Summary
The overexpression of Six1 dramatically promotes CRC tumor growth and metastasis by increasing populations of cancer stem cells, stimulating angiogenesis and recruiting tumor-associated macrophages. MAPK activation may be a common mechanism leading to Six1-associated tumor progression.
Abstract
The homeoprotein Six1 is overexpressed in many human cancers and is associated with increased tumor progression and metastasis. Recent studies have shown that Six1 is associated with poorer overall survival in advanced-stage colorectal cancer (CRC). In the current study, we explored the functional changes and molecular events associated with Six1 overexpression in a mouse model of CRC. An orthotopic model and a splenic injection metastasis model were used to investigate the role of Six1 in CRC tumor growth and metastasis using mouse colon adenocarcinoma MC38 cells overexpressing Six1. We found that overexpression of Six1 dramatically promotes CRC tumor growth and metastasis in vivo. Six1 overexpression in MC38 increased protein levels of aldehyde dehydrogenase-1 and expanded CD44+/CD166+ populations, indicating Six1 increased features of cancer stem cells. In addition, Six1 overexpression stimulated angiogenesis by upregulating the expression of vascular endothelial growth factor (VEGF). Six1-overexpressing tumor cells recruited tumor-associated macrophages (TAM) by increasing the expression of macrophage-specific colony stimulating factor, chemokine (C-C motif) ligand 2/5 and VEGF, further facilitating CRC tumor growth and metastasis. Furthermore, we determined that Six1 activated mitogen-activated protein kinase (MAPK) signaling in CRC cells. In summary, our studies strongly suggest that Six1 overexpression promotes CRC growth and metastasis and remodels tumor stroma by stimulating angiogenesis and recruiting TAM. MAPK activation may be a pivotal event in Six1-associated tumor progression, which may provide opportunities for pharmacologic intervention.
Introduction
Colorectal cancer (CRC) is the third most common malignancy and the third leading cause of cancer-related death in men and women in the USA. According to the American Cancer Society, in 2014, an estimated 71830 men and 65000 women will be diagnosed with CRC; and 26270 men and 24040 women will die of the disease (1).
The homeoprotein Six1, a member of the Six family of homeodomain transcription factors, is essential for the development of numerous organs (2–4). Six1 overexpression has been found in various human cancers and is associated with increased tumor progression and metastasis, and decreased survival (5). Ono et al. found that Six1 overexpression is associated with poorer overall survival in advanced-stage CRC (stages III and IV), where cancer metastasis to regional lymph nodes or distant organs has occurred (6). Moreover, Kahlert et al. have recently shown that Six1 is also overexpressed in non-metastatic CRC (stages I–III) and is associated with poor prognosis in two independent cohorts (7). Studies using RNA interference have demonstrated that inhibition of Six1 expression suppresses CRC cell growth and invasion (8). These findings suggest that Six1 overexpression may promote CRC progression and metastasis. Therefore, in this study, we investigated the role of Six1 on tumor progression and metastasis in mouse and human CRC cells. We found that overexpression of Six1 promoted CRC tumor growth and metastasis in vivo, increased features of cancer stem cells (CSCs) and stimulated angiogenesis by upregulating the expression of vascular endothelial growth factor (VEGF). Furthermore, we determined that Six1 overexpression resulted in the recruitment of tumor-associated macrophages (TAM) by increasing the expression of macrophage-specific colony stimulating factor (CSF-1), chemokine (C-C motif) ligand 2/5 (CCL2/5) and VEGF, further facilitating CRC tumor growth and metastasis.
Materials and methods
Cell culture
Human CRC cell lines HCT116, SW480, SW620, LoVo and SW48 were obtained from American Type Culture Collection (ATCC) from 2006 to 2012. Human CRC cell line HT29 and the C57BL/6-derived mouse colon adenocarcinoma cell line MC38 were kind gifts from Dr Dan Dixon. Human CRC cell lines were authenticated using Short Tandem Repeat analysis in 2015 and 2016. MC38 were validated by IDEXX BioReseach (Columbia, MO) using mouse Short Tandem Repeat profiling in 2016. HCT116, SW480, SW620 and HT29 were maintained in RPMI-1640 medium (Mediatech, Manassas, VA) supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA). MC38, LoVo and SW48 were cultured in Dulbecco’s Modification of Eagle’s Medium (DMEM) with 4.5 g/L glucose (Mediatech) supplemented with 10% FBS. All cells were maintained in a humidified atmosphere of 5% CO2 at 37°C.
Six1 overexpression and knockdown
The mouse Six1 cDNA (Origene, Rockville, MD) was cloned into the EcoRI and NotI sites of pcDNA3.1. This construct is referred to as pcDNA3.1-mSix1. MC38 cells were stably transfected with pcDNA3.1 (control) or pcDNA3.1-mSix1 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. Stable transfectants, MC38-Ctrl and MC38-Six1, were selected in media containing 300 µg/ml Zeocin (Invitrogen). A human Six1 expression plasmid, pcDNA3.1-Six1, has been described previously (9). HT29, HCT116 and SW480 were transfected with pcDNA3.1 (control) or pcDNA3.1-Six1 using FuGENE 6 (Promega, Madison, WI). Stably transfected HT29 (HT29-Ctrl and HT29-Six1) were selected in the presence of 200 µg/ml Zeocin whereas HCT116 (HCT116-Ctrl and HCT116-Six1) and SW480 (SW480-Ctrl and SW480-Six1) were selected in the presence of 100 µg/ml Zeocin. HEK293T cells were transfected with green fluorescent protein (GFP)-tagged lentiviral (pGIPZ) constructs containing Six1 shRNA (shSix1) or scrambled shRNA (shCtrl; Dharmacon, Lafayette, CO), and packaging plasmids (pCMV-∆8.2 and pCMV-VSVG, ADDGENE, Cambridge, MA) using Lipofectamine 2000. The viral supernatants were collected 60 h after transfection and CRC cells were immediately infected in the presence of 10 μg/ml polybrene (Sigma-Aldrich, St. Louis, MO). Cells expressing Six1 shRNA or scrambled RNA were selected with puromycin (5 μg/ml) followed by cell sorting and collection of the top 10–20% GFP-positive cells.
Animal models
The orthotopic CRC mouse model was established as described previously (10). Briefly, 8-week-old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were anesthetized by inhalation of 2% isoflurane in oxygen. A midline incision was made to expose the cecum. Phosphate buffered saline (PBS) containing 2 × 106 MC38-Ctrl or MC38-Six1 cells (10 μl) was injected into the cecum subserosa using a 33-gauge micro-injector (Hamilton Company, Reno, NV). The injection site was sealed with a tissue adhesive (3M, St. Paul, MN) to prevent leakage of cells and washed with 70% alcohol and PBS. The cecum was replaced in the peritoneal cavity, and the abdominal wall and skin closed with 6-0 polyglycolic acid sutures (CP Medical, Portland, OR). Six weeks after implantation, mice were killed, tumor weights measured and tumors processed.
In the CRC metastasis model, liver metastasis was induced by splenic injection of tumor cells (11,12). A lateral incision was made to expose the spleen. PBS containing 2 × 105 MC38-Ctrl or MC38-Six1 cells (10 μl) was injected into the spleen using a 33-gauge micro-injector. The injection site was sealed with a tissue adhesive to prevent leakage of cells and washed with PBS. The spleen was replaced and the abdominal wall and skin closed. Three weeks after implantation, mice were killed and spleens and livers were collected and weighed.
For the subcutaneous model, 2 × 106 MC38-Ctrl or MC38-Six1 cells were suspended in 100 μl of PBS and injected subcutaneously into the flank of C57BL/6 mice. Six weeks after implantation, mice were killed and tumors were stripped and weighed. C57BL/6 mice were maintained at the Mouse Experimentation Core Facility of the Center for Colon Cancer Research at the University of South Carolina. All animal experiments were conducted according to the guidelines and approval of USC Institutional Animal Care and Use Committee.
Histology, immunohistochemistry and immunofluorescence
Tumor sections were processed as described previously (9). Non-specific epitopes were blocked with normal horse serum (Jackson ImmunoResearch, West Grove, PA) for 1 h. Samples were incubated overnight at 4°C with antibodies against the following proteins: proliferating cell nuclear antigen (PCNA; 1:300, Abcam, Cambridge, MA), CD31, lysyl oxidase (LOX), matrix metalloproteinases 9 (MMP9), alpha-smooth muscle actin (α-SMA), VEGF, F4/80 (1:100, Abcam), cleaved caspase-3 (1:100, Cell Signaling Technology, Danvers, MA), aldehyde dehydrogenase-1 (ALDH1; 1:100, Santa Cruz, Santa Cruz, CA) and Ki67 (1:100, OriGene). For immunohistochemistry, after incubation with the appropriate HRP-conjugated secondary antibodies (Bio-Rad, Hercules, CA) for 1 h at room temperature, antigen signals were detected using the 2-Solution Diaminobenzidine (DAB) Kit (Invitrogen), counterstained with hematoxylin and mounted in Acrymount (StatLab, Mckinney, TX). For immunofluorescence, sections were incubated with fluorochrome-conjugated secondary antibodies (Invitrogen) for 1 h at room temperature and stained with 1:10000 dilution of 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) before cells were mounted. Samples were viewed using an Olympus ×81 fluorescence microscope.
Cell proliferation assay
To determine the growth rate of MC38-Ctrl and MC38-Six1 cells, 20000 cells per well were plated in 6-well plates, in 2 ml of DMEM containing 10% FBS. Cells in triplicate wells were counted daily for up to 6 days using a hemocytometer. For human CRC cells, 3000 cells per well in 100 μl of complete culture media were plated in 96-well plates. Four days after plating, cell proliferation was measured by reagent WST-1 (Sigma-Aldrich) according to the manufacturer’s protocol.
Western blot analysis
Whole cells lysates were prepared in RIPA buffer (Pierce, Rockford, IL). Antibodies against the following proteins were used: Six1, ALDH1, β-catenin, cyclin E (1:1000, Santa Cruz), E-cadherin, fibronectin (BD Biosciences, San Jose, CA), p-ERK, p-JNK, p-p38, ERK, p38, cyclin D1, vimentin (1:3000, Cell Signaling Technology), MMP2, MMP9, PCNA, VEGF (1:1000, Abcam), CCL2, CCL5, CSF-1 (1:1000, 1:1000 and 1:10000, R&D Systems, Minneapolis, MN). β-Actin (1:5000, Santa Cruz) was used as a loading control.
Sera from MC38-Ctrl and MC38-Six1 tumor-bearing mice were analyzed by western blotting. Antibodies against the following proteins were used: MMP2, MMP9, VEGF, interleukin (IL)-1β, LOX (1:1000, Abcam), TNFα and IL-6 (1:1000, Cell Signaling Technology). Albumin (1:10000, Santa Cruz) was used as a loading control.
Flow cytometry analysis
MC38-Ctrl and MC38-Six1 were stained with FITC-conjugated anti-CD44 and PE-conjugated anti-CD166. Single-cell suspensions from tumors were obtained using sequential enzymatic digestion as described previously (13). Cell suspensions were filtered through a 70 µm nylon cell strainer (BD Biosciences) and washed twice with PBS. Single-cell suspensions isolated from MC38-Ctrl or MC38-Six1 tumors in the cecum were stained with APC-conjugated CD11b, FITC-conjugated anti-Gr1 and PE-conjugated anti-F4/80 from eBioscience (San Diego, CA). Cells (1 × 106) were incubated with antibodies in 100 μl wash buffer (PBS containing 0.1% BSA) at 4°C for 1 h. Cells were then washed twice with cold wash buffer and fixed with 1% paraformaldehyde. Flow cytometry analysis (30000 cells) was performed using a BD LSRII flow cytometer (BD Biosciences).
In vitro tumorsphere formation assay
MC38-Ctrl, MC38-Six1, HCT116-Ctrl, HCT116-Six1, HT29-Ctrl and HT29-Six1 (10000 per well) were plated in ultra-low attachment 6-well plates (Corning, Lowell, MA) in 2 ml of serum-free media as described previously (9). Cells were cultured in an atmosphere of 5% CO2 at 37°C for 7 days. The number of tumorspheres in each well was manually counted.
Wound healing, cell migration and cell invasion assays
MC38-Ctrl and MC38-Six1 cells were plated into 6-well plates and cultured until 90% confluent. Cell monolayers were wounded with a sterile 200 µl pipet tip and then incubated with DMEM containing 1% FBS for 24 or 48 h in an atmosphere of 5% CO2 at 37°C. Representative areas of wounded monolayers containing wounds of the same width were marked and photographed. After incubation, the same areas were photographed. The extent of wound repair was evaluated by measuring the wound area using the Image J (NIH). Each experiment was performed in triplicate wells and repeated three times.
The invasion and migration capacity of Six1-overexpressing cells and corresponding controls (MC38-Ctrl, MC38-Six1, HCT116-Ctrl, HCT116-Six1, HT29-Ctrl and HT29-Six1) and Six1-knockdown cells and their corresponding controls (SW480-shCtrl, SW480-shSix1, SW620-shCtrl, SW620-shSix1, LoVo-shCtrl, LoVo-shSix1, SW48-shCtrl and SW48-shSix1) was measured as we have described previously (9). Each experiment was performed in triplicate wells and repeated three times.
Real-time PCR
Total RNA was isolated from cells using the Total RNA Isolation Mini Kit (Agilent, Wilmington, DE). Reverse transcription was performed with 1 μg of total RNA using iScript cDNA Synthesis Kit (Bio-Rad), and real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad). The sequence of the primers used for real-time PCR is shown in Supplementary Table 1, available at Carcinogenesis Online. β-Actin was used as an internal control. All samples were assayed in triplicate.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using the student t-test when only two value sets were compared, and one-way ANOVA followed by Dunnett’s test when the data involved three or more groups. P < 0.05, P < 0.01 or P < 0.001 were considered statistically significant and indicated in the figures by *, ** or ***, respectively.
Results
Overexpression of Six1 enhances CRC tumor growth and metastasis
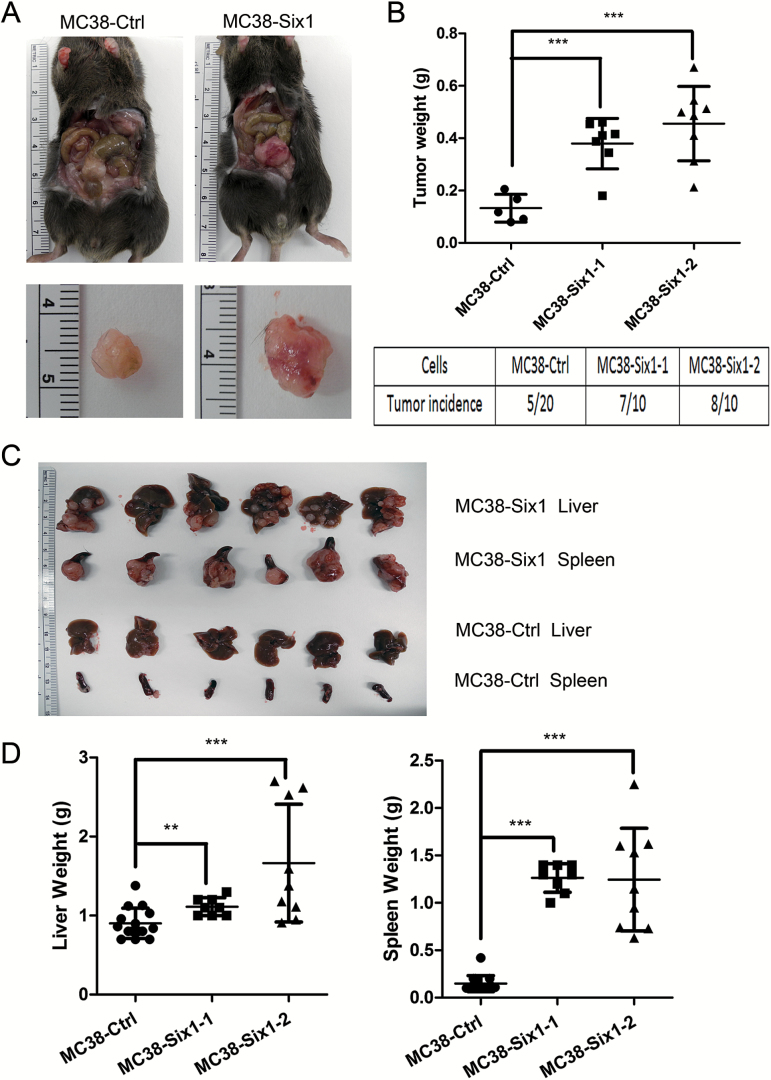
To explore the association between Six1 overexpression and CRC development, we examined the expression of Six1 mRNA in the intestines from C57BL/6 and C57BL/6-ApcMin/+ mice and in adenomas from C57BL/6-ApcMin/+ mice. We observed increased expression of Six1 mRNA in the intestines from C57BL/6-ApcMin/+ mice compared with that from wild type C57BL/6 mice and much higher expression in adenomas from C57BL/6-ApcMin/+ mice (Supplementary Figure 1, available at Carcinogenesis Online). We chose a tumorigenic but poorly metastatic mouse colon cancer cell line MC38 to investigate CRC progression in response to Six1 overexpression (14). A surgical orthotopic mouse model was used to explore the role of Six1 on tumor behavior in vivo by injecting MC38 vector control cells (MC38-Ctrl) and MC38 cells overexpressing Six1 (two independently derived clones, MC38-Six1-1 and MC38-Six1-2) into the cecum of syngeneic C57BL/6 mice. Six1-overexpressing MC38 formed primary tumors in 75% of implanted mice (15 of 20) and the average tumor weight was ~420 mg (Figure 1A and B). In contrast, MC38-Ctrl cells formed tumors in only 25% of the injected mice (5 of 20), and the average tumor weight was ~132 mg. Thus, Six1 overexpression in MC38 significantly increased both tumor incidence and growth.
Figure 1.
Six1 overexpression increases CRC tumor growth and metastasis in vivo. (A) MC38-Six1 or MC38-Ctrl were orthotopically injected into the cecum subserosa of C57BL/6 mice and mice were killed after 6 weeks. Left panels: tumors from MC38-Ctrl. Right panels: tumors from MC38-Six1. (B) The weight of tumors and tumor incidence in mice injected in the cecum with MC38-Ctrl or isolates of MC38-Six1 cells. (C) Upper panel: livers and spleens from MC38-Six1 tumor-bearing mice. Lower panel: livers and spleens from MC38-Ctrl tumor-bearing mice. (D) The weight of livers (left panel) and spleens (right panel) from MC38-Six1 or MC38-Ctrl tumor-bearing mice. Bars indicate SD, and ** and *** indicate P values <0.01 and <0.001, respectively.
The liver is one of the most common sites of metastatic spread of CRC, and autopsies have found that up to 70% of colon cancer patients had liver metastasis at the time of death (15). To determine if Six1 is involved in CRC metastasis, we used a splenic injection model to compare liver metastasis from MC38-Six1 and MC38-Ctrl cells. Mice injected with MC38-Six1 formed larger tumor burden in the spleen and developed more metastases in the liver compared with those injected with MC38-Ctrl (Figure 1C). However, the size of primary tumors in the spleen did not correlate with the extent of metastasis in the liver in either MC38-Ctrl or MC38-Six1 (Figure 1C), suggesting that the increased metastatic burden is not fully due to larger primary tumors. We used liver weight as an indicator of liver metastases and spleen weight as an indicator of the primary tumor size in the spleen. Mice injected with MC38-Six1 had significantly increased spleen and liver weights as compared with those injected with MC38-Ctrl (Figure 1D), suggesting that Six1 overexpression increased primary tumor growth and metastatic spread.
Six1 increases features of CSCs in CRC
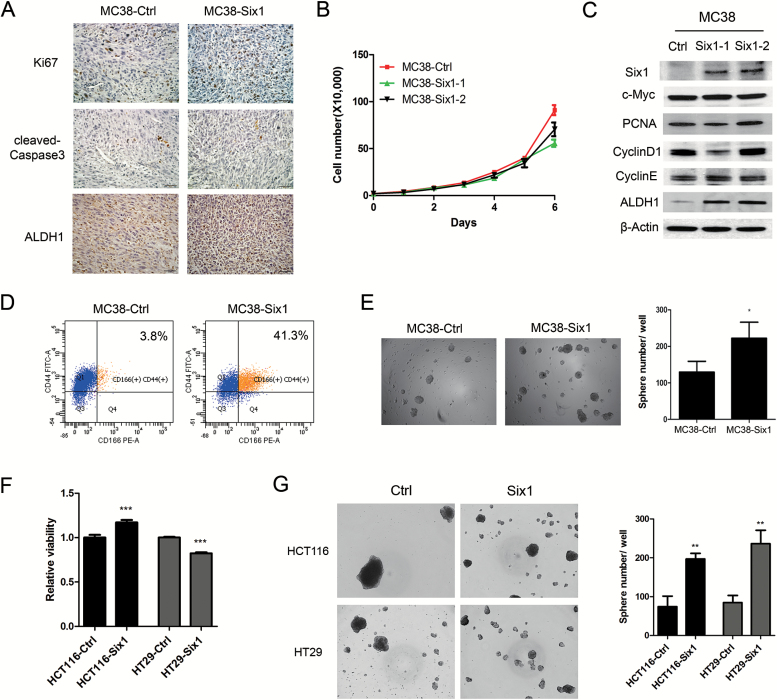
To explore how Six1 overexpression promotes CRC growth and metastatic spread, we analyzed properties of tumors in the cecum arising from either MC38-Ctrl or MC38-Six1. Since the increased tumor size found in MC38-Six1 could be the result of increased proliferation or decreased apoptosis, we used immunohistochemistry to compare the proliferation marker Ki67 and the apoptosis marker cleaved caspase-3 in MC38-Ctrl and MC38-Six1 tumors. MC38-Six1 showed increased levels of Ki67 positive cells but no marked difference in the percentage of cleaved caspase-3 positive cells compared with MC38-Ctrl (Figure 2A), suggesting that the effect of Six1 on tumor growth is associated with increased cell proliferation rather than reduced rates of apoptosis. However, when we compare cell proliferation in vitro, Six1 overexpression slightly suppressed cell growth in MC38 (Figure 2B) rather than increasing cell proliferation as we found in the in vivo cecal studies. Levels of proteins involved in cell proliferation, including c-Myc, PCNA, cyclin D1 and cyclin E, were unchanged in MC38-Six1 compared with MC38-Ctrl (Figure 2C), indicating again that Six1 overexpression did not increase cell proliferation in vitro as it did in vivo. This observation, coupled with our previous finding that Six1 is associated with an increased CSCs population during human papillomavirus (HPV)-16-mediated transformation (9), led us to examine if Six1 could enhance CSC characteristics in MC38. We determined the expression of ALDH1 in MC38-Ctrl and MC38-Six1 in tumors and in lysates from cultured MC38. We found that the protein level of ALDH1 increased dramatically in tumors derived from MC38-Six1 and in cultured MC38-Six1 compared with MC38-Ctrl (Figure 2A and C). Since cell surface antigens CD44+ and CD166+ are CRC CSC markers (16,17), we measured CD44+/CD166+ populations in MC38-Ctrl and MC38-Six1 by flow cytometry. MC38-Six1 had a marked increase in the CD44+/CD166+ population (41.3%) compared with MC38-Ctrl (3.8%) (Figure 2D). We then examined the ability of MC38-Ctrl and MC38-Six1 to form tumorspheres. An increased number of tumorspheres was observed in MC38-Six1 as compared with MC38-Ctrl (Figure 2E). Furthermore, we examined cell proliferation and tumorsphere formation in human CRC cells in response to Six1 overexpression or Six1 knockdown. We overexpressed Six1 in HCT116 and HT29, which have low endogenous expression of Six1 (Supplementary Figure 2, available at Carcinogenesis Online). Six1 overexpression slightly increased cell proliferation in HCT116, but slightly decreased cell proliferation in HT29 (Figure 2F). Similarly, inhibition of Six1 by shRNA in SW480 and SW620 increased cell proliferation, whereas decreased cell proliferation was observed in SW48 and LoVo upon Six1 knockdown (Supplementary Figures 3 and 4, available at Carcinogenesis Online), indicating that the tumor promoting role of Six1 may not directly be associated with cell proliferation in CRC. Consistent with what we observed in MC38, Six1 overexpression in HCT116 and HT29 both significantly increased tumorsphere formation (Figure 2G). Thus, Six1 overexpression increases the CSC-like population in CRC cells.
Figure 2.
Six1 overexpression in MC38 cells increases CSC characteristics. (A) The expression of Ki67, cleaved caspase-3 and ALDH1 in tumors arising from mice injected in the cecum with MC38-Ctrl and MC38-Six1 as detected by immunohistochemistry. (X400) (B) Cell proliferation was determined by directly counting cells at the days indicated in two isolates of MC38-Six1 and MC38-Ctrl. (C) Western blots for Six1, c-Myc, PCNA, cyclin D1, cyclin E and ALDH1 in MC38-Ctrl and two isolates of MC38 overexpressing Six1 (MC38-Six1). β-Actin was used as a loading control. (D) Analysis by flow cytometry of CD44 and CD166 expression in MC38-Ctrl or MC38-Six1. (E) Tumorsphere formation ability in MC38-Ctrl and MC38-Six1. (F) Cell proliferation was measured by WST-1 in HCT116-Ctrl, HCT116-Six1, HT29-Ctrl and HT29-Six1. (G) Tumorsphere formation ability in HCT116-Ctrl, HCT116-Six1, HT29-Ctrl and HT29-Six1. Bars indicate SD and *, ** and *** indicate P values <0.05, <0.01 and <0.001, respectively.
Six1 overexpression stimulates angiogenesis in CRC tumors
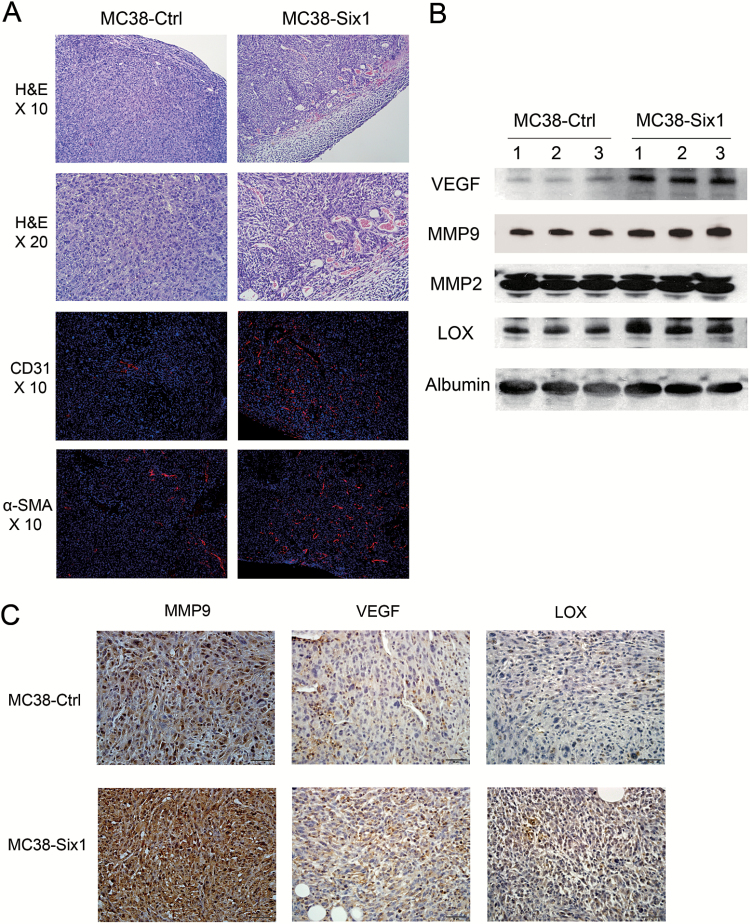
Histopathological analysis showed a hyper-cellular solid carcinoma with high grade atypia and frequent mitosis in both Six1-overexpressing and control tumors (Figure 3A). Interestingly, in MC38-Six1 tumors, there were more vessels, especially blood vessels, located within the tumor mass compared with MC38-Ctrl tumors (Figure 3A). CD31 and α-SMA staining was used to demonstrate the presence of vascular endothelial cells and pericytes in blood vessels. MC38-Six1 tumors had greater numbers of CD31 and α-SMA positive cells compared with tumors from MC38-Ctrl (Figure 3A), indicating that Six1 overexpression promotes tumor angiogenesis. We next analyzed the levels of proteins associated with tumor angiogenesis and metastasis in sera from MC38-Six1 and MC38-Ctrl tumor-bearing mice. Protein levels of VEGF, MMP9 and LOX increased in sera of MC38-Six1 tumor-bearing mice as compared with MC38-Ctrl mice whereas MMP2 levels remained unchanged (Figure 3B). Immunohistochemical analyses showed increased levels of MMP9, VEGF and LOX in MC38-Six1 compared with MC38-Ctrl tumors (Figure 3C) indicating that the increased levels of these proteins we observed in the serum were probably due to the increased expression of these proteins in the MC38-Six1 tumors rather than the larger size of the MC38-Six1 tumors compared with MC38-Ctrl. These data suggest that Six1 overexpression in CRC tumors stimulates angiogenesis and metastasis by increasing the expression of VEGF, LOX and MMP9.
Figure 3.
Six1 overexpression promotes angiogenesis. (A) Upper panels: H&E staining of primary tumors formed in the cecum of mice from MC38-Ctrl and MC38-Six1. Lower panels: immunofluorescent staining of CD31 (red) and α-SMA (red) merged with nuclei (blue) in MC38-Ctrl and MC38-Six1 tumors. (B) Western blots for VEGF, MMP9, MMP2 and LOX in sera from MC38-Six1 tumor-bearing mice and MC38-Ctrl mice. Albumin was used as a loading control. (C) The expression of MMP9, VEGF and LOX in MC38-Six1 and MC38-Ctrl tumors as assessed by immunohistochemistry (×400).
Six1 overexpression promotes CRC cell migration and invasion in vitro
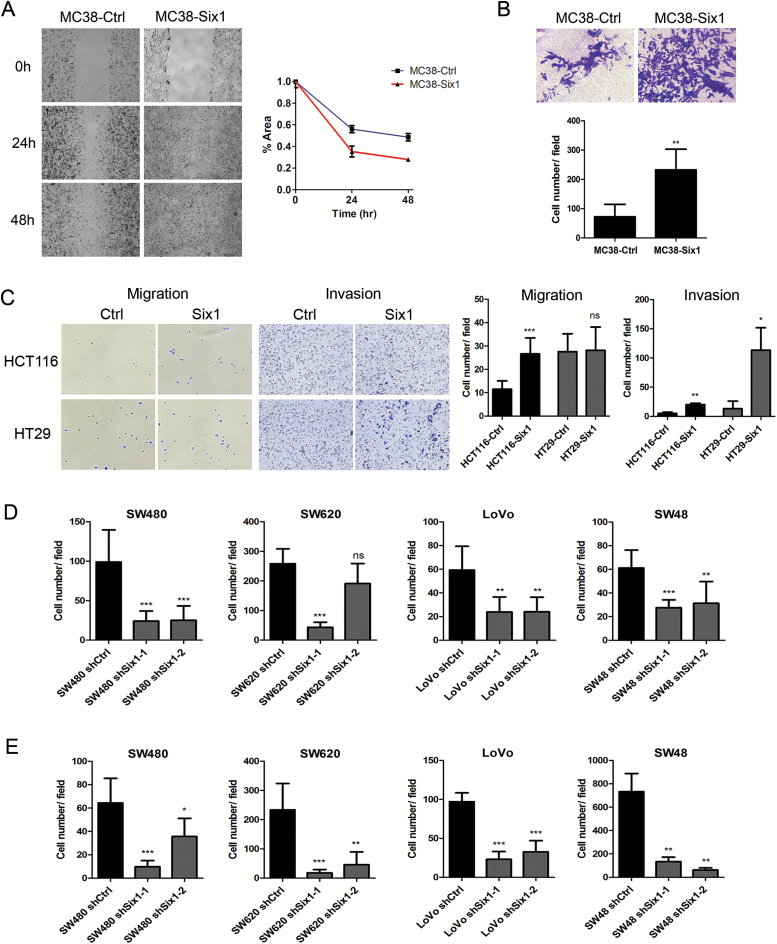
Since Six1 overexpression in MC38 enhanced tumor progression and metastasis in vivo, we sought to identify changes in cell behavior associated with Six1 overexpression. In a wound healing assay, MC38-Six1 exhibited enhanced cell migration compared with MC38-Ctrl (Figure 4A). MC38-Six1 also showed ~3.2-fold increase in cell invasion through Matrigel compared with MC38-Ctrl (Figure 4B). Moreover, the overexpression of Six1 in HCT116 and HT29 also significantly promoted cell invasion (Figure 4C). Also, overexpression of Six1 in HCT116, but not HT29, increased cell migration (Figure 4C). In contrast, the knockdown of Six1 in SW480, SW620, LoVo and SW48 resulted in a decrease in cell migration and cell invasion (Figure 4D and E; Supplementary Figures 5 and 6, available at Carcinogenesis Online). Consistent with our findings in tumor tissue and in MC38-Six1 tumor-bearing mice, MC38-Six1 cells in vitro displayed increased protein levels of MMP9 and VEGF, but not MMP2 compared with MC38-Ctrl (Figure 5A). These results show that Six1 overexpression increases CRC cell migration and invasion in vitro.
Figure 4.
Six1 promotes migration and invasion in CRC cells in vitro. (A) Cell migration was determined in MC38-Ctrl and MC38-Six1 using a wound healing assay. Images are at ×100 magnification. The area of the wound is quantified to determine the extent of wound repair. (B) Cell invasion in MC38-Ctrl and MC38-Six1 was determined using a Matrigel transwell invasion assay. The number of invading cells was quantified after crystal violet staining. Images are shown at ×400 magnification. (C) Cell invasion and migration in HCT116-Ctrl, HCT116-Six1, HT29-Ctrl and HT29-Six1 were determined using a transwell assay with or without Matrigel. Images are shown at ×100 magnification. (D and E) The quantification of numbers of migrating (D) or invading (E) cells after crystal violet staining in non-target shRNA control (shCtrl) and Six1 knockdown (shSix1-1 and shSix1-2) of SW480, SW620, LoVo and SW48. Each column represents the mean of five different fields. Bars indicate SD and *, ** and *** indicate p values < 0.05, 0.01 and 0.001, respectively.
Figure 5.
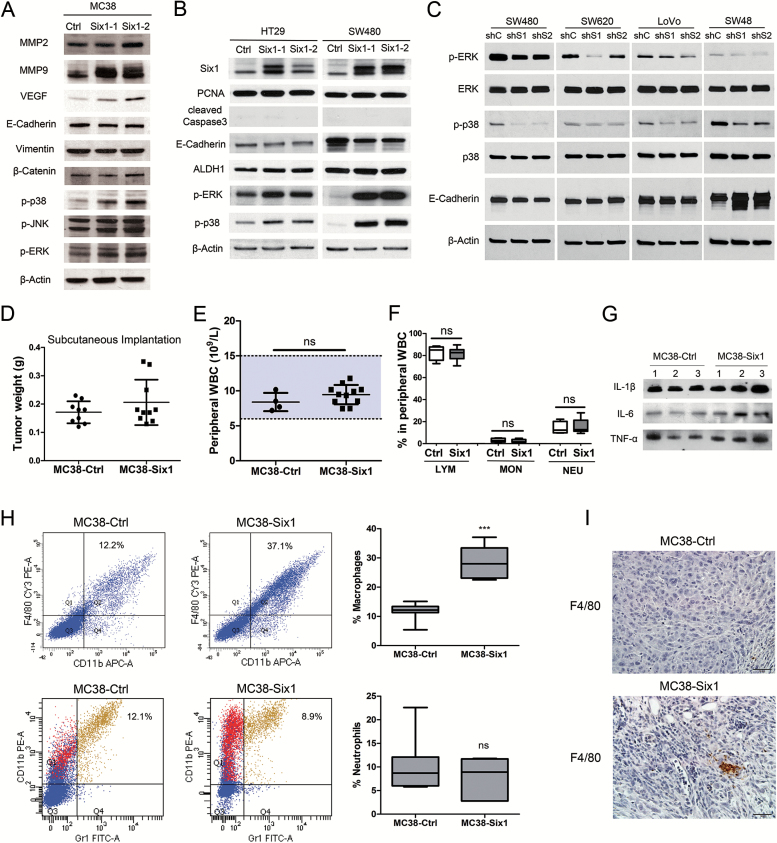
(A–C) Six1 actives MAPK in vitro. (A) Western blots for MMP2, MMP9, VEGF, E-cadherin, vimentin, β-catenin, p-p38, p-JNK and p-ERK in two different isolates of MC38-Six1 and a MC38-Ctrl line. (B) Western blots for Six1, PCNA, cleaved caspase-3, E-cadherin, ALDH1, p-ERK and p-p38 in two different isolates of Six1-overexpressing HT29 (HT29-Six1) and SW480 (SW480-Six1) and corresponding controls (HT29-Ctrl and SW480-Ctrl). (C) Western blots for p-ERK, ERK, p-p38, p38 and E-cadherin in Six1-knockdown SW480 (SW480-shS1 and SW480-shS2), SW620 (SW620-shS1 and SW620-shS2), LoVo (LoVo-shS1 and LoVo-shS2) and SW48 (SW48-shS1 and SW48-shS2) as well as corresponding controls (SW480-shC, SW620-shC, LoVo-shC and SW48-shC). β-Actin was used as a loading control. (D–I) MC38 cells overexpressing Six1 recruit macrophages to tumors. (D) MC38-Six1 or MC38-Ctrl were injected into the flank of C57BL/6 mice and the mice killed 6 weeks later (n = 9–10). The weight of tumors formed from MC38-Ctrl and MC38-Six1 is shown. (E) Peripheral white blood cell (WBC) count from MC38-Ctrl and MC38-Six1 tumor-bearing mice (cecal implantation). (F) Percentage of lymphocytes (LYM), monocytes (MON) and neutrophils (NEU) in peripheral WBC from MC38-Ctrl and MC38-Six1 tumor-bearing mice (cecal implantation). (G) Western blot of IL-1β, IL-6 and TNF-α in sera from MC38-Six1or MC38-Ctrl tumor-bearing mice (cecal implantation). (H) Left panels: Flow cytometry analysis of the expression of CD11b, F4/80 and Gr1 in MC38-Ctrl and MC38-Six1 tumors from the cecum. Right panels: the percentage of total cells positive for either CD11b+/F4/80+ (a marker for macrophages) or CD11b+/Gr1+ (a marker for neutrophils) cells. Results are representative of seven independent experiments. Bars indicate SD and *** indicates P < 0.001. (I) Immunohistochemical analysis of the expression of F4/80 in MC38-Ctrl and MC38-Six1 derived tumors (×400).
Epithelial-mesenchymal transition (EMT) enables epithelial cells to acquire enhanced migratory capacity and invasiveness and is considered a critical step in tumor progression and metastasis (18,19). Six1-induced EMT has been found in various cancers (6,9,20). We compared the expression of EMT-related genes in MC38-Six1 with MC38-Ctrl by western blotting. Surprisingly, we only observed a small decrease in E-cadherin and no marked changes in vimentin or β-catenin levels (Figure 5A); suggesting that EMT is not an important cellular response to Six1 overexpression in MC38. In human CRC cells, Six1 overexpression significantly decreased the expression of E-cadherin in SW480 and only slightly decreased E-cadherin in HT29 (Figure 5B). The inhibition of Six1 by shRNA noticeably increased E-cadherin in SW48, but not in SW480, SW620 and LoVo (Figure 5C). Furthermore, we examined the expression of E-cadherin, vimentin and endogenous Six1 in 12 CRC cell lines. We did not observe a correlation between endogenous Six1 levels and the EMT markers (Supplementary Figure 2, available at Carcinogenesis Online). Taken together, these data indicate that EMT may not be a critical event in response to Six1 overexpression in CRC cells.
Six1 overexpression activates ERK and p38 in mouse and human CRC cell lines
In HPV16-immortalized human keratinocytes, we previously reported that Six1 overexpression leads to activation of mitogen-activated protein kinase (MAPK) signaling, especially phosphorylation of ERK and p38 MAPK (9). Similarly, the phosphorylation of p38 and ERK, but not JNK, increased considerably in MC38-Six1 compared with MC38-Ctrl (Figure 5A).
We also compared some of the molecular changes associated with Six1 overexpression in mouse MC38 to Six1-overexpressing human CRC cell lines HT29 and SW480. Protein level of PCNA remained the same and cleaved caspase-3 was not detectable in Six1-overexpressing and control cells, indicating that Six1 overexpression had no major impact on human CRC cell proliferation and apoptosis in vitro (Figure 5B). ALDH1 protein levels increased in HT29, but not in SW480. Notably, as in MC38, ERK and p38 MAPK phosphorylation was increased in Six1-overexpressing SW480 and HT29 compared with controls (Figure 5B). In contrast, the phosphorylation of ERK and p38 was decreased in SW480-shSix1, SW620-shSix1, LoVo-shSix1 and SW48-shSix1 compared with their corresponding controls (Figure 5C). Thus, Six1 activates the ERK and p38 MAPK pathway in MC38 and human CRC cells.
The overexpression of Six1 in MC38 enhances the recruitment of TAM
In contrast to the results obtained from the orthotopic CRC model, in which MC38-Six1 tumors were significantly larger than MC38-Ctrl tumors in the cecum, in the subcutaneous injection model, Six1-overexpressing MC38 cells formed larger tumors in the flanks of C57BL/6 mice in some animals compared with MC38-Ctrl cells, but the increase in tumor weight was not statistically significant (Figure 5D). This observation suggested that the tumor microenvironment could be critical for Six1-induced tumor progression. The tumor microenvironment, also referred to as the tumor-associated stroma, is composed of non-neoplastic cells such as fibroblasts, infiltrating immune cells, endothelial cells and structural components (21). In CRC, tumor-infiltrating immune cells, cytokines and other immune mediators are involved in most steps of colon tumorigenesis, including initiation, promotion, progression and metastasis (22). We investigated whether Six1 overexpression was associated with systemic inflammation in the orthotopic CRC model, by examining the peripheral white blood cell (WBC) count. The peripheral WBC count from MC38-Six1 and MC38-Ctrl tumor-bearing mice both fall within the normal range and there was no significant difference between them (Figure 5E). Also, the percentage of lymphocytes, monocytes and neutrophils in peripheral WBCs was not significantly different in C57BL/6 mice bearing MC38-Six1 tumors in their cecum compared with control mice (Figure 5F). Furthermore, we examined the protein levels of three proinflammatory cytokines interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α in the sera of these mice and found no consistent differences (Figure 5G).
CRC tumors are infiltrated by various types of immune cells, including T cells, neutrophils, mast cells, natural killer (NK) cells and TAM (22). To identify the altered population of immune cells, we performed flow cytometry analyses to detect major immune cell types, using a combination of immunostaining and found low numbers or small changes in B cells, T cells, mast cells or NK cells (data not shown). A significant increase in the CD11b+/F4/80+ cell population (a marker for macrophages) was observed in MC38-Six1 tumors (37.1%) compared with MC38-Ctrl (12.2%) indicating Six1 overexpression in MC38 increased macrophage infiltration (Figure 5H, upper panel). We also observed a reduction in CD11b+/Gr1+ population (a marker for neutrophils) in some Six1-overexpressing tumors when compared with control tumors, but the reduction was not statistically significant (Figure 5H, lower panel). We could also detect the F4/80 positive cells in tumor samples from cecum by immunohistochemistry. Large clusters of F4/80 positive macrophages were observed in Six1-overexpressing tumors (MC38-Six1) but were infrequently found in control tumors (MC38-Ctrl; Figure 5I). Furthermore, we used immunofluorescence to visualize VEGF and F4/80 positive cells in MC38-Six1 tumor sections. Consistent with the results in Figure 3C, wherein a number of small stromal cells were strongly positive for VEGF, we observed colocalization of VEGF-positive cells and F4/80 positive cells (Supplementary Figure 7, available at Carcinogenesis Online), suggesting that TAM expressed VEGF to further facilitate tumor angiogenesis. These results indicate that Six1 overexpression in MC38 recruits TAM and promotes tumor angiogenesis. The increase in the TAM population in the tumor is most probably due to their recruitment by tumor cells, not due to the systemic inflammation.
Six1 increases the expression of CSF-1 and CCL2/5, to augment the recruitment of TAM
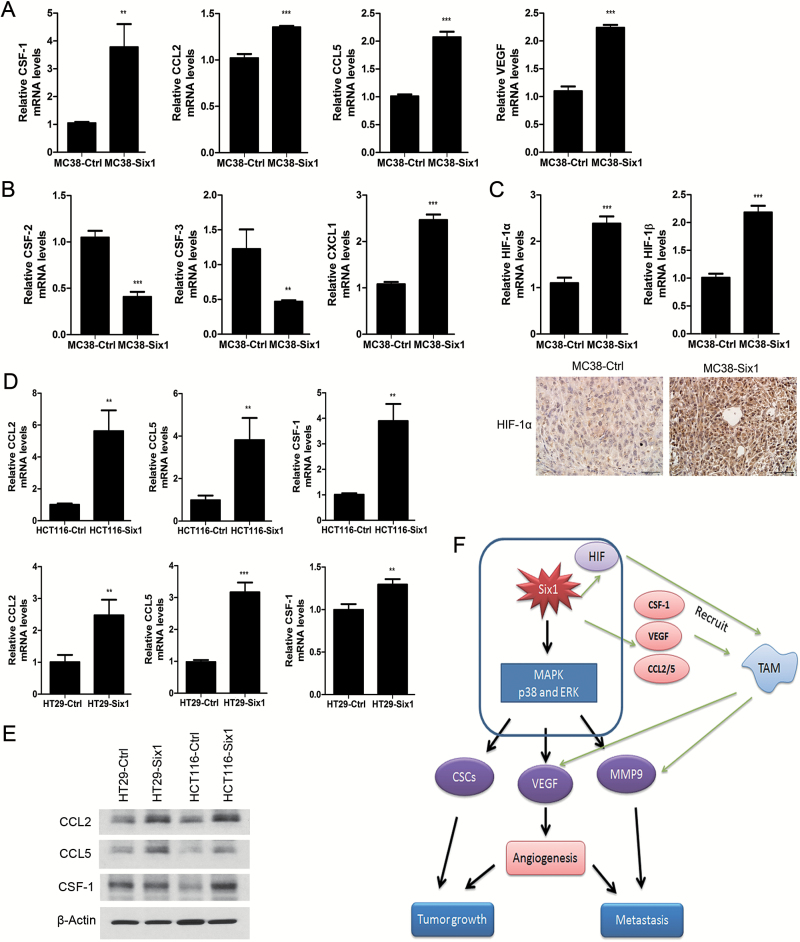
Through the secretion of cytokines, chemokines and growth factors, TAM exert pro-tumor functions by promoting tissue remodeling and angiogenesis rather than cytotoxic activity (23). TAM can be recruited to CRC tumor sites by CCL2 and CCL5, VEGF and CSF-1 (24). We determined that the mRNA expression of CSF-1, CCL2, CCL5 and VEGF was increased in MC38-Six1 compared with MC38-Ctrl (Figure 6A). We also explored mRNA expression of cytokines related to the recruitment of neutrophils to tumors, including colony stimulating factor-2 (CSF-2), CSF-3 and chemokine (C-X-C motif) ligand 1 (CXCL1). We found decreased expression of CSF-2 and CSF-3 in MC38-Six1 and increased expression of CXCL1 (Figure 6B). This may help explain the wide variation in the proportion of neutrophils in tumors in the same group, and our finding that there was no difference in the number of neutrophils in tumors overexpressing Six1 and controls (Figure 5H). Since TAM can be recruited into tumors and accumulate in areas of hypoxia (25), we analyzed the expression of HIF-1α and HIF-1β mRNA in MC38-Ctrl and MC38-Six1. An increase in mRNA for both HIF-1α and HIF-1β was observed in MC38-Six1 (Figure 6C, upper panel). Increased protein level of HIF-1α was observed in MC38-Six1 tumors compared with control tumors (Figure 6C, lower panel). Furthermore, we detected the expression of cytokines related to the recruitment of macrophages in response to Six1 overexpression in human CRC cell lines. We observed increased mRNA (Figure 6D) and protein levels of CCL2 and CCL5 (Figure 6E) in HCT116-Six1 and HT29-Six1 when compared with their corresponding controls. Although CSF-1 mRNA was increased in response to Six1 overexpression in both HCT116 and HT29 (Figure 6D), CSF-1 protein levels were increased in HCT116 but not HT29 (Figure 6E). The knockdown of Six1 in SW480 and SW620 significantly decreased mRNA levels of CCL2 and CCL5 (Supplementary Figure 8, available at Carcinogenesis Online). Taken together, our data suggest that Six1 increases the expression of cytokines that elicit macrophage infiltration into the tumor, which results in CRC tumor growth, progression and metastasis.
Figure 6.
Six1 overexpression in MC38 increases the expression of molecules that induce chemotaxis of TAM. mRNA expression of CSF-1, CCL2, CCL5 and VEGF (A), CSF-2, CSF-3 and CXCL1 (B) and HIF-1α and HIF-1β (C, upper panel) was determined by real-time PCR in MC38-Ctrl and MC38-Six1. Bars indicate SD, and ** and *** indicate P values <0.01 and <0.001, respectively. (C, lower panel) Protein levels of HIF-1α were examined by immunohistochemistry (×400) in MC38-Ctrl and MC38-Six1 derived tumors. (D) mRNA expression of CCL2, CCL5 and CSF-1 in Six1-overexpressing HCT116 (HCT116-Six1) and HT29 (HT29-Six1) and their corresponding controls (HCT116-Ctrl and HT29-Ctrl). (E) Protein levels by Western blot of CCL2, CCL5 and CSF-1 in HT29-Ctrl, HT29-Six1, HCT116-Ctrl and HCT116-Six1. β-Actin was used as a loading control (F) Model illustrating how Six1 overexpression promotes CRC tumor growth and metastasis.
Discussion
Overexpression of Six1 is associated with decreased survival in advanced-stage CRC (6,8). We found Six1 overexpression in the intestines from C57BL/6-ApcMin/+ mice compared with wild type C57BL/6 mice, and much higher levels in tumors from C57BL/6-ApcMin/+ mice. Six1 overexpression in the mouse colon cancer cell line MC38 enhanced their tumorigenic and metastatic potential in vivo. Six1 increased the population of CSCs and stimulated angiogenesis. Moreover, Six1 overexpression induced macrophage chemotaxis, most probably by increasing the expression of CSF-1, CCL2/5 and VEGF, thereby further facilitating CRC tumor growth and metastasis.
Stromal cells, an important component of the tumor microenvironment, are recruited by molecular signals from cancer cells and cooperate with cancer cells to support tumor progression, by enhancing its growth and enabling its metastatic spread (21). Tumor cells produce VEGF, a potent angiogenic factor, to induce endothelial cell proliferation and migration to promote angiogenesis. These new blood vessels help to satisfy the oxygen and other metabolic needs of the tumor, provide an escape route for cancer cells and facilitate tumor progression and metastasis (26,27). We explored the role of Six1 overexpression in CRC progression using an orthotopic CRC mouse model by implanting tumor cells in the cecum of immunocompetent mice. We found that Six1 overexpression not only affects tumor cell malignancy by increasing CSC-like characteristics, but also promotes remodeling of the tumor stroma by stimulating angiogenesis and recruiting TAM.
Metastasis, primarily to the liver, is the leading cause of death for CRC patients (15,28,29).. We used a splenic injection model to facilitate investigation of the role of Six1 in CRC metastasis. The model mimics spontaneous liver metastasis by recapitulating early dissemination and later extravasation and colonization of the liver (30). We found increased liver metastasis in mice bearing tumors from Six1-overexpressing cells compared with mice bearing Six1 control cells, suggesting that Six1 facilitates CRC metastasis.
EMT is a critical step in tumor metastasis (18) and loss of E-cadherin is a marker of EMT (31). We found that Six1-induced EMT during HPV16-mediated transformation (9). Here we observed a significant decrease in E-cadherin in SW480 overexpressing Six1; but only a slight decrease in E-cadherin was found in MC38 and HT29 overexpressing Six1. Similarly, SW48 showed increased E-cadherin after the knockdown of Six1, whereas three other human CRC cell lines SW480, SW620 and LoVo showed no increase in E-cadherin in response to Six1 knockdown. These observations suggest that EMT may be necessary for increased CRC cell migration and invasion associated with Six1 overexpression in some cases; however, the extent of the mesenchymal phenotype varies among different CRC cell lines. We found increased expression of VEGF and MMP9 in response to Six1 overexpression, which is expected, to promote tumor angiogenesis and metastasis (32–35), and may provide the molecular basis by which Six1 promotes CRC progression.
Clinical and experimental evidence have underscored the role of TAM in promoting tumorigenesis (23). During the early stages of tumor development, macrophages promote a proinflammatory environment in the tumor; but during later stages, macrophages stimulate angiogenesis, enhance tumor cell migration and invasion and regulate anti-tumor immunity. For example, overexpression of CSF-1, the major regulator of macrophages, is associated with poor prognosis in various cancers, including CRC (36). Macrophages accumulate in hypoxic areas of the tumor and constitutively express HIF-1α, which regulates the transcription of genes associated with angiogenesis, including VEGF. Tumor cells stimulate the production of MMP9 by macrophages, which disrupts extracellular matrix and promotes cell invasion (23). We found that overexpression of Six1 in CRC grown as orthotopic tumors induce TAM infiltration by enhancing the expression of CSF-1, CCL2/5 and VEGF. The recruited TAM reciprocally secrete molecules that are beneficial for the tumors, such as VEGF and MMP9, and further promote tumor angiogenesis and metastasis.
Enhanced MAPK signaling is common in tumors, and the link between MAPK signaling and cancer cell survival, angiogenesis and metastasis is well established (37,38). Studies using human CRC samples found a significant correlation between VEGF expression and ERK activation (39). The p38 MAPK pathway regulates the expression of MMP9, which is critical for the migration and invasion of various cancers, including CRC (40–42). Activation of p38 is essential for the expression of HIF-1α and VEGF, which promote tumor angiogenesis (43,44). In addition, activation of p38 signaling results in an increase in the CSC population found under hypoxic conditions (45). Similarly, the ERK-dependent signaling pathway regulates MMP9 mediated cell invasion (46). We previously reported that Six1 overexpression results in resistance to differentiation, promotes EMT and induces features of CSC in HPV16-immortalized HKc by activating MAPK signaling (9,47). Others found that Six1 significantly correlates with phosphorylated ERK in human breast cancers, and its overexpression increases the number of tumor initiating cells by activating ERK (48). We demonstrate that overexpression of Six1 leads to ERK and p38 activation in both mouse and human CRC cells, whereas the knockdown of Six1 results in decreased phosphorylation of ERK and p38. We propose that MAPK activation may be a common mechanism leading to tumor progression in Six1-overexpressing tumors; thus, the MAPK pathway could provide potential therapeutic targets.
Supplementary material
Supplementary Figures 1–8 and Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
This work was supported in part by the National Institute on Minority Health and Health Disparities (5P20MD001770 to K.C.); and the National Institute of Health (5R01CA154731-A101 to M.P., 5P30GM103336 to the Center for Colon Cancer Research).
Supplementary Material
Acknowledgements
We would like to thank Dr Chang-uk Lim for technical support of flow cytometry.
Conflict of Interest Statement: None declared.
Abbreviations
- ALDH1
aldehyde dehydrogenase-1
- ANOVA
analysis of variance
- α-SMA
alpha-smooth muscle actin
- CRC
colorectal cancer
- HPV
human papillomavirus
- LOX
lysyl oxidase
- MAPK
mitogen-activated protein kinase
- MMP9
matrix metalloproteinases 9
- PBS
phosphate buffered saline
- PCNA
proliferating cell nuclear antigen
- TAM
tumor-associated macrophages
- VEGF
vascular endothelial growth factor
References
- 1. Siegel R., et al. (2014) Colorectal cancer statistics, 2014. CA Cancer J. Clin., 64, 104–117. [DOI] [PubMed] [Google Scholar]
- 2. Zheng W., et al. (2003) The role of Six1 in mammalian auditory system development. Development, 130, 3989–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ikeda K., et al. (2010) Six1 is indispensable for production of functional progenitor cells during olfactory epithelial development. Int. J. Dev. Biol., 54, 1453–1464. [DOI] [PubMed] [Google Scholar]
- 4. Xu P.X., et al. (2003) Six1 is required for the early organogenesis of mammalian kidney. Development, 130, 3085–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Christensen K.L., et al. (2008) The six family of homeobox genes in development and cancer. Adv. Cancer Res., 101, 93–126. [DOI] [PubMed] [Google Scholar]
- 6. Ono H., et al. (2012) SIX1 promotes epithelial-mesenchymal transition in colorectal cancer through ZEB1 activation. Oncogene, 31, 4923–4934. [DOI] [PubMed] [Google Scholar]
- 7. Kahlert C., et al. (2015) Overexpression of SIX1 is an independent prognostic marker in stage I-III colorectal cancer. Int. J. Cancer, 137, 2104–2113. [DOI] [PubMed] [Google Scholar]
- 8. Li Z., et al. (2014) Targeting Six1 by lentivirus-mediated RNA interference inhibits colorectal cancer cell growth and invasion. Int. J. Clin. Exp. Pathol., 7, 631–639. [PMC free article] [PubMed] [Google Scholar]
- 9. Xu H., et al. (2014) Six1 promotes epithelial-mesenchymal transition and malignant conversion in human papillomavirus type 16-immortalized human keratinocytes. Carcinogenesis, 35, 1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y., et al. (2013) Development and characterization of a reliable mouse model of colorectal cancer metastasis to the liver. Clin. Exp. Metastasis, 30, 903–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brand M.I., et al. (1996) Development of a reliable colorectal cancer liver metastasis model. J. Surg. Res., 63, 425–432. [DOI] [PubMed] [Google Scholar]
- 12. Bouvet M., et al. (2006) In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res., 66, 11293–11297. [DOI] [PubMed] [Google Scholar]
- 13. Giavazzi R., et al. (1986) Metastatic behavior of tumor cells isolated from primary and metastatic human colorectal carcinomas implanted into different sites in nude mice. Cancer Res., 46(4 Pt 2), 1928–1933. [PubMed] [Google Scholar]
- 14. Smith J.J., et al. (2010) Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer. Gastroenterology, 138, 958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schima W., et al. (2005) Liver metastases of colorectal cancer: US, CT or MR? Cancer Imaging, 5(Spec No A), S149–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dalerba P., et al. (2007) Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl Acad. Sci. USA, 104, 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levin T.G., et al. (2010) Characterization odf the intestinal cancer stem cell marker CD166 in the human and mouse gastrointestinal tract. Gastroenterology, 139, 2072–2082.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christiansen J.J., et al. (2006) Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res., 66, 8319–8326. [DOI] [PubMed] [Google Scholar]
- 19. Mani S.A., et al. (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Micalizzi D.S., et al. (2009) The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. J. Clin. Invest., 119, 2678–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanahan D., et al. (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21, 309–322. [DOI] [PubMed] [Google Scholar]
- 22. Terzić J., et al. (2010) Inflammation and colon cancer. Gastroenterology, 138, 2101–2114.e5. [DOI] [PubMed] [Google Scholar]
- 23. Qian B.Z., et al. (2010) Macrophage diversity enhances tumor progression and metastasis. Cell, 141, 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Erreni M., et al. (2011) Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron., 4, 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lewis C., et al. (2005) Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am. J. Pathol., 167, 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu L., et al. (2006) Placenta growth factor overexpression inhibits tumor growth, angiogenesis, and metastasis by depleting vascular endothelial growth factor homodimers in orthotopic mouse models. Cancer Res., 66, 3971–3977. [DOI] [PubMed] [Google Scholar]
- 27. Woodhouse E.C., et al. (1997) General mechanisms of metastasis. Cancer, 80(suppl. 8), 1529–1537. [DOI] [PubMed] [Google Scholar]
- 28. Steeg P.S. (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat. Med., 12, 895–904. [DOI] [PubMed] [Google Scholar]
- 29. Society A.C. (2013) Cancer Facts and Figures 2013. American Cancer Society, Atlanta, GA. [Google Scholar]
- 30. Lavilla-Alonso S., et al. (2011) Optimized mouse model for the imaging of tumor metastasis upon experimental therapy. PLoS One, 6, e26810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perl A.K., et al. (1998) A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature, 392, 190–193. [DOI] [PubMed] [Google Scholar]
- 32. Egeblad M., et al. (2002) New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer, 2, 161–174. [DOI] [PubMed] [Google Scholar]
- 33. López-Otín C., et al. (2007) Emerging roles of proteases in tumour suppression. Nat. Rev. Cancer, 7, 800–808. [DOI] [PubMed] [Google Scholar]
- 34. Martin M.D., et al. (2007) The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev., 26, 717–724. [DOI] [PubMed] [Google Scholar]
- 35. Hanrahan V., et al. (2003) The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J. Pathol., 200, 183–194. [DOI] [PubMed] [Google Scholar]
- 36. Mroczko B., et al. (2007) Serum macrophage-colony stimulating factor levels in colorectal cancer patients correlate with lymph node metastasis and poor prognosis. Clin. Chim. Acta., 380, 208–212. [DOI] [PubMed] [Google Scholar]
- 37. Mansour S.J., et al. (1994) Transformation of mammalian cells by constitutively active MAP kinase kinase. Science, 265, 966–970. [DOI] [PubMed] [Google Scholar]
- 38. Fang J.Y., et al. (2005) The MAPK signalling pathways and colorectal cancer. Lancet. Oncol., 6, 322–327. [DOI] [PubMed] [Google Scholar]
- 39. Cassano A., et al. (2002) Expression of vascular endothelial growth factor, mitogen-activated protein kinase and p53 in human colorectal cancer. Anticancer Res., 22, 2179–2184. [PubMed] [Google Scholar]
- 40. Simon C., et al. (2001) The p38 SAPK pathway regulates the expression of the MMP-9 collagenase via AP-1-dependent promoter activation. Exp. Cell Res., 271, 344–355. [DOI] [PubMed] [Google Scholar]
- 41. Ringshausen I., et al. (2004) Constitutive activation of the MAPkinase p38 is critical for MMP-9 production and survival of B-CLL cells on bone marrow stromal cells. Leukemia, 18, 1964–1970. [DOI] [PubMed] [Google Scholar]
- 42. Wei S.C., et al. (2013) Flt-1 in colorectal cancer cells is required for the tumor invasive effect of placental growth factor through a p38-MMP9 pathway. J. Biomed. Sci., 20, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshino Y., et al. (2006) Activation of p38 MAPK and/or JNK contributes to increased levels of VEGF secretion in human malignant glioma cells. Int. J. Oncol., 29, 981–987. [PubMed] [Google Scholar]
- 44. Duyndam M.C., et al. (2003) Evidence for a role of p38 kinase in hypoxia-inducible factor 1-independent induction of vascular endothelial growth factor expression by sodium arsenite. J. Biol. Chem., 278, 6885–6895. [DOI] [PubMed] [Google Scholar]
- 45. Lin S.P., et al. (2012) Survival of cancer stem cells under hypoxia and serum depletion via decrease in PP2A activity and activation of p38-MAPKAPK2-Hsp27. PLoS One, 7, e49605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lakka S.S., et al. (2002) Downregulation of MMP-9 in ERK-mutated stable transfectants inhibits glioma invasion in vitro. Oncogene, 21, 5601–5608. [DOI] [PubMed] [Google Scholar]
- 47. Xu H., et al. (2015) Six1 overexpression at early stages of HPV16-mediated transformation of human keratinocytes promotes differentiation resistance and EMT. Virology, 474, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iwanaga R., et al. (2012) Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways. Breast Cancer Res., 14, R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.