Abstract
The treatment of metastatic breast cancer has become more complicated due to increasing numbers of new therapies which need to be tested. Therapies are now being developed to treat special clinical or molecular subgroups. Even though intrinsic molecular subtypes play a major role, more and more new therapies for subgroups and histological subtypes are being developed, such as the use of PARP inhibitors to treat patients with BRCA mutations (breast and ovarian cancer). Supportive therapies are also evolving, allowing problems such as alopecia or nausea and vomiting to be treated more effectively. Treatment-related side effects have a direct impact on the prognosis of patients with metastatic breast cancer, and supportive therapy can improve compliance. Digital tools could be useful to establish better patient management systems. This overview provides an insight into recent trials and how the findings could affect routine treatment. Current aspects of breast cancer prevention are also presented.
Key words: breast cancer, treatment, metastasis, CDK4/6, PD1/PDL1, trials, risk, prevention
Zusammenfassung
Die Behandlung des metastasierten Mammakarzinoms hat bei immer neu zu testenden Therapien deutlich an Komplexität zugenommen. Therapien werden nunmehr nur noch für spezielle klinische oder molekulare Subgruppen entwickelt. Hierbei spielen die intrinsischen, molekularen Subtypen zwar immer noch die größte Rolle, jedoch gibt es zunehmend auch Therapien, die subgruppen- oder sogar histologieübergreifend entwickelt werden, wie z. B. der PARP-Inhibitor bei BRCA-mutierten Patientinnen (Mamma- und Ovarialkarzinom). Aber auch Supportivtherapien entwickeln sich weiter, sodass Probleme wie die Alopezie besser behandelt werden können und neue Therapiearten von Übelkeit und Erbrechen etabliert werden. In einem engen Zusammenhang mit den Supportivtherapien stehen die Nebenwirkungen, welche bei Patientinnen mit einem metastasierten Mammakarzinom einen direkten Einfluss auf die Prognose haben. Hier könnten digitale Werkzeuge helfen, um ein besseres Patientinnenmanagement zu etablieren. Diese Übersichtsarbeit soll diese Aspekte vor dem Hintergrund neuer, aktuell publizierter Studien beleuchten und einen Einblick geben, wie sich diese Studien zu etablierten Routinetherapien verhalten. Zusätzlich werden aktuelle Aspekte der Mammakarzinomprävention beleuchtet.
Schlüsselwörter: Mammakarzinom, Behandlung, Metastasen, CDK4/6, PD1/PDL1, Studien, Risiko, Prävention
Introduction
Significant progress has been made in recent years in the treatment of metastatic breast cancer. The establishment of new targets and the introduction of new substance classes such as antibody-drug conjugates have significantly improved progression-free survival rates and sometimes even the overall survival of some subgroups. Interest continues to focus on understanding how side effects occur and how they should be treated as well as on maintaining patientsʼ quality of life. As it is becoming possible to describe personal risks more precisely, prevention is also becoming more individualized. The basic approaches in metastatic breast cancer, supportive therapies and prevention presented as part of new, recently published trials and at recent conferences (including the 2017 San Antonio Breast Cancer Symposium) are discussed in more detail below.
Treatment of Metastatic HER2-positive and Triple-negative Breast Cancer (TNBC)
Data is consolidating on PARP inhibitors
New targeted therapies for metastatic TNBC (mTNBC) are urgently needed to improve the prognosis of this patient population which has shown only a limited response to other lines of therapy. Several therapeutic approaches have recently been presented at conferences and in published articles.
Last year, it was reported that PARP inhibitors yielded promising results in the treatment of TNBC. In the OlympiAD trial, the PARP inhibitor olaparib showed a benefit with regard to progression-free survival in metastatic patients with proven germline mutations in the BRCA gene compared to selected chemotherapies (capecitabine, eribulin, vinorelbine) 1 . These results led to the drug being approved for use in the USA 2 . Patients with mTNBC especially benefitted. The EMBRACA trial presented data on the PARP inhibitor talazoparib 3 , which was used in an almost identical setting as olaparib in the OlympiAD trial. Here too, progression-free survival (PFS) was significantly extended (8.6 vs. 5.6 months; HR 0.54 [0.41 – 0.71]; p < 0.0001). The objective rate of response was 63% and therefore more than double the rate for chemotherapies (27%). Another study 4 investigated the effect of higher concentrations of talazoparib 5 . But higher systemic concentrations only resulted in more side effects but did not improve efficacy. It appears that the use of PARP inhibitors for TNBC is headed for success. It still unclear, however, whether a BRCA mutation is a precondition for this therapy.
Other antibody-drug conjugates to treat mTNBC
At the latest after the introduction of T-DM1, antibody-drug conjugates became a hot topic of discussion. Sacituzumab govitecan is an anti-Trop-2-SN-38 antibody-drug conjugate, which was used after second-line treatment in 110 patients with mTNBC until tumor progression or limiting toxicity 6 . The antibody is directed against the epithelial antigen Trop-2 and is conjugated with the active metabolite of irinotecan. The objective response rate in this heavily treated patient cohort was 34% with a median duration of response of 7.6 months (95% CI: 4.8 – 11.3). The most important side effects were hematotoxicity (neutropenia grade 3/4: 41%; febrile neutropenia: 8%), fatigue and gastrointestinal symptoms. Sacituzumab govitecan appears to be an interesting drug which merits further research even if the response rates and side effects appear to be comparable to those of other monotherapies. A phase-III trial is already underway in the USA and another phase-III trial is expected to kick-off in Europe in 2018.
Immune checkpoint inhibitors headed for approval
There is a growing body of data on immune checkpoint inhibitors. The pembrolizumab (anti-PD-1) and atezolizumab (anti-PD-L1) antibodies have already been tested in numerous studies on metastatic breast cancer, and the reported response rate was around 20% in patients pretreated for mTNBC 7 . Adams and colleagues recently presented results from the KEYNOTE-086 trial, where the first-line treatment consisted of monotherapy with pembrolizumab (n = 52). The response rate was 23% and the duration of response was 10.4 months. Patients who had partial or complete response (PR, CR) showed a significantly longer duration of response and a longer overall survival 8 .
Numerous studies have investigated combinations of immune checkpoint inhibitors and chemotherapy or radiotherapy, and it is hoped that the number of patients who respond to this approach will increase. A phase-I/II study was recently presented which investigated a combination of eribulin mesylate and pembrolizumab in patients with mTNBC 9 . Irrespective of the PD-L1 status, a response rate of 26.4% was reported for the 107 patients treated in the study. If treatment was successful, the duration of response was 8.3 months, with more than half of patients responding for longer than 6 months.
Another study investigated a combination of pembrolizumab and trastuzumab in patients with trastuzumab-resistant advanced HER2-positive breast cancer 10 . Some level of response (15%) was reported for PD-L1-expressing tumors but not for PD-L1-negative tumors. It appears therefore that HER2-pretreated tumors are not very immunogenic.
In summary, a clear trend has been observed with regard to the use of immune checkpoint inhibitors in breast cancer therapy: individual patients appear to respond very well and for an appreciable length of time to immunomodulatory therapy. Cytotoxic therapies might even achieve further improvements in the rate of response. But for the majority of patients, this therapy does not appear to result in any improvement. As neither positive nor negative predictive factors are known which could predict whether patients will respond, it is at present not possible to preselect patients. Further studies, particularly in the neoadjuvant setting, will determine whether the number and composition of tumor-infiltrating lymphocytes might play a role.
Treatment of Hormone Receptor-positive, HER2-negative Advanced Breast Cancer
Data on CDK4/6 inhibitors in premenopausal patients
In recent years, therapies for patients with metastatic, hormone receptor-positive and HER2-negative breast cancer have significantly improved, although a large percentage of patients still receive chemotherapy 11 . This could be changed by the introduction of therapies which are more effective than anti-hormone monotherapy. After the introduction of everolimus, interest has focused on CDK4/6 inhibitors. More and more studies in other patient cohorts are being published. Results from the MONALEESA-7 trial which investigated the CDK4/6 inhibitor ribociclib in a population of premenopausal patients were recently presented 12 . The trial compared an anti-endocrine therapy of choice (tamoxifen, letrozole or anastrozole) plus ovarian function suppression (OFS) with the same therapy plus the addition of ribociclib. The median progression-free survival was 23.8 months in the ribociclib arm compared with 13 months in the placebo arm ( Fig. 1 ). The overall response rate was significantly higher in patients with a measurable lesion at the start of therapy in the ribociclib arm compared with the placebo arm (51% vs. 36%). Grade 3/4 neutropenia was reported in 61% of patients in the ribociclib arm, although this side effect was not symptomatic in most patients. Patients in the ribociclib arm additionally benefited from a prolongation of the time to deterioration of their quality of life. This combination hormone therapy should therefore be recommended to treat premenopausal high-risk patients.
Fig. 1.

Progression-free survival (response determined by the examiner) in the MONALEESA trials. Goserelin was administered in both arms. NR: not achieved, CI: confidence interval (based on 12 ).
Studies with CDK4/6 inhibitors are combined to answer further questions
The American regulatory authority (FDA) recently presented an analysis in which all studies which filed for approval of a CDK4/6 inhibitor were pooled for analysis 13 . This included studies on palbociclib, ribociclib and abemaciclib in combination with an aromatase inhibitor as the initial therapy for metastatic or inoperable breast cancer. The intention-to-treat population included 1992 patients. Progression-free survival was compared for patients aged ≥ 70 years and patients aged < 70 years. The progression-free survival benefit from CDK4/6 inhibitors was similar for both groups of patients. The side effects were only slightly higher in older patients. The conclusion is that higher age is no reason to refuse therapy with CDK4/6 inhibitors to older patients.
Another pooled analysis combined the datasets of the MONARCH-2 and MONARCH-3 trials to look for predictors which could be used to identify patients who would respond particularly well to therapy with abemaciclib and aromatase inhibitors 14 . Out of all the univariate analyses analyzing progression-free survival, only the interval between primary treatment and the start of therapy was found to be a predictor for the efficacy of abemaciclib in the setting of advanced cancer. The number of previous endocrine therapies, previous endocrine therapy for advanced disease, the time from diagnosis to metastasis, and primary metastatic status were not predictors for the efficacy of abemaciclib. However, no confidence intervals were presented in this analysis, which limits the interpretation of the data.
Establishment of new mTOR inhibitors unsuccessful
The current therapy recommendations and guidelines 15 , 16 recommend using three different endocrine therapies in the metastatic setting. One of these therapies includes the mTOR inhibitor everolimus. Vistusertib is a new dual mTOR inhibitor which has been tested in a single study. Vistusertib plus fulvestrant was compared with a therapy consisting of everolimus and fulvestrant 17 . Progression-free survival was longest (12.3 months) following treatment with everolimus and fulvestrant. Both therapy arms with vistusertib were inferior.
Androgen receptor inhibition attempts to prove its worth
The androgen receptor inhibitor enzalutamide has already shown some benefits in the treatment of TNBC 18 , 19 , 20 . Data on the efficacy of this therapy to treat hormone receptor-positive breast cancer have now also been presented 21 . Therapy with enzalutamide and exemestane was compared with therapy using exemestane alone in patients with advanced breast cancer with and without previous endocrine therapy. No difference between the therapy arms was reported for the overall patient population. However, when analysis was limited to a subgroup in which gene expression testing had detected an activation of the androgen receptor pathway, a significant benefit was detected for patients who had not had prior treatment (hazard ratio [HR]: 0.44; PFS 16.5 vs. 4.3 months). Enzalutamide was even found to benefit patients with prior treatment (HR: 0.55; PFS: 6 vs. 4.3 months). This therapy merits investigation in further studies.
Supportive Therapy
Supportive therapies are playing an increasingly important role in oncology and are being investigated in numerous scientific studies. The often significant side effects of modern multimodal therapies can only be controlled by optimal supportive treatment, and supportive therapy is needed to improve the quality of life and long-term compliance of patients.
Benefits of acupuncture and physical exercise in therapy-induced arthralgia
The problem of compliance is still unresolved, with high numbers of patients terminating their adjuvant endocrine treatment because of side effects such as arthralgia 22 , 23 , 24 , 25 . Acupuncture is an alternative, non-drug therapy for arthralgia. However, the data is inconsistent. A study was carried out to compare the use of acupuncture with feigned acupuncture (short needles at non-acupuncture sites) and a control group over a period of 12 weeks (first 6 weeks: 2 × per week, subsequently 1 × per week) 26 . A total of 226 patients with breast cancer who reported ≥ 3 points on the pain scale (Brief Pain Inventory, BPI) at the start of treatment with an aromatase inhibitor (AI) or who reported increasing pain during AI therapy were included in the study. Acupuncture was carried out in 11 centers by licensed therapists who had received extensive training. Multiple measurements showed significant improvements in the strongest level of pain after 6 weeks (primary endpoint) compared to the feigned acupuncture group (p = 0.01) and the control group who received no form of acupuncture intervention (p = 0.01). Significant improvements were also reported with regard to average levels of pain and joint stiffness. The benefit persisted for 12 weeks after the acupuncture intervention.
Another study investigated the effect of physical exercise on arthralgia due to AI therapy 27 . A significant improvement in pain scores was reported after 12 months of exercise (p = 0.067) if patients followed a program of 120 – 150 minutes of walking or running per week and complied with more than 70% of the planned program. This also positively affected patientsʼ compliance with AI therapy (p = 0.03).
Diarrhea control depends on the treating physician
Neratinib, a pan-Her tyrosine kinase inhibitor, is another oral adjuvant therapy for patients with HER-2-positive breast cancer who have already had adjuvant therapy with trastuzumab. However, neratinib requires optimal supportive therapy. The rate of grade III diarrhea reported in the approval study was 39.8%, which led to termination of the therapy by 16.8% of patients. The CONTROL study compared an intensified loperamide regimen (12 mg/day, d1–14, followed by 6 – 8 mg/day, d15–56) with combinations of loperamide and the steroid budesonide or the cholesterol-lowering drug colestipol 28 . Grade III diarrhea occurred in 30.7, 25.0 and 7.7% of patients, and therapy termination rates were 20.4, 9.4 and 0%, respectively. The addition of budesonide or colestipol appears to reduce the duration and number of diarrhea episodes and could therefore improve compliance with neratinib therapy. The final analysis of the CONTROL study will be carried out when all patients have completed the 12-month neratinib therapy.
Alopecia from chemotherapy partially avoidable
Alopecia is an extremely onerous side effect of chemotherapy. Four studies investigated the efficacy of scalp cooling during therapy. The success rate during treatment with anthracyclines was 50 – 62.5% 29 , 30 . Menopausal status, therapy setting (neoadjuvant versus adjuvant) and dose density had no impact on alopecia rates. The problem is the high rate of around 30% of patients who discontinue scalp cooling because of the pain, with about half of these patients subsequently losing all of their hair 29 . It should be noted, however, that grade I loss of hair after scalp cooling was also rated as a success in the studies. Grade I is a loss of hair of up to 50%, which can also represent a significant burden for patients.
Nausea and vomiting rates continue to fall
Although there are excellent antiemetic drugs and standards, there is still potential for improvement. The use of aprepitant is standard during treatment with anthracyclines, but its use in moderately emetogenic chemotherapies has not been investigated much. A randomized, double-blinded phase-III trial which compared fosaprepitant combined with ondansetron and dexamethasone, d1, with ondansetron, d1–3, and dexamethasone, d1, in 231 patients with breast cancer showed a complete response (no vomiting or need of rescue medication during the trial) in 76.4 vs. 68.6% of patients 31 . Another multicenter study investigated a combination of 5-HT3 and NK1-receptor antagonists in a combined capsule (NEPA) to simplify therapy. The interim analysis presented the results for 2384 patients receiving highly or moderately emetogenic chemotherapy (HEC/MEC) 32 . As regards the primary endpoint, over 90% of the study participants who received HEC or MEC reported that their daily life and quality of life was not affected by vomiting. The rate of complete response (no nausea, no rescue medication) was 74%. Efficacy was rated by both the physicians and the study participants as good to very good in more than 90% of cases.
Neuropathy continues to be a big problem
One of the biggest current challenges is chemotherapy-related neuropathy, particularly over the longer term. A study of 238 patients in the adjuvant setting and 442 patients with metastasis showed that 30% of all studied patients were significantly or strongly affected by neuropathies in their daily life 33 . Neuropathies were significantly associated with sleep disorders, pain, impairments of physical function, restricted social life, fatigue, anxiety and depression. 32% had not received any coping strategies from their treating physician. The lack of counseling with regard to supportive therapies was associated with a significantly higher negative impact.
The above-mentioned studies on supportive therapies clearly illustrate the importance of a holistic approach for patients and the importance of targeting potential side effects both prophylactically and therapeutically.
Bone Oncology
The preventive effect of bisphosphonates on bone metabolism and bone density and their contribution to reducing the risk of fractures has been known since many years. An Oxford meta-analysis once again showed that the adjuvant use of bisphosphonates to prevent osteoporosis after menopause additionally resulted in a 34% improvement in breast cancer-specific disease-free survival (DFS) and even a 17% improvement in overall survival 34 . This has led to a positive recommendation for adjuvant bisphosphonate therapy in parallel to treatment AI in the AGO and the current DGGG S-3 guidelines 15 , 16 .
Duration of adjuvant bisphosphonate therapy
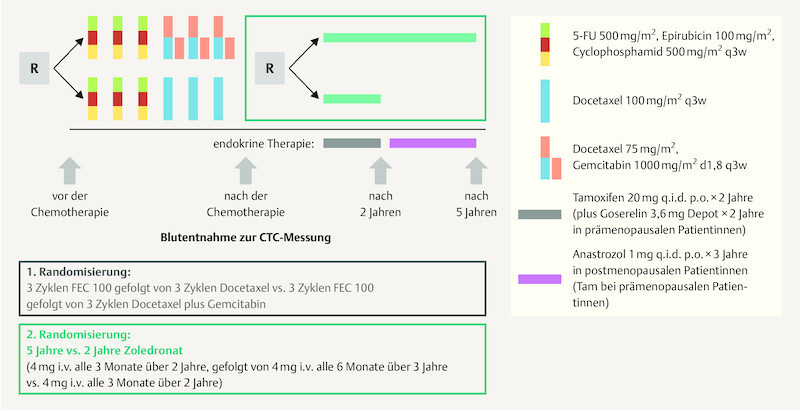
But the question remains how long the respective bisphosphonate therapy should be administered. In this context, the SUCCESS study investigated the impact of 2 yearsʼ (4 mg zoledronic acid every 3 months) vs. 5 yearsʼ (4 mg zoledronic acid every 3 months for 2 years, then every 6 months) bisphosphonate therapy on DFS and overall survival (OS) ( Fig. 2 ) 35 . The results for 2802 patients out of the original 3754 patients were included in the analysis. The two study groups showed no significant differences with regard to their basic characteristics or tumor characteristics. The maximum observation period was 4 years (the median observation period was 2.95 years for DFS and 3 years for OS). DFS and OS were defined based on the STEEP criteria. The results of the analysis showed no significant differences in terms of DFS (p > 0.827) or OS (p > 0.713) between the 2-year and the 5-year bisphosphonate therapy 35 . Multiple Cox regression analysis adjusted for a number of risk factors also showed no significant differences between groups with regard to DFS, bone recurrence or OS in either premenopausal or postmenopausal women with breast cancer. No differences were found between the two groups with regard to circulating tumor cells (CTC). The rate of side effects was significantly higher after 5 years of treatment compared to 2 years of bisphosphonate therapy. Osteonecrosis of the jaw (ONJ) occurred in 11 and 5 cases, respectively, during 5-year or 2-year bisphosphonate therapy. The limitations of the study listed by the authors included the relatively short observation period, the unbalanced rate of “loss to follow up” and the relatively rare events. Unfortunately this study also lacked a control group which did not receive bisphosphonate therapy 35 .
Fig. 2.
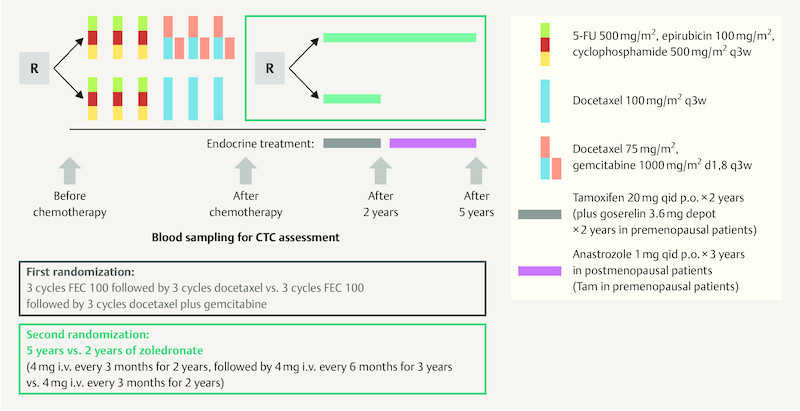
SUCCESS A study design (based on 35 ).
Aromatase inhibitors must be monitored continuously for bone protection
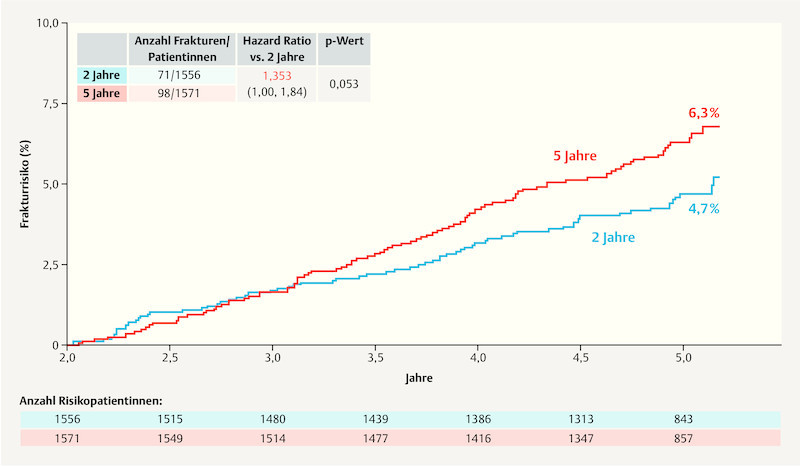
As part of the ABCSG-16 trial, the impact of extending adjuvant endocrine therapy (EAT) with anastrozole by a further 2 vs. 5 years after 4 – 6 years of primary endocrine therapy was investigated. As regards the primary endpoint of disease-free survival, the report 36 found that after 10 years, DFS for the group which had two additional years aromatase inhibitor therapy was 71.1%, while DFS for the group with an additional 5 years was 70.3% and therefore not significantly different (HR: 0.997; 95% CI: 0.86 – 1.15; p = 0.982). As regards the side effect “fractures” the group with 5 years of EAT had a higher rate of fractures compared to the group which received 2 years of anastrozole therapy ( Fig. 3 ) 36 . The figures were not adjusted for potential bone protective therapies.
Fig. 3.
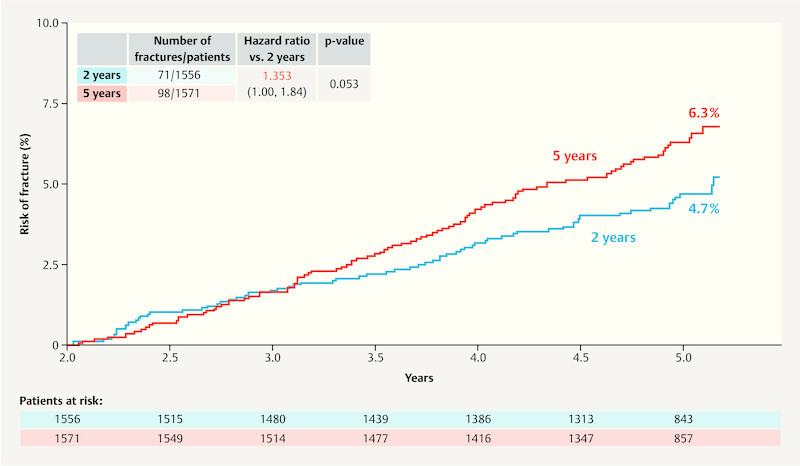
Fracture rates in the ABCSG-16 trial. (2 years: 2 years of therapy with an aromatase inhibitor; 5 years: 5 years of therapy with an aromatase inhibitor) (based on 36 ).
Molecular pathways of breast cancer risk, bone metabolism and breast development are linked
It has been known for some time that the signaling pathways which regulate bone metabolism (RANKL/RANK/OPG) also regulate progesterone-based proliferation in the breast and can have an impact on the pathogenesis and progression of breast cancer 37 , 38 , 39 . It was recently reported that this signaling pathway is particularly important in patients with a BRCA 1 mutation. RANKL appears to be the main mediator in the pathogenesis of breast cancer in this patient population 40 , 41 , 42 , meaning that a blockade of this signaling pathway might be a useful strategy for breast cancer prevention. A study with denosumab is planned to investigate this point further 42 . Other substances such as the selective progesterone receptor modulator telapristone may also be of interest, as preclinical models have shown that these substances inhibit the paracrine expression of RANKL 43 .
Quality of Life and Digitization of Medicine
Quality of life, therapies with few side effects and survival are among the most important therapy goals for patients 44 , 45 , and patient well-being, therapy, compliance and overall well-being are mutually dependent. Side effects, e.g. due to therapy with aromatase inhibitors, can result in the patient terminating their therapy 22 – 25 , which in turn is likely to result in a poorer prognosis 46 . This means that taking the patientʼs quality of life into consideration is also important in the context of improving her prognosis.
Digital medicine to improve communication, quality of life and prognosis
Digitization in medicine is constantly increasing 47 . There are still huge opportunities to optimize the healthcare for patients by improving therapy selection, patient care and the management of related areas. Much has been written in this context about “big and smart data”. Given the rapid advances in machine learning and the interoperability of databases (which has done much to simplify their use), it is expected that central patient care processes will be automated 47 and electronic tools will increasingly be used to communicate with patients 11 , 48 . But it will also be necessary to ensure that interests of the patients (data confidentiality, self-determination and data sovereignty) are maintained 49 .
The field has huge potential and presents many opportunities. To take just one example, it has been shown that the use of electronic tools to provide a constant line of communication between the treating medical staff and patients as a means of monitoring side effects can result in better overall survival 50 . The integration of such platforms in various studies across the German-speaking world is increasing. Electronic patient-reported outcome systems have already been tested since several years in studies such as PRAEGNANT 51 , 52 , Seraphina 53 and Precycle 54 .
Knowledge processing and knowledge exchange for physicians
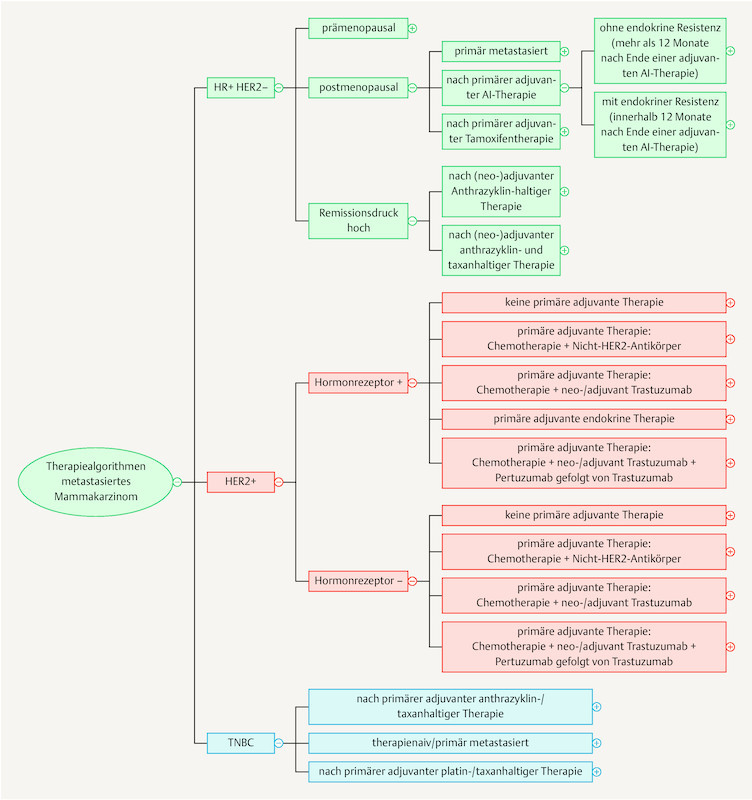
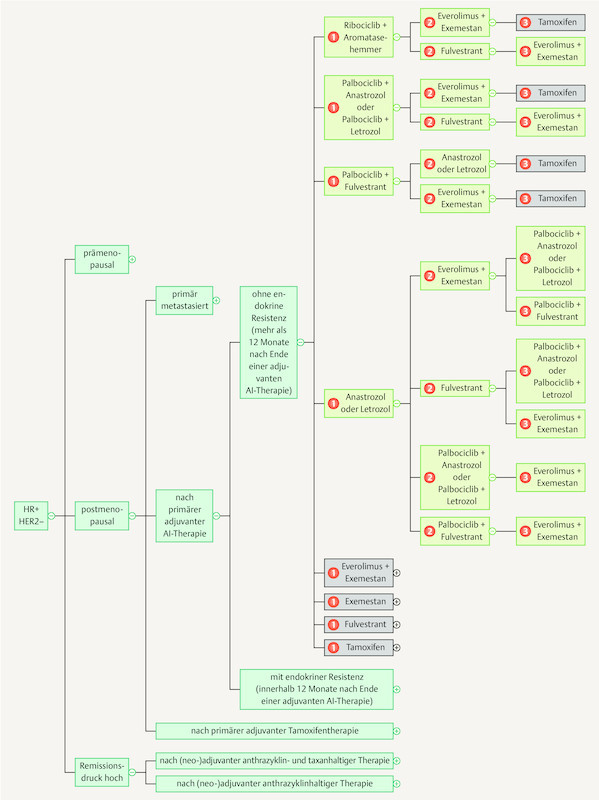
As the breadth of knowledge continues to grow by leaps and bounds, it cannot be ruled out that in the long run knowledge will be permanently managed using digital means. The App “Mammakarzinom Transparent” ( https://mammakarzinom.onkowissen.de/ ; 55 ) is just one attempt to make therapy algorithms available and include additional information on efficacy and side effects. Examples of clickable icons are shown in Figs. 4 to 6 ; clicking on the icon will call up further information about the respective therapy.
Fig. 4.
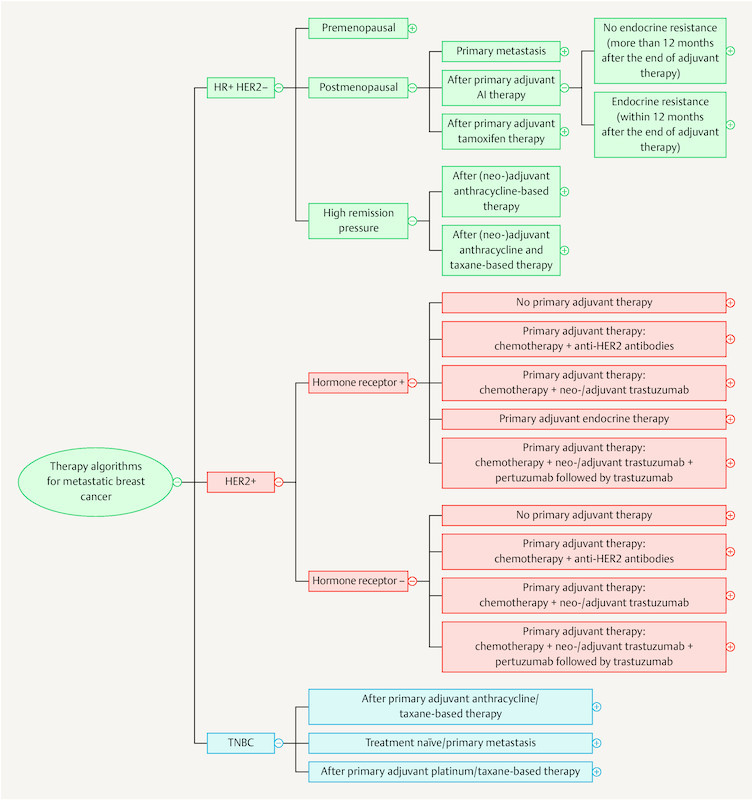
Current therapy algorithms for metastatic breast cancer (schematic) (based on 55 ).
Fig. 6.
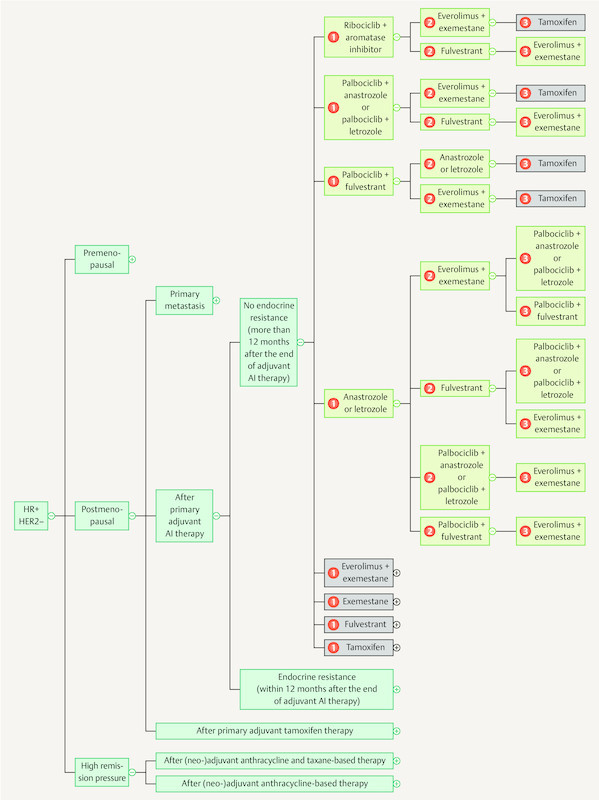
Current therapy algorithms for patients with hormone receptor-positive, HER2-negative breast cancer (schematic) (based on 55 ).
Fig. 5.
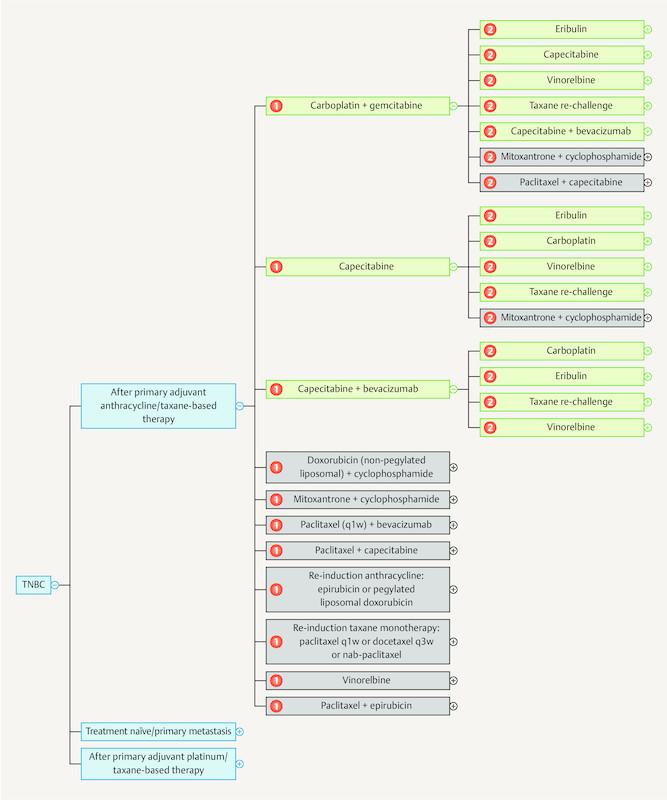
Current therapy algorithms for patients with metastatic triple-negative breast cancer (schematic) (based on 55 ).
Quality of life in the metastatic setting
In Germany, quality of life is an accepted endpoint in addition to overall survival for the assessment of therapies after they have been approved. In recent evaluations, progression-free survival was not accepted for assessments about the additional benefit of a specific therapy. However, it is well known that for the patient disease progression has a negative impact on their quality of life and a delay in disease progression will therefore improve patientsʼ quality of life 56 , 57 . Particularly in the metastatic therapy setting where there are frequent changes of therapy, data about the extent to which therapy sequences influence the further course of disease and quality of life have not been thoroughly investigated. New studies which take a comprehensive look at therapy lines 51 , 58 could help to collect new data and improve the care of these patients.
Prevention
Importance of panel genes
In the last few years, the medical understanding of genetic and non-genetic risk factors has significantly improved 59 , 60 . In addition to the BRCA 1 and BRCA 2 genes, the clinical importance of so-called panel genes (e.g., the following genes which are associated with a high or moderate risk of breast cancer: CHEK2, PALB2, ATM, RAD51D, BARD1, MSH6 and others) is being investigated 61 , 62 , 63 , 64 , 65 , 66 , 67 . One study reported that PALB2 (OR: 7.5) might need to be classified as belonging to the group of high-risk genes while ATM, BARD1 and RAD51D could probably be classified as moderate risk genes 62 . No increased risk was reported for the genes BRIP1, RAD51C, MRE11A, RAD50, NBN, MLH1 and PMS2 62 . It is not yet clear to what extent this information will have an impact on prevention.
Which population is suitable for mutation testing?
The approval of the first PARP inhibitor to treat metastatic breast cancer in the USA 2 raised the question whether genotyping should be carried out in all patients who could be considered for this therapy. A recent study presented the mutation frequencies for BRCA 1 and BRCA 2 and other panel genes irrespective of the usual test criteria used in predictive genetic diagnostics 68 (see chapter “Prognostic and Predictive Factors). The question about the extent to which predictive genetic testing could and should be expanded also includes the debate about whether patients with low mutation rates needed to be informed about any variants of unclear significance (VUS) detected during testing. In the case of genetic changes where not much is known about their clinical relevance, the uncertainty could lead to anxiety, worry and even unjustified measures. At present, more than 3000 of such VUS in the BRCA 1 and BRCA 2 genes are known. Significant progress has also been achieved recently in this area. Studies have shown that functional in vitro analysis of 139 VUS in BRCA 2 was able to identify 13 more pathogenic mutations, while 12 could be classified as harmless variants 69 . More analyses of this type could significantly decrease the diagnostic uncertainty.
Importance of low-penetrance risk genes
In addition to high and moderate risk genes, the importance of common genetic changes with significantly lower risk modifications is becoming increasingly clear. A further 75 variants have just been recently validated 70 , 71 . Other variants and their clinical importance are also gradually being characterized 72 , 73 , 74 , 75 , 76 . The data obtained could be summarized in scores and tested in clinical practice 77 . Combinations with clinical risk factors such as mammographic density, size or BMI could also be of clinical use 78 , 79 , 80 , 81 , 82 . However, such studies still need to be carried out.
Acknowledgements
This work was partly the result of funding received from Riemser and the PRAEGNANT study network, neither of which had any involvement in the compilation of this manuscript. The authors alone are responsible for the contents of this paper.
Danksagung
Diese Arbeit entstand teilweise in Folge von Förderungen der Firma Riemser und des PRAEGNANT Netzwerks, die keinen Anteil bei der Verfassung dieses Manuskripts hatten. Für den Inhalt des Manuskripts sind alleine die Autoren verantwortlich.
Footnotes
Conflict of Interest/Interessenkonflikt A. D. H. received honoraria from AstraZeneca, Genomic Health, Roche, Novartis, Celgene and Pfizer. N. N. received consultancy honoraria from Janssen-Cilag and travel support from Novartis. F. O. received speaker and consultancy honoraria from Amgen, Celgene, TEVA, AstraZeneca, Novartis, Roche, and MSD. F.-A. T. received honoraria from Astra Zeneca, Genomic Health and Novartis. H.-C. K. received honoraria from Carl Zeiss meditec, TEVA, Theraclion, Novartis, Amgen, Astra Zeneca, Pfizer, Janssen-Cilag, GSK, LIV Pharma, Roche and Genomic Health. P. H. received honoraria, unrestricted educational grants and research funding from Amgen, AstraZeneca, Eli Lilly, MSD, Novartis, Pfizer and Roche. P. A. F. received honoraria from Roche, Pfizer, Novartis and Celgene. His institution conducts research for Novartis. H. T. received honoraria from Novartis, Roche, Celgene, TEVA, Pfizer and travel support from Roche, Celgene and Pfizer. J. E. received honoraria from Roche, Celgene, Novartis, Pfizer, Pierre Fabre, TEVA and travel support from Celgene, Pfizer, TEVA and Pierre Fabre. M. P. L. has participated on advisory boards for AstraZeneca, MSD, Novartis, Pfizer, Genomic Health and Roche and has received honoraria for lectures from Lilly, Roche, Novartis, Pfizer, Genomic Health, AstraZeneca, medac and Eisai. M. W. received speaker honoraria from Astra Zeneca, Celgene and Novartis. V. M. received speaker honoraria from Amgen, Astra Zeneca, Celgene, Daiichi-Sankyo, Eisai, Pfizer, Pierre-Fabre, Novartis, Roche, Teva, Janssen-Cilag and consultancy honoraria from Genomic Health, Roche, Pierre Fabre, Amgen, Daiichi-Sankyo and Eisai. E. B. received honoraria from Novartis, Riemser and Hexal for consulting and clinical research management activities. A. S. received honoraria from Roche, Celgene, AstraZeneca, Novartis, Pfizer, Zuckschwerdt Verlag GmbH, Georg Thieme Verlag, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH and promedicis GmbH. W. J. received honoraria and research grants from Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, Sanofi, Daichi, Tesaro. F. S. participated on advisory boards for Novartis, Amgen and Roche and received honoraria for lectures from Roche, Novartis and Pfizer.
A. D. H. hat Honorare von AstraZeneca, Genomic Health, Roche, Novartis, Celgene und Pfizer erhalten. N. N. hat Beraterhonorare von Janssen-Cilag und Reisekostenunterstützung von Novartis erhalten. F. O. hat Sprecher- und Beraterhonorare von Amgen, Celgene, TEVA, AstraZeneca, Novartis, Roche, und MSD erhalten. F.-A. T. hat Honorare von AstraZeneca, Genomic Health und Novartis erhalten. H.-C. K. hat Honorare von Carl Zeiss meditec, TEVA, Theraclion, Novartis, Amgen, AstraZeneca, Pfizer, Janssen-Cilag, GSK, LIV Pharma, Roche und Genomic Health erhalten. P. H. hat Honorare, nicht zweckgebundene Fortbildungszuschüsse und Forschungsförderung von Amgen, AstraZeneca, Eli Lilly, MSD, Novartis, Pfizer und Roche erhalten. P. A. F. hat Honorare von Roche, Pfizer, Novartis und Celgene erhalten. Sein Institut führt Forschungen für Novartis aus. H. T. hat Honorare von Novartis, Roche, Celgene, TEVA und Pfizer und Reisekostenzuschüsse von Roche, Celgene und Pfizer erhalten. J. E. hat Honorare von Roche, Celgene, Novartis, Pfizer, Pierre Fabre, TEVA und Reisekostenzuschüssen von Celgene, Pfizer, TEVA und Pierre Fabre erhalten. M. P. L. hat an Beratungsgremien bei AstraZeneca, MSD, Novartis, Pfizer, Genomic Health und Roche teilgenommen und hat Honorare für Vorträge von Lilly, Roche, Novartis, Pfizer, Genomic Health, AstraZeneca, medac und Eisai erhalten. M. W. hat Sprecherhonorare von AstraZeneca, Celgene und Novartis erhalten. V. M. hat Sprecherhonorare von Amgen, AstraZeneca, Celgene, Daiichi-Sankyo, Eisai, Pfizer, Pierre-Fabre, Novartis, Roche, Teva, Janssen-Cilag und Beraterhonorare von Genomic Health, Roche, Pierre Fabre, Amgen, Daiichi-Sankyo und Eisai erhalten. E. B. hat Honorare von Novartis, Riemser und Hexal für Beratertätigkeiten und klinisches Forschungsmanagement erhalten. A. S. hat Honorare von Roche, Celgene, AstraZeneca, Novartis, Pfizer, Zuckschwerdt Verlag GmbH, Georg Thieme Verlag, Aurikamed GmbH, MCI Deutschland GmbH, bsh medical communications GmbH und promedicis GmbH erhalten. W. J. hat Honorare und Forschungsstipendien von Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, Sanofi, Daichi, und Tesaro erhalten. F. S. hat an Beratergremien für Novartis, Amgen und Roche teilgenommen und hat Vortragshonorare von Roche, Novartis und Pfizer erhalten.
References/Literatur
- 1.Robson M, Im S-A, Senkus E. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 2.United States Food and Drug Administration (FDA) . http:// http://
- 3.Litton J, Rugo H, Ettl J. Abstract GS6-07: EMBRACA: A phase 3 trial comparing talazoparib, an oral PARP inhibitor, to physicianʼs choice of therapy in patients with advanced breast cancer and a germline BRCA mutation. Cancer Res. 2018;78:GS6-07-GS06-07. [Google Scholar]
- 4.Turner N C, Telli M L, Rugo H S.Final results of a phase 2 study of talazoparib (TALA) following platinum or multiple cytotoxic regimens in advanced breast cancer patients (pts) with germline BRCA1/2 mutations (ABRAZO) J Clin Oncol 201735(Suppl.)Abstr.. 1007 [Google Scholar]
- 5.Telli M, Turner N, Mailliez A. Abstract P1-14-03: ABRAZO: Exposure-efficacy and -safety analyses of breast cancer patients with germline BRCA1/2 mutations receiving talazoparib in a phase 2 open-label trial. Cancer Res. 2018;78:P1-14-03-P11-14-03. [Google Scholar]
- 6.Bardia A, Vahdat L, Diamond J. Abstract GS1-07: Sacituzumab govitecan (IMMU-132), an anti-Trop-2-SN-38 antibody-drug conjugate, as ≥ 3-line therapeutic option for patients with relapsed/refractory metastatic triple-negative breast cancer (mTNBC): efficacy results. Cancer Res. 2018;78:GS1-07-GS01-07. [Google Scholar]
- 7.Nanda R, Chow L Q, Dees E C. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams S, Loi S, Toppmeyer D. Abstract PD6-10: KEYNOTE-086 cohort B: Pembrolizumab monotherapy for PD-L1–positive, previously untreated, metastatic triple-negative breast cancer (mTNBC) Cancer Res. 2018;78:PD6-10-PD16-10. [Google Scholar]
- 9.Tolaney S, Kalinsky K, Kaklamani V. Abstract PD6-13: Phase 1b/2 study to evaluate eribulin mesylate in combination with pembrolizumab in patients with metastatic triple-negative breast cancer. Cancer Res. 2018;78:PD6-13-PD16-13. [Google Scholar]
- 10.Loi S, Giobbe-Hurder A, Gombos A. Abstract GS2-06: Phase Ib/II study evaluating safety and efficacy of pembrolizumab and trastuzumab in patients with trastuzumab-resistant HER2-positive metastatic breast cancer: results from the PANACEA (IBCSG 45-13/BIG 4-13/KEYNOTE-014) study. Cancer Res. 2018;78:GS2-06-GS02-06. [Google Scholar]
- 11.Hartkopf A D, Graf J, Simoes E. Electronic-based patient-reported outcomes: willingness, needs, and barriers in adjuvant and metastatic breast cancer patients. JMIR Cancer. 2017;3:e11. doi: 10.2196/cancer.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tripathy D, Sohn J, Im S-A. Abstract GS2-05: First-line ribociclib vs placebo with goserelin and tamoxifen or a non-steroidal aromatase inhibitor in premenopausal women with hormone receptor-positive, HER2-negative advanced breast cancer: results from the randomized phase III MONALEESA-7 trial. Cancer Res. 2018;78:GS2-05-GS02-05. [Google Scholar]
- 13.Singh H, Howie L, Bloomquist E. Abstract GS5-06: A U.S. food and drug administration pooled analysis of outcomes of older women with hormone-receptor positive metastatic breast cancer treated with a CDK4/6 inhibitor as initial endocrine based therapy. Cancer Res. 2018;78:GS5-06-GS05-06. [Google Scholar]
- 14.Goetz M, OʼShaughnessy J, Sledge G. Abstract GS6-02: The benefit of abemaciclib in prognostic subgroups: an exploratory analysis of combined data from the MONARCH 2 and 3 studies. Cancer Res. 2018;78:GS6-02-GS06-02. [Google Scholar]
- 15.Kommission Mamma der Arbeitsgemeinschaft Gynäkologische Onkologie e.V. in der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe e.V. sowie in der Deutschen Krebsgesellschaft e.V. Diagnostik und Therapie von Patientinnen mit primärem und metastasiertem Brustkrebs (2017)Online:https://www.ago-online.de/fileadmin/downloads/leitlinien/mamma/2017-03/AGO_deutsch/PDF_Gesamtdatei_deutsch/Alle_aktuellen_Empfehlungen_2017.pdflast access: 07.01.2018
- 16.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft Deutsche Krebshilfe AWMF) S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.0, 2017 AWMF Registernummer: 032-045OL 2017. Online:http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/last access: 07.01.2018
- 17.Schmid P, Zaiss M, Harper-Wynne C. Abstract GS2-07: MANTA – A randomized phase II study of fulvestrant in combination with the dual mTOR inhibitor AZD2014 or everolimus or fulvestrant alone in estrogen receptor-positive advanced or metastatic breast cancer. Cancer Res. 2018;78:GS2-07-GS02-07. [Google Scholar]
- 18.Traina T A, OʼShaughnessy J, Nanda R. Preliminary results from a phase 2 single-arm study of enzalutamide, an androgen receptor (AR) inhibitor, in advanced AR plus triple-negative breast cancer (TNBC) doi:10.1158/1538-7445.SABCS14-P5-19-09 Cancer Res. 2015;75:Abstract. P5-19-09. [Google Scholar]
- 19.Traina T A, Miller K, Yardley D A. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR plus triplenegative breast cancer (TNBC) doi:10.1200/jco.2015.33.15_suppl.1003 J Clin Oncol. 2015;33(15):1003–1003. [Google Scholar]
- 20.Cortes J, Crown J, Awada A. Overall survival (OS) from the phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) signaling inhibitor, in AR plus advanced triple-negative breast cancer (aTNBC) Eur J Cancer. 2015;51:S265–S265. [Google Scholar]
- 21.Krop I, Abramson V, Colleoni M. Abstract GS4-07: Results from a randomized placebo-controlled phase 2 trial evaluating exemestane ± enzalutamide in patients with hormone receptor–positive breast cancer. Cancer Res. 2018;78:GS4-07-GS04-07. doi: 10.1158/1078-0432.CCR-20-1693. [DOI] [PubMed] [Google Scholar]
- 22.Nabieva N, Kellner S, Fehm T. Patient and tumor characteristics and their influence on early therapy persistence with letrozole in postmenopausal patients with early breast cancer. Ann Oncol. 2017 doi: 10.1093/annonc/mdx630. [DOI] [PubMed] [Google Scholar]
- 23.Hadji P, Jackisch C, Bolten W. COMPliance and Arthralgia in Clinical Therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Ann Oncol. 2014;25:372–377. doi: 10.1093/annonc/mdt513. [DOI] [PubMed] [Google Scholar]
- 24.Hadji P, Ziller V, Kyvernitakis J. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Treat. 2013;138:185–191. doi: 10.1007/s10549-013-2417-1. [DOI] [PubMed] [Google Scholar]
- 25.Hadji P, Blettner M, Harbeck N. The Patientʼs Anastrozole Compliance to Therapy (PACT) Program: a randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol. 2013;24:1505–1512. doi: 10.1093/annonc/mds653. [DOI] [PubMed] [Google Scholar]
- 26.Hershman D, Unger J, Greenlee H. Abstract GS4-04: Randomized blinded sham- and waitlist-controlled trial of acupuncture for joint symptoms related to aromatase inhibitors in women with early stage breast cancer (S1200) Cancer Res. 2018;78:GS4-04-GS04-04. [Google Scholar]
- 27.Tamaki K, Takaesu M, Nagamine S. Abstract P6-11-01: Final results of the randomized trial of exercise intervention vs. usual care for breast cancer patients with aromatase inhibitor to prevent and improve the aromatase inhibitor induced arthralgia. Cancer Res. 2018;78:P6-11-01-P16-11-01. [Google Scholar]
- 28.Hurvitz S, Chan A, Iannotti N. Abstract P3-14-01: Effects of adding budesonide or colestipol to loperamide prophylaxis on neratinib-associated diarrhea in patients with HER2+ early-stage breast cancer: the CONTROL trial. Cancer Res. 2018;78:P3-14-01-P13-14-01. [Google Scholar]
- 29.Silva G, Moreira R, Gimenes D. Abstract P6-11-06: Efficacy of scalp cooling in preventing chemotherapy-induced alopecia in breast cancer patients: a retrospective, comprehensive review of 330 cases of Brazil. Cancer Res. 2018;78:P6-11-06-P16-11-06. [Google Scholar]
- 30.Kurbacher C, Kurbacher A, Herz S. Abstract P6-11-14: Safety and effectiveness of sensor-controlled scalp cooling to prevent alopecia in primary breast cancer patients receiving neoadjuvant or adjuvant epirubicin, taxanes, or both. Cancer Res. 2018;78:P6-11-14-P16-11-14. [Google Scholar]
- 31.Weinstein C, Jordan K, Green S. Abstract P6-11-11: Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer receiving moderately emetogenic chemotherapy regimens. Cancer Res. 2018;78:P6-11-11-P16-11-11. [Google Scholar]
- 32.Schilling J, Klare P, Heilmann V. Abstract P6-11-05: NEPA for CINV prevention in highly or moderately emetogenic chemotherapy – interim results of a German non-interventional study on quality of life and efficacy. Cancer Res. 2018;78:P6-11-05-P16-11-05. [Google Scholar]
- 33.Zaleta A, Miller M, Johnson J. Abstract P6-11-08: Chemotherapy-induced peripheral neuropathy and quality of life among breast cancer survivors. Cancer Res. 2018;78:P6-11-08-P16-11-08. [Google Scholar]
- 34.Early Breast Cancer Trialistsʼ Collaborative Group . Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 35.Janni W, Friedl T, Fehm T. Abstract GS1-06: Extended adjuvant bisphosphonate treatment over five years in early breast cancer does not improve disease-free and overall survival compared to two years of treatment: phase III data from the SUCCESS A study. Cancer Res. 2018;78:GS1-06-GS01-06. [Google Scholar]
- 36.Gnant M, Steger G, Greil R. Abstract GS3-01: A prospective randomized multi-center phase-III trial of additional 2 versus additional 5 years of anastrozole after initial 5 years of adjuvant endocrine therapy – results from 3,484 postmenopausal women in the ABCSG-16 trial. Cancer Res. 2018;78:GS3-01-GS03-01. [Google Scholar]
- 37.Bayer C M, Beckmann M W, Fasching P A. Updates on the role of receptor activator of nuclear factor kappaB/receptor activator of nuclear factor kappaB ligand/osteoprotegerin pathway in breast cancer risk and treatment. Curr Opin Obstet Gynecol. 2017;29:4–11. doi: 10.1097/GCO.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 38.Hein A, Bayer C M, Schrauder M G. Polymorphisms in the RANK/RANKL genes and their effect on bone specific prognosis in breast cancer patients. Biomed Res Int. 2014;2014:842452. doi: 10.1155/2014/842452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Timotheadou E, Kalogeras K T, Koliou G A. Evaluation of the prognostic value of RANK, OPG, and RANKL mRNA expression in early breast cancer patients treated with anthracycline-based adjuvant chemotherapy. Transl Oncol. 2017;10:589–598. doi: 10.1016/j.tranon.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sigl V, Owusu-Boaitey K, Joshi P A. RANKL/RANK control Brca1 mutation-driven mammary tumors. Cell Res. 2016;26:761–774. doi: 10.1038/cr.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiechl S, Schramek D, Widschwendter M. Aberrant regulation of RANKL/OPG in women at high risk of developing breast cancer. Oncotarget. 2017;8:3811–3825. doi: 10.18632/oncotarget.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nolan E, Vaillant F, Branstetter D. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med. 2016;22:933–939. doi: 10.1038/nm.4118. [DOI] [PubMed] [Google Scholar]
- 43.Lee O, Sun L, Karavites L. Abstract P5-14-01: Telapristone acetate abrogates PR-dependent paracrine-mediated mammary cell proliferation. Cancer Res. 2018;78:P5-14-01-P15-14-01. [Google Scholar]
- 44.Lux M P, Bayer C M, Loehberg C R. Shared decision-making in metastatic breast cancer: discrepancy between the expected prolongation of life and treatment efficacy between patients and physicians, and influencing factors. Breast Cancer Res Treat. 2013;139:429–440. doi: 10.1007/s10549-013-2557-3. [DOI] [PubMed] [Google Scholar]
- 45.Thiel F C, Schrauder M G, Fasching P A. Shared decision-making in breast cancer: discrepancy between the treatment efficacy required by patients and by physicians. Breast Cancer Res Treat. 2012;135:811–820. doi: 10.1007/s10549-012-2218-y. [DOI] [PubMed] [Google Scholar]
- 46.Chirgwin J H, Giobbie-Hurder A, Coates A S. Treatment adherence and its impact on disease-free survival in the Breast International Group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34:2452–2459. doi: 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tresp V, Overhage J M, Bundschus M. Going digital: a survey on digitalization and large-scale data analytics in healthcare. Proceedings of the IEEE. 2016;104:2180–2206. [Google Scholar]
- 48.Wallwiener M, Matthies L, Simoes E. Reliability of an e-PRO Tool of EORTC QLQ-C30 for measurement of health-related quality of life in patients with breast cancer: prospective randomized trial. J Med Internet Res. 2017;19:e322. doi: 10.2196/jmir.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dabrock P, Augsburg S.Big Data und Gesundheit – Datensouveränität als informationelle Freiheitsgestaltung (2017)Online:http://www.ethikrat.org/presse/pressekonferenzen/pressekonferenz-30-11-2017last access: 17.01.2018
- 50.Basch E M, Deal A M, Dueck A C.Overall survival results of a randomized trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment J Clin Oncol 201735(Suppl.)Abstr. LBA2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fasching P A, Brucker S Y, Fehm T N. Biomarkers in patients with metastatic breast cancer and the PRAEGNANT Study Network. Geburtsh Frauenheilk. 2015;75:41–50. doi: 10.1055/s-0034-1396215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallwiener M, Heindl F, Brucker S Y. Implementation and feasibility of electronic patient-reported outcome (ePRO) data entry in the PRAEGNANT Real-Time Advanced and Metastatic Breast Cancer Registry. Geburtsh Frauenheilk. 2017;77:870–878. doi: 10.1055/s-0043-116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fasching P A, Wallwiener M, Lux M P. Seraphina – Safety efficacy and patient reported outcomes of advanced breast cancer patients: therapy management with NAB-paclitaxel in daily routine. doi:10.1158/1538-7445.SABCS15-OT3-02-09 Cancer Research. 2016;76:Abstract OT3-02-09.. [Google Scholar]
- 54.West German Study Group Commission for translational Research of the AGO Impact of eHealth-support on quality of life in metastatic breast cancer patients treated with palbociclib and endocrine therapy (PRECYCLE) (2017)Online:https://clinicaltrials.gov/ct2/show/NCT03220178last access: 17.01.2018
- 55.Overkamp F, Kolberg H C, Schütz F.Mammakarzinom Transparent (2017)Online:https://mammakarzinom.onkowissen.de/last access: 24.01.2018
- 56.Muller V, Nabieva N, Haberle L. Impact of disease progression on health-related quality of life in patients with metastatic breast cancer in the PRAEGNANT breast cancer registry. Breast. 2018;37:154–160. doi: 10.1016/j.breast.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Hurvitz S A, Lalla D, Crosby R D. Use of the metastatic breast cancer progression (MBC-P) questionnaire to assess the value of progression-free survival for women with metastatic breast cancer. Breast Cancer Res Treat. 2013;142:603–609. doi: 10.1007/s10549-013-2734-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ClinicalTrials Study to assess the safety and efficacy of ribociclib (LEE011) in combination with letrozole for the treatment of men and pre/postmenopausal women with HR+ HER2- aBC (RIBANNA-study) 2018Online:https://clinicaltrials.gov/ct2/show/NCT02941926last access: 17.01.2018
- 59.Fasching P A, Ekici A B, Adamietz B R. Breast cancer risk – genes, environment and clinics. Geburtsh Frauenheilk. 2011;71:1056–1066. doi: 10.1055/s-0031-1280437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fasching P A, Ekici A B, Wachter D L. Breast cancer risk – from genetics to molecular understanding of pathogenesis. Geburtsh Frauenheilk. 2013;73:1228–1235. doi: 10.1055/s-0033-1360178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Couch F J, Hart S N, Sharma P. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol. 2015;33:304–311. doi: 10.1200/JCO.2014.57.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couch F J, Shimelis H, Hu C. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190–1196. doi: 10.1001/jamaoncol.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Couch F, Shimelis H, LaDuca H. Abstract PD1-01: Triple negative breast cancer predisposition genes. Cancer Res. 2018;78:PD1-01-PD01-01. [Google Scholar]
- 64.Shimelis H, Mesman R LS, Von Nicolai C. BRCA2 hypomorphic missense variants confer moderate risks of breast cancer. Cancer Res. 2017;77:2789–2799. doi: 10.1158/0008-5472.CAN-16-2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Southey M C, Goldgar D E, Winqvist R. PALB2, CHEK2 and ATM rare variants and cancer risk: data from COGS. J Med Genet. 2016;53:800–811. doi: 10.1136/jmedgenet-2016-103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmidt M K, Hogervorst F, van Hien R. Age- and tumor subtype-specific breast cancer risk estimates for CHEK2*1100delC carriers. J Clin Oncol. 2016;34:2750–2760. doi: 10.1200/JCO.2016.66.5844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pelttari L M, Khan S, Vuorela M. RAD51B in familial breast cancer. PLoS One. 2016;11:e0153788. doi: 10.1371/journal.pone.0153788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fasching P, Hu C, Hart S. Abstract PD1-02: Cancer predisposition genes in metastatic breast cancer – association with metastatic pattern, prognosis, patient and tumor characteristics. Cancer Res. 2018;78:PD1-02-PD01-02. [Google Scholar]
- 69.Couch F, Shimelis H, Hart S. Abstract GS4-06: Cancer risks and response to targeted therapy associated with BRCA2 variants of uncertain significance. Cancer Res. 2018;78:GS4-06-GS04-06. [Google Scholar]
- 70.Milne R L, Kuchenbaecker K B, Michailidou K. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat Genet. 2017;49:1767–1778. doi: 10.1038/ng.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michailidou K, Lindstrom S, Dennis J. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghoussaini M, French J D, Michailidou K. Evidence that the 5p12 variant rs10941679 confers susceptibility to estrogen-receptor-positive breast cancer through FGF10 and MRPS30 regulation. Am J Hum Genet. 2016;99:903–911. doi: 10.1016/j.ajhg.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Couch F J, Kuchenbaecker K B, Michailidou K. Identification of four novel susceptibility loci for oestrogen receptor negative breast cancer. Nat Commun. 2016;7:11375. doi: 10.1038/ncomms11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Purrington K S, Slager S, Eccles D. Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis. 2014;35:1012–1019. doi: 10.1093/carcin/bgt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stevens K N, Fredericksen Z, Vachon C M. 19p13.1 is a triple-negative-specific breast cancer susceptibility locus. Cancer Res. 2012;72:1795–1803. doi: 10.1158/0008-5472.CAN-11-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haberle L, Hein A, Rubner M. Predicting triple-negative breast cancer subtype using multiple single nucleotide polymorphisms for breast cancer risk and several variable selection methods. Geburtsh Frauenheilk. 2017;77:667–678. doi: 10.1055/s-0043-111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mavaddat N, Pharoah P D, Michailidou K. Prediction of breast cancer risk based on profiling with common genetic variants. doi:10.1093/jnci/djv036. J Natl Cancer Inst. 2015;107:pii:djv036. doi: 10.1093/jnci/djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vachon C M, Pankratz V S, Scott C G. The contributions of breast density and common genetic variation to breast cancer risk. doi:10.1093/jnci/dju397. J Natl Cancer Inst. 2015;107:pii:dju397. doi: 10.1093/jnci/dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rudolph A, Song M, Brook M N. Joint associations of a polygenic risk score and environmental risk factors for breast cancer in the Breast Cancer Association Consortium. Int J Epidemiol. 2018 doi: 10.1093/ije/dyx242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo Q, Burgess S, Turman C. Body mass index and breast cancer survival: a Mendelian randomization analysis. Int J Epidemiol. 2017;46:1814–1822. doi: 10.1093/ije/dyx131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang B, Shu X O, Delahanty R J. Height and breast cancer risk: evidence from prospective studies and Mendelian randomization. doi:10.1093/jnci/djv219. J Natl Cancer Inst. 2015;107:pii:djv219. doi: 10.1093/jnci/djv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vachon C M, Scott C G, Fasching P A. Common breast cancer susceptibility variants in LSP1 and RAD51L1 are associated with mammographic density measures that predict breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:1156–1166. doi: 10.1158/1055-9965.EPI-12-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]