Summary
We found a significant association between the entire mTOR pathway and breast cancer risk, particularly with overall and ER− breast cancer risk in women of African ancestry, driven primarily by two different genes, PRKAG3 and RPS6KA3, respectively.
Abstract
Functional studies have elucidated the role of the mammalian target of rapamycin (mTOR) pathway in breast carcinogenesis, but to date, there is a paucity of data on its contribution to breast cancer risk in women of African ancestry. We examined 47628 SNPs in 61 mTOR pathway genes in the genome wide association study of breast cancer in the African Diaspora study (The Root consortium), which included 3686 participants (1657 cases). Pathway- and gene-level analyses were conducted using the adaptive rank truncated product (ARTP) test for 10994 SNPs that were not highly correlated (r2 < 0.8). Odds ratio (OR) and 95% confidence interval (CI) were estimated with logistic regression for each single-nucleotide polymorphism. The mTOR pathway was significantly associated with overall and estrogen receptor-negative (ER−) breast cancer risk (P = 0.003 and 0.03, respectively). PRKAG3 (Padj = 0.0018) and RPS6KA3 (Padj = 0.061) were the leading genes for the associations with overall breast cancer risk and ER− breast cancer risk, respectively. rs190843378 in PRKAG3 was statistically significant after gene-level adjustment for multiple comparisons (OR = 0.50 for each T allele, 95% CI = 0.38–0.66, Padj = 3.6E−05), with a statistical power of 0.914. These results provide new insights on the biological relevance of the mTOR pathway in breast cancer progression and underscore the need for more genetic epidemiology studies of breast cancer in the African Diaspora.
Introduction
The mammalian target of rapamycin (mTOR) pathway is well-known for its association with breast cancer recurrence and survival, and has been a focus for the treatment of metastatic breast cancer (1). Its potential functions include response to cellular growth conditions and energy signalling, especially regulating tumor cell growth and proliferation (2). In the context of cancer prevention (3), a few studies have examined the relationship between genetic variants in this pathway and the occurrence of several types of cancers, including breast cancer (4–13). However, the results remain inconclusive. Taking one of the most highly researched genes in the pathway, hypoxia-inducible factor-1α (HIF-1α) as an example, this is a subunit of the key transcription factor (HIF-1) that regulates cellular adaptation to hypoxia (14), the results have been inconsistent across different data sets. In a meta-analysis, one important single-nucleotide polymorphism (SNP) rs11549465 of HIF-1α was associated with significantly increased breast cancer risk in Asians (11,12), while another SNP, rs11549467 within the same gene was more likely to contribute to increased breast cancer susceptibility in Caucasians (10,13). No significant signals were identified in women of African ancestry in the African American Breast Cancer Epidemiology and Risk (AMBER) consortium (4).
Other than exploring the genetic variant at SNP level, it is now widely accepted to explore the association between breast cancer risk and the entire mTOR pathway, considering that the effects of each gene may be small and dependent on each other because of gene–gene interactions (15,16). Most of the previous studies have focused on SNPs in limited number of genes in the mTOR pathway (e.g. HIF-1α, TSC2, mTOR), while some genes, such as EIF4E, a key element of this pathway, have not been studied extensively (14). To date, only AMBER consortium has examined association between the entire mTOR pathway and breast cancer, using the approach of integrating genes within this pathway (4). They did not report significant result for pathway-level or gene-level associations after Bonferroni corrections for multiple tests.
Obesity is a confounding risk factor in African American women (prevalence: about 50%) (17) and several studies have proposed that mTOR pathway dysregulation could be a potential mechanism linking obesity to breast cancer, especially to the association with triple negative breast cancer (18). Given the higher prevalence of triple negative breast cancer in indigenous women of African ancestry with less confounding risk of obesity (prevalence less than 20%) (19), the current study aimed to evaluate gene variants in the mTOR pathway genes as risk factors for breast cancer in The Root Consortium, a cohort enriched for indigenous African women from Nigeria.
Method
Study participants
The study populations of The Root Consortium have been described previously (20,21). Briefly, this study included 3686 participants of African ancestry. Nearly half of the cases and controls were ascertained outside the United States in Nigeria (711 cases and 624 controls), Barbados (92 cases and 229 controls) and four sites in Chicago, Philadelphia, Maryland and Nashville in the USA (854 cases and 1176 controls). Patients were histologically and/or clinically diagnosed as having invasive breast cancer by the clinicians at each study site (20,21). Each participant provided informed consent for genomic analysis of breast cancer at each site.
SNP genotyping and imputation
Information about the genome-wide association study (GWAS) genotyping, quality control, imputation and principal component analysis (PCA) has been previously reported (20). Briefly, genotyping was conducted using the Illumina HumanOmni2.5-8v1 array, including approximately 2.4 million genetic variants. Genotype imputation was conducted with the IMPUTE2 software (22). With the 1000 Genomes Project Phase 1 (1092 individuals from 14 diverse populations) integrated variant set as the reference panel, 23098723 SNPs were imputed and included in the GWAS analyses. To account for population structure, the first 10 principal components were computed with the smartpca program in the EIGENSOFT package (23).
Selection of candidate genes and SNPs in the mTOR pathway
A list of mTOR pathway genes was manually curated in two ways: (a) querying the Molecular Signature Database (MSigDB) (24) and (b) literature review. For each candidate gene, its start and end chromosomal positions plus 10 kb upstream and 10 kb downstream were determined using the UCSC Genome Browser (https://genome.ucsc.edu). SNPs within each gene region were extracted from The Root Consortium GWAS data and defined as ‘total SNPs’. A total of 61 genes with 47628 variants were extracted (Table 1).
Table 1.
P value of pathway- and gene-level tests with risk of overall, ER+ and ER− breast cancer
| Gene | Chromosome | Number of total SNPsa | Number of effective SNPsa | P value | ||
|---|---|---|---|---|---|---|
| Overall | ER+ | ER− | ||||
| Pathway | — | 37658 | 10688 | 0.006 | 0.16 | 0.046 |
| Pathway excluding top gene | — | 37498 | 10623 | 0.54 | — | 0.85 |
| AKT1 | 14 | 348 | 146 | 0.90 | 0.70 | 0.16 |
| AKT2 | 19 | 392 | 90 | 0.61 | 0.06 | 0.14 |
| AKT3 | 1 | 1794 | 289 | 0.06 | 0.42 | 0.36 |
| BRAF | 7 | 1101 | 153 | 0.67 | 0.65 | 0.63 |
| CAB39 | 2 | 877 | 173 | 0.24 | 0.35 | 0.51 |
| CAB39L | 13 | 1111 | 348 | 0.75 | 0.004 | 0.96 |
| DDIT4 | 10 | 114 | 39 | 0.76 | 0.43 | 0.98 |
| EEF2K | 16 | 486 | 125 | 0.87 | 0.60 | 0.31 |
| EIF4B | 12 | 332 | 76 | 0.48 | 0.43 | 0.39 |
| EIF4E | 4 | 517 | 86 | 0.82 | 0.02 | 0.27 |
| EIF4E1B | 5 | 247 | 129 | 0.55 | 0.052 | 0.35 |
| EIF4E2 | 2 | 421 | 109 | 0.63 | 0.12 | 0.72 |
| EIF4EBP1 | 8 | 286 | 82 | 0.38 | 0.95 | 0.55 |
| EIF4G1 | 3 | 190 | 72 | 0.39 | 0.49 | 0.29 |
| FIGF | X | 276 | 91 | 0.20 | 0.87 | 0.19 |
| HIF1A | 14 | 406 | 111 | 0.17 | 0.032 | 0.95 |
| IGF1 | 12 | 477 | 125 | 0.10 | 0.38 | 0.41 |
| INS | 11 | 100 | 59 | 0.76 | 0.36 | 0.77 |
| MAPK1 | 22 | 892 | 154 | 0.63 | 0.29 | 0.53 |
| MAPK3 | 16 | 111 | 47 | 0.82 | 0.60 | 0.79 |
| MLST8 | 16 | 143 | 71 | 0.48 | 0.83 | 0.49 |
| MTOR | 1 | 1163 | 192 | 0.032 | 0.08 | 0.62 |
| PDPK1 | 16 | 235 | 72 | 0.37 | 0.55 | 0.48 |
| PGF | 14 | 204 | 98 | 0.71 | 0.63 | 0.55 |
| PIK3CA | 3 | 562 | 100 | 0.84 | 0.86 | 0.74 |
| PIK3CB | 3 | 660 | 105 | 0.09 | 0.32 | 0.27 |
| PIK3CD | 1 | 585 | 215 | 0.39 | 0.14 | 0.31 |
| PIK3CG | 7 | 347 | 99 | 0.88 | 0.58 | 0.27 |
| PIK3R1 | 5 | 640 | 221 | 0.08 | 0.21 | 0.64 |
| PIK3R2 | 19 | 218 | 68 | 0.13 | 0.33 | 0.61 |
| PIK3R3 | 1 | 797 | 144 | 0.72 | 0.21 | 0.15 |
| PIK3R5 | 17 | 830 | 339 | 0.13 | 0.10 | 0.69 |
| PPM1A | 14 | 460 | 94 | 0.24 | 0.73 | 0.73 |
| PRKAA1 | 5 | 346 | 75 | 0.43 | 0.37 | 0.13 |
| PRKAA2 | 1 | 589 | 137 | 0.97 | 0.43 | 0.18 |
| PRKAB1 | 12 | 191 | 63 | 0.77 | 0.95 | 0.23 |
| PRKAB2 | 1 | 268 | 64 | 0.06 | 0.78 | 0.68 |
| PRKAG1 | 12 | 109 | 41 | 0.16 | 0.32 | 0.39 |
| PRKAG2 | 7 | 2953 | 1239 | 0.53 | 0.48 | 0.98 |
| PRKAG3 | 2 | 160 | 65 | 0.00003b | 0.09 | 0.17 |
| RHEB | 7 | 530 | 158 | 0.35 | 0.75 | 0.87 |
| RICTOR | 5 | 990 | 169 | 0.35 | 0.11 | 0.31 |
| RPS6 | 9 | 225 | 57 | 0.86 | 0.45 | 0.75 |
| RPS6KA1 | 1 | 408 | 132 | 0.47 | 0.10 | 0.18 |
| RPS6KA2 | 6 | 4147 | 1622 | 0.09 | 0.69 | 0.11 |
| RPS6KA3 | X | 417 | 101 | 0.045 | 0.16 | 0.001 |
| RPS6KA6 | X | 541 | 95 | 0.14 | 0.43 | 0.04 |
| RPS6KB1 | 17 | 340 | 72 | 0.77 | 0.85 | 0.67 |
| RPS6KB2 | 11 | 117 | 48 | 0.96 | 0.99 | 0.55 |
| RPTOR | 17 | 4113 | 1110 | 0.75 | 0.94 | 0.82 |
| STK11 | 19 | 205 | 94 | 0.37 | 0.64 | 0.10 |
| STRADA | 17 | 386 | 78 | 0.86 | 0.69 | 0.78 |
| STRADB | 2 | 186 | 38 | 0.09 | 0.006 | 0.65 |
| TSC1 | 9 | 492 | 107 | 0.14 | 0.28 | 0.57 |
| TSC2 | 16 | 301 | 146 | 0.915 | 0.32 | 0.49 |
| ULK1 | 12 | 283 | 104 | 0.28 | 0.34 | 0.51 |
| ULK2 | 17 | 656 | 152 | 0.97 | 0.80 | 0.13 |
| ULK3 | 15 | 118 | 49 | 0.70 | 0.92 | 0.67 |
| VEGFA | 6 | 229 | 102 | 0.69 | 0.96 | 0.12 |
| VEGFB | 11 | 121 | 42 | 0.74 | 0.96 | 0.14 |
| VEGFC | 4 | 915 | 206 | 0.71 | 0.86 | 0.76 |
aFor each candidate gene, its start and end chromosomal positions plus 10 kb upstream and 10 kb downstream were determined using the UCSC Genome Browser. SNPs within each gene region were extracted from The Root Consortium GWAS data and defined as ‘total SNPs’. ‘Effective SNPs’ means the remaining SNPs after excluding the SNPs with MAF < 0.01 and the SNPs with a lower MAF in every pair of SNPs with correlation r2 ≥ 0.8.
bOnly P value for PRKAG3 remained significant after Bonferroni correction.
Statistical analysis
We compared all cases with controls using three approaches to examine the associations between SNPs and breast cancer risk: pathway-, gene- and SNP-based analyses. As an exploratory analysis, we also examined whether SNPs are associated with ER-negative breast cancer or ER-positive breast cancer.
The pathway- and gene-based analyses were performed using the adaptive rank truncated product (ARTP) test with the R package ARTP2. The ARTP method, as one of the most popular self-contained methods, shows power advantage over other similar methods, such as aSPUpath (25,26). The underlying rationale is that it optimizes the selection of truncation points and correction for multiple testing by permutation analyses (15). One SNP with a lower minor allele frequency (MAF) of every pair of SNPs with correlation r2 ≥ 0.8 was excluded from the gene-based tests using the filter in the R package. Thus, allowing us to avoid capturing only a few association signals for some genes due to correlations between their top SNPs. Any SNPs with MAF less than 0.01were also removed from the analysis, and a set of 10994 SNPs were selected from the 61 genes in the end. Statistical significance at the gene-based analysis was declared at the 0.00082 (=0.05/61 genes) level.
The single-SNP association tests, required as input for the ARTP analyses, were performed for SNPs in genes with a nominal P < 0.05 in the ARTP tests. We used logistic regression with case status as the outcome, and an additive model for genotype, adjusting for age (10-year groups), study site and the first four eigenvectors from principal component analysis, using SNPtest (27). The first four eigenvectors were used to control for population stratification, as only the first four eigenvectors were associated with case status (21). We corrected for multiple testing with a Bonferroni correction for the effective number of independent SNPs tested within a gene using Gao’s SimpleM approach (28), and called this the ‘gene-wide’ significance. ‘Effective SNPs’ (Table 1) means the remaining SNPs after excluding SNPs with MAF < 0.01 and SNPs with lower MAF in every pair of SNPs with correlation r2 ≥ 0.8. The adjusted P values after Bonferroni correction were named as Padj for simplicity. We also conducted power calculations for gene-wide significant SNPs, with α as 0.05 divided by the number of effective SNPs within each gene. Furthermore, to be conservative and to better minimize any potential false positives (29), we excluded SNPs with imputation information scores <0.7.
Association signal visualization and SNP functional evaluation
Single marker associations for top genes were plotted with linkage disequilibrium (LD) data using the LocusZoom (30), we checked other 1000 Genome Project Phase 3 SNPs that can be tagged/captured by the most significant SNPs in their corresponding regions with LDlink3.0 (https://analysistools.nci.nih.gov/LDlink/). Information about the functionality of the gene-wide significant SNPs and their tag SNPs were explored in HaploReg v4, in which epigenomic data from the ENCODE (Encyclopedia of DNA Elements), the GTEx pilot and RegulomeDB databases were integrated (31). Searches for eQTLs information in breast tissues were also carried out in the GTEx portal and eQTL Browser (http://eqtl.uchicago.edu/cgi-bin/gbrowse/eqtl/).
Results
The study included 1657 breast cancer cases and 2029 controls from Barbados, Nigeria and the USA. The mean ages of cases and controls were 49.3 and 48.4 years, respectively. About 48.1% of cases with ER status were ER negative. The means of body mass index were 28.4 ± 7.0 years, 29.7 ± 7.0 years for case and control, respectively.
The mTOR pathway was significantly associated with both overall and ER− breast cancer (P = 0.006 and 0.046, respectively) (Table 1). The pathway level association for overall breast cancer risk was attributable to the PRKAG3 gene; after Bonferroni correction for the number of genes tested (n = 61), PRKAG3 remained significant (Padj = 0.0018). The pathway level association for ER− breast cancer risk was attributable to RPS6KA3, with its adjusted P value close to the boundary of significance (Padj = 0.061). Excluding PRKAG3 from the analysis of overall breast cancer risk, and RPS6KA3 from the analysis of ER− breast cancer risk, both pathway-level associations become non-significant (P = 0.54 and 0.85, respectively).
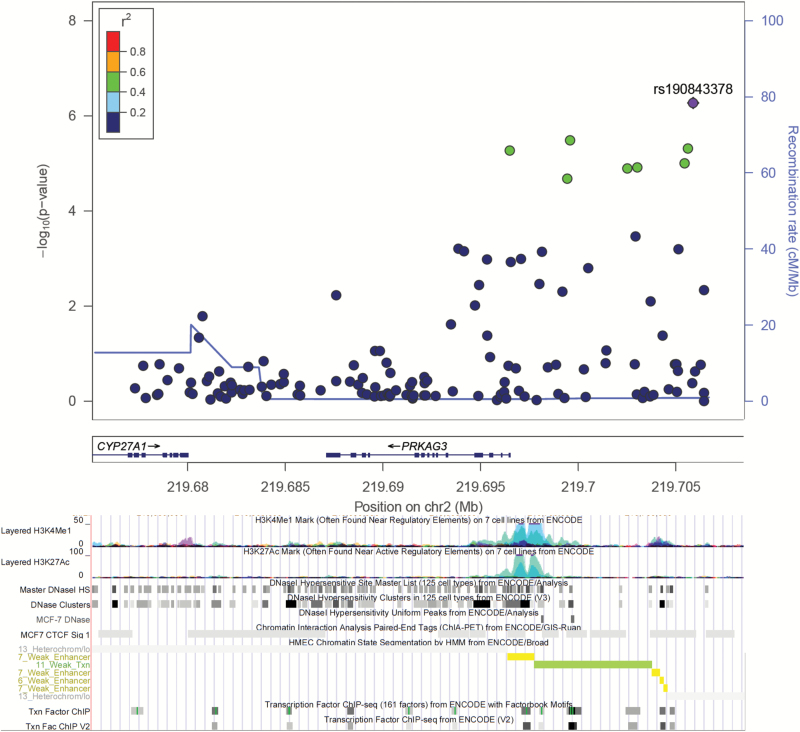
Table 2 shows gene-wide significant variants from the SNP-level analysis that were associated with breast cancer overall, or only with ER+ and ER− disease. The best signal locus was an upstream variant, rs190843378, in the gene PRKAG3 (Figure 1). The T allele of rs190843378 was significantly associated with a 50% decrease in overall breast cancer risk (odds ratio (OR) = 0.50, 95% confidence interval (CI) = 0.38–0.66, Padj = 3.6E−05), with a statistical power of 0.914 (Table 2). In a subgroup analysis, similar odds ratios were observed for the analysis of ER+ and ER− diseases (0.56 and 0.59, respectively).
Table 2.
Gene-wide significant tested SNPs for overall, ER+ and ER− breast cancer
| Gene | SNP | Function | Major/minor alleles | MAFa | Information score | All cases versus controls | ER+ cases versus controls | ER− cases versus controls | Powerd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI)b | P | P adj c | OR (95% CI)b | P | P adj c | OR (95% CI)b | P | P adj c | |||||||
| Overall | |||||||||||||||
| mTOR | rs185024685 | Intron | T/A | 0.011 | 0.730 | 0.42 (0.25–0.69) | 0.00070 | 0.14 | 0.55 (0.26–1.19) | 0.02651 | 1.00 | 0.41 (0.19–0.90) | 0.12790 | 1.00 | — |
| PRKAG3 | rs190843378 | Upstream variant | C/T | 0.036 | 0.842 | 0.50 (0.38–0.66) | 5.3E−07 | 3.60E−05 | 0.56 (0.36–0.88) | 0.01070 | 0.72 | 0.59 (0.39–0.91) | 0.01736 | 1.00 | 0.914 |
| RPS6KA3 | rs111286678 | Near-gene-5 | T/G | 0.076 | 0.978 | 1.37 (1.15–1.64) | 0.00052 | 0.055 | 1.38 (1.00–1.91) | 0.04696 | 1.00 | 1.92 (1.39–2.64) | 0.00006 | 0.006 | — |
| ER+ | |||||||||||||||
| CAB39L | rs75224640 | Near-gene-3 | A/T | 0.027 | 0.967 | 1.42 (1.08–1.86) | 0.01167 | 1.00 | 3.29 (2.03–5.34) | 1.4E−06 | 0.0005 | 0.88 (0.52–1.48) | 0.62676 | 1.00 | 0.021 |
| EIF4E | rs141689493 | Intron | G/A | 0.028 | 0.704 | 1.21 (0.87–1.68) | 0.25851 | 1.00 | 2.98 (1.64–5.42) | 0.00033 | 0.03 | 1.14 (0.63–2.09) | 0.66051 | 1.00 | 0.006 |
| HIF1A | rs73317134 | Intron | G/A | 0.010 | 0.780 | 1.75 (1.05–2.93) | 0.03337 | 1.00 | 8.10 (2.78–23.62) | 0.00013 | 0.02 | 1.19 (0.45–3.17) | 0.73070 | 1.00 | 0.029 |
| HIF1A | rs8019084 | near-gene-3, ncRNA |
C/T | 0.023 | 0.917 | 1.29 (0.96–1.75) | 0.09284 | 1.00 | 2.54 (1.50–4.30) | 0.00053 | 0.06 | 1.39 (0.81–2.37) | 0.23260 | 1.00 | — |
| Untranslated-3 | |||||||||||||||
| STRADB | rs16837635 | Intron | A/G | 0.117 | 1.000 | 1.21 (1.05–1.39) | 0.01012 | 0.44 | 1.61 (1.26–2.06) | 0.00017 | 0.01 | 1.22 (0.95–1.58) | 0.11512 | 1.00 | 0.624 |
| ER− | |||||||||||||||
| RPS6KA3 | rs114874970 | Intron | G/C | 0.102 | 0.985 | 1.22 (1.05–1.42) | 0.01146 | 1.00 | 1.24 (0.95–1.63) | 0.11720 | 1.00 | 1.81 (1.39–2.36) | 0.00001 | 0.0011 | 0.283 |
| RPS6KA6 | rs189075473 | Upstream variant | A/C | 0.032 | 0.854 | 1.23 (0.93–1.62) | 0.14847 | 1.00 | 0.83 (0.50–1.38) | 0.47015 | 1.00 | 2.02 (1.27–3.22) | 0.00292 | 0.28 | — |
aMinor allele frequency among controls.
bAdditive model with each SNP coded as 0, 1 or 2 copies of the minor allele, adjusting for age (10-year groups), study site and the first four eigenvectors from principal component analysis.
c P adj mean P values after Bonferroni correction, which were calculated by multiplying P values with the effective number of independent SNPs tested within a corresponding gene. ‘Effective SNPs’ means the remaining SNPs after excluding the SNPs with MAF < 0.01 and the SNPs with a lower MAF in every pair of SNPs with correlation r2 ≥ 0.8. Bold Padj are significant at the 0.05 level.
dWe did power calculations for gene-wide significant variants only. The gene-wide significant level (α) was calculated through dividing 0.05 by the number of effective SNPs within each gene.
Figure 1.
Plot of log-transformed P values from single marker analysis for the PRKAG3 gene in overall test (generated using the LocusZoom program). The labeled marker with rs# was the most significant SNP (index SNP), and the LD between other markers in the gene and the index SNP was color coded, with red color indicating strong LD (r2 > 0.8) and blue color indicating weak LD (r2 < 0.2). For the bottom part, analysis of regulation enhancer with data from ENCODE through UCSC Genome Browser, including histone modification marks for H3K4Me1 and H3K27Ac of seven cell types, transcription factor binding sites and DNase hypersensitivity sites of human mammary epithelial cells (HMEC), breast cancer cells (MCF7). Chromosomal coordinates are in NCBI build 37.
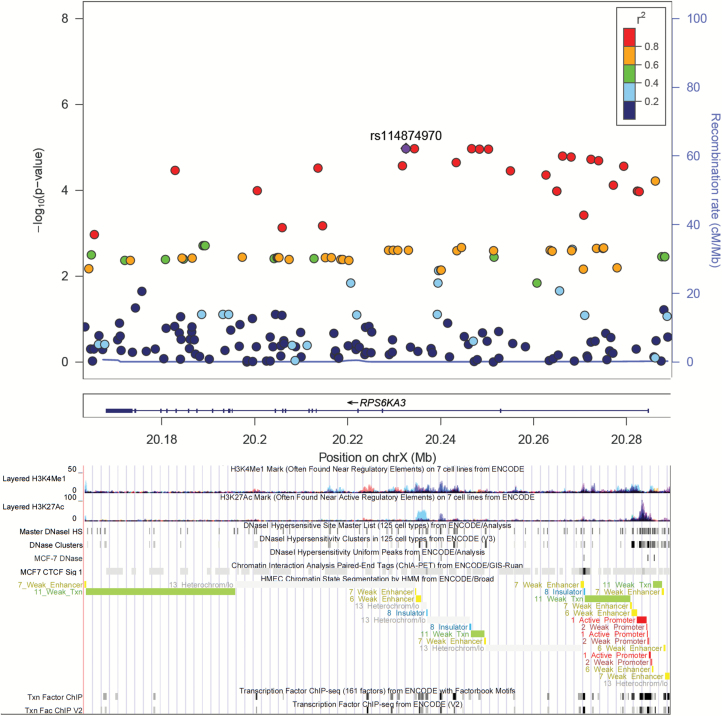
In addition, variants associated with ER+ breast cancer included rs141689493 in EIF4E (intron, OR = 2.98 for each copy of the A allele, 95% CI = 1.64–5.42, Padj = 0.03, Supplementary Figure 1A, available at Carcinogenesis Online), rs73317134 in HIF1A (intron, OR = 8.10 for each copy of the A allele, 95% CI = 2.78–23.62, Padj = 0.02, Supplementary Figure 1B, available at Carcinogenesis Online), rs75224640 in CAB39L (500b downstream variant, OR = 3.29 for each copy of the T allele, 95% CI = 2.03–5.34, Padj = 0.0005, Supplementary Figure 1C, available at Carcinogenesis Online), and rs16837635 in STRADB (intron, OR = 1.61 for each copy of the G allele, 95% CI = 1.26–2.06, Padj = 0.01, Supplementary Figure1D, available at Carcinogenesis Online). Moreover, RPS6KA3 rs114874970 (intron, OR = 1.81 for each copy of the C allele, 95% CI = 1.39–2.36, Padj = 0.0011, Figure 2) was associated with an increased risk of ER− breast cancer.
Figure 2.
Plot of log-transformed P values from single marker analysis for RPS6KA3 in ER− subgroup test (generated using the LocusZoom program). The labeled marker with rs# was the most significant SNP (index SNP), and the LD between other markers in the gene and the index SNP was color coded, with red color indicating strong LD (r2 > 0.8) and blue color indicating weak LD (r2 < 0.2). For the bottom part, analysis of regulation enhancer with data from ENCODE through UCSC Genome Browser, including histone modification marks for H3K4Me1 and H3K27Ac of seven cell types, transcription factor binding sites and DNase hypersensitivity sites of human mammary epithelial cells (HMEC), breast cancer cells (MCF7). Chromosomal coordinates are in NCBI build 37.
The top-ranked SNPs in otherwise non-significant genes are listed in Supplementary Table 1, available at Carcinogenesis Online. Among the SNPs with MAF ≥ 0.01, rs11709841 in PIK3CB and rs116104616 in PRKAB2 were significantly associated with overall breast cancer risk after Bonferroni correction (Padj < 0.05), but only rs34717888 in AKT2 was associated with ER+ breast cancer risk. We did not find any additional eQTL information for these SNPs to further corroborate their association with gene expression and breast cancer.
Discussion
In this Root consortium study, we observed that the entire mTOR pathway was significantly associated with overall and ER− breast cancer risk (P = 0.006 and 0.046 respectively). These associations were driven primarily by two different genes (PRKAG3 and RPS6KA3) for overall and ER− breast cancer, respectively. Furthermore, a SNP within PRKAG3, rs190843378, was significantly associated with overall breast cancer risk on the gene-wide level, and the power was sufficient. To our knowledge, this is the first study to demonstrate an association of the entire mTOR pathway with breast cancer risk in women of African ancestry.
PRKAG3, as the leading gene associated with overall breast cancer risk, also named as AMPKγ3, has been considered a potential target of cancer treatment due to its function in regulating glucose/lipid metabolism and skeletal muscle glycogen content (32,33). However, only two studies have examined its role in the risk of breast cancer. The AMBER study did not report this association (4), while another study suggested an association between PRKAG3 and the recurrence of triple negative breast cancer in the Chinese population (5). We found a leading role for PRKAG3 in the mTOR pathway, and an independent significant SNP, rs190843378 that had a moderately protective effect for its T allele. This SNP was tagged by SNPs that overlap a transcription regulatory (enhancer) mark in breast variant human mammary epithelial cells. Considering PRKAG3’s high specificity for coordinating transcription of genes critical for lipid and glucose metabolism in white glycolytic skeletal muscle (34), our findings suggest a link between obesity/metabolism or lipid/glucose metabolism and breast cancer as a new role for this gene.
RPS6KA3 (RSK2), the most significant gene associated with ER− breast cancer in our study, has also been proposed as a target of cancer therapy (35). It is located at Xp22.2, can phosphorylate Y-box binding protein-1 (YB-1), and works as a transcription factor activated in breast cancer (36). In contrast to results from the AMBER Consortium (4), we found that RPS6KA3 has its strongest effect of the whole pathway on ER− breast cancer through an independent significant SNP, rs114874970, with an almost double risk for its C allele. Previous gene expression studies (37), and functional genomics evidence (Supplementary Table 2 and Figure 2, available at Carcinogenesis Online) support a plausible role of this gene in cancer, however, the association result in this study warrants further confirmation due to the limited power for the SNP-level result.
Other studies have shown the associations of SNPs in mTOR, HIF-1α and EIF4E with breast cancer. However, all significant SNPs in our study were novel, and there was no known information related to the reported SNP of rs181088346 in women of African ancestry (4). To date, at least four Meta analyses reported significant association between HIF-1α and breast cancer, especially in Asian women, but not in women of African ancestry (10–13). The recent AMBER study did not report an association with breast cancer risk (4). Our study did find a gene-level significant SNP of rs73317134 in HIF-1α with a more than 700% higher risk, and rs141689493 in EIF4E, with an almost 200% higher risk for their risk alleles. Both SNPs tag other SNPs that overlap a transcription regulatory (enhancer) mark in breast cells, supporting their role in promoting cellular transformation and tumorigenesis (38,39).
A few studies have also investigated CAB39L, STRADB and RPS6KA6 and breast cancer risk, but their potential roles in breast cancer carcinogenesis remain unclear (40–42). In contrast to the AMBER study, as the only study involving at least one of these genes (4), we did find significant associations for these genes with the occurrence of breast cancer. In consideration of the related expression information that covers transcription regulatory (enhancer) and repressed polycomb in breast tissues, it is biologically plausible that these sequence variants affect their transcription, leading to increased breast cancer susceptibility. Further mechanistic studies are needed to confirm the involvement of these genes in breast carcinogenesis.
In order to test whether the associations reported in this study can be replicated in European populations, the GAME-ON GWAS look up tool (http://gameon.dfci.harvard.edu) was searched for our top six significant SNPs in Table 2. All of these SNPs are monomorphic in European populations. CAB39L and TRADB were reported to have different gene-level significant SNPs in Europeans. A similar result was reported by the AMBER study, which reflects the vastly different genetic backgrounds and LD patterns between women of African and European ancestries (43). While we could not eliminate the possibility that our findings were due to chance, The Root Consortium was specifically assembled to allow us to take advantage of the short LD blocks and the relatively young age at diagnosis of breast cancer in African populations to identify risk alleles for ER− breast cancer. Nonetheless, further replication studies are needed to confirm this result.
Strengths of our study include a more comprehensive evaluation of the mTOR pathway and our inclusion of a unique population of women of African ancestry from Nigeria and Barbados than previous studies. However, several limitations should also be noted. First, despite having almost 4000 participants, the present study had limited power to detect individual SNP associations of small magnitude as well as stronger associations for rare SNPs. Nevertheless, it is one of the largest studies to date on the genetics of breast cancer in women of African ancestry. Second, although we did not have sufficient statistical power, we did an exploratory analysis by breast cancer ER subtypes. Thus, our findings require replication, as many of the gene-level associations were not significant after correction for genome-wide multiple tests. Meanwhile, the lack of functionality of the identified SNPs is a typical limitation of SNP association studies. The identified associations will certainly shed light on future experimental studies to determine their function.
In conclusion, in one of the largest breast cancer studies of women of African ancestry, we found evidence of associations between the mTOR pathway and breast cancer risk, particularly with overall and ER− breast cancer. These associations were driven primarily by two genes (PRKAG3 and RPS6KA3), which may provide new insights into the role of this pathway in breast cancer progression in women of African ancestry.
Supplementary material
Supplementary material is available at Carcinogenesis online.
Funding
This work was in part supported by the American Cancer Society MRSG-13-063-01-TBG (D.H.) and CRP-10-119-01-CCE (O.I.O.), National Cancer Institute CA142996 (O.I.O.) and CA161032 (O.I.O.), Susan G. Komen for the Cure (O.I.O.). The funding agencies have no involvement in the study design, data collection, analysis, interpretation of data, writing of the manuscript or decision to submit the manuscript for publication. S.W. was supported by a University of Chicago Global Health Fellowship.
Conflict of Interest Statement: None declared.
Supplementary Material
Acknowledgements
We thank Dr. Han Zhang from Division of Cancer Epidemiology and Genetics, National Cancer Institute, for helping us set up the R package ARTP2. We also thank Walmy Elisabeth Sveen from University of Chicago, for editorial support.
Abbreviations
- AMBER
African American Breast Cancer Epidemiology and Risk
- ARTP
adaptive rank truncated product
- CI
confidence interval
- ER
estrogen receptor
- GWAS
genome-wide association study
- HIF-1α
hypoxia-inducible factor-1α
- MAF
minor allele frequency
- mTOR
mammalian target of rapamycin
- OR
odds ratio
- SNPs
single-nucleotide polymorphisms
- YB-1
Y-box binding protein-1
References
- 1. Paplomata E., et al. (2014) The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther. Adv. Med. Oncol., 6, 154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pópulo H., et al. (2012) The mTOR signalling pathway in human cancer. Int. J. Mol. Sci., 13, 1886–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Spitz M.R., et al. (2012) Integrative cancer epidemiology–the next generation. Cancer Discov., 2, 1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng T.Y., et al. (2016) Genetic variants in the mTOR pathway and breast cancer risk in African American women. Carcinogenesis, 37, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen L.H., et al. (2011) Identification of prognostic genes for recurrent risk prediction in triple negative breast cancer patients in Taiwan. PLoS One, 6, e28222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao Y., et al. (2016) Impacts of the mTOR gene polymorphisms rs2536 and rs2295080 on breast cancer risk in the chinese population. Oncotarget, 7, 58174–58180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slattery M.L., et al. (2012) Genetic variation in genes involved in hormones, inflammation and energetic factors and breast cancer risk in an admixed population. Carcinogenesis, 33, 1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng T.Y., et al. (2016) Genetic variants in the mTOR pathway and interaction with body size and weight gain on breast cancer risk in African-American and European American women. Cancer Causes Control, 27, 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta M.S., et al. (2011) Polymorphic variants in TSC1 and TSC2 and their association with breast cancer phenotypes. Breast Cancer Res. Treat., 125, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang X., et al. (2013) HIF-1α 1772 C/T and 1790 G/A polymorphisms are significantly associated with higher cancer risk: an updated meta-analysis from 34 case-control studies. PLoS One, 8, e80396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ren H.T., et al. (2014) Associations between C1772T polymorphism in hypoxia-inducible factor-1α gene and breast cancer: a meta-analysis. Med. Sci. Monit., 20, 2578–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y., et al. (2015) The association between the rs11549465 polymorphism in the hif-1α gene and cancer risk: a meta-analysis. Int. J. Clin. Exp. Med., 8, 1561–1574. [PMC free article] [PubMed] [Google Scholar]
- 13. Yan Q., et al. (2014) Association between HIF-1α C1772T/G1790A polymorphisms and cancer susceptibility: an updated systematic review and meta-analysis based on 40 case-control studies. BMC Cancer, 14, 950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yi T., et al. (2013) Hypoxia-inducible factor-1α (HIF-1α) promotes cap-dependent translation of selective mRNAs through up-regulating initiation factor eIF4E1 in breast cancer cells under hypoxia conditions. J. Biol. Chem., 288, 18732–18742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yu K., et al. (2009) Pathway analysis by adaptive combination of P-values. Genet. Epidemiol., 33, 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H., et al. (2014) A fast multilocus test with adaptive SNP selection for large-scale genetic-association studies. Eur. J. Hum. Genet., 22, 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bandera E.V., et al. (2015) Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res. Treat., 150, 655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogden C.L., et al. (2014) Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA, 311, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huo D., et al. (2009) Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J. Clin. Oncol., 27, 4515–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian F., et al. (2016) Genetic variants in microRNA and microRNA biogenesis pathway genes and breast cancer risk among women of African ancestry. Hum Genet., 135, 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huo D., et al. (2016) Genome-wide association studies in women of African ancestry identified 3q26.21 as a novel susceptibility locus for oestrogen receptor negative breast cancer. Hum. Mol. Genet., 25, 4835–4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howie B.N., et al. (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet., 5, e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patterson N., et al. (2006) Population structure and eigenanalysis. PLoS Genet., 2, e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Subramanian A., et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA, 102, 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang H., et al. (2016) A powerful procedure for pathway-based meta-analysis using summary statistics identifies 43 pathways associated with type II diabetes in European populations. PLoS Genet., 12, e1006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cantor R.M., et al. (2010) Prioritizing GWAS results: a review of statistical methods and recommendations for their application. Am. J. Hum. Genet., 86, 6–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marchini J., et al. (2010) Genotype imputation for genome-wide association studies. Nat. Rev. Genet., 11, 499–511. [DOI] [PubMed] [Google Scholar]
- 28. Gao X. (2011) Multiple testing corrections for imputed SNPs. Genet. Epidemiol., 35, 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verma S.S., et al. (2014) Imputation and quality control steps for combining multiple genome-wide datasets. Front. Genet., 5, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pruim R.J., et al. (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics, 26, 2336–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boyle A.P., et al. (2012) Annotation of functional variation in personal genomes using RegulomeDB. Genome Res., 22, 1790–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barnes B.R., et al. (2004) The 5’-AMP-activated protein kinase gamma3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J. Biol. Chem., 279, 38441–38447. [DOI] [PubMed] [Google Scholar]
- 33. Rivera Rivera A., et al. (2016) Anti-breast cancer potential of quercetin via the Akt/AMPK/mammalian target of rapamycin (mTOR) signaling cascade. PLoS One, 11, e0157251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Long Y.C., et al. (2005) Role of AMP-activated protein kinase in the coordinated expression of genes controlling glucose and lipid metabolism in mouse white skeletal muscle. Diabetologia, 48, 2354–2364. [DOI] [PubMed] [Google Scholar]
- 35. Ludwik K.A., et al. (2016) Development of a RSK inhibitor as a novel therapy for triple-negative breast cancer. Mol. Cancer Ther., 15, 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stratford A.L., et al. (2008) Y-box binding protein-1 serine 102 is a downstream target of p90 ribosomal S6 kinase in basal-like breast cancer cells. Breast Cancer Res., 10, R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith J.A., et al. (2005) Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res., 65, 1027–1034. [PubMed] [Google Scholar]
- 38. Byrnes K., et al. (2006) High eIF4E, VEGF, and microvessel density in stage I to III breast cancer. Ann. Surg., 243, 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen X., et al. (2014) XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature, 508, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dümmler B.A., et al. (2005) Functional characterization of human RSK4, a new 90-kDa ribosomal S6 kinase, reveals constitutive activation in most cell types. J. Biol. Chem., 280, 13304–13314. [DOI] [PubMed] [Google Scholar]
- 41. Li Q., et al. (2014) Frequent epigenetic inactivation of RSK4 by promoter methylation in cancerous and non-cancerous tissues of breast cancer. Med. Oncol., 31, 793. [DOI] [PubMed] [Google Scholar]
- 42. Thakur A., et al. (2007) Aberrant expression of X-linked genes RbAp46, Rsk4, and Cldn2 in breast cancer. Mol. Cancer Res., 5, 171–181. [DOI] [PubMed] [Google Scholar]
- 43. Yao S., et al. (2016) Genetic variations in vitamin D-related pathways and breast cancer risk in African American women in the AMBER consortium. Int. J. Cancer, 138, 2118–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.