would like to thank Dr Julie Greenfield of Ataxia UK for her suggestion to the manuscript. PG works at University College London Hospitals/University College London, which receives a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, and receives support from the NIHR Clinical Research Network (CRN).
References
- 1. Vankan P. Prevalence gradients of Friedreich’s Ataxia and R1b haplotype in Europe co-localize, suggesting a common Palaeolithic origin in the Franco-Cantabrian ice age refuge. J Neurochem 2013;126:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Reetz K, Dogan I, Costa AS, et al. Biological and clinical characteristics of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS) cohort: a cross-sectional analysis of baseline data. Lancet Neurol 2015;14:174–82. [DOI] [PubMed] [Google Scholar]
- 3. Friedreich N. Ueber Degenerative Atrophie der Spinalen Hinterstränge. Arch Pathol Anat Phys Klin Med 1863;26:391–419. [Google Scholar]
- 4. Harding AE. Friedreich’s ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 1981;104:589–620. [DOI] [PubMed] [Google Scholar]
- 5. Campuzano V, Montermini L, Moltò MD, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 1996;271:1423–7. [DOI] [PubMed] [Google Scholar]
- 6. Milne SC, Corben LA, Yiu E, et al. Gastrocnemius and soleus spasticity and muscle length in Friedreich’s ataxia. J Clin Neurosci 2016. Jul;29:29–34. [DOI] [PubMed] [Google Scholar]
- 7. Dürr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med 1996;335:1169–75. [DOI] [PubMed] [Google Scholar]
- 8. Fahey MC, Cremer PD, Aw ST, et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain 2008;131:1035–45. [DOI] [PubMed] [Google Scholar]
- 9. Fortuna F, Barboni P, Liguori R, et al. Visual system involvement in patients with Friedreich’s ataxia. Brain 2009;132:116–23. [DOI] [PubMed] [Google Scholar]
- 10. Rance G, Corben LA, Du Bourg E, et al. Successful treatment of auditory perceptual disorder in individuals with Friedreich ataxia. Neuroscience 2010;171:552–5. [DOI] [PubMed] [Google Scholar]
- 11. Delatycki MB, Paris DB, Gardner RJ, et al. Clinical and genetic study of Friedreich ataxia in an Australian population. Am J Med Genet 1999. 19;87:168–74. [DOI] [PubMed] [Google Scholar]
- 12. Corben LA, Ho M, Copland J, et al. Increased prevalence of sleep disordered breathing in Friedreich ataxia. Neurology 2013;81:40–5. [DOI] [PubMed] [Google Scholar]
- 13. Tsirikos AI, Smith G. Scoliosis in patients with Friedreich’s ataxia. J Bone Joint Surg Br 2012;94:684–9. [DOI] [PubMed] [Google Scholar]
- 14. Nieto A, Correia R, de Nóbrega E, et al. Cognition in Friedreich ataxia. Cerebellum 2012;11:834–44. [DOI] [PubMed] [Google Scholar]
- 15. Silva CB, Yasuda CL, D’Abreu A, et al. Neuroanatomical correlates of depression in Friedreich’s ataxia: a voxel-based morphometry study. Cerebellum 2013. Jun;12:429–36. [DOI] [PubMed] [Google Scholar]
- 16. Koeppen AH. Friedreich’s ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci 2011;303:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morral JA, Davis AN, Qian J, et al. Pathology and pathogenesis of sensory neuropathy in Friedreich’s ataxia. Acta Neuropathol 2010;120:97–108. [DOI] [PubMed] [Google Scholar]
- 18. Della Nave R, Ginestroni A, Giannelli M, et al. Brain structural damage in Friedreich’s ataxia. J Neurol Neurosurg Psychiatry 2008;79:82–5. [DOI] [PubMed] [Google Scholar]
- 19. Rezende TJ, Silva CB, Yassuda CL, et al. Longitudinal magnetic resonance imaging study shows progressive pyramidal and callosal damage in Friedreich’s ataxia. Mov Disord 2016;31:70–8. [DOI] [PubMed] [Google Scholar]
- 20. Regner SR, Lagedrost SJ, Plappert T, et al. Analysis of echocardiograms in a large heterogeneous cohort of patients with friedreich ataxia. Am J Cardiol 2012;109:401–5. [DOI] [PubMed] [Google Scholar]
- 21. Pousset F, Legrand L, Monin ML, et al. A 22-year follow-up study of long-term cardiac outcome and predictors of survival in Friedreich Ataxia. JAMA Neurol 2015;72:1334–41. [DOI] [PubMed] [Google Scholar]
- 22. Tsou AY, Paulsen EK, Lagedrost SJ, et al. Mortality in Friedreich ataxia. J Neurol Sci 2011;307:46–9. [DOI] [PubMed] [Google Scholar]
- 23. Parkinson MH, Boesch S, Nachbauer W, et al. Clinical features of Friedreich’s ataxia: classical and atypical phenotypes. J Neurochem 2013;126:103–17. [DOI] [PubMed] [Google Scholar]
- 24. Lecocq C, Charles P, Azulay JP, et al. Delayed-onset Friedreich’s ataxia revisited. Mov Disord 2016;31:62–9. [DOI] [PubMed] [Google Scholar]
- 25. Martinez AR, Moro A, Abrahao A, et al. Nonneurological Involvement in Late-Onset Friedreich Ataxia (LOFA): exploring the phenotypes. Cerebellum 2017. Feb;16:253–6. [DOI] [PubMed] [Google Scholar]
- 26. Cossée M, Schmitt M, Campuzano V, et al. Evolution of the Friedreich’s ataxia trinucleotide repeat expansion: founder effect and premutations. Proc Natl Acad Sci U S A 1997;94:7452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakamoto N, Chastain PD, Parniewski P, et al. Sticky DNA: self-association properties of long GAA.TTC repeats in R.R.Y triplex structures from Friedreich’s ataxia. Mol Cell 1999;3:465–75. [DOI] [PubMed] [Google Scholar]
- 28. Castaldo I, Pinelli M, Monticelli A, et al. DNA methylation in intron 1 of the frataxin gene is related to GAA repeat length and age of onset in Friedreich ataxia patients. J Med Genet 2008;45:808–12. [DOI] [PubMed] [Google Scholar]
- 29. Evans-Galea MV, Carrodus N, Rowley SM, et al. FXN methylation predicts expression and clinical outcome in Friedreich ataxia. Ann Neurol 2012;71:487–97. [DOI] [PubMed] [Google Scholar]
- 30. Yandim C, Natisvili T, Festenstein R. Gene regulation and epigenetics in Friedreich’s ataxia. J Neurochem 2013;126:21–42. [DOI] [PubMed] [Google Scholar]
- 31. Branda SS, Yang ZY, Chew A, et al. Mitochondrial intermediate peptidase and the yeast frataxin homolog together maintain mitochondrial iron homeostasis in Saccharomyces cerevisiae. Hum Mol Genet 1999;8:1099–110. [DOI] [PubMed] [Google Scholar]
- 32. Rötig A, de Lonlay P, Chretien D, et al. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet 1997;17:215–7. [DOI] [PubMed] [Google Scholar]
- 33. Huynen MA, Snel B, Bork P, et al. The phylogenetic distribution of frataxin indicates a role in iron-sulfur cluster protein assembly. Hum Mol Genet 2001;10:2463–8. [DOI] [PubMed] [Google Scholar]
- 34. Schmucker S, Martelli A, Colin F, et al. Mammalian frataxin: an essential function for cellular viability through an interaction with a preformed ISCU/NFS1/ISD11 iron-sulfur assembly complex. PLoS One 2011. 26;6:16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Condò I, Malisan F, Guccini I, et al. Molecular control of the cytosolic aconitase/IRP1 switch by extramitochondrial frataxin. Hum Mol Genet 2010;19:1221–9. [DOI] [PubMed] [Google Scholar]
- 36. Bridwell-Rabb J, Fox NG, Tsai CL, et al. Human frataxin activates Fe-S cluster biosynthesis by facilitating sulfur transfer chemistry. Biochemistry 2014;53:4904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pastore A, Puccio H. Frataxin: a protein in search for a function. J Neurochem 2013;126:43–52. [DOI] [PubMed] [Google Scholar]
- 38. Puccio H, Simon D, Cossée M, et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet 2001;27:181–6. [DOI] [PubMed] [Google Scholar]
- 39. Koeppen AH, Ramirez RL, Yu D, et al. Friedreich’s ataxia causes redistribution of iron, copper, and zinc in the dentate nucleus. Cerebellum 2012;11:845–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koeppen AH, Ramirez RL, Becker AB, et al. Dorsal root ganglia in Friedreich ataxia: satellite cell proliferation and inflammation. Acta Neuropathol Commun 2016;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pandolfo M, Hausmann L. Deferiprone for the treatment of Friedreich’s ataxia. J Neurochem 2013;126:142–6. [DOI] [PubMed] [Google Scholar]
- 42. Abeti R, Parkinson MH, Hargreaves IP, et al. Mitochondrial energy imbalance and lipid peroxidation cause cell death in Friedreich’s ataxia. Cell Death Dis 2016;7:e2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abeti R, Uzun E, Renganathan I, et al. Targeting lipid peroxidation and mitochondrial imbalance in Friedreich’s ataxia. Pharmacol Res 2015;99:344–50. [DOI] [PubMed] [Google Scholar]
- 44. González-Cabo P, Palau F. Mitochondrial pathophysiology in Friedreich’s ataxia. J Neurochem 2013;126:53–64. [DOI] [PubMed] [Google Scholar]
- 45. Corben LA, Lynch D, Pandolfo M, et al. Clinical Management Guidelines Writing Group Consensus clinical management guidelines for Friedreich ataxia. Orphanet J Rare Dis 2014;9:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bonney H, de Silva R, Giunti P, et al. ; Guideline Development Group Management of the ataxias – towards best clinical practice third edition. July 2016. Available at https://www.ataxia.org.uk/research-appendix-references.
- 47. Ilg W, Synofzik M, Brötz D, et al. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology 2009;73:1823–30. [DOI] [PubMed] [Google Scholar]
- 48. Delatycki MB, Holian A, Corben L, et al. Surgery for equinovarus deformity in Friedreich’s ataxia improves mobility and independence. Clin Orthop Relat Res 2005;430:138–41. [DOI] [PubMed] [Google Scholar]
- 49. Olver J, Esquenazi A, Fung VS, et al. Botulinum toxin assessment, intervention and aftercare for lower limb disorders of movement and muscle tone in adults: international consensus statement. Eur J Neurol 2010;17:57–73. [DOI] [PubMed] [Google Scholar]
- 50. Milne SC, Campagna EJ, Corben LA, et al. Retrospective study of the effects of inpatient rehabilitation on improving and maintaining functional independence in people with Friedreich ataxia. Arch Phys Med Rehabil 2012;93:1860–3. [DOI] [PubMed] [Google Scholar]
- 51. Milbrandt TA, Kunes JR, Karol LA. Friedreich’s ataxia and scoliosis: the experience at two institutions. J Pediatr Orthop 2008;28:234–8. [DOI] [PubMed] [Google Scholar]
- 52. Piazzolla A, Solarino G, De Giorgi S, et al. Cotrel-Dubousset instrumentation in neuromuscular scoliosis. Eur Spine J 2011;20:S75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsirikos AI, Smith G. Scoliosis in patients with Friedreich’s ataxia. J Bone Joint Surg Br 2012;94:684–9. [DOI] [PubMed] [Google Scholar]
- 54. Folker J, Murdoch B, Cahill L, et al. Dysarthria in Friedreich’s ataxia: a perceptual analysis. Folia Phoniatr Logop 2010;62:97–103. [DOI] [PubMed] [Google Scholar]
- 55. Yorkston KM, Beukelman DR. Ataxic dysarthria: treatment sequences based on intelligibility and prosodic considerations. J Speech Hear Disord 1981;46:398–404. [DOI] [PubMed] [Google Scholar]
- 56. Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996;11:93–8. [DOI] [PubMed] [Google Scholar]
- 57. Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurol 2006;5:31–7. [DOI] [PubMed] [Google Scholar]
- 58. Thurtell MJ, Leigh RJ. Treatment of nystagmus. Curr Treat Options Neurol 2012;14:60–72. [DOI] [PubMed] [Google Scholar]
- 59. Rance G, Corben LA, Du Bourg E, et al. Successful treatment of auditory perceptual disorder in individuals with Friedreich ataxia. Neuroscience 2010;171:552–5. [DOI] [PubMed] [Google Scholar]
- 60. Lad M, Parkinson MH, Rai M, et al. Urinary, bowel and sexual symptoms in a cohort of patients with Friedreich's ataxia. Orphanet J Rare Dis 2017;12:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Corben LA, Ho M, Copland J, et al. Increased prevalence of sleep-disordered breathing in Friedreich ataxia. Neurology 2013;81:46–51. [DOI] [PubMed] [Google Scholar]
- 62. Fuster V, Rydén LE, Cannom DS, et al. ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 2011;57:e101–98. [DOI] [PubMed] [Google Scholar]
- 63. Lagedrost SJ, Sutton MS, Cohen MS, et al. Idebenone in Friedreich ataxia cardiomyopathy – results from a 6-month phase III study (IONIA). Am Heart J 2011;161:639–45. [DOI] [PubMed] [Google Scholar]
- 64. Pineda M, Arpa J, Montero R, et al. Idebenone treatment in paediatric and adult patients with Friedreich ataxia: long-term follow-up. Eur J Paediatr Neurol 2008;12:470–5. [DOI] [PubMed] [Google Scholar]
- 65. Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology Foundation American Heart Association Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53:e1–e90. [DOI] [PubMed] [Google Scholar]
- 66. Epstein AE, Dimarco JP, Ellenbogen KA, et al. American College of Cardiology American Heart Association Task Force on Practice Guidelines American Association for Thoracic Surgery Society of Thoracic Surgeons ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm 2008;5:e1–62. [Google Scholar]
- 67. Babady NE, Carelle N, Wells RD, et al. Advancements in the pathophysiology of Friedreich’s Ataxia and new prospects for treatments. Mol Genet Metab 2007;92:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. National Institute for Health and Care Excellence (NICE) (2017) Type 2 diabetes in adults: management. NICE Guidelines [NG28]. Available at https://www.nice.org.uk/guidance/ng28
- 69. Ran S, Abeti R, Giunti P. Diabetes in Friedreich Ataxia In: Barbetti F, Ghizzoni L, Guaraldi F. Diabetes Associated with Single Gene Defects and Chromosomal Abnormalities. Frontiers in Diabetes, Vol. 25, Basel: Karger, 2017;172–81. [Google Scholar]
- 70. Bürk K, Schulz SR, Schulz JB. Monitoring progression in Friedreich ataxia (FRDA): the use of clinical scales. J Neurochem 2013;126:118–24. doi:10.1111/jnc.12318. [DOI] [PubMed] [Google Scholar]
- 71. Pandolfo M, Arpa J, Delatycki MB, et al. Deferiprone in Friedreich ataxia: a 6-month randomized controlled trial. Ann Neurol 2014;76:509–21. [DOI] [PubMed] [Google Scholar]
- 72. Parkinson MH, Schulz JB, Giunti P. Co-enzyme Q10 and idebenone use in Friedreich’s ataxia. J Neurochem 2013;126:125–41. [DOI] [PubMed] [Google Scholar]
- 73. Nachbauer W, Hering S, Seifert M, et al. Effects of erythropoietin on frataxin levels and mitochondrial function in Friedreich ataxia—a dose-response trial. Cerebellum 2011;10:763–9. [DOI] [PubMed] [Google Scholar]
- 74. Mariotti C, Nachbauer W, Panzeri M, et al. Erythropoietin in Friedreich ataxia. J Neurochem 2013;126:80–7. [DOI] [PubMed] [Google Scholar]
- 75. Libri V, Yandim C, Athanasopoulos S, et al. Epigenetic and neurological effects and safety of high-dose nicotinamide in patients with Friedreich’s ataxia: an exploratory, open-label, dose-escalation study. Lancet 2014;384:504–13. [DOI] [PubMed] [Google Scholar]