Abstract
Enhanced inflammation has been associated with Alzheimer’s disease (AD) and diseases with Lewy body (LB) pathology, such as Parkinson’s disease (PD) and Dementia with Lewy bodies (DLB). One issue is whether amyloid and tangle pathology, features of AD, or α-synuclein LB pathology have similar or different effects on brain inflammation. An aim of this study was to examine if certain features of inflammation changed in brains with increasing LB pathology. To assess this, we measured levels of the anti-inflammatory protein CD200 and the pro-inflammatory protein intercellular adhesion molecule-1 (ICAM-1) in cingulate and temporal cortex from a total of 143 cases classified according to the Unified Staging System for LB disorders. Changes in CD200 and ICAM-1 levels did not correlate with LB pathology, but with AD pathology. CD200 negatively correlated with density of neurofibrillary tangles, phosphorylated tau and amyloid plaque density. ICAM-1 positively correlated with these AD pathology measures. Double immunohistochemistry for phosphorylated α-synuclein and markers for microglia showed limited association of microglia with LB pathology, but microglia strongly associated with amyloid plaques or phosphorylated tau. These results suggest that there are different features of inflammatory pathology in diseases associated with abnormal α-synuclein compared to AD.
Keywords: Inflammation, ELISA, neuropathology, neuroprotection, plaques, tangles, α-synuclein
1. Introduction
Both Alzheimer’s disease (AD) and diseases with Lewy body (LB) pathology (abnormal deposits of modified α–synuclein) have increased numbers of activated microglia associated with pathological features (Mrak and Griffin, 2007), which is taken as evidence for increased tissue inflammation. In AD brains, the strongest microglial responses are to neuritic plaques containing extracellular amyloid beta (Aβ) peptide deposits [example (Mrak, 2012)]. In LB diseases, inflammatory features of the substantia nigra (SN) of Parkinson’s disease (PD) cases have been the most widely studied, where greatly increased numbers of activated microglia are present (Croisier et al., 2005; Hirsch, 1993; Imamura et al., 2003; Long-Smith et al., 2009; McGeer et al., 1988; Nagatsu and Sawada, 2005; Zucca et al., 2004). With respect to dementia with Lewy bodies (DLB), where cortical LBs are the main pathological feature, some studies have shown significantly increased numbers of activated microglia associated with LB structures (Iseki et al., 2000; Mackenzie, 2000; Mrak and Griffin, 2007; Togo et al., 2001), while others have not confirmed these findings (Rozemuller et al., 2000; Shepherd et al., 2000). These studies also observed a significant role for the amount of concurrent AD pathology in determining the degree of microglial activation in these LB diseases. The presence of coexisting AD is a common feature in LB disease affected brains (Dugger et al., 2014) .
Defining inflammation associated with different pathologies in human autopsy brain tissues by measuring levels of inflammatory cytokines, such as interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, is one approach, but has been problematic as their levels are very low (Imamura et al., 2005; Katsuse et al., 2003; Sawada et al., 2006; Walker et al., 2015). Markers such as CD200 and intercellular adhesion molecule-1 (ICAM-1) also reflect inflammatory changes (Wang et al., 2011) and can be readily measured. Both CD200 and ICAM-1 have been studied in relation to neuroinflammation in AD (Akiyama et al., 1993; Frohman et al., 1991; Verbeek et al., 1994; Walker et al., 2009; Zhang et al., 2005) and PD (Luo et al., 2010, Miklossy et al, 2006). Both proteins share similar structures, but have opposite functions. CD200 is a ligand for myeloid cells expressing CD200 receptor (CD200R). The interaction of CD200 with CD200R results in activation of anti-inflammatory signaling in CD200R-expressing cells (Lyons et al., 2012, 2009; Mihrshahi et al., 2009). In human AD brains, we showed decreased CD200 mRNA and protein compared to controls suggesting a deficit in this anti-inflammatory system (Walker et al., 2009). Human and experimental animal studies have shown that CD200 expression is reduced in inflammatory conditions and aging (Frank et al., 2006, 2007; Maarouf et al., 2011, 2014), while blocking CD200 and CD200R interactions can result in significantly increased tissue inflammation (Meuth et al., 2008; Wang et al., 2011; Zhang et al., 2011). Increasing expression of CD200 in a mouse model of AD had significant neuroprotective effects (Varnum et al., 2015). By comparison, increased expression of ICAM-1 is associated with many inflammatory conditions (Hoarau et al., 2011; Xu and Li, 2009; Yang et al., 2008; Zakynthinos and Pappa, 2009). Cellular ICAM-1 expression is normally low, but is rapidly induced by proinflammatory cytokines (An et al., 2011). In AD-affected brains, ICAM-1 immunoreactivity was associated with plaques, and astrocytes surrounding plaques (Akiyama et al., 1993; Eikelenboom et al., 1994; Frohman et al., 1991; Verbeek et al., 1994), while in PD-affected brains, there was increased immunoreactivity for ICAM-1 in microglia and astrocytes (Imamura et al., 2003; Miklossy et al., 2006). Increased expression of ICAM-1 on cerebral vascular endothelial cells is also a feature of inflammation in brain tissue (Doerck et al., 2010).
There is experimental evidence that aggregated extracellular α-synuclein can induce an activated phenotype in cultured microglia (Béraud et al., 2011; Béraud and Maguire-Zeiss, 2012; Klegeris et al., 2008; Lee et al., 2010; Zhang et al., 2005), similar to what has been observed with aggregated amyloid beta (Aβ) peptide (Walker et al., 2006). Microglial activation by α-synuclein has also been shown in transgenic α-synuclein models of LBD (Gao et al., 2011, 2008; Sekiyama et al., 2012; Su et al., 2008). Overall, there are much less biochemical data on inflammatory events in LB disease-affected human brains compared to experimental animal models (Imamura et al., 2005; Katsuse et al., 2003; Sawada et al., 2006).
We have described a Unified Staging System to define the pathological progression and spread of LB pathology in different brain regions in cases with various LB disorders (Beach et al., 2009), We hypothesized that tissue samples from cases classified according to this system would be well-suited to examine how these inflammatory markers might change with increasing formation of abnormal α-synuclein-containing structures. It was expected that if inflammatory changes were occurring in response to α-synuclein pathology, there should be progressive changes with increasing LB pathology. However, our results demonstrated that coexistent AD pathology in these cases had significant effects on changes in CD200 and ICAM-1 levels, while the magnitude of LB pathology did not.
2. Methods
2.1. Human brain tissue samples and diagnoses criteria
Human brain tissue samples of temporal and cingulate cortex used in this study were obtained from the Banner Sun Health Research Institute Brain and Body Donation Program (Beach et al., 2008)(Beach et al., 2015). The operations of the Brain and Body Donation Program has been continuously reviewed by an Institutional Review Board. Tissue was taken from the right hemisphere of each donor and frozen at autopsy in 1 cm thick coronal slices on dry ice, then stored at −70 to −80°C. Tissues samples from cingulate cortex and temporal cortex from each case were dissected frozen, and then further trimmed so the samples being analyzed contained primarily gray matter.
Details of all cases used in this study are summarized in Table 1. These cases were a subset of a larger series used to develop the Unified Staging System (Beach et al., 2009), and were classified for severity of LB pathology without regard to primary neuropathological diagnosis. The mean Lewy-type synucleinopathy density scores (LTS) derived from Dementia with Lewy bodies (DLB) Consortium templates for the aggregate density of both Lewy bodies and neurites (scale 0–4) were used as a measure of Lewy pathology in 10 brain regions (maximum score 40) (McKeith et al., 2005). Cases were selected to include neurologically normal controls without LB pathology, classified as stage 0, and a range of cases from stage I (Lewy pathology only in olfactory bulb), stage IIa (Lewy pathology predominantly in olfactory bulb and brainstem), stage IIb (Lewy pathology predominantly in olfactory bulb and limbic regions), stage III (Lewy pathology in olfactory bulb, brain stem and limbic regions) and stage IV (significant Lewy pathology extended into neocortex). To obtain adequate sample sizes of each stage, cases were from several different LB disease classifications, including incidental Lewy body disease (ILBD), Alzheimer’s disease with Lewy bodies (ADLB), PD, PD with dementia (PDD) and DLB. To assess severity of AD pathology, sections from 5 brain regions (entorhinal cortex, hippocampus, frontal cortex, temporal cortex and parietal cortex) were stained with Thioflavin S, Gallyas and Campbell-Switzer histological stains and assessed semi-quantitatively for the density of neurofibrillary tangles and amyloid plaques. Each brain region was ranked on a scale of 0–3. By combining the measures across these 5 brain regions, assessment of AD pathology was ranked on a scale of 0–15 for both plaques and tangles (Dugger et al., 2012) Consensus clinical and neuropathological criteria were used to diagnose AD, DLB and PD in these cases (McKeith et al., 2005, 1996; Mirra et al., 1991). Apolipoprotein E (ApoE) genotype was determined for all cases using a PCR restriction fragment polymorphism technique employing DNA extracted from cerebellum (Hixson and Vernier, 1990).
Table 1.
Demographics of combined cases
| Group | Stage 0 | Stage I | Stage IIa | Stage IIb | Stage III | Stage IV |
|---|---|---|---|---|---|---|
| N (143) | 19 | 17 | 5 | 18 | 36 | 48 |
| Male:Female | 12:7 | 8:9 | 3:2 | 9:9 | 22:14 | 28:20 |
| Age at death (years) | 84.1±6.4 | 86.1±4.5 | 87.6±3.5 | 80.7±7.3 | 80.9±7.1 | 78.9±5.4* |
| PMD (hours) | 2.5±0.6 | 3.1±0.9 | 2.5±0. | 4.3±3.0* | 3.3±1.3 | 3.3±0.7 |
| ApoE (ε2: ε3: ε4 (%)) | 10.5:76.3:13.2 | 3.1:68.8:28.1 | 10:70:20 | 2.8:58.3:38.9 | 5.6:73.6:20.8 | 6.3:58.3:35.4 |
| LTS (Cing) | 0 | 0 | 0.2±0.45 | 0.47±0.51 | 1.82±0.83 | 3.19±0.73 |
| LTS (Temp) | 0 | 0 | 0 | 0.26±0.45 | 0.94±0.34 | 2.54±0.77 |
| Plaques | 3.8±4.2 | 12.5±2.1* | 9.3±4.2 | 13.2±2.2* | 6.5±5.1 | 10.6±4.3*@ |
| Tangles | 4.4±2.1 | 11.5±4.7* | 5.8±3.9 | 12.3±3.5* | 4.5±2.9* | 8.0±4.4*@ |
Numbers represent mean ± standard deviation
PMD: Postmortem delay. LTS (Cing): Lewy type density score (cingulate cortex)
LTS (Temp): Lewy type density score (temporal cortex)
significant difference (P< 0.05) between group and Stage 0 samples (ANOVA with post hoc test)
significant difference (P<0.05) between stage III and Stage IV samples (ANOVA with post hoc test)
2.2. Tissue Processing
Detergent soluble extracts were prepared by sonicating each tissue sample in 5 volumes (weight to volume) of RIPA buffer (Pierce-ThermoFisher Scientific; 20 mM Tris-HCl, pH 7.5. 150 mM NaCl, 1% Triton X100, 1% sodium deoxycholate, 0.1 % sodium dodecyl sulfate) supplemented with protease and phosphatase inhibitors (Pierce-ThermoFisher Scientific). Extracted samples were centrifuged at 18,000g in a refrigerated microcentrifuge for 30 minutes and the cleared supernatants aliquoted and stored at −80°C. Total protein concentration in each sample was determined using a MicroBCA assay kit with albumin as standard.
2.3. Enzyme Linked Immunosorbent Assays for CD200 and ICAM-1
ELISAs specific for each protein were used to measure CD200 and ICAM-1 levels in tissue samples (R & D Systems, Minneapolis, MN). Due to the detergent extraction procedures used, the assay detects both membrane released CD200 and ICAM-1 as well as their soluble forms. Sandwich ELISA procedures for both measurements were essentially the same and followed the manufacturer’s recommendations. ELISA plates were prepared by diluting the supplied capture antibody at the recommended dilution in phosphate buffered saline (PBS) and adding it to wells of Nunc high-bind microtiter plates for 18 hours at 4°C. Plates were washed with wash buffer (PBS + 0.05% Tween 20) using an automated plate washer, and blocked for 1 hour in 1% bovine serum albumin diluted in wash buffer. Brain extract samples were diluted to predetermined optimal concentrations (2.5 μg/100 μl for CD200 and 0.5 μg/100 μl for ICAM-1) in blocking buffer and added to wells for 2 hours at room temperature. Dilutions of purified recombinant protein for CD200 or ICAM-1 were included on each plate as standards. Bound protein was detected by sequential incubation with biotinylated detection antibodies to CD200 or ICAM-1, followed by incubation with Streptavidin conjugated horseradish peroxidase (HRP) (1:200), and then tetramethylbenzidine (TMB) substrate. The concentrations of CD200 or ICAM-1 in samples were obtained from absorbance readings at 450 nm converted into ng of CD200/mg protein or ng of ICAM-1/mg of total protein based on the values obtained from the standard curves prepared from the purified proteins. Intra-assay variability for each product was calculated based on values of samples repeated on separate plates.
2.4. Western Immunoblot Analyses
Relative levels of soluble and insoluble phosphorylated tau (serine 202/threonine 205) were measured in temporal cortex samples from most cases. To characterize the properties of the phosphorylated tau (pTAU), brain samples were homogenized with Tris-buffered saline and centrifuged to produce a soluble (cytosolic-enriched) fraction and the resulting pellet was extracted with RIPA buffer and centrifuged to produce a membrane-enriched detergent soluble fraction. These fractions were analyzed by western blot following our published procedure and detected using the mouse monoclonal antibody AT8 (Pierce/ThermoFisher Scientific) that recognizes tau phosphorylated at serine 202/threonine 205. The pTAU positive bands were detected and quantified using a Flurochem Q imaging system (Protein Simple, San Jose, CA). Blots were reprobed with an antibody to β-actin (Abcam, Cambridge, MA), and reimaged for normalization purposes. In addition, Western blots for CD200 and ICAM-1 were carried out on a subset of samples to verify the specificity and changes in expression detected by ELISA, and also with antibodies to detect synaptic proteins synaptophysin, SNAP25 and PSD95.
2.5. Immunohistochemistry
Formaldehyde-fixed tissue sections from temporal cortex were used for cellular localization of phosphorylated α-synuclein, Aβ peptide and phosphorylated tau with activated microglia according to our previously published procedures (Walker et al., 2009). Antibodies used in this study are listed in Table 2. Localization of bound antibody was visualized using avidin-biotin HRP complex (ABC-Vector Laboratories, Burlingame, CA) histochemistry and nickel ammonium sulfate-enhanced diaminobenzidine as substrate to produce a purple reaction product. The second round of histochemistry was carried out using the mouse monoclonal antibody LN3 (Abcam, Inc, Cambridge, MA), which identifies HLA-DR, a marker of activated microglia. This second antibody was detected using the same procedure, but with diaminobenzidine without nickel ammonium sulfate as substrate to produce a brown reaction product.
Table 2.
Primary Antibodies Used in study.
| Name | Epitope | Manufacturer | Host Species | Dilution |
|---|---|---|---|---|
| p-α-syn | Ser 129 α-synuclein | Custom | Rabbit polyclonal | 1:10,000 |
| LN3 | HLA-DR | Abcam, (Cambridge, MA) | Mouse monoclonal | 1:750 |
| 6E10 | Aβ 1–16 | Covance (Dedham, MA) | Mouse monoclonal | 1:2000 |
| AT8 | PHF-tau (Ser 202/Thr205) | Thermo Scientific (Waltham, MA) | Mouse monoclonal | 1:3000 |
| ICAM-1 | ICAM-1 | R&D Systems (Minneapolis, MN) | Goat polyclonal | 1:1000 |
| CD200 | CD200 | R&D Systems | Goat polyclonal | 1:2000 |
| SY38 | Synaptophysin | Abcam | Mouse monoclonal | 1:2000 |
| PSD95 | PSD95 peptide | Abcam | Rabbit monoclonal | 1:5000 |
| SNAP25 | SNAP25 peptide | Abcam | Rabbit monoclonal | 1:2000 |
2.6. Data Analysis
Data of CD200 and ICAM-1 levels in samples classified by Lewy Body Stage or disease diagnoses were analyzed by one-way Analysis of Variance (ANOVA) with Newman Keuls post hoc test for significance between paired groups. Determination of the relationship between levels of CD200 and ICAM-1 and Lewy density scores, plaque scores and tangle scores was performed using Spearman correlation while Pearson’s method was used for the correlation with soluble and insoluble phosphorylated tau. Significant differences were assumed if P values of less than 0.05 were obtained. All statistical analyses were carried out using Graphpad Prism Version 7 software.
3. Results
3.1. Neuropathological characteristics of brain samples used in study
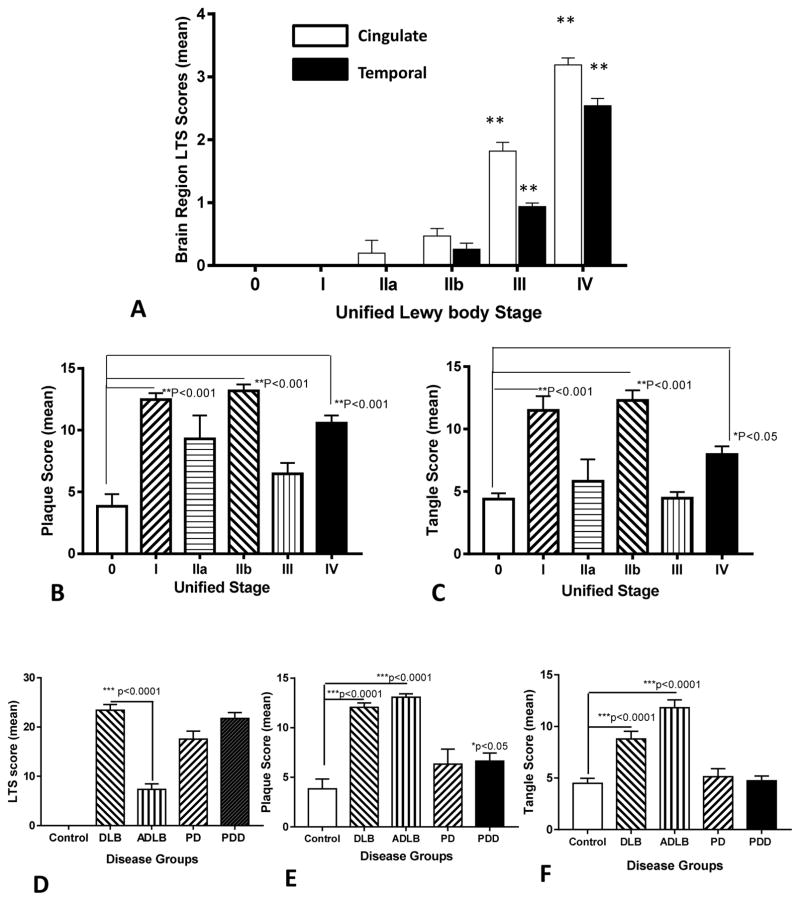
The demographic details of brain tissue samples analyzed in this study are shown in Table 1. These samples were selected to represent a spectrum of cases with varying degrees of LB pathology classified according to the Unified Staging System. In Fig. 1A, the relative progression of changes of LB scores, as the measure of α-synuclein pathology, with Stage in the cingulate cortex and temporal cortex is shown. Consistent with this staging system, where spread of α-synuclein structures occur first to limbic cortex before neocortex, there are higher LB scores (LTS) in the cingulate cortex than temporal cortex. The values presented in Fig. 1A show the mean individual LTS scores for cingulate or temporal cortex (0–4 ranking). These same cases were assessed for the degree of Alzheimer’s disease (AD) pathology as demonstrated by the total brain plaque scores (Fig. 1B) or total tangle scores (Fig. 1C). There were significantly increased numbers of plaques and tangles in the Stage I, IIb, and Stage IV cases. Stage I and Stage IIb cases had a primary neuropathological diagnosis of ADLB, but Stage IIb cases had significantly higher levels of LB pathology in the amygdala (mean LTS score 3.6) and the transentorhinal cortex (mean LTS score 2.61), but with low levels in cingulate and temporal cortex (Fig. 1A). Stage IV cases had neuropathological diagnoses of DLB (57%) or PD (43%). Using the samples described here, the goal of this study was to determine whether cases with progressively increasing LB pathology showed changes in inflammation using altered levels of CD200 and ICAM-1 as the markers for this feature. The different mean levels of total LTS scores (Fig. 1D), total plaque scores (Fig. 1E) and total tangle scores (Fig. 1F) for these staged cases when segregated into disease groups are shown. It can be seen that many of the DLB cases (primarily stage IV) with the highest LTS values also had significant amounts of AD pathology. There were no differences in LTS scores between DLB and PD or PDD cases (Fig. 1D).
Fig. 1. Lewy body and Alzheimer’s disease pathology in cases used in study.
(A). Changes in mean Lewy–type synucleinopathy (LTS) density scores with Unified Stage in cingulate cortex (white bars) and temporal cortex (black bars) in samples used in this study (see Table 1). LTS scores in Stage III and Stage IV were significantly different (** P< 0.01) from Stage 0. Lewy body pathology was significantly greater in Stage III and Stage IV cingulate cortex samples compared to Stage III and Stage IV temporal cortex samples. Results shown on bar graphs are mean ± standard error of mean (SEM). (B). Changes in mean plaque scores with Unified Stage. Results show significantly increased plaque scores in Stage I, Stage IIb and Stage IV cases. Results shown on bar graphs are mean ± standard error of mean (SEM). Significantly increased plaque scores in Stage I, Stage IIb and Stage IV compared to Stage 0. (C). Changes in mean tangle scores with Unified Stage. Results show significantly increased tangle scores in Stage I, Stage IIb and Stage IV cases. Results shown on bar graphs are mean ± standard error of mean (SEM). Significantly increased tangle scores in Stage I, Stage IIb and Stage IV compared to Stage 0. (D–E). Mean total LTS scores (D), plaque scores (E) and tangle scores (F) for samples categorized according to neuropathology diagnosis. Results shown on bar graphs are mean ± standard error of mean (SEM). DLB: Dementia with Lewy bodies: ADLB; Alzheimer’s disease with Lewy bodies. PD: Parkinson’s disease. PDD; Parkinson’s disease with dementia.
3.2 Changes in CD200 and ICAM-1 levels in Unified Staged LBD cases
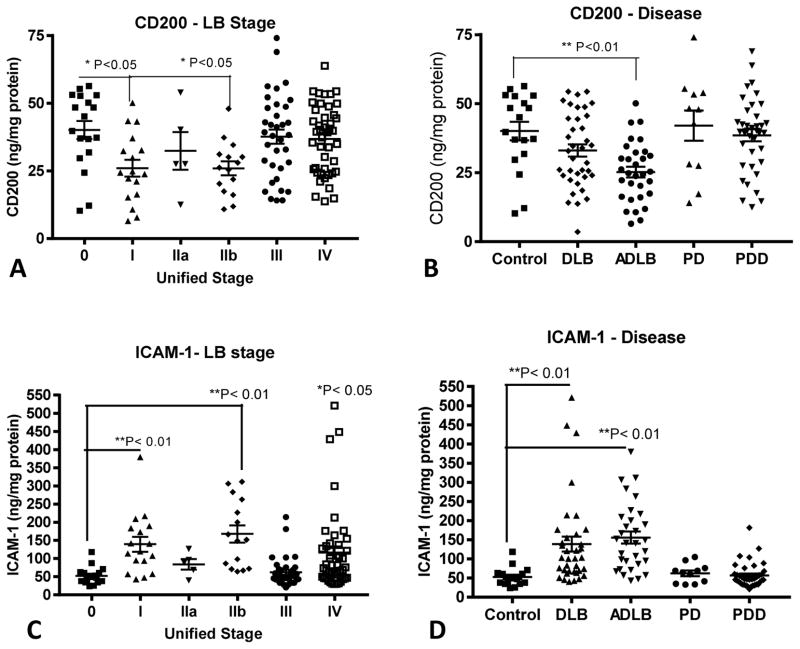
Levels of CD200 were significantly lower in temporal cortex samples of Stage I and Stage IIb samples compared to Stage 0, however levels were unchanged between Stage 0 cases (no temporal cortex LB pathology), Stage III cases (moderate levels of LB pathology) and Stage IV cases (severe LB pathology) (Fig. 2A). The samples with significantly lower levels of CD200 had a primary diagnosis of ADLB (Fig. 2B). By comparison, changes in ICAM-1 expression followed an opposite pattern; the highest levels were present in Stage I and Stage IIb cases (**P< 0.01), but with significantly increased levels in Stage IV cases (*P<0.05) (Fig. 2C) as well. Stage III cases with moderate levels of Lewy body pathology (Fig. 1A) and low levels of AD pathology (Fig. 1B and 1C) had essentially Stage 0 (control) levels of CD200 and ICAM-1 in temporal (Fig. 2) and cingulate cortex (Fig. 3).
Fig. 2. Relative levels of CD200 and ICAM-1 according to Unified Stage and Disease Group in temporal cortex.
Scatter plots showing distributions of CD200 measurements (A and B) with Unified Stage (A) or Disease Group (B), and for ICAM-1 (C and D) with Unified Stage (C) or Disease Group (D). Bars indicate positions of mean ± standard error of mean (SEM). Statistical significances between groups indicated. DLB: Dementia with Lewy bodies: ADLB; Alzheimer’s disease with Lewy bodies. PD: Parkinson’s disease. PDD; Parkinson’s disease with dementia.
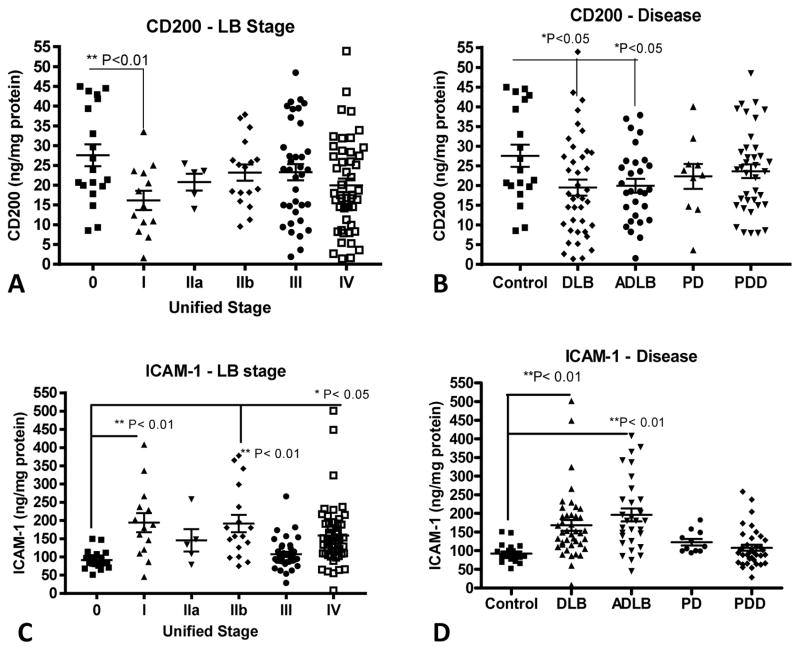
Fig. 3. Relative levels of CD200 and ICAM-1 according to Unified Stage and Disease Group in cingulate cortex.
Scatter plots showing distributions of CD200 measurements (A and B) with Unified Stage (A) or Disease Group (B), and for ICAM-1 (C and D) with Unified Stage (C) or Disease Group (D). Bars indicate position of mean ± SEM. Statistical significances between groups indicated. DLB: Dementia with Lewy bodies: ADLB; Alzheimer’s disease with Lewy bodies. PD: Parkinson’s disease. PDD; Parkinson’s disease with dementia.
Similar patterns of changes in amounts of CD200 and ICAM-1 were seen in cingulate cortex samples, even though Stage III and Stage IV cases from this region had higher levels of LB pathology than temporal cortex (Fig. 3A – 3D). The differences between cingulate cortex and temporal cortex results were that there was no significant decrease in CD200 levels in cingulate cortex Stage IIb samples, but the decrease in CD200 in the DLB samples reached statistical significance (Fig. 3B). There were significant differences between cingulate and temporal cortex in the Stage 0 levels of these proteins. The mean control (Stage 0) levels of CD200 in temporal cortex was 40.3 ng/mg protein and 27.6 ng/mg in cingulate cortex (**P=0.0065, t-test). By comparison, the mean control (Stage 0) levels of ICAM-1 were 52.6 ng/mg in temporal cortex and 92 ng/mg in cingulate cortex (***P=0.0008. t-test). A summary of these measures in all Stages are shown in Table 3. Overall, there were significant negative correlations between CD200 and ICAM-1 levels in temporal cortex and cingulate cortex (supplemental Fig. 1). A representative western blot is shown to illustrate the specificity of changes in values measured by ELISA (supplemental Fig. 2).
Table 3.
Changes in CD200 and ICAM-1 levels with Unified Stage
| CD200 (ng/mg protein ± std. dev.) | Stage 0 | Stage I | Stage IIa | Stage IIb | Stage III | Stage IV |
|---|---|---|---|---|---|---|
| Temporal Cortex (N) | 40.1±14.1 (18) | 26±12.8* (17) | 32.4±15.6 (5) | 26±10* (15) | 37.7+15.5 (35) | 36.8±12.7 (44) |
| Cingulate Cortex (N) | 27.6±12.2 (18) | 16.1±8.9** (16) | 20.8+4.8 (5) | 23.2+8.6 (17) | 23.3+12.4 (36) | 19.9±11.8 (47) |
| ICAM-1 (ng/mg protein ± std. dev.) | ||||||
| Temporal Cortex (N) | 52.6±23.6 (18) | 139.3±83.5** (17) | 84.1±32.2 (5) | 167.8±91.8** (15) | 62.5±40.2 (35) | 116.7±111.3* (44) |
| Cingulate Cortex (N) | 92.1+25.6 (18) | 193.9±97.8** (16) | 145.4±68.5 (5) | 191.9±98.3** (17) | 107.4±42.6 (36) | 158.8±88.6* (47) |
Statistical significance difference (ANOVA) between indicated stage and stage 0.
P<0.01;
P<0.05 (N): number of cases analyzed for each stage
3.3 Correlations of CD200 and ICAM-1 brain levels with Lewy density, plaque or tangle scores
As CD200 and ICAM-1 levels in cingulate cortex and temporal cortex did not change with Unified Stage, we also assessed whether levels correlated positively or negatively with overall LTS. There were no correlations between CD200 levels or ICAM-1 levels and LTS scores in temporal or cingulate cortex (Table 4). Similarly, when comparing CD200 or ICAM-1 levels with individual LTS from temporal cortex or cingulate cortex alone (scale 0–4), there were no correlations (data not shown).
Table 4.
Correlation of CD200 and ICAM-1 levels with Lewy body pathology (LTS), plaque scores and tangle scores
| Factor | Lewy Body LTS | Plaque scores | Tangle scores |
|---|---|---|---|
| Temporal cortex | |||
| CD200 | 0.167 (P=0.06) | −0.21(*P=0.015) | −0.35 (***P=<0.0001) |
| ICAM-1 | 0.024 (P=0.79) | 0.7 (***P=<0.0001) | 0.68 (***P=<0.0001) |
| Cingulate Cortex | |||
| CD200 | 0.005 (P=0.55) | −0.07(P=0.38) | −0.21 (*P=0.015) |
| ICAM-1 | 0.11 (P=0.21) | 0.7 (***P=<0.0001) | 0.57 (***P=<0.0001) |
Results represent Spearman r non-parametric correlations between ELISA values and pathology ranking scores.
A different pattern was observed when levels of CD200 and ICAM-1 were correlated with measures of AD pathology (Table 4). In temporal cortex, levels of CD200 negatively correlated with plaque score (r = −0.21, *P=0.015) and tangle score (r=0.35, ***P< 0.0001), and ICAM-1 levels positively correlated with plaque score (r=0.7, ***P< 0.0001) and tangle score (r= 0.68, ***P< 0.0001). CD200 levels in cingulate cortex did not correlate with plaque score (r=−0.07, P=0.38), but there was a significant negative correlation with tangle score (r=−0.21, *P=0.015). By comparison, ICAM-1 levels in cingulate cortex strongly correlated with both plaque (r=0.60, ***P< 0.0001) and tangle scores (r=0.57, ***P< 0.0001). The results with both CD200 and ICAM-1 suggest that there are progressively increased inflammatory responses occurring with the increasing severity of the AD pathology. The fact that there were control levels of ICAM-1 in samples of Stage III cases in both cingulate and temporal cortex (Table 3), whose level of AD pathology is not significantly different from Stage 0 levels, indicates that increased formation of abnormal α-synuclein structures was not associated with this inflammatory response.
To confirm this, we examined Lewy body staged samples from cases that contained little or low levels of AD pathology (plaque and tangle scores < 5), but excluded Stage 0 cases due to the absence of LB pathology. In these samples, we showed that CD200 or ICAM-1 levels did not correlate with either total or brain region LB scores (Table 5). We also examined whether there were correlations between CD200 and ICAM-1 levels and LB scores in cingulate cortex and temporal cortex using only the measurements from Stage 0 and Stage III samples. Results also showed no significant correlations between these measures (Table 5), confirming our conclusion.
Table 5.
Absence of correlation of CD200 and ICAM-1 levels with Lewy body pathology scores (LTS) in low AD pathology cases.
| Factor | Spearman R | P value | N |
|---|---|---|---|
| Cingulate cortex (All cases/Low AD) | |||
| CD200 | 0.311 | 0.122 NS | 26 |
| ICAM-1 | − 0.266 | 0.188 NS | 36 |
| Temporal cortex (All cases/Low AD) | |||
| CD200 | 0.184 | 0.368 NS | 27 |
| ICAM-1 | − 0.306 | 0.155 NS | 24 |
| Cingulate cortex (Stage 0 and Stage III cases) | |||
| CD200 | 0.094 | 0.497 NS | 54 |
| ICAM-1 | 0.120 | 0.396 NS | 49 |
| Temporal cortex (Stage 0 and Stage III cases) | |||
| CD200 | 0.061 | 0.671 NS | 52 |
| ICAM-1 | − 0.017 | 0.908 NS | 51 |
3.4 Effect of apolipoprotein E 4 allele on CD200 and ICAM-1 levels
The possession of one or two apolipoprotein E4 (apoE 4) alleles increases the risk of AD and also is associated with increased inflammatory changes. We compared CD200 and ICAM-1 protein levels in all cases divided into groups with no apoE 4 allele (apoE 4-) or with one or two alleles (apoE 4+). ICAM-1 levels were significantly higher in apoE4 + cases, while CD200 levels were not significantly different (Table 6).
Table 6.
Influence of apolipoprotein E 4 allele on CD200 and ICAM-1 levels across Unified Staged LB cases.
| Temporal Cortex | apoE 4 (−)(n=77) | apoE 4 (+)(n=59) | P |
|---|---|---|---|
| CD200 (ng/mg protein) | 35.4 (±1.61) | 33.8 (± 1.94) | NS0.50 |
| ICAM-1 (ng/mg protein) | 90.9 (±10.4) | 116.5 (±11.2) | *0.013 |
| Cingulate Cortex | |||
| CD200 (ng/mg protein) | 23.3 (±1.4) | 21.0 (± 1.4) | NS0.77 |
| ICAM-1 (ng/mg protein) | 125.6 (± 6.7) | 164.1 (±12.4) | *0.014 |
Samples were separated into cases with no apoE 4 alleles (apoE 2/3, apoE 3/3) and those with one or two apoE 4 alleles (apoE 2/4, apoE 3/4 and apoE 4/4). Results are expressed as mean values (± standard error of mean). Statistical analyses with Mann Whitney non-parametric U Test.
3.5. Phosphorylated tau levels correlate with CD200 or ICAM-1 levels, not Lewy body pathology
In previous sections, we have presented data to determine if there was correlation between biochemical measurements of CD200 and ICAM-1 with non-parametric histological measures. To extend these findings, we correlated western blot measurements of levels of pTAU in temporal cortex of a subset of the cases, to provide a biochemical measure of tangle load, with CD200 and ICAM-1 levels. We focused on this biochemical measure of tangle pathology as there were stronger correlations with tangle scores than plaque scores. We compared brain samples that had been separated into soluble and insoluble (detergent soluble) fractions to also assess changes in pTAU solubility, a pathological feature of AD, with CD200 and ICAM-1 levels.
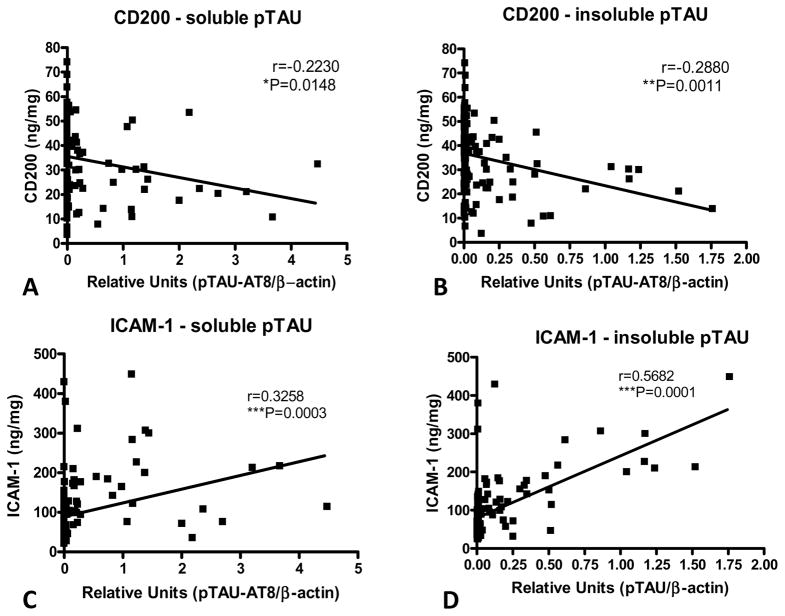
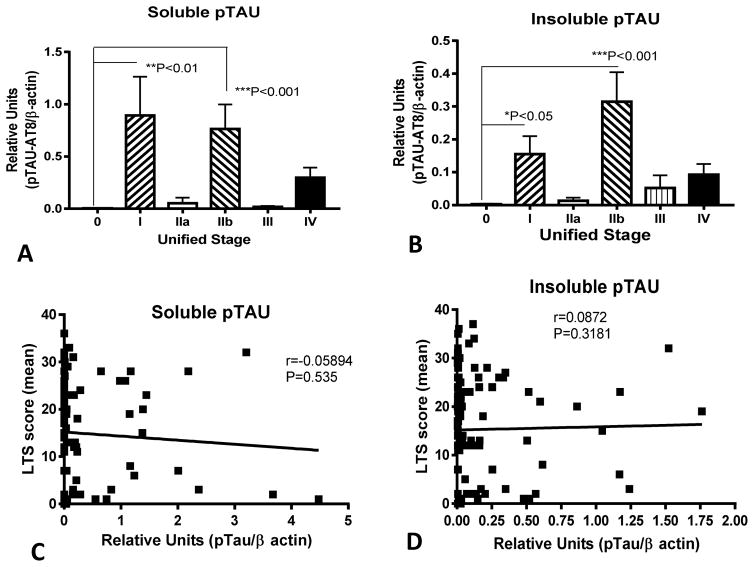
We detected significant negative correlations between soluble (Fig. 4A) and insoluble (Fig. 4B) pTAU levels and CD200, and strong positive correlations between pTAU levels and ICAM-1 (Fig. 4C and 4D). These results generally agree with the analyses of CD200 and ICAM-1 levels with histological tangle scores. Similar to our earlier analyses of Unified Stage, LTS and tangle scores, these results showed significantly higher levels of pTAU (soluble and insoluble) in Stage I and Stage IIb samples, but there were no progressive changes between temporal cortex soluble or insoluble pTAU levels and LB stage (Fig. 5A and 5B), and there was no correlations between soluble and insoluble pTAU levels and LB scores (Fig. 5C and 5D). A representative western blot for pTAU in a series of cases is included as part of supplemental Fig. 2.
Fig. 4. Significant correlations between soluble and insoluble phosphorylated tau and CD200 or ICAM-1 levels in temporal cortex.
Significant negative correlations between soluble pTAU (A) or insoluble pTAU (B) and CD200 levels in temporal cortex confirm association demonstrated using histological tangle scores. Significant positive correlations between soluble pTAU (C) or insoluble pTAU (D) and ICAM-1 levels in temporal cortex confirm association demonstrated using histological tangle scores.
Fig. 5. Lack of association of phosphorylated tau levels with Unified Stage or Lewy Type Synucleinopathy Scores.
In confirmation of histological tangle scores, there was no association of phosphorylated tau levels with unified stage (A and B) or for LTS (C and D). These results confirm that pTAU levels were only increased in cases with higher levels of AD pathology.
3.6. Microglial interactions with phosphorylated α-synuclein in sections from LB staged brain tissue samples
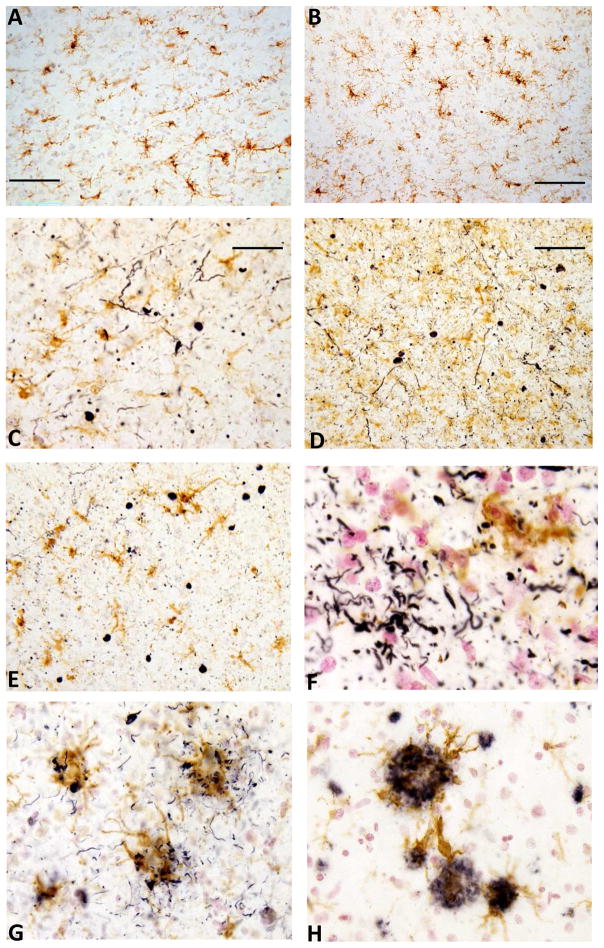
To directly examine the nature of the immune response to LB structures, we used immunohistochemistry to determine if there was evidence of increased microglial responses to phosphorylated α-synuclein positive structures (Fig. 6). Sections of temporal cortex from selected cases used in the biochemical measures were double-stained with antibodies to phosphorylated-α-synuclein and MHCII to determine if there was colocalization of these features. The antibody to phosphorylated-α-synuclein sensitively detected LB structures including Lewy bodies and Lewy neurites (Fig. 6). The levels of activated microglia in a control case with no LB or AD pathological structures (Fig. 6A) and a control case with no LB pathology and low amounts of Aβ plaques and tangles (Fig. 6B) are shown. By comparison, the relative differences in microglial activation in a PD case with limited AD pathology (Fig. 6C) and a similar case with higher levels of AD pathology (Fig. 6D) are shown. Examination of additional cases revealed limited interaction of microglia with LBs (Fig. 6E) or Lewy neurites (Fig. 6F). Both cases had similar levels of LB pathology and moderate to severe levels of AD pathology. For comparison, we also examined matched samples for colocalization of phosphorylated tau positive tangles (Fig. 6G) or Aβ plaques (Fig. 6H). It can be seen that there is considerable colocalization of activated microglia (brown) with phosphorylated tau (Fig. 6G) or Aβ peptide (Fig. 6H).
Fig. 6. Patterns of microglial activation in staged human brain sections.
Double immunohistochemistry was carried out to determine the pattern of colocalization of microglia with phosphorylated α-synuclein (A–F), phosphorylated tau (G) and amyloid beta peptide (H) using temporal cortex from cases with progressive degrees of LB and AD pathology. Sections were stained with antibody to phosphorylated α-synuclein (A–F), AT8 to phosphorylated tau (G) or 6E10 to Aβ (H) in the first round of immunohistochemistry. These proteins were identified by reaction with nickel containing diaminobenzidine to produce dark purple color. All sections were subsequently reacted with antibody LN3 to HLA-DR that recognizes activated microglia, which was localized with diaminobenzidine without nickel to produce a brown color. (A). Double staining of Stage 0 control case (plaque score 0, tangle score 2.75) shows no staining for phosphorylated α-synuclein but identifies a number of activated microglia consistent with aging. (B) Double staining of Stage 0 case with limited amounts of AD pathology (plaque score 6.5, tangle score 4.5) also showing absence of phosphorylated α-synuclein staining and aging associated microglial activation. (C). Double staining of a Parkinson disease dementia (PDD) case (stage IV, plaque score 1.25, tangle score 3.5 and LTS score 24) showing lack of significant association of microglia with Lewy neurites or Lewy bodies. (D). Increased level of overall activation of microglia in an AD case with Lewy bodies (stage IIb, plaque score 14.5, tangle score 14.5, LTS score 20) but no obvious response to phosphorylated α-synuclein structures. (E) Similarly, in a PDD case (stage IV, plaque score 12.5, tangle score 7.25, LTS score 33)), no distinct microglial response to Lewy bodies. (F). Another PDD case (stage III, plaque score 14, tangle score 14, LTS score 23) with no obvious response to immunopositive neuritic structures. (G) and (H): By comparison, in the same case as shown for F, three was evidence of strong microglial reactivity to phosphorylated tau (G) and Aβ (H) structures. Scale bar represent 50 μm.
4. Discussion
One of the goals of this project was to assess whether human brains affected by progressively increasing loads of aggregated α-synuclein as a consequence of different Lewy body (LB) neurodegenerative diseases showed evidence of increased degrees of neuroinflammation. This is a central issue in determining whether human brains structures enriched in abnormal forms of α–synuclein induce inflammatory responses in microglia or astrocytes that might be contributing to disease pathology. To address this, we took the approach of measuring concentrations of the inflammatory-associated proteins CD200 and ICAM-1 in a large series of cingulate cortex and temporal cortex samples classified using the Unified Staging System (Beach et al., 2009) for increasing severity and spread of phosphorylated α-synuclein-immunoreactive Lewy structures. The biochemical distribution and properties of phosphorylated α-synuclein in these samples have been described (Lue et al, 2012; Walker et al, 2013). The major findings from this study were the lack of correlation of CD200 or ICAM-1 levels with LB stage or Lewy body density scores (LTS), but correlation of these changes with the hallmark pathology associated with AD, namely plaque and tangle scores. In these series, we had cases with low levels of LB pathology in the cingulate cortex or temporal cortex, but extensive AD pathology (Stage I and Stage IIb); these cases showed significantly altered expression of CD200 and ICAM-1. We also had cases with moderate to severe levels of LB pathology but low to moderate levels of AD pathology (Stage III and some Stage IV); these cases did not have significantly altered expression of CD200 and ICAM-1. These results would suggest that in human brains, LB structures consisting of abnormal aggregates of phosphorylated α-synuclein do not have the same inflammation-inducing effects compared to AD pathological structures. The presence of mixed pathologies is a feature of most postmortem brains from subjects with neurodegenerative diseases and needs to be considered in human focused studies (Dugger et al., 2012, 2014)
This study has also furthered our understanding of CD200 and ICAM-1 in the human brain. There is now strong data suggesting CD200 – CD200R interactions plays a prominent role in controlling cellular inflammation in many different conditions. We reported significant down regulation of both CD200 and CD200R in AD affected human brains (Walker et al., 2009). This study showed negative correlation of CD200 and CD200R levels with tangle scores. Our findings in this report confirm that AD pathology affects the CD200 regulatory system in a negative way. Altered CD200 expression has also been characterized in human multiple sclerosis brains (Koning et al., 2009). The data also suggested a different mechanism of regulation between CD200 and ICAM-1. The induction of ICAM-1 was more significantly correlated with AD pathology features than was the decline in CD200. This might be due to the major cell types expressing these proteins. In AD brains, neurons appear to be the main cell type expressing CD200 (Walker et al., 2009), while endothelial cells, astrocytes and microglia express increased ICAM-1 under pathological conditions (Akiyama et al., 1993; Miklossy et al., 2006).
Some studies have addressed whether α-synuclein structures are inducing microglia by histological examination for numbers or densities of activated microglia as the marker of inflammation in cortical brain sections from DLB cases (Imamura et al., 2005; Katsuse et al., 2003; Mackenzie, 2000; Rozemuller et al., 2000; Shepherd et al., 2000; Szpak et al., 2001). DLB cases will have significantly higher amounts of abnormal α-synuclein deposits in cortical regions, but these studies have not produced consistent observations on whether there are increased levels of microglial activation. Increased numbers of activated microglia were measured in DLB cases without AD pathology in transentorhinal, cingulate and frontal cortex compared to controls, but there were increased numbers of activated microglia in DLB cases with coexistent AD pathology (Mackenzie, 2000). In these DLB cases, however, the microglia were not specifically associated with the LB/α synuclein structures. A similar finding of enhanced numbers of activated microglia in limbic and neocortex of DLB cases, but at reduced levels compared to AD cases was also reported (Szpak et al., 2001). By comparison, using similar methodology, another study concluded that there were not increased numbers of microglia in DLB cases without AD pathology (Rozemuller et al., 2000). A further study concluded that levels of activated microglia in DLB cases lacking AD associated tau neuritic pathology were similar to non-demented controls (Shepherd et al., 2000), which agrees with our findings.
These findings might be considered unexpected as a number of in vitro studies have established that aggregated α-synuclein activated microglia in a similar manner to aggregated Aβ (Alvarez-Erviti et al., 2011; Austin et al., 2011; Béraud et al., 2011; Klegeris et al., 2008; Lee et al., 2010; Zhang et al., 2005; Qin et al., 2016). Free, extracellular aggregated α-synuclein appears to be highly immunogenic in inducing activation of microglia or astrocytes. Similarly, in vivo models have shown that when different forms of α-synuclein are directly injected into brains of rodents, they can induce inflammation and many pathological features of PD. However, in human LB disorders, the majority of the pathological structures containing α-synuclein are intracellular, which suggests that they may not be accessible to microglia once aggregated into cytoplasmic LB or Lewy neurite inclusions. However, this demonstrates that pathological LB structures are not contributing to inflammation. It was shown that the sequence of α-synuclein between amino acids 62–96 was required for macrophage-activating properties, while peptides with deletion of sequences of this region were not effective at inducing inflammation (Lee et al., 2009). One of the limitations of this study was the fact that we are using histological immunohistochemistry to assess the presence of aggregated and pathological α-synuclein as an inflammatory inducing agent. A number of recent studies have demonstrated the spread of pathological α-synuclein in a prion-like manner involving intracellular spreading and also extracellular secretion of α-synuclein exosomes (Emmanouilidou et al., 2010; Masuda-Suzukake et al, 2013; Sacino et al, 2014; Osterberg et al, 2015). Extracellular forms of soluble oligomeric α-synuclein would not be evident using histological methods, but these forms have been shown to have significant neurotoxic properties (Deas et al., 2016; Kaufman et al, 2016).
In conclusion, we have explored using the proteins CD200 and ICAM-1 as markers in staged human brain samples whether there was evidence for increased inflammatory activation, with increasing severity of α-synuclein containing LB pathology. We can conclude that increased deposition of LB structures did not correlate with altered inflammatory responses caused by LB pathology. It is possible that abnormal α-synuclein in the LB structures found in cingulate and temporal cortex might not be accessible and thus not activate glial cells. However, it was clear that underlying AD pathology had much stronger and significant effects on these inflammatory parameters. As many of our studied cases had both pathologies, distinguishing definitively between the two features in human brains requires further studies in additional brain regions and further cases. However, we can conclude that the mechanisms associated with inflammatory activation in human brains by plaque or tangle pathology differ from those associated with α-synuclein LB pathology.
Supplementary Material
Supplemental Fig. 1. Negative correlations between CD200 and ICAM-1 levels in temporal cortex and cingulate cortex. Results analyzed by Pearson correlation analysis.
Supplemental Fig. 2. Representative Western blots for ICAM-1, CD200, pTAU and IBA-1
A set of cingulate cortex samples used for ELISA were analyzed by western blot for ICAM-1, CD200, pTAU (AT8) and IBA-1. The samples represented different unified stages (0-IV) and different disease diagnoses. The respective sample ELISA values for ICAM-1 and CD200 are annotated above the lanes. Antibodies detected major polypeptide bands with expected molecular weights. Abbreviations: Con; neurologically normal controls: DLB: Dementia with Lewy bodies: ADLB: Alzheimer’s disease with Lewy bodies: PDD: Parkinson’s disease with dementia: PD: Parkinson’s disease.
Supplemental Fig. 3. Representative Western blots for synaptic proteins synaptophysin (SYPH), PSD95 and SNAP25
A set of cingulate cortex samples used for ELISA were analyzed by western blot for synaptophysin (SYPH), PSD 95 and SNAP25. The samples represented different stages (0–IV) and different diseases diagnoses. Antibodies detected major polypeptide bands with expected molecular weights. Abbreviations: Same as in supplemental Fig. 2.
Highlights.
CD200 and ICAM-1 brain levels reflected amount of plaque and tangle pathology
CD200 and ICAM-1 brain levels did not reflect amount of Lewy body pathology
CD200 and ICAM-1 brain levels negatively correlated as a reflection of inflammation
Absence of colocalization of microglia with Lewy body pathology
Inflammatory changes in human brains appear different between amyloid plaques and phosphorylated α-synuclein structures
Acknowledgments
This work was supported by a grant from the Arizona Biomedical Research Commission to the Arizona Parkinson Disease Consortium. The operation of the Banner Sun Health Research Institute Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research (The Prescott Family Initiative). The study sponsors played no role in study design, study execution or data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Kawamata T, Yamada T, Tooyama I, Ishii T, McGeer PL. Expression of intercellular adhesion molecule (ICAM)-1 by a subset of astrocytes in Alzheimer disease and some other degenerative neurological disorders. Acta Neuropathol. 1993;85:628–34. doi: 10.1007/BF00334673. [DOI] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJA. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res. 2011;69:337–42. doi: 10.1016/j.neures.2010.12.020. [DOI] [PubMed] [Google Scholar]
- An Y, Chen Q, Quan N. Interleukin-1 exerts distinct actions on different cell types of the brain in vitro. J Inflamm Res. 2011;2011:11–20. doi: 10.2147/JIR.S15357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin SA, Rojanathammanee L, Golovko MY, Murphy EJ, Combs CK. Lack of alpha-synuclein modulates microglial phenotype in vitro. Neurochem Res. 2011;36:994–1004. doi: 10.1007/s11064-011-0439-9. [DOI] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Serrano G, Shill HA, Walker DG, Lue L, Roher AE, Dugger BN, Maarouf C, Birdsill AC, Intorcia A, Saxon-Labelle M, Pullen J, Scroggins A, Filon J, Scott S, Hoffman B, Garcia A, Caviness JN, Hentz JG, Driver-Dunckley E, Jacobson SA, Davis KJ, Belden CM, Long KE, Malek-Ahmadi M, Powell JJ, Gale LD, Nicholson LR, Caselli RJ, Woodruff BK, Rapscak SZ, Ahern GL, Shi J, Burke AD, Reiman EM, Sabbagh MN. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology. 2015;35:354–89. doi: 10.1111/neup.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béraud D, Maguire-Zeiss KA. Misfolded α-synuclein and Toll-like receptors: therapeutic targets for Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S17–20. doi: 10.1016/S1353-8020(11)70008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béraud D, Twomey M, Bloom B, Mittereder A, Ton V, Neitzke K, Chasovskikh S, Mhyre TR, Maguire-Zeiss KA. α-Synuclein Alters Toll-Like Receptor Expression. Front Neurosci. 2011;5:80. doi: 10.3389/fnins.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RKB, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas E, Cremades N, Angelova PR, Ludtmann MH, Yao Z, Chen S, Horrocks MH, Banushi B, Little D, Wood NW, Gandhi S, Abramov AY. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson's Disease. Antioxid Redox Signal. 2016;24:376–91. doi: 10.1089/ars.2015.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerck S, Göbel K, Weise G, Schneider-Hohendorf T, Reinhardt M, Hauff P, Schwab N, Linker R, Mäurer M, Meuth SG, Wiendl H. Temporal pattern of ICAM-I mediated regulatory T cell recruitment to sites of inflammation in adoptive transfer model of multiple sclerosis. PLoS One. 2010;5:e15478. doi: 10.1371/journal.pone.0015478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger BN, Adler CH, Shill HA, Caviness J, Jacobson S, Driver-Dunckley E, Beach TG. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord. 2014;20:525–529. doi: 10.1016/j.parkreldis.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger BN, Hentz JG, Adler CH, Sabbagh MN, Shill HA, Jacobson S, Caviness JN, Belden C, Driver-Dunckley E, Davis KJ, Sue LI, Beach TG. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. J Neuropathol Exp Neurol. 2014;73:244–252. doi: 10.1097/NEN.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger BN, Serrano GE, Sue LI, Walker DG, Adler CH, Shill HA, Sabbagh MN, Caviness JN, Hidalgo J, Saxon-Labelle M, Chiarolanza G, Mariner M, Henry-Watson J, Beach TG. Presence of Striatal Amyloid Plaques in Parkinson’s Disease Dementia Predicts Concomitant Alzheimer’s Disease: Usefulness for Amyloid Imaging. J Parkinsons Dis. 2012;2:57–65. doi: 10.3233/JPD-2012-11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Zhan SS, Kamphorst W, van der Valk P, Rozemuller JM. Cellular and substrate adhesion molecules (integrins) and their ligands in cerebral amyloid plaques in Alzheimer’s disease. Virchows Arch. 1994;424:421–7. doi: 10.1007/BF00190565. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–22. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frohman EM, Frohman TC, Gupta S, de Fougerolles A, van den Noort S. Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer’s disease. J Neurol Sci. 1991;106:105–11. doi: 10.1016/0022-510x(91)90202-i. [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VMY. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–98. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 2011;119:807–14. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC. Does oxidative stress participate in nerve cell death in Parkinson’s disease? Eur Neurol. 1993;33(Suppl 1):52–9. doi: 10.1159/000118538. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- Hoarau JJ, Krejbich-Trotot P, Jaffar-Bandjee MC, Das T, Thon-Hon GV, Kumar S, Neal JW, Gasque P. Activation and control of CNS innate immune responses in health and diseases: a balancing act finely tuned by neuroimmune regulators (NIReg) CNS Neurol Disord Drug Targets. 2011;10:25–43. doi: 10.2174/187152711794488601. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Ono K, Suzuki H, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Cytokine production of activated microglia and decrease in neurotrophic factors of neurons in the hippocampus of Lewy body disease brains. Acta Neuropathol. 2005;109:141–50. doi: 10.1007/s00401-004-0919-y. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–26. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Iseki E, Marui W, Akiyama H, Uéda K, Kosaka K. Degeneration process of Lewy bodies in the brains of patients with dementia with Lewy bodies using alpha-synuclein-immunohistochemistry. Neurosci Lett. 2000;286:69–73. doi: 10.1016/s0304-3940(00)01090-9. [DOI] [PubMed] [Google Scholar]
- Katsuse O, Iseki E, Kosaka K. Immunohistochemical study of the expression of cytokines and nitric oxide synthases in brains of patients with dementia with Lewy bodies. Neuropathology. 2003;23:9–15. doi: 10.1046/j.1440-1789.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann TJ, Harrison PM, Richardson MJ, Pinheiro TJ, Wall MJ. Intracellular soluble α-synuclein oligomers reduce pyramidal cell excitability. J Physiol. 2016;15:2751–2772. doi: 10.1113/JP271968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29:739–52. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Koning N, Swaab DF, Hoek RM, Huitinga I. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol. 2009;68:159–67. doi: 10.1097/NEN.0b013e3181964113. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, Junn E, Kim HS. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185:615–23. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- Lee S, Park SM, Ahn KJ, Chung KC, Paik SR, Kim J. Identification of the amino acid sequence motif of alpha-synuclein responsible for macrophage activation. Biochem Biophys Res Commun. 2009;381:39–43. doi: 10.1016/j.bbrc.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol. 2009;89:277–87. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Lue LF, Walker DG, Adler CH, Shill HA, Tran H, Akiyama H, Sue LI, Caviness J, Sabbagh MN, Beach TG. Biochemical increase in phosphorylated alpha-synuclein precedes histopathology of Lewy-type synucleinopathies. Brain Pathol. 2012;22:745–756. doi: 10.1111/j.1750-3639.2012.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Zhang JJ, Zhang CD, Liu R, Zheng L, Wang XJ, Chen SD, Ding JQ. Altered regulation of CD200 receptor in monocyte-derived macrophages from individuals with Parkinson’s disease. Neurochem Res. 2010;35:540–7. doi: 10.1007/s11064-009-0094-6. [DOI] [PubMed] [Google Scholar]
- Lyons A, Downer EJ, Costello DA, Murphy N, Lynch MA. Dok2 mediates the CD200Fc attenuation of Aβ-induced changes in glia. J Neuroinflammation. 2012;9:107. doi: 10.1186/1742-2094-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons A, McQuillan K, Deighan BF, O’Reilly JA, Downer EJ, Murphy AC, Watson M, Piazza A, O’Connell F, Griffin R, Mills KHG, Lynch MA. Decreased neuronal CD200 expression in IL-4-deficient mice results in increased neuroinflammation in response to lipopolysaccharide. Brain Behav Immun. 2009;23:1020–7. doi: 10.1016/j.bbi.2009.05.060. [DOI] [PubMed] [Google Scholar]
- Maarouf CL, Daugs ID, Kokjohn TA, Walker DG, Hunter JM, Kruchowsky JC, Woltjer R, Kaye J, Castano EM, Sabbagh MN, Beach TG, Roher AE. Alzheimer’s disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One. 2011;6:e27291. doi: 10.1371/journal.pone.0027291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarouf CL, Kokjohn TA, Walker DG, Whiteside CM, Kalback WM, Whetzel A, Sue LI, Serrano G, Jacobson SA, Sabbagh MN, Reiman EM, Beach TG, Roher AE. Biochemical assessment of precuneus and posterior cingulate gyrus in the context of brain aging and Alzheimer’s disease. PLoS One. 2014;9:e105784. doi: 10.1371/journal.pone.0105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR. Activated microglia in dementia with Lewy bodies. Neurology. 2000;55:132–4. doi: 10.1212/wnl.55.1.132. [DOI] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Brain. 2013;136:128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–91. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VMY, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–24. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Simon OJ, Grimm A, Melzer N, Herrmann AM, Spitzer P, Landgraf P, Wiendl H. CNS inflammation and neuronal degeneration is aggravated by impaired CD200-CD200R-mediated macrophage silencing. J Neuroimmunol. 2008;194:62–9. doi: 10.1016/j.jneuroim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Mihrshahi R, Barclay AN, Brown MH. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J Immunol. 2009;183:4879–86. doi: 10.4049/jimmunol.0901531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197:275–83. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak RE. Microglia in Alzheimer brain: a neuropathological perspective. Int J Alzheimers Dis. 2012;2012:165021. doi: 10.1155/2012/165021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak RE, Griffin WST. Common inflammatory mechanisms in Lewy body disease and Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:683–6. doi: 10.1097/nen.0b013e31812503e1. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- Osterberg VR, Spinelli KJ, Weston LJ, Luk KC, Woltjer RL, Unni VK. Progressive aggregation of alpha synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell reports. 2015;10:1252–1260. doi: 10.1016/j.celrep.2015.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Buckley JA, Li X, Liu Y, Fox TH, Meares GP, Yu H, Yan Z, Harms AS, Li Y, Standaert DG, Benveniste EN. Inhibition of the JAK/STAT Pathway Protects Against α-Synuclein-Induced Neuroinflammation and Dopaminergic Neurodegeneration. J Neurosci. 2016;36:5144–5159. doi: 10.1523/JNEUROSCI.4658-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozemuller AJ, Eikelenboom P, Theeuwes JW, Jansen Steur EN, de Vos RA. Activated microglial cells and complement factors are unrelated to cortical Lewy bodies. Acta Neuropathol. 2000;100:701–8. doi: 10.1007/s004010000225. [DOI] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, McKinney AB, Thomas MA, Shaw G, Golde TE, Giasson BI. Brain injection of alpha-synuclein induces multiple proteinopathies, gliosis, and a neuronal injury marker. J Neurosci. 2014;34:12368–12378. doi: 10.1523/JNEUROSCI.2102-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Imamura K, Nagatsu T. Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transm Suppl. 2006:373–81. doi: 10.1007/978-3-211-45295-0_57. [DOI] [PubMed] [Google Scholar]
- Sekiyama K, Sugama S, Fujita M, Sekigawa A, Takamatsu Y, Waragai M, Takenouchi T, Hashimoto M. Neuroinflammation in Parkinson’s Disease and Related Disorders: A Lesson from Genetically Manipulated Mouse Models of α-Synucleinopathies. Parkinsons Dis. 2012;2012:271732. doi: 10.1155/2012/271732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd CE, Thiel E, McCann H, Harding AJ, Halliday GM. Cortical inflammation in Alzheimer disease but not dementia with Lewy bodies. Arch Neurol. 2000;57:817–22. doi: 10.1001/archneur.57.6.817. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29:1690–701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpak GM, Lechowicz W, Lewandowska E, Bertrand E, Wierzba-Bobrowicz T, Gwiazda E, Schmidt-Sidor B, Dymecki J. Neurones and microglia in central nervous system immune response to degenerative processes. Part 1: Alzheimer’s disease and Lewy body variant of Alzheimer’s disease Quantitative study Folia. Neuropathol. 2001;39:181–92. [PubMed] [Google Scholar]
- Togo T, Iseki E, Marui W, Akiyama H, Uéda K, Kosaka K. Glial involvement in the degeneration process of Lewy body-bearing neurons and the degradation process of Lewy bodies in brains of dementia with Lewy bodies. J Neurol Sci. 2001;184:71–5. doi: 10.1016/s0022-510x(00)00498-6. [DOI] [PubMed] [Google Scholar]
- Varnum MM, Kiyota T, Ingraham KL, Ikezu S, Ikezu T. The anti-inflammatory glycoprotein, CD200, restores neurogenesis and enhances amyloid phagocytosis in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2015;36:2995–3007. doi: 10.1016/j.neurobiolaging.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek MM, Otte-Höller I, Westphal JR, Wesseling P, Ruiter DJ, de Waal RM. Accumulation of intercellular adhesion molecule-1 in senile plaques in brain tissue of patients with Alzheimer’s disease. Am J Pathol. 1994;144:104–16. [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Link J, Lue LF, Dalsing-Hernandez JE, Boyes BE. Gene expression changes by amyloid beta peptide-stimulated human postmortem brain microglia identify activation of multiple inflammatory processes. J Leukoc Biol. 2006;79:596–610. doi: 10.1189/jlb.0705377. [DOI] [PubMed] [Google Scholar]
- Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer’s disease: a potential mechanism leading to chronic inflammation. Exp Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, Akiyama H, Serrano GE, Sue LI, Beach TG. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol. 2013;240:190–204. doi: 10.1016/j.expneurol.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DG, Lue LF, Serrano G, Adler CH, Caviness JN, Sue LI, Beach TG. Altered Expression Patterns of Inflammation-Associated and Trophic Molecules in Substantia Nigra and Striatum Brain Samples from Parkinson’s Disease, Incidental Lewy Body Disease and Normal Control Cases. Front Neurosci. 2015;9:507. doi: 10.3389/fnins.2015.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Zhang S, Yan ZQ, Zhao YX, Zhou HY, Wang Y, Lu GQ, Zhang JD. Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic Biol Med. 2011;50:1094–106. doi: 10.1016/j.freeradbiomed.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li S. Blockade of ICAM-1: a novel way of vasculitis treatment. Biochem Biophys Res Commun. 2009;381:459–61. doi: 10.1016/j.bbrc.2009.02.111. [DOI] [PubMed] [Google Scholar]
- Yang J, Fei M, Gu Y, Sun L, Ben Z, Zhou D, Chen J, Wang Y, Wang P, Shen A, Cheng C. Evaled expression of ICAM-1 and its ligands in the rat spinal cord following lipopolysaccharide intraspinal injection. Neuromolecular Med. 2008;10:385–92. doi: 10.1007/s12017-008-8049-7. [DOI] [PubMed] [Google Scholar]
- Zakynthinos E, Pappa N. Inflammatory biomarkers in coronary artery disease. J Cardiol. 2009;53:317–33. doi: 10.1016/j.jjcc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang XJ, Tian LP, Pan J, Lu GQ, Zhang YJ, Ding JQ, Chen SD. CD200-CD200R dysfunction exacerbates microglial activation and dopaminergic neurodegeneration in a rat model of Parkinson’s disease. J Neuroinflammation. 2011;8:154. doi: 10.1186/1742-2094-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–42. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- Zucca FA, Giaveri G, Gallorini M, Albertini A, Toscani M, Pezzoli G, Lucius R, Wilms H, Sulzer D, Ito S, Wakamatsu K, Zecca L. The neuromelanin of human substantia nigra: physiological and pathogenic aspects. Pigment Cell Res. 2004;17:610–7. doi: 10.1111/j.1600-0749.2004.00201.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Negative correlations between CD200 and ICAM-1 levels in temporal cortex and cingulate cortex. Results analyzed by Pearson correlation analysis.
Supplemental Fig. 2. Representative Western blots for ICAM-1, CD200, pTAU and IBA-1
A set of cingulate cortex samples used for ELISA were analyzed by western blot for ICAM-1, CD200, pTAU (AT8) and IBA-1. The samples represented different unified stages (0-IV) and different disease diagnoses. The respective sample ELISA values for ICAM-1 and CD200 are annotated above the lanes. Antibodies detected major polypeptide bands with expected molecular weights. Abbreviations: Con; neurologically normal controls: DLB: Dementia with Lewy bodies: ADLB: Alzheimer’s disease with Lewy bodies: PDD: Parkinson’s disease with dementia: PD: Parkinson’s disease.
Supplemental Fig. 3. Representative Western blots for synaptic proteins synaptophysin (SYPH), PSD95 and SNAP25
A set of cingulate cortex samples used for ELISA were analyzed by western blot for synaptophysin (SYPH), PSD 95 and SNAP25. The samples represented different stages (0–IV) and different diseases diagnoses. Antibodies detected major polypeptide bands with expected molecular weights. Abbreviations: Same as in supplemental Fig. 2.