Abstract
Problem
SIV model indicates that upon traversing the cervicovaginal mucosa, SIV/SIV-infected cells migrate to regional lymph nodes where active replication occurs prior to systemic virus dissemination. The purpose of the study is to develop a model to study early HIV-1 transmission events that occur after crossing the cervical mucosa into regional lymph nodes.
Methods of study
We developed an organ culture model combining intact cervical tissue explants and tonsil tissue cells as the surrogate draining lymphoid tissue. Viral replication was measured by HIV-1 p24 production, quantification of viral DNA and viral RNA expression in tonsil cells.
Results
In this combined organ culture model transmission of cell-free and cell-associated R5- and X4-tropic HIV-1 through the cervical mucosa to tonsilar cells was observed as determined by HIV-1 p24 in culture supernatant, and the presence of HIV-1 proviral DNA, HIV-1 p24 gag protein in CD4+, CD11c+, CD68+ cells and expression of HIV-1 mRNA expressing CD45RO+ CD4 T cells in tonsil cells. Furthermore, co-receptor usage of HIV-1 in tonsil cells correlated with inoculating virus tropism.
Conclusions
Our combined cervix-tonsil organ culture could serve as an experimental model to study the earliest stages of HIV-1 transmission through cervicovaginal mucosa to its proximal lymph nodes.
Keywords: Organ Culture, Cervix and Tonsil cells, HIV-1, Transmission
Introduction
Heterosexual transmission through vaginal intercourse accounts for the majority of HIV-1 infection in women1. The complex nature of female genital mucosal surfaces presents a major challenge for comprehensive cellular and molecular studies of the early events that occur during HIV-1 transmission through mucosal tissue and the subsequent migration of HIV-1 to regional lymph nodes. Using SIV in the macaque system, Miller et al.2 have shown that penetration of virus through cervicovaginal epithelial cells can occur rapidly within 24 hours of inoculation, after which SIV is amplified in the regional lymph nodes at 3–5 days post-inoculation through continual seeding of virus and virus-infected cells into these draining lymph node sites. Only seven days after inoculation adequate accumulation of virus and HIV-1 infected cells occur in regional lymph nodes to form a critical seed from which SIV is able to establish systemic infection in other distal lymphoid tissue, particularly in gut associated lymphoid tissue (GALT). These results emphasize the migration of infection from the vaginal tract to draining lymph nodes as a critical bottleneck prior to the establishment of systemic HIV-1 infection. Once HIV-1 is disseminated beyond these draining lymph nodes to other tissues, a vaccine or microbicide can do little to curtail the establishment of chronic HIV-1 infection2, 3.
It is difficult to determine precisely whether HIV-1 dissemination from the initial site of infection to the lymph nodes is via cell-free or cell-associated virus or both. However, data from the SIV/macaque model of vaginal transmission of SIV2 indicate that both virus and infected cells are detected within 24 hr at sites distal (presumably lymph node) to the genital tract exposed to live virus. Although transmission of SIV through the vaginal tract has been studied in macaques2, the earliest events of HIV-1 transmission, particularly the spread of HIV-1 to regional lymph nodes after virus inoculation in the cervicovaginal mucosa, remain poorly understood due to the lack of a human experimental model. The palatine tonsil is a secondary lymphoid organ that is routinely used as a surrogate lymph node in tissue studies due to its ready availability and a comparable architecture similar to draining lymph nodes4, 5. The external surface of the tonsil consists of stratified squamous epithelium with invaginations known as tonsillar crypts which are lined by reticulated epithelium populated mainly by epithelial cells and leukocytes. Tonsil tissue-derived organ cultures have been implemented extensively as an in vitro model to study HIV infection in the lymph nodes6–9. As a lymphoid organ, tonsil tissue provides a more natural context than peripheral blood cells for modeling the seeding of HIV-1 infection into a lymph node site9.
In this report we describe the development of a novel combined organ culture model using cervical tissue and palatine tonsil tissue-derived cells to study the earliest events of HIV-1 transmission in a human model, particularly after cervical mucosal transmission and migration of infection into a regional lymph node. In this combined organ culture model we provide evidence for transmission of cell-free and cell-associated R5- and X4-tropic HIV-1 through the cervical mucosa to tonsilar cells with no allogeneic aberration between the two compartments. Our results shed light on the role of co-receptor usage in sexual transmission of HIV-1 and demonstrate the utility of the combined organ culture model toward dissecting the earliest cellular and molecular events preceding establishment of systemic HIV-1 infection in human tissues.
Materials and Methods
Combined organ culture with cervical tissue and tonsil cells
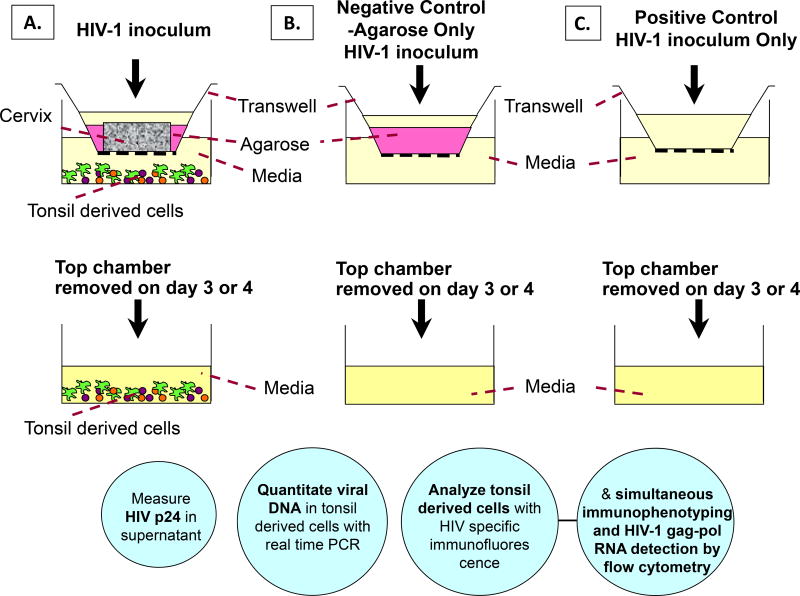
Ectocervical tissues were obtained from HIV-1 negative, premenopausal women aged 50 or below undergoing hysterectomy with no history of sexually transmitted diseases. Tonsil tissues were obtained from routine tonsillectomies from HIV-negative children. Cervical tissues were immersed for 5 minutes in a concentrated antibiotic solution containing 20,000 U/ml Penicillin/Streptomycin, 120 U/ml Nystatin and 250 ug/ml Fungizone in phosphate buffered solution (PBS), then rinsed twice with Dulbecco’s Modified Eagle’s Medium (DMEM). A 6.0 mm-diameter biopsy punch (2–3 mm in thickness) of cervical tissue was placed on the top chamber of a 12-well Transwell with permeable support (3µm membrane pore size) with the epithelial layer facing up (Figure 1). The area surrounding the cervical tissue was sealed with 3% agarose (SeaKem Le Agarose) such that HIV-1 transmission to the bottom transwell chamber proceeds only through the cervical explant. Tonsil cells were isolated by mechanical disaggregation of the tissue using a scalpel, and were washed and pelleted by centrifugation at 500×g for 5 minutes, followed by resuspension in complete IL-2 media. Approximately 4 × 106 tonsil-derived cells in complete IL-2 media (RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 µg/ml Normocin™ (Amaxa) and 500 U of interleukin-2) were placed on the bottom chamber of the transwell. A transwell with agarose only on the top chamber served as a negative control, whereas a Transwell with the membrane-only and media flowing freely between the two chambers served as a positive control (Figure 1).
Figure 1.
In vitro Transwell organ culture model to study HIV transmission from the cervical mucosa to tonsil derived cells. A) Transwell with cervix tissue surrounded with agarose, B) Transwell with agarose only – negative control, C) Transwell only - positive control. Tonsil tissue derived cells were cultured on the bottom well. Cell-free or cell-associated HIV was added to the apical surface of the cervix tissue, on the agarose or onto the Transwell membrane alone and incubated for 3 – 4 days. Tonsil cells were left in culture for an additional 12 days at 37°C. Circles describe methods to be used for HIV detection in culture supernatant or in tonsil cells.
To study virus transmission, 300µl of cell free HIV-1BAL, or HIV-1IIIB (105 to 106 TCID50) or cell-associated HIV-1BAL or HIV-1IIIB (2 × 105 cells, TCID50 of 4×103) was added to the cervix tissue on the top chamber and incubated for 3 days at 37°C. Following incubation, the top chambers were removed and tonsil cells remained in culture for 12 additional days. Culture media was replaced with fresh IL-2 media every three days. To examine the integrity of the cervix organ culture and any leakiness in the system, virus was removed from the top chamber after day 3 and the top chamber was transferred to a new 12-well plate containing 1ml of complete IL-2 media. 300µl of 1% filter-sterilized blue dextran (a 2 × 106-molecular weight polysaccharide), was added to the top chamber containing the cervix and the agarose control and cultured for another 24 h at 37°C. Transmission of blue dextran into the bottom well was then monitored by measuring absorbance at 620nm. The amount of blue dextran transmitted through the cervix was less than 1%, an amount lower than 1% found for the agarose-only control wells.
Immunofluorescence microscopy
For measurement of CD4 and CD11c markers, tonsil cells were fixed for 15 minutes with 2% paraformaldehyde (PFA) and surface stained 1 h with mouse monoclonal antibodies (MAbs) Alexa Fluor 647-labeled anti-CD4 (RPA-T4; BD Pharmingen) (1:20), PE-labeled anti-CD11c (BU15; Caltag™) (1:20). For measurement of CD68, tonsil cells were fixed for 10 minutes with 1% PFA, permeabilized 10 minutes with 0.5% Triton X-100, blocked 1 h with 1% bovine serum albumin (BSA) and stained 1 h with mouse mAb PE-labeled anti-CD68 (Y1/82A; BD Pharmingen) (1:5). Intracellular staining of p24 was performed with mouse mAb FITC anti-p24 (KC-57; Beckman Coulter) (1:100) Samples were visualized with a magnification of 40× and 60× using a Nikon Eclipse E600 microscope and analyzed with the Nuance imaging system (CRI Inc., Woburn, MA).
Simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization
The assay was performed as previously described10–12. Briefly, tonsil cells were washed twice in HBSS, resuspended with 10% DMSO/90% FBS, and stored at −140°C. Cells were thawed, rinsed, pelleted by centrifugation and stained for surface markers with MAbs PC5-labeled anti-CD4, PE-labeled anti-CD45RO, and PE-labeled anti-CD68. For the detection of HIV-1 mRNA, cells were treated with ViroTect VR in-cell HIV-1 detection system (IncellDX, Menlo Park, CA)). Flow cytometry was performed on a FACScalibur (BDIS, San Jose, Calif.).
TaqMan® real-time PCR
DNA was extracted from cell pellets as per manufacture’s protocol (PUREGENE® DNA Purification kit). DNA pellet was resuspended with 20µl of nuclease free water. A TaqMan® PCR reaction (30µl) was performed by using 5µl of DNA and TaqMan® Universal PCR Master Mix (AppliedBiosystems), 333nM each of forward and reverse primer and 250nM FAM/TAMRA labeled probe. Primers used were: BAL-F (5’-TGG GTT ATG AAC TCC ATC CTG AT-3’) and BAL-R (5’-TGT CAT TGA CAG TCC AGC TGT CT-3’). TaqMan probe was BAL-P (5’-FAM-AAT GGA CAG TAC AGC CTA TAG TGC TGC CAG AA-TAMRA-3’). Real-time PCR cycling condition was: 50 °C for 2 min, 95 °C for 10 min, 50 cycles of 95 °C for 15 sec and 60 °C for 1 min using the ABI Prism 7000 Sequence Detection System. Serial diluted pNL4-3 plasmid DNA (1 to 106 copies) was used in each PCR assay to construct the standard curve. To ensure that there was no cross contamination a no template control was included in each assay. Samples were run in triplicate. To analyze PCR data and estimation of HIV-1 copy number, the ABS Prism 7000 SDS Software (Applied Biosystems) was used.
Measurement of Cytokines by Luminex
20 million isolated tonsil cells were infected by 1:5 and 1:20 diluted HIV IIIB (TCID50 of 1×106) respectively, and cultured at 37 degree in RPMI 1640 medium containing 20% FBS, 5% pen-strep and 5% IL-2. PHA and medium only were included as positive and negative control respectively. About half of the culture medium was replaced with fresh medium after 6 days culture. The supernatant was taken at day 0, 6, 9 for testing of p24 and the cytokines production measured by the Luminex test as suggested by the manufacturer (Bio Rad).
Results
Replication of HIV-1 in tonsil cells
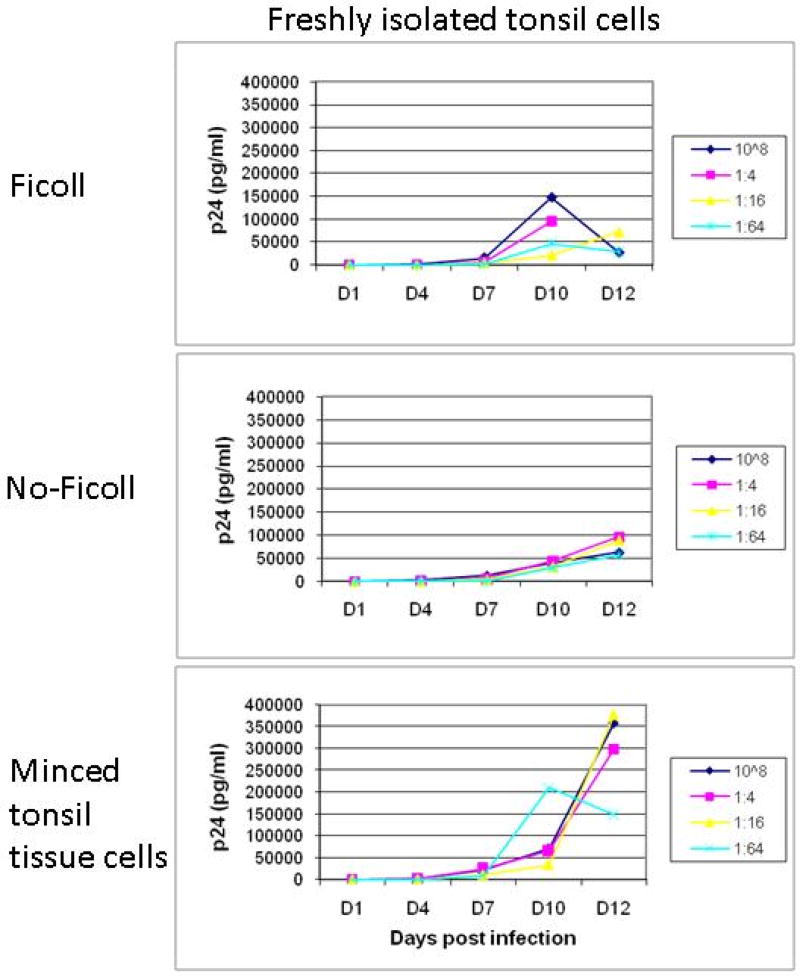
In ex vivo models of oral transmission, HIV-1 transmission into intact human palantine tonsil tissue is known to be highly inefficient using virus inoculums at physiological levels due to the epithelial barrier present in tonsil crypts8. In vitro HIV-1 inoculation of intact cervical tissue typically results in similarly low levels of virus replication and virions release from the basal submucosa13. Thus, an in vitro human organ culture combining cervical explants with intact whole tonsillar tissue is difficult to experimentally model transmission of HIV-1 from cervical mucosa to lymphoid tissue. We therefore investigated whether mechanically disaggregated tonsil tissue could support HIV-1 infections at levels effective for experimental study of vaginal tract to lymphoid tissue transmission in a human model. Tonsil tissue was subjected to mechanical disaggregation and subsequently assayed for HIV-1 replicative capacity. In parallel, we also quantified the HIV-1 replicative capacity of single cell suspensions of tonsil cells prepared by syringe plunging tonsil tissue through a 60 µm mesh sieve, with or without Ficoll-hypaque density gradient purification of the resulting single cell suspensions. While all three tissue manipulations were found to support viral replication, mechanically disaggregated tonsil tissue cells supported the highest level of HIV-1 replication (Figure 2).
Figure 2.
Virus replication from tonsil derived cells. Tonsil cells isolated by 1) pressing the tissue over a sieve and by Ficoll-hypaque density-gradient centrifugation, 2) pressing the tissue over a sieve but without Ficoll-hypaque density-gradient centrifugation, 3) mechanically disaggregated tonsil tissue without any further separation, namely, minced tonsil tissue cells. Two to three million cells were infected with serial dilutions of cell free HIV-1BAL at 1:4, 1:16, 1:64, starting TCID50 of 106.
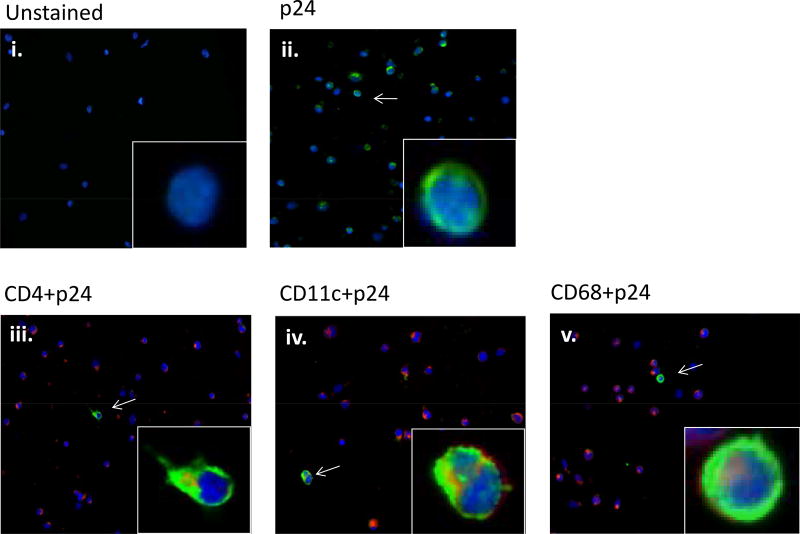
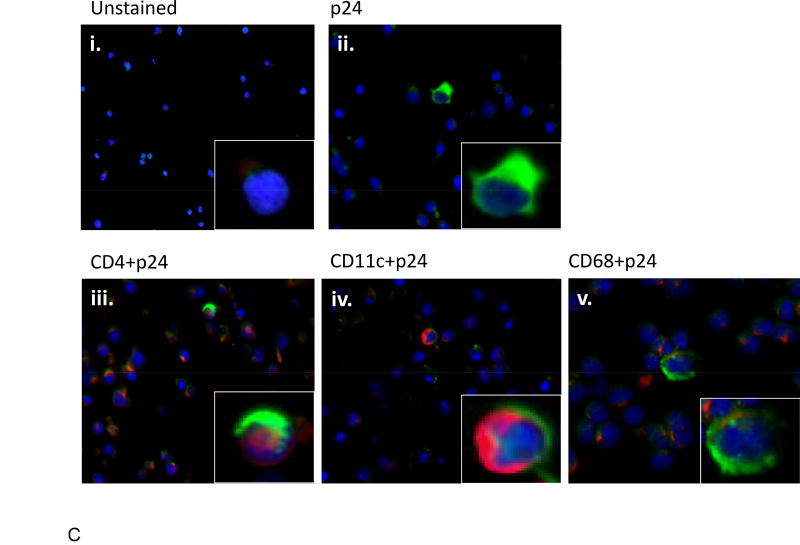
The higher replicative capacity of mechanically disaggregated tonsil tissue cells over tonsil cell suspensions is consistent with the results of Maher et al.8 who demonstrated the role of disrupted epithelial cells in binding and concentrating HIV-1 virions, facilitating in productive infection of underlying lymphoid cells. Mechanically disaggregated tonsil tissue likely retains similar compositional features conducive to productive HIV-1 infection that are presumably lost in single cell suspensions of the tissue. Upon HIV-1 infection, the cell types positive for HIV-1 p24 gag protein were identified to be CD4+, CD11c+, and CD68+ cells as detected by immunofluorescence microscopy (Figure 3), indicating an expected pattern of CD4+ T cell, CD11c+ dendritic cell, and CD68+ monocyte HIV-1 infection2. These results validate the utility of mechanically disaggregated tonsil tissue as a surrogate lymphoid compartment and its application in an in vitro experimental system to study HIV-1 transmission in human tissues.
Figure 3.
Expression of HIV in tonsil cells following cell-free HIV-1BAL infection. HIV-1 p24 antigen (ii, iii, iv, v) (green) and cells positive to p24, CD4+ (iii), CD11c+ (iv), and CD68+ (v) (red), were detected by double-staining of cell markers and intracellular p24 antigens. Insets shows enlarged section of each image
HIV-1 transmission from cell-free and cell-associated virus in combined cervical tissue and tonsil cells
To evaluate HIV-1 dissemination to proximal lymphoid tissue after virus transmission across the cervical mucosa, an organ culture system combining cervix tissue and mechanically disaggregated tonsil tissue was implemented using a 3 µm-pore transwell system with cervical tissue on the top well and tonsil tissue cells in the bottom well (Figure 1A). Cervical explants were oriented in the top chamber with the epithelial layer facing up and underlying stromal tissue in contact with the transwell membrane as described previously14. A physical seal around the cervical explant was made with 3% agarose so that virus transmission only proceeds through the epithelial layer of the cervix into the underlying basal layers of the explant. Separate wells containing agarose-only (Figure 1B) and HIV-1 inoculum-only (Figure 1C) in the top chambers were used as negative and positive controls respectively, to assess specific HIV-1 transmission through the cervical muscosal tissue. Use of a transwell system with a 3µm pore size allows for dissemination of virus from the cervix to the bottom chamber, but not a passage of cells. Cytotoxicity tests using MTT assays revealed tonsillar tissue cells maintained their viability even after 12 days of co-culture (data not shown).
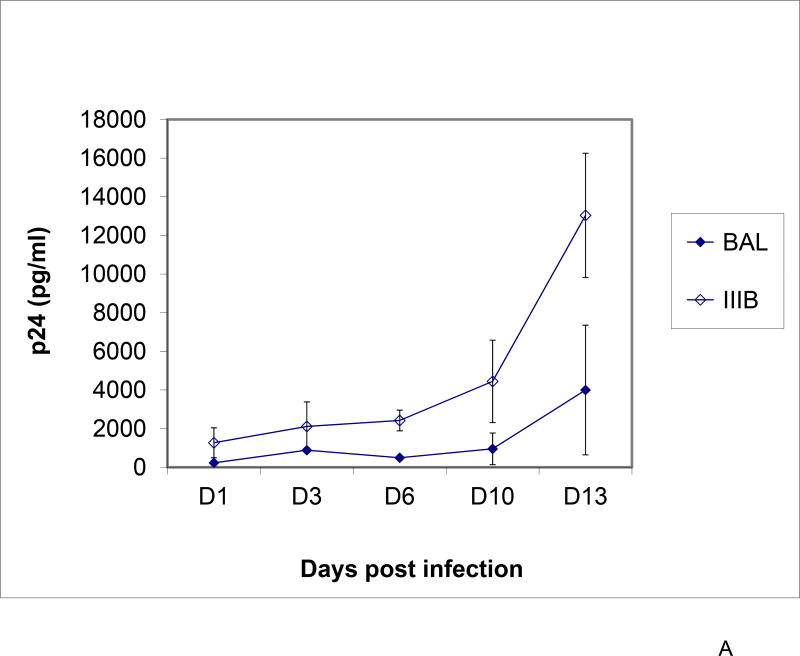
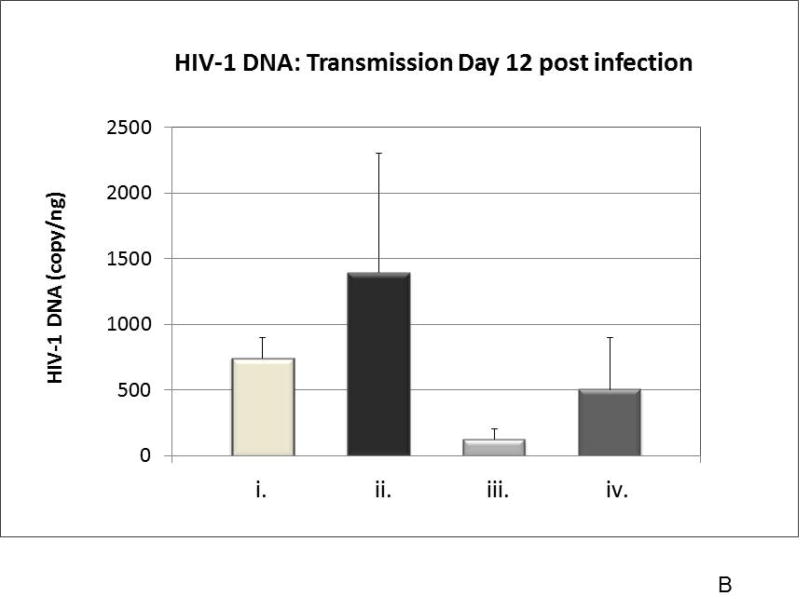
To assess cell-free HIV-1 transmission in the combined cervix tissue/tonsil tissue cell model, virus inoculums of R5-tropic HIV-1BAL or X4-tropic HIV-1IIIB (TCID50 of 105) were topically applied to the cervical tissue in the top well. HIV-1 replication of both HIV-1BAL and HIV-1IIIB in tonsil cells after transmission through the cervical mucosa was demonstrated by detection of increasing HIV-1 p24 production in culture supernatant after removal of the top well containing the cervix explant (Figure 4A). At 12 days post cervical inoculation, higher levels of HIV replication in tonsil cells was observed from HIV-1IIIB compared to HIV-1BAL. Replication of HIV-1 in tonsil cells was further confirmed by quantification of HIV-1 proviral DNA at day 12 by real-time PCR (Figure 4B) and by the presence of HIV-1 p24 gag protein in tonsil cells (Figure 4C). Real-time PCR demonstrated HIV-1 infection of tonsil cells was clearly higher under cervical transmission than when virus was added directly to media containing tonsil tissue cells (Figure 4B), demonstrating the importance of virus amplification in the cervical mucosa for effective transmission downstream to lymphoid tissue. An analysis of tonsil cell types expressing HIV-1 p24 gag antigen demonstrated co-localizations of HIV-1 to CD4+, CD11c+, and CD68+ cells (Figure 4C), a pattern reflected in animal models of transmission2.
Figure 4.
Presence of HIV-1 in tonsil cells. Panel A) Virus replication from culture supernatant of the combined cervix and tonsil cells model. Cell-free HIV-1BAL or HIV-1IIIB (TCID50 of 105) was used as inoculums to the cervix tissue and culture supernatant from the bottom well was tested for HIV-1 p24 production every 2 – 3 days. Data points represent the mean and standard deviation of three replicates at each time, except for IIIB at day 3 and day 6 due to very low p24 values. Panel B) Quantitative analysis of integrated HIV-1 DNA in tonsil cells on day 12 post infection determined by Taq Man® real-time PCR. HIV-1 proviral DNA in tonsil cells from the bottom well from virus crossing the cervical mucosa (cervical tissues infected with cell-free: i) HIV-1BAL and ii) HIV-1IIIB in combined tissue model; iii) and iv) tonsil cells alone (control) infected with cell-free HIV-1BAL and HIV-1IIIB, respectively). Each panel (i–iv) represents mean and standard deviation of three replicates. Panel C) Expression of HIV-1 p24 in tonsil cells after crossing the cervix. Cell phenotypes CD4+ (iii), CD11c+ (iv), and CD68+ (v) (red) expressing HIV-1 p24 (ii, iii, iv, v) (green) detected by immunofluorescence. i) DAPI stained cells served as a control.
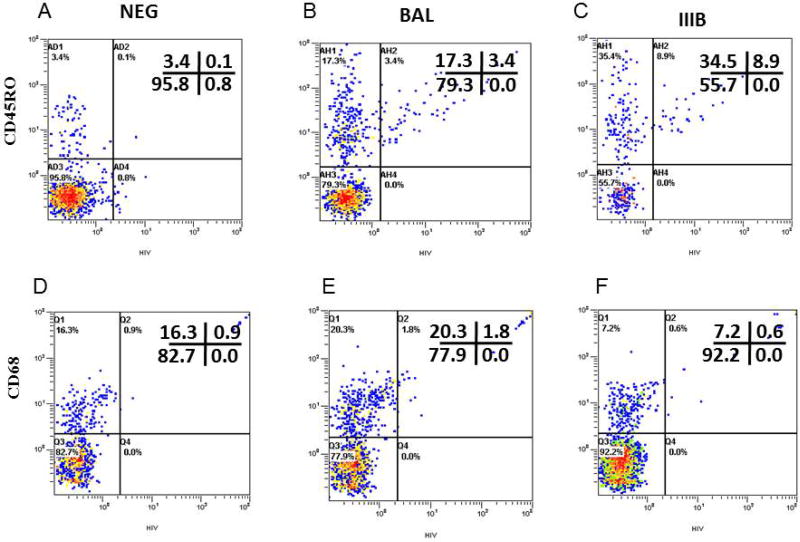
A previous analysis of HIV-1 transmission across cervical mucosa demonstrated CD45RO+ memory CD4 T cells as the earliest detectable HIV-1 infected cells in the cervical mucosa15. Tonsil cells were analyzed by flow cytometry using simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization to detect the extent of productive infection in tonsil-derived memory CD4+ T cells. After 12 days post-cervical inoculation, 16.4% {3.4/(17.3+3.4)} and 20.5% {8.9/8.9+34.5)} of tonsil-derived CD45RO+ CD4 T cells were productively expressing HIV-1 mRNA after cervical inoculation with HIV-1BAL or HIV-1IIIB, respectively (Figure 5 Panel B and C). In the combined cervix-tonsil tissue culture, HIV-1 infection of tonsillar CD4+ T cells was exclusively in CD45RO+ CD4+ T memory cells. Interestingly, an expansion of total memory cells under X4-tropic HIV-1IIIB transmission was observed relative to that of R5-tropic HIV-1BAL transmission. CD45RO+ as a percentage of total cells was 43.4% (34.5%+8.9%) for HIV-1IIIB versus 20.7 % (17.3%+3.4%) for HIV-1BAL. Detection of only 0.03% {0.1/(3.4+0.1)} total CD4+ CD45RO+ T cells in uninfected control tonsil cells (Figure 5A) demonstrated that tonsilar memory CD4+ T cells in Figure 5B and C were specifically infected by HIV-1 transmitting through the cervical mucosa tissue. In contrast, reduced levels of HIV-1 gene expression were detected in tonsilar monocytes where only 8.1% (1.8/(20.3+1.8) and 7.7% (0.6/(7.2+0.6)) of CD68+ cells were infected after cervical inoculations with HIV-1BAL and HIV-1IIIB, respectively (Figure 5E and F). This result was only slightly elevated over background levels of 5.2% {0.9/(16.3+0.9)}CD68+ cells in uninfected control cells (Figure 5D). Together, these results indicate a preferential infection of CD4+ T cells over macrophages that are not explained by lower monocyte levels in this lymphoid tissue compartment.
Figure 5.
Phenotypic characterization of productively infected tonsil cells with cell-free HIV-1 BAL(Panel B and E) or HIV-1 IIIB (Panel C and F) or uninfected negative control (panel A and D) following 12 days in culture from the combined cervical tissue and tonsil cells model by simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization. Cells were stained with anti-CD4 and anti-CD45RO for activated T cells, and anti-CD68 for macrophages. After staining, ultrasensitive fluorescence in situ hybridization was performed and the cells were examined by flow cytometry. Non-specific immunoglobins controls were used to adjust the dot plot quadrants (data not shown). Cells were gated according to the forward and side characteristics of light scatter. CD45RO+ cells were pregated on CD4+ cells.
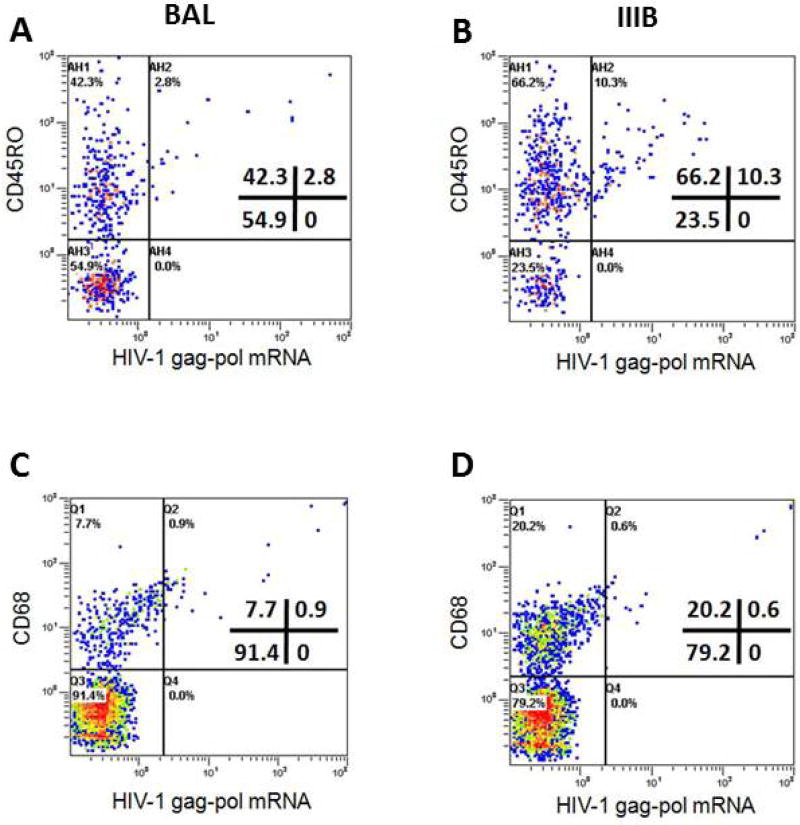
Since in vivo HIV-1 transmission likely involves both cell-free and cell-associated HIV-1 from the semen of seropositive men13, 15–18, we assessed dynamic HIV-1 transmission from cell-associated HIV-1BAL and HIV-1IIIB using the cervical explant/tonsil tissue cell organ culture. CD8-depleted PBMCs infected by either HIV-1BAL or HIV-1IIIB (TCID50 104) were topically added to the epithelial surface of cervical explants in the combined organ culture system. An analysis of cell-associated transmission revealed reduced levels of tonsillar memory CD4+ T cell infection by cell-associated transmission compared to cell-free virus transmission (Figure 6A–D). A CD45RO+ CD4+ T cell infection rate of 6.2% {2.8/(42.3+2.8)} was observed under HIV-1BAL cell-associated transmission compared to 13.5% {10.3/(66.2+10.3)} for HIV-1IIIB. However, expansion of the CD45RO+ memory compartment was also evident under HIV-1IIIB cell-associated transmission compared to HIV-1BAL transmission, where CD45RO+ as a percentage of total cells was 76.5% (66.2%+10.3%) for HIV-1IIIB versus 45.1% (42.3%+2.8%) for HIV-1BAL. HIV-1 infection of tonsilar CD68+ monocytes remained comparatively low under cell-associated transmission with HIV-1BAL transmission showing a 10.4% {0.9/(7.7+0.9)} infection rate over 2.9% {0.6/(20.2+0.6)} for HIV-1IIIB.
Figure 6.
Phenotypic characterization of tonsil cells productively infected with cell-associated HIV-1 (PBMC-HIV-1 BAL; Panel A and C or PBMC-HIV-1 IIIB; Panel B and D) following 12 days in culture in the combined cervical tissue and tonsil cells model by simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization. Cells were stained and analyzed as described in legend to figure 5. Uninfected Negative Control: Same as Figure 5A and D.
Co-receptor usage of replicating HIV-1 in tonsil cells
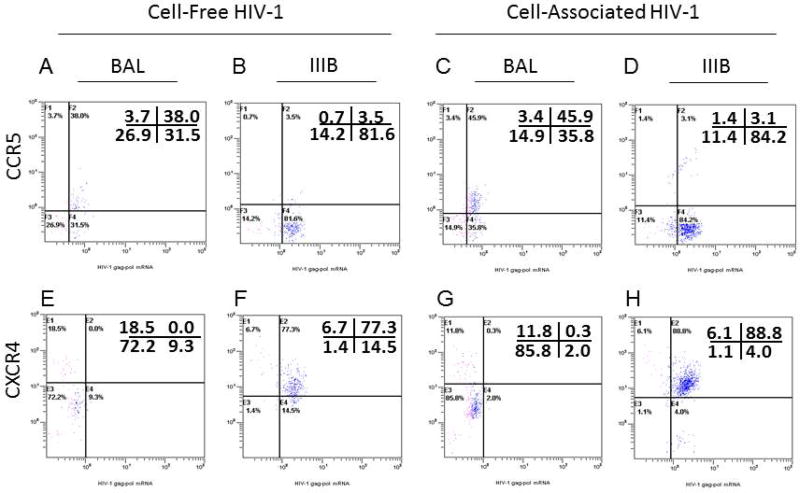
To evaluate the specificity of HIV-1 transmission in the combined organ culture model, we assessed co-receptor usage of HIV-1 in tonsil cells after viral transmission through the cervical mucosa. We found that when cervical tissues were exposed to either cell-free or cell-associated R5-tropic HIV-1BAL, there was preferential infection of CCR5+ tonsil cells (Figure 7A and C) while no CXCR4+ tonsils cell were found to be infected from HIV-1BAL cervical transmission (Figure 7E and G). In contrast, when cervical tissues were exposed to either cell-free or cell-associated X4-tropic HIV-1IIIB, very little infection of CCR5+ tonsil cells was detected (Figure 7B and D), whereas preferential infection of CXCR4+ tonsil cells under HIV-1IIIB cervical transmission was clearly evident (Figure 7F and G). The specificity of inoculating virus tropism with co-receptor usage in tonsil cells indicates HIV-1 specifically crosses the cervical mucosa and seeds itself into the surrogate lymphoid tissue through productive virus replication.
Figure 7.
HIV-1 co-receptor usage in tonsil cells from cell-free and cell-associated HIV-1 IIIB (Panel B, D, F and H) and HIV-1 BAL (Panel A, C, E and G) virus after crossing the cervix mucosa on day 12 was detected by simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization. Cells were stained with antibodies to CCR5 and CXCR4 markers.
Lack of allogeneic immune response between cervix and tonsil
Since the cervix and tonsil tissues are derived from two different donors, we examined whether there is any allogeneic immune response between these tissues that may have impact on HIV transmission. For this purpose a standard combined cervical and tonsil tissue derived organ culture was constructed as described above. As controls, tonsil derived cells and cervical tissues alone were also utilized. Cultures were continued for 12 days and the levels of inflammatory cytokines in the basolateral culture supernatant were measured by a Luminex assay. Results shown in Table 1 indicate that there is no difference on inflammatory cytokines IL1β, IL6, IL8, IFNγ and TNFα response between combined tissue as compared to cervix and tonsil tissues alone. These results indicate there is no significant allogeneic inmmune response between the cervix and tonsil. We also measured induction of inflammatory cytokines IL1β, IL6, IL8, IFNϒ and TNFα in tonsil cells as well as in culture supernatant following HIV infection. We could not find any difference in the level of any of these cytokines between the media control and HIV-1-infected tonsil cells (data not shown).
Table 1.
Cytokine Measurement in Culture Supernatant of Cervix, Tonsil and Combined Cervix/Tonsil Tissues
| Tonsil (ng/ml ± SD) |
Cervix (ng/ml ± SD) |
Cervix/Tonsil (ng/ml ± SD) |
|
|---|---|---|---|
|
|
|||
| Cytokines | |||
| IL-1β | 0.01 ± 0.003 | 0.04 ± 002 | 0.5 ± 0.002 |
| IL-6 | 0.32 ± 0.158 | 1.0 ± 6.0 | 1.1 ± 3.5 |
| IL-8 | 0.98 ± 2.3 | 1.1 ± 0.05 | 1.1 ± 0.08 |
| IFNγ | 0.08 ± 0.04 | 0.002 ± 0.001 | 0.017 ± 0.003 |
| TNFα | 0.01 ± 0.008 | 0.001 ± 0.003 | 0.014 ± 0.007 |
Cytokine measurements from basolateral culture supernatant shown here represent secreted cytokines after 72 hr. in culture. Cytokines measurements at 24 and 48 hr in culture showed similar results as in 72 hr. culture.
Discussion
We have previously described a cervical tissue-derived organ culture model to study HIV-1 transmission across the cervical mucosa isolated from women14. Unlike cell lines, this model provides the natural tissue architecture, including epithelial cells, submucosa and immune cells such as T cells, macrophages (mΦ) and Langerhans cells (LC), and allows for the evaluation of transmission of infectious cell-free and cell-associated HIV-1 across the mucosa. Detailed studies have documented that the HIV-1 transmission observed in our organ culture was not due to leakiness in the system. These data include: i) lack of any discontinuity of the basal layer of the tissue during culture as assayed by transmission electron microscopy, ii) lack of transmission across the mucosa of blue dextran, a polysaccharide of 2×106 Da molecular weight (measured by spectrophotometry) and fluorescent beads of 7.2 micron (slightly bigger than HIV −1 particle) as measured by flow cytometry, and iii) lack of mucosal transmission by UV and heat inactivated HIV-114, 15 and more recently EIAV19, a lentivrus with similar size as HIV. We have also examined the tissue integrity during in vitro culture. Hematoxylin and eosin staining of the paraffin embedded tissue at various times after cultivation indicated that the stratified squamous epithelial layers and especially the basal layer of cells remained largely unchanged after culture14. Measurement of the cellular functions of the tissues during the culture period indicate that they remained unchanged during cultivation in both mock-exposed and HIV-1-exposed tissues14.
The report described here utilizes our previously described cervical tissue based organ culture model and expands to a novel in vitro experimental system to study HIV-1 transmission through cervical mucosa into regional lymph node in a human model. We observed a preferential infection of tonsillar memory CD4+ T cells over monocytes, suggesting the cellular microenvironment of the lymphoid tissue likely plays a role in determining the susceptibility of different cells to HIV-1 infection. Our results place a predominant role of memory CD4+ T cells in mediating HIV-1 transmission. A previous analysis by our group had determined that in human cervical tissue, it is memory CD4+ T cells that are first infected by HIV-115. Clinical and animal studies have shown that during progression of acute infection, memory CD4+ T cells appear as the primary targets of infection, dissemination and subsequently systemic depletion of the CD4+ T cell compartment throughout lymphoid tissue sites, particularly in the GALT2, 3. Thus, our observation of preferential infection of tonsilar memory CD4+ T cells validates the use of tonsil tissue as a surrogate ex vivo lymphoid compartment in analyzing the most formative events of cervicovaginal HIV-1 transmission. Interestingly, both R5 and X4 HIV-1 isolates were efficiently transmitted through cervical tissue at comparable levels in cell-free and cell-associated modes of transmission. This corroborates our previous data demonstrating no selective advantage of R5 over X4 HIV-1 isolates in transmission across the cervical mucosa15. Our current results, therefore, provide further indications that the bottleneck favoring human sexual transmission of R5 HIV-1 isolates20–22 is likely due to predominantly R5 virus present in semen of HIV-1-infected men23–26. Although it will be interesting to use founder virus in addition to the lab strains HIV IIIB and BAL, we have not tested the founder virus yet in this system. Recently we reported the transmission efficiency and viral diversity of the chronic (IIIB, BAL and two clinical isolates) and transmitter/founder virus isolates (CH040 and CH058) using a cervical tissue-derived organ culture19. Our results indicate that transmitter/founder viruses did not have higher transmission efficiency than chronic HIV-1 isolates. Furthermore, no evidence for a difference in diversity was found between the inoculums and transmitted viruses. In addition, the founder/transmitter variants did not have selective advantage of transmission over chronic HIV-1 isolates. Therefore, although we do not anticipate any difference in transmission to tonsil cell between founder virus and HIV IIIB and BAL, further work needs to be done to confirm it.
Our combined in vitro organ culture model represents a novel platform for probing precise time-sequence measurements of cellular and molecular factors important to HIV-1 transmission in vivo. In vitro and animal studies to date reveal HIV-1 transmission to be a highly complex phenomenon involving virion interaction with tight epithelial sheets, numerous infectable cell subsets, cell-free and cell-associated virion transport, as well as a complex array of innate inflammatory responses modulating the actions of immune cells targeted by HIV-1. The precise time-coordinated cellular and molecular elements required for an HIV-1 transmission event to successfully produce systemic infection are far from established. Our previous results and those of others, suggest that events critical to HIV-1 infection occur relatively rapidly on time scales less than 24 hours2, 15. Given the complexity of factors involved, a systems approach will likely be required to unravel the web of events occurring at the portal of HIV-1 sexual transmission. In this regard, animal models may be less feasible than in vitro ones since variability becomes an issue for systems-level experimental approaches involving gene expression, proteomic, and signal cascade profiling. Our organ culture model facilitates multiple experimental replicates and conditions on the same tissue source, mimicking the time course of HIV-1 replication events in the tissue compartments involved in transmission. In addition, molecular dissection of sequential events on the order of minutes to hours is more feasible with an ex vivo tissue system than in live animals. While in vivo non-human primate models have provided much insight on mechanisms underlying lentiviral transmission, HIV-1 likely contains unique molecular properties that are specific for effective transmission via human genital and lymphoid tissues. As such, ex vivo tissue culture models present several experimental advantages not easily controlled for in cell line or animal systems.
While our combined organ culture model provides an experimental platform for dissecting the earliest cellular and molecular events occurring during HIV-1 transmission, it does present certain limitations in its present form. For example, the tonsil is used as a surrogate marker for LN and tonsil cells are used as opposed to tonsil tissue. In addition, tonsils are generally removed because of inflammation. Therefore, it is possible that tonsil cells could be activated and therefore could facilitate HIV-1 infection. Our current system does not attempt to model cell trafficking between the cervical mucosa and regional lymph nodes. Other than directing the trafficking of HIV-1 infected or virion-associated cells to draining lymph node, it is unclear what further role afferent lymphatics play in seeding infection in regional lymph nodes. In our experimental system, we observed that active HIV-1 replication in the cervical mucosa was a sufficient requirement for cell-free dissemination of virons from the cervix to surrogate lymphoid tissue in the transwell system. This emphasizes the potential contribution of cell-free HIV-1 dissemination from an active cervicovaginal replication site in vivo towards the spread of infection into a regional lymph nodes. A corollary to this is that cell migration out of the cervix may not be a necessary requirement for a successful virus transmission event. It is likely that in vivo both cell-free and cell-associated virus transport contribute to the migration of HIV-1 from the cervicovaginal mucosa to proximal lymph nodes preceding more system infection in the GALT8. Implementation of a larger transwell membrane pore size in our combined organ culture can potentially facilitate study of cellular taxis in response to HIV-1 infection of cervicovaginal explants. We are currently attempting technical improvements that will test cell migration in such a system to determine not only the exiting cell types but also their molecular signatures and homing properties.
In summary, we developed an organ culture model combining intact cervical tissue explants and tonsil tissue cells as the surrogate draining lymphoid tissue to study the fate of HIV-1 after crossing cervical mucosa into regional lymph nodes. This combined model supports transmission of cell-free and cell-associated R5- and X4-tropic HIV-1 through the cervical mucosa to tonsilar cells and therefore could be used to study the earliest stages of HIV-1 transmission and its inhibition by microbicides.
Acknowledgments
We thank Drs. Miguel Reyes-Múgica and Ronald Jaffe, and Ms. Lori Schmidt from the Children’s Hospital of the University of Pittsburgh for their generous support for procuring tonsil tissues and Dr. Rajiv Dhir for procuring cervical tissues through Pathology tissue bank of the University of Pittsburgh Medical Center. We also thank Dr. Charles Rinaldo and Ms. Luann Borowski for their help in flow cytometry. This work was supported by a grant R01 HD052436 from the National Institute of Health.
References
- 1.UNAIDS/WHO. Global Summary of AIDS Epidemic 2008. 2008 cited; Available from: www.unaids.gov.
- 2.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. Journal of virology. 2005;79(14):9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reynolds MR, Rakasz E, Skinner PJ, White C, Abel K, Ma ZM, et al. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. Journal of virology. 2005;79(14):9228–35. doi: 10.1128/JVI.79.14.9228-9235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry M, Whyte A. Immunology of the tonsils. Immunol Today. 1998;19(9):414–21. doi: 10.1016/s0167-5699(98)01307-3. [DOI] [PubMed] [Google Scholar]
- 5.Nave H, Gebert A, Pabst R. Morphology and immunology of the human palatine tonsil. Anat Embryol (Berl) 2001;204(5):367–73. doi: 10.1007/s004290100210. [DOI] [PubMed] [Google Scholar]
- 6.Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1(12):1320–2. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 7.Glushakova S, Yi Y, Grivel JC, Singh A, Schols D, De Clercq E, et al. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J Clin Invest. 1999;104(5):R7–R11. doi: 10.1172/JCI7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maher D, Wu X, Schacker T, Larson M, Southern P. A model system of oral HIV exposure, using human palatine tonsil, reveals extensive binding of HIV infectivity, with limited progression to primary infection. J Infect Dis. 2004;190(11):1989–97. doi: 10.1086/425423. [DOI] [PubMed] [Google Scholar]
- 9.Maher DM, Zhang ZQ, Schacker TW, Southern PJ. Ex vivo modeling of oral HIV transmission in human palatine tonsil. J Histochem Cytochem. 2005;53(5):631–42. doi: 10.1369/jhc.4A6534.2005. [DOI] [PubMed] [Google Scholar]
- 10.Patterson BK, Mosiman VL, Cantarero L, Furtado M, Bhattacharya M, Goolsby C. Detection of HIV-RNA-positive monocytes in peripheral blood of HIV-positive patients by simultaneous flow cytometric analysis of intracellular HIV RNA and cellular immunophenotype. Cytometry. 1998;31(4):265–74. doi: 10.1002/(sici)1097-0320(19980401)31:4<265::aid-cyto6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Patterson BK, Behbahani H, Kabat WJ, Sullivan Y, O'Gorman MR, Landay A, et al. Leukemia inhibitory factor inhibits HIV-1 replication and is upregulated in placentae from nontransmitting women. J Clin Invest. 2001;107(3):287–94. doi: 10.1172/JCI11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson BK, McCallister S, Schutz M, Siegel JN, Shults K, Flener Z, et al. Persistence of intracellular HIV-1 mRNA correlates with HIV-1-specific immune responses in infected subjects on stable HAART. AIDS. 2001;15(13):1635–41. doi: 10.1097/00002030-200109070-00005. [DOI] [PubMed] [Google Scholar]
- 13.Coombs RW, Speck CE, Hughes JP, Lee W, Sampoleo R, Ross SO, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177(2):320–30. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 14.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6(4):475–9. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 15.Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. Journal of virology. 2002;76(19):9868–76. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta P, Mellors J, Kingsley L, Riddler S, Singh MK, Schreiber S, et al. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. Journal of virology. 1997;71(8):6271–5. doi: 10.1128/jvi.71.8.6271-6275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Voorhis BJ, Martinez A, Mayer K, Anderson DJ. Detection of human immunodeficiency virus type 1 in semen from seropositive men using culture and polymerase chain reaction deoxyribonucleic acid amplification techniques. Fertil Steril. 1991;55(3):588–94. [PubMed] [Google Scholar]
- 18.Vernazza PL, Eron JJ, Fiscus SA. Sensitive method for the detection of infectious HIV in semen of seropositive individuals. J Virol Methods. 1996;56(1):33–40. doi: 10.1016/0166-0934(95)01899-9. [DOI] [PubMed] [Google Scholar]
- 19.Shen C, Ding M, Ratner D, Montelaro RC, Chen Y, Gupta P. Evaluation of cervical mucosa in transmission bottleneck during acute HIV-1 infection using a cervical tissue-based organ culture. PLoS One. 2012;7(3):e32539. doi: 10.1371/journal.pone.0032539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(21):7552–7. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar-Gonzalez JF, Bailes E, Pham KT, Salazar MG, Guffey MB, Keele BF, et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. Journal of virology. 2008;82(8):3952–70. doi: 10.1128/JVI.02660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS pathogens. 2009;5(1):e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delwart EL, Mullins JI, Gupta P, Learn GH, Jr, Holodniy M, Katzenstein D, et al. Human immunodeficiency virus type 1 populations in blood and semen. Journal of virology. 1998;72(1):617–23. doi: 10.1128/jvi.72.1.617-623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta P, Leroux C, Patterson BK, Kingsley L, Rinaldo C, Ding M, et al. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral Quasi species between blood and semen. J Infect Dis. 2000;182(1):79–87. doi: 10.1086/315644. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Dornadula G, Beumont M, Livornese L, Jr, Van Uitert B, Henning K, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339(25):1803–9. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 26.Pillai SK, Good B, Pond SK, Wong JK, Strain MC, Richman DD, et al. Semen-specific genetic characteristics of human immunodeficiency virus type 1 env. Journal of virology. 2005;79(3):1734–42. doi: 10.1128/JVI.79.3.1734-1742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]