Abstract
The goal of this study was to compare the efficacy of human glial restricted progenitors (hGRPs) in promoting axonal growth of different tracts. We examined the potential of hGRPs grafted into a cervical (C4) dorsal column lesion to test sensory axons, and into a C4 hemisection to test motor tracts. The hGRPs, thawed from frozen stocks, were suspended in a PureCol matrix and grafted acutely into a C4 dorsal column or hemisection lesion. Control rats received PureCol only. Five weeks after transplantation, all transplanted cells survived in rats with the dorsal column lesion but only about half of the grafts in the hemisection. In the dorsal column lesion group, few sensory axons grew short distances into the lesion site of control animals. The presence of hGRPs transplants enhanced axonal growth significantly farther into the transplants. In the hemisection group, coerulospinal axons extended similarly into both control and transplant groups with no enhancement by the presence of hGRPs. Rubrospinal axons did not grow into the lesion even in the presence of hGRPs. However, reticulospinal and raphespinal axons grew for a significantly longer distance into the transplants. These results demonstrate the differential capacity of axonal growth/regeneration of the motor and sensory tracts based on their intrinsic abilities as well as their response to the modified environment induced by the hGRPs transplants. We conclude that hGRP transplants can modify the injury site for axon growth of sensory and some motor tracts, and suggest they could be combined with other interventions to restore connectivity.
Keywords: acute spinal cord injury, human glial progenitor cells, cell transplantation, axon regeneration, motor and sensory tracts
1. Introduction
Spinal cord injury (SCI) results in functional deficits caused by cell death and interruption of motor and sensory axons at the injury site, with minimal or no regeneration to restore connectivity. The failure of axon growth in the adult spinal cord is due to limitation in the intrinsic capacity of the central nervous system (CNS) neurons to regenerate as well as environmental barriers, including the lack of growth support, the presence of inhibitory factors at the lesion area and formation of scar and cavities, which together impede axon growth and connectivity. Strategies that have been used to promote axonal regeneration after SCI at different stages of the injury (Coumans et al., 2001; Jin et al., 2002; Liu et al., 1999; Lorgulescu et al., 2015) include: administration of neurotrophic factors (Elliott Donaghue et al., 2015; Kelamangalath and Smith, 2013), neutralization or blocking inhibitory factors (Brosamle et al., 2000; Fouad et al., 2001; Petrosyan et al., 2013), reducing scar formation (Houle et al., 2006; Yick et al., 2004) and bridging the lesion cavity using cellular transplants (Bonner et al., 2010; David and Aguayo, 1981; Jin et al., 2002; Reier et al., 1986; Santos-Benito and Ramon-Cueto, 2003; Xu et al., 1995b).
Cell transplantation was first done by Cajal and Tello (Ramon y Cajal, 1928; Tello, 1907), which found that providing a cellular environment supported axonal regrowth. Later demonstration of regeneration in the CNS was accomplished by transplantation of a peripheral nerve graft into the injured spinal cord showing that adult mammalian axons can regenerate when a suitable environment is provided at lesion area (David and Aguayo, 1981), which confirmed the finding of Cajal and Tello. Since then, other tissue transplants have been applied to bridge the lesion cavity and promote axonal regeneration including: fetal (Broude et al., 1999; Reier et al., 1988), Schwann cells (Williams et al., 2015; Xu et al., 1995b), olfactory ensheathing cells modified fibroblasts (Jin et al., 2002; Liu et al., 1999), and neural stem cells (Cummings et al., 2005; McDonald et al., 1999; Mitsui et al., 2005; Shihabuddin et al., 2000). In particular, transplants that generate neurons have been shown to form functional relays with the potential to reconnect the injured spinal cord (Bonner et al., 2011; Haas and Fischer, 2014; Lu et al., 2012).
Recently, the focus has shifted to testing the properties of human stem cells to provide therapeutic data for clinical application. Transplants of neurospheres derived from human induced pluripotent stem cells can differentiate into neurons, astrocytes, and oligodendrocytes in the injured spinal cord and improve motor functional recovery after SCI (Nori et al., 2011). Oligodendrocyte progenitor cells derived from human embryonic stem cell grafted into injured spinal cord can remyelinate and restore locomotion (Keirstead et al., 2005). Glial restricted progenitor cells (GRPs) from neural progenitor cells differentiate into astrocytes and oligodendrocytes both in vivo and in vitro (Sandrock et al., 2010). Transplants of GRPs from rodent or human have demonstrated beneficial effects on functional recovery after SCI (Cao et al., 2005; Nout et al., 2011; Walczak et al., 2011). Previous studies from our group showed that human GRPs transplanted into a contusion model survived inside the injury, reduced the glial scar, and improved autonomic function (Jin et al., 2011). Astrocytes derived from human GRPs supported sensory axon regeneration after SCI (Haas and Fischer, 2013). For clinical application, cells that can be stored as frozen stocks and then thawed for transplantation would be particularly beneficial. In the present study, we compared axonal regeneration from different ascending and descending tracts following SCI and acute transplants of human GRPs directly from frozen stocks.
2. Results
2.1 Survival of human GRP cells following acute transplantation into a spinal cord lesion
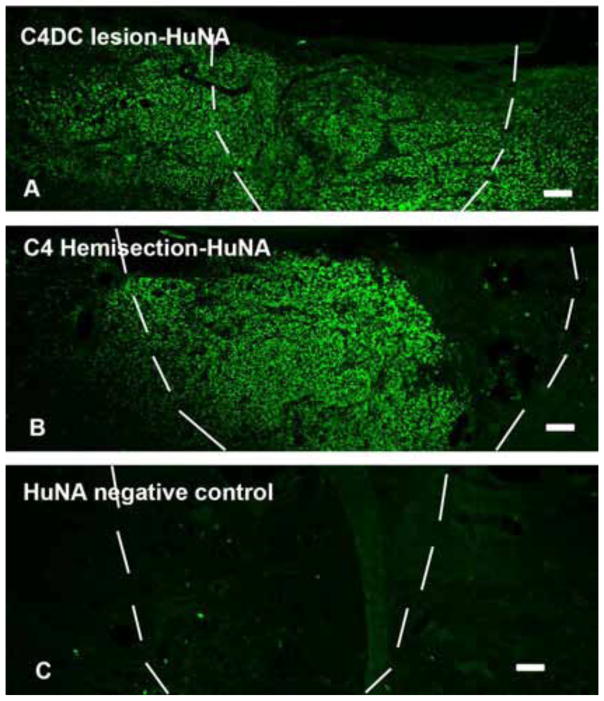
Human GRPs (hGRPs) suspended into a PureCol matrix (Advanced BioMatrix, Calsbad, CA) were transplanted acutely into C4 dorsal column lesion and C4 hemisection site from frozen stocks (Q Therapeutics, INC). Animals were sacrificed at 5 weeks. The hGRP cells were identified by immunohistochemical (IHC) staining with human nuclei, a specific marker for human cells. In C4 dorsal column lesion group, the entire cellular transplant survived at and around lesion area (Figure 1A). In C4 hemisection injury group, only half the rats showed cell survival at/around lesion/transplant. In the rats with cell survival, transplanted cells did not cover the entire the injury area (Figure 1B). We also measured the area of the survived cells inside lesion cavity in both injury models. In C4 dorsal column lesion, transplanted hGRPs covered 92.3 ± 3.7% of the lesion cavity, while in C4 hemisection model, only 57.9 ± 3.7% of hGRPs covered the lesion cavity (Data = mean ± S.E.M.).
Figure 1.
Cell survival after acute transplant hGRPs into C4 dorsal column and hemisection lesion. Transplanted hGRPs were stained with specific antibody for human cells HuNA. Injury/transplant area is outlined with white dash lines. In C4 dorsal column (DC) lesion, all animals showed HuNA positive staining inside and around the lesion area. Panel A demonstrates that hGRPs survived inside the lesion area which is covered almost entire injury region. Transplanted cells also migrated out of the injury site into nearby host spinal cord. While in C4 hemisection, HuNA positive staining did not show in all rats. Panel B shows that in rats with cell survival, most of hGRPs were located inside the lesion close to rostral stump of host cord but not fully covered the entire area. Panel C shows negative control staining without primary antibody for HuNA. DC = dorsal column, scale bar =100μm in all images.
2.2 Sensory axon regeneration
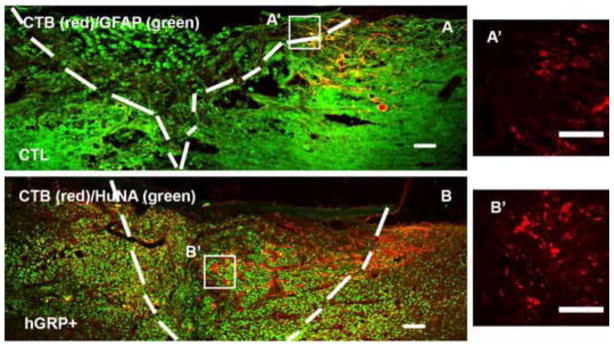
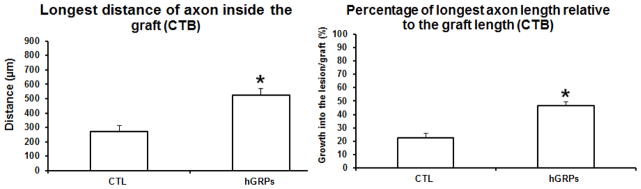
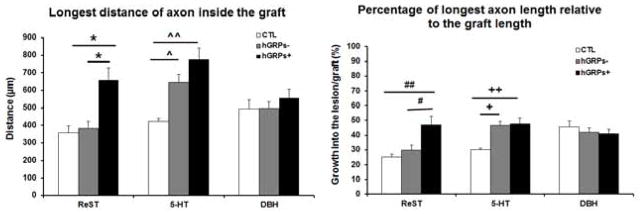
All sections were stained with GFAP to identify the lesion/graft boundary and different tracers were used to assess the length of axons extended into the graft. Animals with a dorsal column lesion received cholera toxic B subunit (CTB) injection into the sciatic nerve 3 days before sacrifice to trace sensory axons growing into the transplants. Double staining with GFAP and CTB showed GFAP+ astrocytes accumulated around lesion border in the control group (white dash lines), with most of CTB+ axons remaining at the caudal to the lesion (Figure 2A) and few growing into the lesion. In the hGRP transplant group, there was a reduced GFAP staining around the lesion site and more CTB+ axons extending into the graft (Figure 2B). Analysis of the longest distance of axon inside the graft showed that CTB+ axons grew a short distance inside the graft in the control injury group for about 270±43 μm from the caudal border of lesion. In the hGRP group, CTB+ axons grew significantly longer distances into lesion/transplant for an average of 522±49 μm (Figure 3 left, *p<0.001, relative to the control group). Comparing the longest axon relative to the graft length (% of distance into the lesion site) showed that animals with hGRP transplants had CTB axons that grew longer distance of 47±3% of the graft length, while in the control group CTB axons only grew for 23±4% of length of lesion cavity (Figure 3 right, *p=0.000, hGRPs vs CTL). Thus hGRP transplants promoted sensory axons growing longer distance inside the graft.
Figure 2.
Regenerating sensory axons growing into lesion and transplant. The lesion/transplant area is identified with GFAP (A, green) and HuNA (B, green) IHC staining and outlined with white dash lines. CTB labeled sensory axons grew a short distance into the injury site (A with red), while transplanted hGRPs promoted CTB axons growing a longer distance into the transplant (B with red) and transplanted cells were stained with HuNA (B, green). Panels A′ and B′ are high magnification of CTB axons within boxes in Figure 2A and B. Scale bar =100 μm in all images.
Figure 3.
Quantitative analysis of the longest distance of sensory axons growing into the lesion/transplant. CTB axons grew into the injury site a short distance (left), reaching only 20% of the length of injury area (right). Transplanted GRP promoted axonal growth for a longer distance (left, *p<0.001) reaching the middle of the transplant about 50% of the length of injury (right, *p=0.000). n = 4 for both CTL and hGRP+ groups. Data = Mean ± S.E.M. Data was analyzed with Student’s t-test.
2.3 Motor axon regeneration
Axonal regeneration from four different motor tracts was examined after acute C4 hemisection. We analyzed 1) RST (rubrospinal tract), labeled by BDA injected into the red nucleus; 2) ReST (reticulospinal tract), labeled by GFP with injection of AAV1-GFP into the gigantocellular reticular nucleus; 3) Raphespinal tract (5-HT), identified by IHC staining of serotonin; and the Coeruleospinal tract, identified by dopamine β-hydroxylase (DβH) staining. Because not all the transplanted cells survived in C4 hemisection model, axonal regeneration was analyzed in three separate groups: control group (CTL), grafts of hGRPs without cell survival (hGRPs−) in which cells might have survived transiently, and grafts of hGRPs with cell survival (hGRPs+).
2.3.1 Axonal regeneration from raphespinal tract
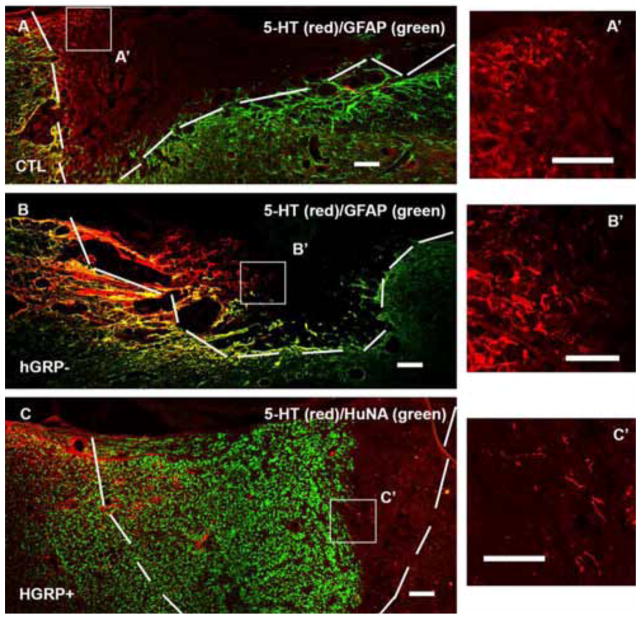
The Raphespinal tract was traced by serotonin (5-HT) labeling. In the control group, whose lesion area was clearly outlined by GFAP staining, some 5-HT+ axons grew into the lesion to an average distance of 424±17μm (Figure 4A). In the hGRP− group, the lesion boundary, also outlined clearly by GFAP staining, the 5-HT+ axons grew into the lesion site at an average 645±46μm (Figure 4B). In the GRP+ group (Figure 4C), while the GFAP staining around the lesion/transplant area was reduced relative to the control injury group, the borders were still clear. Many 5-HT+ axons grew into the lesion/transplant with an average of 776±64μm. Regenerating 5-HT axons significantly increased the distance inside the lesion/transplant in both hGRP− and hGRP+ groups compared to the control group (Figure 5 left, ^p<0.0017, hGRP− vs control; ^^p=0.000, hGRP+ vs control) with no significant difference between hGRP− and hGRP+ groups. Analysis of the percentage of longest length relative to the lesion/graft length showed that 5-HT axons grew only 30% of the lesion length in the control group but increased to 46% and 48% in the hGRP− and hGRP+ groups, respectively (Figure 5 right, +p<0.003, hGRP− vs control; ++p<0.001, hGRP+ vs control). Raphe spinal axons thus extended greater distances in the presence of grafts in which hGRPs had been included even in those in which the hGRPs had not survived at 5 weeks.
Figure 4.
Regeneration of serotonergic axons. The lesion/transplant area is identified with GFAP (A and B, green) and HuNA (C, green) IHC staining and outlined with white dash lines. 5-HT labeled serotonergic axons (labeled with red) grew into control injury (A), hGRP transplant without cell survival (B), and hGRP transplant with cell survival (C) grew further. Panels A′, B′ and C′ are high magnification of 5-HT axons within boxes in Figure 3A, B and C. Scale bar =100 μm in all images.
Figure 5.
Analysis of longest distance of motor axon inside the graft (left) demonstrated that there is a significant increase of ReST growth in the GRP+ group compared to control (CTL) and GRP− groups. For regeneration of 5-HT, the growth distance into the transplant is significantly increased in both GRP− and GRP+ compared to the control group. However, the distance of DβH axons growing into the lesion in all groups was similar without significant differences. Analysis of percentage of longest axons length relative to the graft length (right) showed that the hGRP transplant promoted ReST axons growing half length of the graft, significantly longer than control and GRP− groups. 5-HT axons grew into the graft near half of the graft length in both GRP− and GRP+. The percentage of DBH axons did not show significant differences among all groups. In the left: one way ANOVA, F (2,30) =9.539, p=0.001; BONFERRONI’s post hoc test, *p<0.02, hGRP+ vs hGRP− or CTL; one way ANOVA, F (2,30) = 11.375, p=0.000; BONFERRONI’s post hoc test, ^p<0.0017, hGRP− vs CTL, ^^p= 0.000 hGRP+ vs CTL. In the right: one way ANOVA, F (2,29) = 7.056, p<0.003; BONFERRONI’s post hoc test, #p<0.04 hGRP+ vs hGRP−; ##p<0.004, hGRP+ vs CTL; one way ANOVA, F (2,30) = 9.328, p<0.001; BONFERRONI’s post hoc test, +p<0.003 hGRP− vs CTL; ++p<0.001, hGRP+ vs CTL. n = 3 for CTL group; n = 4 for both hGRP− and hGRP+ groups. Data = Mean ± S.E.M.
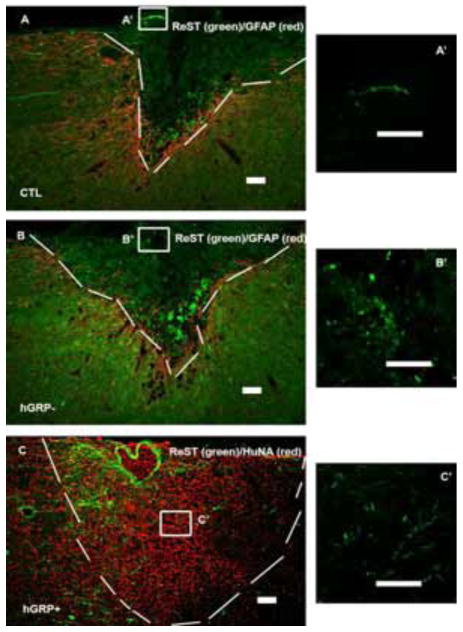
2.3.2 Axonal regeneration from reticulospinal tract
Axons from reticulospinal tract were labeled by AAV1/GFP injected into the gigantocellular reticular nucleus 2 weeks before the end of the experiment. GFP+ axons grew into the lesion/transplant area in the control, hGRP−, and hGRP+ groups (Figures 6A–C). However, GFP+ axons grew a short distance in both the control and hGRP− groups with an average of 361±37μm and 383±71μm, respectively. In the hGRP+ group, GFP+ axons grew an average of 658μm into the transplant (Figure 5 left), which was significantly further compared to the control and hGRP− groups (* p<0.002). There was also a significant increase in the percentage of longest axon length in hGRP+ group compared to the hGRP− group (# p<0.04) and to the control group (## p<0.004, Figure 5 right). The three conditions showed different effects on axonal growth:, greater for hGRP surviving grafts, least for none cell control group, as well as for the grafts in which the cells did not survive at 5 weeks
Figure 6.
Regeneration of reticulospinal axons (ReST). The lesion/transplant area is identified with GFAP (A and B, red) and HuNA (C, red) IHC staining and outlined with white dash lines. ReST axons (green) grew into the injury only a very short distance (A), same as transplant without cell survival (B). However, transplants with cell survival attracted ReST regenerating further into the lesion/transplant (C). Panels A′, B′ and C′ are high magnification of ReST axons within boxes in Figure 4A, B and C. Scale bar =100 μm in all images.
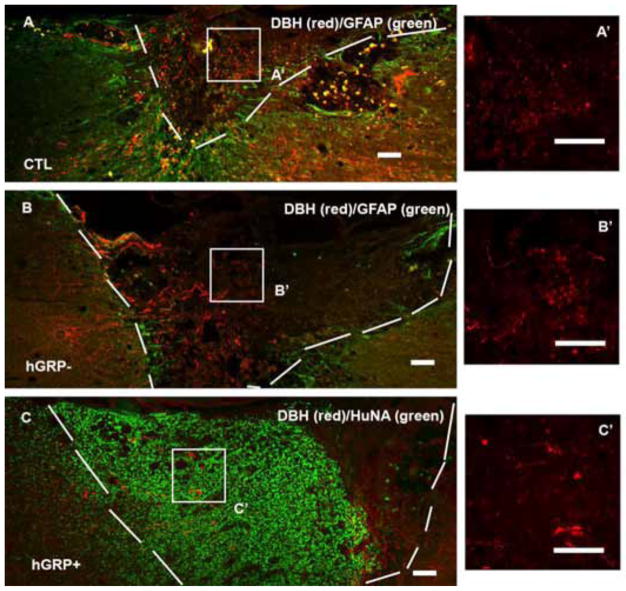
2.3.3 Axonal regeneration from coeruleospinal tract
Axons from the coeuleospinal tract, identified by DβH staining, grew into the lesion/transplant in all groups (Figures 7A–C). The longest distance of axon growth was similar in all groups with no significant differences, with an average of 496±50μm in control group, 498±38μm in hGRP− group, and 556±53μm in hGRP+ group (Figure 5 left). The longest axon length relative the lesion/transplant length was also similar among all 3 groups, with an average of 45%, 42% and 41% in control, hGRP− and hGRP+ groups, respectively (Figure 5 right). Thus coeruleospinal axons grew equally robustly into grafts in all three conditions and thus differenced from regeneration of 5-HT and ReST.
Figure 7.
Regeneration of coerulospinal axons. The lesion/transplant area is identified with GFAP (A and B, green) and HuNA (C, green) IHC staining and outlined with white dash line. Coerulospinal axons identified by DβH staining (red) grew a similar distance into the injury alone (A), as transplants without cell survival (B), or transplants with cell survival (C). Panels A′, B′ and C′ are high magnification of DβH axons within boxes in Figure 5A, B and C. Scale bar =100 μm in all images.
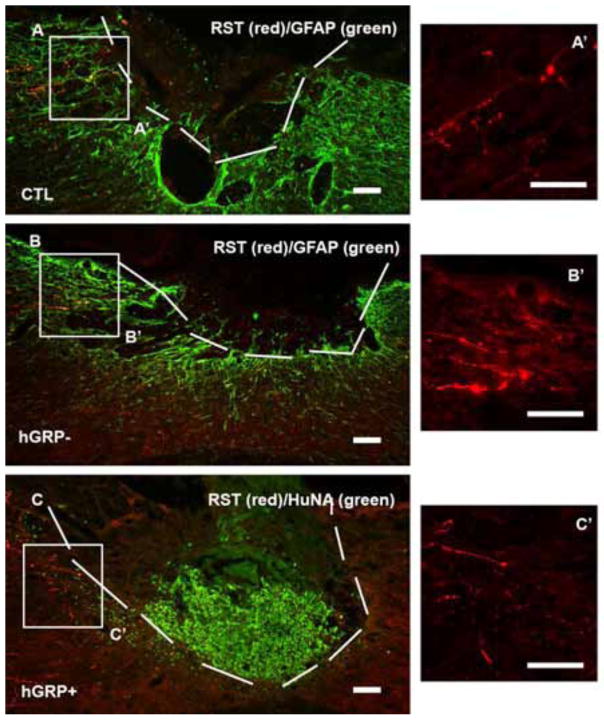
2.3.4 Axonal regeneration from rubrospinal tract
Axons from the rubrospinal tract were traced by injected BDA into the contralateral side of the red nucleus 2 weeks before animal sacrifice. In control group, BDA labeled RST axons were found near the lesion site but they did not grow into the lesion area (Figure 8A). The hGRP− as well as the hGRP+ groups showed similar results (Figures 8B and 8C, respectively). Notice that the hGRP group where the transplants survived at the lesion area showed less GFAP staining compared to the control (Figure 8A) and hGRP− (Figure 8C).
Figure 8.
Regeneration of rubrospinal axons. The lesion/transplant area is identified with GFAP (A and B, green) and HuNA (C, green) IHC staining and outlined with white dash lines. Rubrospinal axons (RST) traced with BDA (red) stopped near the lesion area in the injury alone group (A). RST axons did not grow further into the transplant without cells (B) or with cell survival (C). Panels A′, B′ and C′ are high magnification of RST axons within boxes in Figure 6A, B and C. Scale bar =100 μm in all images.
3. Discussion
We have shown in this study that 1) the survival of the transplanted hGRPs was dependent on the type of injury, with the small injury having better cell survival, 2) the regeneration capacity of sensory and motor axons following SCI varied among the different tracts, 3) The hGRPs transplants promoted axon growth into the transplants from sensory and selective motor tracts (Table 1). In no case did axons exit the graft. However, if the objective is to promote host axon regeneration across the injury, it may be necessary to guide the migration of transplant toward putative targets to extend regenerating axons, or to provide neurons within the transplants in which the regenerating axons can synapse (Bonner et al., 2011; Lu et al., 2012; Yuan et al., 2016) or to increase the regenerative capacity of the axons, as has been demonstrated for a pre-conditioning peripheral nerve injury (Neumann and Woolf, 1999).
Table.
Distance and percentage of axonal growth inside the lesion/transplant
| Tracts | Control (CTL) Percentage/Distance | Transplant (hGRP−) Percentage/Distance | Transplant (hGRP+) Percentage/Distance |
|---|---|---|---|
| Sensory (CTB) | 22.7% (270.2μm) | N/A | 46.5% (521.7μm) |
| Reticulospinal tract (ReST) | 25.2% (360.6μm) | 29.7% (382.8μm) | 47% (658.1μm) |
| Rephaspinal tract (Serotonin, 5-HT) | 30% (423.7μm) | 46.4% (644.7μm) | 47.7% (775.7μm) |
| Coerulospinal tract (DβH) | 45.3% (496μm) | 42.2% (497.7μm) | 41.2% (555.5μm) |
| Rubrospinal tract (RST) | 0 | 0 | 0 |
3.1 Grafted cell survival
Survival and integration are essential for the efficacy of cell transplants, particularly when the objective is to bridge the lesion and promote connectivity. One of the critical tests for the potential efficacy of such transplants is the ability to promote the growth of host injured axons into the lesion/transplant site. Poor transplant survival means that no new bridge is created for axon regeneration through. However, it is still possible that even the transient presence of the transplant will have some effects on promoting regeneration, by secretion of growth factors and other permissive molecules at the early post-injury stages, as was seen in the case of hGRP− transplants for the raphe spinal tract.
The choices for delivery of transplants depends on the specific injury model (e.g., hemisection, transection, contusion), the timing of transplantation (e.g., acute, delayed) and the objective of the study (neuroprotection, restored connectivity). In some studies, cells have been injected, rostral or caudally to the injury, but not directly inside the injury where cell survival is poor (Cao et al., 2005; Karimi-Abdolrezaee et al., 2010). However, if the objective of the transplant is to promote host axons growth into/through the lesion/transplants, the cells may need to be transplanted inside the lesion in order to replace the lost host tissue and bridge the lesion. Our previous studies have shown that transplantation of rat neural progenitor cells into a dorsal column lesion showed good survival in contrast to a complete transection injury, where survival was poor (Bonner et al., 2010; Haas and Fischer, 2013; Medalha et al., 2014). Here, we compared the effects of hGRPs transplants between two injury models, a small dorsal column lesion that severed sensory axons and a larger C4 hemisection that severed in axons in the lateral and ventral funiculi. We found that the hGRPs survived well in the dorsal column lesion, compared to the hemisection injury, where at sacrifice, only half of the animals showed hGRP survival within the graft. These results are consistent with our previous findings that indicated that the survival of the transplants is reduced with the severity of the injury. Recent study (Hou et al., 2018) also indicates that severed spinal cord injury resulted in a large lesion cavity and severe fibrotic scars around the cavity. Therefore, transplanted cells rarely formed a tissue bridge. Small injury by cut or crush caused smaller cavity and transplanted cells survived inside the lesion and integrated well with host tissue. This result is consistent with our finding here which indicates that survival of transplants is not depended on type of cells but on the injury severity.
3.2 Sensory axon regeneration
Regeneration of sensory axons was traced by injection of CTB into the sciatic nerve, which labeled large myelinated Aβ fibers in the dorsal column. Our results showed that CTB+ axons grew only a short distance into the dorsal column lesion area in the control animals, while transplanted hGRPs promoted CTB+ axons to grow longer distance, reaching about half way through the transplant/injury site. These results are consistent with our previous study using hGRPs, which were pre-cultured (Haas and Fischer, 2013), suggesting that these cells can be used effectively from frozen stocks to follow realistic clinical protocols. In both studies, the CTB+ axons grew into but not through the transplant indicating that hGRP provided permissive environment for the growth of sensory axons, but had only a limited effect on the regenerative capacity of these axons. Sensory axons have been shown to regenerate after SCI following other treatments such as administration of neurotrophic factors (Bradbury et al., 1999), reducing glial scar (Lee et al., 2010; Tan et al., 2006), transplants of Schwann cells and modified fibroblasts (Tuszynski et al., 1994; Xu et al., 1995b), neuronal and glial restricted progenitors (Bonner et al., 2011), and human stem cells (Hoeber et al., 2015). The ability of hGRP transplants to promote sensory axon growth into the lesion site can be exploited by combining them with neuronal progenitor cells to form a relay to reconnect the spinal cord as demonstrated for rodent cells (Bonner et al., 2011).
3.3 Motor axon regeneration
Among descending motor axons from the brain stem, raphespinal and reticulospinal tracts demonstrated the highest regeneration capacity, which further improved in the presence of hGRP transplant. In contrast, the rubrospinal and the coerulospinal tract were refractive to the presence of the graft. This regenerative “ranking” is consistent with a recent report that compared the ability of neural progenitor cells (NPCs) transplanted at the site of SCI to receive synaptic connectivity from various descending tracts (measured by PRV tracing) and found the reticulospinal tract at the high end and the rubrospinal tract at the low end (Adler et al., 2017). Interestingly, this study also found high connectivity with the cortical spinal tracts and poor connectivity with the Raphespinal tract. Another study, which examined the regenerative axon growth of multiple tracts in SCI revealed the following ranking of fiber tracts with respect to profile density in the fibrous scar at 5 weeks post-lesion: CGRP (sensory) > 5-HT > TH > RST > CST (Schiwy et al., 2009). This study also found that scar-suppressing treatment increased the number of axons intersecting the lesion border.
Consisted with our results, 5-HT axonal regeneration has been shown using various transplants in different SCI models, such as embryonic spinal cord in the neonatal/adult SCI (Bregman, 1987; Reier et al., 1986) and chronic SCI (Reier et al., 1988), Schwann cells alone (Xu et al., 1995b) or with growth factors (Xu et al., 1995a) or cell modified to secrete growth factors (Menei et al., 1998), genetically modified fibroblast that secrete NGF or BDNF in chronic SCI (Grill et al., 1997; Jin et al., 2000; Karimi-Abdolrezaee et al., 2010), olfactory ensheathing glia (Ramon-Cueto et al., 1998), and neural stem cells (Boido et al., 2009; Hodgetts et al., 2013; Medalha et al., 2014). Here we show that raphespinal axons identified by 5-HT staining regenerated into the hemisection lesion area a short distance, but in the presence of hGRP transplants which survived at lesion site there was a significant increased the distance of 5-HT growth. Interestingly, 5-HT axons also grew a similar distance in the transplanted group, when the cells did not survive at the lesion/transplant area, compared to the control non-cell transplant group. These results suggest that transplanted hGRPs could exert an early effect on the capacity of 5-HT axonal regeneration, likely by secretion of permissive factors.
The coerulospinal tract was identified in our studies by using antibodies for dopamine-β-hydroxylase (DβH). Previous studies showed that transplants of fetal spinal cord or Schwann cells promoted coerulospinal axonal growth into the transplants. When combined with growth factors the regeneration can be enhanced (Bregman et al., 1997; Weidner et al., 1999). Acute or delayed transplants of olfactory ensheathing cells also promote regeneration of coerulospinal axons after SCI (Lopez-Vales et al., 2006). In transected feline spinal cord, coerulospinal axons regenerated into a collagen matrix bridge (de la Torre and Goldsmith, 1994). From our study, we found that coerulospinal axons identified by DβH antibody can grow into cervical hemisection only, or hemisection with hGRP in both hGRP+ and hGRP− conditions. The distance inside the lesion/transplant is not significant difference in any conditions. The regeneration of coerulospinal axons is different from raphespinal axons on hGRP transplant in being refractory to the effects of the hGRP.
The remaining two supraspinal tracts that we have studied were rubrospinal (RST) and reticulospinal (ReST) tracts, which were labeled by anterograde tracer into the red nucleus and gigantocellular reticular nucleus, respectively. The regenerative capacity of these tracts and their response to the hGRP transplant were very different. ReST axons regenerated into hGRP transplant a longer distance compared to the control non-transplant and transplant without cell survival. Transplanted hGRPs without cell survival at lesion/transplant did not increase the length of ReST inside the transplant. In contrast, RST did not show regeneration and stopped at the injury interface in all 3 groups, control, hGRP+ and hGRP−.
3.4 Responses to SCI and hGRP transplants from both sensory and motor system
Our study indicates that the regenerative capacity of different tract following SCI and the response to hGRPs is varied (Table 1). All axons tested in this study, except for rubrospinal, show some regeneration into the lesion area. Sensory axons, reticulospinal and raphespinal axons responded to the presence of hGRP, which promoted their regeneration into the transplants, with the raphespinal axons increasing their growth even when the transplant cells did not survive. Although long-term transplant survival is critical for strategies of cell replacement (e.g., neurons, oligodendrocytes), we observed that whether the hGRPs covered most of lesion cavity (92% in a dorsal column lesion) or just half cavity (57.9% in a hemisection), the regenerating axons from both sensory and motor system did not grow through the entire lesion. We speculate that even a modest survival of permissive cells can promote axon growth during a critical time post injury, and dependent on the intrinsic regenerative properties of different tracts. However, our data indicate that transplants of hGRPs alone are not efficient to promote the injured axons growing through the transplants. Combined strategies will be investigated to promote axon regeneration through the lesion/transplant in our future studies.
4. Conclusions
We have also showed that transplant of hGRPs directly from a frozen stock can promote both sensory and motor axonal regeneration, which indicates that hGRPs are a good candidate for future translational studies. Sensory and motor axons respond differently to hGRP transplants. Thus, using hGRPs alone may not be enough for functional regeneration for both sensory and motor system. Combination with new interventions will be investigated for both axonal regeneration and functional recovery in future.
5. Experimental procedure
5.1 Animals and Experimental design
Adult female Sprague-Dawley (SD, 225–250g) rats were used for two sets of experiments. In the first experiment, 12 rats received a C4 hemisection, with 8 received cell transplant mixed with PureCol (a matrix for cell culture from Advanced BioMatrix, Calsbad, CA) and 4 received PureCol alone for a control. One rat from control was sacrificed earlier due to the autophagia. In the second experiment, 8 rats received a dorsal column lesion at C4, with 4 received cell transplant mixed with PureCol and 4 received PureCol only as control.
5.2 Preparation of human GRPs
Human GRP (obtained from Q therapeutics, Salk lake City, Utah) were prepared directly from frozen stock. After thawed the stock, cells were washed twice with Hank’s Balanced Salt solution (HBSS). Cells were spun down and mixed with PureCol (1:1) at 1.5×106/5ul for hemisection and 1×106/3ul for dorsal column lesion. Cells were kept on ice all the time during surgery. Cell viability was tested by trypan blue after surgery.
5.3 Surgery and cell transplantation
Rats were anesthetized by intraperitoneal injection of an XAK cocktail of Xylazine (10 mg/kg; Webster Veterinary, Sterling, MA), Acepromazine maleate (0.7 mg/kg; Webster Veterinary) and Ketamine (95 mg/kg; Webster Veterinary). The skin was shaved and cleaned with Betadine and 70% ethanol. A laminectomy was performed at C3–4 to expose the spinal cord. In the hemisection model, the dura was incised above the dorsal root entry zone. Micro-scissors cut was made in the rostral and caudal directions to create a cavity. For dorsal column lesion, the dura was cut along the rostral-caudal direction near the midline. A 30 gauge needle was used to make a complete injury in one side of the dorsal column, sparing the descending corticospinal tract and leaving most of the contralateral dorsal column intact in the other side. Sutures (9-0) were placed in the dura on both sides of the lesion but not tightened. After achieving homeostasis of the spinal cord injury, about 5ul of cell (1.5×106 cells) suspension were injected into the lesion site in hemisection, or 3ul of cell (1×106 cells) suspension were injected into dorsal column lesion site. The sutures were quickly tightened to maintain the cell suspension within the lesion site. The muscles were hen sutured and skin was closed with clips. Buprenex (0.015–0.02 mg/kg, 0.3 mg/ml, Reckitt Benckiser, Richmond, VA) was administered subcutaneously post-surgery and twice a day for two days. All animals received subcutaneous injection of cyclosporine A (Sandimmune; Novartis Pharmaceuticals East Hanover, NJ, USA) at a dose of 1 mg/100 g per day beginning 2–3 days before transplantation and continuing to the end of the experiments. Animals survived for 5 weeks after lesion/transplantation.
5.4 Axonal tracing
For axonal tracing, two weeks before sacrificed rats were anesthetized with XAK and placed into a stereotaxic device (David Kopf Instruments, Tujunga, CA). The dorsal surface of the cranium was exposed and a small burr hole was made with a high speed drill at one of two sites: the red nucleus contralateral to the spinal cord injury lesion to label rubrospinal tract (RST) and the medullary gigantocellular reticular nucleus ipsilateral to the spinal cord lesion to trace reticulospinal tract (ReST). The following coordinates were used for injections, using Bregma as the zero point: anterior-posterior 5.8mm, medial-lateral 0.75mm, dorsal-ventral (DV, from surface of dura) 7.0mm for the red nucleus; AP 11.6mm, ML 1.0mm, DV 8.5mm for the medullary gigantocellular reticular nucleus. A 30 gauge needle attached to a 10ul Hamilton syringe was filled with either 10% solution of BDA (10,000MW, Molecular Probes, Eugene, OR, for RST) or AAV-1/GFP (for ReST), 2μl per point. The needle was lowered to the desired level and the solution was injected at 0.2μl/min. The needle was left in the place for additional 2 min to allow for diffusion of injected tracer, and then raised out of the brain slowly. Gel foam was inserted into the hole and skin was closed with wound clips.
For CTB injection, 4ul of 1% CTB solution was injected into the sciatic nerve ipsilateral to the lesion 3 days before sacrificed to trace sensory axons.
5.5 Tissue preparation
Five weeks after SCI with or without cell transplantation, animals were overdosed with Euthosol (J. A. Webster) and then transcardially perfused with 100ml of ice-cold 0.9% saline and 500ml of ice-cold 4% paraformaldehyde in phosphate buffer. The spinal cord and brain were removed and placed in 4% paraformaldehyde overnight, followed by cryoprotection in 30% sucrose/0.1 M phosphate buffer at 4°C for at least 3 days. Spinal cords were embedded in M1 medium (Thermo Fischer Scientific) and cut sagittally in 20μm sections for the cord with lesion/transplant, or cut coronal sections in 30μm for C1 cords. Tissue was collected on the glass slides coated with gelatin. Slides were kept at −20°C for future usage.
5.6 Immunohistochemical staining (IHC)
Sagittal sections were processed for immunohistochemical staining with primary antibodies against human nuclei (HuNA, 1:200, Chemicon) to identify for transplanted hGRP, GFAP (1:1000, Chemicon) for astrocytes, GFP (1:1000, Millipore) for ReST, serotonin (5-HT, 1:20,000 Immuostar) for descending raphespinal tract, anti-dopamine beta hydroxylase (DβH, 1:1000, Chemicon) for descending coeruleospinal tract, anti-cholera toxin B subunit (CTB, 1:2000, List Biological Laboratories, Inc) for ascending sensory axons. Sections were washed with phosphate buffered saline, blocked with 10% goat or donkey serum for 1hr at room temperature, and then incubated in primary antibodies at room temperature overnight. Species-specific secondary antibodies (goat anti-mouse, goat anti-rabbit, or donkey anti-goat conjugated to FITC or rhodamine, 1:400, Jackson ImmunoResearch) were applied for 2 h at room temperature and the slides were cover-slipped with fluoromount-G (SouthernBiotech).
5.7 Measurement of relative area of transplanted hGRPs inside the lesion cavity
HuNA stained tissue sections at injure region were visualized with a 10x objective and images were captured. NIH image J was subsequently used to outline the lesion area. HuNA stained area within the injury site was measured and is presented as percentage of the lesion area.
5.8 Analysis of the longest distance of regenerating axons inside the graft
The images of regenerating axons were captured from double-stained sections of GFAP and the respective axon labeling at X10 using a Leica DM5500B microcopy with SlideBook software (Intelligent Imaging Innovations). All of our samples are collected from three slides obtained from each animal and different stains were performed on sequential slides. We confirmed that the coefficient of variance across samples was equivalent for each animal. The longest distance of axons inside the lesion/transplant was quantified using NIH Image J. Data were presented as the average length of the longest labeled axon inside the transplant as well as percentage of the length of the longest axon relative to the entire length of the graft, to indicate how far labeled axons had grown into the transplant.
5.9 Statistical analysis
We performed a power analysis on our data and confirmed that for our large effect size with a significance level of 0.05, that we could obtain robust results (power = 0.80) with a sample size of 3–4 per group. Statistical analysis was performed by one-way ANOVA or Student’s t-test, where appropriate, and BONFERRONI’s post hoc test with IBM SPSS Statistics 20 software.
Highlights.
Human GRPs can be effectively used directly from a frozen stock for transplantation into spinal cord injury.
Transplanted human GRPs survived following acute dorsal column lesions and to a lesser extent in hemisection lesion underscoring the challenges associated with large lesions.
Transplants of human GRPs could promote axonal growth/regeneration into the lesion/transplants, which varied among the different sensory and motor tracts.
Human GRPs are promising candidates for spinal cord injury transplantation therapy.
Acknowledgments
We thank Julein Bouyer, Maryla Obrocka, and Theresa Connors for technical support and animal care; and Q therapeutics, Salk lake City, Utah for kindly providing the human GRPs. We also thank Dr. Marion Murray for comments on the manuscript. This work was supported by National Institutes of health (NIH, PO1, NS055976) and the Craig H. Neilsen Foundation (160746).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler AF, Lee-Kubli C, Kumamaru H, Kadoya K, Tuszynski MH. Comprehensive Monosynaptic Rabies Virus Mapping of Host Connectivity with Neural Progenitor Grafts after Spinal Cord Injury. Stem Cell Reports. 2017;8:1525–1533. doi: 10.1016/j.stemcr.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boido M, Rupa R, Garbossa D, Fontanella M, Ducati A, Vercelli A. Embryonic and adult stem cells promote raphespinal axon outgrowth and improve functional outcome following spinal hemisection in mice. Eur J Neurosci. 2009;30:833–46. doi: 10.1111/j.1460-9568.2009.06879.x. [DOI] [PubMed] [Google Scholar]
- Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88:1182–92. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–86. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Khemani S, Von R, King Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–83. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Bregman BS. Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 1987;431:265–79. doi: 10.1016/0165-3806(87)90214-8. [DOI] [PubMed] [Google Scholar]
- Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148:475–94. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- Brosamle C, Huber AB, Fiedler M, Skerra A, Schwab ME. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000;20:8061–8. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broude E, McAtee M, Kelley MS, Bregman BS. Fetal spinal cord transplants and exogenous neurotrophic support enhance c-Jun expression in mature axotomized neurons after spinal cord injury. Exp Neurol. 1999;155:65–78. doi: 10.1006/exnr.1998.6964. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multineurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–57. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–44. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–74. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–3. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Goldsmith HS. Coerulospinal fiber regeneration in transected feline spinal cord. Brain Res Bull. 1994;35:413–7. doi: 10.1016/0361-9230(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Elliott Donaghue I, Tator CH, Shoichet MS. Sustained delivery of bioactive neurotrophin-3 to the injured spinal cord. Biomater Sci. 2015;3:65–72. doi: 10.1039/c4bm00311j. [DOI] [PubMed] [Google Scholar]
- Fouad K, Dietz V, Schwab ME. Improving axonal growth and functional recovery after experimental spinal cord injury by neutralizing myelin associated inhibitors. Brain Res Brain Res Rev. 2001;36:204–12. doi: 10.1016/s0165-0173(01)00096-0. [DOI] [PubMed] [Google Scholar]
- Grill RJ, Blesch A, Tuszynski MH. Robust growth of chronically injured spinal cord axons induced by grafts of genetically modified NGF-secreting cells. Exp Neurol. 1997;148:444–52. doi: 10.1006/exnr.1997.6704. [DOI] [PubMed] [Google Scholar]
- Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma. 2013;30:1035–52. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C, Fischer I. Transplanting neural progenitors to build a neuronal relay across the injured spinal cord. Neural Regen Res. 2014;9:1173–6. doi: 10.4103/1673-5374.135321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts SI, Simmons PJ, Plant GW. A comparison of the behavioral and anatomical outcomes in sub-acute and chronic spinal cord injury models following treatment with human mesenchymal precursor cell transplantation and recombinant decorin. Exp Neurol. 2013;248:343–59. doi: 10.1016/j.expneurol.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Hoeber J, Trolle C, Konig N, Du Z, Gallo A, Hermans E, Aldskogius H, Shortland P, Zhang SC, Deumens R, Kozlova EN. Human Embryonic Stem Cell-Derived Progenitors Assist Functional Sensory Axon Regeneration after Dorsal Root Avulsion Injury. Sci Rep. 2015;5:10666. doi: 10.1038/srep10666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Saltos TM, Iredia IW, Tom VJ. Surgical techniques influence local environment of injured spinal cord and cause various grafted cell survival and integration. J Neurosci Methods. 2018;293:144–150. doi: 10.1016/j.jneumeth.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–15. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Tessler A, Fischer I, Houle JD. Fibroblasts genetically modified to produce BDNF support regrowth of chronically injured serotonergic axons. Neurorehabil Neural Repair. 2000;14:311–7. doi: 10.1177/154596830001400407. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–75. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Jin Y, Neuhuber B, Singh A, Bouyer J, Lepore A, Bonner J, Himes T, Campanelli JT, Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J Neurotrauma. 2011;28:579–94. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–76. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelamangalath L, Smith GM. Neurotrophin treatment to promote regeneration after traumatic CNS injury. Front Biol (Beijing) 2013;8:486–495. doi: 10.1007/s11515-013-1269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:3340–5. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19:4370–87. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vales R, Fores J, Verdu E, Navarro X. Acute and delayed transplantation of olfactory ensheathing cells promote partial recovery after complete transection of the spinal cord. Neurobiol Dis. 2006;21:57–68. doi: 10.1016/j.nbd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Lorgulescu B, Patel S, Louro J, Andrade C, Sanchez A, Pearse D. 182 Acute Putrescine Supplementation With Schwann Cell Transplantation Improves Sensory and Serotonergic Axon Growth and Functional Recovery in Spinal Cord Injury. Neurosurgery. 2015;62(Suppl 1):226–7. doi: 10.1155/2015/186385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–73. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–2. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- Medalha CC, Jin Y, Yamagami T, Haas C, Fischer I. Transplanting neural progenitors into a complete transection model of spinal cord injury. J Neurosci Res. 2014;92:607–18. doi: 10.1002/jnr.23340. [DOI] [PubMed] [Google Scholar]
- Menei P, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci. 1998;10:607–21. doi: 10.1046/j.1460-9568.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Shumsky JS, Lepore AC, Murray M, Fischer I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005;25:9624–36. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, Ikeda E, Toyama Y, Yamanaka S, Nakamura M, Okano H. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A. 2011;108:16825–30. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nout YS, Culp E, Schmidt MH, Tovar CA, Proschel C, Mayer-Proschel M, Noble MD, Beattie MS, Bresnahan JC. Glial restricted precursor cell transplant with cyclic adenosine monophosphate improved some autonomic functions but resulted in a reduced graft size after spinal cord contusion injury in rats. Exp Neurol. 2011;227:159–71. doi: 10.1016/j.expneurol.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosyan HA, Hunanyan AS, Alessi V, Schnell L, Levine J, Arvanian VL. Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats. J Neurosci. 2013;33:4032–43. doi: 10.1523/JNEUROSCI.4702-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–15. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal S. In: Degeneration and Regeneration of the Nervous System. May RM, editor. New York: Hafner Publishing Co; 1928. p. 1. [Google Scholar]
- Reier PJ, Bregman BS, Wujek JR. Intraspinal transplantation of embryonic spinal cord tissue in neonatal and adult rats. J Comp Neurol. 1986;247:275–96. doi: 10.1002/cne.902470302. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Houle JD, Jakeman L, Winialski D, Tessler A. Transplantation of fetal spinal cord tissue into acute and chronic hemisection and contusion lesions of the adult rat spinal cord. Prog Brain Res. 1988;78:173–9. doi: 10.1016/s0079-6123(08)60280-0. [DOI] [PubMed] [Google Scholar]
- Sandrock RW, Wheatley W, Levinthal C, Lawson J, Hashimoto B, Rao M, Campanelli JT. Isolation, characterization and preclinical development of human glial-restricted progenitor cells for treatment of neurological disorders. Regen Med. 2010;5:381–94. doi: 10.2217/rme.10.24. [DOI] [PubMed] [Google Scholar]
- Santos-Benito FF, Ramon-Cueto A. Olfactory ensheathing glia transplantation: a therapy to promote repair in the mammalian central nervous system. Anat Rec B New Anat. 2003;271:77–85. doi: 10.1002/ar.b.10015. [DOI] [PubMed] [Google Scholar]
- Schiwy N, Brazda N, Muller HW. Enhanced regenerative axon growth of multiple fibre populations in traumatic spinal cord injury following scar-suppressing treatment. Eur J Neurosci. 2009;30:1544–53. doi: 10.1111/j.1460-9568.2009.06929.x. [DOI] [PubMed] [Google Scholar]
- Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20:8727–35. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–39. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello F. Degeneration et regeneration des plaques motrices apres la section des nerfs. Travaux du Laboratorire de Recherches Biologiques de I’Universit6 de Madrid. 1907;5:117–149. [Google Scholar]
- Tuszynski MH, Peterson DA, Ray J, Baird A, Nakahara Y, Gage FH. Fibroblasts genetically modified to produce nerve growth factor induce robust neuritic ingrowth after grafting to the spinal cord. Exp Neurol. 1994;126:1–14. doi: 10.1006/exnr.1994.1037. [DOI] [PubMed] [Google Scholar]
- Walczak P, All AH, Rumpal N, Gorelik M, Kim H, Maybhate A, Agrawal G, Campanelli JT, Gilad AA, Kerr DA, Bulte JW. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia. 2011;59:499–510. doi: 10.1002/glia.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner N, Blesch A, Grill RJ, Tuszynski MH. Nerve growth factor-hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1. J Comp Neurol. 1999;413:495–506. doi: 10.1002/(sici)1096-9861(19991101)413:4<495::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Williams RR, Henao M, Pearse DD, Bunge MB. Permissive Schwann cell graft/spinal cord interfaces for axon regeneration. Cell Transplant. 2015;24:115–31. doi: 10.3727/096368913X674657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995a;134:261–72. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995b;351:145–60. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Yick LW, So KF, Cheung PT, Wu WT. Lithium chloride reinforces the regeneration-promoting effect of chondroitinase ABC on rubrospinal neurons after spinal cord injury. J Neurotrauma. 2004;21:932–43. doi: 10.1089/neu.2004.21.932. [DOI] [PubMed] [Google Scholar]
- Yuan XB, Jin Y, Haas C, Yao L, Hayakawa K, Wang Y, Wang C, Fischer I. Guiding migration of transplanted glial progenitor cells in the injured spinal cord. Sci Rep. 2016;6:22576. doi: 10.1038/srep22576. [DOI] [PMC free article] [PubMed] [Google Scholar]