Abstract
Introduction
The escalation in the prevalence of obesity throughout the world has led to an upsurge in the number of obese surgical patients to whom perioperative care needs to be delivered.
Sources of data
After determining the scope of the review, the authors used PubMed with select phrases encompassing the words in the scope. Both preclinical and clinical reports were considered.
Areas of agreement
There were no controversies regarding preoperative management and the intraoperative care of the obese surgical patient.
Areas of controversy
Is there a healthy obese state that gives rise to the obesity paradox regarding postoperative complications?
Growing points
This review considers how to prepare for and manage the obese surgical patient through the entire spectrum, from preoperative assessment to possible postoperative intensive care.
Areas timely for developing research
What results in an obese patient developing ‘unhealthy’ obesity?
Keywords: obesity, surgery, intensive care
Introduction
Over the 150-year period between 1850 and 2000, life expectancy in the US more than doubled;1 however, with the start of obesity epidemic, beginning in the late 1970s,2 the rate of increase in life expectancy has plateaued and in 2015 it began to decline. This decline is due to a significant increase in deaths related to the obesity complications of cardiac disease, diabetes, chronic liver disease, and stroke.3 These, as well as other obesity-related diseases, such as musculoskeletal disorders, and several cancers (endometrial, breast, ovarian, prostate, liver, gall bladder, kidney and colon) frequently require surgical interventions and therefore the need for perioperative and critical care of the obese surgical patient is set to increase remarkably. These concerning demographic trends are likely to be replicated throughout the developed world2 and provide a good reason for a topical review on the clinical management of the obese surgical patient.
Though obesity is associated with greater morbidity than smoking, alcoholism and poverty and will overtake cigarette smoking as the leading cause of preventable deaths,4 there exists an ‘obesity paradox’ in which obesity is somehow protective against mortality that was reported in retrospective analyses of several chronic wasting diseases. The obesity paradox was first identified in the setting of systolic heart failure5 and has now been found in chronic renal failure,6 chronic obstructive pulmonary disease7 and cancer.8 Recently, the obesity paradox has been challenged as an artifact arising from the ‘single snapshot’ body mass index (BMI) measurement that does not longitudinally represent the subject’s long-term adiposity when contemporaneously measured.9 However, when obesity-induced diabetes supervenes the paradoxical survival benefit is eliminated.10 Furthermore a recent study involving more than 0.5 million subjects reported that even in the absence of metabolic derangements, obese patients are more likely to develop cardiovascular complications.11
Definition and diagnostic criteria
Obesity can be established according to either anthropometric or body composition diagnostic criteria.
The BMI, defined as weight in kilograms divided by the height in meters, squared, provides a value in units of kg/m2, although these units are often omitted. According to the NIH,12 overweight encompasses subjects between 25.0 and 29.9 while Obesity (I) is defined as 30.0–34.9 and Obesity (II) as 35–39.9. Patients with a BMI ≥ 40 are referred to as having extreme or morbid obesity (III) (Table 1).13 The weakness of the BMI is that it cannot discriminate between adipose and non-adipose tissue in the individual although it does correlate with total body adiposity at the population level.14
Table 1.
Categories of BMI
| Underweight | 15–19.9 |
| Normal weight | 20–24.9 |
| Pre-obesity | 25–29.9 |
| Class I obesity | 30–34.9 |
| Class II obesity | 35–39.9 |
| Class III obesity | ≥40 |
Reprinted with permission from Nuttall FQ. Body Mass Index: obesity, BMI, and health: a critical review. Nutr Today. 2015 May;50(3):117–128.13
Waist circumference (WC) is used to identify the relative risk of developing obesity-associated comorbidities in subjects with a BMI between 25 and 3515 because it correlates with the presence of visceral adipose tissue that is the key abnormality that results in cardiovascular disease.16
The waist to hip ratio (WHR) is used to reduce the sex dimorphism of adipose tissue distribution (males in the abdominal cavity and females in the legs) with lower limit of normal being 0.85 for women and 1.00 for men. While the WHR does reflect relative adipose tissue distribution,17 its prognostic value for obesity-associated morbidity is not high because loss of weight may change both the waist and hip circumference proportionally resulting in no change in the ratio.
Because various ethnic groups have different relationship between the BMI and body fat percentage,18 there can be no universal threshold BMI values for the diagnoses of overweight/obesity/morbid obesity. Using Caucasians as the standard, African-Americans have a 1.3 kg/m2 higher BMI and Indonesian and Thai people have a 3 kg/m2 lower BMI at the same level of body fat.19 In Caucasian populations, obesity is defined as a body fat compartment of 25% in adult males and 35% for adult females. Densitometry, using water displacement, underwater weighing or air displacement, is a laboratory-based technique for deducing the fat mass (0.9 kg/L) from the fat-free mass (1.1 kg/L) based upon the differences in density in these two compartments. Dual energy X-ray absorptiometry (DXA) scans can distinguish mineral from soft tissue, and within soft tissue, of lean mass from fat mass based upon their different energy attenuation coefficients. Other scanning techniques including computed tomography (CT) and magnetic resonance imaging (MRI) identify adipose tissue directly and then assume that 80% of adipose tissues is made up of fat. Because of the radiation and costs of whole body scans, these techniques are usually only employed for assessment of the distribution of fat. Outside the laboratory setting, skinfold thickness, usually from four different sites (biceps, triceps, subscapular and supra-iliac), are used for estimating total body fat because the relationship between subcutaneous fat and total fat is relatively constant. Bioelectrical impedance may also be used for assessing total body fat.
Prevalence
In the United States, the National Health and Nutritional Examination Survey (NHANES) reports annually on the prevalence of obesity using the BMI. Between 1960 and 1980 the overall prevalence of obesity (BMI ≥ 30) was fairly constant at approximately 14% for men and women aged 20–74 years.20 By 1994, the prevalence of obesity had increased to 21%. In the most recent analysis of NHANES data for 2014, the prevalence of age-adjusted obesity amongst US women was 40.4%, which represents a significant increase from the data surveyed a decade earlier when controlled for age, race, smoking status, and educational attainment.21 For men the prevalence was 35% with no significant trend from the prior decade. The prevalence of morbid obesity (BMI ≥ 40), had reached 10% in women by 2014, again a significant increase from the previous decade, while in men it was relatively constant at 5.5%.21
In 2- to 19-year-olds in the US, the prevalence of obesity was 17.0% and morbid obesity was 5.8%.22 Regarding trends, the rates have decreased in children aged 2–5 years since 2003–04, stabilized in 6- to 11-year-olds since 2007–08, but steadily increased among adolescents since 1988.23
In the EU, approximately 40–50% of men and 25–35% of women are overweight and 15–25% of men and women are obese.24
According to the WHO, 41 million children >5 years had a BMI > 25%.25 Worldwide, the prevalence of obesity in adults will reach 18% in men and surpass 21% in women; severe obesity will surpass 6% in men and 9% in women.26
Causes of obesity
Weight gain can be explained as an energy imbalance between calories consumed and calories expended. A coordinated network of central mechanisms, that include the hypothalamic arcuate nucleus, and peripheral signals, including from the microbiome and cells within adipose tissue, stomach, and pancreas, control short-term and long-term energy balance.27 Emphasis on exercise, better dietary choices and nutritional content labeling of foods have been advocated as methods for reversing the energy imbalance.22 Yet, these strategies have not resulted in a reversal of the trend in the obesity epidemic possibly because of lack of compliance or because of the advent of counter-regulatory central orexigenic signals that increase appetite and food intake thereby limiting the degree of predicted weight loss that is associated with interventions such as exercise programs.28 Alternately, some have advocated that reliance exclusively on only diet (energy intake) and exercise (energy expenditure) ignores many other factors including the contributing role of sleep disturbance.29
Occupational factors may also contribute to the obesity epidemic as the daily occupation-related energy expenditure has declined by >100 calories over the past 50 years.30 Environmental influences for weight gain are evidenced by the inverse relationship between obesity and socioeconomic class as well as the trend toward increasing obesity in developing countries associated with urbanization.31 Furthermore, there has been a displacement of leisure-time physical activities with sedentary activities dominated by the use of electronic devices.30 Another factor is thought to be the plethora of drugs that have, as a side-effect, weight gain.32
Genetics may also be a possible cause for the dysregulation between caloric intake and expenditure.27 A combination of family, twin and adoption studies have demonstrated that the heritability of BMI is between 0.71 and 0.8633 although the specific genes have not been identified. An overarching concept of genetic influence suggests that the easy availability of food has rendered superfluous ‘thrifty genes’, that favor survival during periods of famine; this theory has been challenged.34 There are 11 rare monogenic obesity diseases that are caused by mutations in the leptin/melanocortin-4 receptor pathways that can produce severe obesity especially in children.35 Recently, epigenetic factors have also been mooted as a cause of the energy imbalance that results in obesity.36
Sequelae of obesity
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is characterized by repetitive partial or complete airway collapse during sleep causing hypoxemia and/or hypercarbia. Standard diagnosis usually requires overnight polysomnography and is defined as cessation of airflow of greater than 10 s with continued ventilatory effort, five or more times per hour of sleep, with a decrease in arterial oxygen saturation.37 Associated symptoms include snoring, daytime somnolence, sleep disruption, altered cardiovascular function, systemic and pulmonary hypertension, cardiac arrhythmias, myocardial ischemia, ventricular hypertrophy and failure.38–40 The prevalence of OSA is approximately 20% in the general population.41 Based on two large population-based studies, the Sleep Heart Health Study and the Wisconsin Sleep Cohort, up to 80% of individuals with less severe forms of OSA are undiagnosed42 while severe OSA is undiagnosed in approximately 10–20% of patients with BMI > 35.43 Undiagnosed and hence unsuspected OSA may lead to perioperative complications including difficult mask ventilation and/or intubation, postoperative reintubation, cardiac dysrhythmias and increased hospital length of stay.44–46
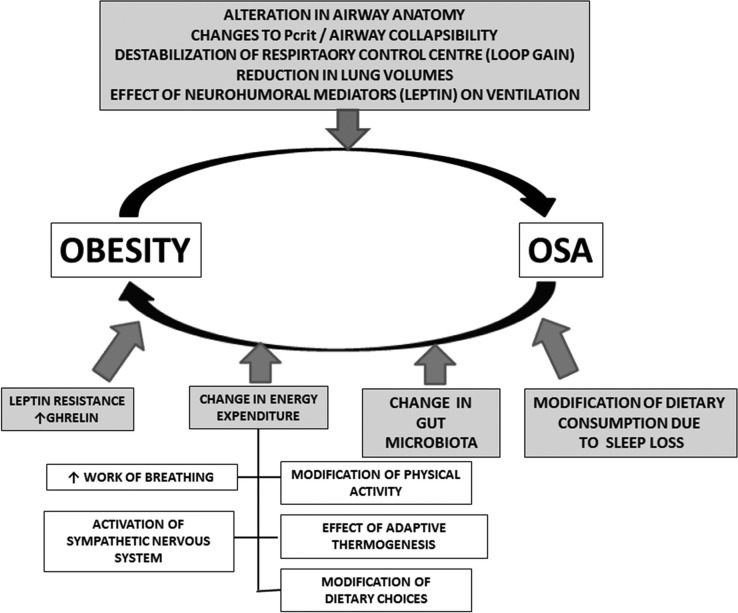
Among the risk factors for the development of OSA, obesity has the most influence especially in those <50 years and this is bidirectional (Fig. 1).47 Studies on the relationship between weight change and progression and regression of OSA have found a strong correlation.48
Fig. 1.
This figure illustrates the bidirectional role of obesity and OSA. Note particularly the impact of OSA upon the changes in gut microbiome as a consequence of chronic intermittent hypoxemia.47 Reprinted with permission from Joosten SA, et al. Impact of weight loss management in OSA. Chest 2017 [Epub ahead of print]. doi:10.1016/j.chest.2017.01.027.
The treatment of choice for OSA is the use of devices that can maintain continuous positive airway pressure (CPAP) to ensure airway patency. Ill-fitting masks and discomfort from the devices cause a significant degree of non-adherence,49 defined by the CMS as use the CPAP device for <4 h per night for <70% of the nights in a consecutive 30-day period. In the search for higher patient compliance advances in design have resulted in newer masks such as the Bilevel positive airway pressure (BiPAP) devices which allow for distinct inspiratory and expiratory pressure settings.50
Metabolic syndrome
A variety of criteria have been used to define the metabolic syndrome in the literature. The influential National Cholesterol Education Program (NCEP) defines metabolic syndrome as occurring when three or more of the following are present: (i) abdominal obesity (waist circumference, >102 cm in men or >88 cm in women); (ii) glucose intolerance (fasting glucose level, ≥100 mg/dL); (iii) hypertension, ≥130 mm Hg systolic and/or ≥85 mm Hg diastolic; and (iv) hypertriglyceridemia, ≥150 mg/dL or high-density lipoprotein cholesterol <40 mg/dL in men or <50 mg/dL in women.51
In 2009, several major organizations, including the International Diabetes Federation and the American Heart Association (AHA), required at least three of five criteria with hypertriglyceridemia now split into two separate elements and with the waist circumference cutoff being defined not only by sex but by ethnicity, and region (Table 2).52
Table 2.
Criteria for clinical diagnosis of the metabolic syndrome
| Measure | Categorical cut points |
|---|---|
| Elevated waist circumference* | Population- and country-specific definitions |
| Elevated triglycerides (drug treatment for elevated triglycerides is an alternate indicator†) | ≥150 mg/dL (1.7 mmol/L) |
| Reduced HDL-C (drug treatment for reduced HDL-C is an alternate indicator†) | <40 mg/dL (1.0 mmol/L) in males; <50 mg/dL (1.3 mmol/L) in females |
| Elevated blood pressure (antihypertensive drug treatment in a patient with a history of hypertension is an alternate indicator) | Systolic ≥130 and/or diastolic ≥85 mm Hg |
| Elevated fasting glucose‡ (drug treatment of elevated glucose is an alternate indicator) | ≥100 mg/dL |
HDL-C indicates high-density lipoprotein cholesterol.
*It is recommended that the IDF cut points be used for non-Europeans and either the IDF or AHA/NHLBI cut points used for people of European origin until more data are available.
†The most commonly used drugs for elevated triglycerides and reduced HDL-C are fibrates and nicotinic acid. A patient taking 1 of these drugs can be presumed to have high triglycerides and low HDL-C. High-dose ω-3 fatty acids presumes high triglycerides.
‡Most patients with Type 2 diabetes mellitus will have the metabolic syndrome by the proposed criteria.
Reprinted with permission from Alberti KG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009 Oct 20;120(16):1640–45.52
The prevalence of metabolic syndrome in US adults, using a revised AHA/NCEP definition, has increased from 28% in the period between 1988 and 1994 to 34% in the period between 1999 and 2006.53 Using NCEP criteria the prevalence of metabolic syndrome in bariatric surgical patients was 80%.54
While several studies had shown that obesity is not associated in increased perioperative complications, morbidity or mortality (‘Obesity Paradox’),55–59 obese non-cardiac surgical patients with ‘modified’ metabolic syndrome (presence of hypertension and diabetes) were at increased risk for mortality, cardiac adverse events, pulmonary complications, acute kidney injury, stroke, wound complications and postoperative sepsis.60 Using the data from the National Inpatient Sample from the Hospital Cost and Utilization Project, posterior lumbar spine fusion patients with metabolic syndrome (presence of three out of the four conditions: obesity, hypertension, diabetes mellitus and dyslipidemia) had significantly increased rates of length of hospital stay and cardiac complications compared to patient without metabolic syndrome.61 Using the National Surgical Quality Improvement Program (NSQIP) database, hepatic resection patients with metabolic syndrome (BMI ≥ 30 kg/m2 in the setting of concomitant diabetes and hypertension) had a greater risk for reintubation, >48 h of ventilator dependence, myocardial infarction and superficial surgical-site infections compared with patient without metabolic syndrome.62
As both acute illness and the perioperative period are associated with insulin resistance, surgical patients with metabolic syndrome are likely to develop hyperglycemia which increases the risk for postoperative complications including surgical-site infection.63 Thus far, there is little consensus regarding the blood glucose threshold for initiating therapy, the targeted blood glucose level, which therapies to use and for how long these should be maintained.64
Preoperative interventions
The importance of a preoperative assessment and management of the obese surgical patient population is highlighted by the associated increased risk of morbidity and mortality secondary to associated comorbidities including hypertension, diabetes, dyslipidemia and cardiovascular disease.60
Obstructive sleep apnea
The Snoring, Tiredness, Observed apnea, high blood Pressure (STOP)-Body mass index, Age, Neck Circumference and Gender (Bang) screening tool has been extensively validated in surgical patients.65–67 In the obese, a STOP-BANG score >3 has a sensitivity of >90% for detecting OSA with a positive predictive value of 85%. A score of >5 has the sensitivity of 53%, specificity of 70% of predicting moderate to severe OSA.68
There is a correlation between OSA severity and each of advanced age, cardiovascular disease and left ventricular dysfunction.43 The American Society of Anesthesiologists Task Force on Perioperative Management of Patient with OSA recommend that patients with confirmed or suspected OSA undergo preoperative assessment prior to the day of surgery with sufficient time to allow preparation and execution of a perioperative management plan.69 The preoperative assessment should include a comprehensive review of medical records including sleep studies, patient interview and physical exam. A focused interview in the setting of suspected OSA may include questions on snoring, apneic episodes, frequent arousals during sleep, morning headaches and daytime somnolence. The physical exam should include the airway, nasopharyngeal anatomy, neck circumference and tongue volume.
As the diagnosis of OSA is associated with a significantly increased incidence of postoperative complications including respiratory failure, postoperative cardiac events and unplanned intensive care admission, the preoperative preparation for patients with confirmed OSA may include CPAP or BiPAP, preoperative oral appliances70 and preoperative weight loss. Preoperative initiation and perioperative use of CPAP or BiPAP can reduce postoperative complications such as hypercarbia, hypoxemia and pulmonary artery vasoconstriction.71,72 Preoperative initiation of CPAP is recommended particularly if OSA is severe. There is insufficient evidence to support canceling or delaying surgery to perform sleep studies for definitive OSA diagnosis.73
OSA is associated with increased sensitivity to central and peripheral effects of opioids,74,75 including the respiratory depressant effects of opioids.76 Sleep disruption and nocturnal intermittent hypoxemia may enhance pain by acting directly or by activating inflammatory pathways.77,78
Cardiovascular disease
Obesity produces changes in cardiac morphology and ventricular function as an adaptation to excess body mass and increased metabolic demands.79 The excess body mass leads to an increase in cardiac output which progresses over time from left ventricular (LV) hypertrophy, to dilation, and ultimately to LV failure. Risk factors for coronary artery disease in the obese patient include diabetes, hypertension, dyslipidemia, inflammation, and a hypercoagulable state. A complete preoperative cardiac assessment should be obtained including a history and physical examination, and functional capacity. A patient’s functional status can be inferred from their ability to perform activities of daily living and higher cardiorespiratory fitness (established by metabolic equivalents [METS]) is associated with a lower risk of adverse cardiac events.80 However, the assessment of functional status can be difficult due to limited mobility in obese patients. A cardiac risk assessment should be performed using the American Heart Association (AHA) guidelines.81 According to the 2004 AHA guidelines, for patients who have elevated cardiac risk and poor or unknown functional capacity, exercise testing and/or cardiac imaging are reasonable management options. In the obese population, barriers to testing may result from body habitus and weight limitations of diagnostic equipment. Overall, the need for further cardiac testing depends on consideration of the patient’s risk factors, cardiac risk determined by the planned surgery and the patients functional status. The American College of Surgeons NSQIP Risk Calculator and the Revised Cardiac Risk Index are validated tools for estimating perioperative risk.82
Several population-based studies have demonstrated a direct relationship between obesity and hypertension.83 Some evidence exists to suggest that the use of angiotensin-converting-enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) may be beneficial secondary to their ability to increase insulin sensitivity.84
Obese patients have multiple risk factors for developing pulmonary hypertension, LV dysfunction, and pulmonary thromboembolism. Preoperative assessment for patients with pulmonary hypertension should include ECG and an echocardiogram to assess ventricular and valvular structure and function. Preoperative management should be conducted in conjunction with pulmonary and/or cardiology specialists to optimize the patient’s cardiopulmonary disease. The obesity surgery mortality risk score (OR-MRS) is a validated tool to stratify risk in the bariatric population. This tool uses five preoperative variable, including BMI, male gender, hypertension, known risk factors for pulmonary embolism, and age ≥45 years, to classify groups into low, intermediate, and high risk.85
Pharmacologic considerations of the obese surgical patient
While anesthetic drug-dosing is typically based on patient weight and clinical condition, in obesity the pharmacokinetic parameters of medications are altered. In obese patients, the amount of adipose tissue increases in proportion to the increase in total body weight, while the amount of lean body weight remains relatively constant resulting in a reduced proportion of lean body weight per kilogram. Consequently, the volume of distribution for lipophilic drugs is increased in obesity (Table 3).86 Changes in total blood volume and cardiac output also occur in obesity and can also affect pharmacokinetic parameters.87 Regional blood flow must also be taken into account in obese patients as cardiac output remains directed at lean muscle and visceral organs. Thus, dosing hydrophilic drugs by total body weight instead of a measurement directed at lean body mass (LBM). The James formula is perhaps the most widely used for calculating LBM in the perioperative period and its practical use for target-controlled infusion in obese patients is well-explained by Absalom and colleagues.88
Table 3.
Pharmacologic concerns in obesity
| Greater than normal amount adipose tissue |
| Increased lean body mass (LBW = IBW + 20–30%) |
| Increased blood volume |
| Smaller than normal fraction total body water |
| Increased cardiac output |
| Decreased pulmonary function |
| Increased proteins, free fatty acids |
| Increased renal blood flow |
| Increased glomerular filtration rate |
| Abnormal liver function |
Reprinted with permission from Leykin Y, et al. Pharmacokinetic considerations in the obese. Best Pract Res Clin Anaesthesiol. 2011 Mar;25(1):27–36.86
In addition to changes in pharmacokinetics, obese patients may have comorbid conditions that also determine the ultimate clinical effect of medication administration. For example, changes in plasma protein binding and hepatic metabolism due to obesity-induced hepatic steatosis may alter drug clearance.89,90 Patients with OSA, a frequent accompaniment of obesity (see above), have pharmacodynamic alterations with an increased sensitivity to certain sedatives.91
Individual drugs
Benzodiazepines
As these lipophilic medications have an increased volume of distribution (Vd) in obese patients,92 dosing should be determined by total body weight (TBW).
Opioids
In the distribution and clearance models used, the appropriate remifentanil infusion rate is governed by either ideal or lean body weight as dosing by total body weight may result in opioid overdose.93 Similarly, fentanyl dosing by total body weight also results in overdose; thus fentanyl should be dosed by IBW or LBW.94
Propofol
This lipophilic sedative-hypnotic is commonly used for the induction and maintenance of general anesthesia, as well as for procedural sedation has a short half life (t 1/2α) due primarily to rapid redistribution. The appropriate dosing scalar for the ‘induction’ of anesthesia is lean body weight, as opposed to total body weight.95 Dosing propofol induction dose to a targeted bispectral index has also been advocated.96 For ‘maintenance’ of general anesthesia, propofol dosing by a total body weight has been found to be appropriate, without evidence of increased drug accumulation in obese patients.97
Dexmedetomidine
This alpha-2 agonist is used for sedation, anxiolysis and pain relief. When used intraoperatively as an adjunct with general anesthesia for bariatric surgery, its use resulted in opioid reduction, reduced recovery stay, reduced antiemetic dose.98 One study examined the influence of obesity on dexmedetomidine pharmacokinetics, and found that dosing dexmedetomidine based on TBW resulted in increased serum concentrations. This was due to volume of distribution increases dependent on lean body tissue, and a reduced clearance.99
Neuromuscular blockers
The non-depolarizing muscle relaxant rocuronium should be dosed based on IBW which results in a similar time to adequate intubation conditions, while minimizing the duration of action.100 Similarly, rocuronium should also be dosed by ideal body weight.101 Conversely, in order to achieve optimal intubating conditions with the depolarizing relaxant succinylcholine TBW should be used for dosing.102
Volatile anesthetics
Volatile anesthetic uptake is determined in part by solubility of the inhaled agent, which is confounded by the increased fat stores in obese patients. Therefore, it would be expected that recovery from relatively insoluble volatile agents would be more rapid than from soluble agents which is what was demonstrated when the less soluble desflurane was compared to sevoflurane in morbidly obese patients undergoing gastric bypass surgery that was supplemented with epidural analgesia.103 However, in another comparative study of sevoflurane vs. desflurane in morbidly obese patients without the use of epidural analgesia there was no difference in recovery.104
Technical considerations
Complications related to airway management is a major cause of morbidity and mortality related to anesthesia in the obese population. Obesity is an independent predictor of difficult mask ventilation and difficult laryngoscopy.87,105–108 Additionally, neck circumference and OSA are independent predictors of difficult airway.109 The ASA’s Difficult Airway Algorithm provides a guideline to management of the difficult airway. In the obese population where difficult mask ventilation and intubation are more likely, the ASA’s Difficult Airway Algorithm suggests use of techniques such as awake induction, recruitment of additional personnel as well as the immediate availability of additional advanced airway devices, including tracheotomy equipment.110
Standard operating room tables have a safe weight capacity of approximately 450 lbs. Specific operating tables designed to hold up to 1 000 lbs should be allocated as needed to prevent patient falls and ensure intraoperative safety. Nerve injury is a known complication related to surgery and patient positioning. Studies have demonstrated an increased risk of nerve injury in the obese surgical population.111–112 Venous access is more difficult in this population, making both intravenous line insertion and laboratory blood sampling quite challenging.113
Perioperative complications
Pulmonary system
Respiratory mechanics are significantly altered in obesity, and these changes are further exacerbated with general anesthesia. These obesity-related changes are characterized by a reduced functional residual capacity (FRC), primarily via reduction in expiratory reserve volume (ERV) resulting in atelectasis and shunt physiology.114 Obesity also results in reduced lung and chest wall compliance, increased lung resistance, reduced oxygenation and increased work of breathing.115 The reduced FRC results in a shorter period of apnea tolerated by obese patients before desaturation.116
Overweight (BMI > 25) and obese (BMI > 30) patients have also been noted to have an increased incidence of asthma.117 The mechanism for this association is not clear, but may be related to changes in lung mechanics as outlined above, as well as inflammation and immune function.118
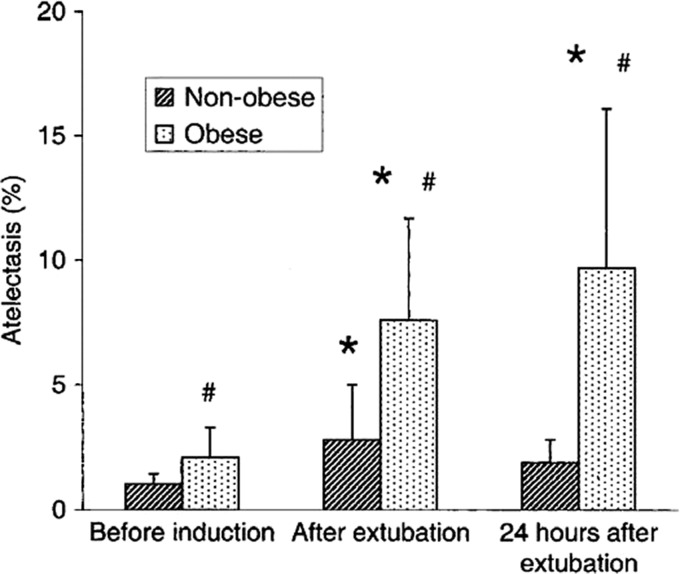
These changes in lung volumes increase the likelihood of morbidly obese surgical patients developing pulmonary complications. In a study of postoperative pulmonary events following bariatric surgery, atelectasis and pneumonia were found to be the two leading adverse events, though the overall incidence of pulmonary complications in this group was <1%.119 The presence of metabolic syndrome in these patients further increased the likelihood of pulmonary complications.119 In addition to an increase in atelectasis during general anesthesia and abdominal surgery, morbidly obese patients have persistent atelectasis postoperatively compared to their non-obese counterparts (Fig. 2).120
Fig. 2.
Comparison of the percentage of pulmonary atelectasis between morbidity obese and non-obese patients at the three study times (before anesthesia induction, after extubation, and 24 h later).120 *P < 0.05 compared with before induction (within group); #P < 0.05 compared with the control group (between groups). Reprinted with permission from Eichenberger A, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg 2002;95:1788–92.
A number of strategies has been suggested to reduce the development of atelectasis in obese patients undergoing anesthesia and surgery. One such intervention is the application of positive end-expiratory pressure (PEEP). Pelosi et al found that a PEEP of 10 cm H20 resulted in improved oxygenation and compliance in obese patients compared with no PEEP; no improvement was noted with this amount of PEEP in lean surgical patients.121 Preoxygenation with head of bed elevation has been found to prolong the post-apneic time until desaturation occurs.122
Noninvasive positive pressure ventilation during anesthesia induction followed by an early lung recruitment maneuver following intubation has been demonstrated to improve oxygenation, and this improvement in oxygenation is correlated with an increase in ERC.123 A meta-analysis of ventilation strategies in obesity found that lung recruitment maneuvers combined with PEEP also improved oxygenation and compliance during the maintenance of anesthesia.124
Cardiovascular system
Obesity is associated with a number of cardiovascular system comorbidities that may influence perioperative management and outcomes. These comorbidities include atherosclerotic cardiovascular disease, hypertension, cardiomyopathy, pulmonary hypertension, arrhythmias, thromboembolic disease and poor exercise tolerance.125 However, as noted elsewhere in this review, obesity itself is not associated with an increased incidence of cardiovascular morbidity and in fact, may be protective. An exception may be in patients that are morbidly obese and have additional insulin resistance. For example, in patients undergoing coronary artery bypass grafting (CABG), those with a BMI > 40 did exhibit an increased risk of perioperative myocardial infarction and morbidity.126 However, a recent report suggested that even in the non-metabolically-deranged obese patient, cardiovascular complications are in fact more likely.11
In the most recent authoritative guidelines, the potential for perioperative cardiovascular risk is determined primarily by functional status, rather than the traditional risk factors outlined by the revised cardiac risk index.81 Furthermore, as discussed elsewhere in this review, the metabolic syndrome is associated with an increased mortality rate after CABG.127 Thus while obesity is associated with various cardiovascular risk factors, and body habitus may limit functional status, obesity in and of itself is not necessarily a risk factor for perioperative cardiovascular morbidity.
Venous thromboebolism
Several studies have reported that obesity is an independent risk factor for perioperative venous thromboebolism (VTE).126,128–134 The already high risk for VTE is further exacerbated with progressively higher BMI, prolonged surgical time, older age, male sex, history of OSA, obesity hypoventilation syndrome and a previous history of VTE.129;134,135 According to the 2013 Clinical Practice guidelines for bariatric surgical patients, it is recommended that VTE prophylaxis, including sequential compression devices and chemoprophylaxis, to be administered perioperatively.136 The American Society for Bariatric and Metabolic Surgery in association with American Association of Clinical Endocrinologists and The Obesity Society issued guidelines which recommend that all bariatric surgical patients receive mechanical prophylaxis, early ambulation and chemoprophylactic interventions with low molecular weight heparin (LMWH) or unfractionated heparin.137 However, chemoprophylactic dosing will need to consider that adipose tissue has a lower blood volume than lean tissue; hence, the volume of distribution of heparin will differ in obese patients. Even though heparin dosing requirements do not increase linearly with body weight,138,139 the 2012 American College of Chest Physicians guidelines state that weight-based dosing is preferred to fixed dosing in obese patients.134 In the treatment of VTE the total body weight is used to calculate the initial bolus dose and infusion rate to achieve a therapeutic PTT with dosing adjusted accordingly.140,141
The dose of LMWH should also be adjusted based on total body weight.142 It is suggested that in bariatric surgical patients, enoxaparin dose is adjusted as follows: (i) BMI = <50 kg/m2 – enoxaparin 40 mg BID SQ; (ii) BMI > 50 kg/m2 – enoxaparin 60 mg BID SQ.143,144
Postoperative cognitive decline
Both postoperative delirium (POD) as well as postoperative cognitive dysfunction (POCD) results in increased morbidity and even mortality.145,146 As risk factors may differ between the encompassed entities of POCD and POD,147 there is no consensus regarding a unifying etiology and pathophysiology for the constellation of PCD conditions. Over the last decade investigations have focused on the putative role of neuroinflammation in the development of PCD, and have systematically addressed how pro-inflammatory cytokines and immune cells propagate PCD in animal models.148–155 Recently, similar inflammatory changes in surgical patients have been noted.156–159
Obesity is associated with both a low-grade chronic inflammatory state160 as well as overactivity of the sympathetic nervous system,161 and these may be related because cholinergic stimulation is required to terminate the inflammatory response.162 Furthermore obesity increases the risk for the development of diabetes,163–165 an insulin-resistant state in which elevated levels of pro-inflammatory cytokines supervene.166 As pro-inflammatory cytokines interfere with long-term potentiation, the neurobiologic correlate for learning and memory,167,168 it is unsurprising that obesity increases dementia risk by 64%.169 Hudetz and colleagues were the first to draw attention to the link between the metabolic syndrome and the increased likelihood for the development of PCD (Table 4).170,171 The pathophysiologic basis for the relationship between the metabolic syndrome and exaggerated postoperative cognitive decline have further emphasized the role of unresolved inflammation.172,173 Furthermore, obese patients are more likely to develop OSA (considered elsewhere in this review) that has an increased risk for postoperative cognitive decline.174
Table 4.
Neurocognitive scores of surgical patients
| Variables | Baseline | −Metabolic N = 28 | Baseline | +Metabolic N = 28 | P | z Score difference | ||
|---|---|---|---|---|---|---|---|---|
| 1 Week | z Scores | 1 Week | z Scores | |||||
| Nonverbal memory | ||||||||
| Figure reconstruction | 21 ± 7 | 17 ± 8 | −2.3 ± 2 | 21 ± 7 | 17 ± 7 | −2.5 ± 2.1 | 0.77 | −0.2 |
| Delayed figure reproduction | 8 ± 3 | 6 ± 4 | −0.7 ± 1.5 | 8 ± 3 | 6 ± 3 | −1.3 ± 1.2 | 0.11 | −0.6 |
| Verbal memory | ||||||||
| Immediate story recall | 19 ± 5 | 18 ± 7 | −1.2 ± 2.18 | 18 ± 4 | 15 ± 5 | −2.6 ± 2.1 | 0.02 | −1.4 |
| Delayed story recall | 10 ± 3 | 9 ± 4 | −1.4 ± 2.2 | 9 ± 2 | 7 ± 3 | −2.9 ± 2.3 | 0.02 | −1.5 |
| Immediate word list recall | 29 ± 8 | 25 ± 9 | −2.6 ± 1.8 | 23 ± 6 | 20 ± 5 | −2.5 ± 2.3 | 0.96 | 0.1 |
| Delayed word list recall | 7 ± 3 | 5 ± 3 | −2.6 ± 2.2 | 5 ± 3 | 3 ± 2 | −3.7 ± 2.2 | 0.06 | −1.1 |
| Executive functions | ||||||||
| Digit span | 9 ± 2 | 9 ± 3 | −1.1 ± 1.3 | 8 ± 2 | 7 ± 2 | −1.9 ± 1.1 | 0.02 | −0.8 |
| Semantic fluency | 17 ± 5 | 14 ± 4 | −0.8 ± 1 | 15 ± 4 | 11 ± 3 | −0.8 ± 1 | 0.89 | 0.0 |
| Phonemic fluency | 13 ± 4 | 11 ± 5 | −0.7 ± 1.4 | 10 ± 4 | 8 ± 4 | −0.9 ± 1.1 | 0.52 | −0.2 |
| Stroop | 43 ± 13 | 37 ± 16 | −1.4 ± 1.2 | 36 ± 13 | 29 ± 12 | −1.7 ± 1.2 | 0.49 | −0.3 |
| GDS-15 | 2 ± 2 | 3 ± 3 | 0.2 ± 0.7 | 3 ± 3 | 3 ± 3 | −0.1 ± 0.6 | 0.09 | −0.3 |
Data are expressed as mean ± standard deviation.
Abbreviations: pB, between-group significance under baseline conditions (Student t-test); p1W, between-group significance after 1 week (repeated measures analysis of variance).
Reprinted with permission from Hudetz JA, et al. Metabolic syndrome exacerbates short-term postoperative cognitive dysfunction in patients undergoing cardiac surgery: results of a pilot study. J Cardiothorac Vasc Anesth. 2011 Apr;25(2):282–287.170
As obese patients are at increased risk for PCD, the application of ‘Care Bundles,’ designed to reduce the modifiable precipitating factors for delirium, are advocated.175–180
Wound infection
Obesity increases the risk of postoperative wound infection. Interrogation of the Veterans Affairs Surgical Quality Improvement Program (VASQIP) database showed that in total joint arthroplasty, a BMI > 40 kg/m2 is an independent predictor for superficial infection.181 Similarly, obese patients undergoing open reduction and internal fixation of distal humeral fractures or total elbow arthroplasty132 total ankle arthroplasty or ankle arthrodesis,131 total shoulder arthroplasty133 fixation for intertrochanteric femur fractures,182 and fixation of a fracture of an ankle183 all have an increased incidence of surgical-site infections when compared to non-obese patients undergoing the same surgery. Other non-orthopedic surgeries are also complicated by increased incidence of surgical-site infection in obese patients including abdominal hysterectomy, coronary bypass graft and large bowel surgery.184 Morbidly obese patients have 6.5-fold increase in deep sternal wound infection.185 Interrogation of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database for abdominal surgery showed that obesity and morbid obesity are independently associated with overall surgical-site infections development in clean and clean-contaminated cases.186 Further analysis of the same database addressing 16 major cardiovascular, orthopedic and oncologic surgeries showed that morbid obesity significantly increased the odds of wound complications in all surgeries except for pneumonectomy.126 A retrospective study on lower extremity vascular surgery also found that obesity and morbid obesity were independent predictors of infectious wound complications, including dehiscence, surgical-site infections, seroma and hematoma at 30 days.187
The pathophysiologic mechanisms leading to surgical wound infections in obese patients could include relative hypoperfusion/ischemia, dysregulated immune and inflammatory responses, coupled with decreased delivery of antibiotics.188 Furthermore, tension on the wound edges is often increased in obese patients resulting in decreased oxygen supply to the wound. Hypovascularity and the difficulty that obese patients have of repositioning themselves may increase the risk of pressure ulcers or pressure-related injuries. Also, redundant skin folds serve as a moist area for micro-organisms to thrive, which may lead to infection and tissue breakdown, and the skin to skin friction may cause ulceration.189 Adipose tissue macrophages produce excessive cytokines (TNF-alpha, IL-1, IL-6, IL-8, IL-10) and chemokines (IL-8, MCP-1, IP-10, MCP, monocyte chemoattractant protein-1; IP-10 interferon-gamma-inducible protein 10) that act as endocrines, while impaired peripheral blood mononuclear cell function and decreased lymphocyte proliferation significantly disrupt the innate immune response.188 Apart from these local events, obesity can be associated with other comorbidities including diabetes, anxiety and depression that result in impaired wound healing through impaired immune response.188–189
Strategies designed to mitigate the high likelihood of surgical-site infections include weight-adjusted antibiotics dosing,136 the use of minimally invasive approaches when possible,126 and layered closure of incisions.135
Critical care considerations
The care of the critically ill obese surgical patient requires a number of special considerations. For example, technical aspects related to positioning, monitoring, and vessel cannulation can all be challenging due to increased body habitus. As discussed above, drug-dosing in the obese patient also deserves special consideration given changes in drug distribution and clearance. The data for pharmacokinetic-guided dosing in obese patients are quite limited. Commonly used drugs in the ICU that may require additional consideration include antibiotics, sedative/analgesics and anticoagulants.
Acute respiratory distress syndrome
A recent meta-analysis of BMI and acute respiratory distress syndrome (ARDS) found that obese patients have lower mortality,190 as noted in our discussion of the Obesity Paradox. Despite mechanical changes associated with obesity in healthy patients, as outlined above, obese patients with ARDS show no additional changes in lung elastance or recruitment ability.191 Furthermore, obese patients do not fare worse than normal weight patients with ARDS on ECMO, and obesity is not a contraindication for ECMO initiation.192 When choosing ventilator settings for obese patients, the tidal volume should be based on ideal and not total body weight.193 The addition of PEEP improves alveolar recruitment and oxygenation in morbidly obese anesthetized postoperative patients.121
Proning in obesity
Prone position has been shown to improve mortality in patients with severe ARDS.194 Given the technical difficulties associated with achieving prone positioning in the obese population, the utility of this intervention in obese patients with ARDS has been questioned. One retrospective review found that proning in patients with abdominal obesity increased risk of mortality, renal failure, hepatic failure.195 However, another case-controlled study found that proning improves oxygenation and mortality in morbidly obese patients with ARDS compared with non-obese patients with ARDS.196 A difference in the definition of obesity may account for differing conclusions, as the first study focused on abdominal obesity, as determined by sagittal abdominal diameter on imaging, and the latter determined obesity based on traditional BMI (see elsewhere).
Nutrition
Nutritional support of critically ill obese patients is a necessary component of recovery, as obesity does not mitigate the development of acute ICU-related malnutrition. The recommendations from American Society of Parenteral and Enteral nutrition include early enteral nutrition, as for all patients with critical illness. Caloric requirements should be determined by indirect calorimetry and protein requirements determined by weight-based calculations and urine nitrogen to achieve positive nitrogen balance. The nutritional goal for obese patients can be achieved with high-protein, hypocaloric feeding.197
Conclusion
Perioperative care of the obese surgical patient remains a challenging proposition because of the metabolic, pharmacologic, and system-wide disorders that are the foundational basis for the complications that can ensue. In order to understand the Obesity Paradox, in which complication rates may actually be lower than normal weight surgical patients, it will be necessary to distinguish ‘healthy’ obesity from those obese states in which insulin resistance comes to the fore. Further surrogate biomarkers that characterize the different types of obesity will be needed to focus particular attention on those obese surgical patients for whom the perioperative period is particularly hazardous.
Conflict of interest statement
The authors have no potential conflicts of interest.
Acknowledgements
Each of the authors is a California-licensed physician and an American Board of Anesthesia-certified Anesthesiologist. Dr. Parekh received further training as an Intensivist. Dr. Tsui received further training in interventional chronic pain management and in clinical trials. Dr. Lang is the Director of the Preoperative Assessment Clinic at the Zuckerberg San Francisco General Hospital and Trauma Center. Dr. Maze is a funded investigator with awards from the National Institutes of Health (R01GM104194) Dr. Maze’s research involving both preclinical and clinical studies addresses postoperative cognitive decline, and was the first to demonstrate the neuroinflammatory basis for this condition.
References
- 1. Ludwig DS. Lifespan weighed down by diet. JAMA 2016;315:2269–70. [DOI] [PubMed] [Google Scholar]
- 2. Popkin BM, Doak CM. The obesity epidemic is a worldwide phenomenon. Nutr Rev 1998;56:106–14. [DOI] [PubMed] [Google Scholar]
- 3. Ahmad FB. Quarterly provisional estimates for selected indicators of mortality, 2015-Quarter 4, 2016. 2017. https://www.cdc.gov/nchs/products/vsrr/mortality-dashboard.htm (accessed 12 Jun 2017).
- 4. Lavie CJ, Milani RV. Obesity and cardiovascular disease: the hippocrates paradox? J Am Coll Cardiol 2003;42:677–9. [DOI] [PubMed] [Google Scholar]
- 5. Lavie CJ, Osman AF, Milani RV, et al. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol 2003;91:891–4. [DOI] [PubMed] [Google Scholar]
- 6. Kovesdy CP, Czira ME, Rudas A, et al. Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant 2010;10:2644–51. [DOI] [PubMed] [Google Scholar]
- 7. Kalantar-Zadeh K, Horwich TB, Oreopoulos A, et al. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care 2007;10:433–42. [DOI] [PubMed] [Google Scholar]
- 8. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7. [DOI] [PubMed] [Google Scholar]
- 9. Yu E, Ley SH, Manson JE, et al. Weight history and all-cause and cause-specific mortality in three prospective cohort studies. Ann Intern Med 2017;166:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adamopoulos C, Meyer P, Desai RV, et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity-matched study. Eur J Heart Fail 2011;13:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lasalle C, Tzoulaki I, Moons K, et al. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan-European case-cohort analysis. Eur Heart J 2017;ehx448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Executive Summary. Obes Res 1998;6:51S–179S.
- 13. Nuttall FQ. Body Mass Index: obesity, BMI, and health: a critical review. Nutr Today 2015;50:117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 2000;72:694–701. [DOI] [PubMed] [Google Scholar]
- 15. Berentzen TL, Angquist L, Kotronen A, et al. Waist circumference adjusted for body mass index and intra-abdominal fat mass. PLoS One 2012;7:e32213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Demerath EW, Reed D, Rogers N, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr 2008;88:1263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan DC, Watts GF, Barrett PH, et al. Waist circumference, waist-to-hip ratio and body mass index as predictors of adipose tissue compartments in men. QJM 2003;96:441–7. [DOI] [PubMed] [Google Scholar]
- 18. Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002;3:141–6. [DOI] [PubMed] [Google Scholar]
- 19. Deurenberg P, Yap M. The assessment of obesity: methods for measuring body fat and global prevalence of obesity. Baillieres Best Pract Res Clin Endocrinol Metab 1999;13:1–11. [DOI] [PubMed] [Google Scholar]
- 20. Flegal KM, Carroll MD, Kuczmarski RJ, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord 1998;22:39–47. [DOI] [PubMed] [Google Scholar]
- 21. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016;315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zylke JW, Bauchner H. The unrelenting challenge of obesity. JAMA 2016;315:2277–8. [DOI] [PubMed] [Google Scholar]
- 23. Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 yhrough 2013–2014. JAMA 2016;315:2292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berghofer A, Pischon T, Reinhold T, et al. Obesity prevalence from a European perspective: a systematic review. BMC Public Health 2008;8:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization Obesity and overweight: fact sheet. Updated Jun 2016. http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed 12 Jun 2017).
- 26. Risk NCD. Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016;387:1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell 2015;161:119–32. [DOI] [PubMed] [Google Scholar]
- 28. Thomas DM, Bouchard C, Church T, et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev 2012;13:835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McAllister EJ, Dhurandhar NV, Keith SW, et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr 2009;49:868–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Church TS, Thomas DM, Tudor-Locke C, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One 2011;6:e19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr 2006;84:289–98. [DOI] [PubMed] [Google Scholar]
- 32. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017;376:254–66. [DOI] [PubMed] [Google Scholar]
- 33. Silventoinen K, Magnusson PK, Tynelius P, et al. Heritability of body size and muscle strength in young adulthood: a study of one million Swedish men. Genet Epidemiol 2008;32:341–9. [DOI] [PubMed] [Google Scholar]
- 34. Speakman JR. Thrifty genes for obesity and the metabolic syndrome—time to call off the search? Diab Vasc Dis Res 2006;3:7–11. [DOI] [PubMed] [Google Scholar]
- 35. Pigeyre M, Yazdi FT, Kaur Y, et al. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond) 2016;130:943–86. [DOI] [PubMed] [Google Scholar]
- 36. Bray MS, Loos RJ, McCaffery JM, et al. NIH working group report-using genomic information to guide weight management: From universal to precision treatment. Obesity (Silver Spring) 2016;24:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc 2011;86:549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Epstein LJ, Kristo D, Strollo PJ Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 39. Korcarz CE, Peppard PE, Young TB, et al. Effects of obstructive sleep apnea and obesity on cardiac remodeling: the Wisconsin. Sleep Cohort Study Sleep 2016;39:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep 2015;38:677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217–39. [DOI] [PubMed] [Google Scholar]
- 42. Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5. [DOI] [PubMed] [Google Scholar]
- 43. Gottlieb DJ, Somers VK, Punjabi NM, et al. Restless legs syndrome and cardiovascular disease: a research roadmap. Sleep Med 2017;31:10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sato S, Hasegawa M, Okuyama M, et al. Mask ventilation during induction of general anesthesia: influences of obstructive sleep apnea. Anesthesiology 2017;126:28–38. [DOI] [PubMed] [Google Scholar]
- 45. Kondo YJ, Okutomi T, Hoka S. Beware of gastric tube! Anesth Analg 2002;95:1122. [DOI] [PubMed] [Google Scholar]
- 46. Mooe T, Gullsby S, Rabben T, et al. Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis 1996;7:475–8. [PubMed] [Google Scholar]
- 47. Joosten SA, Hamilton GS, Naughton MT. Impact of weight loss management in OSA. Chest 2017;152:194–203. doi:10.1016/j.chest.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 48. Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med 2005;165:2408–13. [DOI] [PubMed] [Google Scholar]
- 49. Ward K, Hoare KJ, Gott M. What is known about the experiences of using CPAP for OSA from the users’ perspective? A systematic integrative literature review. Sleep Med Rev 2014;18:357–66. [DOI] [PubMed] [Google Scholar]
- 50. Gay PC, Herold DL, Olson EJ. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep 2003;26:864–9. [DOI] [PubMed] [Google Scholar]
- 51. National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- 52. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 53. Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care 2011;34:216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Purnell JQ, Selzer F, Smith MD, et al. Metabolic syndrome prevalence and associations in a bariatric surgery cohort from the Longitudinal Assessment of Bariatric Surgery-2 study. Metab Syndr Relat Disord 2014;12:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dindo D, Muller MK, Weber M, et al. Obesity in general elective surgery. Lancet 2003;361:2032–5. [DOI] [PubMed] [Google Scholar]
- 56. Klasen J, Junger A, Hartmann B, et al. Increased body mass index and peri-operative risk in patients undergoing non-cardiac surgery. Obes Surg 2004;14:275–81. [DOI] [PubMed] [Google Scholar]
- 57. Smith PW, Wang H, Gazoni LM, et al. Obesity does not increase complications after anatomic resection for non-small cell lung cancer. Ann Thorac Surg 2007;84:1098–105. ; discussion105-6. [DOI] [PubMed] [Google Scholar]
- 58. Mullen JT, Davenport DL, Hutter MM, et al. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol 2008;15:2164–72. [DOI] [PubMed] [Google Scholar]
- 59. Adamiak T, Gheller-Rigoni A, Arca M, et al. Perianal disease as the initial presentation of autoimmune neutropenia. J Pediatr Gastroenterol Nutr 2010;50:99–102. [DOI] [PubMed] [Google Scholar]
- 60. Glance LG, Wissler R, Mukamel DB, et al. Perioperative outcomes among patients with the modified metabolic syndrome who are undergoing noncardiac surgery. Anesthesiology 2010;113:859–72. [DOI] [PubMed] [Google Scholar]
- 61. Memtsoudis SG, Kirksey M, Ma Y, et al. Metabolic syndrome and lumbar spine fusion surgery: epidemiology and perioperative outcomes. Spine (Phila Pa 1976) 2012;37:989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bhayani NH, Hyder O, Frederick W, et al. Effect of metabolic syndrome on perioperative outcomes after liver surgery: A National Surgical Quality Improvement Program (NSQIP) analysis. Surgery 2012;152:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kao LS, Meeks D, Moyer VA, et al. Peri-operative glycaemic control regimens for preventing surgical site infections in adults. Cochrane Database Syst Rev 2009;3:CD006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bagry HS, Raghavendran S, Carli F. Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology 2008;108:506–23. [DOI] [PubMed] [Google Scholar]
- 65. Tan A, Yin JD, Tan LW, et al. Predicting obstructive sleep apnea using the STOP-Bang questionnaire in the general population. Sleep Med 2016;27-28:66–71. [DOI] [PubMed] [Google Scholar]
- 66. Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev 2016; doi:10.1016/j.smrv.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 67. Doshi V, Walia R, Jones K, et al. STOP-BANG questionnaire as a screening tool for diagnosis of obstructive sleep apnea by unattended portable monitoring sleep study. Springerplus 2015;4:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chung F, Yang Y, Liao P. Predictive performance of the STOP-Bang score for identifying obstructive sleep apnea in obese patients. Obes Surg 2013;23:2050–7. [DOI] [PubMed] [Google Scholar]
- 69. American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology 2014;120:268–86. [DOI] [PubMed] [Google Scholar]
- 70. Hamoda MM, Kohzuka Y, Almeida FR. Oral Appliances for the management of OSA: an updated review of the literature. Chest 2017. [DOI] [PubMed] [Google Scholar]
- 71. American Society of Anesthesiologists Task Force on Management of the Difficult Airway Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003;98:1269–77. [DOI] [PubMed] [Google Scholar]
- 72. Gupta RM, Parvizi J, Hanssen AD, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc 2001;76:897–905. [DOI] [PubMed] [Google Scholar]
- 73. Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016;123:452–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kundermann B, Spernal J, Huber MT, et al. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med 2004;66:932–7. [DOI] [PubMed] [Google Scholar]
- 75. Onen SH, Alloui A, Gross A, et al. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res 2001;10:35–42. [DOI] [PubMed] [Google Scholar]
- 76. Ramachandran SK, Haider N, Saran KA, et al. Life-threatening critical respiratory events: a retrospective study of postoperative patients found unresponsive during analgesic therapy. J Clin Anesth 2011;23:207–13. [DOI] [PubMed] [Google Scholar]
- 77. Haack M, Lee E, Cohen DA, et al. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain 2009;145:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Doufas AG, Tian L, Davies MF, et al. Nocturnal intermittent hypoxia is independently associated with pain in subjects suffering from sleep-disordered breathing. Anesthesiology 2013;119:1149–62. [DOI] [PubMed] [Google Scholar]
- 79. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 80. Kokkinos PF, Faselis C, Myers J, et al. Cardiorespiratory fitness and incidence of major adverse cardiovascular events in US veterans: a cohort study. Mayo Clin Proc 2017;92:39–48. [DOI] [PubMed] [Google Scholar]
- 81. Fleisher LA, Fleischmann KE, Auerbach AD, et al. ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130:2215–45. [DOI] [PubMed] [Google Scholar]
- 82. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–9. [DOI] [PubMed] [Google Scholar]
- 83. Kannel WB, Brand N, Skinner JJ Jr., et al. The relation of adiposity to blood pressure and development of hypertension. The Framingham study. Ann Intern Med 1967;67:48–59. [DOI] [PubMed] [Google Scholar]
- 84. Sharma AM, Pischon T, Engeli S, et al. Choice of drug treatment for obesity-related hypertension: where is the evidence? J Hypertens 2001;19:667–74. [DOI] [PubMed] [Google Scholar]
- 85. DeMaria EJ, Murr M, Byrne TK, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg 2007;246:578–82. ; discussion83-4. [DOI] [PubMed] [Google Scholar]
- 86. Leykin Y, Miotto L, Pellis T. Pharmacokinetic considerations in the obese. Best Pract Res Clin Anaesthesiol 2011;25:27–36. [DOI] [PubMed] [Google Scholar]
- 87. Wada DR, Bjorkman S, Ebling WF, et al. Computer simulation of the effects of alterations in blood flows and body composition on thiopental pharmacokinetics in humans. Anesthesiology 1997;87:884–99. [DOI] [PubMed] [Google Scholar]
- 88. Absalom AR, Mani V, De Smet T, et al. Pharmacokinetic models for propofol–defining and illuminating the devil in the detail. Br J Anaesth 2009;103:26–37. [DOI] [PubMed] [Google Scholar]
- 89. Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 2000;39:215–31. [DOI] [PubMed] [Google Scholar]
- 90. Merrell MD, Cherrington NJ. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab Rev 2011;43:317–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. den Herder C, Schmeck J, Appelboom DJ, et al. Risks of general anaesthesia in people with obstructive sleep apnoea. BMJ 2004;329:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. van Rongen A, Vaughns JD, Moorthy GS, et al. Population pharmacokinetics of midazolam and its metabolites in overweight and obese adolescents. Br J Clin Pharmacol 2015;80:1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Egan TD, Huizinga B, Gupta SK, et al. Remifentanil pharmacokinetics in obese versus lean patients. Anesthesiology 1998;89:562–73. [DOI] [PubMed] [Google Scholar]
- 94. Shibutani K, Inchiosa MA Jr., Sawada K, et al. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: derivation of dosing weight (‘pharmacokinetic mass. Anesthesiology 2004;101:603–13. [DOI] [PubMed] [Google Scholar]
- 95. Ingrande J, Brodsky JB, Lemmens HJ. Lean body weight scalar for the anesthetic induction dose of propofol in morbidly obese subjects. Anesth Analg 2011;113:57–62. [DOI] [PubMed] [Google Scholar]
- 96. Subramani Y, Riad W, Chung F, et al. Optimal propofol induction dose in morbidly obese patients: a randomized controlled trial comparing the bispectral index and lean body weight scalar. Can J Anaesth 2017;64:471–79. [DOI] [PubMed] [Google Scholar]
- 97. Servin F, Farinotti R, Haberer JP, et al. Propofol infusion for maintenance of anesthesia in morbidly obese patients receiving nitrous oxide. A clinical and pharmacokinetic study. Anesthesiology 1993;78:657–65. [DOI] [PubMed] [Google Scholar]
- 98. Tufanogullari B, White PF, Peixoto MP, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesth Analg 2008;106:1741–8. [DOI] [PubMed] [Google Scholar]
- 99. Cortinez LI, Anderson BJ, Holford NH, et al. Dexmedetomidine pharmacokinetics in the obese. Eur J Clin Pharmacol 2015;71:1501–8. [DOI] [PubMed] [Google Scholar]
- 100. Meyhoff CS, Lund J, Jenstrup MT, et al. Should dosing of rocuronium in obese patients be based on ideal or corrected body weight? Anesth Analg 2009;109:787–92. [DOI] [PubMed] [Google Scholar]
- 101. van Kralingen S, van de Garde EM, Knibbe CA, et al. Comparative evaluation of atracurium dosed on ideal body weight vs. total body weight in morbidly obese patients. Br J Clin Pharmacol 2011;71:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lemmens HJ, Brodsky JB. The dose of succinylcholine in morbid obesity. Anesth Analg 2006;102:438–42. [DOI] [PubMed] [Google Scholar]
- 103. Strum EM, Szenohradszki J, Kaufman WA, et al. Emergence and recovery characteristics of desflurane versus sevoflurane in morbidly obese adult surgical patients: a prospective, randomized study. Anesth Analg 2004;99:1848–53. , table of contents. [DOI] [PubMed] [Google Scholar]
- 104. Arain SR, Barth CD, Shankar H, et al. Choice of volatile anesthetic for the morbidly obese patient: sevoflurane or desflurane. J Clin Anesth 2005;17:413–9. [DOI] [PubMed] [Google Scholar]
- 105. Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology 2000;92:1229–36. [DOI] [PubMed] [Google Scholar]
- 106. Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology 2006;105:885–91. [DOI] [PubMed] [Google Scholar]
- 107. Kheterpal S, Healy D, Aziz MF, et al. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the multicenter perioperative outcomes group. Anesthesiology 2013;119:1360–9. [DOI] [PubMed] [Google Scholar]
- 108. Juvin P, Lavaut E, Dupont H, et al. Difficult tracheal intubation is more common in obese than in lean patients. Anesth Analg 2003;97:595–600. , table of contents. [DOI] [PubMed] [Google Scholar]
- 109. Gonzalez H, Minville V, Delanoue K, et al. The importance of increased neck circumference to intubation difficulties in obese patients. Anesth Analg 2008;106:1132–6. , table of contents. [DOI] [PubMed] [Google Scholar]
- 110. Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013;118:251–70. [DOI] [PubMed] [Google Scholar]
- 111. Werner BC, Fashandi AH, Chhabra AB, et al. Effect of obesity on complication rate after elbow arthroscopy in a medicare population. Arthroscopy 2016;32:453–7. [DOI] [PubMed] [Google Scholar]
- 112. Al-Temimi MH, Chandrasekaran B, Phelan MJ, et al. Incidence, risk factors, and trends of motor peripheral nerve injury after colorectal surgery: analysis of the National Surgical Quality Improvement Program database. Dis Colon Rectum 2017;60:318–25. [DOI] [PubMed] [Google Scholar]
- 113. Abir F, Bell R. Assessment and management of the obese patient. Crit Care Med 2004;32:S87–91. [DOI] [PubMed] [Google Scholar]
- 114. Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest 2006;130:827–33. [DOI] [PubMed] [Google Scholar]
- 115. Pelosi P, Croci M, Ravagnan I, et al. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg 1998;87:654–60. [DOI] [PubMed] [Google Scholar]
- 116. Jense HG, Dubin SA, Silverstein PI, et al. Effect of obesity on safe duration of apnea in anesthetized humans. Anesth Analg 1991;72:89–93. [DOI] [PubMed] [Google Scholar]
- 117. Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med 2007;175:661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dixon AE, Holguin F, Sood A, et al. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc 2010;7:325–35. [DOI] [PubMed] [Google Scholar]
- 119. Schumann R, Shikora SA, Sigl JC, et al. Association of metabolic syndrome and surgical factors with pulmonary adverse events, and longitudinal mortality in bariatric surgery. Br J Anaesth 2015;114:83–90. [DOI] [PubMed] [Google Scholar]
- 120. Eichenberger A, Proietti S, Wicky S, et al. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg 2002;95:1788–92. , table of contents. [DOI] [PubMed] [Google Scholar]
- 121. Pelosi P, Ravagnan I, Giurati G, et al. Positive end-expiratory pressure improves respiratory function in obese but not in normal subjects during anesthesia and paralysis. Anesthesiology 1999;91:1221–31. [DOI] [PubMed] [Google Scholar]
- 122. Dixon BJ, Dixon JB, Carden JR, et al. Preoxygenation is more effective in the 25 degrees head-up position than in the supine position in severely obese patients: a randomized controlled study. Anesthesiology 2005;102:1110–5. ; discussion 5A. [DOI] [PubMed] [Google Scholar]
- 123. Futier E, Constantin JM, Pelosi P, et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients: a randomized controlled study. Anesthesiology 2011;114:1354–63. [DOI] [PubMed] [Google Scholar]
- 124. Aldenkortt M, Lysakowski C, Elia N, et al. Ventilation strategies in obese patients undergoing surgery: a quantitative systematic review and meta-analysis. Br J Anaesth 2012;109:493–502. [DOI] [PubMed] [Google Scholar]
- 125. Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation 2009;120:86–95. [DOI] [PubMed] [Google Scholar]
- 126. Sood A, Abdollah F, Sammon JD, et al. The effect of body mass index on perioperative outcomes after major surgery: results from the National Surgical Quality Improvement Program (ACS-NSQIP) 2005-2011. World J Surg 2015;39:2376–85. [DOI] [PubMed] [Google Scholar]
- 127. Echahidi N, Pibarot P, Despres JP, et al. Metabolic syndrome increases operative mortality in patients undergoing coronary artery bypass grafting surgery. J Am Coll Cardiol 2007;50:843–51. [DOI] [PubMed] [Google Scholar]
- 128. Wang L, Pryor AD, Altieri MS, et al. Perioperative rates of deep vein thrombosis and pulmonary embolism in normal weight vs obese and morbidly obese surgical patients in the era post venous thromboembolism prophylaxis guidelines. Am J Surg 2015;210:859–63. [DOI] [PubMed] [Google Scholar]
- 129. Vandiver JW, Ritz LI, Lalama JT. Chemical prophylaxis to prevent venous thromboembolism in morbid obesity: literature review and dosing recommendations. J Thromb Thrombolysis 2016;41:475–81. [DOI] [PubMed] [Google Scholar]
- 130. Caprini JA, Tapson VF, Hyers TM, et al. Treatment of venous thromboembolism: adherence to guidelines and impact of physician knowledge, attitudes, and beliefs. J Vasc Surg 2005;42:726–33. [DOI] [PubMed] [Google Scholar]
- 131. Werner BC, Burrus MT, Looney AM, et al. Obesity Is associated with increased complications after operative management of end-stage ankle arthritis. Foot Ankle Int 2015;36:863–70. [DOI] [PubMed] [Google Scholar]
- 132. Werner BC, Griffin JW, Yang S, et al. Obesity is associated with increased postoperative complications after operative management of proximal humerus fractures. J Shoulder Elbow Surg 2015;24:593–600. [DOI] [PubMed] [Google Scholar]
- 133. Werner BC, Burrus MT, Browne JA, et al. Superobesity (body mass index >50 kg/m2) and complications after total shoulder arthroplasty: an incremental effect of increasing body mass index. J Shoulder Elbow Surg 2015;24:1868–75. [DOI] [PubMed] [Google Scholar]
- 134. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e227S–e77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Chen SY, Stem M, Schweitzer MA, et al. Assessment of postdischarge complications after bariatric surgery: A National Surgical Quality Improvement Program analysis. Surgery 2015;158:777–86. [DOI] [PubMed] [Google Scholar]
- 136. Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21:S1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. American Society for Metabolic and Bariatric Surgery Clinical Issues Committee ASMBS updated position statement on prophylactic measures to reduce the risk of venous thromboembolism in bariatric surgery patients. Surg Obes Relat Dis 2013;9:493–7. [DOI] [PubMed] [Google Scholar]
- 138. Myzienski AE, Lutz MF, Smythe MA. Unfractionated heparin dosing for venous thromboembolism in morbidly obese patients: case report and review of the literature. Pharmacotherapy 2010;30:324. [DOI] [PubMed] [Google Scholar]
- 139. Schaefer DC, Hufnagle J, Williams L. Rapid heparin anticoagulation: use of a weight-based nomogram. Am Fam Physician 1996;54:2517–21. [PubMed] [Google Scholar]
- 140. Bauer SR, Ou NN, Dreesman BJ, et al. Effect of body mass index on bleeding frequency and activated partial thromboplastin time in weight-based dosing of unfractionated heparin: a retrospective cohort study. Mayo Clin Proc 2009;84:1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Al-Yaseen E, Wells PS, Anderson J, et al. The safety of dosing dalteparin based on actual body weight for the treatment of acute venous thromboembolism in obese patients. J Thromb Haemost 2005;3:100–2. [DOI] [PubMed] [Google Scholar]
- 142. Doherty C. Vertical banded gastroplasty. Surg Clin North Am 2001;81:1097–112. [DOI] [PubMed] [Google Scholar]
- 143. Maynard EP. State residency regulation: a view from out of state. Bull N Y Acad Med 1991;67:355–8. [PMC free article] [PubMed] [Google Scholar]
- 144. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis 2008;4:625–31. [DOI] [PubMed] [Google Scholar]
- 145. Yang FM, Marcantonio ER, Inouye SK, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics 2009;50:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 2009;110:548–55. [DOI] [PubMed] [Google Scholar]
- 147. Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care 2011;17:376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wan Y, Xu J, Ma D, et al. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology 2007;106:436–43. [DOI] [PubMed] [Google Scholar]
- 149. Cibelli M, Fidalgo AR, Terrando N, et al. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol 2010;68:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Terrando N, Monaco C, Ma D, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 2010;107:20518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Fidalgo AR, Cibelli M, White JP, et al. Systemic inflammation enhances surgery-induced cognitive dysfunction in mice. Neurosci Lett 2011;498:63–6. [DOI] [PubMed] [Google Scholar]
- 152. Terrando N, Eriksson LI, Ryu JK, et al. Resolving postoperative neuroinflammation and cognitive decline. Ann Neurol 2011;70:986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Degos V, Vacas S, Han Z, et al. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology 2013;118:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Terrando N, Yang T, Ryu JK, et al. Stimulation of the alpha7 nicotinic acetylcholine receptor protects against neuroinflammation after tibia fracture and endotoxemia in mice. Mol Med 2015;20:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Feng X, Valdearcos M, Uchida Y, et al. Microglia mediate postoperative hippocampal neuroinflammation and cognitive decline in mice. JCI Insight 2017;2:e91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hirsch J, Vacas S, Terrando N, et al. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation 2016;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fragiadakis GK, Gaudilliere B, Ganio EA, et al. Patient-specific immune states before surgery are strong correlates of surgical recovery. Anesthesiology 2015;123:1241–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gaudilliere B, Fragiadakis GK, Bruggner RV, et al. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med 2014;6:255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Forsberg A, Cervenka S, Fagerlun MJ, et al. The immune response of the human brain to abdominal surgery. Ann Neurol 2017;84:572–82. [DOI] [PubMed] [Google Scholar]
- 160. Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013;17:644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hall JE, da Silva AA, do Carmo JM, et al. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 2010;285:17271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res 2015;63:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997;146:214–22. [DOI] [PubMed] [Google Scholar]
- 164. Resnick HE, Valsania P, Halter JB, et al. Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. J Epidemiol Community Health 2000;54:596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–9. [DOI] [PubMed] [Google Scholar]
- 166. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014;2014:943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167. Balschun D, Wetzel W, Del Rey A, et al. Interleukin-6: a cytokine to forget. FASEB J 2004;18:1788–90. [DOI] [PubMed] [Google Scholar]
- 168. Lynch MA. Neuroinflammatory changes negatively impact on LTP: a focus on IL-1beta. Brain Res 2015;1621:197–204. [DOI] [PubMed] [Google Scholar]
- 169. Anstey KJ, Cherbuin N, Budge M, et al. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev 2011;12:e426–37. [DOI] [PubMed] [Google Scholar]
- 170. Hudetz JA, Patterson KM, Iqbal Z, et al. Metabolic syndrome exacerbates short-term postoperative cognitive dysfunction in patients undergoing cardiac surgery: results of a pilot study. J Cardiothorac Vasc Anesth 2011;25:282–7. [DOI] [PubMed] [Google Scholar]
- 171. Hudetz JA, Patterson KM, Amole O, et al. Postoperative cognitive dysfunction after noncardiac surgery: effects of metabolic syndrome. J Anesth 2011;25:337–44. [DOI] [PubMed] [Google Scholar]
- 172. Feng X, Degos V, Koch LG, et al. Surgery results in exaggerated and persistent cognitive decline in a rat model of the Metabolic Syndrome. Anesthesiology 2013;118:1098–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Su X, Feng X, Terrando N, et al. Dysfunction of inflammation-resolving pathways is associated with exaggerated postoperative cognitive decline in a rat model of the metabolic syndrome. Mol Med 2013;18:1481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology 2012;116:788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Siddiqi N, Stockdale R, Britton AM, et al. Interventions for preventing delirium in hospitalised patients. Cochrane Database Syst Rev 2007;2:CD005563. [DOI] [PubMed] [Google Scholar]
- 176. Bjorkelund KB, Hommel A, Thorngren KG, et al. Reducing delirium in elderly patients with hip fracture: a multi-factorial intervention study. Acta Anaesthesiol Scand 2010;54:678–88. [DOI] [PubMed] [Google Scholar]
- 177. Chen CC, Lin MT, Tien YW, et al. Modified hospital elder life program: effects on abdominal surgery patients. J Am Coll Surg 2011;213:245–52. [DOI] [PubMed] [Google Scholar]
- 178. Balas MC, Devlin JW, Verceles AC, et al. Adapting the ABCDEF bundle to meet the needs of patients requiring prolonged mechanical ventilation in the long-term acute care hospital setting: historical perspectives and practical implications. Semin Respir Crit Care Med 2016;37:119–35. [DOI] [PubMed] [Google Scholar]
- 179. Avidan MS, Fritz BA, Maybrier HR, et al. The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study: protocol for an international multicentre randomised controlled trial. BMJ Open 2014;4:e005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at seven California community hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med 2017;45:171–78. [DOI] [PubMed] [Google Scholar]
- 181. Ward DT, Metz LN, Horst PK, et al. Complications of morbid obesity in total joint arthroplasty: risk stratification based on BMI. J Arthroplasty 2015;30:42–6. [DOI] [PubMed] [Google Scholar]
- 182. Kempegowda H, Richard R, Borade A, et al. Obesity is associated with high peri-operative complications among surgically treated intertrochanteric fracture of the femur. J Orthop Trauma 2017;31:352–357. [DOI] [PubMed] [Google Scholar]
- 183. Olsen LL, Moller AM, Brorson S, et al. The impact of lifestyle risk factors on the rate of infection after surgery for a fracture of the ankle. Bone Joint J 2017;99-B:225–30. [DOI] [PubMed] [Google Scholar]
- 184. Thelwall S, Harrington P, Sheridan E, et al. Impact of obesity on the risk of wound infection following surgery: results from a nationwide prospective multicentre cohort study in England. Clin Microbiol Infect 2015;21:1008 e1–8. [DOI] [PubMed] [Google Scholar]
- 185. Ghanta RK, LaPar DJ, Zhang Q, et al. Obesity increases risk-adjusted morbidity, mortality, and cost following cardiac surgery. J Am Heart Assoc 2017;6:e003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Winfield RD, Reese S, Bochicchio K, et al. Obesity and the risk for surgical site infection in abdominal surgery. Am Surg 2016;82:331–6. [PubMed] [Google Scholar]
- 187. Arnaoutakis DJ, Scully RE, Sharma G, et al. Impact of body mass index and gender on wound complications after lower extremity arterial surgery. J Vasc Surg 2017;65:1713–18 e1. [DOI] [PubMed] [Google Scholar]
- 188. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Wilson JA, Clark JJ. Obesity: impediment to postsurgical wound healing. Adv Skin Wound Care 2004;17:426–35. [DOI] [PubMed] [Google Scholar]
- 190. Ni YN, Luo J, Yu H, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care 2017;21:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Chiumello D, Colombo A, Algieri I, et al. Effect of body mass index in acute respiratory distress syndrome. Br J Anaesth 2016;116:113–21. [DOI] [PubMed] [Google Scholar]
- 192. Kon ZN, Dahi S, Evans CF, et al. Class III obesity is not a contraindication to venovenous extracorporeal membrane oxygenation support. Ann Thorac Surg 2015;100:1855–60. [DOI] [PubMed] [Google Scholar]
- 193. Acute Respiratory Distress Syndrome Network Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–8. [DOI] [PubMed] [Google Scholar]
- 194. Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159–68. [DOI] [PubMed] [Google Scholar]
- 195. Weig T, Janitza S, Zoller M, et al. Influence of abdominal obesity on multiorgan dysfunction and mortality in acute respiratory distress syndrome patients treated with prone positioning. J Crit Care 2014;29:557–61. [DOI] [PubMed] [Google Scholar]
- 196. De Jong A, Molinari N, Sebbane M, et al. Feasibility and effectiveness of prone position in morbidly obese patients with ARDS: a case-control clinical study. Chest 2013;143:1554–61. [DOI] [PubMed] [Google Scholar]
- 197. McClave SA, Kushner R, Van Way CW 3rd, et al. Nutrition therapy of the severely obese, critically ill patient: summation of conclusions and recommendations. JPEN J Parenter Enteral Nutr 2011;35:88S–96S. [DOI] [PubMed] [Google Scholar]