Abstract
Objective
To evaluate the efficacy and safety of gemcitabine (GEM) at 30 min standard-dose infusion (30 min-SDI) compared with prolonged low-dose infusion (P-LDI) in patients with advanced non-small-cell lung cancer (NSCLC).
Methods
Electronic databases including Pubmed, EMbase, Cochrane Library, CNKI, CBM, and VIP were searched using keywords “GEM”, “P-LDI”, and “NSCLC”. Review Manager 5.3 was used to perform the meta-analysis. Primary endpoints were overall response rate (ORR) and 1-year survival rate (1-year SR). Secondary endpoints were grade 3/4 hematotoxicity and nausea/vomiting. In association. GRADE quality of evidence system was used to assess the results of meta-analysis.
Results
Six randomized controlled trials (RCTs) with a total of 637 patients were included and no statistical heterogeneity was found among the studies. The results showed that P-LDI was superior in ORR (RD = 0.09, 95% CI: 0.02 to 0.16, P = 0.02), but had a similar 1-year SR (RD = 0.05, 95% CI: -0.02 to 0.12, P = 0.18) as compared with 30 min-SDI. For grade 3/4 adverse events, there was no significant difference in anemia (RD = 0.02, 95% CI: -0.01 to 0.04, P = 0.27) and nausea/vomiting (RD = 0.01, 95% CI: -0.04 to 0.06, P = 0.64) between the two treatments. However, patients with P-LDI experienced less leukopenia (RD = -0.08, 95% CI: -0.15 to -0.01, P = 0.03) and thrombocytopenia ((RD = -0.05, 95% CI: -0.09 to –0.01, P = 0.006). The GRADE profile showed that the included RCTs had low quality of evidences.
Conclusion
P-LDI was superior in terms of ORR, experienced less grade 3/4 thrombocytopenia and leukopenia compared with 30 min-SDI, and could be a viable treatment option for advanced NSCLC. However, the results need to be further verified by high quality trials and large samples owing to the low quality of evidences.
Introduction
Lung cancer is the most common cause of cancer-related deaths, and NSCLC accounts for most of these cases [1] (85% to 90%). Many patients with NSCLC have locally advanced or metastatic disease at the time of diagnosis, and the overall survival is poor [2]. For patients with targetable mutations (such as EGFR and ALK), tyrosine kinase inhibitor (TKI) is considered as the first-line treatment regimens. On the other hand, for patients with no targetable mutations, platinum drugs combined with the third-generation antineoplastic agents, such as paclitaxel, docetaxel, GEM, vinorelbine and pemetrexed, is considered the standard of care for patients with unresectable or advanced NSCLC [3–5].
GEM is a pyrimidine antimetabolite, structurally related to cytosine arabinoside (Ara-C) [6], and is effective in treating a wide range of solid tumors. Currently, GEM combined with platinum is one of the standard chemotherapy regimens for patients with advanced NSCLC [4,5]. In clinical practice, GEM at 1000 mg/m2 is given as a 30-min infusion. Another dose schedule is prolonged infusion of GEM at a fixed dose rate of 10 mg/m2/minute, and both of these dose schedules have been demonstrated to be effective and tolerable. However, several phase I and phase II clinical trials [7–10] have shown that GEM with P-LDI has significant antitumor activity and fewer side effects for patients with advanced NSCLC.
Due to the small sample size of each clinical trials, it is not clear that whether P-LDI is superior to 30 min-SDI for advanced NSCLC. Therefore, a meta-analysis was performed to compare the efficacy and safety of P-LDI with 30 min SDI for the treatment of advanced NSCLC.
Materials and methods
Literature search strategy
Electronic databases including Pubmed, EMbase,Cochrane Library, CNKI, CBM, VIP were queried, and the most recent search was performed on January 3, 2017. The search was limited to articles published in English and Chinese. Keywords included “gemcitabine”, “GEM”, “prolonged low-dose infusion”, “prolonged infusion”, “long infusion”, “low dose”, “30-min infusion”, “standard dose”, ‘‘non-small-cell lung cancer”, and ‘‘NSCLC”. The references from the included studies and the websites of clinical trials was also examined for additional eligible publications.
Inclusion criteria
The inclusion criteria were as follows: RCTs with full articles; patients eligible for the trial had cytologically confirmed inoperable or unresectable NSCLC of stageⅠ–Ⅳ; the follow-up time was more than 1 years; studies comparing GEM at P-LDI with 30 min-SDI; endpoints of ORR (PR+CR); 1-year SR; and hematotoxicity and non-hematotoxicity was reported. Response was assessed by using the response evaluation criteria in solid tumors (RECIST)[11], and National Cancer Institute Common Toxicity Criteria (CTC) version 2.0 were used for grading the toxicity[12]. Two investigators selected the eligible trials based on the inclusion criteria independently. Disagreement was addressed by discussion until consensus was achieved.
Data abstraction
Two investigators extracted data from eligible studies independently, and the items extracted from each study included first author, publication date, journal, intervention group, control group, chemotherapy regimens, number of patients, age, percentage male, ORR, overall survival (OS), progression free survival (PFS), 1-year SR, hematotoxicity, and non-hematotoxicity. We contacted the authors of the primary studies for missing data. If we were unable to contact the authors, we excluded the study.
Quality assessment
Two investigators used the risk of bias tool (Cochrane Handbook V5.1.0) to assess the quality of trials independently. Sequence generation, allocation concealment, blinding, incomplete data, selective reporting and other sources of bias were assessed. Disagreements between the two investigators were resolved by discussion with a third investigator.
Statistical analysis
Two investigators used Review Manager 5.3 to perform the statistical analyses. A fixed-effect model was used to calculate risk difference (RD) for ORR, 1-year SR, and side effects, together with a 95% confidence interval (CI) for dichotomous results. OS and PFS were not included because of insufficient data. A RD>0 indicates that P-LDI is associated with a higher ORR, 1-year SR, and more toxicities than 30 min-SDI. The presence of statistical heterogeneity between the studies was assessed by I2 statistic using Q statistic. A P≥0.05 or I2≤50% indicated that trials are without heterogeneity, and a fixed-effect model was used to perform the meta-analysis. A P<0.05 or I2>50% led us to consider a random-effect model to perform the meta-analysis. Publication bias was assessed by the construction of funnel plots.
Quality evaluation of evidence
GRADE pro 3.2 Software was used to classify the quality of evidence. All of the included studies were RCTs, and the RCT was set as the highest level of evidence. Five factors could reduce the quality of evidence, including risk of bias, inconsistency, indirectness, imprecision and publication bias.
Results
Eligible studies
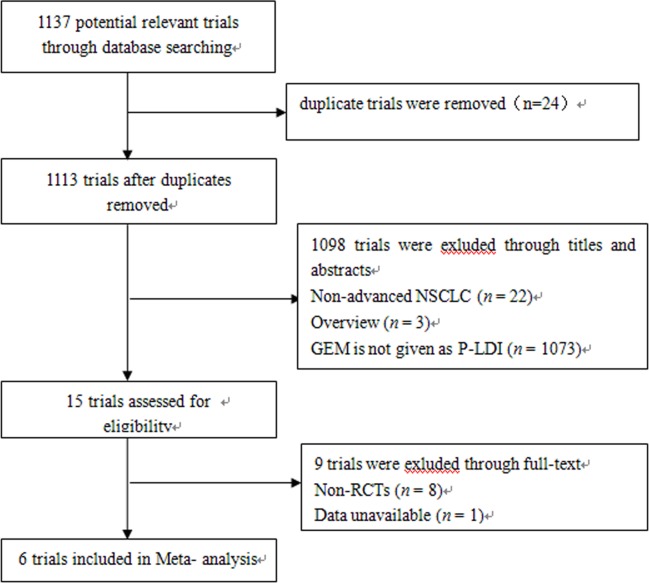
A total of 1137 articles were identified by the initial search strategy. After examining the titles and full-text, six identified RCTs [7–9,13–15] were selected for the meta-analysis (Fig 1). Nine trials were excluded because they were not randomized [6,10,16–21] or because the data was unavailable[22]. The characteristics of the eligible studies are summarized in Table 1.
Fig 1. Flowchart of included and excluded trials.
Table 1. Characteristics of eligible trials.
| Trials | No. | Male (%) |
Age | Stage (No.) |
Chemotherapy regimens | Line | PS | Radiation given |
|---|---|---|---|---|---|---|---|---|
| Beniwal SK, 2012[13] | 30 | 86.6 | 53.3 35–65 |
ⅢB /Ⅳ 17/13 |
GEM (1000 mg/m2 in 30 min d1, d8) +CBP (AUC 5 d1). 21 d cycle, 4–6cycles. |
First-line | 0~2 | No |
| 30 | 93 | 54.5 40–70 |
ⅢB /Ⅳ 15/15 |
GEM (350 mg/m2 in 6 h d1, d8) +CBP (AUC 5 d1). 21 d cycle, 4–6 cycles. |
First-line | 0~2 | No | |
| Vrankar M, 2014[7] | 52 | 75 | 58 42–72 |
Ⅰ~Ⅱ/ⅢA /ⅢB 3/19/30 | GEM (1250 mg/m2 in 30 min d1, d8) +DDP (75 mg/m2 d2). 21 d cycle, 3 cycles. Followed with radiotherapy concurrent with DDP + VP16 | First-line | 0~1 | After Chemotherapy |
| 54 | 81.5 | 57 30~77 |
Ⅰ~Ⅱ/ⅢA /ⅢB 2/31/21 | GEM (250 mg/m2 in 6 h d1, d8) +DDP (75 mg/m2 d2). 21 d cycle, 3 cycles. Followed with radiotherapy concurrent with DDP + VP16 | First-line | 0~1 | After Chemotherapy | |
| Zwitter M, 2009[8] | 125 | 76 | 58 41–77 |
ⅢB /Ⅳ 9/116 |
GEM (1250 mg/m2 in 30 min d1, d8) +DDP (75 mg/m2 d2). 22 d cycle, 4 cycles. continued with two additional cycles of GEM as monotherapy. | Unclear | 0~2 | No |
| 124 | 75 | 59 40–79 |
ⅢB /Ⅳ 11/113 |
GEM (250 mg/m2 in 6 h d1, d8) +DDP (75 mg/m2 d2). 22 d cycle, 4 cycles. continued with two additional cycles of GEM as monotherapy. | Unclear | 0~2 | No | |
| Zwitter M, 2010[9] | 57 | 80.7 | 66 41–81 |
ⅢB /Ⅳ 2/55 |
GEM (1250 mg/m2 in 30 min d1, d8) +DDP (60 mg/m2 d2). 21 d cycle, 2–6 cycles. |
First-line | 2~3 | No |
| 55 | 67.3 | 65 49–80 |
ⅢB /Ⅳ 3/52 |
GEM (200 mg/m2 in 6 h d1, d8) +DDP (60 mg/m2 d2). 21 days cycle, 2~6cycles. |
First-line | 2~3 | No | |
| Shang, ZT, 2010[14] | 30 | 21 | 58 30–64 | ⅢB /Ⅳ 19/11 |
GEM (1000 mg/m2 in 30 min d1, d8) +DDP (75 mg/m2 d1). 21 days cycle, 2~6 cycles. |
First-line | 0~2 | No |
| 30 | 20 | 52 32–75 |
ⅢB /Ⅳ 20/10 |
GEM (250 mg/m2 in 6 h d1, d8) + DDP (75 mg/m2 d1). 21 d cycle, 2–6 cycles. |
First-line | 0~2 | No | |
| Xiong JP, 2005[15] | 25 | 16 | 52 32–68 | ⅢB /Ⅳ 9/16 |
GEM (1000 mg/m2 in 30 min d1, d8) +DDP (75 mg/m2 d1). 21 d cycle, 4 cycles. |
First-line | 0~2 | No |
| 25 | 15 | 56 28–70 | ⅢB /Ⅳ 8/17 |
GEM (250 mg/m2 in 6 h d1, d8) +DDP (75 mg/m2 d1). 21 d cycle, 4 cycles. |
First-line | 0~2 | No |
CBP: Carboplatin; DDP: Cisplatin; etoposide: VP16
Quality and publication bias of included trials
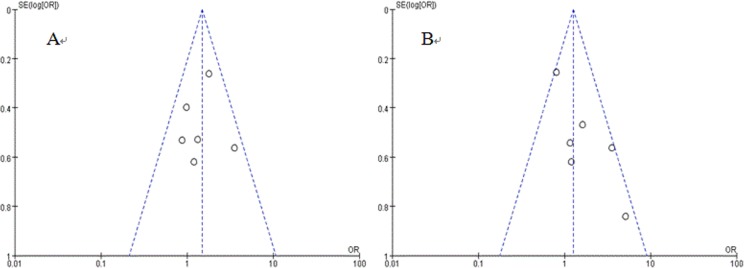
Although participants were randomized into different treatment arms in each trial, there were only two trials presented the detail of sequence generation and blinding, and none of them presented details of allocation concealment, selective reporting, or other sources of bias (Table 2). In summary, the risk of bias and the methodology quality of the included trials were acceptable, and no significant publication bias was detected by using funnel plots (Fig 2).
Table 2. Quality evaluation of included trials.
| Included trials |
Sequence generation |
Allocation concealment |
Blinding | Incomplete data | Selective reporting |
Other sources of bias |
|---|---|---|---|---|---|---|
| Beniwal SK, 2012[11] | Unclear | Unclear | Unclear | No | Unclear | Unclear |
| Vrankar M, 2014[7] | Unclear | Unclear | Unclear | Yes | Unclear | Unclear |
| Zwitter M, 2009[8] |
Computer-generated sequence of random numbers | Unclear | Single- blind | Yes | Unclear | Unclear |
| Zwitter M, 2010[9] |
Computer-generated sequence of random numbers | Unclear | Single- blind | Yes | Unclear | Unclear |
| Shang ZT, 2010[13] |
Unclear | Unclear | Unclear | No | Unclear | Unclear |
| Xiong JP, 2005[14] | Unclear | Unclear | Unclear | No | Unclear | Unclear |
Fig 2. Funnel plots of meta-analysis.
A: funnel plots for ORR; B: funnel plots for 1-year SR.
Overall response rate (ORR)
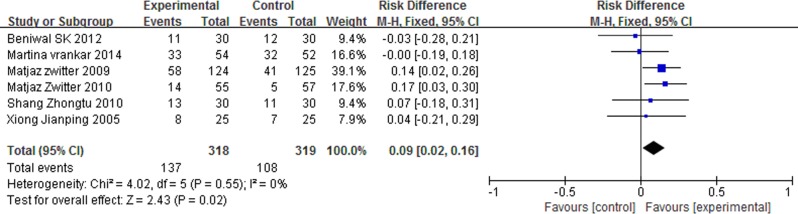
The ORR was defined as the patients who achieved a complete remission (CR) or partial remission (PR). No statistical heterogeneity between studies was found (I2 = 0%, P = 0.55). We used a fixed-effect model for meta-analysis, and the results indicated that P-LDI was superior in ORR (RD = 0.09, 95% CI: 0.02 to 0.16, P = 0.02) as compared with 30 min-SDI (Fig 3).
Fig 3. Forest plot of meta-analysis for ORR.
1-year survival rate (1-year SR)
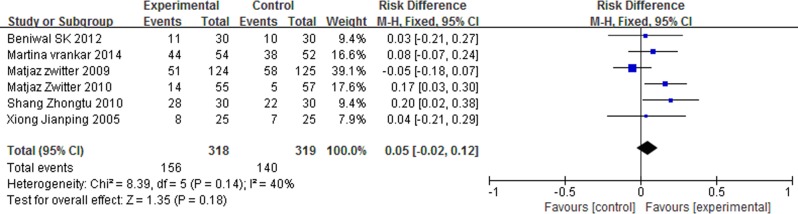
No statistical heterogeneity between studies was found (I2 = 40%, P = 0.14), and we used a fixed-effect model. Meta-analysis results indicated that P-LDI had a similar 1-year SR (RD = 0.05, 95% CI: -0.02 to 0.12, P = 0.18) compared with 30 min-SDI (Fig 4). This indicated that there was no statistical difference of 1-year SR between the two arms.
Fig 4. Forest plot of meta-analysis for 1-year SR.
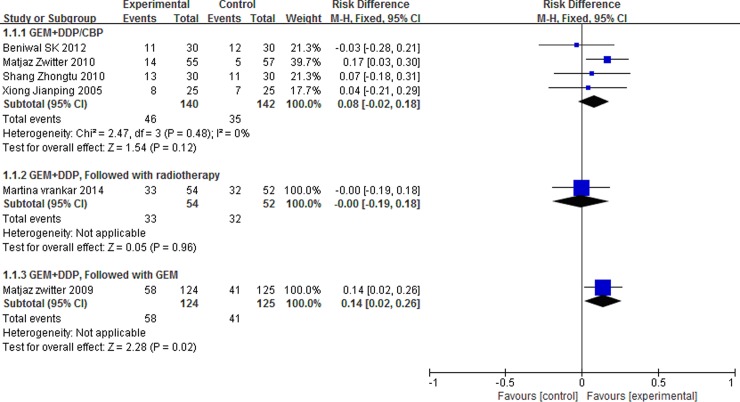
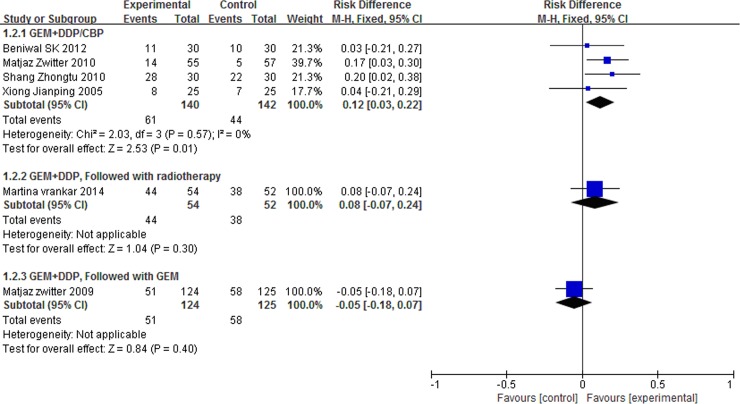
Subgroup analysis
The were three different schedules for the treatment of advanced NSCLC, including GEM combined with DDP/CBP, GEM combined with DDP and followed with radiotherapy, GEM combined with DDP and followed GEM. So we did a subgroup analysis, and the subgroup analysis showed that P-LDI was superior in ORR as compared with 30 min-SDI for patients who accepted GEM combined with DDP and followed GEM (Fig 5). On the other hand, P-LDI was superior in 1-year SD as compared with 30 min-SDI for patients who accepted GEM combined with DDP/CBP (Fig 6).
Fig 5. Forest plot of subgroup analysis for ORR.
Fig 6. Forest plot of subgroup analysis for 1-year SR.
Grade 3/4 adverse events
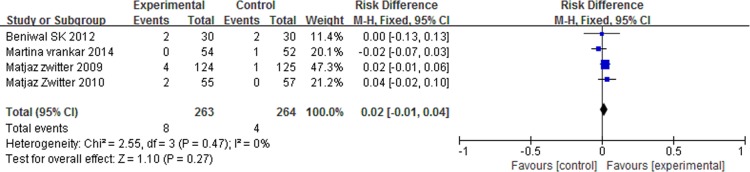
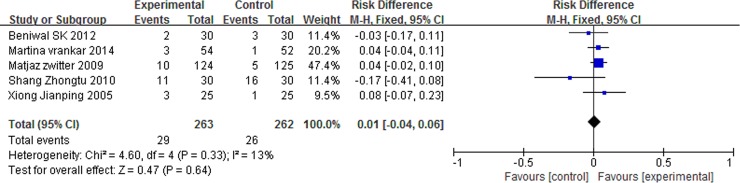
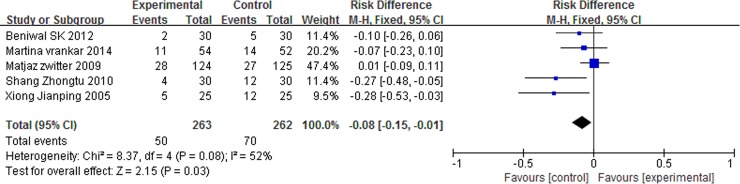
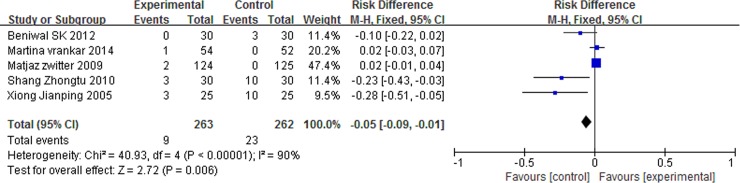
There was no significant differences in anemia (RD = 0.02, 95% CI: -0.01 to 0.04, P = 0.27) and nausea/vomiting (RD = 0.01, 95% CI: -0.04 to 0.06, P = 0.64) between the two arms. However, patients with P-LDI experienced less leucopenia (RD = -0.08, 95% CI: -0.15 to -0.01, P = 0.03) and thrombocytopenia (RD = -0.05, 95% CI: -0.09 to -0.01, P = 0.006) than did patients with 30 min-SDI. (Figs 7 and 8 and 9 and 10).
Fig 7. Forest plot of meta-analysis for anemia (grade 3/4).
Fig 8. Forest plot of meta-analysis for nausea/vomiting (grade 3/4).
Fig 9. Forest plot of meta-analysis for leukopenia (grade 3/4).
Fig 10. Forest plot of meta-analysis for thrombocytopenia (grade 3/4).
Quality evaluation of evidence
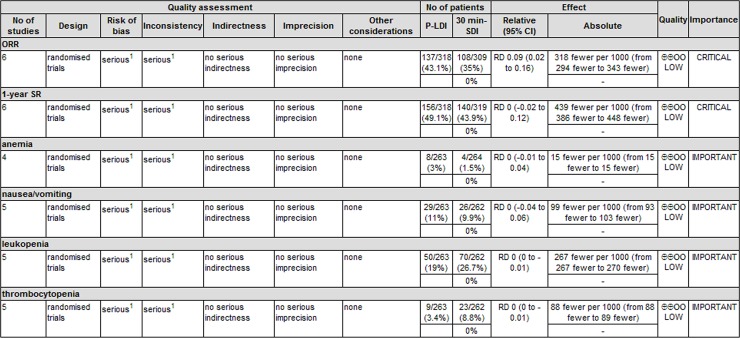
When used GRADE profiler software to assess the quality of evidence. According to the GRADE system, it was clearly that all of the outcomes were low in the GRADE system for grading evidence (Fig 11), indicated that the results need to be further verified by high quality trials and large samples.
Fig 11. GRADE system for grading the quality of evidence.
Discussion
GEM combined with platinum has been proven to be effective and well tolerated for patients with advanced NSCLC [2]. In several large phase Ⅲ trials [1–3], the ORR ranged from 22 to 40.6%, PFS from 4.2 to 9.8 months, OS from 8.1 to 9.8 months, and 1-year SR from 32 to 39% [2,3].
GEM is transported across the plasma membrane by specific nucleoside transporters and phosphorylated to the triphosphate (dFdCTP) by deoxycytidine kinase (DK) [23]. However, the DK is saturated at concentration of 10–20 μmol/L of GEM, and there is no linear dose-activity relationship between the dFdCTP and the AUC of GEM [24,25]. GEM is usually administered as a 30 min infusion of 1000–1250 mg/m2 on days 1, 8, 15, ever 28 d afterwards, and is effective and well tolerated for patients with advanced NSCLC [2]. However, the plasma concentration following 30 min infusion of 1000 mg/m2 often exceeds the saturation concentration of DK [23,24]. Thus, by prolonging the infusion time, the plasma concentration of dFdCTP may be increased to achieve better efficiency.
There are three types of infusion for the administration of GEM, including 30 min-SDI, fixed-dose rate (FDR) of 10 mg/m2/min infusion, and P-LDI. The 30 min infusion of GEM is the standard regimen. However, some studies [26] have investigated the feasibility and efficacy of FDR in the treatment of NSCLC, and controversial conclusions have been drawn from these trials. A meta-analysis of 6 RCTs [21] demonstrated that FDR of GEM had an equal ORR and 1-year SR as 30 min infusion in patients with advanced NSCLC. Otherwise, FDR was associated with more grade 3/4 hematotoxicity and non-hematotoxicity than 30 min-SDI was.
Another type of infusion is P-LDI, and several clinical trials [7–9] were established to evaluate the efficacy and safety of GEM at 30 min-SDI compared with P-LDI in patients with advanced NSCLC. In a phase I–II trial, GEM with a 6 h infusion in combination with cisplatin was used to the treat advanced NSCLC [10]. During the phase I trial, the dose of GEM ranged from 130 to 250 mg/m2, and there was no dose–response relationship in this range. In a phase II trial, the remaining patients received GEM at 250 mg/m2 in a 6-h infusion, and the ORR, PFS, OS and 1-year SR were 46%, 6 months, 9.5 months and 40%, respectively [10]. Matjaz Zwitter [9] presented a phase II randomized clinical trial of two schedules of chemotherapy for patients with NSCLC. The response rate was 26.9% and 9.4%, the median PFS was 3.8 and 5.6 months, the median OS was 4.3 and 6.8 months for 30 min-SDI and P-LDI, respectively (P<0.05). Another study from Beniwal SK [13] reported that GEM (P-LDI) in combination with carboplatin had an equal activity and low toxicity as compared with 30 min-SDI. In order to evaluate the efficacy and safety of GEM at 30 min-SDI compared with P-LDI in patients with advanced NSCLC, a meta-analysis was performed.
Limitations of this meta-analysis should also be acknowledged. At first, we excluded non-English articles, and most studies included in this meta-analysis included a small size, thus this may lead to a small study effect. Secondly, due to insufficient data of OS and PFS, we did not pool the survival data of OS or PFS. Instead, we utilized other survival metrics, the 1-year SR, to address this limitation. Thirdly, there is no significant difference in 1-year SR, which may be caused by the small number of original studies. Therefore, more studies with large sample sizes are required to answer this question.
Conclusion
Compared with 30 min-SDI, GEM with P-LDI was superior in ORR and resulted in less grade 3/4 thrombocytopenia and leukopenia in patients with advanced NSCLC. Thus, GEM with P-LDI is a viable treatment option for patients with advanced NSCLC. However, the results need to be further verified by high quality trials and large samples owing to the limited number of RCTs and the poor quality among the included studies.
Supporting information
(DOC)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013; 49: 1374–403. doi: 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 2.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Natl Compr N Canc Netw. 2017; 15(4):504–535. [DOI] [PubMed] [Google Scholar]
- 3.Richardet E, Acosta L, Hernandez P A, Molina M, Richardet M, et al. P1.40: Comparative Study of Chemotherapy Regimens Based on Platinum and Its Different Toxicities in Patients With Advanced NSCLC: Track: Advanced NSCLC. Journal of Thoracic Oncology. 2016; 11(10): S207–S207. [Google Scholar]
- 4.Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S, ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology Official Journal of the European Society for Medical Oncology. 2014; 25: 27–39. [DOI] [PubMed] [Google Scholar]
- 5.Vansteenkiste J, De R D, Eberhardt W E, Lim E, Senan S, Felip E, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology Official Journal of the European Society for Medical Oncology. 2013; 24:89–98. [DOI] [PubMed] [Google Scholar]
- 6.Cattel L., Airoldi M., Delprino L., Passera R, Milla P, Pedani F. Pharmacokinetic evaluation of gemcitabine and 2’,2’-difluorodeoxycytidine-5’-triphosphate after prolonged infusion in patients affected by different solid tumors. Annals of Oncology. 2006; 17:142–147. [DOI] [PubMed] [Google Scholar]
- 7.Vrankar Martina, Zwitter Matjaz, Bavcar Tanja, Milic A, Kovac V. Induction gemcitabine in standard dose or prolonged low-dose with cisplatin followed by concurrent radiochemotherapy in locally advanced non-small cell lung cancer:a randomized phase II clinical trial. Radiol Oncol. 2014; 48(4): 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zwitter Matjaz, Kovac Viljem, Smrdel U, Vrankar M, Zadnik V. Gemcitabine in Brief versus Prolonged Low-Dose Infusion,both Combined with Cisplatin, for Advanced Non-smallCell Lung Cancer:A Randomized Phase II Clinical Trial. Thorac Oncol. 2009; 4: 1148–1155. [DOI] [PubMed] [Google Scholar]
- 9.Zwitter M, Kovac V, Rajer M, Vrankar M, Smrdel U. Two schedules of chemotherapy for patients with non-small cell lung cancer in poor performance status: a phase II randomized trial. Anti-Cancer Drugs. 2010, 21:662–668. doi: 10.1097/CAD.0b013e32833ab7a0 [DOI] [PubMed] [Google Scholar]
- 10.Zwitter M, Kovac V, Smrdel U, Kocijancic I, Segedin B, Vrankar M. Phase I–II trial of low-dose gemcitabine in prolonged infusion and cisplatin for advanced non-small cell lung cancer. Anti-Cancer Drugs. 2005; 16:1129–1134. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). European Journal of Cancer. 2009; 45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Kaba H, Fukuda H, Yamamoto S, Ohashi Y. Reliability at the National Cancer Institute-Common Toxicity Criteria version 2.0. Gan to Kagaku Ryoho Cancer & Chemotherapy. 2004; 31(8): 1187–1192. [PubMed] [Google Scholar]
- 13.Beniwal SK, Patel KM, Shukla S, Parikh BJ, Shah S, Patel A. Gemcitabine in brief versus prolonged low-dose infusion,both combined with carboplatin for advanced non-small cell lung cancer.indian journal of cancer. 2012; 49(2):202–208. doi: 10.4103/0019-509X.102861 [DOI] [PubMed] [Google Scholar]
- 14.Zhongtu Shang, Shangwu Chen. Clinical Evaluation of delayed infusion of low dose gemcitabine combined with cisplatin maintenance chemotherapy regimens for treatment of advanced NSCLC patients. Chin J Cancer Prev Treat. 2010; 17(19):1568–1570. [Google Scholar]
- 15.Jianping Xiong, Jun Xu, Luxing Zhong. Clinical observation on gem citabine 6 h prolonged infusion regmi en in the treatment of advanced non-small cell lung cancer. Chinese Clinical Oncology. 2005; 10(6):643–646. [Google Scholar]
- 16.Jianping Xiong, Ling Zhang, Luxing Zhong. PhaseⅡTrial of Prolonged Infusion of Low-dose Gemcitabine in Advanced Non-Small Cell Lung Cancer Treatment Chinese Journal of Cancer. 2006; 25(8): 995–998. [PubMed] [Google Scholar]
- 17.Hongkai Yang. Low Dose Gemcitabine Combined Nedaplatin for Advanced Nonsmall-cell Lung Cancer:Observation of Curative Effect. Evaluation and analysis of drug-use in hospitals of china. 2008; 8(5):380–381. [Google Scholar]
- 18.Wu ZY, Guan HH, Lin ZX, Yang HK, Zhou L, Cai QC. Combination of low-dose gemcitabine in 6-hour infusion and carboplatin is a favorable option for patients in poor performance status with advanced non-small cell lung cancer. Journal of Chemotherapy. 2014; 26(5): 306–311. doi: 10.1179/1973947813Y.0000000139 [DOI] [PubMed] [Google Scholar]
- 19.Anderson H, Thatcher N, Walling J, Hansen H. A phase I study of a 24 hour infusion of gemcitabine in previously untreated patients with inoperable non-small-cell lung cancer. British Journal of Cancer. 1996; 74:460–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jun Chen, Qingsong Tao, Linrong Pang. Prolonged infusion of low-dose gemcitabine in maintenance treatment for advanced non-small cell lung cancer: a phase II trial. Zhejiang Medical Journal. 2013; 35(20):1823–1826. [Google Scholar]
- 21.Qiu MT, Ding XX, Hu JW, Tian HY, Yin R, Lin Xu. Fixed-dose rate infusion and standard rate infusion of gemcitabine in patients with advanced non-small-cell lung cancer:a meta-analysis of six trials. Cancer Chemother Pharmacol. 2012; 70: 861–873. doi: 10.1007/s00280-012-1974-z [DOI] [PubMed] [Google Scholar]
- 22.Huihong Guan, Hongkai Yang, Lan Zhou, Jianfang Xu, Chen Y. clinical Efficacy of Low-dose Gemcitabine in Prolonged Infusion plus Nedaplatin for Advanced/Metastatic Non-small Cell Lung Cancer Patients. Anti-tumor Pharmacy. 2014;4(4):277–281. [Google Scholar]
- 23.Plunkett W, Huang P, Xu Y, et al. Gemcitabine: metabolism, mechanism of action and self potentiation. Semin Oncol. 1995; 22: 3–10. [PubMed] [Google Scholar]
- 24.Grunewald R, Kantarjian H, Du M, Faucher K, Tarassoff P, Plunkett W. Gemcitabine in leukemia: a phase I clinical, plasma, and cellular pharmacology study. Clin Oncol. 1992; 10:406–413. [DOI] [PubMed] [Google Scholar]
- 25.Patel SR, Gandhi V, Jenkins J, Papadopolous N, Burgess MA, Plager C. Phase II clinical investigation of gemcitabine in advanced soft tissue sarcomas and window evaluation of dose rate on gemcitabine triphosphate accumulation. Clin Oncol. 2001; 19(15): 3483–3489. [DOI] [PubMed] [Google Scholar]
- 26.Soo RA, Wang LZ, Tham LS, Yong WP, Boyer M, Lim HL. A multicentre randomised phase II study of carboplatin in combination with gemcitabine at standard rate or fixed dose rate infusion in patients with advanced stage non-small-cell lung cancer. Ann Oncol. 2006; 17(7): 1128–1133. doi: 10.1093/annonc/mdl084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.