Summary
Increased personal exposure to PM2.5 and BaP from household coal combustion may be related to decreased leukocyte mitochondrial DNA copy number, a marker of oxidative stress, in non-smoking women residing in rural China.
Abstract
Households in Xuanwei and Fuyuan, China, possess hazardous levels of fine particulate matter with an aerodynamic diameter <2.5 microns (PM2.5) and polycyclic aromatic hydrocarbons (PAHs) from coal combustion. Previous studies found that increased exposure to PM2.5 and benzo[a]pyrene (BaP; a PAH) were associated with decreased mitochondrial DNA copy number (mtDNAcn), a marker of oxidative stress. We further evaluated these associations in a cross-sectional study of 148 healthy non-smoking women from Xuanwei and Fuyuan. Personal exposure to PM2.5 and BaP was measured using portable devices. MtDNAcn was measured using qPCR amplification of leukocyte DNA that was collected after air measurements. Linear regression models were used to estimate the associations between personal exposure to PM2.5 and BaP, and mtDNAcn adjusted for age, body mass index (BMI) and fuel type. We found inverse associations between exposure to PM2.5 and BaP, and mtDNAcn. Each incremental log-μg/m3 increase in PM2.5 was associated with a significant decrease in mtDNAcn of −10.3 copies per cell [95% confidence interval (95% CI): −18.6, −2.0; P = 0.02]. Additionally, each log-ng/m3 increase in BaP was associated with a significant decrease in mtDNAcn of −5.4 copies per cell (95% CI: −9.9, −0.8, P = 0.02). Age, BMI, fuel type and coal mine type were not significantly associated with mtDNAcn. Exposure to PM2.5 and BaP may alter mitochondrial dynamics in non-smoking Chinese women. MtDNAcn may be a potential mediator of indoor air pollution on chronic disease development.
Introduction
Indoor air pollution is a substantial global health burden in low- and middle-income nations. Residential combustion of smoky (bituminous) coal for heating and cooking in households of rural Xuanwei and Fuyuan, China, has led to hazardous levels of fine particulate matter with an aerodynamic diameter <2.5 microns (PM2.5) and polycyclic aromatic hydrocarbons (PAHs) (1–3). Primarily formed through combustion, the composition of PM2.5 varies depending on physical environment, atmospheric conditions and source of emissions. Inhalation of PM2.5 is a substantial health concern because it can be deposited into the alveoli of the lung, where it can cause local inflammation, in addition to systemic inflammation if its components diffuse into the blood stream (4). PM2.5 can influence health outcomes through mechanisms involving lipid peroxidation of cell membranes, oxidative damage to the genome and DNA adduct formation (5–8). Indeed, exposure to PM2.5 in residential, occupational and public settings has been shown to be associated with increased risk of chronic respiratory conditions, cardiovascular disease and all-cause mortality (9–13). PAHs are organic compounds composed of multiple benzene rings that are emitted from the combustion of fossil fuels. A common type of PAHs is benzo[a]pyrene (BaP), which is often used as a surrogate for overall levels of PAHs. The carcinogenic and genotoxic potential of reactive PAH/ BaP metabolites is well established (14). Upon enzymatic conversion by cytochrome P-450, metabolites such as benzo[a]pyrene diol epoxide can form adducts with guanine, resulting in the disruption of normal DNA replication (14).
The burden of indoor air pollution is compounded for women of Xuanwei and Fuyuan, who because of a traditional agrarian lifestyle, spend considerable time indoors preparing food for their families. Further, despite government sanctioned stove improvement/ventilation programs, many households still use unvented fire pits for cooking (1,15). We previously found that the geometric mean average indoor PM2.5 and BaP exposure concentrations for female smoky coal users were 148.0 μg/m3 and 44.7 ng/m3, respectively (1,15). Although outdoor PM2.5 levels in rural Xuanwei and Fuyuan are still being characterized, the indoor PAH concentrations exceeded outdoor levels that were reported in a previous study and were beyond the national outdoor criteria of 10.0 ng/m3 (16).
Mitochondria are the eukaryotic organelles that serve two prominent roles in the cell: (i) energy production through the synthesis of adenosine triphosphate via the Krebs cycle and electron transport chain and (ii) production of reactive oxygen species, which are released in unison with cytochrome c to initiate apoptosis. Human mitochondrial DNA consists of a 16 569 bp circular double-stranded DNA molecule. Mitochondrial DNA copy number (mtDNAcn) is normally in steady state, which is related to the energy demand of the host cells (17). However, due to the lack of DNA repair machinery, mitochondria compensate for mtDNA damage by altering its copy number (18). Indeed, oxidative stress and mtDNA damage have been found to be associated with increased mtDNAcn in leukocytes (18,19). In various somatic cells, mitochondria possess 2–10 000 copies of their genomes (20–23). Further, population-based estimates of leukocyte mtDNAcn vary from ~50 copies per cell in Danish adults (24), to about 110–120 copies per cell in Sardinian and U.S. adults (25,26).
Given the genotoxic potential of PM2.5 and PAHs, these pollutants may influence mitochondrial dynamics. MtDNAcn has been found to be associated with risk of various cancers (27–30), including that of the lung, in case–control and nested case–control studies (20,26,31,32). However, few studies have examined the relations between environmental exposure to PM2.5 and PAHs, and mtDNAcn. The dynamics between PM2.5 and PAH exposure and mtDNAcn are complex within the current literature. A study of an elderly Flemish population from Genk, Belgium, found that increased exposure to PM2.5 was associated with decreased mtDNAcn (33). A study of truck drivers in Beijing did not find a significant association between PM2.5 and mtDNAcn (34). Other studies found that PAH exposure was associated with both higher and lower mtDNAcn (35,36). Perturbations in mtDNAcn in either direction may be reflective of cumulative oxidative damage. To unravel these relationships further, we conducted a cross-sectional molecular epidemiological study of healthy non-smoking women in Xuanwei and Fuyuan, China. Our objective was to assess the associations between personal exposure to PM2.5 and BaP from household air pollution and leukocyte mtDNAcn.
Materials and methods
Study population and design
The study population has been described previously in detail (15). In brief, the Xuanwei Exposure Assessment Study characterized household air pollutants and exposures related to the combustion of solid fuels for cooking and heating. We enrolled 148 healthy (disease-free) female heads of household from 30 villages across Xuanwei and Fuyuan during the first visit in August 2008 to February 2009. Up to five households were preferentially selected in each village based on (i) having a stove that used solid fuel; (ii) the residence was more than 10 years old; (iii) use of the same cooking or heating equipment for the past 5 years; and (iv) presence of a non-smoking healthy woman aged 20−80 years, who was primarily responsible for cooking. We conducted two sequential 24 h air measurements and whole blood samples were collected on the second day.
Personal PM2.5 and PAH exposure assessment
Personal PM2.5 measurements across 24 h were collected on pre-weighed 37 mm Teflon filters using portable devices with an aerodynamic cutoff of 2.5 μm (Model BGI, GK2.05SH) at a flow rate of 3.5 l/min (±20%) as described previously (15). The pump was packed in a hip bag and the portable device was attached near the breathing zone of each participant while they were awake. The sampling bag was placed next to the women’s bed while they were sleeping. All exposed filters were individually placed in Petri slides, sealed in plastic bags and stored at −80°C before post-weighing. Particulate mass was determined by pre- and post-weighing of the filters in duplicate. PM2.5 concentrations (μg/m3) were calculated by dividing the weights by the total volume of air drawn through the filters. Approximately, 10% of households were randomly selected to have duplicate PM2.5 measurements to assess reproducibility. The coefficient of variation (CV) for the PM2.5 measurements was 13%. Particle-bound PAHs were collected with 37 mm Teflon filters in the cyclones, whereas personal gas phase PAHs were measured with XAD-2 sorbent tubes at a median air flow rate of 63 ml/min (1). PAH species (ng/m3) were identified using gas chromatograph connected to a mass spectrometer (Shimadzu QP2010 plus) (1). The median CV for PAH readings from 13 duplicate samples was 25% (1).
Household interviews and measurement
In-person interviews were conducted by two trained personnel. Information on household, demographic and anthropometric characteristics was collected. Body mass index (BMI; kg/m2) was derived from weight (kg) and height (m). The women’s activities during the sampling periods were recorded using an activity questionnaire. This survey collected information on household stove and ventilation type, cooking activities, secondhand smoke exposure, heating practices, coal mine type that supplied household fuel and fuel usage. Coal type was reported as either smoky or smokeless and confirmed via petrochemical analysis of collected coal samples (37). Other categories of fuel included wood, plants, mixed coal (combinations of briquettes, smoky and smokeless coal) and mixed use of fuel (combinations of wood, plant materials and coal). Household stove and ventilation characteristics for this study population were described previously in detail (1,15). Written informed consent was obtained from all eligible participants. This study was approved by the National Cancer Institute Special Studies Institutional Review Board (#06CN092).
Leukocyte mtDNAcn
Total DNA was isolated from the whole blood using phenol–chloroform extraction. A modified version of a previously described SYBR green-based qPCR method was used to determine mtDNAcn on a Roche LC480 System (Roche Molecular Biochemicals, Pleasanton, CA) (19,38). Briefly, the qPCR assay was performed using LightCycle-FastStart DNA Master SYBR Green I at Changhua Christian Hospital. Each reaction used 20 ng of total DNA template and was performed in triplicate. The single copy nuclear gene [human β-globin (HB)] was amplified using the following primers—forward: 5′-GAAGAGCCAAGGACAGGTAC-3′ and reverse: 5′-CAACTTCATCCACGTTCACC-3′. Amplification of the HB gene was performed under the following qPCR cycling conditions: 95°C for 300 s, followed by 40 cycles of 10 s at 95°C, 5 s at 58°C and 18 s at 72°C. The single copy mitochondrial gene (ND1) was amplified using the following primers—forward: 5′-AACATACCCATGGCCAACCT-3 ′ and reverse: 5′-AGCGAAGGGTTGTAGTAGCCC-3′, under the follow conditions: 95°C for 300 s, followed by 40 cycles of 10 s at 95°C, 5 s at 58°C and 8 s at 72°C. Standard curves were performed for each batch for both the HB and ND1 reactions to assess qPCR efficiency. Linear correlations (R2) ≥ 0.98 and qPCR efficiencies between 1.95 and 2.00 were considered acceptable, or else the reactions were repeated. The relative ratio of ND1 and HB threshold cycle numbers, estimated using a linear regression model, is proportional to the mtDNAcn in each cell (26,39). mtDNAcn was interpolated from the linearity of the dosage-dependently constructed standard plasmids of the ND1 and HB genes as described previously (19,38). The average CV from 10 quality control samples for mtDNAcn was 9.8%. The intraclass correlations from the same quality control samples with two to four replicates were 79% [95% confidence interval (95% CI): 58–90].
Analysis
The distribution and normality of continuous variables were assessed using histograms and Shapiro-Wilks tests, respectively. Spearman correlation coefficients were used to assess relations among ordinal and continuous variables. Multivariable-adjusted linear regression models were used to assess the associations between log-transformed personal PM2.5 and BaP exposure averaged from the two sequential collection days, and leukocyte mtDNAcn (copies per cell). Separate models were performed for PM2.5 and BaP as main exposures due to high collinearity (rho = 0.72, P < 0.0001). The models were adjusted for age (continuous), BMI (continuous) and fuel type (smoky coal, smokeless coal, other coal, other fuels and wood/plant) via stepwise inclusion. Since lung cancer risk was found to differ by fuel type (40), we stratified the analyses by smoky coal users and users of all other fuels (non-smoky coal users). Further, we assessed effect modification using interaction terms between PM2.5 X fuel type and BaP X fuel type. We also conducted stratified analyses for PM2.5 and BaP, dichotomized as high and low by each other’s median values (PM2.5: <167 and ≥167 µg/m3; BaP: <39 and ≥39 ng/m3). P-values <0.05 and P-interactions <0.10 were considered statistically significant. All analyses were performed using SAS v9.3 (SAS Institute Inc., Cary, NC).
Results
Personal and household characteristics
With respect to personal characteristics, the average age was 55.4 (14.4 SD) years and the average BMI was 21.8 (3.4 SD) kg/m2 (Table 1). Additionally, there was an average of 119 (30 SD) copies of mitochondrial DNA per cell in leukocytes. When examining air pollutants, the median personal PM2.5 and BaP exposure averaged from the two sequential 24 h periods were 167 µg/m3 (IQR: 118, 235) and 40 ng/m3 (IQR: 18, 77), respectively. During the first visit, half of the women used smoky coal for heating and cooking in their homes (50.7%), whereas 8.8% used smokeless coal, 5.4% used wood/plant biomass, and 35.1% used a mix or other fuel types.
Table 1.
Characteristics of the Xuanwei Exposure assessment study
| Air pollutants/demographic/anthropometric | ||
| Age (years), mean, SD | 55.4 | (14.4) |
| BMI (kg/m2), mean, SD | 21.8 | (3.4) |
| Average personal PM2.5 exposure (μg/m3), median (IQR) | 167 | (118, 235) |
| Average personal BaP exposure (ng/m3), median (IQR) | 40 | (18, 77) |
| County of residence, n (%) | ||
| Xuanwei | 74 | (50.0) |
| Fuyuan | 74 | (50.0) |
| Molecular biomarkers | ||
| Leukocyte mtDNA copy number (count per cell), mean, SD | 119 | 30 |
| Fuel type, n (%) | ||
| Smoky coal | 75 | (50.7) |
| Smokeless coal | 13 | (8.8) |
| Wood | 4 | (2.7) |
| Plant | 4 | (2.7) |
| Other | 52 | (35.1) |
| Type of coal mine which supplied fuel, n (%) | ||
| Coking coal | 73 | (49.3) |
| Gas Fat coal | 23 | (15.5) |
| Smokeless coal | 20 | (13.5) |
| One-third coking coal | 10 | (6.8) |
| Other | 20 | (13.5) |
| Unknown | 2 | (1.4) |
Associations between PM 2.5 and BaP exposure, personal and fuel characteristics and leukocyte mtDNAcn
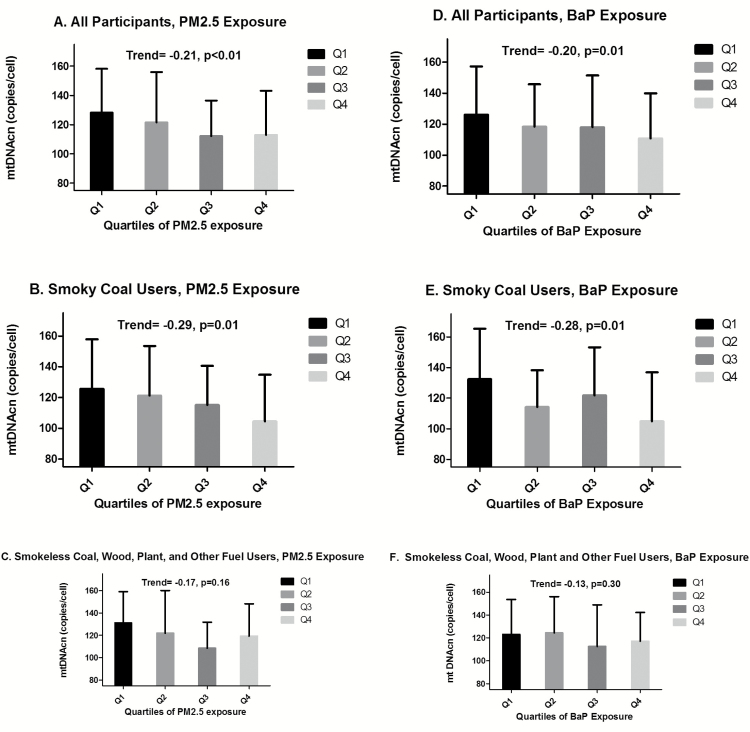
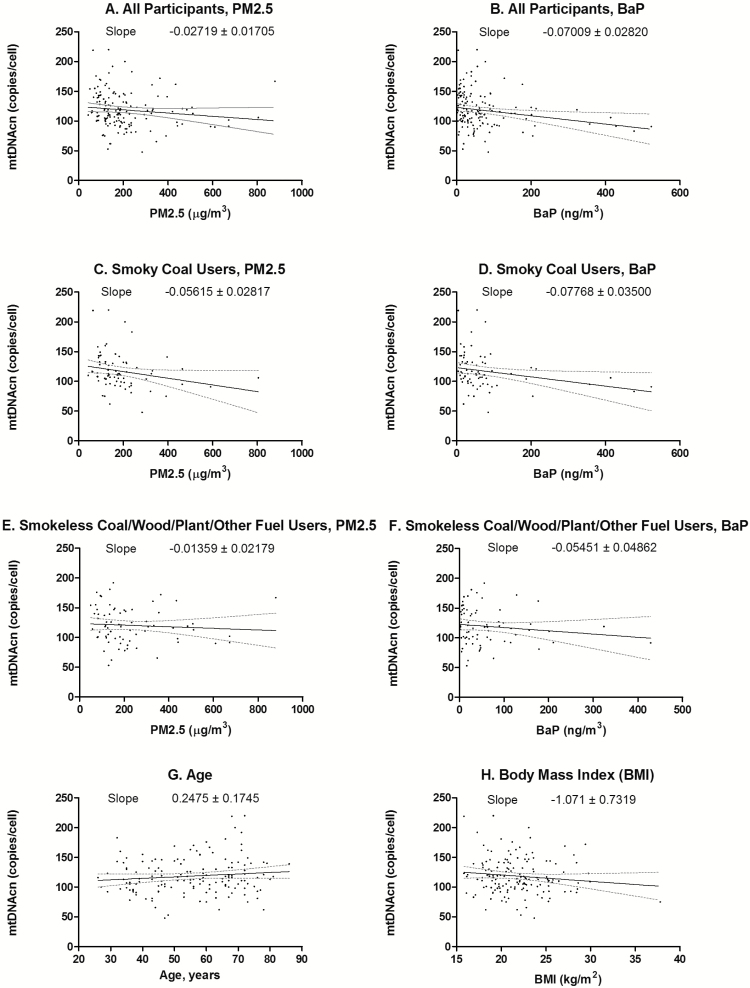
The distributions of leukocyte mtDNAcn across quartiles of PM2.5 and BaP exposure are presented in Supplementary Table 1, available at Carcinogenesis Online. We found significant inverse correlations between mtDNAcn and ordinal quartiles of personal PM2.5 (rho = −0.21, P < 0.01) and BaP (rho = −0.20, P = 0.01) (Figure 1). Stronger trends were observed when analyses were restricted to smoky coal users. The correlations were non-significant in analyses that were restricted to users of other fuel types. Similar trends were observed when categorizing PM2.5 and BaP as continuous (Figure 2).
Figure 1.
Mean mtDNA copy number by quartiles of personal PM2.5 and BaP. (A) In all participants, by quartiles of PM2.5; (B) in all participants, by quartiles of BaP; (C) in smoky coal users, by quartiles of PM2.5; (D) in smoky coal users, by quartiles of BaP; (E) in users of smokeless coal, wood, plant and other fuels, by PM2.5; and (F) in users of smokeless coal, wood, plant and other fuels, by BaP. Whiskers represent standard deviations. Trends between quartiles of exposure and mtDNAcn were analyzed with Spearman correlations. For PM2.5: Q1 = 41–117 μg/m3, Q2 = 118–166 μg/m3, Q3 = 167–233 μg/m3, and Q4 = ≥234 μg/m3. For BaP: Q1= 2–17 ng/m3, Q2= 18–38 ng/m3, Q3 = 39–76 ng/m3, Q4= ≥77 ng/m3. P-values <0.05 were considered statistically significant .
Figure 2.
Scatter plots between PM2.5, BaP, age, BMI and continuous leukocyte mtDNA copy number. Red dotted lines represented 95% CIs of the slope.
Age (β = 0.2 copies per cell; 95% CI: −0.1, 0.1; P = 0.16) and BMI (β = −1.1 copies per cell; 95% CI: −2.5, 0.4; P = 0.15) were not significantly associated with mtDNAcn. Additionally, the type of coal mine that supplied household fuel was not significantly associated with mtDNAcn, compared with smokeless coal mine (coking coal: β = −8.1 copies per cell, 95% CI: −22.8, 6.7, P = 0.28; gas fat coal: β = 13.3 copies per cell, 95% CI: 4.6, 31.2, P = 0.14; one-third coking coal: β = 10.2 copies per cell, 95% CI: −12.5, 32.9, P = 0.37; other: β = −11.2 copies per cell, 95% CI: −29.2, 6.9, P = 0.22). Furthermore, smoky coal use (β = 0.4 copies per cell; 95% CI: −10.0, 10.8; P = 0.95) and smokeless coal use (β = 13.6 copies per cell, 95% CI: −4.8, 32.0; P = 0.15) were not significantly associated with mtDNAcn compared with other fuel types, adjusted for age and BMI.
Each incremental log-μg/m3 increase in personal PM2.5 exposure was associated with a statistically significant decrease in leukocyte mtDNAcn of −10.3 copies per cell (95% CI: −18.6, −2.0; P = 0.02), adjusted for age, BMI, and fuel type (Table 2). When stratified by fuel type, similar statistically significant inverse associations with PM2.5 were observed when analyses were restricted to smoky coal users but not users of other fuel types. When stratified by median BaP levels, PM2.5 was significantly inversely associated with mtDNAcn when BaP was below the median (<39 ng/m3: β = −16.2 copies per cell; 95% CI: −30.6, −1.8; P = 0.03) but was not statistically significant when BaP was above the median (≥39 ng/m3: β = −6.8 copies per cell; 95% CI: −23.2, 9.7; P = 0.41).
Table 2.
Associations between personal exposure to PM2.5 and BaP, and leukocyte mtDNAcn in non-smoking women of the Xuanwei exposure assessment study
| All participants (n = 148) | Smoky coal users (n = 75) | Smokeless coal, wood, plant, coal cakes and other users (n = 73) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | |||||||||
| Estimate | Lower | Upper | P-value | Estimate | Lower | Upper | P-value | Estimate | Lower | Upper | P-value | |||
| PM2.5, log (μg/m3) | ||||||||||||||
| Unadjusted main effect | −10.0 | −18.2 | −1.8 | 0.02 | * | −15.0 | −27.7 | −2.3 | 0.02 | * | −7.1 | −18.2 | 4.0 | 0.20 |
| Adjusted main effect | ||||||||||||||
| Including age | −10.4 | −18.6 | −2.2 | 0.01 | * | −15.3 | −28.1 | −2.5 | 0.02 | * | −7.5 | −18.4 | 3.5 | 0.18 |
| Including age and BMI | −10.6 | −18.8 | −2.5 | 0.01 | * | −16.0 | −28.2 | −3.8 | 0.01 | * | −7.3 | −18.2 | 3.7 | 0.19 |
| Including age, BMI, and fuel type | −10.3 | −18.6 | −2.0 | 0.02 | * | — | — | — | — | — | — | — | − | |
| BaP, log (ng/m3) | ||||||||||||||
| Unadjusted main effect | −5.8 | −10.1 | −1.5 | 0.01 | * | −9.6 | −16.4 | −2.8 | 0.01 | * | −3.3 | −9.1 | 2.5 | 0.27 |
| Adjusted main effect | ||||||||||||||
| Including age | −5.8 | −10.1 | −1.6 | 0.01 | * | −9.7 | −16.5 | −2.8 | 0.01 | * | −3.4 | −9.1 | 2.4 | 0.25 |
| Including age and BMI | −5.8 | −10.1 | −1.6 | 0.01 | * | −9.3 | −15.9 | −2.7 | 0.01 | * | −3.2 | −8.9 | 2.6 | 0.27 |
| Including age, BMI, and fuel type | −5.4 | −9.9 | −0.8 | 0.02 | * | — | — | — | — | — | — | — | — | |
* P-values <0.05 were considered statistically significant.
Each incremental log-ng/m3 increase in personal BaP exposure was associated with a statistically significant decrease in leukocyte mtDNAcn of −5.4 copies per cell (95% CI: −9.9, −0.8; P = 0.02), adjusted for covariates (Table 2). When stratified by fuel type, similar statistically significant inverse associations with BaP were observed when analyses were restricted to smoky coal users but not users of other fuel types. When stratified by median PM2.5 levels, significant associations between BaP and mtDNAcn were not found in either low or high PM2.5 levels (<167 µg/m3: β = −3.6 copies per cell, 95% CI: −12.7, 5.5, P = 0.43; ≥167 µg/m3: β = −5.7 copies per cell, 95% CI: −13.5, 2.2, P = 0.16). The interaction terms between PM2.5× fuel type and BaP and fuel type were not statistically significant and thus not included in the models.
Discussion
In our cross-sectional molecular epidemiological study, we found that increased personal exposure to PM2.5 and BaP was associated with decreased leukocyte mtDNAcn in non-smoking women from Xuanwei and Fuyuan, China. The inverse relationships were observed in smoky coal users but not users of other fuels.
Several factors may explain our results; therefore, sensitivity analyses were conducted to assess the robustness of the findings. Potential confounders such as age, BMI and fuel type may have introduced bias to the results. However, we found that sequential stepwise inclusion of these potential confounders to the models did not appreciably change the estimates. Furthermore, fuel type may be a potential effect modifier given the compositional differences between emissions. When the analyses were stratified by fuel type, similar directions of associations were observed in both smoky coal and non- smoky coal users. The associations were only significant among smoky coal users, albeit the interaction terms with fuel type were not significant, which may be due to limited statistical power. However, these findings may be attributed to compositional difference in PM2.5 between fuel types. For instance, a previous geologic study found elemental differences in transition metals including nickel, silicon, aluminum, titanium, iron, and manganese between smoky and smokeless coal (37). When stratified by median BaP levels, the inverse association between PM2.5 and mtDNAcn was more pronounced at low BaP levels; suggesting that the relationship was unlikely to be driven by high BaP levels. Additionally, BaP was not significantly associated with mtDNAcn when stratified by median PM2.5 levels.
Few population-based studies have examined the relationships between PM2.5 and PAH exposure, and mtDNAcn. Similar to our study, Pieters et al. in 2016 (33) found that increased PM2.5 exposure was associated with decreased mtDNAcn in an elderly Flemish population from Genk, Belgium. Furthermore, a short-term repeated-measures study of 35 Type 2 diabetes patients in Shanghai, China, found non-significant inverse associations between various fractions of PM0.25–10 and mtDNAcn (41). However, a repeated-measures study of 60 truck drivers and 60 office workers from Beijing, China, did not find significant associations between average cross-shift shift PM2.5 exposure and mtDNAcn; but inverse associations were observed with PM10 (34). A cross-sectional study of 63 male healthy steel workers from Brescia, Italy, found that ultrafine PM1, which are subsumed by PM2.5, was positively associated with mtDNAcn. The differences in findings between studies may be attributed to variability in the toxicities of PM2.5 components and other compounds between geographic regions and seasons (42). For instance, compositional components that may be different between diesel and coal combustion include organic and elemental carbon, NO3-, SO42-, NH4+, and heavy and transition metals (43–47). Furthermore, the amount of energy in the combustion process, degree of incomplete combustion and atmospheric conditions can greatly affect the size and composition of the components.
With respect to BaP, results from our study were in the same direction as those reported by Pieters et al. in 2013 (36), which found inverse associations with mtDNAcn. However, our results and those of Pieters et al. (2013) were in the opposite direction of findings by Pavanello et al. in 2013 in 46 Polish non-smoking men (35). These discordances may be attributed to sex and geographic differences between the study populations. Additionally, we cannot discount the contribution of other lifestyle, diet and environmental factors that were unique to each study. Differences in PAH exposure levels and exposure time-windows among studies may also be influential. Given the limited number of studies that examined the associations between exposure to PM2.5 and PAHs, and mtDNAcn, firm relationships have yet to be established.
Previous case–control and cohort studies found positive associations of both smoky coal use and mtDNAcn, to risk of lung cancer mortality (20,26,32,40,48). However, a recent pooled analysis did not find associations between mtDNAcn and lung cancer risk (49). Furthermore, higher PM2.5 levels were previously reported in smoky coal users compared with smokeless coal users in Xuanwei (15). Given the inverse associations between PM2.5 and mtDNAcn found in our study, it was possible that the elevated risks of lung cancer mortality associated with smoky coal use found in previous studies may be due to other components emitted from its combustion or unrelated to mtDNAcn pathways all together.
This study had strengths in addressing the study question. First, the sample population was exclusively non-smoking Chinese women, which removes potential confounding by active cigarette smoking, race/ethnicity and sex. Further, nearly all participants were exposed to secondhand smoke from cohabitating family members. Second, differential misclassification of the exposure and outcome was unlikely because PM2.5 and BaP, and mtDNAcn were independently measured and could not possibly affect each other. Moreover, laboratory personnel were blinded to characteristics of the participants. However, there were important assumptions and caveats of note. First, we assumed that the PM2.5 measurements were representative of exposure spanning further into the past and that the effect on mtDNAcn would be captured within the study’s time frame. Second, the sample size of this study was limited, which hindered our statistical power to detect subtle associations. Third, white blood cell differentials and platelet concentrations, which may be sources of mtDNAcn variability, were not assessed.
In summary, we found that increased personal exposure to indoor PM2.5 and BaP produced via smoky coal combustion may be associated with decreased leukocyte mtDNAcn in non-smoking Chinese women. These findings suggest that exposure to PM2.5 and BaP may alter mitochondrial dynamics and that mtDNAcn may be a potential mediator of indoor air pollution on chronic disease development. However, given the aforementioned limitations, we cannot discount the possibility of chance findings. Caution is recommended when interpreting the results. Subsequent investigations of air pollution and mtDNAcn should utilize large prospective cohorts with numerous repeated-measurements to characterize their intricate dynamics throughout the lifecourse.
Supplementary material
Supplementary data are available at Carcinogenesis online.
Funding
This study was supported by intramural funding from the National Cancer Institute.
Conflict of Interest Statement: None declared.
Supplementary Material
Abbreviations
- BaP
benzo[a]pyrene
- BMI
body mass index
- mtDNAcn
mitochondrial DNA copy number
- 95% CI
95% confidence interval
- CV
coefficient of variation
- HB
human β-globin
- PAH
polycyclic aromatic hydrocarbon
- PM2.5
fine particulate matter with an aerodynamic diameter <2.5 microns
References
- 1. Downward G.S., et al. (2014) Polycyclic aromatic hydrocarbon exposure in household air pollution from solid fuel combustion among the female population of Xuanwei and Fuyuan counties, China. Environ. Sci. Technol., 48, 14632– 146–41.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mumford J.L., et al. (1987) Lung cancer and indoor air pollution in Xuan Wei, China. Science, 235, 217–220. [DOI] [PubMed] [Google Scholar]
- 3. Lan Q., et al. (2002) Household stove improvement and risk of lung cancer in Xuanwei, China. J. Natl Cancer Inst., 94, 826–835. [DOI] [PubMed] [Google Scholar]
- 4. Laohaudomchok W., et al. (2010) Assessment of occupational exposure to manganese and other metals in welding fumes by portable X-ray fluorescence spectrometer. J. Occup. Environ. Hyg., 7, 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nuernberg A.M., et al. (2008) Urinary 8-isoprostane and 8-OHdG concentrations in boilermakers with welding exposure. J. Occup. Environ. Med., 50, 182–189. [DOI] [PubMed] [Google Scholar]
- 6. Yang L., et al. (2014) Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ. Toxicol., 31, 923–936. doi:10.1002/tox.22102. [DOI] [PubMed] [Google Scholar]
- 7. Valko M., et al. (2005) Metals, toxicity and oxidative stress. Curr. Med. Chem., 12, 1161–1208. [DOI] [PubMed] [Google Scholar]
- 8. Chang J., et al. (1993) Bulky DNA-adduct formation induced by Ni(II) in vitro and in vivo as assayed by 32P-postlabeling. Mutat. Res., 291, 147–159. [DOI] [PubMed] [Google Scholar]
- 9. Puett R.C., et al. (2014) Particulate matter air pollution exposure, distance to road, and incident lung cancer in the nurses’ health study cohort. Environ. Health Perspect., 122, 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hystad P., et al. (2013) Long-term residential exposure to air pollution and lung cancer risk. Epidemiology, 24, 762–772. [DOI] [PubMed] [Google Scholar]
- 11. Raaschou-Nielsen O., et al. (2011) Lung cancer incidence and long-term exposure to air pollution from traffic. Environ. Health Perspect., 119, 860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hart J.E., et al. (2011) Long-term ambient multipollutant exposures and mortality. Am. J. Respir. Crit. Care Med., 183, 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Young M.T., et al. (2014) Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am. J. Respir. Crit. Care Med., 190, 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moorthy B., et al. (2015) Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol. Sci., 145, 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu W., et al. (2014) Personal and indoor PM2.5 exposure from burning solid fuels in vented and unvented stoves in a rural region of China with a high incidence of lung cancer. Environ. Sci. Technol., 48, 8456– 84–64.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lv J., et al. (2009) Indoor and outdoor air pollution of polycyclic aromatic hydrocarbons (PAHs) in Xuanwei and Fuyuan, China. J. Environ. Monit., 11, 1368–1374. [DOI] [PubMed] [Google Scholar]
- 17. Capps G.J., et al. (2003) A model of the nuclear control of mitochondrial DNA replication. J. Theor. Biol., 221, 565–583. [DOI] [PubMed] [Google Scholar]
- 18. Shokolenko I., et al. (2009) Oxidative stress induces degradation of mitochondrial DNA. Nucleic Acids Res., 37, 2539–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu C.S., et al. (2003) Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic. Res., 37, 1307–1317. [DOI] [PubMed] [Google Scholar]
- 20. Hosgood H.D., 3rd, et al. (2010) Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis, 31, 847–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gahan M.E., et al. (2001) Quantification of mitochondrial DNA in peripheral blood mononuclear cells and subcutaneous fat using real-time polymerase chain reaction. J. Clin. Virol., 22, 241–247. [DOI] [PubMed] [Google Scholar]
- 22. Barthelemy C., et al. (2001) Late-onset mitochondrial DNA depletion: DNA copy number, multiple deletions, and compensation. Ann. Neurol., 49, 607–617. [PubMed] [Google Scholar]
- 23. Miller F.J., et al. (2003) Precise determination of mitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: lack of change of copy number with age. Nucleic Acids Res., 31, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mengel-From J., et al. (2014) Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet., 133, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ding J., et al. (2015) Assessing mitochondrial DNA variation and copy number in lymphocytes of ~2,000 sardinians using Tailored Sequencing Analysis Tools. PLoS Genet., 11, e1005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hofmann J.N., et al. (2014) A nested case-control study of leukocyte mitochondrial DNA copy number and renal cell carcinoma in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Carcinogenesis, 35, 1028–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng S., et al. (2016) Pre-diagnostic leukocyte mitochondrial DNA copy number and skin cancer risk. Carcinogenesis, 37, 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemnrau A., et al. (2015) Mitochondrial DNA copy number in peripheral blood cells and risk of developing breast cancer. Cancer Res., 75, 2844–2850. [DOI] [PubMed] [Google Scholar]
- 29. Thyagarajan B., et al. (2013) Mitochondrial DNA copy number is associated with breast cancer risk. PLoS One, 8, e65968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhou W., et al. (2014) Peripheral blood mitochondrial DNA copy number is associated with prostate cancer risk and tumor burden. PLoS One, 9, e109470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim C., et al. (2015) Mitochondrial DNA copy number and chronic lymphocytic leukemia/small lymphocytic lymphoma risk in two prospective studies. Cancer Epidemiol. Biomarkers Prev., 24, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonner M.R., et al. (2009) Mitochondrial DNA content and lung cancer risk in Xuan Wei, China. Lung Cancer, 63, 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pieters N., et al. (2016) Biomolecular markers within the core axis of aging and particulate air pollution exposure in the elderly: a cross-sectional study. Environ. Health Perspect., 124, 943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hou L., et al. (2013) Inhalable particulate matter and mitochondrial DNA copy number in highly exposed individuals in Beijing, China: a repeated-measure study. Part Fibre Toxicol., 10, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pavanello S., et al. (2013) Mitochondrial DNA copy number and exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol. Biomarkers Prev., 22, 1722–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pieters N., et al. (2013) Decreased mitochondrial DNA content in association with exposure to polycyclic aromatic hydrocarbons in house dust during wintertime: from a population enquiry to cell culture. PLoS One, 8, e63208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Downward G.S., et al. (2014) Heterogeneity in coal composition and implications for lung cancer risk in Xuanwei and Fuyuan counties, China. Environ. Int., 68, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang C.C., et al. (2014) Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry Clin. Neurosci., 68, 551–557. [DOI] [PubMed] [Google Scholar]
- 39. Moore A., et al. (2017) A prospective study of mitochondrial DNA copy number and the risk of prostate cancer. Cancer Causes Control, 28, 529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barone-Adesi F., et al. (2012) Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ, 345, e5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xia Y., et al. (2015) Ambient air pollution, blood mitochondrial DNA copy number and telomere length in a panel of diabetes patients. Inhal. Toxicol., 27, 481–487. [DOI] [PubMed] [Google Scholar]
- 42. Cao J.J., et al. (2012) Winter and summer PM2.5 chemical compositions in fourteen Chinese cities. J. Air Waste Manag. Assoc., 62, 1214–1226. [DOI] [PubMed] [Google Scholar]
- 43. Cui M., et al. (2016) Chemical composition of PM from two tunnels with different vehicular fleet characteristics. Sci. Total Environ., 550, 123–132. [DOI] [PubMed] [Google Scholar]
- 44. Yang J., et al. (2015) [Study on the relationship between the inhalable fine particulate matter of Xuanwei coal combustion and lung cancer]. Zhongguo Fei Ai Za Zhi, 18, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tian L., et al. (2008) Nanoquartz in Late Permian C1 coal and the high incidence of female lung cancer in the Pearl River Origin area: a retrospective cohort study. BMC Public Health, 8, 398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu Y.C., et al. (2008) Characteristics of water-soluble ionic species in fine (PM2.5) and coarse particulate matter (PM10-2.5) in Kaohsiung, southern Taiwan. J. Air Waste Manag. Assoc., 58, 1579–1589. [DOI] [PubMed] [Google Scholar]
- 47. Large D.J., et al. (2009) Silica-volatile interaction and the geological cause of the Xuan Wei lung cancer epidemic. Environ. Sci. Technol., 43, 9016–9021. [DOI] [PubMed] [Google Scholar]
- 48. Kim C., et al. (2014) Smoky coal, tobacco smoking, and lung cancer risk in Xuanwei, China. Lung Cancer, 84, 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim C., et al. (2014) Pooled analysis of mitochondrial DNA copy number and lung cancer risk in three prospective studies. Cancer Epidemiol. Biomarkers Prev., 23, 2977–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.