Abstract
Disablement affects over 40% of patients with advanced stage cancer, devastates their quality of life (QoL), and increases their healthcare costs. Proactively treating the causes of disablement; physical impairments, pain, and immobility, can prolong functional independence, improve QoL and, potentially, reduce utilization. However rehabilitation service delivery models are reactive in nature and focus on catastrophic rather than incipient disability. A validated collaborative approach, the three component model (TCM), optimizes important clinical outcomes and may provide an ideal framework to overcome barriers to proactively integrating rehabilitation into cancer care. A novel expansion of the TCM that targets disablement by engaging local physical therapists to address physical impairments and immobility, the TCM-Rehabilitation Services (TCM-RS), benefits and is well received by patients. However, its effectiveness has not been rigorously assessed. The 3-arm randomized COllaborative Care to Preserve PErformance in Cancer (COPE) Trial compared: 1) enhanced usual care, 2) rehabilitation services targeting physical impairments and immobility via the TCM-RS, and 3) TCM-RS plus conventional TCM pain management TCM-RS+Pain. Of the 516 participants, those randomized to arms 2 and 3 underwent an initial 4-week intervention period and were then followed for 6 months with remote monitoring and monthly telephone calls. The trial’s primary outcome, functional status, and secondary outcomes were assessed at baseline, 3, and 6 months. Utilization was abstracted from clinical records. By estimating the effectiveness and cost-utility implications of the TCM-RS and TCM-RS+Pain, COPE will inform future delivery research, practice and policy in the means to reduce disablement in chronically diseased populations.
1. Introduction
The COllaborative Care to Preserve PErformance in Cancer (COPE) trial was designed to address a gap in clinical knowledge regarding the potential benefits and costs of proactively delivering rehabilitation services to patients with late stage cancer and serious, progressive hematological conditions. Although functional loss is nearly universal among patients with advanced illness, relatively simple rehabilitation measures known to be effective in preserving patients’ independence are almost never proactively included in their care. Rather, they are typically delivered only after patients have lost so much function that they often require costly and prolonged institutional-level care.1,2 The failure to address this gap is striking since not only is functional decline most easily addressed early in its course, but its neglect is linked to lost autonomy, shorter survival, poorer quality of life (QoL), and increased health care utilization.1–3,4,5
The lack of a proactive approach to the care of patients with advanced disease is not unique to their functional needs and parallels, for example, the historic under-treatment of mood disorders. In fact, the recognition that depression was widely under-diagnosed and poorly managed led to the development of collaborative telecare models that have proven capable of delivering effective guideline concordant treatments for mood disorders and other adverse symptoms such as pain in diverse populations. Until recently this model had not been tested in a cancer population for whom function degradation, mood disorders, and adverse symptoms are uniquely prevalent and problematic.
This situation changed in 2010 with the landmark Indiana Cancer Pain and Depression (INCPAD) trial’s demonstration that the benefits of tele-collaborative care are robust in this population.6 Specifically, INCPAD delivered a collaborative care approach, the Three Component Model (TCM),7–10 which focused on the pharmacological treatment of pain and mood disorders via telecare and found that it significantly improved the patients’ pain and depression relative to usual care in a clinically and cost effective manner.6,11
The INCPAD intervention offers many of the key ingredients needed to ensure the timely, efficient, and effective delivery of functionally-directed rehabilitation services to patients with cancer: patient-centricity, low cost, and broad geographic reach. The latter is particularly critical since the specialists capable of delivering these services are predominantly situated in tertiary/quaternary medical centers where barely 15% of patients with cancer receive their care.12,13 However, INCPAD’s focus on at-a-distance pharmacological management is not directly generalizeable to the in-person interactions that are frequently required for the delivery of rehabilitation services.
The collaborative intervention tested in the COPE trial was designed to retain the desirable attributes of the INCPAD while adapting them to meet the unique exercise and physical activity requirements of rehabilitation. This adaption was accomplished by having physical therapists specialized in cancer rehabilitation serve as fitness care managers and coordinators, in lieu of nurses who were central in INCPAD and, in fact, collaborative care in general. This is illustrated in Figure 1. As pain contributes to disablement and a barrier to effective rehabilitation,14 we incorporated an arm that supplemented COPE’s telerehabilitation efforts with TCM-based pain management program modeled on INCPAD’s efforts.
Figure 1.
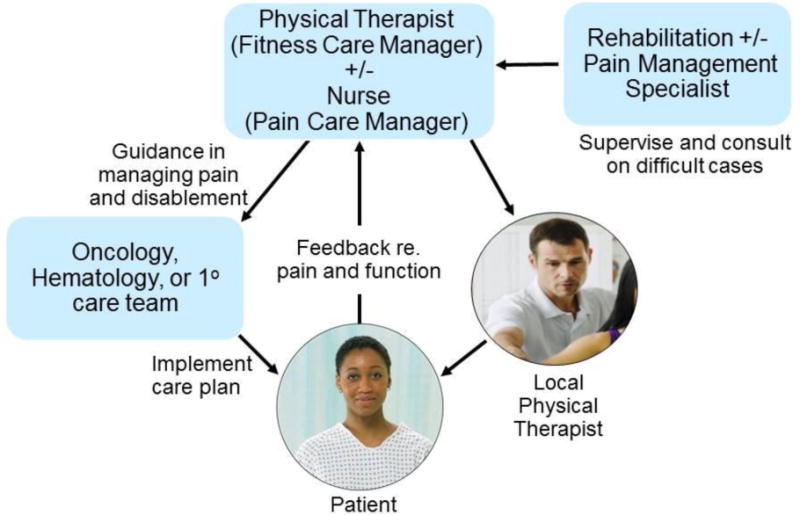
The Three Component Model adapted to include local physical therapists for the COPE trial.
2. Methods
2.1. Design and Overview
This 3-arm randomized, controlled trial was designed to test whether the proactive and coordinated delivery of guideline-endorsed treatments to address the primary mediators of cancer-related disablement, physical impairments, pain, and immobility, could preserve a patient’s function, reduce health care utilization, and improve quality of life (QoL). Enrollment began December 13, 2012 and was completed 38 months later on March 3, 2016.
Participants were randomly assigned to one of three arms as illustrated in the study schema, Figure 2. Arm I served as an active control and involved what was termed “enhanced usual care,” i.e., participants received their usual medical and oncological care with the addition of twice monthly remote telephonic or web-based monitoring. The remaining two arms assessed the COPE interventions. Arm II consisted of the enhanced usual care of Arm I augmented by the tele-delivery of TCM modeled rehabilitation services (i.e., “TCM-RS”). This involved the coordination of rehabilitation services by a physical therapist (PT) Fitness Care Manager (FCM); treatment and instruction in the cancer-validated Rapid Easy Strength Training (REST) program (described in more detail below),15 and provision of a pedometer-based walking First Step Program (FSP)16,17 by both the FCM and a PT who was local to where the participant resided. Arm III was identical to Arm II with the exception that it also included the provision of TCM-based optimization of pain management (termed TCM-RS + Pain). Participants were followed for 6 months.
Figure 2.
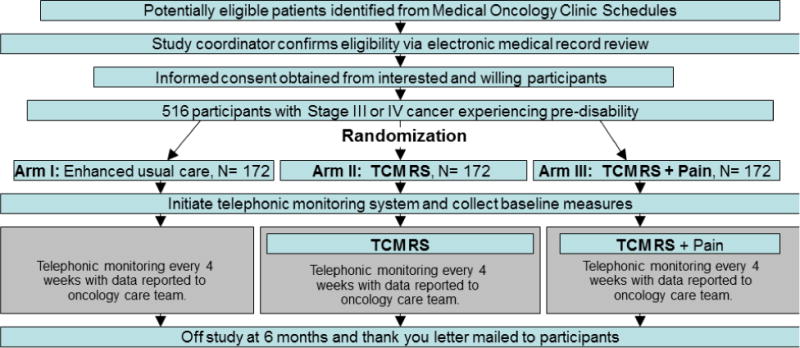
COPE study schema
The intervention schedule is outlined in Table 1. During the 4-week initial phases of COPE Arms II and III, the FCM identified and supervised the management of physical impairments with ≤ 8 sessions of locally-administered PT, and immobility through the REST and pedometer-based step programs. Pain in participants randomized to Arm III was addressed by a nurse Pain Care Manager (PCM) using a pharmacological algorithm validated in the INCPAD trial.6,9 Pain management in Arms I and II was at the discretion of participants’ oncology, hematology, or primary care team, who were provided participants’ e-PRO pain ratings without further therapeutic guidance. In Arm III, the care team also received the patient pain e-PRO pain ratings but, in this case, accompanied with the PCM’s treatment recommendations. As part of the enrollment process, participants identified which care team (primary, hematologic, or oncologic) should receive their electronic-Patient Reported Outcome (e-PRO) ratings based upon overall participant preference.
Table 1.
Schedule of COPE Arm II and III interventions
| Week 1 | Week 2 | Week 3 | Week 4 | Weeks 5-24 | ||
|---|---|---|---|---|---|---|
| TCM-RS | FCM | Initial call to introduce FCM role, FSP, REST, and remote monitoring | Follow-up call | Follow-up call | Monthly follow-up calls | |
| Contact per remote monitoring-triggered alerts | ||||||
| Local PT | Local PT evaluation & treatment | Resume PT as needed | ||||
| TCM-Pain | PCM | Call to introduce PCM role | Contact per remote monitoring-triggered alerts | |||
The structure and incremental nature of the COPE study arms is depicted in Figure 3. All participants underwent twice monthly automated telephonic or web-based assessments, per their preference, to assess their function and pain. Function was assessed with a five item-short form derived from the Activity Measure for Post-Acute Care (AM PAC).18,19 Pain was assessed with the validated 3-item PEG pain scale derived from the Brief Pain Inventory.20 Pain scores from Arm I participants were provided electronically to participants’ oncology, hematology, or primary care teams without collaborative care guidance. Data collected from Arm II participants were provided to the care teams as well as to the FCM who in turn utilized the information to coordinate, optimize, and individualize participants’ PT, REST exercise, and FSP pedometer programs. Data collected from Arm III participants were provided not only to the oncology, hematology, or primary care team and the FCM (as in Arm II) but also to the PCM, who suggested analgesic modifications to participants’ chosen care teams.
Figure 3.
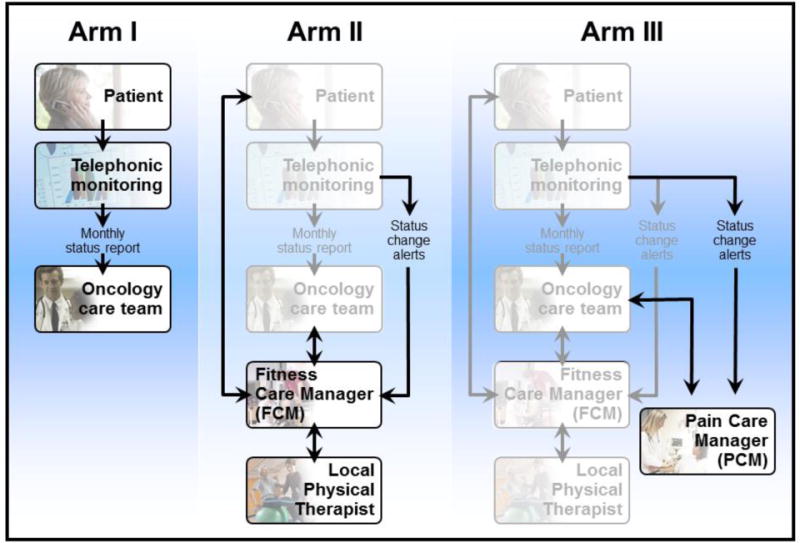
Incremental nature of COPE study arms
The COPE trial utilized two independently operating TCM-based care delivery systems – one addressed physical impairments and immobility (i.e., Arm II’s “TCM+RS”) and the other these functional issues as well as pain (Arm III’s “TCM-RS+Pain”). Figure 3 illustrates the parallel operation of these systems. Separate administration was felt to be essential in order to detect the specific effect of each approach. The basic structure of TCM was expanded for the TCM-RS by adding a local PT who was essential to provide the in-person care required to individualize the REST exercises and FSP in order to address physical impairments and implement precautions, e.g., weight-bearing status for patients with symptomatic pelvic or femoral bone metastases. The four providers of the TCM-RS team were therefore the oncology, hematology, or primary care team; a PT FCM; a supervising cancer-specialized Physical Medicine and Rehabilitation (PM&R) physician; and a local PT. The relationships between these providers and the patient are represented in Figure 1 which also reflects the FCM’s critical role as an intermediary between the patient, their regular care team, the PM&R physician specialist, and the local PT.
The primary roles of the team members were as follows: 1) the FCM identified physical impairments, coordinated care delivery, promoted adherence to the exercise program, and adjusted the program as indicated per ongoing monitoring; 2) the local PT collaborated with the FCM in identifying physical impairments, treating physical impairments, and individualizing the REST and FSP programs, as required to address pre-morbid and cancer-related impairments; 3) the PM&R physician supervised the FCM and provided advice on complex or non-responding cases; and 4) the oncology, hematology, or primary care team assisted the FCM with the implementation of local PT and, in Arm III, pain management. The pain-directed collaborative care of Arm III was effectively identical to the TCM approach utilized in the INCPAD trial.6 TCM-Pain team members included the oncology, hematology or primary care team, a nurse PCM, and a supervising physician pain specialist.
2.2. Participants and recruitment
A total of 516 patients with Stage IIIC or IV solid tumors, multiple myeloma, myelodysplastic syndrome, or Stage IIIC or IV lymphoma were enrolled. All were required to be manifesting pre-disability, defined as an AM PAC Computer Adaptive Test (AM PAC CAT) score of 53-66,21,22 and having at least a 6-month anticipated survival. Patients within 2 months of a major surgical procedure or receiving neoadjuvant chemotherapy in anticipation of cure-oriented surgery were not eligible. Participants were also required to be fluent in the English language, have a Folstein mini-mental score > 25, and auditory acuity sufficient to permit effective information exchange.
Exclusion criteria were developed to avoid the recruitment of patients unlikely to benefit from rehabilitation services and included: a) pain or fatigue numerical rating scale scores > 8/10; b) paralysis of > 2 limbs; c) cerebellar ataxia; d) chronic (> 1 year) non-cancer pain of either >5/10 severity or resulting in the patient applying for/receiving disability payments; e) severe medical or psychiatric co-morbidities such as major depression or unstable angina that could impede the rehabilitation process or produce symptoms not amenable to cancer-related guidelines were also the basis for exclusion. Patients who endorsed anhedonia underwent screening with the Patient Health Questionnaire (PHQ-9); those with depression of moderate or worse severity (PHQ-9 >10) were ineligible.23
Potentially eligible patients who had attended at least 2 medical oncology or hematology appointments at a Mayo Clinic Rochester, MN; Scottsdale, AZ; or Jacksonville, FL location within the previous 6-months were identified using an electronic health record (EHR) search tool. Queries using ICD-9 and -10 codes, text words, and appointment and provider type were constructed and run at 3-month intervals to generate new lists of potential participants. The electronic health records (EHRs) of identified patients (who per Mayo regulations had indicated willingness for their records to be used for research) were manually reviewed to ensure accuracy of cancer and hematological diagnoses and stages. Uncertainty regarding diagnoses or prognoses was resolved through discussions with a patient’s oncology or hematology provider.
Eligible subjects were mailed an opt-out post-card with a toll free call-in number, a professionally designed brochure describing the trial, and consent/HIPPA forms. Patients who mailed in signed consents were contacted within 3 business days by the trial’s study coordinator. The study coordinator also contacted potentially eligible patients who had not responded within 2 weeks to inquire about their interest in participating in the trial. Receptive patients were screened for eligibility. Eligible patients were telephonically enrolled once signed, printed consent and HIPPA forms had been received. Candidates who were ineligible due to AM PAC CAT scores >66 were asked if they were willing to be re-contacted in 6 months to reassess their eligibility.
2.3. Randomization
The study coordinator registered participants on the password protected Mayo Clinic Cancer Center remote registration/randomization application. A dynamic allocation procedure balanced the marginal distributions of the stratification factors (cancer type, presence of an in-home caregiver, and gender, and baseline AM PAC CAT score and pain ratings) as part of the patient study arm randomization procedures.24 Rationales for these choices included the fact that: 1) cancer type impacts rate of progression and symptom burden;25 2) the availability of an in-home caregiver reduces the risk of hospitalization and institutionalization;26 3) the willingness to report well-being differs across genders;27 and 4) baseline functional status and pain correlate with subsequent disablement.28–30
2.4. Impairment- and Immobility-directed Intervention
The sequence of the COPE intervention’s initial (weeks 1-4) care delivery period and subsequent monitoring phase (weeks 5 – 24) is outlined in Table 1. The Arm II intervention was initiated, coordinated and monitored by the FCM. The FCM contacted the patient and conducted a scripted telephonic evaluation during the first week following randomization. The call consisted of screening for physical impairments, identification of a convenient local rehabilitation facility, development of a PT prescription, and the scheduling of the participants’ initial PT visit. In addition, the FCMs helped participants to formulate individual goals and to identify potential barriers to enhancing their physical activity. The FCMs also introduced REST exercises and FSP pedometer program. The FCMs made efforts to emphasize the linkage between a participant’s achieving their goals and their regular performance of REST and adherence to their FSP targets. FCMs used motivational interviewing (MI) techniques learned prior to the initiation of the study during two 1-hour sessions with an MI-certified psychologist. Standardized PT prescriptions were signed and faxed to the local PT following review by the oncology, hematology, or primary care team.
The FCMs identified PTs situated near a participant’s home with experience in cancer rehabilitation, ascertained through review of the American Physical Therapy Association’s website, as well as other web-based resources.31 FCMs contacted the local PTs to explain the COPE trial, introduce REST, and answer any questions they may have had prior to a participant’s initial PT session. The COPE PT prescription included individualization of the REST exercises and FSP, as well as the evaluation and management of any impairments identified by the FCM during EHR review and/or their initial telephone contact with a participant, as described below. The FCMs also alerted the local PTs of the relevant details of participants’ cancer and cancer treatment, and precautions related to the presence of bone or brain metastases, as well as other premorbid or treatment-related impairments. The local PTs were provided access to a website with these materials and additional REST instructions.
The local PT evaluation included a comprehensive function-oriented physical examination to confirm the presence of physical impairments identified by the FCM, and to screen for additional disabling flexibility, sensory, motor or coordination deficits. In addition, local PTs instructed participants in the REST exercises as adapted to accommodate and address their cancer-related and premorbid impairments. A maximum of 8 sessions, depending on the patient’s needs, were permitted. If a local PT believed that more care was needed, their requests to continue treatment beyond 8 sessions or to treat additional impairments were reviewed on a case by case basis through a consensus process that included both FCM and the PM&R physician.
The monitoring phase began in week 5 (i.e., after Arms II and III had completed their 4-week period of training) and extended for an additional 22 weeks. If a participant’s logging of their REST exercises or step counts fell below the recommended weekly frequency, or if they performed fewer than the recommended 4 REST sessions (2 upper and 2 lower extremity) per week for two consecutive weeks, they were contacted by the FCM. Additionally, if a participant’s AM PAC score dropped by >1.5 point (the AM PAC CAT’s minimally important difference (MID)),14 relative to their 4th week score, email alerts were automatically sent to the FCM. The FCM contacted the participant by phone to identify potential medical problems that might indicate the need for evaluation by their oncologist or hematologist and new or progressive physical impairments. If the FCM believed that further local PT treatments were indicated, they obtained the approval of the PM&R physician prior to generating a PT prescription. The FCM responded to all participant-generated requests for contact submitted via the remote monitoring system throughout the 6 month duration of the TCM-RS intervention. In addition 1) the FCM met weekly with the PM&R physician to review complex and non-responding cases; 2) the PM&R physician was available to the FCM at all times to answer questions. A similar approach was used in Arm III, but in this case the PCM forwarded an optimized analgesic regimen to the patient’s primary care, hematology, or oncology team in lieu of the mere relaying of pain data to the team that occurred in Arms I and II.
2.5. Intervention components to address impairments and immobility
2.5.1. Education
The FCM educated participants in the following: 1) the role of exercise/physical activity in symptom management, 2) the benefits of REST, 3) the consequences of cancer- and cancer-treatment-related loss of muscle bulk and power, and 4) the implications of adverse symptoms experienced during physical activity/exercise.
2.5.2. Rapid Easy Strength Training (REST)
Rapid Easy Strength Training (REST) was included to address participants’ immobility-related loss of lean muscle mass and has been shown to improve functional status and reduce adverse symptoms in 3 RCTs of late stage cancer.15,32,33 The program includes an upper and a lower body routine, each of which consists of five exercises performed with resistive elastic bands. The REST routines can be performed by patients with mild disabilities in <10 minutes and are grounded in established techniques known to enhance the muscle quality and power of frail individuals.34,35 Local PTs adapted the routines to accommodate participant fitness levels, impairments, and cancer-related precautions. Participants were instructed to perform both routines, upper and lower extremity, twice per week. The FCM assisted participants in increasing REST intensity following a schedule that was based on strength training experience for cancer-related fatigue.34,35 In general, participants began REST with 1 set of 12 repetitions for each exercise using the level of resistance recommended by the local PT with the goal of gradually increasing to 15 repetitions before advancing to an exercise band that offered greater resistance. Participants logged their REST sessions in their print or web-based COPE intervention manuals. Participants also received the REST DVD and the COPE intervention manual which included detailed exercise instructions.
2.5.3. Pedometer-based step counting
The First Step Program (FSP) is a validated pedometer-facilitated, walking regimen that is designed to increase the participant’s activity levels and address their immobility-related loss of aerobic conditioning. The approach relies on the theoretical principles of self-efficacy and social support and uses the common clinical practices of goal-setting, self-monitoring and feedback.16,17 In the FSP, pedometers were used to establish baseline level of physical activity, show participants how many steps they normally take in a given amount of time, and facilitate their personal goal-setting, self-monitoring and feedback. Participants logged their step counts in their COPE intervention manuals and reported them via the IVR or web remote monitoring systems. Pedometers use correlates well (r’s =0.80-0.93) with more expensive accelerometers in controlled and field conditions.36,37
2.5.4. Treatment of physical impairments
If FCMs identified physical impairments during their telephone-based evaluation, they expanded the standardized COPE PT prescription, which outlined the REST exercises and FSP, with treatment specifics based on standardized, guideline-congruent PT treatment plans for the 20 most common physical impairments detected in late stage cancer patients as developed by an expert panel of cancer rehabilitation specialists under the auspices of the American Physical Therapy Association.31 Common elements of these plans included impairment-specific: 1) therapeutic exercise to address flexibility, strength, balance, and coordination deficits; 2) physical modalities; and 3) compensatory strategies for mobility and the performance of activities of daily living (ADLs). As per conventional practice, and in accordance with established reimbursement criteria, the local PTs developed treatment plans and completed session summaries after all visits. The FCMs obtained and reviewed these documents to ensure that they were consistent with participants’ original prescriptions, the REST framework, and care standards. Concerns regarding the appropriateness of treatment were reviewed by both the FCM and the PM&R physician specialist.
2.6 Pain-directed Intervention
Previous work suggested that ≥80% of the cohort would experience activity-limiting pain.1 Pain management in the trial’s Arm III mirrored that of the telecare approach validated in the INCPAD trial.6 More specifically, telephone-based pain management was delivered by a nurse PCM trained in assessing treatment responses with standardized pain scales; evaluating medication adherence; providing brief pain-specific patient education; and in forwarding adjustments according to evidence-based cancer pain treatment guidelines to the participant’s care team.9,38 The PCM contacted Arm III participants, see Table 1, during week 1 to describe their role and to determine whether pain was an impediment to their performance of REST or the FSP. The PCM met weekly with the physician pain specialist to review cases.
The PCM assessed a participant’s current and previously trialed pain treatments, with a focus on activity-related incident pain, to determine whether inadequate dosage, scheduling, or adherence had been a problem. If pain was either perceived as problematic or rated ≥5/10, the PCM recommended upward titration of the current analgesic with appropriate dosing and scheduling, or trial of a novel analgesic. A pain intensity threshold of 5/10 for initial intervention was selected since pain of this and worse intensity has been shown to significantly interfere with patients’ functionality.39–41
Changes in pain medication dosage/choice were made in accordance with the National Comprehensive Cancer Network Cancer Pain Clinical Practice Guidelines as adapted for INCPAD.9,42,6 The PCM was thoroughly trained in this guideline, and during the COPE trial start-up and early enrollment phases consultation with the physician pain specialist occurred for all Arm III participants.
Arm II participants who reported increasing levels of pain or exercise-associated pain to their FCM were referred to their oncology, hematology, or primary care team. No analgesic recommendations were provided to Arm II participants as part of the COPE Trial intervention, with the sole exception being recommendations to seek care from pain specialists for refractory or complex pain that was unresponsive to first line therapies.
2.7. Assuring Fidelity of the COPE Arms II and III
The PM&R physician (AC) 1) trained the FCMs extensively prior to starting the study; 2) monitored the first 20 calls and reviewed the first 20 prescriptions with the FCMs; and 3) discussed cases weekly with the FCMs to assure that their scripted telephone screening for physical impairments and PT prescriptions were appropriate and reflected current best practices. In addition, a second cancer PM&R physician audited 3 participants’ intervention records per month for the first 2 years of the trial and provided feedback to the FCMs and Dr. Cheville.
Measures to assure the fidelity of REST instruction by local PTs included: 1) provision of REST specifics on a website; 2) telephone calls or email exchange from the FCMs to the PTs to discuss the REST and FSP programs prior to a participants’ initial visit; 3) ongoing availability of the FCM to answer questions, and 4) availability of the REST DVD content and exercise instruction on a website. The FCM and the PM&R physician reviewed PT treatment plans and session summaries in their weekly conferences.
Procedures to assure PCM fidelity to the NCCN guidelines included: 1) extensive training in the pain guidelines by the INCPAD trialist (KK) prior to starting the study; 2) monitoring of 20 PCM calls by Dr. Kroenke with feedback; 3) weekly pain case management conferences with the physician pain specialist (TM) during which subjects experiencing pain or inadequately responding to treatment were reviewed; and 4) completion of a PCM checklist for each telephone contact. A randomly selected 10% sample of all checklists was audited over the 3.5 years of the Arm III COPE intervention delivery.
2.8. Enhanced Usual Care Arm
Participants randomized to enhanced usual care underwent baseline and monthly telephonic or web-based assessments of their pain and function with these data being provided electronically to their oncology, hematology, or primary care teams. No attempts were made to influence their behavior. Some care teams had COPE Arm II and III, as well as enhanced usual care participants (Arm I) in their practices. However, numerous primary care effectiveness trials have shown that there is little spillover of the intervention to usual care patients in the absence of a collaborative care model.6,43–45 As a result, contamination was not considered a major concern particularly as any spillover that occurred would result in a more conservative estimate of intervention effectiveness.
2.9. Automated Symptom and Function Monitoring and Telephonic Care Management
All participants utilized an automated monitoring system created and maintained by Interactive Performance Technologies® (IPT) that allowed self-reporting via either a telephone-based interactive voice response (IVR) functionality or a web-based interface. The system collected pain and function patient-reported outcomes (PROs) monthly from all participants who chose a convenient day and time to receive their scheduled calls or email-based reminders. Those not completing a scheduled assessment received an automated call/email reminding them to initiate a call to complete the PROs. Participants also had the capability to initiate toll-free calls or web entry in order to provide PRO information between scheduled contacts and/or request a call back.
Arm II and III participants also used the IPT system to log their REST sessions and step counts on a weekly basis. This capability was not available to Arm I participants. The system concluded a logging session by providing automated “pop-up” messages that offered randomly varying performance-matched encouragement and congratulations. Participants who exceeded the recommended number of REST sessions received “pop-up” messages advising them to attend to any exercise-associated discomfort and contact their FCM for guidance in advancing their program. Participants were queried after recording their step counts if they wanted to advance their FSP goal, with a 10% increase being suggested as a target. The system also allowed Arm II and III participants to leave a voice or text message for their FCM, or to request a call back.
The IPT system also allowed the FCM and PCM to track participant interactions and to view the trend of a participant’s self-reports over time in either a graphical or tabular format. Criteria-based alerts triggered by predefined events and scores with notifications were automatically emailed to the FCMs or PCM. Specifically, an AM PAC score drop of ≥1.5 points, established as the MID by our prior work,14 by an Arm II or III participant triggered an FCM telephone call. REST and/or the FSP were modified, and/or additional local PT sessions were prescribed contingent on the FCM’s assessment of the participant’s situation. Pain intensity rating of > 5, in participants randomized to Arm III, triggered a PCM telephone call for assessment and potential adjustment of the participant’s analgesic regimen forwarded to their primary, oncologic, or hematologic care team as indicated.
2.10. Data Collection and Outcomes
Data sources included participants’ EHRs; the IPT automated monitoring system; telephonically administered baseline, 3- and 6-month PROs; participant-reported care utilization at 3- and 6-months; medical records describing hospitalizations and Emergency Department visits at Mayo and non-Mayo institutions; and their local therapist PT summaries. The following information was abstracted from participants’ Mayo Clinic EHRs: demographics and education; medical comorbidities, and disabilities; and cancer histology, staging, anatomical location, and treatment. Staging was based on the seventh edition of the TNM classification of Malignant Tumors, introduced in 2009.46 Data were abstracted to calculate a baseline Charlson Index.47
2.10.1. Patient-Reported Outcomes (PRO) and health information
Table 2 outlines the PRO collection schedule. Primary and secondary research outcomes were collected telephonically at 3 and 6 months, independent of the IPT reporting system, by an interviewer from the Mayo Clinic Survey Research Center (MCSRC) who was blinded to study arm. Baseline outcomes were collected by the COPE trial study coordinator. The outcomes included 15 items from the AM PAC Basic Mobility item bank administered via a computer adaptive testing (CAT) platform (primary outcome) as well as the secondary variables of the Euroqol 5-Dimensions (EQ-5D), the FACIT fatigue questionnaire,48,49 the Godin Leisure-Time Exercise questionnaire (GLTEQ),50,51 the Linear Analogue Self-Assessment (LASA) Scale for QOL,52 and the Care Utilization Report (CUR). A total of 5 attempts were made to contact non-responding participants. Participants were compensated $25 for completing the 3- and 6-month telephone interviews.
Table 2.
Schedule of COPE trial patient reported outcome administration
| Outcome | Domain | Measure | Items | Baseline | Month 3 | Month 6 |
|---|---|---|---|---|---|---|
| 1° | Function | Activity Measure for Post Acute Care (AM-PAC), Basic Mobility | ≤ 15 | ✓ | ✓ | ✓ |
| 2° | Pain | Brief Pain Inventory | 11 | ✓ | ✓ | ✓ |
| 2° | Health utility | EQ-5D | 5 | ✓ | ✓ | ✓ |
| 2° | Quality of life | Linear analogue self-assessment | 1 | ✓ | ✓ | ✓ |
| 2° | Fatigue | Facit Fatigue | 13 | ✓ | ✓ | ✓ |
| 2° | Utilization | Care Utilization Report | 3 | ✓ | ✓ | |
| Moderator | Exercise behaviors | Stanford Patient Education Research Center Questionnaire | 6 | ✓ | ||
| Moderator | Receptivity to change | The Stages of Change Questionnaire | 4 | ✓ |
PROs used to direct the clinical intervention– pain, function, and REST/FSP adherence data– were collected via the IPT telephone IVR/web-based self-reporting system as outlined above. These latter PROs were the basis for monitoring the Arm II and III participants’ adherence to the REST and FSP programs, as well as for triggering automated alerts to the FCMs and PCM. Data collected by the IPT system included the 5-item AM PAC short form, pain scores, and the weekly logging of the subjects pedometer step counts and REST sessions. This data collection approach has been used successfully in several previous studies, including the INCPAD trial.6
2.10.2. Specific PRO Measures
AM PAC Basic Mobility (BM)
The AM PAC BM measure is currently the only function-oriented computer adaptive test (CAT) that has both undergone extensive validation and has been shown to discriminate across a broad range of functional abilities in patients with late stage cancer.18, 14,53 The basic mobility domain was established through Rasch, confirmatory, factor, and modified parallel analysis.18,54 The AM PAC BM is responsive to functional status changes in patients with late stage cancer with a MID of ~1.5.14
PEG
The PEG is a 3-item version of the Brief Pain Inventory (BPI) that has proven to be as sensitive to change in treatment trials as the BPI itself and other longer pain measures.20,55 The BPI items included in the PEG are: average pain intensity (P), interference with enjoyment of life (E), and interference with general activity (G). Brief Pain Inventory Short Form (BPI-SF). The BPI-SF scale normally has 13-items, however the 2 items (degree of relief from pain treatments and time to relief) that are not utilized to compute patients’ global pain scores were eliminated to reduce respondent burden.56 The BPI scale is a multidimensional measurement tool with demonstrated reliability in patients with cancer as well as other pain conditions57.
FACIT fatigue
The FACIT fatigue is a 13-item questionnaire that assesses self-reported tiredness, weakness, and difficulty conducting usual activities due to fatigue. It has been validated and found to be responsive in diverse cancer populations.58
Euroqol -5D (EQ-5D)
The EQ-5D is a widely used, well-validated instrument to assess utilities.59 EQ-5D scoring was based on the U.S. weighting system developed by Shaw et al.60
Linear Analogue Self-Assessment (LASA) Scale for QOL
Single item linear analogue QOL assessments have been utilized extensively in previous oncology trials.52 LASAs were rated on a scale from 0 (as bad as it can be) to 10 (as good as it can be). All scores were subsequently converted to a 0-100 point scale for comparability.
Care Utilization Report (CUR)
The CUR was used to record care utilization during the prior 3 months and involved the use of direct requests, when appropriate, for a participant’s outside medical records including: hospitalizations and rehabilitation/skilled nursing facility institutionalizations, as well as visits to the emergency room, primary physician, oncology care provider, and pain/symptom management specialists. CURs have been successfully used in wide ranging populations including patients with cancer.61
Stanford Exercise Behaviors Questionnaire
This six-item questionnaire queries respondents about the amount of time they spend engaging in specific types of physical activity, e.g., stretching, bicycling, and offers 5 ordinal response options ranging from none to more than 3 hours per week. The questionnaire has been shown to be valid in diverse clinical populations including patients with cancer.62
Stages of change (SoC) questionnaire
The SoC for exercise were assessed using the physical activity staging questionnaire described by Nigg et al.63 Four questions that offer binary response options classify individuals according to their intention to be physically fit and their actual participation in in physical activity. The approach has been widely utilized in cancer populations.64
2.10.3. Validation of health information
Information obtained from the CURs was validated by collecting and reviewing health records to corroborate all emergency department visits, hospitalizations, and office visits to primary and specialty providers. Under reporting of health care utilization was estimated by ascertaining whether the 52 residents of Olmsted County, MN, who participated in the COPE trial omitted any episodes of care from their CURs. Olmsted residents receive all their care at either Olmsted Medical Center or the Mayo Clinic which allowed for the identification of visits and hospitalizations missing from the CURs.
2.11. Statistical Analyses
2.11.1. Sample size
The sample size was estimated using the subset of a standing cohort of Stage III and IV lung cancer patients that had AM PAC CAT Basic Mobility scores between 53 and 66, at least 6 months of follow-up, and at least 3 measurements in that six month time period. A mixed effects model fit to this data gave an estimated standard deviation for the key regression coefficient of where n is the number of subjects. Formative work by our group estimated the minimal clinically important difference (MCID) of the AM PAC CAT, among patients with advanced stage cancer, to be between 1 and 2.14 Table 3 outlines the sample sizes needed to detect varying effects at alpha=.05 and power=.8. Given the need to increase the sample substantially to allow for drop out, we selected an effect of 1.85. To detect an inter-group difference of 1.85 for a one-sided test, the number per group would be (q(.8) + q(.95))^2 (7.17/1.75)^2 = 93 participants per group, where q(x) is the quantile function for a normal distribution. For a 2-sided test the sample size is (q(.8) + q(.975))^2 (7.17/2)^2 = 118 per group. If we add further adjustment for 2 tests (Arm 1 versus Arm 2 and Arm 1 versus Arm 3) using the Bonferroni method this gives an alpha of 1/120 and leads to a sample size of (q(.8) + q(119/120))^2(7.17/2)^2 = 143 subjects per group. The target sample was by 20% to 172 to allow for death and drop out given the ill and disabled nature of the study sample.
Table 3.
Sample sizes needed to detect varying effects at alpha=.05 and power=.8
| Effect | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha | 1.5 | 1.55 | 1.6 | 1.65 | 1.7 | 1.75 | 1.8 | 1.85 | 1.9 | 1.95 | 2 |
| .1 | 141 | 132 | 124 | 117 | 110 | 104 | 98 | 93 | 88 | 84 | 79 |
| .05 | 179 | 168 | 158 | 148 | 140 | 132 | 125 | 118 | 112 | 106 | 101 |
| .05/2 | 217 | 203 | 191 | 179 | 169 | 160 | 151 | 143 | 135 | 129 | 122 |
2.11.2. Analytic Plan
All primary analyses will be intention-to-treat and will analyze each subject regardless of their adherence to recommended intervention activities. The primary outcome will be based on difference in AM PAC CAT scores between enrollment and month 6 between the groups. We will apply standard diagnostic techniques to assess model adequacy.65 A key assumption for regression modeling is the absence of outliers in either the response or predictors. The primary predictors, e.g., treatment arm, age, are known to be well behaved a priori, the primary and secondary outcome variables may, however, have a highly skewed distribution due to subjects with a large deficit at end of life. We will tabulate the presence of such values, by treatment arm, and then repeat the analyses using rank(y) as the response to verify that tests or estimates are not adversely affected. Given the sample size it will require a significant number and/or imbalance of extreme values to have a major impact.
Missing data due to dropout and/or death will be addressed by comparing participants across the study arms with missing and observed outcomes with respect to baseline characteristics in order to gauge the missing data mechanism (e.g., missing at random vs potentially significant). We will utilize sophisticated sensitivity analyses66,67 to determine the robustness of study results and conclusions. For example, in addition to multiple imputation, we will assume that outcomes among participants with missing data were among the lowest decile, quartile, etc. We will use modern techniques for multiple imputation to generate predicted values for missing outcomes and incorporate them into the primary analyses, presenting these results with those of the analyses using ranks and raw data. If assessments of missingness do not indicate a non-random mechanism, and if the degree of missingness is less than 15% and equally distributed across all study arms, then analyses using the raw data will be our primary analysis.
Secondary outcomes including PEG, GLTEQ, FACIT Fatigue, LASA, and EQ-5D, will be analyzed in a manner similar to that of the primary analysis. We will use adherence variables (logged pedometer step counts and REST sessions) by time interactions to assess whether change in AM PAC CAT differs based on participants’ ”dose” of TCM-RS components to identify potential mediators of TCM-RS benefits. In addition, we will examine moderator effects to assess whether participant demographic (age, gender, pre-enrollment exercise patterns) and clinical (cancer/hematological condition type and stage, presence of metastases, comorbidities) were associated with intervention response as measured with the primary and secondary outcomes.
To assess differential health care utilization between the study arms, counts of emergency department visits, hospital days, and admissions to post-acute care facilities will be included as dependent variables in Poisson, zero-inflated Poisson, or negative binomial models depending on the frequency of service utilization. All analyses will be performed using SAS version 9.2 (SAS Institute, Cary, North Carolina)
A societal perspective will be adopted for the cost-utility analysis. The net cost of the interventions includes the cost of 1) developing and maintaining the IPT monitoring system, 2) local PT sessions, 3) REST-related supplies, 4) the FCM’s and PCM’s time in monitoring and responding to patient symptoms, 5) the PM&R and pain management physicians’ time, less any medical care cost savings derived from the effectiveness of the intervention. The potential cost savings from reduced medical care will be determined by difference in utilization as reflected in clinical records. Utilization outside the Mayo Clinic Health System will be monitored via the CURs, and billing records obtained with costs estimated using Medicare unit prices.
3. Results
3.1. Participants
Of the 516 participants enrolled, 41 (7.9%) died while on study, 31 (6.0%) dropped out, and 3 (0.6%) were lost to follow up. Table 4 lists the characteristics of the total study cohort. Patient characteristics were similar among the 3 arms.
Table 4.
COPE trial participants’ demographic and clinical characteristics
| Total (N=516) |
|
|---|---|
| Age | |
| Mean (SD) | 65.6 (11.1) |
| Gender | |
| Female | 257 (49.8%) |
| Marital Status | |
| Married | 404 (78.3%) |
| Widowed | 33 (6.4%) |
| Divorced | 35 (6.8%) |
| Single | 37 (7.2%) |
| Separated | 1 (0.2%) |
| Partnered | 6 (1.2%) |
| Non-clinical caregiver | |
| In home - w/o disability | 511 (99.0%) |
| In home - disabled | 4 (0.8%) |
| None | 1 (0.2%) |
| Race | |
| White | 492 (95.3%) |
| Black or African American | 5 (1.0%) |
| Native Hawaiian or Other Pacific Islander | 1 (0.2%) |
| Asian | 2 (0.4%) |
| Not reported: patient refused or not available | 11 (2.1%) |
| Unknown: Patient unsure | 5 (1.0%) |
| COPD or Asthma | |
| Yes | 11 (2.1%) |
| Bone metastasis | |
| Missing | 1 |
| Yes | 264 (51.3%) |
| Coronary Artery Disease | |
| Yes | 64 (12.4%) |
| Chronic LBP | |
| Yes | 107 (20.7%) |
| Inflammatory arthopathy or autoimmune conditions | |
| Yes | 67 (13.0%) |
| Joint Replacement | |
| Missing | 1 |
| Yes | 53 (10.3%) |
| Chemotherapy-induced neuropathy | |
| Yes | 112 (21.7%) |
| Osteoarthritis | |
| Yes | 44 (8.5%) |
| Radiculopathy cervical or lumbar | |
| Yes | 23 (4.5%) |
| Spinal stenosis | |
| Yes | 17 (3.3%) |
3.2. Usability of the IPT IVR and web-based self-reporting system
At least one IPT survey was completed by 163 (95%), 160 (93%), and 160 (93%) members of Arms I, II, and III, respectively. In all, 5001 surveys were completed over the course of the COPE trial. The total number of surveys and the proportion of web-based (versus IVR) surveys for Arms I, II and III were 1648 (66%), 1721 (74%), and 1632 (69%), respectively. More participants from all study arms who completed IVR surveys did so in an “outbound” manner such that the IPT system initiated the scheduled call. Among Arm I participants, 321 of 554 (60%) IVR calls were “outbound,” while among Arm II and III participants, respectively, the proportion of outbound calls were 218 of 454 (48%) and 345 of 504 (68%). Over the course of the study 579 calls were triggered by the IPT system from the FCMs (437) and the PCM (142) for non-adherence or concerning changes in function and/or pain.
Although not formally recorded, a majority of participants reported little to no difficulty in inputting their information. A majority (83%) required minimal direction in how to use either the website or IVR system. Among the quarter of participants less versed in IT, half required a few sessions with either the study coordinator or their FCM to master data entry. Over the course of the study 579 calls were triggered from the FCMs (437) and the PCM 142 for non-adherence or concerning changes in function and/or pain.
3.3. Intervention adaptations
The original design was to have Arms II and III function entirely separate from one another, however this proved logistically infeasible. However, declines in function and increases in pain were highly correlated which resulted in Arm III participants often receiving duplicate calls from the FCMs and PCM for episodes of worsening pain and declining mobility. The participants found these redundant and burdensome. Therefore, based on qualitative data, the decision was made within the first two months of COPE intervention delivery to integrate the FCM- and PCM-based interventions for Arm III. This involved creating shared dashboards for these participants, and developing a system by which the FCMs and PCM coordinated efforts to respond to alerts. If the change in pain was more concerning then the PCM responded. Conversely, if the decline in function was larger, then the FCM responded. Communication via the IPT dashboard, email, telephone calls, and in-person discussions occurred regarding Arm III participants between the FCMs and PCM, with mode depending on the degree of change and acuity of need. Thus, rather than the clean separation between Arms II and Arm III suggested by Figure 2, a hybrid approach that involved extensive coordination characterized the Arm III intervention.
3.4. Tolerability of the COPE intervention and adverse events
A noteworthy finding in Arms II and III was the resistance of many patients regarding the need to attend at least one local PT session. Many participants expressed a strong preference to simply perform the REST exercises independently. Ultimately 30.2% (N=104) of Arms II and III participants did not attend a local PT session. Of note, anecdotally, virtually all patients who attended local PT sessions reported that the sessions were useful and most led to modifications of their REST program.
All Arm II and III participants were, with the assistance of the FCMs and the REST DVD, able to mimic the REST exercises independently, prior to their first local PT session; the FCMs needed to modify the programs for 65% of the participants due to the presence of known bone metastases or premorbid musculoskeletal conditions. Pain and fatigue were the most common barriers to REST performance. The FCMs anecdotally noted that most participants preferred the FSP component of the trial to REST, and that if a participant’s adherence waned it was overwhelmingly in REST performance rather than in the FSP. Over the course of the study, 22% of Arm II and III patients either temporarily or permanently stopped their exercise programs due to medical reasons.
Hospitalizations, as well as the initiation of aggressive cancer treatment regimens, often caused setbacks for Arm II and III participants in terms of their ability and willingness to perform REST and, were generally associated with reductions in their step counts. The trial protocol did not specify a structured reduction in exercise intensity or frequency in the event of increased medical morbidity or treatment toxicity. Rather, FCMs helped participants negotiate their disappointment at their loss of fitness; adapted REST and FSP to accommodate reduced strength and balance; and used motivational interviewing to help participants to reframe their goals and re-engage in their programs. The FCMs frequently received unsolicited feedback from participants expressing their appreciation for their support and patience. Many participants, in fact, were disappointed if their FCM was unable to contact them as scheduled.
3.5. Pain-directed treatment
All COPE trial participants, including those in the pain intervention Arm III, reported less intense pain on average than the cohort of patients with Stage III and IV lung cancer whose data were used to power the trial. Average baseline pain was 2.4 in all study arms, with rating ranging from 0 to 8 in Arms I and II, and from 0 to 9 in Arm III. Over the course of the trial the PCM made 235 calls to Arm III participants for pain-related issues, not including the initial introductory call to explain the PCM role. Because an interface between the FCM and PCM was permitted for Arm III participants, pain management recommendations were more function-targeted than they might be in other situations.
3.6. Interaction with oncology, hematology, and primary care teams
Non-protocol, spontaneous interactions between the trial team and the participants’ oncologic, hematologic, and primary care teams were minimal. However, participants’ frequently expressed reassurance regarding the fact that their care teams were aware and involved. Roughly 10%, per FCM estimation, of Arm II and Arm III participants expressed a strong preference that their oncology or hematology care team be included in decisions regarding the initiation and upward titration of their exercise programs.
On only two occasions were participants advised by their oncology care teams not to perform the REST exercises and FSP. These recommendations were generally precipitated by a participant’s development of new bone metastases or unexplained pain. On one occasion, a participant was advised by their medical oncologist that exercise was not necessary. The responses of oncology/hematology/primary care teams to information from the IPT self-monitoring system regarding participants’ pain and function varied. Most did not respond at all. On 2 occasions, teams expressed annoyance at the information, generally via email, and requested that it not be sent. A few, roughly 5%, expressed appreciation and reported that they found the information to be clinically useful.
4. Discussion
The COPE collaborative care trial is the first to focus on maintaining physical function in patients with advanced stage cancer and hematological conditions. It is also unique in its addition of a face-to-face component (i.e., local PTs) to the TCM, and to engage specialist, tertiary-center based PTs in lieu of nurses as the nexus for care coordination. In addition, the trial’s design was spurred by several recent findings relevant to its interpretation. First, a majority of patients with cancer and hematological conditions prefer to exercise at home without the need for additional visits to health care facilities/providers.68–72 Although some published reports of this preference for home-based exercise preceded our trial, an increasing frequency since the trial’s start make the decision to emphasize remote web- and telephone-interfaces an important and patient-centric dimension of the COPE intervention. Second, provision of PRO scores directly to clinicians in the absence of collaborative care support appears to result in little to no impact on their care.73–75 Third, the lack of impact of depression screening and the provision of functional information to clinicians at the point of care documented in other studies,76 served as a justification for use of our trial’s “reporting only” Arm I as the control condition.
4.1. Unexpected Findings and Barriers
The ambivalence of roughly half the Arm II and III participants regarding attending at least one out patient PT session was striking and, with hindsight, perhaps not unexpected. The trial requirement that participants’ insurance cover the cost of outpatient PT along with any necessary co-payments proved to be a disincentive. For this reason, after two participants refused PT due to co-payments, the decision was made that the COPE trial would cover all Arm II and III participants’ co-payments. While this measure was needed to optimize intervention integrity, it constrains the external validity of our findings. Ultimately, 30% of Arm II and III participants did not attend PT. Consistent with reports,68 participants cited inconvenience, a perception that they “already knew how to exercise,” and the competing demands and expense of other medical care.
Techniques to encourage participants to attend PT were moderately successful. These included the FCMs noting that other participants had found the PT sessions beneficial and that their attendance would enable participants to individualize their exercises to ensure that they performed them safely and received the greatest return on their investment of time and effort. Many participants, even those who initially expressed strong ambivalence, ultimately valued their PT experiences and requested to continue beyond the study’s 8 sessions.
The reluctance of participants to attend PT contrasted starkly with their willingness to engage with the FCMs by telephone, particularly once a relationship had been established, as well as their enthusiasm despite high levels of medical morbidity for the FSP and, to a lesser extent, the REST exercises.
Our efforts to communicate IPT-collected pain and function information to the oncology, hematology and primary care teams, for the most part, elicited minimal response. A common reaction was uncertainty regarding how to interpret/respond to the data since its delivery was not linked to a scheduled visit. Two clinicians requested that no further reports be sent to them. This response is not surprising given the high work load and burnout detected in over half of physicians,77 and the fact that, despite our efforts to contextualize the scores, their relevance to near-term clinical care was not always clear. Data were not collected regarding treatment alterations prompted by reporting to the oncologic, hematologic, and primary care teams. However, most were receptive and appreciative of the FCMs’ and PCM’s efforts to coordinate and enhance their patients’ supportive care. In light of the anticipated increasing shortage of oncologists and hematologists,78 the acceptance of clinicians for PRO-related information is likely to shrink and their need for collaborative supportive care to grow.
A notably positive finding was the usability of IPT IVR and web-based reporting system for patients with advanced stage cancer and hematologic conditions: about 80% of participants chose to report via the IPT website. Those that chose the telephonic IVR system found it to be usable and did not report difficulty in inputting their information. A majority of participants required minimal direction in how to use either the website or IVR system. However, those less versed in IT, roughly 25%, required several sessions with either the study coordinator or their FCM to master data entry. Several participants expressed annoyance at being repeatedly contacted by the IPT system, particularly those who used IVR, but for the most part this was not perceived as an issue.
4.2. Conclusion
Although main effects results from the COPE trial are pending, it appears that the trial successfully leveraged the lessons learned from a highly successful model of collaborative telecare in depression and pain management to address the heretofore highly neglected functional losses of people with late stage cancer and hematological conditions. The COPE’s incorporation of collaborative telecare was able to connect the expertise concentrated in tertiary and quaternary cancer centers to provide consistent access for even the most remotely located patients to tailored, personalized, and validated treatments designed to improve their mobility, strength, pain control and independence.
Acknowledgments
NCI 4R01CA163803-05
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheville AL, Troxel AB, Basford JR, Kornblith AB. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. J Clin Oncol. 2008;26:2621–9. doi: 10.1200/JCO.2007.12.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarna L. Fluctuations in physical function: adults with non-small cell lung cancer. J Adv Nurs. 1993;18:714–24. doi: 10.1046/j.1365-2648.1993.18050714.x. [DOI] [PubMed] [Google Scholar]
- 3.Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. Jama. 2000;284:2907–11. doi: 10.1001/jama.284.22.2907. [DOI] [PubMed] [Google Scholar]
- 4.Tang ST, Hsieh CH, Chiang MC, et al. Impact of high self-perceived burden to others with preferences for end-of-life care and its determinants for terminally ill cancer patients: a prospective cohort study. Psycho-oncology. 2016 doi: 10.1002/pon.4107. [DOI] [PubMed] [Google Scholar]
- 5.Lee JE, Shin DW, Cho J, et al. Caregiver burden, patients’ self-perceived burden, and preference for palliative care among cancer patients and caregivers. Psycho-oncology. 2015;24:1545–51. doi: 10.1002/pon.3827. [DOI] [PubMed] [Google Scholar]
- 6.Kroenke K, Theobald D, Wu J, et al. Effect of telecare management on pain and depression in patients with cancer: a randomized trial. Jama. 2010;304:163–71. doi: 10.1001/jama.2010.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietrich AJ, Oxman TE, Williams JW, Jr, et al. Re-engineering systems for the treatment of depression in primary care: cluster randomised controlled trial. Bmj. 2004;329:602. doi: 10.1136/bmj.38219.481250.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroenke K, Bair M, Damush T, et al. Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) study: design and practical implications of an intervention for comorbid pain and depression. Gen Hosp Psychiatry. 2007;29:506–17. doi: 10.1016/j.genhosppsych.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke K, Theobald D, Norton K, et al. The Indiana Cancer Pain and Depression (INCPAD) trial Design of a telecare management intervention for cancer-related symptoms and baseline characteristics of study participants. General hospital psychiatry. 2009;31:240–53. doi: 10.1016/j.genhosppsych.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxman TE, Dietrich AJ, Williams JW, Jr, Kroenke K. A three-component model for reengineering systems for the treatment of depression in primary care. Psychosomatics. 2002;43:441–50. doi: 10.1176/appi.psy.43.6.441. [DOI] [PubMed] [Google Scholar]
- 11.Choi Yoo SJ, Nyman JA, Cheville AL, Kroenke K. Cost effectiveness of telecare management for pain and depression in patients with cancer: results from a randomized trial. General hospital psychiatry. 2014;36:599–606. doi: 10.1016/j.genhosppsych.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NCI Community Cancer Centers Program Pilot: 2007-2010. (Accessed August 29, 2010, at http://ncccp.cancer.gov/Media/FactSheet.htm.)
- 13.Cancer Rehabilitation Special Interest Group Membership list. 2009 at http://www.aapmr.org/
- 14.Cheville AL, Yost KJ, Larson DR, et al. Performance of an item response theory-based computer adaptive test in identifying functional decline. Arch Phys Med Rehabil. 2012;93:1153–60. doi: 10.1016/j.apmr.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. Journal of pain and symptom management. 2013;45:811–21. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tudor-Locke CE, Myers AM, Rodger NW. Development of a theory-based daily activity intervention for individuals with type 2 diabetes. Diabetes Educ. 2001;27:85–93. doi: 10.1177/014572170102700110. [DOI] [PubMed] [Google Scholar]
- 17.Tudor-Locke CE, Myers AM, Bell RC, Harris SB, Wilson Rodger N. Preliminary outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type 2 diabetes. Patient Educ Couns. 2002;47:23–8. doi: 10.1016/s0738-3991(01)00169-0. [DOI] [PubMed] [Google Scholar]
- 18.Jette AM, Haley SM, Tao W, et al. Prospective evaluation of the AM-PAC-CAT in outpatient rehabilitation settings. Physical therapy. 2007;87:385–98. doi: 10.2522/ptj.20060121. [DOI] [PubMed] [Google Scholar]
- 19.Haley SM, Andres PL, Coster WJ, Kosinski M, Ni P, Jette AM. Short-form activity measure for post-acute care. Archives of physical medicine and rehabilitation. 2004;85:649–60. doi: 10.1016/j.apmr.2003.08.098. [DOI] [PubMed] [Google Scholar]
- 20.Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24:733–8. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss CO, Hoenig HM, Fried LP. Compensatory strategies used by older adults facing mobility disability. Arch Phys Med Rehabil. 2007;88:1217–20. doi: 10.1016/j.apmr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Tao W, Haley SM, Coster WJ, Ni P, Jette AM. An exploratory analysis of functional staging using an item response theory approach. Archives of physical medicine and rehabilitation. 2008;89:1046–53. doi: 10.1016/j.apmr.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 25.Chang JA, Curtis JR, Patrick DL, Raghu G. Assessment of health-related quality of life in patients with interstitial lung disease. Chest. 1999;116:1175–82. doi: 10.1378/chest.116.5.1175. [DOI] [PubMed] [Google Scholar]
- 26.Weaver C, Schiech L, Held-Warmkessel J, et al. Risk for unplanned hospital readmission of patients with cancer: results of a retrospective medical record review. Oncol Nurs Forum. 2006;33:E44–52. doi: 10.1188/06.ONF.E44-E52. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med. 1998;60:150–5. doi: 10.1097/00006842-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Williamson GM, Schulz R. Activity restriction mediates the association between pain and depressed affect: a study of younger and older adult cancer patients. Psychol Aging. 1995;10:369–78. doi: 10.1037//0882-7974.10.3.369. [DOI] [PubMed] [Google Scholar]
- 29.Given B, Given C, Azzouz F, Stommel M. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nurs Res. 2001;50:222–32. doi: 10.1097/00006199-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B. Predictors of physical functioning among geriatric patients with small cell or non-small cell lung cancer 3 months after diagnosis. Support Care Cancer. 1999;7:328–31. doi: 10.1007/s005200050270. [DOI] [PubMed] [Google Scholar]
- 31.American, Physical, Therapy, Association. Cancer Rehabilitation: Principles and Practice. Alexandria, VA: American Physical Therapy Association; 2009. [Google Scholar]
- 32.Cheville AL, Girardi J, Clark MM, et al. Therapeutic exercise during outpatient radiation therapy for advanced cancer: Feasibility and impact on physical well-being. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2010;89:611–9. doi: 10.1097/PHM.0b013e3181d3e782. [DOI] [PubMed] [Google Scholar]
- 33.Clark MM, Rummans TA, Atherton PJ, et al. Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer. 2013;119:880–7. doi: 10.1002/cncr.27776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:1653–9. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 35.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:344–51. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 36.Leenders NY, Sherman WM, Nagaraja HN, Kien CL. Evaluation of methods to assess physical activity in free-living conditions. Med Sci Sports Exerc. 2001;33:1233–40. doi: 10.1097/00005768-200107000-00024. [DOI] [PubMed] [Google Scholar]
- 37.Bassett DR, Jr, Ainsworth BE, Swartz AM, Strath SJ, O’Brien WL, King GA. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471–80. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31:206–19. doi: 10.1016/j.genhosppsych.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–84. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 40.Paul SM, Zelman DC, Smith M, Miaskowski C. Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain. 2005;113:37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Li KK, Harris K, Hadi S, Chow E. What should be the optimal cut points for mild, moderate, and severe pain? J Palliat Med. 2007;10:1338–46. doi: 10.1089/jpm.2007.0087. [DOI] [PubMed] [Google Scholar]
- 42.NCCN Clinical Practice Guidelines in Oncology: Adult Cancer Pain. (Accessed January 14, 2011, at http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf.)
- 43.Kroenke K, Taylor-Vaisey A, Dietrich AJ, Oxman TE. Interventions to improve provider diagnosis and treatment of mental disorders in primary care. A critical review of the literature. Psychosomatics. 2000;41:39–52. doi: 10.1016/S0033-3182(00)71172-8. [DOI] [PubMed] [Google Scholar]
- 44.Gilbody S, Whitty P, Grimshaw J, Thomas R. Educational and organizational interventions to improve the management of depression in primary care: a systematic review. Jama. 2003;289:3145–51. doi: 10.1001/jama.289.23.3145. [DOI] [PubMed] [Google Scholar]
- 45.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. Jama. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 46.Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4–9. [PubMed] [Google Scholar]
- 47.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 48.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 49.Victorson D, Barocas J, Song J, Cella D. Reliability across studies from the functional assessment of cancer therapy-general (FACT-G) and its subscales: a reliability generalization. Qual Life Res. 2008;17:1137–46. doi: 10.1007/s11136-008-9398-2. [DOI] [PubMed] [Google Scholar]
- 50.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 51.Plotnikoff RC, Taylor LM, Wilson PM, et al. Factors associated with physical activity in Canadian adults with diabetes. Med Sci Sports Exerc. 2006;38:1526–34. doi: 10.1249/01.mss.0000228937.86539.95. [DOI] [PubMed] [Google Scholar]
- 52.Sloan JA, Symonds T, Vergas-Chanes D, et al. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Inf J. 2003;37:23–31. [Google Scholar]
- 53.Haley SM, Siebens H, Coster WJ, et al. Computerized adaptive testing for follow-up after discharge from inpatient rehabilitation: I. Activity outcomes. Arch Phys Med Rehabil. 2006;87:1033–42. doi: 10.1016/j.apmr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 54.Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Medical care. 2004;42:I49–61. doi: 10.1097/01.mlr.0000103520.43902.6c. [DOI] [PubMed] [Google Scholar]
- 55.Krebs EE, Bair MJ, Damush TM, Tu W, Wu J, Kroenke K. Comparative responsiveness of pain outcome measures among primary care patients with musculoskeletal pain. Med Care. 48:1007–14. doi: 10.1097/MLR.0b013e3181eaf835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cleeland C. Measurement of pain by subjective report. In: Foley KM, editor. Advances in Pain Research and Therapy. 1989. pp. 391–403. [Google Scholar]
- 57.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 58.Cella D, Lai JS, Stone A. Self-reported fatigue: one dimension or more? Lessons from the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) questionnaire. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2011;19:1441–50. doi: 10.1007/s00520-010-0971-1. [DOI] [PubMed] [Google Scholar]
- 59.Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365–84. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 60.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–20. doi: 10.1097/00005650-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Reed SD, Anstrom KJ, Bakhai A, et al. Conducting economic evaluations alongside multinational clinical trials: toward a research consensus. Am Heart J. 2005;149:434–43. doi: 10.1016/j.ahj.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 62.Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent DD, Lynch J. Outcome measures for the health education and other health care interventions. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- 63.Nigg C, Hellsten L, Norman G, et al. Physical activity staging distribution: establishing a heuristic using multiple studies. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2005;29(Suppl):35–45. doi: 10.1207/s15324796abm2902s_7. [DOI] [PubMed] [Google Scholar]
- 64.Lee MK, Yun YH, Park HA, Lee ES, Jung KH, Noh DY. A Web-based self-management exercise and diet intervention for breast cancer survivors: pilot randomized controlled trial. Int J Nurs Stud. 2014;51:1557–67. doi: 10.1016/j.ijnurstu.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis Hoboken. New Jersey: Wiley; 2004. [Google Scholar]
- 66.Troxel A. An index of local sensitivity to nonignorability. Statistica Sinica. 2004;14:1221–37. [Google Scholar]
- 67.Ma G, Troxel A, Heitjan D. An index of local sensitivity to nonignorable dropout in longitudinal modeling. Statist Med. 2005;24:2129–50. doi: 10.1002/sim.2107. [DOI] [PubMed] [Google Scholar]
- 68.Cheville AL, Rhudy L, Basford JR, Griffin JM, Flores AM. How receptive are patients with late stage cancer to rehabilitation services and what are the sources of their resistance? Archives of physical medicine and rehabilitation. 2016 doi: 10.1016/j.apmr.2016.08.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Craike M, Hose K, Courneya KS, Harrison SJ, Livingston PM. Physical Activity Preferences for People Living With Multiple Myeloma: A Qualitative Study. Cancer nursing. 2016 doi: 10.1097/NCC.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 70.Harrington JM, Schwenke DC, Epstein DR. Exercise preferences among men with prostate cancer receiving androgen-deprivation therapy. Oncology nursing forum. 2013;40:E358–67. doi: 10.1188/13.ONF.E358-E367. [DOI] [PubMed] [Google Scholar]
- 71.McGowan EL, Speed-Andrews AE, Blanchard CM, et al. Physical activity preferences among a population-based sample of colorectal cancer survivors. Oncology nursing forum. 2013;40:44–52. doi: 10.1188/13.ONF.44-52. [DOI] [PubMed] [Google Scholar]
- 72.Murnane A, Geary B, Milne D. The exercise programming preferences and activity levels of cancer patients undergoing radiotherapy treatment. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:957–62. doi: 10.1007/s00520-011-1167-z. [DOI] [PubMed] [Google Scholar]
- 73.Carpenter JS, Rawl S, Porter J, et al. Oncology outpatient and provider responses to a computerized symptom assessment system. Oncology nursing forum. 2008;35:661–9. doi: 10.1188/08/ONF.661-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yount SE, Rothrock N, Bass M, et al. A randomized trial of weekly symptom telemonitoring in advanced lung cancer. Journal of pain and symptom management. 2014;47:973–89. doi: 10.1016/j.jpainsymman.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boyce MB, Browne JP. Does providing feedback on patient-reported outcomes to healthcare professionals result in better outcomes for patients? A systematic review. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2013;22:2265–78. doi: 10.1007/s11136-013-0390-0. [DOI] [PubMed] [Google Scholar]
- 76.Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178:997–1003. doi: 10.1503/cmaj.070281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shanafelt TD, Hasan O, Dyrbye LN, et al. Changes in Burnout and Satisfaction With Work-Life Balance in Physicians and the General US Working Population Between 2011 and 2014. Mayo Clinic proceedings. 2015;90:1600–13. doi: 10.1016/j.mayocp.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 78.Warren JL, Mariotto AB, Meekins A, Topor M, Brown ML. Current and future utilization of services from medical oncologists. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3242–7. doi: 10.1200/JCO.2007.14.6357. [DOI] [PubMed] [Google Scholar]


