Abstract
Background/objectives:
Thyroid nodule (TN) is a common thyroid disorder globally, and the incidence has been increasing in recent decades. The objective of this study was to determine the contribution of thyroid-stimulating hormone (TSH), ultrasound (US), and cytological classification system for predicting malignancy among the surgically excised nodules.
Design and methods:
A retrospective analysis was performed between January 2012 and December 2014, using data drawn from 1188 patients (15-90 years), who had 1433 TN and fine-needle aspiration in Prince Sultan Military Medical City, Saudi Arabia. After reviewing all the thyroid cytopathological slides and US reports, classification was done based on the Bethesda System for Reporting Thyroid Cytology and the Thyroid Imaging Reporting and Data System (TI-RADS).
Results:
A total of 1188 patients’ medical records were reviewed for this study, among them 311 patients had undergone surgical intervention (253 patients had single nodule and 58 had 2 nodules), with a total of 369 nodules. However, as 54 nodules on the US were either unavailable or unclear, the 315 remaining nodules were analyzed, revealing 30.2% (n = 95) malignancy overall. Patients with TSH values of >4.5 mIU/L (38.2%), TN <1 cm (48.8%), TI-RADS category 5 (75.6%), and Bethesda category VI (88.9%) revealed a higher percentage of malignancy. From the univariate analysis using the χ2 test, significant relationship between the TSH, nodule size, TI-RADS, and the Bethesda category between the malignant and benign nodules emerged. The regression analysis showed that patients with a TSH value of 0.5 to 4.5 mIU/L (odds ratio [OR]: 2.96), TSH >4.5 mIU/L (OR: 6.54) had higher risk for malignancy than those with a TSH value of ≤0.4 mIU/L. Thyroid nodules with sizes of 1 to 1.9 cm (OR: 1.12), 2 to 2.9 cm (OR: 0.74), 3-3.9 cm (OR: 1.21), and ≥4 cm (OR: 0.52) were found to have no association with the risk of malignancy. Compared with TI-RADS 2 patients, those with categories 4B (OR: 1.35) and 5 (OR: 2.3) were found to be at higher risk of malignancy. Similarly, Bethesda IV (OR: 2.72), V (OR: 8.47), and VI (OR: 20; P < .02) category patients had a higher risk for malignancy than those in Bethesda class I. Among the study population, the papillary thyroid carcinoma was the most common type of thyroid cancer (86, 90.5%) followed by 7.4% (n = 7) of follicular thyroid carcinoma, 1.05% (n = 1) of anaplastic carcinoma, and 1.05% (n = 1) of medullary thyroid carcinoma.
Conclusions:
A predictive model for risk of malignancy using a combining characteristic of the TSH, US, and cytological classification systems could assist the clinicians in minimizing exposing the patients with TNs to nonessential invasive procedures.
Keywords: Bethesda, thyroid nodules, risk of malignancy, TI-RADS, TSH, fine-needle aspiration
Introduction
Thyroid nodules (TNs) are increasingly becoming a clinical problem, with an estimated prevalence of 4% to 7% via palpation, whereas identification through ultrasound (US) has risen from 19% to 68%, as technological advancements have made the imaging equipment more sophisticated.1–4 Most of the TNs are benign (noncancerous), and only less than 10% are malignant.5,6 However, TNs are normally the first indicators of thyroid cancer; therefore, accurate diagnosis is required to circumvent needless surgical interventions in patients having benign nodules. Therefore, identifying suitable tools to assess the risk for malignancy has become a vital need.5,6
The risk factors for thyroid cancer have been clearly identified as age, male sex, history of neck irradiation, as well as the clinical features of the nodule (hardness or fixity to the adjoining structures and occurrence of a cervical lymph node).7,8 Some patients may also possess a family history of differentiated thyroid cancer, as most differentiated thyroid cancers are irregular, familial, and nonmedullary and which account for more than 5% of the instances.9,10
Some of the tools that could prove effective in predicting malignancy in TN assessment include the assessment of the TN size, thyroid-stimulating hormone (TSH) level at the time of diagnosis, Bethesda System for Reporting Thyroid Cytopathology (BSRTC) and Thyroid Imaging Reporting and Data System (TI-RADS).11,12 Consequently, establishing a concordance between the diagnostic procedures (fine-needle aspiration [FNA] and US) might reduce the health care costs as well as numbers of nonessential procedures in the population at risk could be reduced. Findings from various researches also reported that the FNA biopsy must be interpreted precisely to generate a common language among pathologists and surgeons, to enable them to establish a uniform TN management strategy.12
Over the past few eras, thyroid cancer has been considerably rising in frequency, globally, although the mortality rate has gradually decreased, in Saudi Arabia as well.13–15 The decreased mortality in thyroid cancer is an indication of the variations in exposure to the risk factors, as well as to the modifications in the techniques employed in disease diagnosis and treatment, whereas the spike in the incidence is possibly a reflection of the increased rate of detection of this neoplasm over the past few decades.16 However, compared with the developed countries, studies on the prevalence, incidence, and type of thyroid cancer in Saudi Arabia continue to be inadequate because of the absence of pertinent studies performed in these specific areas.17 Hence, this study aimed to determine the risk of malignancy in TN, employing nodule size, TSH level, as well as the Bethesda system, which enhances the evaluations of the findings from FNA cytology (FNAC) and the TI-RADS, and in turn, the analysis of the US reports among the surgically excised nodules.
Methods
Study design and setting
A retrospective analysis was conducted between January 2012 and December 2014 (36 months) using data from 1188 patients (15-90 years) on 1433 TNs at Prince Sultan Military Medical City (PSMMC), a tertiary care center in Riyadh, Saudi Arabia. The PSMMC studied the patients referred from different parts of the Kingdom, which overall, was a good representation of Saudi Arabia. The Research and Ethics Committee of PSMMC, Riyadh, Saudi Arabia, approved the study protocol.
Data collection
Data were gathered from the patients’ medical charts and cytopathology reports in terms of age, sex, TSH level, cytological features, US reports of the TN, and histological types of the population under study. Once the thyroid cytopathological slides were reviewed, they were classified based on the BSRTC system. The TN characteristics from the US images were reviewed in patients who had undergone surgical interventions and had clear US images that enable the classification of TN, based on the French TI-RADS. The TN dimensions were assessed using the US findings.
TSH level (mIU/L)
In all the samples, the serum TSH (normal range: 0.4-4.5 mIU/L) was determined using the electrochemiluminescence immunoassay technique (Roche Corporation, Indianapolis, IN, USA).
Bethesda system
At present, the BSRTC is employed to distinguish among the US-FNAC thyroid specimens.18 Cibas19 cites that this system, introduced in 2007, includes 6 categories as mentioned: (I) Unsatisfactory (UNS) or nondiagnostic (ND), (II) Benign and nonneoplastic, (III) Atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS), (IV) Follicular neoplasm or suspicious for follicular neoplasm (FN/SFN), (V) Suspicious for malignancy (SM) although not diagnostic, and (VI) Malignant. One of the 5 interventional radiologists performed all the FNAs performed under US guidance, with 25-gauge needles, doing 3 to 5 passes. The FNAs were stained on-site using the Diff-Quik stain and all the samples were subjected to adequacy assessment. Five accredited cytopathologists interpreted all the slides.
Thyroid Imaging Reporting and Data System
The 315 available US images of the patients who had undergone surgical intervention were stored on the picture archiving and communication system/radiology information system. The various TNs were reviewed and described by the same radiologist, well experienced in thyroid imaging, according to the French TI-RADS classification.20 The French TI-RADS assessment involved a 6-point scale, namely, 1—normal (absence of nodule); 2—benign (simple cyst, septated cyst, isolated macrocalcification, isoechoic spongiform nodule); 3—very probably benign (oval-shaped, regular borders, isoechoic, or hyperechoic); 4A—low suspicion of malignancy (oval-shaped, regular borders, mildly hypoechoic); 4B—high level of suspicion for malignancy (1 or 2 features indicative of high suspicion: taller-than-wide/taller-than-long, spiculated or lobulated borders, pronounced hypoechogenicity, microcalcifications, high stiffness on elastography); and 5—effectively positively malignant (exhibiting 3-5 features of high level of suspicion and/or the appearance of a presumably metastatic lymph node, of thyroid origin). The radiologist, however, is not responsible for filing the numbers nor can he access the final histological result.
Echogenicity is distinguishable into several classes, namely, hyperechogenicity (higher echogenicity of the TN than in the neighboring thyroid parenchyma), isoechogenicity (similar degree of echogenicity in the TN and surrounding thyroid parenchyma), hypoechogenicity (echogenicity lesser than that of the adjacent thyroid parenchyma but higher than that of the strap muscle around it), and marked hypoechogenicity (echogenicity lower than that of the surrounding strap muscle). The margins too were classified as irregular (with indistinct and rough interface between the lesion and the adjacent thyroid parenchyma) and regular (with a smooth, clear outline).
Calcifications were categorized as microcalcifications (≤1 mm in diameter and visible as minute, punctate, hyperechoic foci, possessing or lacking acoustic shadowing, and absent comet tail artifacts) or macrocalcifications (with hyperechoic foci >1 mm). Shape was another means of categorization, as taller-than-wide (anteroposterior measurement being larger than the transverse one) or wider-than-tall (greater transverse dimension than the anteroposterior measurement).20 As elastography was not performed for all the TNs, these criteria were considered only when present.
Histological examination
The histological examination of TNs was categorized into benign and nonneoplastic and malignant. For papillary thyroid carcinoma (PTC), subtype variants were categorized as follicular variant, classical variant, conventional variant, and tall cell variant. Furthermore, follicular thyroid carcinoma (FTC) was subdivided to widely invasive FTC, and minimally invasive FTC.
Statistical analysis
The Microsoft Excel 2010 (Microsoft Corporation, Seattle, WA, USA) and IBM SPSS Statistics (IBM SPSS Statistics for Windows, Version 22, SPSS Inc., an IBM Company) program were used for all the statistical analysis. The descriptive analysis of the epidemiological data was expressed in terms of frequency and percentages and mean ± SD. The significant difference between malignant and benign was determined using the Pearson χ2 test. The risk estimates (odds ratio [OR]) were assessed and listed using 95% confidence interval. The OR was calculated to quantify the intensity of the presence or absence of certain characteristics linked with benignity or malignancy in the study population. The value of P < .05 was selected to imply statistical significance.
Results
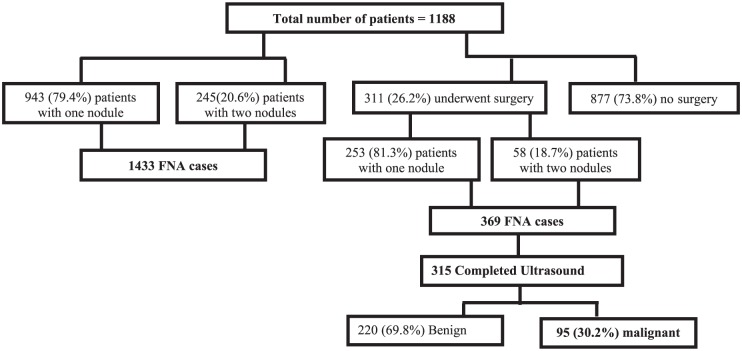
From among the 1188 patients in the study (mean: 46.3 ± 15.1; range: 15-90 years, n = 976 women), 943 patients revealed the presence of a single TN, whereas 245 patients showed 2 TNs, accounting to 1433 nodules in total (FNA instances). Among the total 311 patients (26.2%) who were subjected to surgical intervention, 253 patients showed the presence of 1 TN, whereas 58 patients had 2 TNs, which culminated in a total (FNA) of 369 cases. However, 54 of the 369 nodules were either unavailable or unclear on the US; therefore, only 315 were taken for analysis (Figure 1). The histological study revealed 220 of the nodules benign (69.8%) and 95 were malignant giving an overall 30.2% surgical yield of malignancy.
Figure 1.
Flowchart of thyroid nodules description among 1188 patients and the risk of malignancy among 311 surgically excised nodules. FNA indicates fine-needle aspiration.
TSH, TN size, TI-RADS, and Bethesda
The TSH value of ≤0.4 mIU/L indicated a 16% risk for malignancy, whereas TSH value of 0.5 to 4.5 mIU/L implied 30.5% and >4.5 mIU/l was 38.2%. For TN size of <1 cm, the risk for malignancy was 48.8%, whereas for TN size of 1 to 1.9 cm, it was 40%; for 2 to 2.9 cm, it was 28.8%; for 3 to 3.9 cm, it was 29.8%; and for ≥4 cm, it was 12.7%. For the TI-RADS categories 2, 3, 4A, 4B, and 5, the risks for malignancy were 15.4%, 13.3%, 26.4%, 48.3%, and 75.6%, respectively. The Bethesda distribution for malignancy in the TNs was category V (SM) diagnoses (20 cases), in which 6 cases were benign but 14 cases (70%) histologically confirmed the presence of carcinoma. Finally, in category VI diagnoses (45 cases), 5 cases were benign but 40 cases were histologically verified as having carcinoma (88.9%; Table 1).
Table 1.
Factors associated with risk of malignancy (n = 315).
| Variables | No. of nodules | Benign (n = 220, %) | Malignant (n = 95, %) | P value |
|---|---|---|---|---|
| Age, y | ||||
| <45 | 177 | 123 (69.5) | 54 (30.5) | .489 |
| ≥45 | 138 | 97 (70.3) | 41 (29.7) | |
| Gender | ||||
| Male | 62 | 46 (74.2) | 16 (25.8) | .251 |
| Female | 253 | 174 (68.8) | 79 (31.2) | |
| TSH, mIU/L | ||||
| ≤0.4 | 25 | 21 (84) | 4 (16) | .036 |
| 0.5-4.5 | 256 | 178 (69.5) | 78 (30.5) | |
| >4.5 | 34 | 21 (61.8) | 13 (38.2) | |
| Nodule size, cm | ||||
| <1 | 43 | 22 (51.2) | 21(48.8) | .0001 |
| 1-1.9 | 70 | 42 (60) | 28 (40) | |
| 2-2.9 | 66 | 47 (71.2) | 19 (28.8) | |
| 3-3.9 | 57 | 40 (70.2) | 17 (29.8) | |
| ≥4 | 79 | 69 (87.3) | 10 (12.7) | |
| TI-RADS | ||||
| 2 | 13 | 11 (84.6) | 2 (15.4) | .0001 |
| 3 | 150 | 130 (86.7) | 20 (13.3) | |
| 4A | 53 | 39 (73.6) | 14 (26.4) | |
| 4B | 58 | 30 (51.7) | 28 (48.3) | |
| 5 | 41 | 10 (24.4) | 31 (75.6) | |
| Bethesda | ||||
| I | 4 | 3 (75) | 1 (25) | .0001 |
| II | 169 | 151 (89.3) | 18 (10.7) | |
| III | 37 | 30 (81.1) | 7 (18.9) | |
| IV | 40 | 25 (62.5) | 15 (37.5) | |
| V | 20 | 6 (30) | 14 (70) | |
| VI | 45 | 5 (11.1) | 40 (88.9) | |
Abbreviations: TI-RADS, Thyroid Imaging Reporting and Data System; TSH, thyroid-stimulating hormone.
Pearson χ2 test, P < .05 considered as significant.
Correlation of TN size, TSH, TI-RADS, and Bethesda
In Table 2, the relationship between the TN size, TSH, TI-RADS, and Bethesda values is demonstrated. From among the 315 nodules, 79 (25%) were ≥4 cm in size. Among these 79 nodules, 4 were classified as Bethesda 5 and 6 categories, 14 fell under Bethesda III and IV categories, and 60 were benign (Bethesda II). Of the 43 (13.7%) with size <1-cm nodules, 19 belonged to Bethesda categories V and VI, 10 were included in Bethesda III and IV categories, and 13 were benign in FNA but were surgically excised as the patient also had a second and bigger nodule. Greater numbers of nodules (n = 256) were noted at the TSH 0.5 to 4.5 level and most of them belonged to Bethesda VI category (n = 36; Table 2). TI-RADS 3 was higher in the Bethesda 2 category (n = 112) at TSH 0.5 to 4.5 mIU/L higher among Bethesda 2 (n = 132).
Table 2.
Correlation between thyroid nodule size, TI-RADS, TSH level, and Bethesda.
| Cytology | TSH level, mIU/L |
Thyroid nodule size, cm |
TI-RADS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.4 | 0.5-4.5 | >4.5 | <1 | 1-1.9 | 2-2.9 | 3-3.9 | ≥4 | 2 | 3 | 4A | 4B | 5 | |
| Malignant histological results (n = 95) | |||||||||||||
| Bethesda I | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bethesda II | 1 | 15 | 2 | 2 | 6 | 1 | 4 | 5 | 1 | 10 | 2 | 3 | 2 |
| Bethesda III | 0 | 6 | 1 | 0 | 3 | 2 | 1 | 1 | 0 | 4 | 0 | 1 | 2 |
| Bethesda IV | 1 | 13 | 1 | 3 | 2 | 4 | 4 | 2 | 1 | 2 | 6 | 4 | 2 |
| Bethesda V | 1 | 12 | 1 | 5 | 4 | 4 | 1 | 0 | 0 | 1 | 1 | 8 | 4 |
| Bethesda VI | 1 | 31 | 8 | 10 | 13 | 8 | 7 | 2 | 0 | 3 | 5 | 12 | 20 |
| Total | 4 | 78 | 13 | 21 | 28 | 19 | 17 | 10 | 2 | 20 | 14 | 28 | 31 |
| Benign histological results (n = 220) | |||||||||||||
| Bethesda I | 0 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 |
| Bethesda II | 17 | 117 | 17 | 11 | 23 | 32 | 30 | 55 | 9 | 102 | 21 | 18 | 1 |
| Bethesda III | 0 | 29 | 1 | 6 | 12 | 2 | 5 | 5 | 1 | 14 | 10 | 3 | 2 |
| Bethesda IV | 2 | 21 | 2 | 1 | 3 | 11 | 4 | 6 | 0 | 10 | 6 | 5 | 4 |
| Bethesda V | 2 | 4 | 0 | 2 | 3 | 0 | 0 | 1 | 0 | 2 | 2 | 1 | 1 |
| Bethesda VI | 0 | 5 | 0 | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 2 | 2 |
| Total | 21 | 178 | 21 | 22 | 42 | 47 | 40 | 69 | 11 | 130 | 39 | 30 | 10 |
Abbreviations: TI-RADS, Thyroid Imaging Reporting and Data System; TSH, thyroid-stimulating hormone.
Factors associated with malignancy
A comparison of the various parameters studied in both the TN groups (malignant and benign TNs) is reported in Table 1. Significant differences between the 2 groups (benign and malignant) were evident when a comparison was made of the TSH level, TN size, Bethesda, and TI-RADS categories (P < .001). Factors that achieved statistical significance in the univariate analysis (χ2 test) were analyzed to determine any independent associations with malignancy using the logistic regression model.
Patients with TSH levels of 0.5 to 4.5 mIU/L (OR: 2.96) and >4.5 mIU/L (OR: 6.54) were at a higher risk for malignancy than those with TSH levels of ≤0.4 mIU/L. Patients having nodules of 1 to 1.9 cm (OR: 1.12), 2 to 2.9 cm (OR: 0.74), 3 to 3.9 cm (OR: 1.21), and ≥4 cm (OR: 0.52) in size established that the TN size had no relationship with the risk of malignancy. Patients in Bethesda categories IV (OR: 2.72), 5 (OR: 8.47), and 6 (OR: 20; P < .02) had higher risks for malignancy compared with those in Bethesda category I. When compared with patients in TI-RADS 2, those in categories 4B (OR: 1.35) and 5 (OR: 2.3) had higher risk of malignancy (Table 3).
Table 3.
Factors associated with risk of malignancy (n = 315) (logistic regression).
| Variables | Variable | OR (95% CI) (lower-upper) | P value |
|---|---|---|---|
| TSH, mIU/L | ≤0.4 | 1 | |
| 0.5-4.5 | 2.96 (0.66-13.1) | .15 | |
| >4.5 | 6.54 (1.08-40) | .04 | |
| Nodule size, cm | <1 | 1 | |
| 1-1.9 | 1.12 (0.37-3.33) | .83 | |
| 2-2.9 | 0.74 (0.23-2.36) | .61 | |
| 3-3.9 | 1.21 (0.36-4.13) | .75 | |
| ≥4 | 0.52 (0.15-1.76) | .30 | |
| TI-RADS | 2 | 1 | |
| 3 | 0.56 (0.96-3.32) | .52 | |
| 4A | 0.76 (0.12-4.59) | .76 | |
| 4B | 1.35 (0.22-8.37) | .74 | |
| 5 | 2.30 (0.34-15.4) | .38 | |
| Bethesda | I | 1 | |
| II | 0.70 (0.63-7.90) | .77 | |
| III | 0.99 (0.81-12.2) | .99 | |
| IV | 2.72 (0.22-32.2) | .42 | |
| V | 8.47 (0.63-112) | .10 | |
| VI | 20 (1.57-255) | .02 |
Abbreviations: CI, confidence interval; OR, odds ratio; TI-RADS, Thyroid Imaging Reporting and Data System; TSH, thyroid-stimulating hormone.
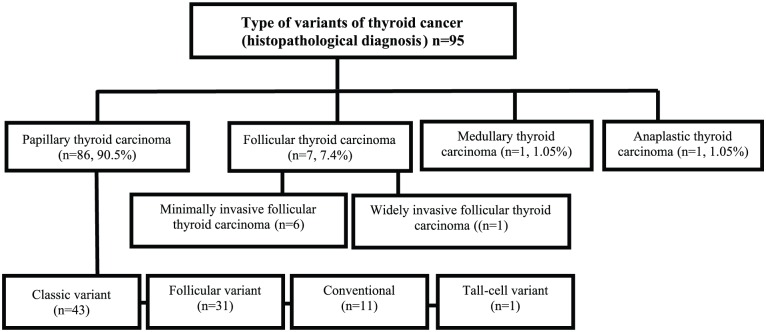
The histopathological examination of type and variants of thyroid cancer is shown in Figure 2. Among the study population, the papillary carcinoma was the most common form of thyroid cancer (n = 86, 90.5%). Among PTC, 4 histologic variants exist, with classic variant PTC reported for higher number (n = 43, 50%) of PTC followed by follicular variant PTC (n = 31, 36%). Besides, 7 of malignancies were FTC (including 1 of the highest risk widely invasive phenotype), 1 of anaplastic thyroid carcinoma, and 1 of medullary thyroid carcinoma.
Figure 2.
Types of variants of thyroid cancer (histopathological diagnosis).
Discussion
Nodules that are malignant or symptomatic and cause compression to the adjoining structures necessitate surgical excision. However, as most TNs are asymptomatic and benign, the thyroid surgeon is dependent on diagnostic studies to decide when surgery is necessary.21 This study aimed at a clear understanding of the roles of TSH, US, and the cytological classification system in predicting malignancy. In this study, we found that 79 (25%) of the 315 nodules were ≥4 cm in size. Four of these belonged to Bethesda categories V and VI, whereas 14 were included in Bethesda categories III and IV and 60 were benign (Bethesda II). Ten nodules ≥4 cm (12.7%; 10/79) were malignant in the final histology, and regression analysis finding reported that the patients with nodule size 1 to 1.9 cm (OR: 1.12), 2 to 2.9 cm (OR: 0.74), 3 to 3.9 cm (OR: 1.21), and ≥4 cm (OR: 0.52) clearly demonstrated that the TN size was not associated with the risk of malignancy. Previous research shows that nodule size may contribute in cancer risk assessment, although data are conflicting with other studies showing that there is no correlation between nodule size and risk of malignancy of TN.22–24 Moreover, it is most often recommended that the larger TNs (≥4 cm) undergo surgical ablation as they are of concern for cancer, even if signs of compression or esthetic concerns or structural infringement on the adjacent neck structures or suspicious characteristics are completely absent.25 This could be partly because size has been established as a strong predictor of malignancy in other nodules like those of the adrenal gland. Furthermore, Kamran et al26 in a retrospective study including 7348 TNs recorded that 13% were malignant (927), of which were those with sizes of 1.0 to 1.9 cm diameter, 10.5% were cancerous. On the contrary, of the nodules >2.0 cm in size, 15% were cancerous (P = .01). However, nodules showing dimensions of 2.0 to 2.9, 3.0 to 3.9, and 4 cm were cancerous in 14%, 16%, and 15% of the instances (P = .14), respectively, revealing the absence of a graded rise in the risk of cancer once the 2-cm threshold was crossed.
Overall, more than one-third of the surgically excised nodules were identified as malignant (95/315) in the current population under study; of those <1 cm, nearly 50% showed malignancy, whereas 40% of those in the range of 1.0 to 1.9 cm in diameter were cancerous. This finding was possibly because such nodules are recommended for surgical intervention, only if suspicions of malignancy arise. These nodules (<2 cm), in our observations, revealed that most of them in their FNA report classified as having Bethesda categories III, IV, and V. On the contrary, nodules >2.0 and <4 cm (2.0-2.9, 3.0-3.9) showed almost 30% cancerous symptoms. Between 2 and 4 cm, the risk becomes more stable, although one-third of the nodules are malignant. In nodules >4 cm, the risk is reduced due to the other indicators for surgery, such as compressive symptoms or esthetic reasons as well as the decision taken by specific surgeons and practices. However, the regression analysis of this study showed that the risk for malignancy was not related to size of the TN. Previous studies also reported that the nodule size was not a variable linked with malignancy risk; it had to be examined cautiously and included with the other clinical symptoms and TN investigation tools, which concurs with the earlier results.27,28
A large number of the patients (81.3%) in this study reported TSH values between 0.5 and 4.5 mIU/L; of these, 10.8% had TSH level >4.5 mIU/L, whereas 7.9% had TSH level ≤0.4 mIU/L. At the TSH values of ≤0.4 mIU/L, the risk for malignancy was 16%, compared with 38.2% when the value was >4.5 mIU/L. The preoperative mean TSH levels were notably higher in the patients having malignant TNs compared with the TSH concentrations in the benign nodules (4.87 ± 1.2 mIU/L versus 1.23 ± 0.76 mIU/L). The TSH was a reliable predictor for malignancy when TSH levels rose above >4.5 mIU/L, as confirmed by the findings of this study using regression analysis. The results of several studies concur with the findings of this study and even relate the higher TSH to the more aggressive form of thyroid cancer, being connected with the extrathyroidal spread of the disease. When compared with the untreated patients, those with multinodular goiter and receiving thyroxine exhibited notably lower serum TSH and occurrence of PTC. There was a positive correlation between nodule size and the TSH levels; however, some studies did not report any relationship between the TSH level and risk of malignancy.29–32 It is, therefore, important to note that several TNs could in reality be toxic thyroid adenomas, especially when low TSH levels are observed. In this context, some authors recommend scintigraphy to be done using 99mTC-sodium pertechnetate to assess the uptake of the TN greater than 1 cm in size and to refrain from unnecessary surgical interventions in the event of a hyperfunctioning or “hot” nodule.33
The percentages of the risk of malignancy for each of the Bethesda categories found in this study are similar to the values reported in the American Thyroid Association Management Guidelines and other studies. The comparisons are as follows: 25% versus 9% to 32% (“nondiagnostic or unsatisfactory” category), 10.7% versus 1% to 10% (“benign and nonneoplastic” category), 18.9% versus 6% to 48% (AUS/FLUS), 70% versus 53% to 97% (“suspicious for malignancy” category), and 88.9% versus 94% to 100% (“malignant” category). Of those in the Bethesda IV class, 37.5% faced the risk of malignancy, which bears close similarity to the meta-analysis recently published by Bongiovanni et al, with a 14% to 34% reported value (FN/SFN). However, in many studies, the greatest variation for the risk of malignancy was seen in category 4, some with the higher malignancy rate (50%-67%) than in the present results.34
From the correlation performed of the TI-RADS with the final histological findings, the probability of malignancy was found for the TNs in TI-RADS category 2 to be 15.4%, whereas for those with TI-RADS 3, it was 13.3%; it was 26.4% for TI-RADS 4A and 48.3% for TI-RADS 4B, whereas the probability of malignancy for TI-RADS 5 was 75.6%. These results concur with the positive predictive value of the French TI-RADS (bearing a close similarity to the classification of the New American Thyroid Association) for stages 4B and 5 alone. The disparity in the risk of malignancy from stages 2 to 4A could possibly be due to the few numbers of patients possessing the final histology, difference in ethnic origins, and retrospective character of the study, which can cause difficulties in interpreting the US images, and the lack of elastography. However, when the regression analysis of this study was compared with the TI-RADS 2 patients of categories 4B (OR: 1.35) and 5 (OR: 2.3), they revealed a higher degree of risk than those with TI-RADS category 2. The TI-RADS 3 findings (13.3%) are comparable with the reports of Hovrath et al35, who recorded a 14.1% risk of malignancy in those with TI-RADS 3. However, the current TI-RADS 3 findings are lower than the ones cited by Park et al, in 2009 (31.1%), and Chandramohan et al, in 2017 (32%), but higher than the results of the French TI-RADS (0.25%).15,20,35,36 Identical results were reported for the 4A, 4B, and 5 TI-RADS categories. In this study, only a single radiologist interpreted all the US recordings to circumvent such interobserver variations, especially of those ignorant of the FNA/histology findings. Several authors have separately stratified the risks for each TI-RADS category, although as in this study, they all revealed a common pattern, with the malignancy risk rising from the categories of TI-RADS 2 to TI-RADS 5. In this cohort study, the TI-RADS 4A and 5 were identified as strong predictors of malignancy (Table 4).
Table 4.
Comparison of diagnostic performance of the ultrasound classification systems.
| TI-RADS 2 | TI-RADS 3 | TI-RADS 4A | TI-RADS 4B | TI-RADS 5 | |
|---|---|---|---|---|---|
| Russ20 | ≈0 | 0.25 | 6 | 69 | ≈100 |
| Chandramohan et al36 | 6.6 | 32 | 36 | 64 | 91 |
| Horvath et al35 | 0 | 14.1 | 45 | 89.6 | |
| Park et al15 | 9.6 | 31.1 | 76.8 | 100 | |
| Current study | 15.5 | 13.3 | 26.4 | 48.3 | 75.6 |
Abbreviation: TI-RADS, Thyroid Imaging Reporting and Data System.
This study stated PTC (90.5%) as the most commonest type of thyroid cancer among the study population. This result confirmed by the previous findings reported that overall PTC as the commonest type of thyroid cancer denotes 80% of all the thyroid malignancies and more than 90% of the differentiated thyroid cancers. A spurt in the occurrence of PTC over the earlier eras has triggered greater interest in this disease.14 The FTC is not being identified as frequent, although there is an growing incidence of well-differentiated thyroid carcinomas everywhere else,14 concurring with the findings of this study.
Strength and limitations
This study is robust as it is simple and practical for a predictive score as a record-based tool that uses a combination of easily acquired predictors. When used as a decision aid, this category could potentially enable clinicians to reduce the number of nonessential invasive interventions done in low-risk individuals of those having unconfirmed or insufficient FNAs. Furthermore, the data for this study were drawn from a tertiary care center in Saudi Arabia and as it includes patients referred from various parts of the Kingdom, it is regarded as an acceptable representation of the country in general. The 30.2% malignancy rate seen in this sample concurs with the occurrence of malignancy in the tertiary care referral milieu.
We acknowledge the limitations inherent in this study, which principally include some degree of lack of histological confirmation for all the patients and its retrospective character. A greater number of histologically confirmed patients and a prospective study in future will enhance the findings. Also, although most of the cytologically benign nodules were categorized as benign, most frequently they were not subjected to a repeat FNA or surgical resection to verify the precision of this cytological detection. Nevertheless, in Saudi Arabia, this cohort constitutes the largest obtainable analysis of consecutive patients recommended for US evaluation and US-guided FNA of all the clinically pertinent nodules.
Conclusions
The management of TNs could be improved if an effective predictive model for malignancy could be drawn up, using the combined characteristics of the TSH, US, and cytological classification system, to enable clinicians across the world to minimize the invasive surgical procedures in patients with low-risk TNs.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MAAD and RB conceived and designed the experiments. MAAD and AAR wrote the first draft of the manuscript. AAR, MAAD, MAT, and RB contributed to the writing of the manuscript and agreed with manuscript results and conclusions. MAAD made critical revisions and approved final version. All authors reviewed and approved the final manuscript.
Ethical Approval: The study protocol was approved by the Research and Ethics Committee of Prince Sultan Military Medical City, Riyadh, Saudi Arabia.
References
- 1. Li XY, Zhang B, Lin YS. The interpretation of 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;52:309–315. [DOI] [PubMed] [Google Scholar]
- 2. Gamme G, Parrington T, Wiebe E, et al. The utility of thyroid ultrasonography in the management of thyroid nodules. Can J Surg. 2017;60:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ezzat S, Sarti DA, Cain DR, Braunstein GD. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–1840. [DOI] [PubMed] [Google Scholar]
- 4. Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699–706. [DOI] [PubMed] [Google Scholar]
- 5. Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–595. [DOI] [PubMed] [Google Scholar]
- 6. Papini E, Guglielmi R, Bianchini A, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. [DOI] [PubMed] [Google Scholar]
- 7. Onder A, Aycan Z. Approach to thyroid nodules in children and adolescents. Turk J Pediatr. 2014;56:219–225. [PubMed] [Google Scholar]
- 8. Pervaiz R, Tulay P, Faisal F, Serakinci N. Incidence of cancer in the Turkish Republic of Northern Cyprus. Turk J Med Sci. 2017;47:523–530. [DOI] [PubMed] [Google Scholar]
- 9. Rago T, Fiore E, Scutari M, et al. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol. 2010;162:763–770. [DOI] [PubMed] [Google Scholar]
- 10. Nose V. Familial follicular cell tumors: classification and morphological characteristics. Endocr Pathol. 2010;21:219–226. [DOI] [PubMed] [Google Scholar]
- 11. Al Dawish MA Robert AA Muna A et al. Bethesda System for Reporting Thyroid Cytopathology: a three-year study at a tertiary care referral center in Saudi Arabia. World J Clin Oncol. 2017;8:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acar Y, Doğan L, Güven HE, et al. Bethesda made it clearer: a review of 542 patients in a single institution. Oncol Res Treat. 2017;40:277–280. [DOI] [PubMed] [Google Scholar]
- 13. Alzahrani AS, Alomar H, Alzahrani N. Thyroid cancer in Saudi Arabia: a histopathological and outcome study. Int J Endocrinol. 2017;2017:8423147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hussain F, Iqbal S, Mehmood A, Bazarbashi S, ElHassan T, Chaudhri N. Incidence of thyroid cancer in the Kingdom of Saudi Arabia, 2000-2010. Hematol Oncol Stem Cell Ther. 2013;6:58–64. [DOI] [PubMed] [Google Scholar]
- 15. Park JY, Lee HJ, Jang HW, et al. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid. 2009;19:1257–1264. [DOI] [PubMed] [Google Scholar]
- 16. Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res. 2015;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alghamdi IG, Hussain, Alghamdi MS, Dohal AA, Almalki SS, El-Sheemy MA. The incidence rate of thyroid cancer among women in Saudi Arabia: an observational descriptive epidemiological analysis of data from Saudi Cancer Registry 2001-2008. J Immigr Minor Health. 2015;17:638–643. [DOI] [PubMed] [Google Scholar]
- 18. Wesola M, Jelen M. Bethesda system in the evaluation of thyroid nodules: review. Adv Clin Exp Med. 2017;26:177–182. [DOI] [PubMed] [Google Scholar]
- 19. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658–665. [DOI] [PubMed] [Google Scholar]
- 20. Russ G. Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: description and reflections. Ultrasonography. 2016;35:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bomeli SR, LeBeau SO, Ferris RL. Evaluation of a thyroid nodule. Otolaryngol Clin North Am. 2010;43:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raparia K, Min SK, Mody DR, Anton R, Amrikachi M. Clinical outcomes for “suspicious” category in thyroid fine-needle aspiration biopsy: patient’s sex and nodule size are possible predictors of malignancy. Arch Pathol Lab Med. 2009;133:787–790. [DOI] [PubMed] [Google Scholar]
- 23. Mendelson AA, Tamilia M, Rivera J, et al. Predictors of malignancy in preoperative nondiagnostic biopsies of the thyroid. J Otolaryngol Head Neck Surg. 2009;38:395–400. [PubMed] [Google Scholar]
- 24. Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–3417. [DOI] [PubMed] [Google Scholar]
- 25. Alexopoulou O, Beguin C, Buysschaert M, et al. Predictive factors of thyroid carcinoma in non-toxic multinodular goitre. Acta Clin Belg. 2004;59:84–89. [DOI] [PubMed] [Google Scholar]
- 26. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98:564–570. [DOI] [PubMed] [Google Scholar]
- 27. Ibrahim Y, Mohamed SE, Deniwar A, et al. The impact of thyroid nodule size on the risk of malignancy in follicular neoplasms. Anticancer Res. 2015;35:1635–1639. [PubMed] [Google Scholar]
- 28. Shrestha M, Crothers BA, Burch HB. The impact of thyroid nodule size on the risk of malignancy and accuracy of fine-needle aspiration: a 10-year study from a single institution. Thyroid. 2012;22:1251–1256. [DOI] [PubMed] [Google Scholar]
- 29. Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91:4295–4301. [DOI] [PubMed] [Google Scholar]
- 30. Haymart MR, Repplinger DJ, Leverson GE, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zafon C, Obiols G, Baena JA, Castellví J, Dalama B, Mesa J. Preoperative thyrotropin serum concentrations gradually increase from benign thyroid nodules to papillary thyroid microcarcinomas then to papillary thyroid cancers of larger size. J Thyroid Res. 2012;2012:530721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fiore E, Rago T, Latrofa F, et al. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocrine Relat Cancer. 2011;18:429–437. [DOI] [PubMed] [Google Scholar]
- 33. Fiore E, Vitti P. Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab. 2012;97:1134–1145. [DOI] [PubMed] [Google Scholar]
- 34. Bongiovanni M, Crippa S, Baloch Z, et al. Comparison of 5-tiered and 6-tiered diagnostic systems for the reporting of thyroid cytopathology: a multi-institutional study. Cancer Cytopathol. 2012;120:117–125. [DOI] [PubMed] [Google Scholar]
- 35. Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748–1751. [DOI] [PubMed] [Google Scholar]
- 36. Chandramohan A, Khurana A, Pushpa BT, et al. Is TIRADS a practical and accurate system for use in daily clinical practice? Indian J Radiol Imaging. 2016;26:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]