Curcumin sensitized chemoresistant pancreatic cancer cells to gemcitabine and prevented the formation of spheroids, a hallmark of CSCs, by inhibiting the expression of the PRC2 subunit EZH2 and its related lncRNA PVT1.
Abstract
Development of resistance to chemotherapeutic drugs is a major challenge in the care of patients with pancreatic ductal adenocarcinoma (PDAC). Acquired resistance to chemotherapeutic agents in PDAC has been linked to a subset of cancer cells termed ‘cancer stem cells’ (CSCs). Therefore, an improved understanding of the molecular events underlying the development of pancreatic CSCs is required to identify new therapeutic targets to overcome chemoresistance. Accumulating evidence indicates that curcumin, a phenolic compound extracted from turmeric, can overcome de novo chemoresistance and re-sensitize tumors to various chemotherapeutic agents. However, the underlying mechanisms for curcumin-mediated chemosensitization remain unclear. The Enhancer of Zeste Homolog-2 (EZH2) subunit of Polycomb Repressive Complex 2 (PRC2) was recently identified as a key player regulating drug resistance. EZH2 mediates interaction with several long non-coding RNAs (lncRNAs) to modulate epithelial–mesenchymal transition and cancer stemness, phenomena commonly associated with drug resistance. Here, we report the re-sensitization of chemoresistant PDAC cells by curcumin through the inhibition of the PRC2-PVT1-c-Myc axis. Using gemcitabine-resistant PDAC cell lines, we found that curcumin sensitized chemoresistant cancer cells by inhibiting the expression of the PRC2 subunit EZH2 and its related lncRNA PVT1. Curcumin was also found to prevent the formation of spheroids, a hallmark of CSCs, and to down-regulate several self-renewal driving genes. In addition, we confirmed our in vitro findings in a xenograft mouse model where curcumin inhibited gemcitabine-resistant tumor growth. Overall, this study indicates clinical relevance for combining curcumin with chemotherapy to overcome chemoresistance in PDAC.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies and the fourth leading cause of cancer-related deaths in the United States (1). While curative resection and chemotherapy is the standard of care for PDAC patients, in most cases it only provides a short-term survival benefit. Furthermore, the majority of these patients will eventually develop resistance to therapeutic drugs through several mechanisms including the activation of multidrug resistance and pro-survival pathways (2–4). There is accumulating evidence that cancer stem cells (CSCs) present in PDAC tumors, which have high tumorigenic abilities to self-renew and produce differentiated progeny, contribute to chemoresistance (5–10). It is speculated that conventional chemotherapy reduces the tumor mass by affecting rapidly dividing PDAC cells that constitute the bulk of the tumor, but fails to target CSCs. This results in treatment failure and tumor recurrence (11). Therefore, a better understanding of the molecular events characterizing pancreatic CSCs is necessary to identify improved therapeutic targets to overcome chemoresistance.
Enhancer of Zeste Homolog-2 (EZH2), a catalytic subunit of polycomb repressive complex 2 (PRC2), is a histone methyltransferase that epigenetically maintains CSCs by regulating gene expression (12,13). A recent study found that EZH2 was overexpressed in the nucleus of ~70% of PDACs, highlighting its key oncogenic role in PDACs (14). Furthermore, overexpression of EZH2 in a PDAC cell line resulted in enhanced resistance to gemcitabine, a first-line drug for treatment of PDACs (14). One of the mechanisms by which EZH2 acts as an oncogene in PDAC is by modulating long non-coding RNA (lncRNA) PVT1 (15–17), a known inducer of drug resistance in PDAC (18). While the roles of other non-coding RNAs such as microRNAs have been well established in cancers over the last decade, the functional roles of lncRNAs in cancers have only recently started to come to light. Several studies have therapeutically targeted EZH2 and PVT1 individually (13,18–21); however, whether co-targeting these two genes could further attenuate chemoresistance in pancreatic cancer remains to be determined.
Molecular inhibitors that specifically target certain genes or cellular pathways can only provide a short delay to almost inevitable cancer progression; cancer cells will eventually acquire resistance to these inhibitors by activating alternative cellular pathways. Multi-gene inhibitors can significantly prolong chemosensitivity over conventional therapeutic agents by simultaneously suppressing several oncogenic pathways. However, the likelihood of off-target effects as well as the complexities involved in the assessment of drug efficacy have hindered the development of such drugs. Over the past decades, numerous studies have shown that curcumin, a phenolic compound extracted from Curcuma longa, has potent anti-inflammatory, anti-oxidant and anti-tumor properties (22,23). Furthermore, curcumin has been shown to sensitize chemotherapeutic agents in multiple cancers. In colorectal cancer, curcumin was shown to enhance cytotoxicity of 5-fluorouracil as well as FOLFOX (24,25). Similarly, curcumin sensitized cisplatin in head and neck squamous cell carcinoma and ovarian cancer, further confirming chemosensitizing potential of curcumin (26,27). In PDACs, curcumin is known to simultaneously suppress multiple oncogenes, including vascular endothelial growth factor (VEGF) and cytochrome c oxidase subunit II, as well as genes involved in chemoresistance such as Akt, Erk and EZH2 (22,28). Understanding the underlying mechanisms of this potent natural compound that targets multiple signaling pathways without noticeable side-effects has become a major avenue for the development of new cancer drugs. Many of the established chemoresistance-associated genes such as EZH2 have been confirmed to be targeted by curcumin (25,29). However, whether curcumin also modulates non-coding RNAs such as lncRNAs remains unexplored.
In this study, we generated a gemcitabine-resistant PDAC cell line. We examined acquired chemoresistance in these pancreatic cancer cells by comparing its properties to the gemcitabine-sensitive parental cell line as well as to a PDAC cell line with inherent gemcitabine resistance. We found that in gemcitabine-resistant PDAC cell lines PVT1 is up-regulated and that curcumin sensitizes some PDAC cells to gemcitabine. We noted that curcumin down-regulates the expression of EZH2, PVT1 and their down-stream targets in gemcitabine-resistant cells. Moreover, curcumin also suppresses the spheroid-forming ability of gemcitabine-resistant PDAC cells, indicating that curcumin specifically targets CSCs. Overall, this study establishes a potential clinical application for combining curcumin with chemotherapy to overcome chemoresistance in PDAC.
Materials and methods
Cell lines and materials
BxPC3, MiaPaCa2 and Panc1 PDAC cells were purchased from ATCC (Manassas, VA). BxPC3 cells were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA) containing 10% fetal bovine serum, 1% penicillin, 1% streptomycin (Gibco) and 2 mM L-glutamine (Thermo Fisher Scientific, Waltham, MA). MiaPaCa2 and Panc1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) containing 10% fetal bovine serum and 1% penicillin and streptomycin (Gibco). Gemcitabine-resistant BxPC3 (BxPC3-GemR) cell lines were established by treating BxPC3 cells with increasing concentrations of gemcitabine (Sigma-Aldrich, St Louis, MO) over a duration of several months. This gemcitabine-resistant cell line was maintained in RPMI 1640 medium with 10% fetal bovine serum, 1% penicillin, 1% streptomycin, 2 mM L-glutamine and 1 µM gemcitabine. All cell lines were maintained at 37°C in a humidified incubator (5% CO2) and routinely tested and authenticated using a panel of genetic and epigenetic markers. Curcumin (BCM-95, Dolcas Biotech, NJ) was dissolved in DMSO and diluted to appropriate concentrations in culture medium. Gemcitabine was diluted in disulfide water.
Viability, cell cycle, apoptosis and clonogenic assays
To assess the viability of cells after treatments, cancer cells were seeded in 96-well plates (2–5 × 103 cells/well) and incubated for 72 h with curcumin and/or gemcitabine. MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was then conducted as described previously (30). In order to assess the synergism between curcumin and gemcitabine on viability, the combination index (CI) was calculated using the Chou–Talalay equation (31) at 50% inhibitory concentration. A CI index of less than 1 was considered to be a synergistic interaction. The percentages of cells in the G0/G1, S and G2/M phases of the cell cycle were determined using a Muse Cell Cycle Assay Kit /MCH10016 (Millipore, Billerica, MA), and the apoptotic cell fraction was measured using a Muse Annexin V and Dead Cell Assay Kit (Millipore) on a Muse™ Cell Analyzer (Millipore) according to the manufacturer’s instructions. Clonogenic assays were performed as described previously (30). The number of colonies (>50 cells) was counted using GeneTools (Syngene, Cambridge, UK). All experiments were conducted in triplicate on at least three different occasions.
RNA isolation and qRT-PCR analysis
Cells in 12-well plates were treated with an IC50 concentration of curcumin and/or gemcitabine, as determined by MTT assays (for BxPC3 and BxPC3-GemR: 8 µM curcumin and 20 nM gemcitabine; for Panc1 cells: 20 µM curcumin and 50 nM gemcitabine). After 48 h of treatment, total RNA was isolated using the miRNeasy Mini Kit (Qiagen, Hilden, Germany) and converted to cDNA using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). 5 ng of the cDNA samples were mixed with 0.5 µl of 10 µM each of forward and reverse primers specific for the target genes, 5 µl SYBR GREEN Master Mix (Thermo Fisher Scientific) and 3.5 µl nuclease-free water (except for MALAT1 and HOTAIR gene expression analysis). For MALAT1 and HOTAIR, 5 ng cDNA samples were mixed with 1 µl TaqMan RNA Assay (20×) specific for target genes, 10 µl TaqMan Universal PCR Master Mix (2×) (Applied Biosystems, Foster City, CA) and 8 µl nuclease-free water. β-actin was used as an endogenous control for both assays. Sequences of all primer sets used are shown in Supplementary Table 1, available at Carcinogenesis Online. For all experiments, each sample was run in duplicate.
Western blotting
Following 48-h treatment with curcumin and/or gemcitabine, total cellular protein was extracted and western immunoblotting was performed as described previously (32). Supplementary Table 2, available at Carcinogenesis Online, lists the primary antibodies that were used. Secondary anti-mouse or anti-rabbit antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX). β-actin (Sigma-Aldrich) was used as a reference protein. All protein bands on the membranes were visualized using G:Box (Syngene), and the signal intensity of each band was calculated with GeneTools (Syngene).
Spheroid formation
Single-cell suspensions of the pancreatic cancer cells were prepared by mild enzymatic dissociation using TrypLE (Gibco). Cells were then seeded in 96-well Costar® ultra-low attachment plates (Corning, Corning, NY) at 3000 cells/well in serum-free medium (DMEM/F12) supplemented with 1% penicillin, 1% streptomycin (Gibco), 1% B27, 1% N2 (Gibco), 10 ng/ml human recombinant basic fibroblast growth factor (bFGF) (Gibco) and 20 ng/ml epidermal growth factor (EGF, Sigma-Aldrich) and the cells were then incubated at 37°C with 5% CO2 to allow spheroid formation for 48 h. The spheroids were then treated with 8 µM curcumin and/or 20 nM gemcitabine for 48 h and collected for further analysis.
Animal experiments
Five-week-old male athymic nude mice (Harlan Laboratories, Houston, TX) were housed under controlled light conditions and provided food and water ad libitum. Xenograft tumors were generated by subcutaneous injection of 1 × 106 BxPC3-GemR cells. Tumor volume was calculated using the formula: (π/6)(length × width × height). Once the average tumor size reached 50 mm3, animals were randomly divided into four groups with 10 animals in each group: (1) control vehicle (PBS), (2) 100 mg curcumin/kg body weight daily, (3) 25 mg gemcitabine/kg body weight once every 4 days or (4) gemcitabine and curcumin together at the concentrations listed above. All treatments were injected intraperitoneally daily for 28 days, followed by euthanasia. Tumor samples were dissected, weighed and stored in RNAlater (Sigma-Aldrich) for further analysis. The animal protocol was approved by the Institutional Animal Care and Use Committee, Baylor Scott& White Research Institute, Dallas, Texas.
Statistical analysis
All analyses were performed using GraphPad Prism Ver.6.0 (GraphPad Software Inc., San Diego, CA). All data were expressed as mean ± SEM with statistical significance indicated when P < 0.05. Statistical comparisons were determined using unpaired t-test or one-way ANOVA with Tukey’s post hoc tests.
Results
Generation of gemcitabine-resistant BxPC3 cells
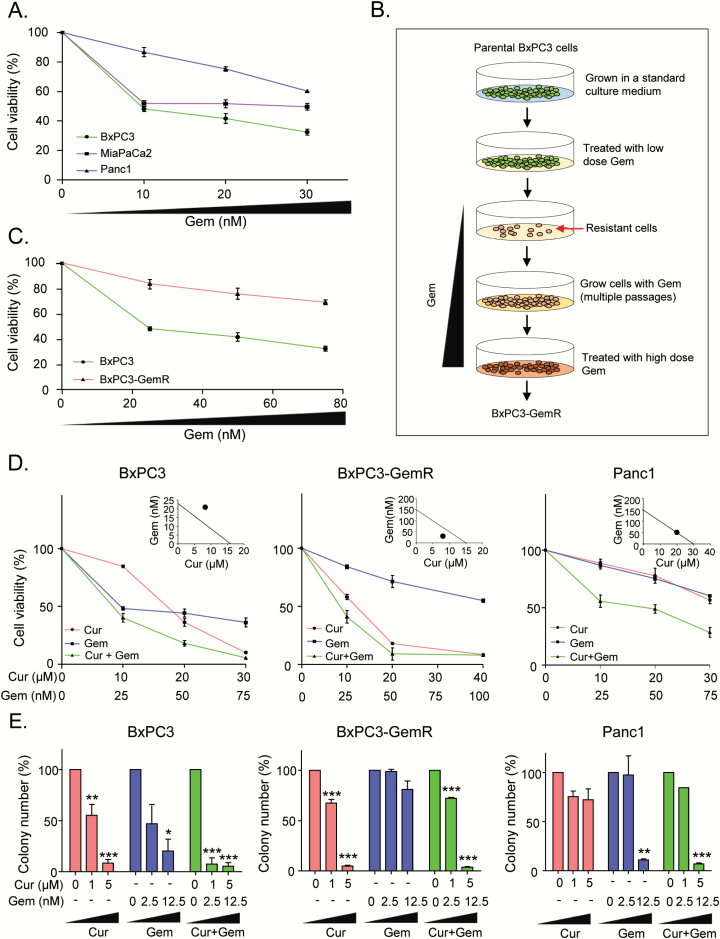
In order to select the appropriate cell lines in which to study drug resistance in pancreatic cancer, we evaluated the chemoresistance of various PDAC cell lines to gemcitabine, a first-line chemotherapeutic drug used in PDAC. Among the three cell lines tested for changes in cellular proliferation using the MTT assay, BxPC3 cells were the most sensitive and Panc1 cells were the least sensitive to gemcitabine (Figure 1A). Therefore, we chose BxPC3 cells and Panc1 as models of inherently chemosensitive and chemoresistant pancreatic cancer cell lines, respectively, for further analysis.
Figure 1.
Curcumin sensitizes gemcitabine-resistant cells. (A) MTT assay was performed to determine the effect of gemcitabine on three different PDAC cell lines in culture. (B) Scheme for establishment of gemcitabine-resistant BxPC3 cells. Briefly, the parental BxPC3 cells were cultured in RPMI medium containing increasing concentrations of gemcitabine until they acquired resistance to gemcitabine. (C) MTT assay was performed to compared cell viability against gemcitabine in resistant BxPC3 cells (BxPC3-GemR) and the parental BxPC3 cells. (D) MTT assay was used to assess cell of BxPC3, BxPC3-GemR and Panc1 cells. Chou-Talalay combination index (CI) (right corner) shows whether combination of curcumin and gemcitabine is synergistic. (E) colony formation assay was used to assess clonogenicity of BxPC3, BxPC3-GemR and Panc1 cells following treatment with different concentrations of curcumin and/or gemcitabine for 2 days. BxPC3-GemR, gemcitabine-resistant BxPC3 cell line; Cur, curcumin; Gem, gemcitabine. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
We then generated a chemoresistant cell line against gemcitabine by culturing BxPC3 cells in media containing increasing concentrations of gemcitabine and then maintained them at 1 µM gemcitabine (Figure 1B). To confirm the acquired chemoresistance of these cells, we analyzed their viability at various gemcitabine concentrations compared to their parental counterparts. We found that BxPC3 cells continually cultured in gemcitabine were able to proliferate in higher concentrations of gemcitabine than the parental cell line (Figure 1C). Next, we compared clonogenicity after treatment with gemcitabine between the two BxPC3 cell lines as well as the inherently gemcitabine-resistant Panc1 cells. The clonogenic ability of the gemcitabine-maintained cells was impacted significantly less by gemcitabine than were the parental cells or even the Panc1 cells (Supplementary Figure 1A, available at Carcinogenesis Online). This suggests that we had indeed established a gemcitabine-resistant BxPC3 cell line. Hereafter, we will refer to this gemcitabine-resistant BxPC3 cell line as ‘BxPC3-GemR’ and use it as a model to study acquired gemcitabine resistance.
Curcumin sensitizes BxPC3-GemR cells to gemcitabine
We evaluated the effect of curcumin on the gemcitabine sensitivity in BxPC3, BxPC3-GemR and Panc1 cell lines by determining changes in cellular proliferation after treatment with curcumin or gemcitabine alone, or in combination. Both gemcitabine and curcumin independently suppressed cell proliferation of parental BxPC3 cells, and the combination of the two compounds further inhibited cell proliferation (Figure 1D). The Chou–Talalay CI (31) showed no synergism between curcumin and gemcitabine in parental BxPC3 cells (CI = 1.37), suggesting that the inhibitory effects of curcumin and gemcitabine in the parental BxPC3 cells were independent. In contrast, BxPC3-GemR cells appear to show resistance to gemcitabine, but not curcumin. However, combined treatment did further enhance cellular cytotoxicity. The CI indicated that gemcitabine and curcumin synergistically enhanced cytotoxicity in BxPC3-GemR cells (CI = 0.67; Figure 1D). On the other hand, both gemcitabine and curcumin inhibited cellular proliferation of Panc1 cells. Based on the Chou–Talalay CI, the combined treatment enhanced cellular cytotoxicity additively (Figure 1D).
Next, we performed a colony formation assay to examine the effects of curcumin and/or gemcitabine on cell proliferation and survival. In parental BxPC3 cells, treatment with either compound effectively inhibited formation of colonies in a dose-dependent manner, and the combination of the two compounds further inhibited the clonogenic capacity (Figure 1E). However, gemcitabine treatment alone did not attenuate the number of colonies in the gemcitabine-resistant cell line BxPC3-GemR at lower doses (Figure 1E). In contrast, curcumin treatment alone effectively suppressed colony formation of BxPC3-GemR cells but not Panc1 cells (Figure 1E). Interestingly, the response to the combination of curcumin and gemcitabine by BxPC3-GemR cells did not differ significantly from that of curcumin treatment alone, indicating that curcumin plays a primary role in inhibiting colonization in these cancer cells (Figure 1E). Taken together, these results suggest that not only is curcumin cytotoxic to both types of BxPC3 cells but this natural compound also sensitizes gemcitabine-resistant cells to gemcitabine and suppresses cellular proliferation.
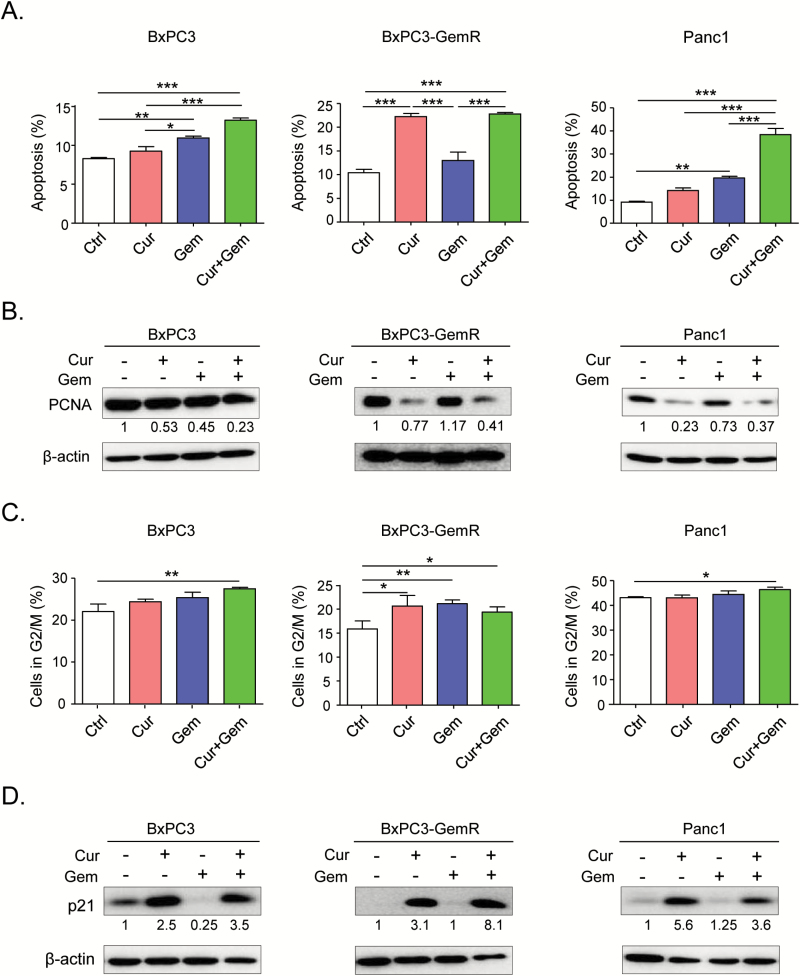
Curcumin induces apoptosis and cell cycle arrest in BxPC3-GemR cells
At the molecular level, gemcitabine suppresses cell proliferation by inhibiting DNA synthesis and inducing apoptosis (33,34). Therefore, we evaluated the effects of curcumin on gemcitabine-induced cell cycle arrest and apoptosis. We used the IC50 concentrations of curcumin and gemcitabine obtained from our MTT assay results (Figure 1D) to establish treatment concentrations of these compounds for each cell line. The concentrations were as follows: BxPC3 and BxPC3-GemR: 8 µM curcumin and 20 nM gemcitabine; Panc1: 20 µM curcumin and 50 nM gemcitabine. Gemcitabine treatment alone did not induce apoptosis in BxPC3-GemR cells. However, consistent with the results of our MTT and colony formation assays, curcumin treatment alone as well as in combination with gemcitabine induced apoptosis in BxPC3-GemR cells (Figure 2A). The cell cycle analysis showed that both curcumin and gemcitabine induced G2/M phase arrest in BxPC3-GemR cells, while the combination of curcumin and gemcitabine caused G2/M phase arrest in all three cell lines (Figure 2C). In addition, to confirm that curcumin inhibited cellular proliferation, we assessed the protein expression of a proliferative marker, proliferating cell nuclear antigen (PCNA) and a well-known cell cycle regulating tumor suppressor, p21. Western blot analysis showed that curcumin, alone or in combination with gemcitabine, suppressed the expression of PCNA, while up-regulating p21 (Figure 2B and D). These results are consistent with a previous publication (34), which reported that curcumin not only induces apoptosis but also modulates the cell cycle in human pancreatic cancer cells.
Figure 2.
Curcumin sensitizes gemcitabine-resistant cells by inducing apoptosis and cell cycle arrest. (A) Apoptosis assay was performed to determine the percentage of apoptotic cells in each cell line after treatment with IC50 concentrations of curcumin and/or gemcitabine for 2 days. (B) Western blot analysis of the cell proliferation marker proliferation marker, PCNA, in cell extracts from pancreatic cancer cell lines after treatment with curcumin and/or gemcitabine. β-actin was used as a loading control. The values are relative to control treatment for each cell line. (C) Cell cycle analysis was conducted to determine whether curcumin induces G2/M arrest. (D) Western blot analysis of the tumor suppressor protein p21 in cell extracts from pancreatic cancer cell lines treated with curcumin and/or gemcitabine. β-actin was used as a loading control. The values are relative to control treatment for each cell line. BxPC3-GemR, gemcitabine resistant-BxPC3 cell line; Ctrl, control; Cur, curcumin; Gem, gemcitabine; PCNA, proliferating cell nuclear antigen. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
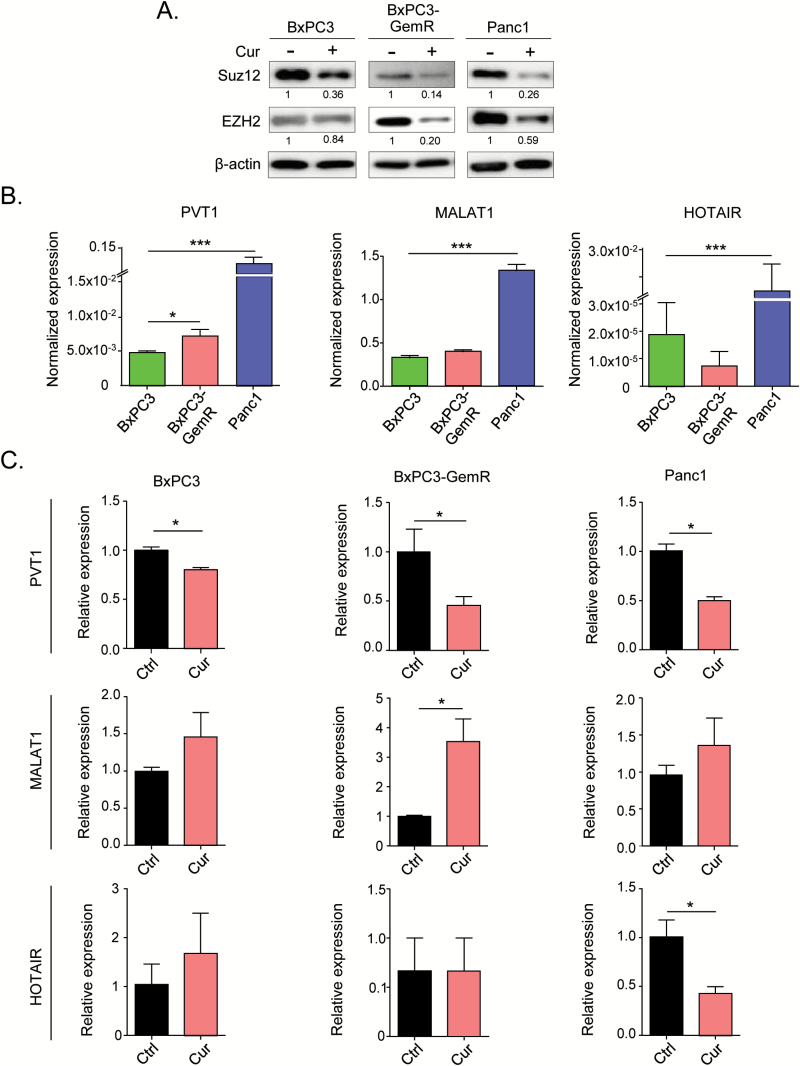
Curcumin inhibits PRC2 and lncRNA-PVT1 expression
One of the theories as to why some PDACs become resistant to chemotherapeutic drugs such as gemcitabine is that these tumors acquire a high portion of CSCs (5–8). PRC2 plays a key role in CSC maintenance through epigenetic regulation of self-renewal-associated genes (8). This function of PRC2 has been reported in several cancer types, including pancreatic cancer cells, and two subunits of PRC2 (SUZ12 and EZH2) have been identified as the key oncogenes (5–8). Therefore, we investigated the effects of curcumin on the expression levels of these PRC2 subunits. Western blot analysis showed that the protein expression of both SUZ12 and EZH2 was markedly down-regulated by curcumin treatment in all three cell lines, especially in the gemcitabine-resistant BxPC3-GemR and Panc1 cells (Figure 3A). These results suggest that curcumin regulates PRC2 activity by regulating the expression of its constituent proteins.
Figure 3.
Curcumin modulates chemoresistance in gemcitabine-resistant cells by inhibiting PVT1 and PRC2. (A) Western blot analysis of the PRC2 sub-components SUZ12 and EZH2 in cell extracts from pancreatic cell lines treated with vehicle or curcumin. β-actin was used as loading control. The intensity values relative to control treatment for each cell line were calculated using GeneTools. (B) qRT-PCR analysis of lncRNAs PVT1, MALAT1 and HOTAIR expression in pancreatic cancer cell lines and (C) qRT-PCR analysis of the lncRNAs following the cells were treated with curcumin. BxPC3-GemR, gemcitabine-resistant BxPC3 cell line; Ctrl, control; Cur, curcumin. Statistical significance: *P < 0.05, ***P < 0.001.
PRC2 has been reported to bind to several oncogenic lncRNAs, including PVT1, metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and HOX transcript antisense RNA (HOTAIR), to function as a repressive complex (15–17). Based on this, we investigated the expression of these PRC2-associated lncRNAs in the three cell lines and their possible regulation by curcumin. Our results showed that of the three lncRNAs, only the expression of PVT1 was significantly higher in BxPC3-GemR than in BxPC3, suggesting that PVT1 is involved in acquisition of chemoresistance (Figure 3B). All three lncRNAs were expressed at a significantly higher level in Panc1 cells than in the other two cell lines (Figure 3B). Interestingly, PVT1 was the only lncRNA significantly down-regulated by curcumin treatment in all three cell lines, while HOTAIR was down-regulated significantly in Panc1 cells only (Figure 3C).
One of the main mechanisms by which PVT1 induces chemoresistance and maintains cancer stemness is through a down-stream effector, c-Myc (35,36,37). C-Myc is a well-recognized oncogene and one of the original genes used to derive induced pluripotent stem cells (iPSCs) (38). PVT1 has been identified as an enhancer of MYC and also acts as a stabilizer of c-Myc protein (39). Interestingly, curcumin treatment suppressed MYC expression in the parental BxPC3 and BxPC3-GemR cells (Supplementary Figure 2, available at Carcinogenesis Online). Taken together, these results suggest that curcumin sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine by down-regulating the PRC2-PVT1-c-Myc axis.
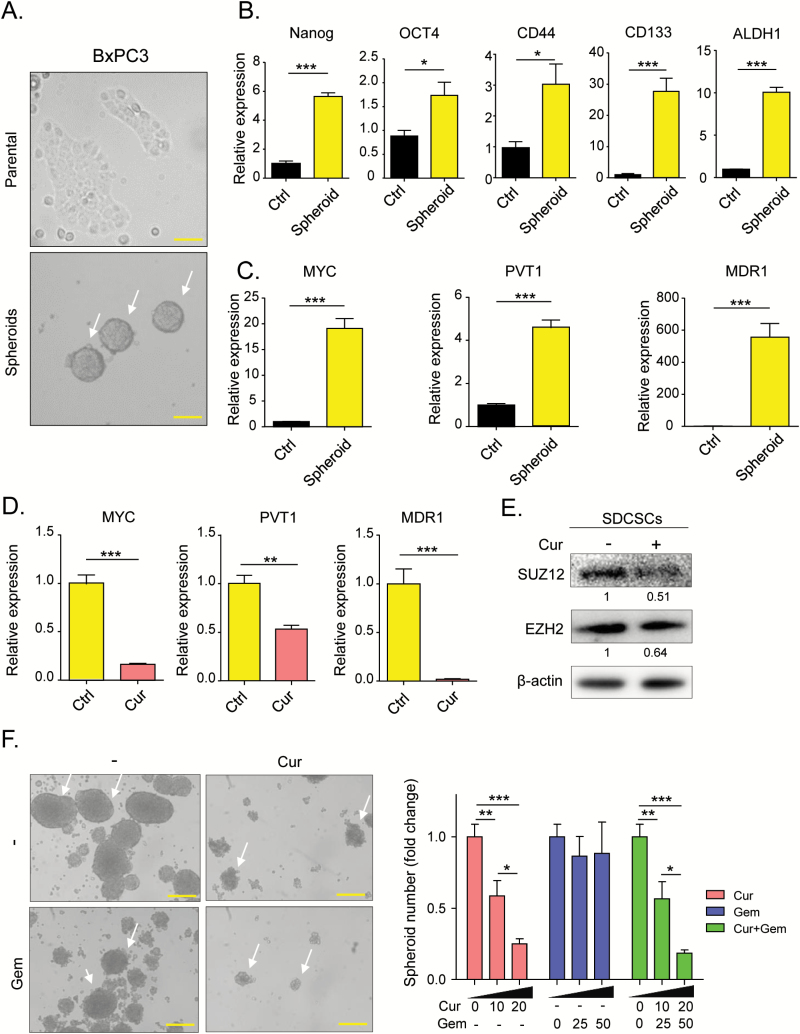
Curcumin inhibits spheroid-derived cancer stem cell (SDCSC) formation
Since PRC2 and c-Myc are involved in the maintenance of cancer stemness, we generated spheroid-derived cancer stem cells (SDCSCs) from BxPC3 (Figure 4A). We evaluated the effects of curcumin on these cells. First, we confirmed that SDCSCs have a high stem cell population by assessing the expression of stemness markers Nanog, Oct4, CD44, CD133 and ALDH1 (40,41) in comparison to its parental cells. As expected, all stemness markers were significantly up-regulated in SDCSCs in comparison to BxPC3 cells, indicating that these spheroids are enriched in CSCs (Figure 4B). We then assessed the expression of Myc and found that it was also significantly up-regulated in spheroids compared to the parental cells (Figure 4C), as was the expression of PVT1. This up-regulation in SDCSCs indicates that PVT1 plays a key role in the maintenance of cancer stemness (Figure 4C). In addition, we assessed the expression of multi-drug resistance 1 (MDR1) and found that it is expressed at higher levels in SDCSCs, indicating that these stem cells have higher drug resistance than their parental cell line (Figure 4C). We then assessed the expression of Myc and PVT1 in SDCSCs following curcumin treatment. Interestingly, the expression of both PVT1 and Myc was significantly inhibited by curcumin treatment, restoring the expression levels almost to those seen in the parental cell line (Figure 4D). To determine whether curcumin treatment enhances sensitivity to chemotherapeutic agents, we investigated the expression of MDR1 in SDCSCs and found that curcumin treatment resulted in significant down-regulation of MDR1 (Figure 4D). Furthermore, western blot analysis showed that both PRC2 complex subunits, EZH2 and SUZ12, were significantly down-regulated in SDCSCs following curcumin treatment (Figure 4E).
Figure 4.
Curcumin modulates cancer stemness by inhibiting PVT1, PRC2 and c-Myc. (A) Comparison of cell morphology between BxPC3 parental cells (above) and spheroids derived from BxPC3 (below). White allows show BxPC3-derived spheroids. Yellow bars represent 100 µm (above) and 50 µm (below). (B) qRT-PCR analysis of the key transcription factors Nanog, OCT4, CD44, CD133 and ALDH1 in BxPC3 cells and BxPC3-derived spheroids. (C) qRT-PCR analysis of PVT1, Myc and MDR1 in BxPC3 cells and BxPC3-derived spheroids. (D) qRT-PCR analysis was performed to determine the effect of curcumin treatment on the expression levels of PVT1, Myc and MDR1 in BxPC3-derived spheroids. (E) Western blot analysis of PRC2 sub-components SUZ12 and EZH2 in cell extracts from BxPC3-derived spheroids treated with vehicle or curcumin. (F) BxPC3-Spheroid formation assessed in media containing various concentrations of curcumin and/or gemcitabine. White allows show BxPC3-derived spheroids. Yellow bars represent 50 µm (all panels). BxPC3-GemR, gemcitabine-resistant BxPC3 cell line; Ctrl, control; Cur, curcumin; Gem, gemcitabine; SDCSCs, spheroid-derived cancer stem cells before. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we tested whether curcumin impacts SDCSC formation by culturing BxPC3 cells in stem cell media with curcumin and/or gemcitabine. We found that curcumin treatment alone and in combination with gemcitabine significantly attenuated spheroid formation in a dose-dependent manner (Figure 4F). Collectively, these data show that curcumin specifically targets cancer stem cells by inhibiting the PRC2-PVT1-c-Myc axis, which was also consistent with our observation in the BxPC3-GemR cell line. Hence, suppression of cancer stem cells appears to be one of the key mechanisms by which curcumin enhances gemcitabine sensitivity in chemoresistant PDAC.
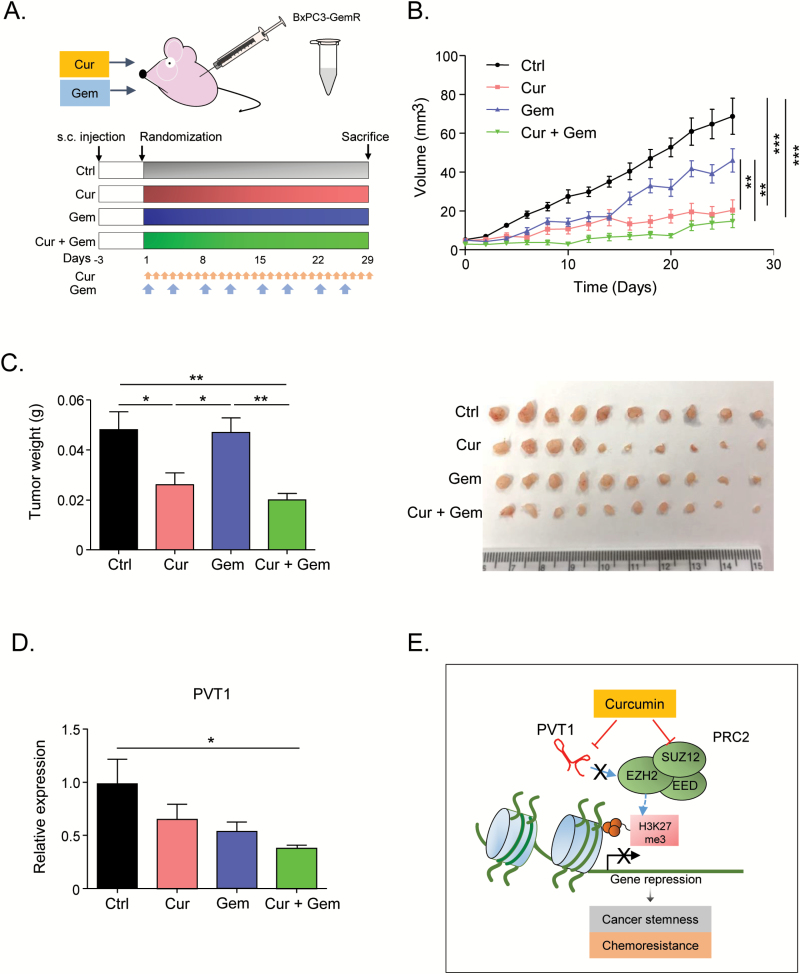
Curcumin enhances sensitivity to gemcitabine in GemR cells in vivo
Finally, we evaluated the ability of curcumin to sensitize gemcitabine-resistant tumors in a xenograft model. We generated chemoresistant xenograft tumors by injecting BxPC3-GemR cells into athymic nude mice and then treated them with intraperitoneal injections of curcumin and/or gemcitabine for 28 days (Figure 5A). Based on tumor weight and volume the BxPC3-GemR-derived tumors showed high tolerance to gemcitabine and proliferated regardless of gemcitabine treatment (Figure 5B and C). However, daily curcumin treatment significantly suppressed tumor growth, and combined treatment with curcumin and gemcitabine further inhibited tumor proliferation (Figure 5B and C). We then assessed the effect of these treatments on the expression of PVT1 in the xenograft tumors and found that combined curcumin and gemcitabine treatment downregulated the expression of PVT1, Myc and MDR1 in the xenograft tumors (Figure 5D and Supplementary Figure 3, available at Carcinogenesis Online). Collectively, these data support our in vitro findings that curcumin re-sensitizes gemcitabine-resistant cancer cells to gemcitabine by attenuating PVT1 (Figure 5E).
Figure 5.
Curcumin enhances sensitivity to gemcitabine in GemR cells in vivo. (A) The timeline for generation of xenograft model in athymic mice and the treatment groups. Blue arrows—gemcitabine injections, red arrows—curcumin injections. (B) Progressive tumor volume increase with treatments. (C) (right) final tumor weight (left) an image of xenograft tumors. (D) qRT-PCR analysis of PVT1 expression levels in tumors from the different treatment groups. (E) Schematic of the mechanism of how curcumin sensitizes gemcitabine and decreasing cancer stemness through attenuation of expression of the PRC2 complex and the lncRNA PVT1. BxPC3-GemR, gemcitabine-resistant BxPC3 cell line; Ctrl, control; Cur, curcumin; Gem, gemcitabine; s.c. injection, subcutaneous injection. Statistical significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The mechanisms by which pancreatic cancers acquire resistance to chemotherapeutic agents are complex and poorly understood. There is accumulating evidence that PDAC is a CSC-driven disease and that CSCs could be involved in the development of chemoresistance that is seen in PDAC (5). Curcumin has been shown in several studies to be a promising sensitizer to chemotherapeutic agents, including gemcitabine, in cancer treatment. Considering that curcumin is a safe and cost-effective natural agent, it could have enormous clinical benefits (42,43). In this study, using a series of in vitro and in vivo experiments, we showed that curcumin enhances chemosensitivity to gemcitabine by modulating the PRC2-PVT1-c-Myc axis in pancreatic cancer. We used gemcitabine-resistant pancreatic cancer cells to demonstrate that curcumin significantly alters cellular proliferation by inducing cell cycle arrest and apoptosis. While BxPC3-GemR cell lines showed high tolerance to gemcitabine alone, combining it with curcumin was more effective at inhibiting their growth. In addition, curcumin inhibited formation of stem-cell-enriched spheroids and sensitized them to gemcitabine.
PRC2 is a key epigenetic mediator that maintains the CSC population by modulating several stemness-associated genes (8). EZH2 and SUZ12 are key subcomponents of PRC2 that are expressed at high levels in CSCs (21). PRC2 silences CDKN2A, E-cadherin and FOXC1 and suppresses differentiation by repressing lineage-specifying factors such as ZBTB16, MX1 and FHL1 in mesenchymal stem cells (44). Furthermore, nuclear accumulation of EZH2 has been identified as a clinical marker of poorly differentiated pancreatic adenocarcinoma, and shRNA-based knock-down of EZH2 enhanced gemcitabine sensitivity in pancreatic cancer (45). Therefore, EZH2 appears to be a key regulatory factor that governs both cancer stemness and drug resistance. Our study shows that by treating chemoresistant cells with curcumin we were able to attenuate the expression of EZH2 in gemcitabine-resistant cells.
Previous studies have demonstrated that PRC proteins such as EZH2 and SUZ12 interact with specific lncRNAs to enhance their oncogenic role in cancers. For example, HOTAIR was the first lncRNA to be identified to form a repressive complex with PRC2, which suppresses the expression of downstream targets through chromatin remodeling (46). Similarly, both MALAT1 and PVT1 have been shown to interact with PRC2 in prostate cancer and thyroid cancer (15,16). Interestingly, PVT1 is located in the well-known cancer-related region 8q24, also known as the ‘gene desert’ (20). Mechanistically, PVT1 is known to participate in DNA rearrangements, encode miRNAs and interact with c-Myc (20). Dysregulation of c-Myc is a common feature in cancer, and c-Myc is constitutively overexpressed in various cancers (47). PVT1 has been identified as a key MYC enhancer and is involved in cell proliferation in a wide variety of cancers. PVT1 is also a key regulator in multi-drug resistance, including resistance against the plutinum-based drugs, docetaxel and gemcitabine (18,21,39,48–52). Using genome-wide screening, You et al. (18) identified PVT1 as a regulator of gemcitabine-sensitivity in human pancreatic cancer cells. In line with these studies, we observed that the expression of PVT1 was higher in gemcitabine-resistant BxPC3-GemR and Panc1 cell lines than in the gemcitabine-sensitive BxPC3 parental cell line, affirming the involvement of PVT1 in resistance to gemcitabine in pancreatic cancer. In addition, our data indicate that curcumin can sensitize BxPC3-GemR and Panc1 cells to gemcitabine by simultaneously suppressing PVT1 and PRC2 expression. Considering that PVT1 affects Myc expression, curcumin is likely to attenuate PRC2-PVT1-c-Myc, which will induce sensitization to gemcitabine in chemoresistant cancers.
Interestingly, in the current study, the PRC2-PVT1-c-Myc axis appears to be involved in CSC formation. PVT1 expression was 4-fold higher in BxPC3 SDCSCs than in its parental cell line. MYC and MDR1, which are key genes known to enhance cancer stemness and the down-stream targets of PVT1, were also significantly up-regulated in the spheroids. These results suggest that the mechanism of gemcitabine resistance in CSCs is consistent with those in gemcitabine chemoresistant cell lines. Moreover, our study demonstrated that the characteristics of acquired gemcitabine-resistant cells resembled those of pancreatic cancer stem-like cells. Collectively, these results highlight the possibility of using curcumin as a sensitizer to chemotherapeutic drugs in chemoresistant PDACs in the clinical settings.
One of the limitations of the current study is that we did not show how curcumin alters the expression of PVT1 and PRC2. Considering that curcumin influences several signaling pathways, it is difficult to determine whether PVT1 was suppressed through inhibition of PRC2 or other independent mechanisms. Nevertheless, we demonstrated that curcumin was able to suppress a number of individual genes involved in the PRC2-PVT1-c-Myc axis, making curcumin a unique compound that suppresses this critical signaling pathway. Currently, EZH2 is one of the most popular genes for a cancer therapeutic target. Several EZH2-specific inhibitors have already been developed and are currently being tested in human phase 1 trials (19). However, many of these drugs have shown relatively high toxicity in animal models (19). Although curcumin has been used in traditional medicine for thousands of years in India and Southeast Asia, it has only recently become a popular supplement in Western countries. We have demonstrated that curcumin was able to not only inhibit EZH2 but also its down-stream target PVT1 and ultimately c-Myc. Therefore, it is possible that botanicals such as curcumin could be used therapeutically as chemosensitizers to conventional chemotherapeutic agents that cannot suppress cancer-stemness-inducing genes. Another limitation of our study is that we derived a gemcitabine resistant cell line from KRAS wild type cells; considering that most PDAC have mutations in KRAS it would be important to determine whether curcumin can re-sensitize KRAS mutant cells.
In summary, using a series of in vitro and in vivo experiments, we have demonstrated that curcumin inhibits PRC2-PVT1-c-Myc, which enhances sensitivity of cancer cells to chemotherapeutic agents by targeting CSCs. Our data is consistent with previous studies and highlights the potential of curcumin as a promising therapeutic agent in pancreatic cancer. Moreover, mechanistic investigation of natural compounds such as curcumin could result in the development of safer and more potent chemotherapeutic agents. Further investigations including clinical trials are needed to confirm the efficacy of this compound as an adjuvant to chemotherapeutic regimens.
Supplementary material
Supplementary material is available at Carcinogenesis Online.
Funding
The present work was supported by the grants R01 CA72851, CA181572, CA184792, CA187956 and CA202797 from the National Cancer Institute, National Institute of Health, a grant (RP140784) from the Cancer Prevention Research Institute of Texas (CPRIT), pilot grants from the Baylor Sammons Cancer Center and Foundation, as well as funds from the Baylor Research Institute.
Supplementary Material
Acknowledgement
We thank Dr. Carson Harrod for carefully proofreading and editing this manuscript.
Conflict of Interest Statement: None declared.
Abbreviations
- CI
combination index
- CSC
cancer stem cell
- EZH2
Enhancer of Zeste Homolog-2
- PDAC
pancreatic ductal adenocarcinoma
- PRC2
polycomb repressive complex 2
- SDCSC
spheroid-derived cancer stem cell
References
- 1. Siegel R.L., et al. (2015)Cancer statistics, 2015. CA. Cancer J. Clin., 65, 5–29. [DOI] [PubMed] [Google Scholar]
- 2. König J., et al. (2005)Expression and localization of human multidrug resistance protein (ABCC) family members in pancreatic carcinoma. Int. J. Cancer, 115, 359–367. [DOI] [PubMed] [Google Scholar]
- 3. Nath S., et al. (2013)MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis, 2, e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z., et al. (2011)Pancreatic cancer: understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol. Hepatol., 8, 27–33. [DOI] [PubMed] [Google Scholar]
- 5. Avan A., et al. (2012)Molecular mechanisms involved in the synergistic interaction of the EZH2 inhibitor 3-deazaneplanocin A with gemcitabine in pancreatic cancer cells. Mol. Cancer Ther., 11, 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong S.P., et al. (2009)CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int. J. Cancer, 125, 2323–2331. [DOI] [PubMed] [Google Scholar]
- 7. Ottinger S., et al. (2012)Targeting of pancreatic and prostate cancer stem cell characteristics by Crambe crambe marine sponge extract. Int. J. Cancer, 130, 1671–1681. [DOI] [PubMed] [Google Scholar]
- 8. Rajeshkumar N.V., et al. (2010)A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol. Cancer Ther., 9, 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma N., et al. (2015)PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget, 6, 32039–32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xia P., et al. (2015)PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am. J. Cancer Res., 5, 1602–1609. [PMC free article] [PubMed] [Google Scholar]
- 11. Sergeant G., et al. (2009)Role of cancer stem cells in pancreatic ductal adenocarcinoma. Nat. Rev. Clin. Oncol., 6, 580–586. [DOI] [PubMed] [Google Scholar]
- 12. Cao R., et al. (2002)Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science, 298, 1039–1043. [DOI] [PubMed] [Google Scholar]
- 13. Viré E., et al. (2006)The Polycomb group protein EZH2 directly controls DNA methylation. Nature, 439, 871–874. [DOI] [PubMed] [Google Scholar]
- 14. Bardeesy N., et al. (2002)Pancreatic cancer biology and genetics. Nat. Rev. Cancer, 2, 897–909. [DOI] [PubMed] [Google Scholar]
- 15. Zhou Q., et al. (2016)Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR). Tumour Biol., 37, 3105–13. [DOI] [PubMed] [Google Scholar]
- 16. Wang D., et al. (2015)LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget, 6, 41045–41055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang K., et al. (2015)Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget, 6, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. You L., et al. (2011)Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem. Biophys. Res. Commun., 407, 1–6. [DOI] [PubMed] [Google Scholar]
- 19. Kim K.H., et al. (2016)Targeting EZH2 in cancer. Nat. Med., 22, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cui M., et al. (2016)Long non-coding RNA PVT1 and cancer. Biochem. Biophys. Res. Commun., 471, 10–14. [DOI] [PubMed] [Google Scholar]
- 21. Huang C., et al. (2015)Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med., 106, 143–149. [PubMed] [Google Scholar]
- 22. Lev-Ari S., et al. (2007)Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest., 25, 411–418. [DOI] [PubMed] [Google Scholar]
- 23. Goel A., et al. (2010)Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr. Cancer, 62, 919–930. [DOI] [PubMed] [Google Scholar]
- 24. Howells L.M., et al. (2011)Curcumin ameliorates oxaliplatin-induced chemoresistance in HCT116 colorectal cancer cells in vitro and in vivo. Int. J. Cancer, 129, 476–486. [DOI] [PubMed] [Google Scholar]
- 25. Toden S., et al. (2015)Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis, 36, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fetoni A.R., et al. (2015)Molecular targets for anticancer redox chemotherapy and cisplatin-induced ototoxicity: the role of curcumin on pSTAT3 and Nrf-2 signalling. Br. J. Cancer, 113, 1434–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He M., et al. (2016)Re-purposing of curcumin as an anti-metastatic agent for the treatment of epithelial ovarian cancer: in vitro model using cancer stem cell enriched ovarian cancer spheroids. Oncotarget, 7, 86374–86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bao B., et al. (2012)Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One, 7, e50165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bao B., et al. (2012)Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res., 72, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi M., et al. (2012)Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis, 33, 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chou T.C. (2010)Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res., 70, 440–446. [DOI] [PubMed] [Google Scholar]
- 32. Jascur T., et al. (2011)N-methyl-N’-nitro-N-nitrosoguanidine (MNNG) triggers MSH2 and Cdt2 protein-dependent degradation of the cell cycle and mismatch repair (MMR) inhibitor protein p21Waf1/Cip1. J. Biol. Chem., 286, 29531–29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Sousa Cavalcante L., et al. (2014)Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur. J. Pharmacol., 741, 8–16. [DOI] [PubMed] [Google Scholar]
- 34. Sahu R.P., et al. (2009)Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br. J. Cancer, 100, 1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saiki Y., et al. (2009)Comprehensive analysis of the clinical significance of inducing pluripotent stemness-related gene expression in colorectal cancer cells. Ann. Surg. Oncol., 16, 2638–2644. [DOI] [PubMed] [Google Scholar]
- 36. Porro A., et al. (2010)Direct and coordinate regulation of ATP-binding cassette transporter genes by Myc factors generates specific transcription signatures that significantly affect the chemoresistance phenotype of cancer cells. J. Biol. Chem., 285, 19532–19543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liao D.J., et al. (2007)Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit. Rev. Oncog., 13, 93–158. [DOI] [PubMed] [Google Scholar]
- 38. Takahashi K., et al. (2006)Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 39. Tseng Y.Y., et al. (2014)PVT1 dependence in cancer with MYC copy-number increase. Nature, 512, 82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hou Y.C., et al. (2014)Coexpression of CD44-positive/CD133-positive cancer stem cells and CD204-positive tumor-associated macrophages is a predictor of survival in pancreatic ductal adenocarcinoma. Cancer, 120, 2766–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mizukami T., et al. (2014)Immunohistochemical analysis of cancer stem cell markers in pancreatic adenocarcinoma patients after neoadjuvant chemoradiotherapy. BMC Cancer, 14, 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ali S., et al. (2010)Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res., 70, 3606–3617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Kunnumakkara A.B., et al. (2007)Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res., 67, 3853–3861. [DOI] [PubMed] [Google Scholar]
- 44. Hemming S., et al. (2016)Identification of novel EZH2 targets regulating osteogenic differentiation in mesenchymal stem cells. Stem Cells Dev., 25, 909–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ougolkov A.V., et al. (2008)Regulation of pancreatic tumor cell proliferation and chemoresistance by the histone methyltransferase enhancer of zeste homologue 2. Clin. Cancer Res., 14, 6790–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsai M.C., et al. (2010)Long noncoding RNA as modular scaffold of histone modification complexes. Science, 329, 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gabay M., et al. (2014)MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med., 4, pii:a014241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhuang C., et al. (2015)Tetracycline-inducible shRNA targeting long non-coding RNA PVT1 inhibits cell growth and induces apoptosis in bladder cancer cells. Oncotarget, 6, 41194–41203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cui D., et al. (2015)Long non-coding RNA PVT1 as a novel biomarker for diagnosis and prognosis of non-small cell lung cancer. Tumour Biol., 37, 4127–4134. [DOI] [PubMed] [Google Scholar]
- 50. Takahashi Y., et al. (2014)Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br. J. Cancer, 110, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang X.W., et al. (2015)Overexpression of long non-coding RNA PVT1 in gastric cancer cells promotes the development of multidrug resistance. Biochem. Biophys. Res. Commun., 462, 227–232. [DOI] [PubMed] [Google Scholar]
- 52. Liu E., et al. (2015)Carboplatin-docetaxel-induced activity against ovarian cancer is dependent on up-regulated lncRNA PVT1. Int. J. Clin. Exp. Pathol., 8, 3803–3810. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.