Summary
By reversing emphasis from precursor uptake to product accumulation and release, we posit that lactate production (‘lactagenesis’) supports carcinogenesis and it is the explanation and purpose of the Warburg effect. We posit that in carcinogenesis, aberrant cell signaling due to exaggerated and continually high lactate levels yields an inappropriate positive feedback loop that increases glucose uptake, glycolysis, lactate production and release, decreases mitochondrial function and clearance and upregulates glycolytic enzyme and monocarboxylate transporter expression thereby supporting angiogenesis, immune escape, cell migration, metastasis and self-sufficient metabolism, all of which encourage progression to cancer.
Abstract
Herein, we use lessons learned in exercise physiology and metabolism to propose that augmented lactate production (‘lactagenesis’), initiated by gene mutations, is the reason and purpose of the Warburg Effect and that dysregulated lactate metabolism and signaling are the key elements in carcinogenesis. Lactate-producing (‘lactagenic’) cancer cells are characterized by increased aerobic glycolysis and excessive lactate formation, a phenomenon described by Otto Warburg 93 years ago, which still remains unexplained. After a hiatus of several decades, interest in lactate as a player in cancer has been renewed. In normal physiology, lactate, the obligatory product of glycolysis, is an important metabolic fuel energy source, the most important gluconeogenic precursor, and a signaling molecule (i.e. a ‘lactormone’) with major regulatory properties. In lactagenic cancers, oncogenes and tumor suppressor mutations behave in a highly orchestrated manner, apparently with the purpose of increasing glucose utilization for lactagenesis purposes and lactate exchange between, within and among cells. Five main steps are identified (i) increased glucose uptake, (ii) increased glycolytic enzyme expression and activity, (iii) decreased mitochondrial function, (iv) increased lactate production, accumulation and release and (v) upregulation of monocarboxylate transporters MTC1 and MCT4 for lactate exchange. Lactate is probably the only metabolic compound involved and necessary in all main sequela for carcinogenesis, specifically: angiogenesis, immune escape, cell migration, metastasis and self-sufficient metabolism. We hypothesize that lactagenesis for carcinogenesis is the explanation and purpose of the Warburg Effect. Accordingly, therapies to limit lactate exchange and signaling within and among cancer cells should be priorities for discovery.
Introduction
In 1923, Otto Warburg observed that cancer cells were characterized by accelerated glycolysis and excessive lactate formation even under fully oxygenated conditions (1,2). His discovery was subsequently named the ‘Warburg Effect’ by Efraim Racker in 1972 (3). Significance of Warburg’s discovery is still apparent in the common cancer diagnostic test using 18F-deoxyglucose positron emission tomography (18F-FDG-PET) (4) which has a high diagnostic accuracy (5–7). Long ago, Warburg observed that when cultured in 13 mM glucose, cancer cells produced a 70-fold increase in lactate accumulation (1). Warburg also observed that blood lactate concentration was higher in blood vessels leaving tumor tissues than the lactate concentration in blood vessels entering tumors (8). Although common thought has been that Warburg’s discovery was one of exacerbated glucose uptake and glycolysis by tumor cells, his main finding may be that of increased lactate production, accumulation and release. According to his calculations from decades ago, arterial glucose uptake in tumor cells was about 47–70% compared to 2–18% in normal tissues and tumor cells converted 66% of glucose uptake to lactate (8). The finding of atypical lactate production led Warburg to propose that the primary lesion common to cancer cells was in the respiratory chain that caused cancer cells to rely on glycolysis instead of oxidative phosphorylation for energy (9,10). Warburg’s work lead to the hypothesis that cancer was a disease of abnormal cell metabolism, and although some researchers support the idea that mitochondrial malfunction is the beginning of cancer (11), there is contemporary consensus that mutations leading to metabolic dysregulation are first steps in progression to carcinogenesis (12). Still, the role of the Warburg Effect in cancer has neither been explained nor understood for nearly a century.
While the Warburg Effect is a hallmark of cancer, the study of cancer cell metabolism was diverted when investigators began to employ genomic techniques to better understand cancer biology. We lament that the lack of understanding about the meaning and role of the Warburg Effect in cancer did not progress in parallel, a history that may have impeded the full comprehension of cancer biology, and, consequently, the development of effective therapeutic approaches abased on understanding of the roles of lactate in promoting carcinogenesis and tumorigenesis. Although there have been important advances in the identification of oncogenes, tumor suppressor mutations and epigenetics as well as some therapeutic applications, the cure for cancer through gene-based research has yet to come to fruition (13). We still do not know the ‘why’ or the ‘purpose’ of the Warburg Effect, its role in cancer growth and carcinogenesis, or how to halt or reverse metabolic dysregulation in cancer cells. Fortunately, recent efforts of many investigators referenced herein are bringing cancer metabolism to a renaissance which may lead to new insights and methods crucial to winning the war against ‘the emperor of all maladies’ as Dr Siddhartha Mukherjee wrote in his best-seller book (14).
Not only has there been a resurgence of interest in understanding the role of lactate in cancer, there is growing appreciation for the role of lactate in normal physiology and it’s use in the treatment of injuries and illnesses (15). In better understanding the regulation and integration of glycolytic and oxidative metabolism in normal physiology, and by comparing and contrasting normal and pathophysiological lactate responses, we seek to dissect out aberrations in cancer. For nearly a century, lactate was viewed as a waste product of anaerobic metabolism when, in fact, via the Lactate Shuttle (16–19), lactate is now recognized to be one of the most important energy fuels, the major gluconeogenic precursor, and a highly active signaling molecule with ‘hormone-like properties’ (18). Aerobic glycolysis and lactate production constantly occur in skeletal muscles, not only during high intensity exercise, but also at rest because lactate is the obligatory product of glycolysis (17–19). And, of course, the rate of lactate production is greatly enhanced in working skeletal muscles (20). Hence, during high-intensity exercise, working muscles display some of the same metabolic characteristics as do cancer cells. Lactate production and accumulation in exercise results in changes in metabolic gene expression (21), but with acute exercise and exercise training, lactate exposures are intermittent and result in favorable metabolic adaptations controlled via negative feedback mechanisms (21). In contrast, herein we propose that continual and dysregulated, as opposed to intermittent and regulated metabolism, lactagenesis in cancer results in positive, feed-forward responses that are maladaptive. In this article, we view cancer cell biology from perspective of the Lactate Shuttle concept proposed by Brooks in the 80’s, wherein lactate produced at one intracellular site can elicit a host of autocrine, paracrine and endocrine responses (17,19). Because there are so many metabolic characteristics in common between working muscles and cancer cells, we think it is reasonable to assess whether new knowledge in skeletal muscle metabolism during exercise can be useful to cancer biology researchers (22). And finally, in this review we emphasize the effects of lactate anion from those of pH. In our studies on normal and transformed cells, buffered media were used (21,23–25). In our studies on healthy human subjects (26–28), and in our studies (15) and those of others (29,30) on traumatic brain-injured patients sodium-lactate treatment has been applied. Such treatment is pH neutral or has, if anything, slightly alkalinizing effect (26). Hence, our document is a plea to consider the effects of lactate anion, as opposed to hydrogen ion, on cancer cell metabolism. Hopefully, this new perspective will lead to a broad discussion of lactate metabolism in cancer that could lead to the development of new diagnoses and therapeutics.
The Warburg Effect: cancer cells thriving on ‘metabolic inefficiency’?
Herein, we propose that the first step by which lactagenesis leads to carcinogenesis is when candidate cells begin to transform themselves to become highly glycolytic and poorly oxidative in a fashion that resembles the bioenergetics of primitive eukaryotic and prokaryotic organisms like yeast or bacteria. Viewed from the perspective of contemporary cell energetics, these newly transformed cancer cells possess an ‘inefficient’ mechanism to produce ATP that favors aerobic glycolysis and lactate production in the cytosol instead of glucose oxidation progressing through mitochondrial oxidative phosphorylation. Again, from a contemporary perspective of cell metabolic efficiency, it is difficult to comprehend why, despite fully aerobic conditions, cancer cells move away from an ‘efficient’ metabolic mechanism and ‘choose’ an inefficient pathway producing two cytosolic ATPs per molecule of glucose instead of ~36–38 ATPs via coupled mitochondrial respiration (Figure 1). Proliferating cells need ATP for growth and scarce levels of ATP can compromise proliferation and result in apoptosis or necrosis (31). In contrast, healthy mammalian cells and organisms have evolved and adapted to environmental stress by emphasizing aerobic metabolism. For example, in response to endurance exercise training mass of the muscle mitochondrial reticulum can double (32,33) resulting in increased mitochondrial capacity and thus improved substrate utilization (32,34–37), an effect that not only raises aerobic capacity and athletic performance, but also increases respiratory control and facilitates metabolic flexibility, which is the ability to switch between lipid and carbohydrate-derived energy fuels depending on the metabolic power needed. But, could it be that our contemporary puzzlement over the fact of aerobic glycolysis in cancer cells contains a bias based on our understanding of efficient metabolism in non-transformed cells? That cancer cells expropriate glucose from an otherwise healthy host is accomplished because the host maintains euglycemia so there is no glucose deficit or other nutritive stress imposed on the transformed cells; the metabolic cost is simply passed onto the host. The stress of accelerated lactate production in cancer cells is mitigated by overexpression of lactate transporters, symporters for lactate anions and protons that export lactic acid into the host.
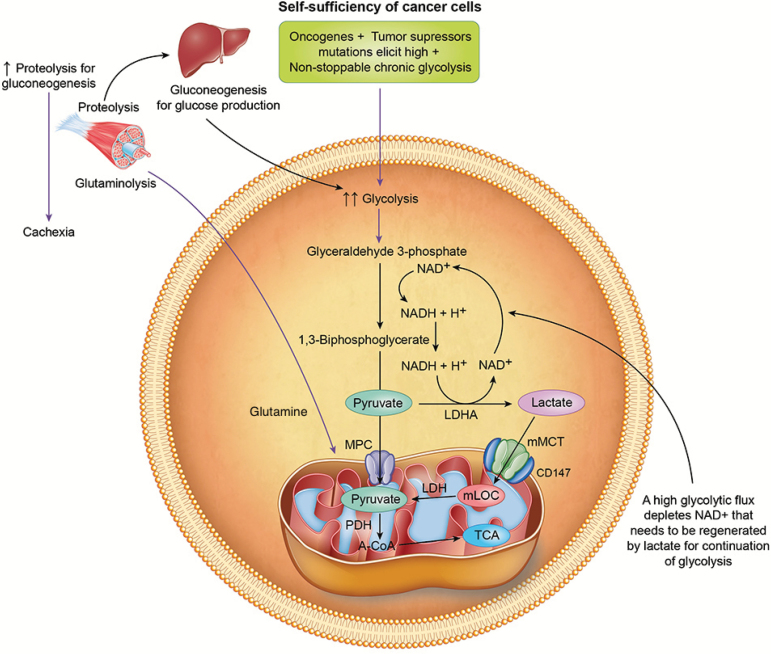
Figure 1.
Representation of self-sufficiency in cancer cells. Accelerated glycolysis elicited by oncogenes and tumor suppression mutations depletes nicotinamide adenine dinucleotide (NAD+). The reduction of pyruvate to lactate replenishes cytosolic levels of NAD+ and regulates the status of the equilibrium of the cytoplasmic redox pair (NADH/NAD+) for continuation of glycolysis. Lactate enters mitochondria and is oxidized to pyruvate and then acetyl CoA (A-CoA) through mitochondrial lactate oxidation complex (mLOC) comprised of mitochondrial monocarboxylate transporters (mMCT), its stabilizer, CD147, mitochondrial lactate dehydrogenase (LDH) and cytochrome oxidase (COx). Pyruvate can also enter mitochondria through mitochondrial pyruvate carrier (MPC) for oxidation to A-CoA. In glycolytic cancers, increased glycolysis is chronic which may deplete glycogen stores leading to increased proteolysis for gluconeogenesis, as well as for glutaminolysis to increase cytosolic pyruvate for lactate production. Chronic increased proteolysis for gluconeogenesis and glutaminolysis could explain cachexia in cancer.
In contrast to metabolic regulation in the healthy heart and skeletal muscles of trained individuals, among cancer cells there occurs a glucose to lactate shunt in which the host bears the burden of providing a relatively limitless glucose supply as well as a sink for disposal of lactate and hydrogen ions. That cancer cells proliferate, and tumors grow and metastasize because of host exploitation may explain why the actual cause of death due to cancer appears to be multifactorial with multiple organ failures, rather than the tumor itself.
Oncogenes, metabolic dysregulation and tumor suppressors: a highly orchestrated performance to produce lactate
Through an orchestrated oncogene activation, tumor suppressor mutations and epigenetics, cancer cells in glycolytic tumors undergo a metabolic reprogramming transforming themselves into highly glycolytic and poorly oxidative cells with lactate formation as the end product despite normoxic conditions (12,38–40).
Glycolytic enzyme expression in tumors: parallels between the lactate shuttle in normal physiology and the Warburg Effect in cancer
Glycolytic enzymes are overexpressed in many tumors with a wide 2–500 fold range increase (41). Of all the genetic components, there is triad of transcription factors involved in cancer cellular metabolic dysregulation comprised of hypoxia-inducible transcription factor-1 (HIF-1), c-Myc and p53 (42). Current thinking is that upregulation of two of these transcription factors (HIF-1 and c-Myc) is instigated by growth factor binding to plasma membrane receptors that stimulate receptor tyrosine kinases (RTKs) that activate the PI3K/Akt pathway and Ras (43). In contrast to HIF-1 and c-Myc, the tumor and glycolytic pathway suppressor p53 is repressed in cancer (44–46). In aggregate, upregulation of HIF-1 and c-Myc and suppression of p53 are likely responsible for the metabolic switch to glycolysis in cancer cells.
In cancer, as elsewhere, HIF-1 responds to low oxygen tension and activates the transcription of genes encoding glycolytic enzymes, glucose and lactate transporters (47–49) and it is correlated with cancer aggressiveness and poor prognosis (50). A key enzyme in glycolysis is pyruvate dehydrogenase (PDH), normally responsible for the oxidation of pyruvate to acetyl-CoA for mitochondrial oxidation. HIF-1 activates pyruvate dehydrogenase kinase-1 (PDK-1) that phosphorylates and inactivates PDH that limits oxidative disposal of pyruvate and, thereby favors diversion of the glycolytic flux to lactate. Inactivation of PDH by HIF-1 is well known in exercise metabolism characterized by high glycolytic flux and lactate production (19), but in human exercise, lactate production is accompanied by well-regulated and rapid disposal via oxidation and gluconeogenesis under the Lactate Shuttle mechanism (19). As with PI3K/Akt activation, RTK signaling to c-Myc results in transcriptional activation of numerous genes involved in glycolysis and lactate production (43). c-MYC cooperates with HIF-1 in activating several genes that encode glycolytic proteins, including lactate dehydrogenase-A (LDHA) (51).
Cytosolic lactate has two key functions in glycolysis (Figure 1). The first, an appropriate physical response, is to regulate status of the equilibrium of the cytoplasmic redox pair (NADH/NAD+) for the continuation of glycolysis by restoring nicotinamide adenine dinucleotide (NAD+). The second important function of lactate is to overcome, via mass action and the Intracellular Lactate Shuttle mechanism, the HIF-1-induced inhibition of PDH, thus on balance, favoring oxidative disposal of the glycolytic flux. This metabolic characteristic of aerobic lactate production followed by oxidative disposal is typical of what happens in working skeletal muscles during moderate and high intensity exercise. However, whereas muscle is adapted to managing the glycolytic stress of intermittent, high-intensity physical exercise, the chronic stimulation of glycolysis in non-skeletal muscle tissue may be maladaptive, setting a course to pathogenesis. Hence, the chronic self-sufficiency of cancer bioenergetics that requires abundant hexose sources coming from glucose, cellular glycogenolysis or proteolysis followed by gluconeogenesis is a concern for the host in vivo. Consequently, the chronic and excessive need to fuel cancer cells with glucose can lead to cachexia (Figure 1), a consequence typical of many cancer patients.
Another key enzyme in cytosolic glycolysis and lactate production is lactate dehydrogenase, isoform A (LDHA) that reduces pyruvate to lactate under conditions or high glycolytic flux (Figure 2). The LDHA isoform in cancer cells is the same isoform expressed in Type IIB and II-X (fast twitch, white) skeletal muscle fibers that favor pyruvate reduction to lactate. LDHA overexpression in cancer cells is stimulated by HIF-1 (52,53) and the c-Myc oncogene (54–57). Moreover, HIF-1 is a canonical regulator of lactate transporters, MCTs (vide infra).
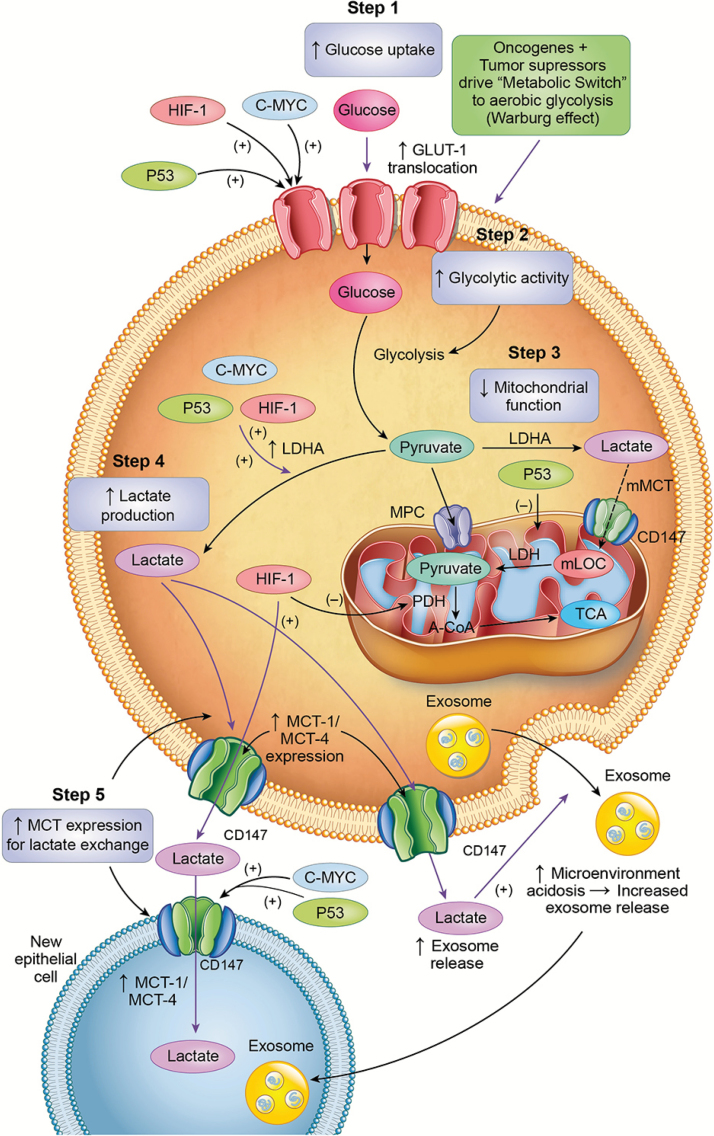
Figure 2.
Lactagenesis is a highly orchestrated effort from oncogenes and tumor suppressor mutations for continuous glucose utilization to produce lactate involving five major steps: (i) increased glucose uptake through increased expression and translocation of glucose transporters GLUT by the transcription factors hypoxia-inducible factor 1 (HIF-1) and c-Myc oncogene as well as repression of tumor suppression factor p53 expression; (ii) increased glycolytic enzyme expression and activity, especially Lactate Dehydrogenase A (LDHA) by HIF-1, c-MYC and p53 downregulation; (iii) decreased mitochondrial function mainly by p53 dysregulation; (iv) increased lactate production, accumulation and release due to mass effect of accelerated glycolysis, mitochondrial dysfunction and increased LDHA expression and (v) Upregulation of monocarboxylate transporters MTC1 and MCT4 and their stabilizer, CD147, for lactate export and instigation of carcinogenesis in susceptible cancer candidate cells.
From the above discussion, it should be clear why PDK, PDH and LDHA are currently targeted among new and promising therapeutic approaches in cancer research. Dichloroacetate inhibits mitochondrial PDK increasing PDH activity and, therefore, pyruvate oxidation to acetyl-CoA for oxidative disposal in mitochondria (58) which also results in decreased lactate formation in normal physiology (59). Dichloroacetate administration lowers lactate accumulation in exercising humans and has been shown to suppress tumor growth in vitro and in vivo (60) probably by promoting pyruvate oxidation in the mitochondrial reticulum. Oxamate and short interfering RNA technique inhibit LDHA activity, and have been shown to be effective in inhibiting carcinogenesis (61–65). Not surprisingly, LDHA knockdown interference inhibits cancer cells proliferation (66,67).
Role of glucose transport
A high glycolytic activity cannot be sustained without a matching and increased glucose uptake. Of all glucose transporters (GLUT1–GLUT12), GLUT-1 is the isoform that is expressed the most in cancer cells such as breast, lung, colorectal, prostate cancers and hepatocellular carcinoma (68–73). GLUT-1 over expression has been associated with tumor aggressiveness and poor survival in several cancers (72,74–76). HIF-1 increases GLUT-1 expression (77–81) allowing a higher capacity for increased glycolysis (Figure 2). As well, c-Myc upregulates GLUT-1 expression in cancer cells (82). Furthermore, mutation of p53 results in a dysregulation of GLUT transporters increasing their expression in cancer (12,83) contributing to the facilitation of glucose utilization of cancer cells. Overexpression of GLUT-3 in non-malignant human breast cells activated known oncogenic signaling pathways to induce malignant phenotype (84).
Divergent roles of mitochondrial function in exercise and cancer
Throughout avian and mammalian animal kingdoms, high muscle mitochondrial density engenders capabilities for both endurance and high metabolic power (85). Indeed, one of the most impressive adaptations to metabolic stress in mammalian biology is doubling of mass of the muscle mitochondrial reticulum due to endurance exercise training (33,86,87). Increased muscle mitochondrial density due to endurance training allows for increased respiratory control, increased fat metabolism and greater lactate clearance in muscles working at a given exercise power output (88). As well, by increasing lactate clearance via oxidation (20,89–91) and gluconeogenesis (89,92), high muscle mitochondrial density and other physiological and metabolic adaptations allow for high rates of muscle glycolysis to be tolerated because of correspondingly high lactate clearance rates. However, while high mitochondrial density provides a metabolic underpinning for exercise capacity, mitochondrial dysfunction is disastrous for lactate clearance in cancer.
In the field of exercise physiology and metabolism, a relationship between the production of lactate and energy substrate partitioning has long been suspected. For example strong, inverse relationship between lactatemia in high intensity exercise and inhibition of lipolysis has long been observed (19,93,94). Recently, GPR81, a receptor for lactate has been identified (95,96). This G-protein coupled receptor is expressed not only in adipocytes in which lactate inhibits lipolysis, but GPR81 has also been found in skeletal muscle (97), brain (98), as well as several cancer cell lines (99). Action of GPR81 is mediated through c-AMP and CREB signaling pathways. In cultured myocytes, we (21) have shown that high lactate concentration affects the expression of CREB, PGC-1α, MCT1, and plasma membrane glycoprotein CD147 (basigin). Thus, it is now possible to posit that lactate affects cancer cell survival by activation of GPR81 leading to increased expression of MCTs, CD147 and PGC-1α (99).
Earlier reports that muscle contraction results in muscle reactive oxygen species (ROS) production have been confirmed (100), and so we tested the effects of lactate stimulation on cellular ROS production (21). Via ROS and CREB-mediated mechanisms, lactate incubation increased the expression of hundreds of genes responsible muscle adaption to exercise in vivo. As well, in similar studies on isolated myocytes we found that the ROS generator H2O2 resulted in fragmentation of the mitochondrial reticulum. Thus, the expression of key proteins of intermediary metabolism responsible for cancer cell survival may be upregulated by two mechanisms: (i) ROS generation for redox control (21) and (ii) cAMP via CREB (21,99).
As already mentioned, Warburg’s hypothesis on cancer pathogenesis posited dysfunctional cellular respiration (9,101) leading to accelerated glycolysis and lactate production. Mitochondrial ultrastructure and decreased oxidative capacity differs significantly from that of normal cells in many types of cancers (11,102). It has also been observed that other different ultrastructure disruptions in cancer lead to respiratory dysfunction and decreased oxidative capacity and ATP synthesis (11,103–106) wherein the degree of mitochondrial dysfunction is correlated to the severity of some cancers such as breast cancer (106). Different genetic mutations are responsible for mitochondrial dysfunction in cancer cells. Among them, p53 has been shown to alter mitochondrial respiration by interfering with transcription of Cytochrome c oxidase 2 (SCO2) (107,108). Undoubtedly, a dysfunctional mitochondrial reticulum contributes to increased lactate production due to a decreased capacity for lactate clearance via oxidation.
The role of lactate transporters in cancer
Despite lactate being the end product of the Warburg Effect, lactate does not just ‘sit around’ as lactate turnover is significant in resting individuals, and very high during exercise far exceeding the glucose turnover rate (20,90,109). Lactate shuttling in normal human physiology parallels that in cancer cells and tumors (22). In cancer, most lactate is exported outside the cells, but internally, lactate increases MCT expression that facilitates the efflux of lactate, ultimately allowing divergent metabolic signaling in target cancer cell candidates.
Lactate is transported into and out of cells by a family of monocarboxylate transporters (MCTs) with different isoforms (MCT1–4) (110–114). Although all MCTs are bidirectional symports, in fast, glycolytic type II skeletal muscle fibers, MCT4 is highly expressed and located to the sarcolemma thus facilitating lactate export through the interstitium for disposal in highly oxidative (slow-twitch red, type I), fast, oxidative glycolytic, types IIA and IIx fibers, and the heart and liver (113,115). In contrast, MCT1 is highly expressed and located to sarcolemmal and mitochondrial membranes of type I and type IIA fibers and heart; hence, in normal physiology MCT1 plays a role in cellular lactate uptake and oxidative disposal (112,115,116). MCT1 and MCT4 transporter proteins have been shown to be overexpressed in many types of cancers (25,117–120) and associated with poor prognosis and high mortality (118). In cancer, HIF-1 activates gene transcription for MCT4 (121) increasing the abundance of MCT4 transporters favoring lactate extrusion from the cell. MCT1 expression is regulated by c-MYC in different types of cancer (122–124) and p53 also increases the expression of MCT1 (125) which is also involved in lactate exportation and uptake in several cancers (124). Moreover, lactate itself rapidly upregulates MCT1 expression. In cultured L6 myocytes, lactate concentrations of either 10 or 20 mM upregulated MCT1 mRNA expression within 1 h (21).
The fact that oncogenes and tumor suppressor mutations directly increase the expression of MCTs shows that the highly orchestrated metabolic reprogramming in cancer cells to glycolysis and lactate production does not just stop at the end of the Warburg Effect. Rather, in cancer, MCTs allow lactate to continue its journey outside the cell fulfilling its role as a mediator of carcinogenesis, which is a key concept we propose herein. In view of their lactate export roles, MCTs have also been targeted for possible therapeutics. Inhibition of MCT1 inhibits growth and carcinogenesis (22,124,126,127). This recognition has led to efforts at targeted inhibition of MCT4 in the treatment of cancer (124,128), the hypothesis being that increased lactate accumulation in cancer cells would lead to acidosis and apoptosis.
As a summary on this section, we compared and contrasted what happens in healthy human exercise and cancer. During healthy submaximal (‘aerobic’) exercise aerobic glycolysis leads to lactate production. There is interplay between the uptake of lipid and carbohydrate-derived fuels (88), and lactate is produced and oxidized in working muscle in situ (20,90), or exported for oxidation in heart, other working red muscles or for splanchnic gluconeogenesis (89,92). In contrast, in glycolytic cancers the combination of increased glucose uptake, glycolytic activity and decreased mitochondrial function promote lactate production, accumulation and export. To reiterate, in cancer it seems plausible to think that many of, or all the highly orchestrated oncogene and tumor suppressor mutations promoting glycolysis, lactate formation and distribution, while at the same time suppressing oxidative disposal of lactate, are all actors in the same play, the subtitle of which is ‘The Warburg Effect.’ In other words, we posit that lactagenesis for carcinogenesis is the purpose of the Warburg Effect in cancer cells and tumors.
If lactate is the final product of the Warburg Effect, why has it been the forgotten traveler at the end of the road?
Lactate has been the great unknown in human metabolism. Lactate has historically been embedded in textbooks as a ‘waste product,’ the result of anaerobic exercise and cause of muscle fatigue. Similarly, in cancer lactate has been historically considered as the ‘end of the road’ of aerobic lactate production and the Warburg Effect. Lactate has been subject of study for many decades and by important scientists including several Nobel Laureates. Lactate studies date back from the 19th Century when Louis Pasteur observed that the absence of air would result in glucose fermentation in some facultative cells (129). Fletcher and Hopkins in 1907 demonstrated that lactate accumulated when frog muscles were stimulated to contract, and that when fatigued muscles were placed in oxygen-rich environments, lactate disappeared (130). In 1920, Nobel Laureate Otto Meyerhof identified glycogen precursor to lactate formed in frog muscles electrically stimulated to fatigue (131) with much of the lactate (~4/5) restored to glycogen during aerobic recovery. In 1923 another Nobel Laureate, Archibald Vivian (AV) Hill and his colleague Walter Morley Lupton described the term ‘O2 Debt’ in which they linked lactate production during human exercise to oxygen-limited lactate production (132). Subsequently, Warburg described that lactate was the end of glycolysis in cancer, but he did not delve into the meaning of lactate production and accumulation in cancer (2,101). Because of the stature of the first investigators and lack of more advanced methodologies to study lactate metabolism, the concept of lactate production as a result of O2 lack was immortalized in textbooks of physiology and biochemistry for over a century. This concept started to change in the mid 1980s when, based on results of isotope tracer studies in rodents and humans, George Brooks proposed the Lactate Shuttle (17-19,133,134). As such, his proposal represented the first hypothesis of functional roles of lactate production and exchange under fully aerobic conditions in healthy functioning individuals (17,133). An effect of that proposal has been to revise thinking around the role of lactate in human health and disease (15,135–137). The production of lactate under fully aerobic conditions has been fully demonstrated (20,138) debunking the belief that lactate was the product of anaerobic exercise and metabolism. In fact, during exercise, ~75–80% of the lactate produced in the muscle is oxidized to pyruvate for ATP synthesis in mitochondria of working muscles as well as in distant highly oxidative organs like the heart (139). The brain also oxidizes lactate (140) and the Astrocyte–Neuron Lactate Shuttle has also been proposed wherein astrocytes produce lactate that is oxidized by neurons (141). Through his Cell–Cell Lactate Shuttle and Intracellular Lactate Shuttle hypotheses, Brooks and colleagues demonstrated that lactate is actively exchanged for purposes of providing an oxidative energy source and gluconeogenic precursor as well as for cell signaling. Because of the latter, autocrine-, paracrine- and endocrine-like properties, lactate has been referred to as a ‘lactormone’ (18,19).
Lactate is actively oxidized in red skeletal muscle. In view of glucose uptake and oxidation, in working muscle, lactate oxidation to pyruvate is unlikely to occur in the cytosol, where the revers occurs. Rather, in skeletal muscle lactate is oxidized in mitochondria (19,142–145). We believe that in muscle mitochondria, lactate is oxidized through a complex we call mitochondrial lactate oxidation complex, comprising monocarboxylate transporter-1 (MCT1), its cell surface chaperone (CD147), mitochondrial lactate dehydrogenase (mLDH) and cytochrome oxidase (COx) (Figure 1) (23,146). Because of the stimulus from training, well trained endurance athletes have the most developed mitochondrial capacity characterized by an increased lactate clearance and oxidation of any humans (147,148).
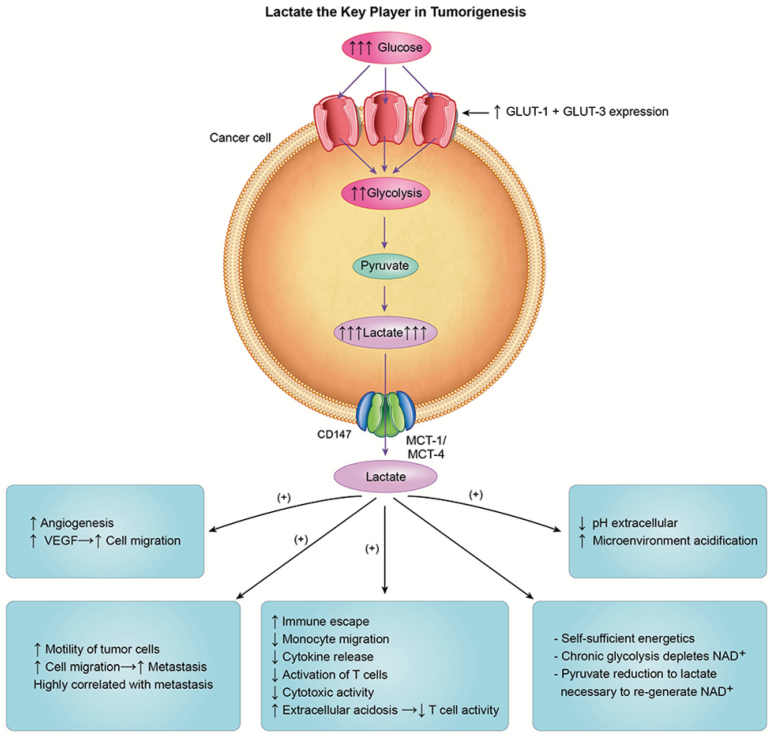
Lactate, the key player in carcinogenesis
In glycolytic tumors, lactate levels of cancer cells are remarkably elevated up to a 40-fold (149,150) and are highly correlated with cancer aggressiveness and poor survival (150). Hence, it is apparent that in cancer, lactate’s journey does not finish at the end with a Warburg Effect of lactate production, but rather the journey of lactate continues as lactate is continuously released from transformed cells to instigate carcinogenesis in susceptible cells and tissues. Within and beyond the bounds of transformed cells, in candidate cancer cells, lactate upregulates MCT4 and MCT1 expression thereby making lactate a key element in the regulation of tumor growth and carcinogenesis. Hanahan and Weinberg, in the most cited paper in cancer research history, described in an elegant way the hallmarks of cancer which include signaling for proliferation, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis and activating invasion and metastasis (151,152). However, little or no attention was given to lactate which has been already shown to be involved in all major steps in carcinogenesis (153). Probably, lactate is the only organic compound that could be involved in all the just mentioned major steps in carcinogenesis of glycolytic cancers as described below.
The role of lactate in angiogenesis
Angiogenesis is a major step in tumorigenesis. It is well known that lactate is a key player in angiogenesis, cell migration, stimulation of VEGF, wound healing and repair (154–157). In cancer, lactate plays an important role in angiogenesis stimulating VEGF protein expression in endothelial cells (158–160). Lactate can enter tumor endothelial cells and lactate released from tumor cells through MCT4 is enough to stimulate angiogenesis and tumor growth (161). Stroma surrounding cancer cells possess elevated levels of hyaluronan which increases cancer growth and motility of cancer cells (162,163). Lactate increases hyaluronan production therefore assisting angiogenesis (164,165). Inhibiting lactate production and transport decreases or inhibits angiogenesis. Oxamate, a LDHA inhibitor, and therefore, lactate inhibitor, greatly reduces angiogenesis (157) and LDH knock out inhibits cancer cell proliferation as well (56,66,67). New approaches to target MCTs and lactate transport across cells in cancer are being currently explored showing effectiveness in decreased angiogenesis and cell migration (124,128,166).
The role of lactate in promoting cell migration and metastasis
Cell migration is another essential step in carcinogenesis and metastasis and lactate seems to be a key element to increase endothelial cell migration (156,163). In glioma cells, lactate induces the expression of transforming growth factor-β2 (TGF-β2), a key regulator of glioma cells migration (167). The addition of exogenous lactate increases cell motility and random migration of different cancer cell lines in a concentration-related manner (168). It has been also known for about two decades that lactate level is highly correlated with metastasis in different forms of cancers (150,159,169–172). Although the mechanisms of lactate’s involvement in metastasis are not fully understood, high concentrations of lactate are correlated with a high incidence of distant metastasis in early stage of cancer (163). Exposure to lactate by cells has been shown to rapidly increase both MCT1 mRNA and protein expression (21). Therefore, by stimulating expression of MCT1 lactate may encourage carcinogenesis in susceptible, candidate cancer cells.
The role of lactate in ‘immune escape’
Lactate contributes to the immune escape in different ways. Monocytes are highly motile cells and precursors of tumor-associated macrophages. Lactate inhibits monocyte migration and release of cytokines tumor necrosis factor and interleukin-6 (IL-6) (168). Furthermore, lactate strongly inhibits the activation of T-cells (173) as well as the differentiation of monocytes to dendritic cells (174,175). Lactate elicits a decrease in cytokine production of human T-cells up to 95% and decreases cytotoxic activity by 50% (173). Lactate also inhibits natural killer cell function directly by inhibiting cytolytic function and indirectly by increasing the numbers of myeloid-derived suppressor cells that inhibit natural killer cytotoxicity (176).
Acidosis could be another way for lactate to suppress immune system (177). Intracellular pH levels in cancer cells are neutral or slightly alkaline, due to lactate and H+ exporting action of MCTs to the microenvironment (178,179). When activated for cell growth and proliferation, T-cells switch to highly glycolytic activity (180) thus producing and exporting important amount of lactate through MCTs. However, because MCTs are symports and move solute and protons down concentration gradients (181), if the extracellular lactate and proton contents are higher than the respective levels within T-cells, then lactate anion and hydrogen ion levels within T-cells may become too high decreasing cytotoxic activity and activating apoptosis within those cells (173).
The role of lactate in cancer cell self-sufficiency and sustained glycolysis
Lactate plays a central role in the bioenergetics, self-sufficiency and sustainability of cancer cells. Above, and in Figures 1 and 2, we have described the mechanisms by which cancer cells are sustained by aerobic glycolysis. This self-sufficiency, depending upon high glycolytic flux, also allows cancer cells to produce lactate necessary for angiogenesis, immune escape, cell migration and metastasis. Furthermore, as pointed earlier, this self-sufficiency and chronic glucose utilization cannot be derived from cancer cell glycogenolysis alone due to the limited glycogen storage in the human body (~400–500 g or 1,600–2,000 kcal). Therefore, gluconeogenic mechanisms within precursors derived mainly from body corpus amino acid and protein reserves, ensure adequate glucose supply to cancer cells (Figure 1). In cancer, gluconeogenesis is supported by the degradation of glutamine. Glutaminolysis is a common metabolic pathway in cancer and it is upregulated in many types of cancers (182,183). Glutamine is converted to glutamate by glutaminase which is overexpressed in cancers and regulated by c-MYC (184). Although glutamine utilization by cancer cells has been historically contemplated as a bioenergetic substrate and biosynthetic precursor, glutaminolysis also produces lactate. Glutamine is oxidized to malate and then to pyruvate by malic enzyme which is overexpressed in different cancers and regulated by p53 (185). Higher rates of conversion of malate to pyruvate in cancer cells were observed already in 1973 in Lehninger’s laboratory (186). As discussed throughout this manuscript, the overexpression of LDHA in glycolytic cancers leads to an increase in the reduction of pyruvate to lactate, thus glutaminolysis in cancer can be regarded as a secondary carbon source for lactagenesis. Further, it seems that in oxidative cancer cells, there is a reciprocal relationship between glutamate and lactate, as lactate promotes glutamate uptake and catabolism by increasing the expression of glutamine transporter ASCT2 and of glutaminase 1 (GLS1) (187). The main source of glutamine in the body is skeletal muscle as it makes up for approximately 50–60% of the free amino acid pool in skeletal muscle (188). Increased glutaminolysis could also explain cachexia in cancer (Figure 1), which in the same manner as glucose utilization and lactate production, is highly correlated with cancer aggressiveness and mortality.
The role of lactate in shaping the tumor microenvironment
Due to its key role in carcinogenesis, tumor-associated microenvironment is currently receiving considerable attention in cancer research. The tumor microenvironment consists of malignant cells, immune cells, non-cancer cell stromas, fibroblasts as well as the vasculature and lymphatics of the tumor. As Chen et al. describe, the tumor microenvironment is a pathologically active niche that shapes tumor evolution (189). A typical characteristic of tumor microenvironment is its acidic environment, which seems to be key for the interaction and signaling of the different players involved in carcinogenesis in the tumor microenvironment. Lactate is the main element responsible for acidosis of the extracellular microenvironment due to the constant lactate and proton shuttling from cancer cells to the extracellular space. In fact, the intracellular pH in cancer cells tends to be slightly alkaline compared to the extracellular space (178,179) where pH of 5.5–7.0 is common in cancers (190)
Lactate and exosomes
Exosomes are emerging players in cancer metastasis and carcinogenesis. Exosomes are microvesicles, 30–100 nm in size of endocytic origin that contain microRNA’s, proteins, metabolic enzymes and structural proteins, which are a representation of the cell they are originated from. Secreted cancer-derived exosomes can be transferred to other cells and may induce epigenetic changes and elicit cancer phenotype in target cells by transferring genetic information including oncogenes and onco-miRNA’s (191). It has been recently shown that cancer-associated fibroblasts exosomes elicit metabolic reprogramming in other cancer cells by inhibiting mitochondrial function and upregulating glucose metabolism of other cancer cells (192). Tumor-derived exosomes have been detected in a wide variety of cancers and can play a key role in cancer carcinogenesis and metastasis (191). Patients with cancer show a higher number of secreted exosomes (193,194), which also correlates with poor prognosis (195).
Lactate may play a key role in exosome release, uptake and physiology. Low pH in tumor microenvironment, caused by lactate, is a major regulator of exosome release and uptake by candidate cells. A low pH increases exosomes release as well as uptake from recipient cells (196). Consequently, treatment with proton pump inhibitors results in a marked inhibition of exosomes release by tumor cells (197) and inhibition of exosomes uptake in melanoma cells (196). Low pH can also be crucial in exosome physiology. Exosomes incubated at a pH4 yield a total exosomal content for both protein and RNA that was 5 times higher than when incubated at a pH7 (198). Furthermore, microglia-derived exosomes express MCT1 and therefore they take up lactate (199). CD147 (basigin), a plasma membrane glycoprotein that facilitates cellular surface expression of MCT1 and -4, has also been identified in exosomes derived from ovarian cancer cells (200). To resolve these seemingly divergent findings additional studies need to be done to identify the roles of lactate in exosome participation in carcinogenesis.
Lactate as a transcription factor?
As already stated, lactate uptake by cancer cell candidates could be for more important purposes than cellular energy. Lactate could be a genetic regulator and elicit ‘cancer phenotype’. In MCF7 cells incubated in 10 mM lactate, a concentration observed in many cancer cells, ~4,131 genes were upregulated (201). Brooks’ laboratory has also shown how exposure to 10–20 mM of lactate upregulated 673 genes in L6 cells (21). As presented vide supra, lactate increases MCT1 expression which is enhanced by c-Myc and P53. Finally, the master transcription factor involved in tumor cell glycolysis, HIF-1, has been shown to be activated by lactate in different cancer lines (47,160,202), Hence, it is plausible to think that the thousands of genes upregulated by lactate may collectively represent a transcriptional network involved in reprogramming cells for lactagenesis and carcinogenesis.
Beyond acting as a transcription switch, lactate could have further mitochondrial metabolism in new cells (Figure 2). Hussien and Brooks showed that mitochondrial LDH isoforms were equally expressed in cancer breast cell lines compared to controls (25) which shows that mitochondrial lactate oxidation complex may remain operative for energy purposes. A study from Bouzier et al. (203) using nuclear magnetic resonance showed how lactate entered the tricarboxylic acid cycle to produce glutamate and suggested that lactate was a better precursor for alanine and glutamate than glucose.
Lactate and cancer relapse
Lactate could also play an important role in cancer recurrence. Cancer stem cells are found in various cancer types and have been implicated in the resistance to therapeutic interventions in various cancers (204,205). Apoptotic blebs of cancer cells fuse together to form novel structures called ‘blebbishields’ (206) which can generate stem cell spheres that are tumorigenic. Lactate enhances sphere formation from blebbishields by 87% and when treated with a proton pump inhibitor, sphere formation is reduced by 80% (206).
‘Lactagenesis,’ the purpose of the Warburg Effect
Because lactate is sine qua non the end product of the Warburg Effect and a key element in all processes involved in carcinogenesis, we believe that lactate production, ‘lactagenesis’, is the purpose of the Warburg Effect, an explanation that has remained elusive for almost a century. As explained throughout this manuscript, lactagenesis is a highly orchestrated effort from oncogenes and tumor suppressor mutations for continuous and non-stoppable glucose utilization to produce lactate involving five major steps (Figure 2): (i) increased glucose uptake through upregulation of GLUT transporter expression, (ii) upregulation of glycolytic enzyme expression, (iii) decreased mitochondrial respiration, (iv) increased lactate production, accumulation and release and (v) upregulation of MCT expression for further lactate shuttling and mediation in cancer growth, proliferation and carcinogenesis purposes (Figure 3).
Figure 3.
Lactate is necessary for all the major steps in carcinogenesis; Lactate increases the expression of vascular endothelial growth factor (VEGF) stimulating angiogenesis, increases motility and migration of cancer cells. Lactate is directly involved in the ‘immune escape’ by decreasing monocyte migration and decreased activation of T cells as well as cytokine release and cytotoxic activity. Lactate increases extracellular acidosis of tumor microenvironment decreasing capacity of T-cell to export lactate, thus decreasing T-cell activity. Finally, lactate is necessary for the self-sufficiency of cancer cells by replenishing cytosolic levels of nicotinamide adenine dinucleotide (NAD+) and regulating the status of the equilibrium of the cytoplasmic redox pair (NADH/NAD+) for continuation of glycolysis.
Lactate is necessary for all the major steps in carcinogenesis: angiogenesis, immune escape, cell migration, metastasis and self-sufficiency of cancer cells. We believe that these well differentiated and at same time interrelated steps of lactagenesis shed new light in cancer growth and carcinogenesis and also provide a new metabolic map that can create targets for new therapeutics. The weakness of cancer resides in its complexity and the highly orchestrated processes that are rare events in nature. Therefore, it should be possible to alter some metabolic links and events necessary for lactagenesis and lactate shuttling and therefore carcinogenesis. However, it is key to understand and explain in the first place the processes involved in such complex process and we believe we explain them herein.
Important views of others: consistencies and contrasts
To reiterate from our Introduction, there is a renaissance of interest in the Warburg Effect in cancer research. However, the contemporary views offer that the Warburg Effect is the result of aberrant cell signaling with lactate as a byproduct of carcinogenic processes, rather than one of the causes. Indeed, as our knowledge in physiology grows, it is possible to note that the many stress and strain signals are correlated. For instance, hypoxia, acidosis and lactate accumulation typically occur simultaneously in normal physiology and cancer. HIF-1 activation can be associated with lactate accumulation, and both hypoxia (52) and lactate (160) activate HIF-1 with a number of down-stream effects such as increased MCT1 expression (52,160). And, as already noted, HIF-1 and c-Myc increase expression of glycolytic enzymes including LDHA (Figure 2). As well, interpretation of results is complicated by the presence of redundant control mechanisms. For example, both HIF-1 and lactate anion upregulate MCT1 expression (21,160). Then there are examples of mixed signals; for instance, the effects of AMP-activated protein kinase (AMPK), the ‘master regulator (governor) of cell energy state’ and, in the long-term, mitochondrial biogenesis. In non-cancerous cells, lactate activates CREB and AMPK simulating MCT1 expression and mitochondrial biogenesis (21); but in cancer AMPK activates phosphofructokinase, but mitochondrial biogenesis is down regulated (43). Many processes are complicated and interpretation of data can be difficult. Our view is that dysregulated and chronic lactate exposure can be both cause and effect in carcinogenesis.
As an example of contemporary views holding that lactate is the result of the ‘cancer phenotype’ we can look to Cairns et al. (43). To simplify aspects of their outstanding review, growth factor binding to plasma membrane receptors stimulates RTKs to activate PI3K/Akt. Akt promotes glucose transporter activity and stimulates glycolysis and lactate production through activation of several glycolytic enzymes including hexokinase and phosphofructokinase. AKT activation also activates mTOR, which in turn, activates HIF-1. As described above, without activation of RTKs, lactate alone can mimic many of the same effects. For instance, lactate upregulates HIF-1 expression which in turn increases the expression of glucose transporters, glycolytic enzymes and PDK1, which blocks the entry of pyruvate into the tricarboxylic acid cycle. As well, c-MYC cooperates with HIF-1 in activating several genes encoding for glycolytic proteins including LDHA. However, to reiterate, the lions of contemporary cancer biology think of lactate production merely in terms of an effect of aberrant metabolism, the ‘cancer phenotype’, with no consideration to the possibility that lactate production and accumulation are causal and regulatory in provoking the overall phenotype. Respectfully, we and others (160) offer a different interpretation based around the role of aberrant lactate metabolism is supporting carcinogenesis and angiogenesis.
Future directions: the importance of targeting lactate production and shuttling in cancer
Different approaches in interfering with lactate metabolism have already been successful in vitro and even in vivo (Figure 4). LDHA inhibition by oxamate, short interfering RNA or knockdown have been already shown to inhibit carcinogenesis and proliferation (56,61–67,207). Dichloroacetate has been shown to be efficient at halting carcinogenesis by increasing PDH activity, therefore interfering with cytosolic lactate production (208). The development of MCT1 and MCT4’s inhibitors has enormous potential as an approach in cancer treatment as shown in different studies (22,126–128,166,209,210). However lack of MCT specificity has been a problem. Recently, AstraZeneca developed a specific MCT1 and MCT2 inhibitor named AR-C155858 (211) inhibiting MCT1 and MCT2 expression in Ras-transformed fibroblasts. However, the cells developed resistance to the inhibition of MCT1 and MCT2 and increased carcinogenesis by overexpressing MCT4 (212). New generations of MCT-1 inhibitors like SR13800 and ACD3965 (currently in Phase 1/2 in the UK) have shown promising results in Raji cells (124) and small cell lung cancer (213) respectively. Furthermore, Draoui et al, showed that a new compound called 7-aminocarboxycoumarin (7ACC) inhibited lactate influx, but not efflux as well as cell proliferation in tumor cells expressing both MCT1 and MCT4 (127).
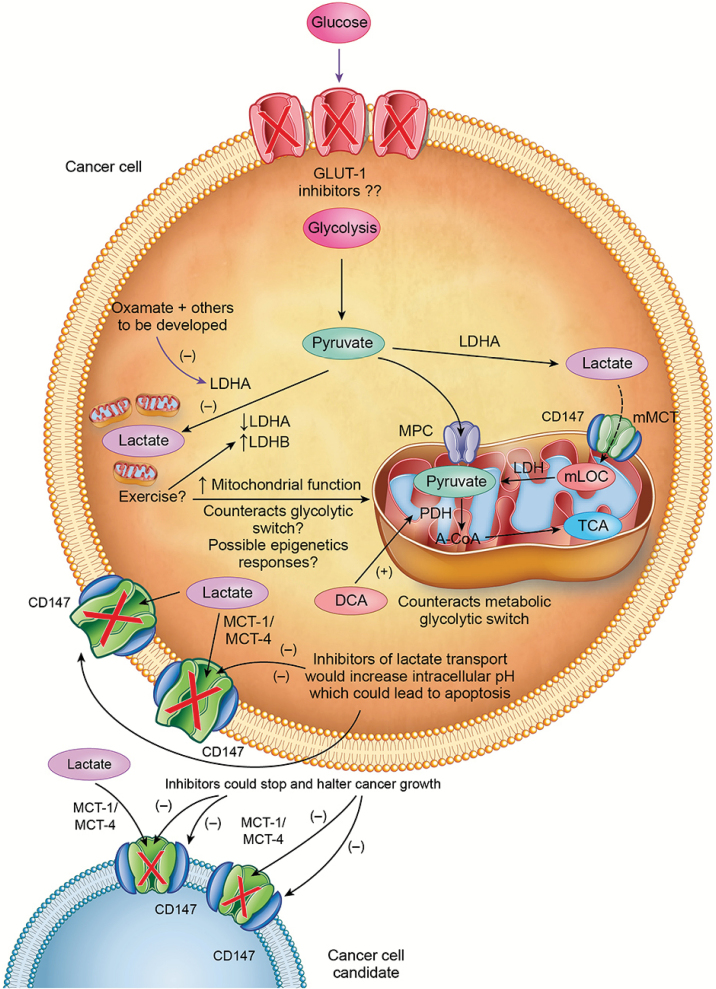
Figure 4.
Proposed mechanisms by which lactagenesis can be halted. Oxamate decreases activity of LDHA and aerobic exercise increases expression of LDHB which oxidizes lactate to pyruvate and may counteract deleterious action of LDHA. Dichloroacetate (DCA) increases the activity of pyruvate dehydrogenase (PDH) which directs glycolytic carbon flux to oxidation and away from lactate production and accumulation. Different inhibitors of MCT1-4 and CD147 are currently being evaluated which would inhibit lactate shuttling between, within and among cancer cells and susceptable candidate cells.
As mentioned earlier, CD147, a plasma membrane glycoprotein that facilitates the cellular surface expression of MCT1 and MCT4 (214) is overexpressed in a large number of cancers (215,216). Not surprisingly, CD147 has been targeted as a potential means to interfere with lactate transport outside the cell (217,218). Silencing CD147 by RNA interference (RNAi) has also shown to reduce pancreatic malignant activity both in vivo and in vitro (219–221). Deleting CD147 gene with ‘zinc finger nucleases’ (ZFN’s) has also shown to decreases MCT1 and MCT4 expression and decrease lactate export in non-small cell lung cancer (222).
Aerobic exercise may counteract the oxidative regression of cancer cells. Anecdotal evidence shows that exercising cancer patients seem to cope with treatments better (223) as well as to increase cancer survivorship (224), although the mechanisms of why exercise may contribute to survival are not well identified yet. It is well documented that aerobic exercise increases mitochondrial function and lactate clearance capacity as well as increases fat oxidation and decreases glycolysis (32,34–37), thus decreasing reliance on glycogen and glucose, an anti-Warburg Effect affect. Furthermore, exercise could attenuate deleterious activity of c-Myc as has been previously hypothesized (51). Therefore, aerobic exercise could contribute to counteract the metabolic switch to glycolytic metabolism in cancer cells and create epigenetic responses that could help in the restoration of oxidative phenotypes. Further research is necessary to identify the exact mechanisms of why exercise may be an effective approach to increase survival as well as an effective approach to prevent cancer as shown in many studies (225).
Conclusion
Although the Warburg Effect has been recognized as a hallmark for cancer, over the course of almost a century, the role of the Warburg Effect in the pathogenesis of cancer has not been established. This lack of understanding may have been an impediment to the development of new approaches to cancer treatment. By means of this manuscript, we bring thinking from the fields of exercise physiology and metabolism, where aerobic glycolysis and lactate metabolism have been extensively studied and the Lactate Shuttle concept was developed by Brooks. Hence, by comparing and contrasting what we know of lactate metabolism in healthful human exercise to what we have learned about ‘lactagenesis’ in cancer, the aim of our effort is to achieve better understanding of carcinogenesis. We believe that we have provided a plausible explanation of role and purpose of the Warburg Effect in carcinogenesis. Our perspectives are offered in the expectation that they may lead to development of means to either correct errors in lactate metabolism or otherwise disrupt steps in progression to cancer. Carcinogenesis is the resultant of a complex orchestrated chain of genetic and metabolic events in which lactate production and accumulation play critical roles. Specifically, we posit that in carcinogenesis aberrant cell signaling due to exaggerated and continually high lactate levels yields an (inappropriate) positive feedback loop that increases glucose uptake and glycolysis, increases lactate production, accumulation and release, decreases mitochondrial function, upregulates monocarboxylate transporter expression thereby supporting angiogenesis, immune escape, cell migration and metastasis all of which encourage carcinogenesis and progression to cancer. If true, then new and novel methods may be found to disrupt the scenario in which the Warburg Effect leads to cancer (Figure 4).
Acknowledgements
Dr W. Thomas Purcell, from the University of Colorado Cancer Center, is thanked for reading and commenting on the initial manuscript draft.
Conflict of Interest Statement: None declared.
Abbreviations
- HIF
hypoxia-inducible transcription factor
- LDHA
lactate dehydrogenase, isoform A
- MCT
monocarboxylate transporter
- PDH
pyruvate dehydrogenase
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
References
- 1. Warburg O., et al. (1923) Versuche an überlebendem carcinom-gewebe. Klin Wochenschr, 2, 776–777. [Google Scholar]
- 2. Warburg O. (1924) Über den stoffwechsel der carcinomzelle. Naturwissenschaften, 12, 1131–1137. [Google Scholar]
- 3. Racker E. (1972) Bioenergetics and the problem of tumor growth: an understanding of the mechanism of the generation and control of biological energy may shed light on the problem of tumor growth. Am. Sci., 60, 56–63. [PubMed] [Google Scholar]
- 4. Burt B.M., et al. (2001) Using positron emission tomography with [(18)F]FDG to predict tumor behavior in experimental colorectal cancer. Neoplasia, 3, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gillies R.J., et al. (2007) Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J. Bioenerg. Biomembr., 39, 251–257. [DOI] [PubMed] [Google Scholar]
- 6. Kroemer G., et al. (2008) Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell, 13, 472–482. [DOI] [PubMed] [Google Scholar]
- 7. Gambhir S.S., et al. (2001) A tabulated summary of the FDG PET literature. J. Nucl. Med., 42(5 suppl), 1S–93S. [PubMed] [Google Scholar]
- 8. Warburg O., et al. (1927) The metabolism of tumors in the body. J. Gen. Physiol., 8, 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warburg O. (1931) The Metabolism of Tumors. RR Smith, New York, NY. [Google Scholar]
- 10. Warburg O. (1956) On respiratory impairment in cancer cells. Science, 124, 269–270. [PubMed] [Google Scholar]
- 11. Seyfried T.N., et al. (2014) Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis, 35, 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levine A.J., et al. (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science, 330, 1340–1344. [DOI] [PubMed] [Google Scholar]
- 13. Joyner M.J., et al. (2016) What happens when underperforming big ideas in research become entrenched? JAMA, 316, 1355–1356. [DOI] [PubMed] [Google Scholar]
- 14. Mukherjee S. (2010) The Emperor of All Maladies: A Biography of Cancer. Simon & Shuster, New York, NY. [Google Scholar]
- 15. Brooks G.A., et al. (2014) Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front. Neurosci., 8, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brooks G.A. (1985) Lactate: glycolytic end product and oxidative substrate during sustained exercise in mammals — the “lactate shuttle.” In Gilles, R. Circulation, Respiration, and Metabolism. Springer, Berlin, pp. 208–218. [Google Scholar]
- 17. Brooks G.A. (1986) The lactate shuttle during exercise and recovery. Med. Sci. Sports Exerc., 18, 360–368. [DOI] [PubMed] [Google Scholar]
- 18. Brooks G.A. (2002) Lactate shuttles in nature. Biochem. Soc. Trans., 30, 258–264. [DOI] [PubMed] [Google Scholar]
- 19. Brooks G.A. (2009) Cell-cell and intracellular lactate shuttles. J. Physiol., 587(Pt 23), 5591–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergman B.C., et al. (1999) Active muscle and whole body lactate kinetics after endurance training in men. J. Appl. Physiol. (1985)., 87, 1684–1696. [DOI] [PubMed] [Google Scholar]
- 21. Hashimoto T., et al. (2007) Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J., 21, 2602–2612. [DOI] [PubMed] [Google Scholar]
- 22. Sonveaux P., et al. (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest., 118, 3930–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hashimoto T., et al. (2006) Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am. J. Physiol. Endocrinol. Metab., 290, E1237–E1244. [DOI] [PubMed] [Google Scholar]
- 24. Hashimoto T., et al. (2008) Evidence for the mitochondrial lactate oxidation complex in rat neurons: demonstration of an essential component of brain lactate shuttles. PLoS One, 3, e2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hussien R., et al. (2011) Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol. Genomics, 43, 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller B.F., et al. (2005) Hematological and acid-base changes in men during prolonged exercise with and without sodium-lactate infusion. J. Appl. Physiol., 98, 856–865. [DOI] [PubMed] [Google Scholar]
- 27. Miller B.F., et al. (2002) Lactate-glucose interaction in men during rest and exercising using lactate clamp procedure. J. Physiol., 544, 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller B.F., et al. (2002) Metabolic and cardiorespiratory responses to “the lactate clamp”. Am. J. Physiol. Endocrinol. Metab., 283, E889–E898. [DOI] [PubMed] [Google Scholar]
- 29. Bouzat P., et al. (2014) Hypertonic lactate and the injured brain: facts and the potential for positive clinical implications. Intensive Care Med., 40, 920–921. [DOI] [PubMed] [Google Scholar]
- 30. Patet C., et al. (2016) Cerebral lactate metabolism after traumatic brain injury. Curr. Neurol. Neurosci. Rep., 16, 31. [DOI] [PubMed] [Google Scholar]
- 31. Izyumov D.S., et al. (2004) “Wages of fear”: transient threefold decrease in intracellular ATP level imposes apoptosis. Biochim. Biophys. Acta, 1658, 141–147. [DOI] [PubMed] [Google Scholar]
- 32. Holloszy J.O. (1967) Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem., 242, 2278–2282. [PubMed] [Google Scholar]
- 33. Davies K.J., et al. (1981) Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch. Biochem. Biophys., 209, 539–554. [DOI] [PubMed] [Google Scholar]
- 34. Gollnick P.D., et al. (1969) Effect of exercise and training on mitochondria of rat skeletal muscle. Am. J. Physiol., 216, 1502–1509. [DOI] [PubMed] [Google Scholar]
- 35. Morgan T.E., et al. (1971) Effects of long-term exercise on human muscle mitochondria. In Pernow, B. et al. Muscle Metabolism During Exercise. Springer, pp. 87–95. [Google Scholar]
- 36. Gollnick P.D., et al. (1972) Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J. Appl. Physiol., 33, 312–319. [DOI] [PubMed] [Google Scholar]
- 37. Turcotte L.P., et al. (1992) Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am. J. Physiol., 262(6 Pt 1), E791–E799. [DOI] [PubMed] [Google Scholar]
- 38. DeBerardinis R.J. (2008) Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet. Med., 10, 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vogelstein B., et al. (2004) Cancer genes and the pathways they control. Nat. Med., 10, 789–799. [DOI] [PubMed] [Google Scholar]
- 40. Soga T. (2013) Cancer metabolism: key players in metabolic reprogramming. Cancer Sci., 104, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moreno-Sánchez R., et al. (2007) Energy metabolism in tumor cells. FEBS J., 274, 1393–1418. [DOI] [PubMed] [Google Scholar]
- 42. Yeung S.J., et al. (2008) Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol. Life Sci., 65, 3981–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cairns R.A., et al. (2011) Regulation of cancer cell metabolism. Nat. Rev. Cancer, 11, 85–95. [DOI] [PubMed] [Google Scholar]
- 44. Rivlin N., et al. (2011) Mutations in the p53 tumor suppressor gene: important milestones at the various steps of tumorigenesis. Genes Cancer, 2, 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hainaut P., et al. (2000) p53 and human cancer: the first ten thousand mutations. Adv. Cancer Res., 77, 81–137. [DOI] [PubMed] [Google Scholar]
- 46. Levine A.J. (1997) p53, the cellular gatekeeper for growth and division. Cell, 88, 323–331. [DOI] [PubMed] [Google Scholar]
- 47. Semenza G.L. (2010) HIF-1: upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev., 20, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pouysségur J., et al. (2006) Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature, 441, 437–443. [DOI] [PubMed] [Google Scholar]
- 49. Semenza G.L. (2007) HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J. Bioenerg. Biomembr., 39, 231–234. [DOI] [PubMed] [Google Scholar]
- 50. Unruh A., et al. (2003) The hypoxia-inducible factor-1 alpha is a negative factor for tumor therapy. Oncogene, 22, 3213–3220. [DOI] [PubMed] [Google Scholar]
- 51. Gohil K., et al. (2012) Exercise tames the wild side of the Myc network: a hypothesis. Am. J. Physiol. Endocrinol. Metab., 303, E18–E30. [DOI] [PubMed] [Google Scholar]
- 52. Semenza G.L., et al. (1996) Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J. Biol. Chem., 271, 32529–32537. [DOI] [PubMed] [Google Scholar]
- 53. Firth J.D., et al. (1995) Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J. Biol. Chem., 270, 21021–21027. [DOI] [PubMed] [Google Scholar]
- 54. Tavtigian S.V., et al. (1994) Cloning of mid-G1 serum response genes and identification of a subset regulated by conditional myc expression. Mol. Biol. Cell, 5, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lewis B.C., et al. (1997) Identification of putative c-Myc-responsive genes: characterization of rcl, a novel growth-related gene. Mol. Cell. Biol., 17, 4967–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shim H., et al. (1997) c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl. Acad. Sci. USA, 94, 6658–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dang C.V., et al. (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res., 15, 6479–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bonnet S., et al. (2007) A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell, 11, 37–51. [DOI] [PubMed] [Google Scholar]
- 59. Stacpoole P.W., et al. (1988) Dichloroacetate in the treatment of lactic acidosis. Ann Intern Med, 108, 58–63. [DOI] [PubMed] [Google Scholar]
- 60. Michelakis E.D., et al. (2008) Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer, 99, 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thornburg J.M., et al. (2008) Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res., 10, R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fiume L., et al. (2010) Impairment of aerobic glycolysis by inhibitors of lactic dehydrogenase hinders the growth of human hepatocellular carcinoma cell lines. Pharmacology, 86, 157–162. [DOI] [PubMed] [Google Scholar]
- 63. Zhai X., et al. (2013) Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol. Rep., 30, 2983–2991. [DOI] [PubMed] [Google Scholar]
- 64. Liu X., et al. (2015) Effects of the suppression of lactate dehydrogenase A on the growth and invasion of human gastric cancer cells. Oncol. Rep., 33, 157–162. [DOI] [PubMed] [Google Scholar]
- 65. Le A., et al. (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl. Acad. Sci. USA, 107, 2037–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fantin V.R., et al. (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell, 9, 425–434. [DOI] [PubMed] [Google Scholar]
- 67. Vander Heiden M.G. (2011) Targeting cancer metabolism: a therapeutic window opens. Nat. Rev. Drug Discov., 10, 671–684. [DOI] [PubMed] [Google Scholar]
- 68. Barron C., et al. (2012) Expression of the glucose transporters GLUT1, GLUT3, GLUT4 and GLUT12 in human cancer cells. BMC Proc., 6, P4. [Google Scholar]
- 69. Medina R.A., et al. (2002) Glucose transporters: expression, regulation and cancer. Biol. Res., 35, 9–26. [DOI] [PubMed] [Google Scholar]
- 70. Brown R.S., et al. (1993) Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study. Cancer, 72, 2979–2985. [DOI] [PubMed] [Google Scholar]
- 71. Grover-McKay M., et al. (1998) Role for glucose transporter 1 protein in human breast cancer. Pathol. Oncol. Res., 4, 115–120. [DOI] [PubMed] [Google Scholar]
- 72. Haber R.S., et al. (1998) GLUT1 glucose transporter expression in colorectal carcinoma. Cancer, 83, 34–40. [DOI] [PubMed] [Google Scholar]
- 73. Jun Y.J., et al. (2011) Clinicopathologic significance of GLUT1 expression and its correlation with Apaf-1 in colorectal adenocarcinomas. World J. Gastroenterol., 17, 1866–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Younes M., et al. (1997) Overexpression of Glut1 and Glut3 in stage I nonsmall cell lung carcinoma is associated with poor survival. Cancer, 80, 1046–1051. [DOI] [PubMed] [Google Scholar]
- 75. Kawamura T., et al. (2001) Expression of glucose transporter-1 in human gastric carcinoma. Cancer, 92, 634–641. [DOI] [PubMed] [Google Scholar]
- 76. Kunkel M., et al. (2003) Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer, 97, 1015–1024. [DOI] [PubMed] [Google Scholar]
- 77. Chen J.Q., et al. (2012) Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim. Biophys. Acta, 1826, 370–384. [DOI] [PubMed] [Google Scholar]
- 78. Hayashi M., et al. (2004) Induction of glucose transporter 1 expression through hypoxia-inducible factor 1 alpha under hypoxic conditions in trophoblast-derived cells. J. Endocrinol., 183, 145–154. [DOI] [PubMed] [Google Scholar]
- 79. Ren B.F., et al. (2008) Hypoxia regulation of facilitated glucose transporter-1 and glucose transporter-3 in mouse chondrocytes mediated by HIF-1 alpha. Joint. Bone. Spine, 75, 176–181. [DOI] [PubMed] [Google Scholar]
- 80. Mobasheri A., et al. (2005) Hypoxia inducible factor-1 and facilitative glucose transporters GLUT1 and GLUT3: putative molecular components of the oxygen and glucose sensing apparatus in articular chondrocytes. Histol. Histopathol., 20, 1327–1338. [DOI] [PubMed] [Google Scholar]
- 81. Baumann M.U., et al. (2007) Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am. J. Physiol. Cell Physiol., 293, C477–C485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Osthus R.C., et al. (2000) Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem., 275, 21797–21800. [DOI] [PubMed] [Google Scholar]
- 83. Johnson R.F., et al. (2012) Nuclear factor-κB, p53, and mitochondria: regulation of cellular metabolism and the Warburg effect. Trends Biochem. Sci., 37, 317–324. [DOI] [PubMed] [Google Scholar]
- 84. Onodera Y., et al. (2014) Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J. Clin. Invest., 124, 367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Brooks G.A., et al. (2000) Exercise Physiology: Human Bioenergetics and Its Applications. Mcgraw-Hill, New York, NY. [Google Scholar]
- 86. Holloszy J.O. (2008) Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J. Physiol. Pharmacol., 59 (suppl. 7), 5–18. [PubMed] [Google Scholar]
- 87. Kirkwood S.P., et al. (1987) Effects of endurance training on a mitochondrial reticulum in limb skeletal muscle. Arch. Biochem. Biophys., 255, 80–88. [DOI] [PubMed] [Google Scholar]
- 88. Brooks G.A., et al. (1994) Balance of carbohydrate and lipid utilization during exercise: the “crossover” concept. J. Appl. Physiol., 76, 2253–2261. [DOI] [PubMed] [Google Scholar]
- 89. Bergman B.C., et al. (2000) Endurance training increases gluconeogenesis during rest and exercise in men. Am. J. Physiol. Endocrinol. Metab., 278, E244–E251. [DOI] [PubMed] [Google Scholar]
- 90. Messonnier L.A., et al. (2013) Lactate kinetics at the lactate threshold in trained and untrained men. J. Appl. Physiol., 114, 1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Emhoff C.A., et al. (2013) Direct and indirect lactate oxidation in trained and untrained men. J. Appl. Physiol., 115, 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Emhoff C.A., et al. (2013) Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J. Appl. Physiol., 114, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Issekutz B., et al. (1962) Plasma free fatty acids during exercise and the effect of lactic acid. Exp Biol Med, 110, 237–239. [Google Scholar]
- 94. Henderson G.C., et al. (2007) Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J. Physiol., 584(Pt 3), 963–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu C., et al. (2009) Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem., 284, 2811–2822. [DOI] [PubMed] [Google Scholar]
- 96. Ahmed K., et al. (2010) An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab., 11, 311–319. [DOI] [PubMed] [Google Scholar]
- 97. Ge H., et al. (2008) Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J. Lipid Res., 49, 797–803. [DOI] [PubMed] [Google Scholar]
- 98. Lauritzen K.H., et al. (2013) Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb. Cortex, 24, bht136. [DOI] [PubMed] [Google Scholar]
- 99. Roland C.L., et al. (2014) Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res., 74, 5301–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Davies K.J., et al. (1982) Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun., 107, 1198–1205. [DOI] [PubMed] [Google Scholar]
- 101. Warburg O. (1956) On the origin of cancer cells. Science, 123, 309–314. [DOI] [PubMed] [Google Scholar]
- 102. Arismendi-Morillo G. (2009) Electron microscopy morphology of the mitochondrial network in human cancer. Int. J. Biochem. Cell Biol., 41, 2062–2068. [DOI] [PubMed] [Google Scholar]
- 103. Seyfried T.N. (2012) Respiratory Dysfunction in Cancer Cells. Wiley, Hoboken, NJ. [Google Scholar]
- 104. Arismendi-Morillo G. (2011) Electron microscopy morphology of the mitochondrial network in gliomas and their vascular microenvironment. Biochim. Biophys. Acta, 1807, 602–608. [DOI] [PubMed] [Google Scholar]
- 105. Arismendi-Morillo G.J., et al. (2008) Ultrastructural mitochondrial pathology in human astrocytic tumors: potentials implications pro-therapeutics strategies. J. Electron Microsc. (Tokyo)., 57, 33–39. [DOI] [PubMed] [Google Scholar]
- 106. Elliott R.L., et al. (2012) Mitochondria organelle transplantation: introduction of normal epithelial mitochondria into human cancer cells inhibits proliferation and increases drug sensitivity. Breast Cancer Res. Treat., 136, 347–354. [DOI] [PubMed] [Google Scholar]
- 107. Matoba S., et al. (2006) p53 regulates mitochondrial respiration. Science, 312, 1650–1653. [DOI] [PubMed] [Google Scholar]
- 108. Lago C.U., et al. (2011) p53, aerobic metabolism, and cancer. Antioxid. Redox Signal., 15, 1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stanley W.C., et al. (1988) Glucose and lactate interrelations during moderate-intensity exercise in humans. Metabolism, 37, 850–858. [DOI] [PubMed] [Google Scholar]
- 110. Halestrap A.P. (2012) The monocarboxylate transporter family–structure and functional characterization. IUBMB Life, 64, 1–9. [DOI] [PubMed] [Google Scholar]
- 111. Juel C. (1988) Intracellular pH recovery and lactate efflux in mouse soleus muscles stimulated in vitro: the involvement of sodium/proton exchange and a lactate carrier. Acta Physiol. Scand., 132, 363–371. [DOI] [PubMed] [Google Scholar]
- 112. Bonen A. (2001) The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur. J. Appl. Physiol., 86, 6–11. [DOI] [PubMed] [Google Scholar]
- 113. Wilson M.C., et al. (1998) Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J. Biol. Chem., 273, 15920–15926. [DOI] [PubMed] [Google Scholar]
- 114. Roth D.A., et al. (1990) Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch. Biochem. Biophys., 279, 377–385. [DOI] [PubMed] [Google Scholar]
- 115. Fox J.E.M., et al. (2000) Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol, 529, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bonen A., et al. (2000) Isoform-specific regulation of the lactate transporters MCT1 and MCT4 by contractile activity. Am. J. Physiol. Endocrinol. Metab., 279, E1131–E1138. [DOI] [PubMed] [Google Scholar]
- 117. Kennedy K.M., et al. (2010) Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol., 6, 127–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pértega-Gomes N., et al. (2011) Monocarboxylate transporter 4 (MCT4) and CD147 overexpression is associated with poor prognosis in prostate cancer. BMC Cancer, 11, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pinheiro C., et al. (2010) Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology, 56, 860–867. [DOI] [PubMed] [Google Scholar]
- 120. Pinheiro C., et al. (2012) Role of monocarboxylate transporters in human cancers: state of the art. J. Bioenerg. Biomembr., 44, 127–139. [DOI] [PubMed] [Google Scholar]
- 121. Ullah M.S., et al. (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J. Biol. Chem., 281, 9030–9037. [DOI] [PubMed] [Google Scholar]
- 122. Schuhmacher M., et al. (2001) The transcriptional program of a human B cell line in response to Myc. Nucleic Acids Res., 29, 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Coller H.A., et al. (2000) Expression analysis with oligonucleotide microarrays reveals that MYC regulates genes involved in growth, cell cycle, signaling, and adhesion. Proc. Natl. Acad. Sci. USA, 97, 3260–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Doherty J.R., et al. (2014) Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Res., 74, 908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Boidot R., et al. (2012) Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res., 72, 939–948. [DOI] [PubMed] [Google Scholar]
- 126. Mathupala S.P., et al. (2004) Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery, 55, 1410–9; discussion 1419. [DOI] [PubMed] [Google Scholar]
- 127. Draoui N., et al. (2014) Antitumor activity of 7-aminocarboxycoumarin derivatives, a new class of potent inhibitors of lactate influx but not efflux. Mol. Cancer Ther., 13, 1410–1418. [DOI] [PubMed] [Google Scholar]
- 128. Draoui N., et al. (2011) Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis. Model. Mech., 4, 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pasteur L. (1863) Nouvel exemple de fermentation déterminée par des animalcules infusoires pouvant vivre sans gaz oxygène libre, et en dehors de tout contact avec l’air de l’atmosphere. CR Acad. Sci., 56, 416. [Google Scholar]
- 130. Fletcher W.M. (1907) Lactic acid in amphibian muscle. J. Physiol., 35, 247–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Meyerhof O. (1920) Die Energieumwandlungen im Muskel. III. Pflügers Arch., 185, 11–32. [Google Scholar]
- 132. Hill A.V., et al. (1924), Muscular exercise, lactic acid, and the supply and utilisation of oxygen. Proc. R Soc. Lond. B Biol. Sci., 681, 84–138. [Google Scholar]
- 133. Brooks G.A. (1985) Circulation, Respiration, and Metabolism: Current Comparative Approaches. Springer, Berlin, pp. 208–218. [Google Scholar]
- 134. Brooks G.A. (2000) Intra- and extra-cellular lactate shuttles. Med. Sci. Sports Exerc., 32, 790–799. [DOI] [PubMed] [Google Scholar]
- 135. Garcia-Alvarez M., et al. (2014) Stress hyperlactataemia: present understanding and controversy. Lancet. Diabetes Endocrinol., 2, 339–347. [DOI] [PubMed] [Google Scholar]
- 136. Nalos M., et al. (2013) Revisiting lactate in critical illness. In Annual Update in Intensive Care and Emergency Medicine. Springer, Berlin, pp.413–423. [Google Scholar]
- 137. Duburcq T., et al. (2014) Hypertonic sodium lactate improves fluid balance and hemodynamics in porcine endotoxic shock. Crit. Care, 18, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Brooks G.A., et al. (1991) Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J. Appl. Physiol., 71, 333–341. [DOI] [PubMed] [Google Scholar]
- 139. Stanley W.C. (1991) Myocardial lactate metabolism during exercise. Med. Sci. Sports Exerc., 23, 920–924. [PubMed] [Google Scholar]
- 140. Glenn T.C., et al. (2015) Lactate: brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J. Neurotrauma, 32, 820–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pellerin L., et al. (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA, 91, 10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jacobs R.A., et al. (2013) Lactate oxidation in human skeletal muscle mitochondria. Am. J. Physiol. Endocrinol. Metab., 304, E686–E694. [DOI] [PubMed] [Google Scholar]
- 143. Dubouchaud H., et al. (2000) Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab., 278, E571–E579. [DOI] [PubMed] [Google Scholar]
- 144. Brooks G.A., et al. (1999) Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J. Appl. Physiol., 87, 1713–1718. [DOI] [PubMed] [Google Scholar]
- 145. Brooks G.A., et al. (1999) Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Natl. Acad. Sci. USA, 96, 1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hashimoto T., et al. (2008) Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med. Sci. Sports Exerc., 40, 486–494. [DOI] [PubMed] [Google Scholar]
- 147. Jacobs R.A., et al. (2013) Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J. Appl. Physiol. (1985)., 114, 344–350. [DOI] [PubMed] [Google Scholar]
- 148. San Millán I.S., et al. (2009) Physiological differences between road cyclists of different categories. A new approach. Med. Sci. Sports Exerc., 41, 64–65. [Google Scholar]
- 149. Holm E., et al. (1995) Substrate balances across colonic carcinomas in humans. Cancer Res., 55, 1373–1378. [PubMed] [Google Scholar]
- 150. Brizel D.M., et al. (2001) Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys., 51, 349–353. [DOI] [PubMed] [Google Scholar]
- 151. Hanahan D., et al. (2000) The hallmarks of cancer. Cell, 100, 57–70. [DOI] [PubMed] [Google Scholar]
- 152. Hanahan D., et al. (2011) Hallmarks of cancer: the next generation. Cell, 144, 646–674. [DOI] [PubMed] [Google Scholar]
- 153. Hirschhaeuser F., et al. (2011) Lactate: a metabolic key player in cancer. Cancer Res., 71, 6921–6925. [DOI] [PubMed] [Google Scholar]
- 154. Kumar V.B., et al. (2007) Endothelial cell response to lactate: implication of PAR modification of VEGF. J. Cell. Physiol., 211, 477–485. [DOI] [PubMed] [Google Scholar]
- 155. Trabold O., et al. (2003) Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen., 11, 504–509. [DOI] [PubMed] [Google Scholar]
- 156. Beckert S., et al. (2006) Lactate stimulates endothelial cell migration. Wound Repair Regen., 14, 321–324. [DOI] [PubMed] [Google Scholar]
- 157. Hunt T.K., et al. (2008) Lactate, with oxygen, incites angiogenesis. In Oxygen Transport to Tissue XXIX. Springer, Berlin, pp. 73–80. [DOI] [PubMed] [Google Scholar]
- 158. Polet F., et al. (2013) Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J. Intern. Med., 273, 156–165. [DOI] [PubMed] [Google Scholar]
- 159. Dhup S., et al. (2012) Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr. Pharm. Des., 18, 1319–1330. [DOI] [PubMed] [Google Scholar]
- 160. De Saedeleer C.J., et al. (2012) Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS One, 7, e46571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Végran F., et al. (2011) Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res., 71, 2550–2560. [DOI] [PubMed] [Google Scholar]
- 162. Stern R. (2008) Hyaluronidases in cancer biology. Semin. Cancer Biol., 18, 275–280. [DOI] [PubMed] [Google Scholar]
- 163. Walenta S., et al. (2004) Lactate: mirror and motor of tumor malignancy. Semin. Radiat. Oncol., 14, 267–274. [DOI] [PubMed] [Google Scholar]
- 164. Formby B., et al. (2003) Lactate-sensitive response elements in genes involved in hyaluronan catabolism. Biochem. Biophys. Res. Commun., 305, 203–208. [DOI] [PubMed] [Google Scholar]
- 165. Stern R., et al. (2002) Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp. Cell Res., 276, 24–31. [DOI] [PubMed] [Google Scholar]
- 166. Sonveaux P., et al. (2012) Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS One, 7, e33418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Baumann F., et al. (2009) Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro. Oncol., 11, 368–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Goetze K., et al. (2011) Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int. J. Oncol., 39, 453–463. [DOI] [PubMed] [Google Scholar]
- 169. Schwickert G., et al. (1995) Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res., 55, 4757–4759. [PubMed] [Google Scholar]
- 170. Walenta S., et al. (1997) Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am. J. Pathol., 150, 409–415. [PMC free article] [PubMed] [Google Scholar]
- 171. Walenta S., et al. (2000) High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res., 60, 916–921. [PubMed] [Google Scholar]
- 172. Walenta S., et al. (2003) Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? J. Cancer Res. Clin. Oncol., 129, 321–326. [DOI] [PubMed] [Google Scholar]
- 173. Fischer K., et al. (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood, 109, 3812–3819. [DOI] [PubMed] [Google Scholar]
- 174. Puig-Kröger A., et al. (2003) Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J. Leukoc. Biol., 73, 482–492. [DOI] [PubMed] [Google Scholar]
- 175. Gottfried E., et al. (2006) Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood, 107, 2013–2021. [DOI] [PubMed] [Google Scholar]
- 176. Husain Z., et al. (2013) Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J. Immunol., 191, 1486–1495. [DOI] [PubMed] [Google Scholar]
- 177. Choi S.Y., et al. (2013) Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J. Pathol., 230, 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Stubbs M., et al. (2000) Causes and consequences of tumour acidity and implications for treatment. Mol. Med. Today, 6, 15–19. [DOI] [PubMed] [Google Scholar]
- 179. Gillies R.J., et al. (2002) MRI of the tumor microenvironment. J. Magn. Reson. Imaging, 16, 430–450. [DOI] [PubMed] [Google Scholar]
- 180. Michalek R.D., et al. (2010) The metabolic life and times of a T-cell. Immunol. Rev., 236, 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Halestrap A.P., et al. (1999) The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem. J., 343 Pt 2, 281–299. [PMC free article] [PubMed] [Google Scholar]
- 182. Medina M.A. (2001) Glutamine and cancer. J. Nutr., 131(9 suppl), 2539S–42S. [DOI] [PubMed] [Google Scholar]
- 183. Daye D., et al. (2012) Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin. Cell Dev. Biol., 23, 362–369. [DOI] [PubMed] [Google Scholar]
- 184. Wise D.R., et al. (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA, 105, 18782–18787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Jiang P., et al. (2013) Reciprocal regulation of p53 and malic enzymes modulates metabolism and senescence. Nature, 493, 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Hansford R.G., et al. (1973) Active oxidative decarboxylation of malate by mitochondria isolated from L-1210 ascites tumor cells. Biochem. Biophys. Res. Commun., 51, 480–486. [DOI] [PubMed] [Google Scholar]
- 187. Pérez-Escuredo J., et al. (2016) Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle, 15, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Roth E., et al. (1990) Glutamine: an anabolic effector? JPEN. J. Parenter. Enteral Nutr., 14(4 suppl), 130S–136S. [DOI] [PubMed] [Google Scholar]
- 189. Chen F., et al. (2015) New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med., 13, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Justus C.R., et al. (2013) Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol., 4, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kharaziha P., et al. (2012) Tumor cell-derived exosomes: a message in a bottle. Biochim. Biophys. Acta, 1826, 103–111. [DOI] [PubMed] [Google Scholar]
- 192. Zhao H., et al. (2016) Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife, 5, e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Taylor D.D., et al. (2008) MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol., 110, 13–21. [DOI] [PubMed] [Google Scholar]
- 194. Rosell R., et al. (2009) Circulating microRNA signatures of tumor-derived exosomes for early diagnosis of non-small-cell lung cancer. Clin. Lung Cancer, 10, 8–9. [DOI] [PubMed] [Google Scholar]
- 195. Kim H.K., et al. (2003) Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur. J. Cancer, 39, 184–191. [DOI] [PubMed] [Google Scholar]
- 196. Parolini I., et al. (2009) Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem., 284, 34211–34222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Federici C., et al. (2014) Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One, 9, e88193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Ban J.J., et al. (2015) Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun., 461, 76–79. [DOI] [PubMed] [Google Scholar]
- 199. Potolicchio I., et al. (2005) Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol., 175, 2237–2243. [DOI] [PubMed] [Google Scholar]
- 200. Millimaggi D., et al. (2007) Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia, 9, 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Martinez-Outschoorn U.E., et al. (2011) Ketones and lactate increase cancer cell “stemness,” driving recurrence, metastasis and poor clinical outcome in breast cancer. Cell Cycle, 10, 1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lu H., et al. (2002) Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J. Biol. Chem., 277, 23111–23115. [DOI] [PubMed] [Google Scholar]
- 203. Bouzier A.K., et al. (1998) Glucose and lactate metabolism in C6 glioma cells: evidence for the preferential utilization of lactate for cell oxidative metabolism. Dev. Neurosci., 20, 331–338. [DOI] [PubMed] [Google Scholar]
- 204. Klonisch T., et al. (2008) Cancer stem cell markers in common cancers - therapeutic implications. Trends Mol. Med., 14, 450–460. [DOI] [PubMed] [Google Scholar]
- 205. Maugeri-Saccà M., et al. (2011) Cancer stem cells and chemosensitivity. Clin. Cancer Res., 17, 4942–4947. [DOI] [PubMed] [Google Scholar]
- 206. Jinesh G.G., et al. (2013) Blebbishields, the emergency program for cancer stem cells: sphere formation and tumorigenesis after apoptosis. Cell Death Differ., 20, 382–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Arora R., et al. (2015) Inhibition of the Warburg effect with a natural compound reveals a novel measurement for determining the metastatic potential of breast cancers. Oncotarget, 6, 662–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kankotia S., et al. (2014) Dichloroacetate and cancer: new home for an orphan drug? Biochim. Biophys. Acta, 1846, 617–629. [DOI] [PubMed] [Google Scholar]
- 209. Miranda-Gonçalves V., et al. (2012) Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro Oncol., 15, 298–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Joanne R Doherty J.L.C. (2013) Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest., 123, 3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Ovens M.J., et al. (2010) AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7-10. Biochem. J., 425, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Le Floch R., et al. (2011) CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. USA, 108, 16663–16668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Polański R., et al. (2014) Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res., 20, 926–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kirk P., et al. (2000) CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J., 19, 3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Marchiq I., et al. (2015) Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H+ symporters. J. Mol. Med., 94, 155–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Riethdorf S., et al. (2006) High incidence of EMMPRIN expression in human tumors. Int. J. Cancer, 119, 1800–1810. [DOI] [PubMed] [Google Scholar]
- 217. Baba M., et al. (2008) Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem. Biophys. Res. Commun., 374, 111–116. [DOI] [PubMed] [Google Scholar]
- 218. Su J., et al. (2009) A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis. Cancer Lett., 273, 140–147. [DOI] [PubMed] [Google Scholar]
- 219. Schneiderhan W., et al. (2009) CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut, 58, 1391–1398. [DOI] [PubMed] [Google Scholar]
- 220. Chen W., et al. (2009) Inhibition of CD146 gene expression via RNA interference reduces in vitro perineural invasion on ACC-M cell. J. Oral Pathol. Med., 38, 198–205. [DOI] [PubMed] [Google Scholar]
- 221. Zou W., et al. (2007) Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett., 248, 211–218. [DOI] [PubMed] [Google Scholar]
- 222. Granja S., et al. (2015) Disruption of BASIGIN decreases lactic acid export and sensitizes non-small cell lung cancer to biguanides independently of the LKB1 status. Oncotarget, 6, 6708–6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Courneya K.S., et al. (2001) Framework PEACE: an organizational model for examining physical exercise across the cancer experience. Ann. Behav. Med., 23, 263–272. [DOI] [PubMed] [Google Scholar]
- 224. Courneya K.S. (2003) Exercise in cancer survivors: an overview of research. Med. Sci. Sports Exerc., 35, 1846–1852. [DOI] [PubMed] [Google Scholar]
- 225. Friedenreich C.M., et al. (2002) Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J. Nutr., 132(11 suppl), 3456S–3464S. [DOI] [PubMed] [Google Scholar]