Abstract
Introduction or background
With a prevalence of 2–5%, amblyopia is the most common vision deficit in children in the UK and the second most common cause of functional low vision in children in low-income countries.
Sources of data
Pubmed, Cochrane library and clinical trial registries (clinicaltrials.gov, ISRCTN, UKCRN portfolio database).
Areas of agreement
Screening and treatment at the age of 4–5 years are cost efficient and clinically effective. Optical treatment (glasses) alone can improve visual acuity, with residual amblyopia treated by part-time occlusion or pharmacological blurring of the better-seeing eye. Treatment after the end of the conventional ‘critical period’ can improve vision, but in strabismic amblyopia carries a low risk of double vision.
Areas of controversy
It is not clear whether earlier vision screening would be cost efficient and associated with better outcomes. Optimization of treatment by individualized patching regimes or early start of occlusion, and novel binocular treatment approaches may enhance adherence to treatment, provide better outcomes and shorten treatment duration.
Growing points
Binocular treatments for amblyopia.
Areas timely for developing research
Impact of amblyopia on education and quality of life; optimal screening timing and tests; optimal administration of conventional treatments; development of child-friendly, effective and safe binocular treatments.
Keywords: amblyopia*/diagnosis, amblyopia*/therapy, child, humans, treatment outcome, vision screening, visual acuity
Background
What is amblyopia?
Amblyopia (‘lazy eye’) is the most common vision deficit in children, affecting 2–5% of children in the UK1,2 and the second most common cause of functional low vision in children in low-income countries.3 Unilateral amblyopia is a developmental defect of vision, and has two main causes: (i) a difference in the optical properties of the two eyes, reflected in a different spectacle prescription for the right and the left eye (anisometropia) and (ii) strabismus (misalignment of the visual axes) (Fig. 1). Some children have both anisometropic and strabismic amblyopia (‘combined’ or ‘mixed mechanism’ amblyopia). Rarely, congenital or early childhood obstruction of the visual axis, for example by lid ptosis or by opacities of the cornea, crystalline lens or vitreous, can give rise to amblyopia by deprivation, as the retina does not receive a clear image. Deprivation amblyopia can affect both eyes. A high degree of refractive errors (long- or short-sightedness (hypermetropia/myopia), astigmatism) in both eyes can also cause bilateral amblyopia.
Fig. 1.
Children with amblyopia can have straight eyes (left, anisometropic amblyopia), strabismus (strabismic amblyopia) or both. Small degrees of strabismus can go unnoticed and may only be discovered by orthoptic assessment (centre). In the UK, commissioning of vision screening at primary school entry is variable (right); commissioning in England has recently changed from Clinical Commissioning Groups to Local Authorities, resulting in boundary changes and further development of local protocols. An updated map is currently in preparation. Purple: pre-school orthoptic-led and delivered with orthoptic assessment, pre-school in community clinics; yellow: orthoptic-led and delivered with orthoptic assessment, in school at age 4–5 years; blue: orthoptic-led, other profession delivered, visual acuity assessment in school at age 4–5 years; green: other profession-led and delivered, visual acuity assessment only in school at age 4–5 years; blue: orthoptic-led visual acuity assessment only; red: no primary screening commissioned; white: unknown, no response to British and Irish Orthoptic Society questionnaire
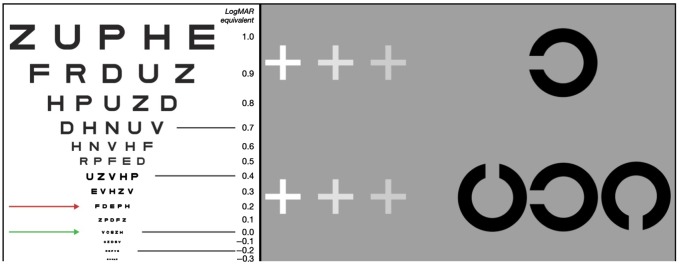
Clinically, unilateral amblyopia is conventionally defined as a difference in best-corrected visual acuity (BCVA) between the two eyes of 0.20 logMAR (2 lines on an acuity chart, Fig. 2).4 BCVA is a measure of the smallest level of detail that can be resolved in an image, typically measured on a chart with letters or pictures of reducing size (optotypes) while wearing full spectacle correction. Smaller differences between the eyes (e.g. a difference of 0.10 logMAR) can be normal, as this is within typically measured levels of test–retest variability. Similarly, bilateral amblyopia is defined as a reduction of 0.20 logMAR or more compared with the developmental norms for BCVA at a given age—e.g. for 4-year olds, a level of 0.10 logMAR is ‘normal’ whereas 6-year olds are expected to have a BCVA of 0.00 logMAR. For the purpose of treatment decisions, two levels of severity are distinguished: moderate amblyopia, with BCVA of the amblyopic eye of 0.60 logMAR or better, and severe amblyopia, with acuity worse than 0.60 logMAR.
Fig. 2.
Left: The visual acuity, measured on a logMAR chart, of an amblyopic eye is two or more lines (=0.20 logMAR, red line) less than the acuity in the better-seeing eye (green line: 0.00 logMAR, normal visual acuity expected from the age of around 6 years on a crowded logMAR test). Right: Here, the reader can simulate the effects of amblyopic vision on acuity and crowding, using their peripheral vision. By fixating on the innermost grey cross in the top row, the isolated Landolt-C element should be reasonably identifiable. The effect of acuity losses can be experienced by fixating the increasingly distant crosses (though the magnitude of losses will depend on viewing distance). Fixating the crosses in the lower row allows visualization of the effects of crowding. Here, the same Landolt-C is flanked to either side by ‘distractor’ elements. Where previously the isolated element was visible (e.g. when fixating the innermost cross), it should now be considerably more difficult to identify. Crowding can also be increased by fixating the more distant crosses and moving the central target further into peripheral vision.
In unilateral amblyopia, the imbalanced input from the two eyes to the primary visual cortex causes deficits in visual processing. Because of this imbalance in image quality between the two eyes stereovision (3D vision) can be strongly reduced or even absent altogether, particularly in strabismus.5,6 This mismatch in image quality is also associated with a frequent suppression of the central part of the visual field of the amblyopic eye7 (Fig. 2). In anisometropic amblyopia, there are additional reductions in contrast sensitivity, while strabismic amblyopia leads to a range of spatial disruptions including vulnerability to crowding in the central visual field, a difficulty identifying relevant information when it is surrounded by clutter,6 as well as perceptual distortions,8 and deficits in positional acuity, the ability to localize the relative position of an object in space. Higher-order deficits in eye-hand motor co-ordination and global-motion processing have also been reported (reviewed in Ref.9).
A neural basis for these visual deficits is slowly beginning to emerge. Changes in the sensitivity of neurons in the primary visual cortex of the brain (V1) have been most extensively examined in this regard. There are suggestions that amblyopia causes ocular dominance preferences to be re-allocated from the amblyopic eye to the better-seeing eye, resulting in an under-representation of the amblyopic eye (reviewed in Ref.5). In addition, anisometropia causes blurred vision in one eye and defocused input to the cortex, leading to suggestions that there is a selective loss of neurons sensitive to fine detail (high spatial frequencies) in the amblyopic eye. In strabismus, the misalignment of the visual axes also disrupts the input to binocular cortical neurons in V1, which only mature when receiving balanced input from both eyes. However, the observed magnitude of these physiological changes in V1 does not appear to wholly account for the magnitude of the sensory deficits described above. It is therefore likely that in addition to disturbances in V1 amblyopia also alters processing in extrastriate areas of the brain.
What is the ‘critical period’, and why does it matter?
The development of the functional architecture of the visual cortex occurs in stages.10 The maturation phase is called the ‘critical period’; imbalance or disruption during this phase can profoundly alter the selectivity of neurons to visual input. Different aspects of visual processing have slightly different critical periods, though they may overlap. It was long thought that treatment for amblyopia was only possible during these early critical periods of visual development. However, newer observations have challenged the concept of a complete loss of plasticity in the visual processing areas even in adulthood, though the quality of plasticity in adulthood may differ from that in childhood. Recent reports indicate that suppression can be reversed and vision successfully improved even after the end of the conventional critical period, though early intervention delivers better outcomes (reviewed in Refs11,12).
Does amblyopia affect other aspects of life?
The reduction in stereopsis can be associated with reduced fine and gross motor skills.13,14 Children treated for amblyopia may have lower social acceptance scores than their peers, and low self-esteem, negative self image, feelings of depression, frustration and embarrassment have been reported.15
What are the economical aspects? What is the burden of amblyopia for the individual, the family and society?
If amblyopia persists into adulthood, then affected individuals may be unable to take up professions that require depth perception. Accidents affecting the better-seeing eye can lead to a loss of quality of life and independence; the estimated lifetime risk of bilateral visual impairment may be as high as 18%, compared with 10% for people without amblyopia.16 Recent utility analysis studies and systematic review found amblyopia screening and treatment to be cost effective, but dependent on the long-term utility effects of unilateral vision loss.1,17,18
Aim of this review
This review aims to provide an update on clinical management of amblyopia in children, including an outlook on new treatment forms that may enter clinical practice over the next few years.
Sources of data
We searched Pubmed (http://www.ncbi.nlm.nih.gov/pubmed), the Cochrane Eyes and Vision Group Trials Register, the ISRCTN registry (www.isrctn.com/editAdvancedSearch), and ClinicalTrials.gov (www.clinicaltrials.gov). We did not use any date or language restrictions in the electronic searches. As this review is limited to 40 references, we narrowed our selection to work published between 2005 and 2015, then prioritized references based on the pyramid of evidence-based medicine, i.e. we first selected systematic reviews and reports of randomized clinical trials. For topic areas not covered by randomized controlled trials (RCTs) evidence, we then selected case series and other publications of high quality. For the background section, we allowed important publications outside these limits.
Areas of agreement
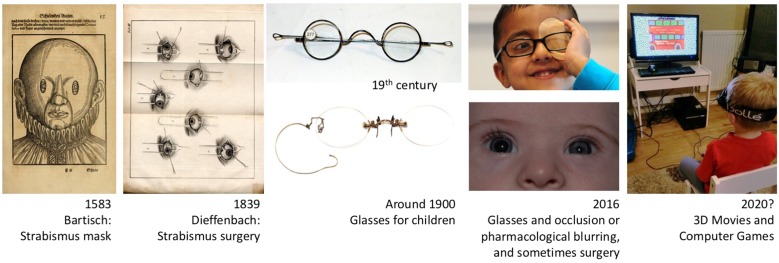
Treatment for strabismus has been attempted for hundreds of years, but surgical correction has only been practiced since 1839.19 It was soon observed that vision can be improved by straightening an amblyopic eye.19 While glasses for presbyopia were first described at the end of the 13th century, and bifocal glasses were worn since the second half of the 18th century, refractive errors could only be accurately measured when the ophthalmoscope was invented in 1850. Glasses for children were first dispensed at the end of the 19th century (Fig. 3). The first glasses for anisometropia are mentioned in 1913, though these were for adults who had undergone unilateral cataract extraction. However, glasses only came into wider use in the 1950s (personal communication from Neil Handley, Curator, British Optical Association Museum).
Fig. 3.
In 1583, Bartisch documented the first conservative treatment of strabismus; in 1839, Dieffenbach first published a surgical method. Glasses for children were introduced at the end of the 19th century (images of spectacles courtesy of the College of Optometrists, London). Current amblyopia treatment includes glasses, occlusion or pharmacological blurring of the better-seeing eye (atropine paralysis of the ciliary muscle/accommodation, with dilation of the pupil as associated effect), and occasionally strabismus surgery. Future treatment may involve binocular strategies balancing the input from the two eyes to the visual cortex.
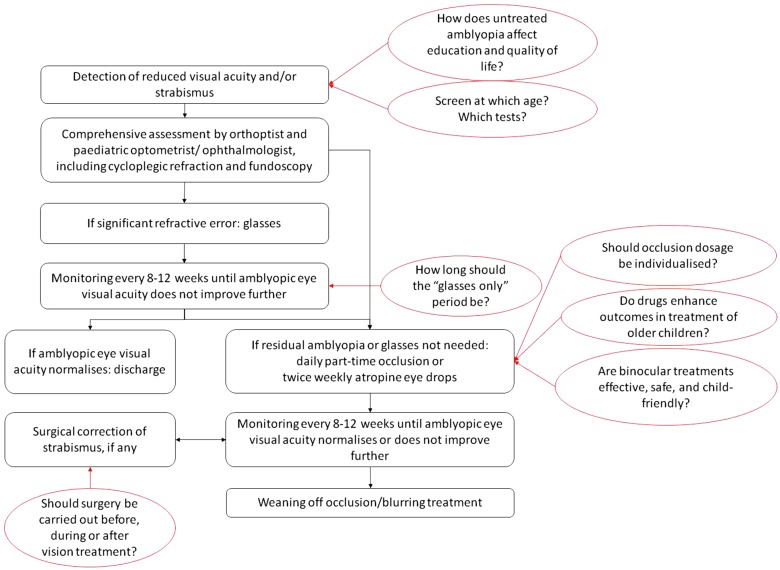
Over the past 15 years, a number of RCTs have explored the role of glasses and patching in the treatment of amblyopia, and form the basis of today's amblyopia management (Fig. 4).
Fig. 4.
Current management of amblyopia in childhood (black) and areas of controversy (red).
The first step is the correction of any refractive error by prescribing glasses. This addresses both differences in refractive error between the two eyes (anisometropia) and high degrees of hypermetropia (longfar-sightedness) in both eyes, which can be a cause of strabismus. Only some children with amblyopia do not have significant refractive errors and will not benefit from glasses. This first phase is called ‘optical treatment’ or ‘refractive adaptation’. Visual acuity typically improves over several months, with greatest improvement over the first few weeks of wearing glasses.11 Visual acuity in the amblyopic eye normalizes with glasses only in a fifth to a third of children,20,21 and no further treatment is needed.
A secondary form of treatment is started if the visual acuity in the amblyopic eye reaches a plateau and fails to improve further, with a persistent difference of 0.20 logMAR or more compared with the better-seeing eye or the level of visual acuity normal for the child's age. Parents and carers are offered a choice between patching (occlusion) or blurring (pharmacological) treatment. The dose of occlusion depends on the severity of the residual amblyopia: 2 h for moderate and 6 h for severe amblyopia (reviewed in Refs11,12,22). If amblyopia persists after a period of two-hourly patching, then a dose increase to 6 h can improve visual acuity further.23 Other doses may be effective, but have not been tested in recent RCTs. Pharmacological blurring of the better-seeing eye with one drop of G Atropine 1% twice a week can be used instead of patching. Children are monitored at eight- to twelve-weekly intervals until the visual acuity in the amblyopic eye has normalized, or until no further improvement is noted.
Children who present or are referred late, after the end of the conventional ‘critical period’ of visual development are typically offered these treatments after an informed discussion about the reduced treatment success rates in older children, and the increased risk of adverse events such as double vision.24
Can medication enhance outcomes in the treatment of older children with amblyopia?
Certain neurotropic medicines such as levodopa enhance neural plasticity after the end of the critical period of vision development. However, a recent RCT found that levodopa does not enhance visual improvement in children aged 7–12 years with residual amblyopia after occlusion treatment.25 A phase I study of donezepil, a medication used in Alzheimer's disease, has not yet published results (NCT01584076).
Areas of controversy
In 2012, the Sight Loss and Vision Priority Setting Partnership asked patients, carers and eye health professionals to identify unanswered questions about the prevention, diagnosis and treatment of sight loss and eye conditions that they wished to see answered. A total of 2220 people responded to the survey. At workshops held in 2013 the top 10 priorities for 12 categories of eye conditions were agreed. Two priorities concern amblyopia: ‘How do we improve screening and surveillance from the ante-natal period through to childhood to ensure early diagnosis of impaired vision and eye conditions?’ and ‘Can the treatment of amblyopia be improved to produce better short- and long-term outcomes than are possible with current treatments?’ These and other questions remain important areas of both innovation and controversy (Fig. 4).
Vision screening in childhood: when and which tests?
The two main factors underlying the development of amblyopia are strabismus and anisometropia. Children with anisometropia usually have ‘straight’ eyes (Fig. 1), and their amblyopia can therefore go unnoticed until well after the critical period of visual development. Obvious Sstrabismus, on the other hand, is usually noticed early, but small degrees of strabismus may go unnoticed. In the UK, the National Screening Committee (NSC) recommends that all children should have an orthoptist-led screening assessment of their vision at the age of 4–5 years (http://legacy.screening.nhs.uk/vision-child). This recommendation is based on a review of currently available evidence, which indicates that optotype-based screening is the preferred screening method; most children are able to co-operate with this type of test by the age of 4–5 years. The current NSC recommendation means that while the screening assessment does not need to be carried out by orthoptists, staff delivering the programme (school nurses, health technology care assistants or other health care professionals) should be trained, supervised, monitored and audited by orthoptists. However, in the UK commissioning of vision screening at primary school entry is variable (Fig. 1).26 Screening is often also not universal, as ‘free’ or ‘independent’ school may not be included. Recently from 2015, commissioning of vision screening in England has changed from Clinical Commissioning Groups to Local Authorities, resulting in boundary changes and further development of local protocols.
The type of screening tests used also varies. While assessment by an orthoptist may be the most reliable test,27 it is also the most expensive cost-effectiveness needs to be considered. Tests that involve the identification of letters (optotypes) are most reliable to detect vision deficits, but most children can only master these from the age of 4 years onwards. Accuracy of detection may be enhanced by the use of ‘crowded’ as opposed to ‘single letter’ acuity charts, i.e. the presentation of optotypes surrounded by other letters or flanking bars, and by additional tests, for example auto-refractors, which estimate the need for glasses, and can be applied to children at younger ages. However, published evidence about autorefractor accuracy in children is limited to hospital-based rather than population-based studies. A change in screening practice would be justified if earlier detection and treatment of amblyopia were to result in better outcomes. Current evidence is conflicting and of variable quality (reviewed in Refs18,28,29). A recently completed population-based vision screening study (two large studies population-based are currently ongoing which will inform this debate: ‘Vision Screening for Amblyopia’ (NCT01430247)) has enroled 15 648 children aged 4–4.5 years, 7000 children in Zagreb to evaluate screening and treatment the efficacy of an optotype-based screening protocol and reported high testability, sensitivity and specificity.30 An ongoing population-based study, the ‘Disinvestment Study of Population-Based Vision Screening in Children’ (NCT01675193), in the Netherlands aims to determine the optimal screening intervals and cost-effectiveness of population-based vision screening in preverbal children.
How does amblyopia affect education, activities of daily living and quality of life?
The effect of amblyopia on fine motor skills and confidence in social interactions is well known.13,14,15 Current work with children focuses on the educational impact, and has shown that children with amblyopia read more slowly.31 Previous studies did not demonstrate an effect of amblyopia on educational attainment, occupational status and social functioning and quality of life (reviewed in Refs18,32). The ongoing study ‘Impact of amblyopia: reasons for not accessing treatment and the effect on developing literacy skills in young children’ (UKCRN ID 16018) explores the educational impact in a cohort of 12 000 children as part of the ‘Born in Bradford’ Project.
What are the economical aspects? What is the burden of amblyopia for the individual, the family and society?
If amblyopia persists into adulthood, then affected individuals may be unable to take up professions that require depth perception. Accidents affecting the better-seeing eye can lead to a loss of quality of life and independence; the estimated lifetime risk of bilateral visual impairment may be as high as 18%, compared with 10% for people without amblyopia.16 Recent utility analysis studies and systematic review found amblyopia screening and treatment to be cost effective, but dependent on the long-term utility effects of unilateral vision loss.1,17,18
Occlusion treatment: when to start, and how much and when and how to stop?
Previous RCTs have shaped the dosage regime of optical, occlusion and pharmacological blurring treatment. However, while studies evaluating optical treatment have shown that vision can gradually improve for several months after glasses have been started,33 many RCTs did not include a period of full refractive adaptation before starting a secondary treatment with patching or blurring. The question therefore arises whether early patching or blurring in addition to glasses would shorten overall treatment duration, possibly increasing adherence with treatment and clinic visits. The current ‘European Paediatric Amblyopia Treatment study for Children: Role of glasses wearing in amblyopia treatment’ (EUPATCH, ISRCTN51712593) addresses this question. It is also not clear whether a shorter treatment duration and better adherence could be achieved by offering an individualized treatment approach, i.e. variable dosage schedules based on clinical findings, tolerance and dose response, and this is the topic of the current RCT ‘Personalizing dosing strategy for amblyopia treatment’ (ISRCTN12292232). Once visual acuity reaches a plateau and fails to improve further on two consecutive visits despite optimal treatment, occlusion or blurring treatment is stopped, typically by gradual tapering to reduce the risk of regression of the treatment effect. A recent study of 15-year-old adolescents treated for amblyopia in childhood indicates that the treatment effect is maintained.34 However, even if regression occurs, visual acuity can often be regained should vision in the better-seeing eye be reduced due to trauma or age-related degenerative changes.
Surgical correction of strabismus: before, during or after amblyopia treatment?
Many practitioners begin the management of strabismic amblyopia by first correcting any refractive error, then adding a secondary type of treatment (patching or blurring) if required, and finally deciding whether surgical correction of the strabismus would be beneficial. With glasses only, one study showed an improvement in amblyopic eye visual acuity of 0.26 logMAR over 18 weeks, and resolution of amblyopia in a third of children; this was independent from any improvement in eye alignment.20 With this approach, the number of strabismus operations in the UK has been declining.35 However, some clinicians prefer to surgically align the eyes early in the management. No RCT evidence is available to inform whether one approach is superior to the other.36
Can medication enhance outcomes in the treatment of older children with amblyopia?
Certain neurotropic medicines such as levodopa enhance neural plasticity after the end of the critical period of vision development. A recent RCT found that levodopa does not enhance visual improvement in children aged 7–12 years with residual amblyopia after occlusion treatment.25 A phase I study of donezepil, a medication used in Alzheimer disease, has not yet published results (NCT01584076).
Can treatments be developed that are more effective and more child-friendly than current treatments?
The main drawbacks of occlusion and pharmacological blurring are poor adherence to treatment and suboptimal treatment outcomes. Lack of adherence to occlusion treatment is common. Patching the better-seeing eye may functionally incapacitate the child, and children often attempt to remove the patch. Adherence with occlusion is typically <50% of the prescribed dose,37 though educational interventions can increase the adherence rate for a 12-week treatment period to 80%.38 Even with the best current treatment, only around half of children achieve near-normal visual acuity in the amblyopic eye. Around 25% of eyes with severe amblyopia and 58% of eyes with moderate amblyopia improve to a level of 0.20 logMAR or better with occlusion treatment over the first 4 months of treatment (reviewed in Refs11,12,22). After treatment is discontinued, visual acuity typically regresses, and a low-dose maintenance treatment is often used to ‘wean’ children off treatment. Two years after stopping treatment and at 15 years of age, BCVA in the amblyopic eye is 0.20 logMAR worse than in the better-seeing eye in up to half of children.39,34 Based on an increased understanding of the cortical processes underlying amblyopia, new treatment approaches have been developed. Most of these have been explored in prospective case series; some are currently being tested in randomized controlled trials. These new approaches are based on simultaneous binocular visual stimulation and aim not only to improve visual acuity in the amblyopic eye, but also to promote binocularity. At the same time, efforts are being made to make these treatments appealing to children. The following sections describe these approaches in more detail.
Growing points
In amblyopia, the fastest area of growth in terms of publications per year, allocation of research grants and open randomized controlled trials is that of binocular treatment. These new methods involve playing computer games or watching movies on digital displays, with manipulation of the images shown to each eye by means of liquid crystal display (LCD) glasses or prism overlays. Presenting different images to each eye is called ‘dichoptic presentation’. To date, three systems have been used in children, albeit in case series only. The first system is known as ‘anti-suppression therapy’, which focuses on the observation that the amblyopic eye has reduced contrast sensitivity relative to the better-seeing eye. In order to balance the cortical input and overcome the interocular suppression (reviewed in Refs5,7,40), images with reduced contrast are presented to the better-seeing eye, and images with higher contrast are shown to the amblyopic eye. The child then has to carry out a task that requires combination of the information from the two eyes. As performance improves, the image contrast shown to the better-seeing eye is gradually increased until contrast is equal for both eyes. A frequently used task is the Tetris game in which a series of falling blocks have to be fit together to form complete lines. The prescribed training dose is typically 1–2 h a day. Improvement of visual acuity and binocular vision can occur within 1–4 weeks of training.7,41 A second treatment approach is ‘balanced binocular viewing (BBV)’, which blurs the image seen by the better-seeing eye to achieve the same aim of balancing input to the primary visual cortex.42 The resolution of the image shown to the better-seeing eye is matched to the resolution perceived by the amblyopic eye. Instead of playing a computer game, children watch movies at home for an hour a day while wearing LCD shutter glasses (Fig. 3). A third method is the ‘Interactive binocular treatment (I-BiT™)’ system, which presents different parts of a two-dimensional visual scene to either eye via shutter glasses, combined with a task that requires combination of the two images.43,44 Images are viewed with both eyes, but parts of the image can only be seen with the amblyopic eye. The material viewed consists of videos and interactive games. Dichoptic treatments have so far only been used in prospective case series without control groups; RCTs are currently under way. The evaluation of results published so far is complicated by the use of different testing protocols, the enrolment of participants from different age groups, and those with and without prior treatment. However, the new treatments may be as effective as conventional patching or blurring treatment: with patching, visual acuity generally improves by around 0.22 logMAR over 6 months34; across dichoptic treatment studies, acuity improvement ranges from 0.08 to 0.26 logMAR (reviewed in Ref.40). As dichoptic treatments balance the input to the primary visual cortex, they may also have a greater effect on binocular function than conventional treatments, as reflected in an improvement in stereoacuity in the range of 200 s of arc (reviewed in Ref.40). In addition, binocular treatments may have advantages other than improvements in visual function. In particular, the use of computer games or videos in these approaches is likely to engage children's attention and may thus improve adherence to treatment. Indeed, high adherence of 80.6–93% has been reported (reviewed in Ref.40). Lastly, through treating the fundamental binocular imbalance of amblyopia, dichoptic approaches may reduce the recurrence of amblyopia after treatment is stopped. So far, recurrence of amblyopia after cessation of treatment is reportedly low, for example 0.055 logMAR at 10 weeks with I-BiT™ and 0.02 logMAR at 14 weeks with BBV, but long-term outcomes are unknown (reviewed in Ref.40).
Areas timely for developing research
With advances in research methods, including advances in functional magnetic resonance imaging and in psychophysical methodologies, amblyopia research is moving from studies mostly conducted with adult participants to studies involving children. The most innovative area of clinical research concerns the development and evaluation of binocular amblyopia treatments. In order to provide a robust evaluation of dichoptic treatments, three multicenter and one single-centre randomized controlled trials are currently open to recruitment. The UK ‘interactive binocular treatment (i-BiT™)’ trial (NCT01702727)44 randomizes children aged 4–7 years to either playing an interactive computer game for 30 min once a week for 6 weeks with or without dichoptic presentation, or to watch a DVD using dichoptic image presentation, in a hospital setting. The Glasgow-based pilot trial ‘Perceptual learning in enhanced amblyopia treatment (PLEAT)’ (ISRCTN14022536) involves playing a dichoptic contrast balancing game while wearing LCD shutter goggles. In New Zealand/Australia/Hong Kong/Singapore, the ‘Binocular Treatment of Amblyopia using Video games (BRAVO)’ trial (ACTRN12613001004752) also uses a form of anti-suppression treatment, delivered as a Tetris game on an iPod touch, for children over the age of 7 years. Lastly, the US ‘Study of Binocular Computer Activities for Treatment of Amblyopia’ (ATS18, NCT02200211) uses the Tetris game on an iPad in children aged 5–17 years, with randomization to either binocular game play for 1 h a day or to occlusion treatment for 2 h a day. The acceptability of these treatments to children and families is still unknown. There are concerns that weekly hospital-based treatments will face problems with adherence, and families will find it difficult to attend frequent appointments. The Tetris game, while popular with adults, may be too repetitive to maintain a child's attention for an hour a day over several weeks. Watching movies is therefore a treatment platform explored by several research teams42,45; this requires modification of existing movies for graded 3D viewing. The spectrum of potential content is vast. Future studies will need to explore not only efficacy and safety, but also acceptability and usage.
Glossary
- Anisometropia
a difference in the optical properties of both eyes, requiring different corrective lenses for the right and left eye
- BCVA
best-corrected visual acuity
- Dichoptic
presenting images to each eye separately, viewing two images with each eye separately
- DVD
digital video disc
- LCD
liquid crystal display
- logMAR
logarithm of minimal angle of resolution, the current standard for measuring visual acuity on optotype charts
- RCT
randomized controlled trial
- Strabismus
a misalignment of the visual axes
- UK
United Kingdom
- US
United States of America
Funding
This review was not supported by a specific funding source. AHDN and VT are employed by the NIHR Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and UCL Institute of ophthalmology. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
References
- 1. Carlton J, Karnon J, Czoski-Murray C, et al. . The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4-5 years: a systematic review and economic evaluation. Health Technol Assess 2008;12:iii, xi–194. [DOI] [PubMed] [Google Scholar]
- 2. Powell C, Hatt SR. Vision screening for amblyopia in childhood. Cochrane Database Syst Rev 2009;3:CD005020. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert CE, Ellwein LB. Prevalence and causes of functional low vision in school-age children: results from standardized population surveys in Asia, Africa, and Latin America. Invest Ophthalmol Vis Sci 2008;49:877–81. [DOI] [PubMed] [Google Scholar]
- 4. Norgett Y, Siderov J. Crowding in children's visual acuity tests--effect of test design and age. Optom Vis Sci 2011;88:920–7. [DOI] [PubMed] [Google Scholar]
- 5. Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res 2013;33:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greenwood JA, Tailor VK, Sloper JJ, et al. . Visual acuity, crowding, and stereo-vision are linked in children with and without amblyopia. Invest Ophthalmol Vis Sci 2012;53:7655–65. [DOI] [PubMed] [Google Scholar]
- 7. Hess RF, Thompson B, Baker DH. Binocular vision in amblyopia: structure, suppression and plasticity. Ophthalmic Physiol Opt 2014;34:146–62. [DOI] [PubMed] [Google Scholar]
- 8. Barrett BT, Bradley A, McGraw PV. Understanding the neural basis of amblyopia. Neuroscientist 2004;10:106–17. [DOI] [PubMed] [Google Scholar]
- 9. Hamm LM, Black J, Dai S, et al. . Global processing in amblyopia: a review. Front Psychol 2014;5:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron 2012;75:230–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor K, Elliott S. Interventions for strabismic amblyopia. Cochrane Database Syst Rev 2014;7:CD006461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor K, Powell C, Hatt SR, et al. . Interventions for unilateral and bilateral refractive amblyopia. Cochrane Database Syst Rev 2012;4:CD005137. [DOI] [PubMed] [Google Scholar]
- 13. Grant S, Moseley MJ. Amblyopia and real-world visuomotor tasks. Strabismus 2011;19:119–28. [DOI] [PubMed] [Google Scholar]
- 14. Webber AL, Wood JM, Gole GA, et al. . The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci 2008;49:594–603. [DOI] [PubMed] [Google Scholar]
- 15. Webber AL, Wood JM, Gole GA, et al. . Effect of amblyopia on self-esteem in children. Optom Vis Sci 2008;85:1074–81. [DOI] [PubMed] [Google Scholar]
- 16. van Leeuwen R, Eijkemans MJ, Vingerling JR, et al. . Risk of bilateral visual impairment in individuals with amblyopia: the Rotterdam study. Br J Ophthalmol 2007;91:1450–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Konig HH, Barry JC. Cost effectiveness of treatment for amblyopia: an analysis based on a probabilistic Markov model. Br J Ophthalmol 2004;88:606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solebo AL, Cumberland PM, Rahi JS. Whole-population vision screening in children aged 4-5 years to detect amblyopia. Lancet 2015;385:2308–19. [DOI] [PubMed] [Google Scholar]
- 19. Loudon SE, Simonsz HJ. The history of the treatment of amblyopia. Strabismus 2005;13:93–106. [DOI] [PubMed] [Google Scholar]
- 20. Writing Committee for the Pediatric Eye Disease Investigator Group. Cotter SA, Foster NC, et al. . Optical treatment of strabismic and combined strabismic-anisometropic amblyopia. Ophthalmology 2012;119:150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tailor V, Glaze S, Khandelwal P, et al. . Prescribed computer games in addition to occlusion versus standard occlusion treatment for childhood amblyopia: a pilot randomised controlled trial. Pilot Feasibility Studies 2015;2015:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li T, Shotton K. Conventional occlusion versus pharmacologic penalization for amblyopia. Cochrane Database Syst Rev 2009;CD006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pediatric Eye Disease Investigator Group. Wallace DK, Lazar EL, et al. . A randomized trial of increasing patching for amblyopia. Ophthalmology 2013;120:2270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheiman MM, Hertle RW, Beck RW, et al. . Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol 2005;123:437–47. [DOI] [PubMed] [Google Scholar]
- 25. Pediatric Eye Disease Investigator Group. Repka MX, Kraker RT, et al. . A randomized trial of levodopa as treatment for residual amblyopia in older children. Ophthalmology 2015;122:874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baxter L, McNamara D, McCallum A, et al. Mapping of vision screening services for 4 to 5 year olds to ensure Outcome 1 of the UK Vision Strategy. Vision UK 2014. London, 2014
- 27. Jarvis SN, Tamhne RC, Thompson L, et al. . Preschool vision screening. Arch Dis Child 1991;66:288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmucker C, Grosselfinger R, Riemsma R, et al. . Effectiveness of screening preschool children for amblyopia: a systematic review. BMC Ophthalmol 2009;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmucker C, Kleijnen J, Grosselfinger R, et al. . Effectiveness of early in comparison to late(r) treatment in children with amblyopia or its risk factors: a systematic review. Ophthalmic Epidemiol 2010;17:7–17. [DOI] [PubMed] [Google Scholar]
- 30. Busic M, Bjelos M, Petrovecki M, et al. . Zagreb Amblyopia Preschool Screening Study: near and distance visual acuity testing increase the diagnostic accuracy of screening for amblyopia. Croat Med J 2016;57:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelly KR, Jost RM, De La Cruz A, et al. . Amblyopic children read more slowly than controls under natural, binocular reading conditions. J AAPOS 2015;19:515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carlton J, Kaltenthaler E. Amblyopia and quality of life: a systematic review. Eye (Lond) 2011;25:403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stewart CE, Moseley MJ, Fielder AR, et al. . Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol 2004;88:1552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Repka MX, Kraker RT, Holmes JM, et al. . Atropine vs patching for treatment of moderate amblyopia: follow-up at 15 years of age of a randomized clinical trial. JAMA Ophthalmol 2014;132:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arora A, Williams B, Arora AK, et al. . Decreasing strabismus surgery. Br J Ophthalmol 2005;89:409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Korah S, Philip S, Jasper S, et al. . Strabismus surgery before versus after completion of amblyopia therapy in children. Cochrane Database Syst Rev 2014;10:CD009272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wallace MP, Stewart CE, Moseley MJ, et al. . Compliance with occlusion therapy for childhood amblyopia. Invest Ophthalmol Vis Sci 2013;54:6158–66. [DOI] [PubMed] [Google Scholar]
- 38. Pradeep A, Proudlock FA, Awan M, et al. . An educational intervention to improve adherence to high-dosage patching regimen for amblyopia: a randomised controlled trial. Br J Ophthalmol 2014;98:865–70. [DOI] [PubMed] [Google Scholar]
- 39. Repka MX, Wallace DK, Beck RW, et al. . Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol 2005;123:149–57. [DOI] [PubMed] [Google Scholar]
- 40. Tailor V, Bossi M, Bunce C, et al. . Binocular versus standard occlusion or blurring treatment for unilateral amblyopia in children aged three to eight years. Cochrane Database Syst Rev 2015;8:CD011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knox PJ, Simmers AJ, Gray LS, et al. . An exploratory study: prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci 2012;53:817–24. [DOI] [PubMed] [Google Scholar]
- 42. Bossi M, Anderson E, Tailor V, et al. . An exploratory study of a novel home-based binocular therapy for childhood amblyopia. ARVO 2014;55:5981. [Google Scholar]
- 43. Herbison N, Cobb S, Gregson R, et al. . Interactive binocular treatment (I-BiT) for amblyopia: results of a pilot study of 3D shutter glasses system. Eye (Lond) 2013;27:1077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Foss AJ, Gregson RM, MacKeith D, et al. . Evaluation and development of a novel binocular treatment (I-BiT) system using video clips and interactive games to improve vision in children with amblyopia (‘lazy eye’): study protocol for a randomised controlled trial. Trials 2013;14:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li SL, Reynaud A, Hess RF, et al. . Dichoptic movie viewing treats childhood amblyopia. J AAPOS 2015;19:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]