Abstract
Pancreatic cancer has remained refractory to treatment. In large part, this results from the lack of an animal model that mimics pancreatic cancer in man. We describe a novel experimental model of pancreatic cancer that shares the genetic background, histologic features and natural history of human mixed acinar–ductal carcinoma. Adult wild-type mice received an injection into the pancreatic duct of lentivirus coding two molecules, KrasG12D mutation and shRNA p53, which recapitulate the mechanisms of pancreatic cancer in humans. The lentivirus constructs also co-expressed the luciferase gene for in vivo imaging by bioluminescence using the Xenogen IVIS imaging system. Weeks post-injection wild-type mice develop pancreatic cancer with the same histologic characteristics and metastases observed with human pancreatic mixed acinar–ductal carcinoma. This novel approach represents the first pancreatic cancer model that does not involve alterations of embryonic development, which is inherent with transgenic mice or knockout mice models. This novel experimental human pancreatic cancer model can be used to more effectively test new anti-cancer drug to inhibit tumor progression in situ and to retard metastases. Furthermore, our method of injecting lentivirus containing oncogenes and molecules implicated in the development of pancreatic can be employed in diabetic and obese mice, two common metabolic conditions characterized by an increased incidence of pancreatic cancer.
Introduction
Pancreatic cancer in man is one of the most intractable to treat and least understood malignancies. Obesity and diabetes are major risk factors for pancreatic cancer. The relative risk of pancreatic cancer is increased 1.5-fold in obese subjects and 2- to 3-fold in type 2 diabetic individuals (1). Approximately, 25% and 40%, respectively, of patients with pancreatic cancer have diabetes and impaired glucose tolerance at the time of diagnosis (2). The prevalence of obesity and type 2 diabetes mellitus has reached epidemic proportions during the last two decades in the US and worldwide, and this may explain, in part, why mortality from pancreatic cancer has not declined in the same way as lung cancer, cancer of the upper digestive tract, bladder cancer and other cancers (1). Advances in the treatment of pancreatic cancer have been negligible over the last two decades, and survival has not improved, with 5 year survival <5% (3). Type 2 diabetes mellitus is the third most modifiable risk factor for pancreatic cancer, after cigarette smoking and obesity (4). The current therapy for pancreatic cancer is gemcitabine plus nab-paclitaxel or 5-flurouacil, but these combinations extend life by only a few months (5, 6).
Oncologic drug development relies heavily on mouse models bearing transplanted tumors to test the efficacy of agents. However, the ability of such models to predict the utility of cancer therapeutic drugs has been disappointing. Transplanted tumors behave differently from tumors in situ, and they fail to recapitulate the behavior of the original malignant cells fully. Unfortunately, neither cell-based assays nor xenograft models have been especially successful in predicting drug responses in humans. Transgenic mice (embryonic overexpressed proteins) and embryonic knockout mice do not mimic the pathogenic mechanisms responsible for the development of cancer that develops in the post-natal period. There are significant and important differences between the development of cancer in humans and in genetically engineered mouse models (7). Both knockout models and overexpression of mutated proteins in transgenic mice have proven to be useful for modeling hereditary tumor syndromes that affect embryonic development. However, they are not well suited to the development of cancers that develop spontaneously with advancing age. Conditional recombinant transgenic mice with drug-sensitive promoter elements can be used to achieve time-dependent expression of oncogenes to induce tumors after development, i.e. in adults. However, models relying on a tissue-specific promoter result in the creation of mutated cells surrounded by other mutant cells. In a spontaneous human tumor, the initiating mutation most probably occurs in a cell that is surrounded by normal cells. Therefore, studies utilizing a tissue-specific promoter to create a pancreatic cancer model have to be interpreted with caution. The Pdx-1 promoter has been used to express Cre and the loxP-flanked DNA segments by Cre recombinase into the pancreas (8). However, utilization of the Pdx-1 promoter to express Cre in the pancreas not only targets the pancreatic beta cells but also the brain tissues (9), which have important functions in the regulation of glucose homeostasis (10). Pdx-1 is also expressed in all pancreatic cells during embryonic development, in endocrine cells in post-developmentally (10), in somatostatin-producing, pancreatic polypeptide-producing and insulin-producing cells in the adult pancreas (10) and in the intestine (11). Thus, use of the Pdx-1 promoter to overexpress a protein in the mouse will target many pancreatic as well as non-pancreatic cell types and tissues. Therefore, the timing of deletion of a protein involved in two different functions in the developmental and post-developmental period can result in completely different phenotypes. Another potential problem in conditional gene disruption experiments using Cre recombinase is Cre toxicity (8). Although most Cre-transgenic mice lines develop normally, some studies have demonstrated that Cre can damage genomic DNA, which results from the recombinase activity of Cre. As the authors (8) suggest, many Cre-expressing mouse lines are not completely normal but may largely overcome Cre toxicity through developmental selection and adaptation processes. If Cre toxicity induces a DNA mutation that causes activation of an oncogene, this could confound results obtained with a Cre-created model of pancreatic cancer. Chou et al. (12) induced pancreatic cancer by retrogradely injecting lentivirus and Cre protein directly into the pancreatic duct in adult transgenic mice with constitutive Cas9 expression to create in vivo CRISPR/Cas9-mediated somatic genome editing. However, the propensity for Cas9 off-target mutations in transformed cell lines highlights the need for evaluation of nuclease precision in vivo applications (13). The generation of DNA breaks at unintended (off-target) sites by imprecise Cas9 nucleases has the potential to alter gene expression and function through direct mutagenesis or the generation of genomic rearrangement (13). The combination of the toxic effect of Cre protein, and off-target mutations associated with the CRISPR/Cas9 method in a transgenic mouse model is unlikely to mimic the spontaneous development of pancreatic cancer in adult humans. In contrast, we used wild-type adult mice, thus obviating the influence of the transgenic model, Cre protein toxicity and CRISPR/Cas9 off-target mutations to develop a model of pancreatic cancer in adult mice. To avoid these problems, we developed a novel approach, which utilizes viral injection into the adult mice pancreas (14, 15) to recreate the pathophysiology of pancreatic cancer that develops in adulthood and thus avoids the development of compensatory mechanisms that occur when a gene is deleted during embryonic development.
Most (95%) pancreatic cancers arise in the exocrine pancreas (16). Pancreatic ductal adenocarcinoma comprises more than 90% of pancreatic cancers; the remaining 10% of pancreatic cancers include cystic neoplasms, acinar carcinomas and islet endocrine tumors (17). All of these pancreatic cancers are lethal. Acinar cell carcinoma of the pancreas is a rare pancreatic neoplasm accounting for ~1–2% of pancreatic tumors in adults and about 15% in pediatric subjects (18–19). Oncogenes can be activated through a variety of mechanisms, including point mutations within the gene and amplification of the gene itself. A growing number of oncogenes have been identified in pancreatic cancer. The most common activating point mutation involves the Kras oncogene, which is present in >90% of human pancreatic cancers (20). Oncogenic mutation of Ras remains constitutively active in the GTP-bound form with impaired GTPase activity. Activation of Ras induces cell cycle progression. In addition to Kras, the tumor suppressor p53 is frequently inactivated in human pancreatic cancers (21). The TP53 tumor-suppressor gene on chromosome 17p encodes the p53 protein (22), which is inactivated in 55–75% of human pancreatic cancers (22). The TP53 gene mutation is a late genetic event in pancreatic cancer progression (23), whereas the Kras mutation is an early event in pancreatic cancer progression (20) and results in the dysregulation of cell division and cell apoptosis. However, the Kras mutation by itself is not sufficient to develop an invasive pancreatic cancer. In the mouse knockout model, TP53 signaling, in the absence of oncogenic Kras, does not lead to the development of pancreatic carcinoma (24). However, in an embryonic genetically engineered mouse model, when mutations in both Kras and TP53 are present simultaneously, pancreatic cancer develops (24). Therefore, we have used the shRNA targeting p53 protein with overexpression of the Kras mutation of the constitutively active form to create a post-embryonic or post-developmental pancreatic cancer model in mice that recapitulate many aspects of the human disease. In a study by Hruban et al. (25), 68 of 82 (83%) human pancreatic carcinomas were shown to harbor an activating point mutation in codon 12 of the Kras oncogene (25). The most frequent Kras mutation is a guanine-to-adenine transition (GGT → GAT); this mutation has been incorporated into our cDNA Kras plasmid construct to create our novel experimental model for human pancreatic cancer.
The ideal pancreatic cancer model should reflect the human disease and produce controlled mutations in relevant endogenous genes in targeted cells while leaving the wild-type genotype in non-targeted cells. Injection of the lentiviral vector in vivo allows transfer of the genetic material randomly in the pancreas and creates mutant cells surrounded by non-mutant cells. The lentivirus vector does not activate dendritic cells, which are activated by the adenovirus vector. Furthermore, lentivirus vectors infect and integrate into both dividing and non-dividing cells, providing high transduction efficiency and sustained gene expression in vivo, they do not induce a significant host immune response or inflammation (the hallmark of an immune response) (26) and they can be successfully re-administered (14, 26). Most importantly, our method of lentiviral vector injection in vivo directly into the adult murine pancreas (14–15) allows one to recreate the spontaneous human pancreatic cancer that appears in post-developmental stage by intraductal injection of KrasG12D mutation and shRNA p53.
Methodology
Viral vector construct
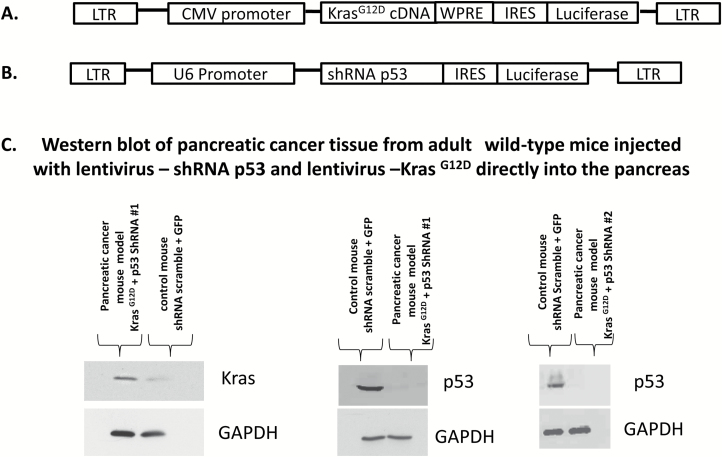
We designed a lentivirus vector construct expressing the KrasG12D mutation gene under control of the cytomegalovirus promoter (Figure 1A). We incorporated into our lentiviral vector construct a woodchuck hepatic virus post-transcriptional regulatory element at the 3’ untranslated region of coding sequence that substantially increased the level of expression of the transgene. The woodchuck hepatic virus post-transcriptional regulatory element functions within the nucleus to stimulate gene expression post-transcriptionally by increasing the level of nuclear transcripts and increasing the RNA half-life. The human KrasG12D mutation gene was subcloned to plasmid cytomegalovirus–woodchuck hepatic virus post-transcriptional regulatory element vector, and the insert was verified by DNA sequencing (Figure 1B). The plasmid–KrasG12D was treated with LR Clonase II enzyme (Invitrogen) and ligated to a Lentivirus plasmid. The recombinant product was transformed into Escherichia coli cells. After overnight incubation, the positive clones were selected and plasmid DNA was purified. The plasmid–KrasG12D was transfected into 293 cells. Forty-eight h after transfection, the cells were lysed in SDS-PAGE buffer and subjected to 4–20% SDS-PAGE gel electrophoresis and western blot analysis. The western blot of pancreatic cancer tissue from adults wild-type mice injected with lentivirus—shRNA p53 and lentivirus—KrasG12D directly to the pancreas was carried out using the anti-Kras antibody and p53 antibody from Santa Cruz Biotechnology at 1:1000 dilution, followed by a horseradish peroxidase-conjugated secondary antibody. The western blot membrane was detected by ECL reagents (Figure 1C). Two different shRNA p53 were introduced into a lentivirus construct using the same method described above for lenti-KrasG12D under the control of the U6 polymerase promoter (Figure 1C). The shRNA 1 was directed against the mouse p53 sequence 5’-GTACTCTCCTCCCCTCAAT-3’ (22). The shRNA 2 was directed against the mouse p53 sequence 5’-GTACATGTGTAATAGCTCC-3’ (23). The polymerase III promoter U6 is active ubiquitously in all cells because of the housekeeping function of polymerase III. Welgen (Worcester, MA) generated the lentivirus. Both lentivirus KrasG12D and shRNA p53 co-express the luciferase gene for tumor detection by the Xenogen IVIS system, which allows examinations of tumors with bioluminescence in vivo.
Figure 1.
(A) Lentiviral vector construct expressing KrasG12D mutation under the control of the cytomegalovirus promoter. We incorporated into our lentiviral vector construct a woodchuck hepatitis virus post-transcriptional regulatory element at the 3’ untranslated region of coding sequence; this substantially increased the level of expression of the transgene. (B) The shRNA p53 was introduced into a lentivirus construct under the control of U6 promoter. Both constructs co-express the luciferase gene. (C) Western blots of pancreatic tissues 30 weeks post-injection with lentivirus shRNA p53 and Lentivirus KrasG12D and non-cancer control mice. Non-pancreatic cancer mouse model (control) was injected with lentivirus shRNA-scramble and with lentivirus-expressing GFP at the same concentration and volume as the pancreatic cancer mouse model.
In vivo method for targeted gene delivery to the adult pancreas
Eight-week-old male mice CFW Swiss Webster (Charles River, Wilmington, MA) were maintained on an ad libitum diet of water and normal chow for all experiments. The two lentivirus vector oncogene(s) (50 µl each at 1 × 108 TU/ml) were injected together directly into the pancreas using a previously described method (12, 13). The control group was injected with lentivirus shRNA-scramble and lentivirus-green fluorescent protein (GFP) co-expressing luciferase protein for comparison with the experimental group. Briefly, the lentiviral construct is introduced the mouse pancreas via the pancreatic duct as follows: a 32-gauge catheter is inserted into the cystic duct through a small opening in the gallbladder. The catheter is then advanced into the common bile duct and secured in place with a slipknot of 0/0 suture around the bile duct and catheter to prevent vector reflux into the liver. With a micro-clamp placed around the sphincter of Oddi to avoid leakage of the vector into the duodenum, 100 μl of total lentiviral vector cocktail expressing cDNA KrasG12D and shRNA p53 at 108 to 109 TU/ml is slowly injected into the pancreatic duct through the catheter. The control placebo cocktail was composed of Lentivirus shRNA-scramble with Lentivirus expressing GFP at the same concentration and volume as the experimental cocktail. We have previously shown that 48 h after the injection of Lentivirus coding for GFP is detected only in pancreatic tissues (14). We demonstrated previously using quantitative morphometric analysis of pancreatic transduction by the lentivirus vector (based on GFP expression) that 60% of the tissues expressed GFP (14, 15) and this was confirmed in the present study with the expression of green fluorescent protein in the control group. Lentivirus vesicular stomatitis virus-glycoprotein envelope transduced both exocrine and endocrine tissues (14, 15). Importantly, expression was detected in the pancreas even after 4 weeks (14, 15). The lentivirus vector expressed GFP was not found in any other tissues in the body, including heart, lung, liver, small intestine, brain, leg muscle and kidney by histology (14, 15), and this was confirmed in the present study. Consistent with previous studies for our lab (14, 15), no evidence of inflammation was observed in the present study with our method of lentivirus injection.
In vivo tumor growth imaging
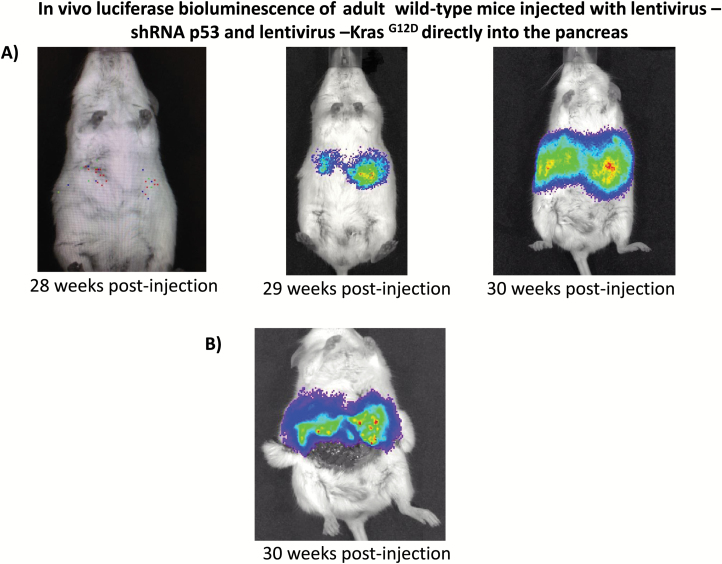
Tumor growth was followed with the Xenogen IVIS imaging system (Xenogen Corp., Alameda, CA). With spontaneous cancer mouse models, it is unclear which animals will develop overt tumors. Simply choosing a time point at which to enroll the animals can result in treating numerous mice that do not harbor tumors. By using the luciferase reporter system in combination with Xenogen IVIS imaging (Xenogen Corp., Alameda, CA), our study was based on a time point at which the tumor was detected with bioluminescence. Pancreatic tumor progression was evaluated in vivo with bioluminescence using the Xenogen IVIS imaging system (Figure 2) and at post-mortem with histological analysis of pancreatic cancer. All images were taken 10 min after intraperitoneal injection of luciferin (225 mg/kg; Xenogen Corp.) using a 60 s acquisition period. During image acquisition, mice were sedated via inhalation of ~3% isoflurane (Abbott Laboratories Ltd, Kent, UK). Ex vivo bioluminescence imaging (BLI) of the isolated pancreas was performed immediately after euthanasia of the animal with CO2 and 10 min after intraperitoneal injection of luciferin, as described above (Figure 2B). Image analysis and bioluminescent quantification was performed using Living Image Software (Xenogen Corp.). Tumor growth in mice injected (n = 10) with Lenti-shRNA p53 and Lenti-KrasG12D and in control mice (n = 10) was evaluated every week for months (Figure 3A). Endpoint criteria included the development of abdominal ascites, severe cachexia, weight loss in excess of 20% of initial body weight or extreme weakness or inactivity.
Figure 2.
In vivo luciferase bioluminescence of adult wild-type mice injected with lentivirus KrasG12D and lentivirus shRNA p53 directly into the pancreas. BLI of spontaneous pancreatic cancer arising from lentivirus—shRNA p53 and Lentivirus—KrasG12Din vivo. Both lentivirus KrasG12D and shRNA p53 co-express the luciferase gene for tumor detection by Xenogen IVIS system that allows visualization of tumors with bioluminescence in vivo. (A)In vivo imaging demonstrated luminescence signals from pancreatic tumors, which developed following intraductal pancreatic injection of lentivirus KrasG12D and shRNA p53 in wild-type adult mice at weeks 28, 29 and 30. (B) Imaging of internal organs after abdominal incision. Wild-type adult mice showed bioluminescence signals in the pancreas injected with lentivirus KrasG12D and shRNA p53.
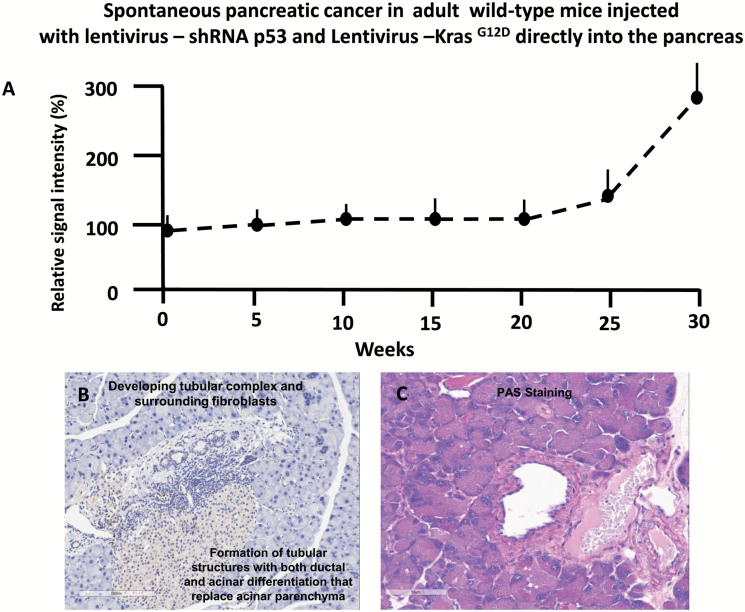
Figure 3.
(A) Tumor growth monitoring in a cohort of four mice began at 8 weeks of age [time zero] and 1 week before injection of lentivirus oncogenes KrasG12D and shRNA p53 and was followed over 30 weeks by in vivo bioluminescence. The signal intensity was normalized for each mouse individually, with 100% representing the geometrical mean of all values obtained per mouse during the 30 weeks observation period. For quantitation of light intensity in time course experiments, a constant analysis gate was defined and individual tumor photos were determined by centering the gate on the highest signal intensity for each time point. The mean ± SEM of the four mice is shown. (B) and (C) Pancreatic tumor progression in adult wild-type mice injected with lentivirus expressing, shRNA p53 and KrasG12D, directly into the pancreas. (B) Pancreatic tissue demonstrated the formation of a tubular complex with surrounding fibroblasts. (C) Periodic acid–Schiff staining is demonstrating mucin accumulation.
Histopathological analysis
Tissues were fixed in 10% formalin solution for 24 h and transferred to 70% ethanol. Tissues were paraffin embedded, sectioned and rehydrated. Hematoxylin/eosin and periodic Acid–Schiff staining were performed according to basic procedures. Sections were stained with anti-phospho-Erk1/2 (New England Biolabs, Ipswich, MA), anti-Ki67 (abcam, Cambridge, MA), anti-chromogranin A (abcam, Cambridge, MA), anti-cytokeratin 7 (Invitrogen, Rockford, IL), anti-cytokeratin 19 (Cell Signaling Technology, Danvers, MA) and with Trypsin, Bcl10, Mucin4 from Santa Cruz Biotechnology, Santa Cruz, CA. For each time point, histology of four sections separated by 200 µm from at least three individual animals was evaluated. Metastasis quantification: for each mouse, two lobes of the liver and one lung were sliced and embedded in paraffin. Gross and microscopic examination of H&E stained sections was used to identify the percentage of mice with at least one metastasis to the liver and lung.
RNA isolation and quantitative real-time PCR analysis
We examined the expression of Mucin1 (MUC1) in pancreatic cancer model compared with the control group. RNA was isolated from tissues using the RNeasy kit (Qiagen). The integrity of each RNA sample was confirmed post-extraction using denaturing (glyoxal) agarose gel electrophoresis. Reverse transcription was carried out with 0.5 µg total RNA using the ImProm II reverse transcription system (Promega, Madison, WI) according to the manufacturer’s instructions. Real-time quantitative PCR was then performed using 2 µl cDNA with a primer and 5’-terminal 6-carboxyflurescein (FAM)-labeled TaqMan probe mix from Applied Biosystems (assay ID Mm00449604_m1; Foster City, CA). Relative expression values were calculated from a standard curve, which consisted of a 2-fold dilution series from a pooled sample of each cDNA, and they were normalized to actin mRNA.
Results
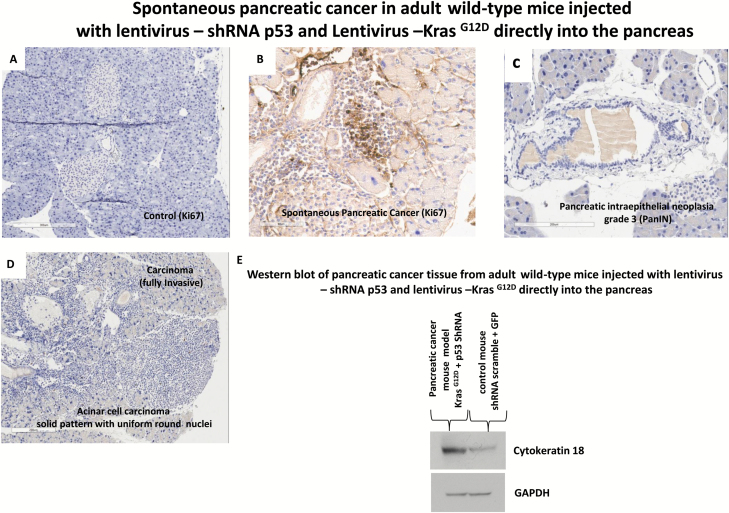
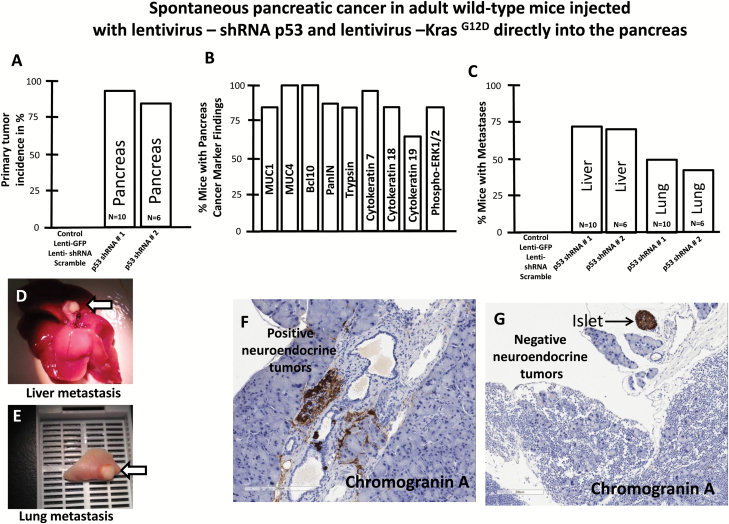
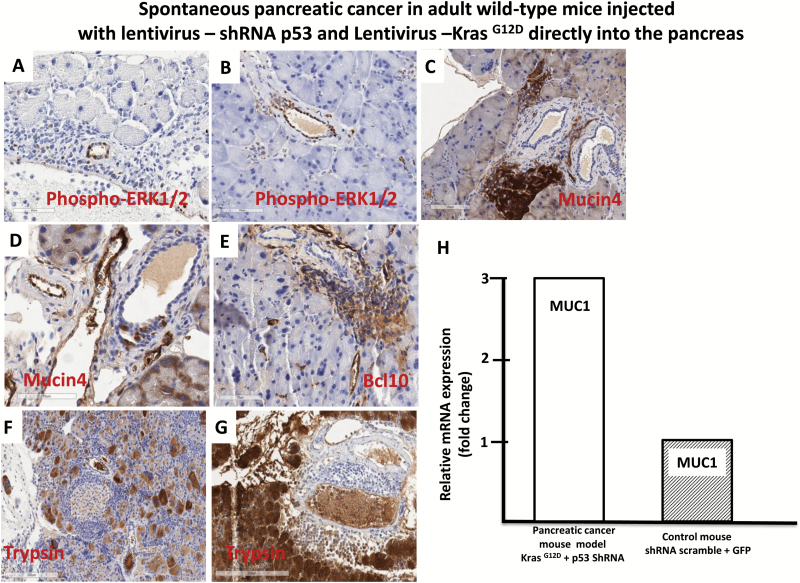
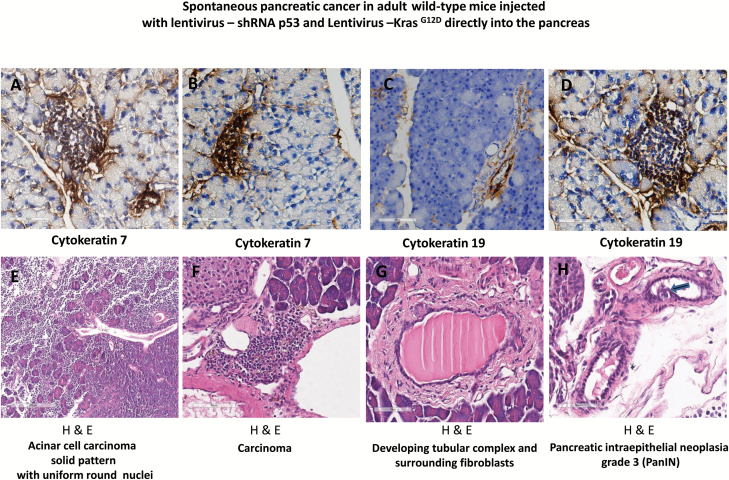
Tumor growth was measured beginning on day zero of injection of lentivirus expressing shRNA p53 and KrasG12D and weekly thereafter by BLI. Twenty-eight weeks post-injection mice developed pancreatic cancer (Figure 2A). The relative BLI signal intensity demonstrated constant progression beginning in week 28 and reaching a peak at 30 weeks (Figure 3A). BLI data demonstrated that tumors growth increased rapidly and exponentially between the short intervals of 28–30 weeks (Figure 2A). Following the final in vivo imaging at week 30, an incision of the abdomen was performed and the pancreas was imaged in situ (Figure 2B). Figure 2B demonstrates that BLI specifically localized into the pancreas. Histological examination of pancreas demonstrated that pancreatic cancer developed tubular complexes and surrounding fibroblasts (Figure 3B). We observed the formation of a tubular structure with both ductal and acinar cell differentiation replacing acinar parenchyma, as present in human pancreatic cancer. Histology section with periodic acid–Schiff staining demonstrated ductal adenocarcinomas containing mucin (Figure 3C). The normal epithelium was replaced by a flat, columnar, mucinous epithelium and grade 3, pancreatic intraepithelial neoplasia (PanIN) was observed in histological sections of the spontaneous cancer model (Figure 4C). PanIN represents microscopic proliferations of the smaller pancreatic ducts and are present in a spectrum ranging from the common and benign PanIN-1a lesion through PanIN-3, a carcinoma in situ (24). Histologic sections showed invasive acinar cell carcinoma with a solid pattern and uniformed round nuclei common in pancreatic cancer (Figure 4D). Ki67, a marker of cell proliferation, was present in histologic sections of mouse pancreatic cancer model but not in control mice (Figure 4A and 4B). Cytokeratin 18, a protein marker of ductal adenocarcinoma, was increased in 80% of the mice, which developed pancreatic cancer (Figures 4E and 6B). The ERK1/2 signaling pathway is upregulated in human pancreatic tumors (27). In our mouse pancreatic cancer model, we also observed activation of the MAP/ERK kinase pathway, measured by phopho-ERK1/2 immunohistochemistry (Figure 5A and 5B); no phosphor-ERK1/2 immunostaining was observed in the control group. BCL10 also was expressed in pancreatic tumors (Figure 5E). Trypsin staining was present in some, but not all pancreatic tumors (Figure 5F and 5G). Immunohistochemistry staining for mucin 4 was observed in all pancreatic tumors (Figures 5C, 5D and 6B). mRNA expression of the pancreatic cancer marker, MUC1, was increased 2-fold in mice with pancreatic cancer compared with the control group (Figure 5H). The incidence of pancreatic cancer was more than 80% in adult wild-type mice injected with lentivirus shRNA p53 and lentivirus KrasG12D directly into the pancreas (Figure 6A). Cytokeratin 7 was observed in mice which developed pancreatic cancer (Figure 7A and 7B) and has been shown to be present in 92% of humans with pancreatic adenocarcinoma (28). Cytokeratin 19 was observed in pancreatic mouse tumors (Figure 7C and 7D) and also has been shown to be expressed in a high percentage of humans with pancreatic ductal adenocarcinoma (29). However, cytokeratin 19 staining was absent in islets, excluding a neuroendocrine origin of the tumor. H&E staining (Figure 7E–7H) demonstrated the characteristic mixed acinar–ductal pancreatic cancer observed in humans. Although it is not possible to anticipate all of the morphologic patterns and pancreatic cancer markers in this newly emerging mouse model, the more common forms found in human pancreatic cancer were observed, including mixed acinar–ductal carcinoma (Figure 6B). About one-third of acinar cell carcinomas show a significant neuroendocrine component (>30%) and these cases are defined as mixed acinar–neuroendocrine carcinomas (30). The neuroendocrine component is very difficult to identify morphologically on H&E stained sections, and the use of immunohistochemistry employing antibodies directed against a general neuroendocrine marker chromogranin A is mandatory. To establish the diagnosis, histologic staining for the chromagranin A marker of neuroendocrine-derived tumors was absent in 11 of 12 mice, which were studied (Figure 6G); chromogranin A staining was present in only one mouse (Figure 6F). Thus, the formation of neuroendocrine derived tumors in our pancreatic cancer mouse model was a rare event. Plasma insulin, glucagon and glucose concentrations were normal in all mice, thus excluding insulinoma and glucagonoma (data not shown). No hyperplastic islets, a characteristic feature of endocrine tumors, were observed by histology. Per pancreatic tissue section, an average of 2.9 ± 0.6 (SEM) carcinomas per mouse was detectable in seven mice. Our mouse pancreatic cancer model develops metastasis in liver and lung (Figure 6C–6E). Furthermore, mice with advanced disease presented significant cachexia and ascites, mimicking the systemic effect observed in patients.
Figure 4.
Progression of pancreatic cancer in adult wild-type mice injected with lentivirus expressing, shRNA p53 and KrasG12D, directly into the pancreas. (A) Histology of pancreas staining for Ki67 in control mice injected with lentivirus shRNA-scramble and GFP. (B) Histology staining of pancreatic cancer for ki67 (brown) in mice injected with lentivirus shRNA p53 and krasG12D. (C) PanIN formation in spontaneous pancreatic cancer in adult wild-type mice injected with lentivirus expressing shRNA p53 and KrasG12D, directly into the pancreas. Histologic section showed the formation of PanIN grade 3. (D) Histology of pancreatic carcinoma in mice injected with shRNA p53 and KrasG12D. (E) Western blot detection of cytokeratin 18 in pancreatic tissues 30 weeks post-injection with lentivirus shRNA p53 and Lentivirus KrasG12D and in non-cancer control mice.
Figure 6.
(A) Percent (%) of wild-type mice injected with lentivirus-shRNA p53 and lentivirus-KrasG12D directly into the pancreas, which developed primary pancreatic tumors. (B) Percentage of mice with pancreatic cancer marker findings (n = 7). (C) Gross and microscopic examination of H&E stained sections were used to identify the percentage of mice with at least one metastasis to the liver and lung. (D) and (E) Metastatic progression in spontaneous pancreatic cancer in adult wild-type mice injected with lentivirus expressing, shRNA p53 and KrasG12D, directly into the pancreas. (D) Liver metastases (arrow). (E) Lung metastases (arrow). (F) and (G) Immunohistochemical staining for Chromagranin A (brown) in the pancreatic tissue section from the mouse cancer model.
Figure 5.
Progression of pancreatic cancer in adults wild-type mice injected with lentivirus expressing, shRNA p53 and KrasG12D, directly into the pancreas. (A) and (B) Activated Erk1/2 pancreatic cancer in adult wild-type mice with lentivirus expressing, shRNA p53 and KrasG12D, injected directly into the pancreas. (A) and (B) Immunohistochemical staining for activated Erk1/2 in the pancreatic tissues section cancer model. The brown color indicates immunoreactivity for activated (phosphorylated) Erk1/2. (C) and (D) Immunohistochemical staining for mucin 4 (brown) in pancreatic tissue section from the mouse cancer model. (E) Immunohistochemical staining for Bcl10 (brown) in the pancreatic tissue section from the mouse cancer model. (F) and (G) Immunohistochemical staining for trypsin (brown) in the pancreatic tissue section from the mouse cancer model. (H) Quantitative reverse transcription–polymerase chain reaction demonstrating increased mRNA level of MUC1 in pancreatic tissues from the mouse cancer model compared with control group.
Figure 7.
Progression of pancreatic cancer in adult wild-type mice injected with lentivirus expressing, shRNA p53 and KrasG12D, directly into the pancreas. (A) and (B) Immunohistochemical staining for cytokeratin 7 (brown) in adult wild-type mice with lentivirus expressing, shRNA p53 and KrasG12D, injected directly into the pancreas. (C) and (D) Immunohistochemical staining for cytokeratin 19 (brown) in adult wild-type mice with lentivirus expressing, shRNA p53 and KrasG12D, injected directly into the pancreas. (E) H&E stained demonstrated acinar cell carcinoma solid pattern with uniform round nuclei. (F) H&E stained of pancreatic carcinoma. (G) H&E stained demonstrated developing tubular complex and surrounding fibroblasts. (H) H&E stained section showed the formation of PanIN grade 3. H&E = Hematoxylin and eosin stain.
Discussion
Pancreatic cancer was responsible for ≈37000 deaths in 2011, making it the fourth leading cause of cancer-related deaths in the US (31), and the 5 year survival rate has not improved significantly over the last two decades. A suggested reason for the low survival includes late diagnosis, highly invasive and metastatic nature of cancer and lack of effective therapies (31). The failure to develop of effective therapies results, in part, from the lack of an appropriate animal model that mimics human pancreatic cancer and which can be used to test new anti-cancer drugs. The use of xenografts in immunodeficient mice and genetically modified cancer mouse models has failed to lead to the development of effective therapies for human pancreatic cancer (32–34). Cancer develops randomly in tissues with cancer cells surrounded by non-cancer cells. In the present study, injection of the lentivirus, containing the oncogene KrasG12D and shRNA p53, into the pancreas resulted in integration of the transgenes into the genomes by targeting the cell in a mosaic pattern that closely mimics human cancer (35) and differs from Cre-Lox recombination systems from a tissue-specific promoter that result in mutated cells are surrounded by other mutant cells and that poorly mimic naturally random occurring genetic development of human pancreatic cancer (8). By using the lentivirus, cells are randomly targeted (36), creating a pattern in which cancer cells are surrounded by non-cancer cells as in human cancer development (8). Thus, this approach differs from the use of a specific promoter in transgenic mice, which targets all cells uniformly without reproducing the mosaic pattern observed in human cancer development. The tumor micro-environment, composed of non-cancer cells and their stroma, has become recognized as a major factor, which influences the growth of cancer (37). The present pancreatic cancer model, which utilizes lentivirus KrasG12D and shRNA p53 injection in vivo to specifically target the adult pancreas, is the first model, which mimics the mosaic pattern of spontaneous cancer development and allows one, for the first time, to test anti-cancer drugs in the context of a model that reproduces the in situ tumor micro-environment.
Another important disadvantage of currently available pancreatic mouse models is that either they do not develop metastases or, if they do, the metastases target different tissues than those observed in human cancer (38). In our model, metastases were consistently present in the lungs and liver, a pattern similar to that observed in human pancreatic cancer (Figure 6B). In human pancreatic cancer, metastatic sites frequently included the liver and lung (39). We observed a 2-fold increase in MUC1 mRNA level in the mice with cancer compared with the control group (Figure 5H). MUC1, a transmembrane mucin glycoprotein, is associated with the most invasive forms of pancreatic ductal adenocarcinomas (40) and is overexpressed in >90% of metastatic pancreatic ductal adenocarcinomas in human (41). Pancreatic cancers containing MUC1 have been shown to demonstrate enhanced invasiveness by inducing epithelial to mesenchymal transition and this correlates with an increased incidence of lung and liver metastasis (40). Cell migration is a fundamental process in cancer metastases. Interestingly, the metastatic lesions in the liver and lung did not display BLI signal, suggesting that cancer cell migration to the lung or liver did not contain the luciferase gene and this was confirmed by reverse transcription–polymerase chain reaction (data not shown). In contrast, injection of cultured xenograft cells containing the luciferase gene allows one to follow migration of the cancer cells to another organ using BLI (42). Our spontaneous in vivo pancreatic cancer model differs from the xenograft cell culture model in that the metastases in the liver and lung did not express luciferase gene. This suggests that the metastases originated from endogenous pancreatic tumor cells and not from the initial cancer cells that integrated the luciferase gene following lentivirus injection, whereas the persistent BLI of the transplanted cultured xenograft cancer cells suggests that endogenous pancreatic cells are not the ones implicated in the metastases development. Rather, the xenograft cancer cells have proliferated in vivo, similar to what these cells do in the Petri dish.
A fundamental feature of cancer is tumor clonality, the development of tumors from single cells that begin to proliferate abnormally. The clonal origin of tumors does not mean that the original progenitor cell that gives rise to the tumor possesses all of the characteristics of the original cancer cell. To the contrary, the development of cancer is a multistep process in which cells gradually become malignant through a progressive series of alterations (43–45). In pancreatic cancer, lung and peritoneal metastases were shown to descend from different clones than the primary tumor (46–47). An emerging, based upon the analysis of metastatic tumor specimens is that the mechanism of action of the metastasis-associated gene products is not likely to be metastasis-specific (47). Analysis of human cancers and mouse circulating tumor cells demonstrated a strong effect of non-genetic events in the development of liver metastasis, suggesting that early disseminated cancer cells may evolve independently of, and in parallel with, cancer cells from the primary tumor (48). Consistent with this concept, phenotypically identical metastatic cells are not identical genetically (45). Our spontaneous pancreatic cancer model in which the metastatic cells did not contain the luciferase gene is consistent with this scenario that spreading tumor need not necessarily keep the same genotype as the original tumor.
Tumor cells are subjected to in situ signals from multiple sources, including stromal cells, matrix proteins, endothelial cells, immune cells, and neighboring epithelial cells (37). When a tumor is removed from its native site, these complex interactions are interrupted. Xenograft cancer cells do not reproduce tumor development from their native site. Our novel spontaneous pancreatic cancer model preserves the in situ developmental interaction between developing cancer and its environment, more closely mimicking the development of pancreatic cancer in man, and provides another advantage over currently available mouse models of pancreatic cancer.
Most pancreatic cancers are diagnosed between the ages of 60–80 years (49). Although uncommon, pancreatic cancer can occur in subjects under the age of 40 (50–51). Our novel pancreatic cancer model permits the introduction of oncogenes into adult mice and, thus, can mimic the spontaneous development of pancreatic cancer in man. In our studies, eight-week-old adult wild-type mice were infected with the lentivirus containing KrasG12D and shRNA p53. An advantage of our method is that it allows one to examine the effect of age on the development and progression of pancreatic cancer. Cigarette smoking, diabetes mellitus and obesity are established risk factors for human pancreatic cancer (2, 51–53). Another advantage of our novel pancreatic cancer model is the ability to study the impact of diabetes and obesity on the natural history of the disease.
Following injection of the lentivirus containing KrasG12D and shRNA p53, 80% of the mice develop pancreatic cancer, with PanIN development, histologic morphology, and metastasis in lung and liver that closely mimic human mixed acinar–ductal pancreatic cancer (Figure 6). The high penetrance of pancreatic cancer development in our model is both time and cost advantageous and promotes more effective preclinical drug screening.
In conclusion, we have developed a novel spontaneous adult in vivo pancreatic cancer model that can be a valuable tool to monitor the formation of tumor development following injection of lentivirus containing KrasG12D and shRNA p53. This pancreatic cancer model can be used to follow formation and progression of metastases and offers the opportunity to test novel anti-cancer drugs in an animal model of pancreatic cancer that closely resembles its human counterpart.
Funding
The study was supported by funds from the Diabetes Division, UTHSCSA.
Acknowledgements
We thank Nikhil A. Acharya from the Diabetes Division of UTHSCSA for assistance with animal experiments. B.D. initiated and directed the project, developed the new pancreatic cancer model, designed and performed the experiments (histology; animal research; molecular analyses), analyzed the data and wrote the manuscript; R.A.D. was involved in the analysis and interpretation of the data and writing of the manuscript. R.A.D’s salary is supported in part by the South Texas Veterans Health Care System.
Conflict of Interest Statement: None declared.
Abbreviations
- BLI
bioluminescence imaging
- GFP
green fluorescent protein
- PanIN
pancreatic intraepithelial neoplasia
References
- 1. Bosetti C., et al. (2012)Pancreatic cancer: overview of descriptive epidemiology. Mol. Carcinog., 51, 3–13. [DOI] [PubMed] [Google Scholar]
- 2. Chari S.T., et al. (2008)Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology, 134, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma C., et al. (2011)Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J. Gastroenterol., 17, 867–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li D. (2012)Diabetes and pancreatic cancer. Mol. Carcinog., 51, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burris H.A., 3rd, et al. (1997)Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol., 15, 2403–2413. [DOI] [PubMed] [Google Scholar]
- 6. Matsuno S., et al. (2001)Trends in treatment for pancreatic cancer. J. Hepatobiliary. Pancreat. Surg., 8, 544–548. [DOI] [PubMed] [Google Scholar]
- 7. Rangarajan A., et al. (2003)Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat. Rev. Cancer, 3, 952–959. [DOI] [PubMed] [Google Scholar]
- 8. Schimidt-Supprian M., et al. (2007)Vagaries of conditional gene targeting. Nat. Immunol., 8, 665–668. [DOI] [PubMed] [Google Scholar]
- 9. Honig G., et al. (2010)Precise pattern of recombination in serotonergic and hypothalamic neurone in a Pdx1-cre transgenic mouse line. J. Biomed.Sci.,17:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Habener J.F., et al. (2005)Minireview: transcriptional regulation in pancreatic development. Endocrinology, 146, 1025–1034. [DOI] [PubMed] [Google Scholar]
- 11. Chen C., et al. (2009)Pdx1 inactivation restricted to the intestinal epithelium in mice alters duodenal gene expression in enterocytes and enteroendocrine cells. Am. J. Physiol. Gastrointest. Liver Physiol., 297, G1126–G1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiou S.H., et al. (2015)Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev., 29, 1576–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bolukbasi M.F., et al. (2016)Creating and evaluating accurate CRISPR-Cas9 scalpels for genomic surgery. Nat. Methods, 13, 41–50. [DOI] [PubMed] [Google Scholar]
- 14. Doiron B., et al. (2012)Lentivirus shRNA Grb10 targeting the pancreas induces apoptosis and improved glucose tolerance due to decreased plasma glucagon levels. Diabetologia, 55, 719–728. [DOI] [PubMed] [Google Scholar]
- 15. Doiron B., et al. (2016)Beta cell formation in vivo through cellular networking, integration and processing (CNIP) in wild type adult mice. Curr. Pharm. Biotechnol., 17, 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson K., et al. (2006)Pancreatic cancer. In Schottenfeld D, Fraumeni J.F. Jr (eds) Cancer Epidemiology and Prevention. Oxford University Press, New York, pp. 721–762. [Google Scholar]
- 17. Tuveson D.A., et al. (2005)Ductal pancreatic cancer in humans and mice. Cold Spring Harb. Symp. Quant. Biol., 70, 65–72. [DOI] [PubMed] [Google Scholar]
- 18. La Rosa S., et al. (2015)Acinar cell carcinoma of the pancreas: overview of clinicopathologic features and insights into the molecular pathology. Front. Med. (Lausanne)., 2, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klimstra D.S. (2007)Nonductal neoplasms of the pancreas. Mod. Pathol., 20 (Suppl 1), S94–112. [DOI] [PubMed] [Google Scholar]
- 20. Almoguera C., et al. (1988)Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell, 53, 549–554. [DOI] [PubMed] [Google Scholar]
- 21. Barton C.M., et al. (1991)Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br. J. Cancer, 64, 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Redston M.S., et al. (1994)p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res., 54, 3025–3033. [PubMed] [Google Scholar]
- 23. Moore P.S., et al. (2001)Genetic profile of 22 pancreatic carcinoma cell lines. Analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch., 439, 798–802. [DOI] [PubMed] [Google Scholar]
- 24. Koorstra J.B., et al. (2008)Morphogenesis of pancreatic cancer: role of pancreatic intraepithelial neoplasia (PanINs). Langenbecks. Arch. Surg., 393, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hruban R.H., et al. (1993)K-ras oncogene activation in adenocarcinoma of the human pancreas. Am. J. Pathol., 143, 545–554. [PMC free article] [PubMed] [Google Scholar]
- 26. Kafri T., et al. (1997)Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat. Genet., 17, 314–317. [DOI] [PubMed] [Google Scholar]
- 27. Chadha K.S., et al. (2006)Activated Akt and Erk expression and survival after surgery in pancreatic carcinoma. Ann. Surg. Oncol., 13, 933–939. [DOI] [PubMed] [Google Scholar]
- 28. Chu P., et al. (2000)Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod. Pathol., 13, 962–972. [DOI] [PubMed] [Google Scholar]
- 29. Jain R., et al. (2010)The use of Cytokeratin 19 (CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver. Appl. Immunohistochem. Mol. Morphol., 18, 9–15. [DOI] [PubMed] [Google Scholar]
- 30. Rindi G, et al. , (2010)Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In Bosmanet al. (eds) WHO Classification of Tumors of the Digestive System. IARC Press, Lyon. [Google Scholar]
- 31. American Cancer Society(2011)Cancer Facts and Figures. American Society, Atlanta. [Google Scholar]
- 32. Ventura A., et al. (2004)Cre-lox-regulated conditional RNA interference from transgenes. Proc. Natl. Acad. Sci. U.S.A., 101, 10380–10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tiscornia G., et al. (2004)CRE recombinase-inducible RNA interference mediated by lentiviral vectors. Proc. Natl. Acad. Sci. U.S.A., 101, 7347–7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olive P.K., et al. (2006)The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin. Cancer Res., 12, 5277–5287. [DOI] [PubMed] [Google Scholar]
- 35. Singer O., et al. (2008)Applications of lentiviral vectors for shRNA delivery and transgenesis. Curr. Gene Ther., 8, 483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weinberger L.S., et al. (2005)Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell, 122, 169–182. [DOI] [PubMed] [Google Scholar]
- 37. Li H., et al. (2007)Tumor microenvironment: the role of the tumor stroma in cancer. J. Cell. Biochem., 101, 805–815. [DOI] [PubMed] [Google Scholar]
- 38. Abate-Shen C. (2006)A new generation of mouse models of cancer for translational research. Clin. Cancer Res., 12, 5274–5276. [DOI] [PubMed] [Google Scholar]
- 39. Saur D., et al. (2005)CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology, 129, 1237–1250. [DOI] [PubMed] [Google Scholar]
- 40. Roy L.D., et al. (2011)MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene, 30, 1449–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lan M.S., et al. (1990)Cloning and sequencing of a human pancreatic tumor mucin cDNA. J. Biol. Chem., 265, 15294–15299. [PubMed] [Google Scholar]
- 42. Yoshimura H., et al. (2013)In vivo bioluminescence imagine of pancreatic cancer xenografts in NOG mice. J. Carcinogene Mutagenes, S9, 003. [Google Scholar]
- 43. van Oijen M.G., et al. (2000)The origins of multiple squamous cell carcinomas in the aerodigestive tract. Cancer, 88, 884–893. [DOI] [PubMed] [Google Scholar]
- 44. El-Naggar A.K., et al. (1996)Microsatellite instability in preinvasive and invasive head and neck squamous carcinoma. Am. J. Pathol., 148, 2067–2072. [PMC free article] [PubMed] [Google Scholar]
- 45. Chung K.Y., et al. (1993)Discordant p53 gene mutations in primary head and neck cancers and corresponding second primary cancers of the upper aerodigestive tract. Cancer Res., 53, 1676–1683. [PubMed] [Google Scholar]
- 46. Campbell P.J., et al. (2010)The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature, 467, 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vanharanta S., et al. (2013)Origins of metastatic traits. Cancer Cell, 24, 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Klein C.A. (2009)Parallel progression of primary tumours and metastases. Nat. Rev. Cancer, 9, 302–312. [DOI] [PubMed] [Google Scholar]
- 49. Matsuno S., et al. (2004)Pancreatic cancer registry in Japan: 20 years of experience. Pancreas, 28, 219–230. [DOI] [PubMed] [Google Scholar]
- 50. Lüttges J., et al. (2004)Rare ductal adenocarcinoma of the pancreas in patients younger than age 40 years. Cancer, 100, 173–182. [DOI] [PubMed] [Google Scholar]
- 51. Hruban R.H., et al. (2007)Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod. Pathol., 20 Suppl 1, S61–S70. [DOI] [PubMed] [Google Scholar]
- 52. Hruban R.H., et al. (2001)Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am. J. Surg. Pathol., 25, 579–586. [DOI] [PubMed] [Google Scholar]
- 53. Gold E.B., et al. (1998)Epidemiology of and risk factors for pancreatic cancer. Surg. Oncol. Clin. N. Am., 7, 67–91. [PubMed] [Google Scholar]