Abstract
Nephrogenesis is dependent on the input of several transcriptional regulatory networks. However, the details of how these networks operate and converge to facilitate nephron progenitor specific programmes are largely unknown. To this end, recent studies have focused on identifying the precise regulatory mechanisms that modulate progenitor maintenance and induction. Continued focus on this area of research will help identify nephrogenic programmes which could be manipulated for therapeutic intervention of kidney disease.
Keywords: nephrogenesis, transcriptional networks, nephron progenitor programmes
The eloquent progression of nephrogenesis during embryonic kidney development requires a careful balance of nephron progenitor self-renewal and differentiation. This ensures a sufficient number of nephrons are formed to carry out their essential roles in waste filtration and body fluid homeostasis. In mammals this is a terminal process; no resident progenitors remain after fetal or early neo-natal stages. De novo nephron formation does not appear to be an option for the adult mammalian kidney, necessitating repair of existing nephrons following injury or disease. In this light, developing alternative, knowledge-based strategies to de novo nephrogenesis is an important therapeutic goal. As a first step, we need to develop a thorough understanding of the nephron progenitor population and the underlying regulatory programs governing its maintenance and nephron specific capabilities. Leveraging this knowledge base will spur the development of new strategies to treat the damaged and diseased kidney.
The mammalian kidney develops through reciprocal interactions of the ureteric epithelium with adjacent mesenchymal nephron progenitors. Signals from nephron progenitors support ureteric epithelial branching and the arborization of the urine transporting collecting duct network derived from this epithelium. In turn, the transition of multi-potent nephron progenitors into epithelial renal vesicles, the nephron precursor, requires signals from the ureteric bud. Over the last few decades, research efforts have uncovered a number of factors with integral roles in kidney development. In particular, the transcriptional regulators and associated components including: Six1, Pax2, Hox11 paralogs, Osr1, Sall1, Six2, Eya1 and Wt1 are all expressed within the nephron progenitors, and the depletion of each from the murine kidney results in insufficient kidney development1–8. Loss of any factor other than Six2 leads to a loss of nephron progenitors at the expense of nephron formation. In contrast, the entire nephrogenic mesenchyme of Six2 mutants commits to nephron formation at the onset of kidney development, prematurely terminating the nephrogenic program with only a small number of renal vesicles in place7,8. Thus, Six2 has a unique regulatory activity amongst these factors: promoting the self-renewal of the nephron progenitor population.
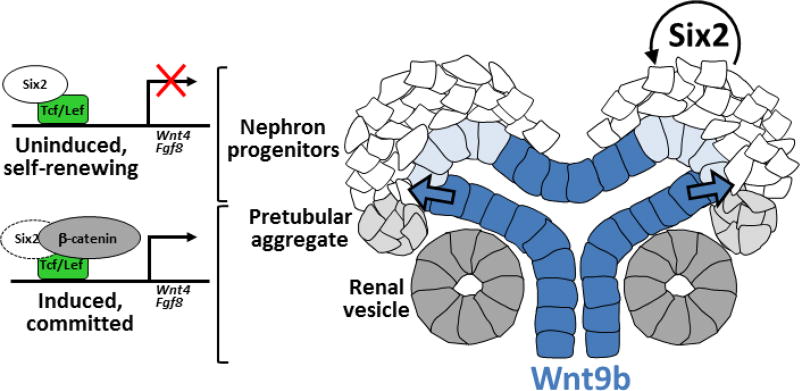
Self-renewal of nephron progenitors is normally opposed by Wnt signaling from the adjacent branching tips of the ureteric epithelium. Here, Wnt9b is expressed in a graded fashion with higher levels beneath the tips where induced mesenchyme cells first aggregate then epithelialize to generate renal vesicles, and at lower levels above the tip where the ureteric epithelium directly contacts the main body of the nephron progenitor pool9. Wnt9b-directed canonical Wnt signaling mediated by a β-catenin containing transcriptional complex induces renal vesicle formation10. Together, these genetic-based data highlight a complex regulatory network underpinning specification, maintenance, and commitment of nephron progenitors. What is not clear is how the transcriptional pathways direct these events.
The majority of functional studies have examined gene knockouts to infer function rather than directly addressing the transcriptional networks at play. A combination of in vivo and in vitro analysis has defined regulatory modules, uncovering some of the basic networks underpinning Six2 regulation11. However, a broader insight requires unbiased genome-scale methodology, integrating a full complement of the regulatory factors to take our understanding to a deeper, systems level. Combining advances in next generation sequencing with chromatin immunoprecipitation-mediated enrichment of transcriptional components at their target sites (ChIP-seq) has resulted in exciting new insights into critical control mechanisms regulating complex biological processes. Similarly, integrating ChIP-seq analysis with gene expression data in nephron progenitors is expected to lead to a new level of insight into transcriptional targets and modules of regulation, and to generate a clearer picture of how nephron progenitor status is programmed, maintained then lost on progenitor commitment to nephron fates.
We have recently taken advantage of such experimental analyses to identify the gene regulatory networks engaged by Six2 and canonical Wnt-directed transcriptional complexes. Six2+ nephron progenitors were isolated from embryonic mouse kidneys and subjected to ChIP-seq either immediately (Six2 ChIP) or after treatment with a Wnt pathway agonist to induce β-catenin transcriptional engagement and epithelial commitment (β-catenin ChIP). While each factor was bound to an independent set of regulatory elements, a subset of genomic regions was directly engaged by both factors suggestive of overlapping regulatory functions. Potential targets included factors expressed within the nephron progenitors such as Six2 and Eya1, as well as Wnt4 and Fgf8, genes activated on induction of progenitors that are essential themselves for the transition to epithelial renal vesicles. Further analyses showed that Six2 likely engages in a complex with Lef/TCF factors, the DNA binding component of the β-catenin-dependent Wnt signaling transcriptional machinery, but that the entry of β-catenin into this complex is restricted to newly induced and differentiating cells. These data suggest a model wherein Six2 action at these sites inhibits Wnt4 and Fgf8 expression in the nephron progenitors. Upon Wnt9b induction, β-catenin entry into the complex turns on the expression of Wnt4, Fgf8, and other targets, promoting commitment of these cells to a nephrogenic program (Figure 1)12.
Figure 1.
Kidney development requires a balance of nephron progenitor self-renewal and induction. See text for further description.
While our analyses have shed new light on the regulatory mechanisms that balance nephron progenitor self-renewal vs. differentiation, a host of transcriptional regulators have integral roles in kidney development and progenitor function. Future studies will employ a combination of ChIP-seq, expression analyses, biochemistry and in vitro and in vivo modeling to identify the regulatory modules employed by these factors. We expect to find independent regulatory networks utilized by each factor but hypothesize that a significant overlap will be identified with any combination of factors. The exploration of shared gene regulatory networks will undoubtedly uncover new mechanisms that help maintain nephron progenitor multi-potency. This knowledge will be critical to future research aimed at exploiting the potential of the nephron program for therapeutic intervention.
References
- 1.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–91. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 2.Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–65. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- 3.Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat. Genet. 1999;23:113–17. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- 4.Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, et al. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development. 2001;128:3105–15. doi: 10.1242/dev.128.16.3105. [DOI] [PubMed] [Google Scholar]
- 5.Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev. 2002;16:1423–32. doi: 10.1101/gad.993302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133:2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- 7.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006;25:5214–28. doi: 10.1038/sj.emboj.7601381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–81. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell. 2005;9:283–92. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007;134:2533–39. doi: 10.1242/dev.006155. [DOI] [PubMed] [Google Scholar]
- 11.Gong KQ, Yallowitz AR, Sun H, Dressler GR, Wellik DM. A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol. Cell. Biol. 2007;27:7661–68. doi: 10.1128/MCB.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, et al. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev. Cell. 2012;23:637–51. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]