Abstract
To assess factors that influence the choice of induction regimen in contemporary kidney transplantation, we examined center-identified, national transplant registry data for 166,776 US recipients (2005–2014). Bi-level hierarchical models were constructed, wherein use of each regimen was compared pairwise with use of interleukin-2 receptor blocking antibodies (IL2rAb). Overall, 81.8% of patients received induction, including thymoglobulin (TMG, 46.0%), IL2rAb (21.9%), alemtuzumab (ALEM, 12.5%), and other agents (1.3%). However, proportions of patients receiving induction varied widely across centers (0%–100%). Recipients of living donor transplants and self-pay patients were less likely to receive induction treatment. Clinical factors associated with use of TMG or ALEM (vs. IL2rAb) included age, black race, sensitization, retransplant status, non-standard deceased donor, and delayed graft function. However, these characteristics explained only 10%–33% of observed variation. Based on intraclass correlation analysis, “center effect” explained most of the variation in TMG (58%), ALEM (66%), other (51%), and no induction (58%) use. Median odds ratios generated from case-factor adjusted models (7.66–11.19) also supported large differences in the likelihood of induction choices between centers. The wide variation in induction therapy choice across US transplant centers is not explained by differences in patient or donor characteristics; rather, it reflects center choice and practice.
Keywords: Induction, Immunosuppression, Kidney transplantation, Practice patterns
INTRODUCTION
Induction therapy in kidney transplantation is a therapeutic strategy to induce a rapid and profound reduction in immune responses against an allograft to mitigate the higher risk of acute rejection in the early posttransplant period. This reduction in immune response is achieved by eliminating T and B lymphocytes that initiate and maintain the immune response, by cell depleting agents, or by blocking interleukin-2 activity critical to activation and sustenance of immunologic injury (interleukin-2 receptor blocking antibodies; IL2rAb) (1). While induction therapy has been demonstrated to reduce the risk of acute rejection and improve long-term allograft survival, it can increase the risk of immunosuppression-related complications such as infections or malignancies (2–6). Commonly used agents have varying risk profiles, and choosing among the available agents requires clinicians to balance the patient’s risk of rejection with his or her expected rate of complications given clinical and donor organ characteristics (e.g., deceased vs. living donor).
In 2009, the Kidney Disease Improving Global Outcomes guideline for “Care of Kidney Transplant Recipients” recommended induction therapy in all kidney transplant recipients (1A) (7). This guideline also recommended IL2rAb for first line induction therapy (1B), while offering a class 2B recommendation for use of cell depleting agents in patients considered “high risk” for acute rejection. Increased immunological risk has been associated with black race, allosensitization, retransplantation, and younger age (7–10). Additionally, recipients of organs believed to be at greater risk of delayed graft function or rejection, such as more donor-recipient human leukocyte antigen (HLA) mismatches, longer cold ischemic time, and higher kidney donor profile index, may warrant stronger induction therapy (8). In addition to considering immunological risks, clinicians modify their choice of induction agent to facilitate steroid-free or belatacept-based maintenance therapy, to reduce the risk of steroid- or calcineurin-inhibitor-related complications, or to mitigate concerns about patient compliance.
Until recently, IL2rAb were the only induction agents approved by the US Food and Drug Administration (FDA) for induction immunosuppression in kidney transplantation. Cell depleting agents including thymoglobulin (TMG) and alemtuzumab (ALEM) have been used off label for this indication (11), although TMG received an induction indication in April 2017 (12). In 2014, 90% of kidney transplant recipients received induction therapy. Despite the FDA approval status, use of IL2rAb fell from 35% in 2004 to 20% in 2014, while use of T-cell depleting agents (including TMG, ALEM) continued to increase, from 39 % in 2004 to 62 % in 2015 (13).
Current national data suggest greater use of cell depleting agents than would be expected based on international guidelines. To better understand factors that contribute to the choice of induction therapy, we examine center-level variation after adjusting for differences in donor and recipient characteristics, using data from the Scientific Registry of Transplant Recipients (SRTR). This study extends work by our group quantifying variation in maintenance immunosuppression in US practice using a similar analytic framework (14).
METHODS
Data Source
The SRTR includes data on all transplant candidates, recipients, and donors in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). Additional data are drawn from the Centers for Medicare & Medicaid Services and the Social Security Death Master File. The Health Resources and Services Administration, US Department of Health and Human Services, provides oversight of the activities of the OPTN and SRTR contractors. We included patients who underwent kidney transplant in the United States from 2005 to 2014.
Sample and Induction Regimens
Induction immunosuppression was defined by center reporting to the registry, and categorized as IL2rAb, TMG, ALEM, other induction (ATGAM, OKT3, rituximab), or no induction. Induction use in the registry is recorded as a binary indication (given or not), including the indication (discriminating use for induction versus treatment of acute rejection) but information on dose and days of treatment is not available. If use of two induction agents were reported, precedence was given to depleting agents per our prior methodology (15). IL2rAb was chosen as the reference given its FDA approval during the study period.
Case Factors
Donor (age, donor type, cold ischemic time), recipient (age, sex, race, body mass index, cause of end-stage renal disease, time on dialysis, panel reactive antibody level [PRA]), and transplant (HLA mismatch, previous transplant, year of transplant, primary payer) characteristics were extracted from the SRTR data for incorporation into multivariate models. We categorized maintenance immunosuppression based on data at discharge using the following taxonomy: Triple therapy – tacrolimus (Tac), mycophenolic acid (mycophenolate mofetil, mycophenolate sodium), or azathioprine (MPA/AZA), and prednisone (Pred); steroid-sparing – Tac+MPA/AZA; MPA/AZA-sparing – Tac, Tac+Pred; mammalian target of rapamycin inhibitor (mTORi)-based – sirolimus (SRL) or everolimus (ERL), with or without Tac or cyclosporine (CsA); CsA-based: CsA without SRL or ERL; other maintenance regimens (Table 1).
Table 1.
Recipient, donor and transplant characteristics according to kidney transplant induction immunosuppression regimen.
| IL2rAb (N = 36,600) |
Thymoglobulin (N = 76,726) |
Alemtuzumab (N = 20,874) |
Other Induction (N = 2,094) |
No Induction (N = 30,422) |
|
|---|---|---|---|---|---|
| Baseline Recipient Factors | % | % | % | % | % |
| Maintenance regimen | ‡ | ‡ | ‡ | ‡ | |
| Tac+MPA + Pred | 68.8 | 59.0 | 24.3 | 59.8 | 45.5 |
| Tac+MPA | 11.8 | 27.9 | 62.3 | 30.0 | 14.1 |
| Tac alone or Tac + Pred | 1.5 | 1.4 | 7.4 | 1.9 | 2.8 |
| mTORi-based | 4.6 | 4.9 | 1.0 | 2.2 | 5.3 |
| CsA-based | 9.7 | 3.6 | 1.8 | 3.2 | 3.0 |
| Other maintenance | 3.6 | 3.3 | 3.3 | 2.9 | 29.4 |
| Female | 35.3 | 41.1‡ | 40.1‡ | 42.6‡ | 38.0‡ |
| Age, yrs | ‡ | ‡ | * | ‡ | |
| <18 | 8.3 | 4.3 | 3.2 | 4.3 | 6.9 |
| 18–30 | 8.4 | 8.3 | 8.6 | 9.5 | 9.0 |
| 31–45 | 17.9 | 21.3 | 21.9 | 22.7 | 20.8 |
| 46–60 | 33.7 | 37.9 | 38.9 | 38.1 | 35.7 |
| >60 | 31.7 | 28.2 | 27.4 | 25.3 | 27.6 |
| Race | ‡ | ‡ | ‡ | ‡ | |
| White | 56.2 | 51.0 | 54.3 | 52.0 | 52.5 |
| Black | 18.0 | 27.4 | 26.1 | 18.5 | 26.3 |
| Hispanic | 17.1 | 14.5 | 14.7 | 14.9 | 14.7 |
| Other | 8.7 | 7.0 | 4.8 | 14.6 | 6.5 |
| Body mass index, kg/m2 | ‡ | ‡ | ‡ | ||
| <18.5 | 5.8 | 3.8 | 3.3 | 4.4 | 4.5 |
| 18.5 to <25 | 31.4 | 29.8 | 28.0 | 33.6 | 26.4 |
| 25 to <30 | 31.7 | 31.2 | 31.3 | 30.4 | 26.0 |
| ≥30 | 28.2 | 31.1 | 35.2 | 28.4 | 24.6 |
| Unknown | 2.8 | 4.0 | 2.3 | 3.3 | 18.6 |
| Cause of ESRD | ‡ | ‡ | ‡ | ‡ | |
| Diabetes | 23.5 | 22.7 | 23.8 | 23.8 | 20.9 |
| Glomerulonephritis | 23.7 | 23.9 | 23.6 | 27.3 | 21.9 |
| Hypertension | 21.4 | 26.2 | 25.0 | 17.1 | 27.5 |
| Polycystic kidney disease | 9.9 | 9.6 | 10.6 | 8.8 | 9.8 |
| Other cause | 21.5 | 17.7 | 17.1 | 23.1 | 19.9 |
| Hypertension | 76.9 | 81.6‡ | 80.6‡ | 83.0‡ | 78.1‡ |
| Dialysis duration, mos. | ‡ | ‡ | ‡ | ‡ | |
| None | 19.8 | 15.6 | 19.3 | 16.3 | 18.0 |
| 0–24 | 34.5 | 29.1 | 31.5 | 29.2 | 30.7 |
| 25–60 | 28.3 | 32.0 | 29.1 | 31.4 | 27.3 |
| >60 | 16.4 | 22.3 | 19.4 | 22.0 | 19.0 |
| Missing | 1.1 | 0.9 | 0.8 | 1.1 | 5.1 |
| Most recent PRA | ‡ | † | ‡ | ||
| 0 to 9 | 71.8 | 56.8 | 60.8 | 52.9 | 61.7 |
| 10 to 79 | 19.3 | 25.9 | 25.6 | 29.3 | 23.3 |
| ≥80 | 4.2 | 13.5 | 11.1 | 15.1 | 9.5 |
| Missing | 4.7 | 3.8 | 2.5 | 2.7 | 5.5 |
| Previous transplant | 8.9 | 16.2‡ | 12.5‡ | 16.3‡ | 13.1‡ |
| Primary payer | ‡ | ‡ | ‡ | ||
| Private | 40.6 | 36.1 | 40.6 | 36.3 | 36.9 |
| Public | 59.1 | 63.5 | 59.1 | 63.4 | 53.7 |
| Self/Other | 0.4 | 0.4 | 0.4 | 0.3 | 9.3 |
| Donor & transplant factors | |||||
| HLA mismatch | ‡ | ‡ | ‡ | ‡ | |
| Zero mismatch | 10.2 | 8.0 | 7.7 | 10.3 | 11.0 |
| 1 to 3 mismatches | 31.4 | 27.0 | 31.5 | 25.3 | 29.9 |
| 4 to 5 mismatches | 45.1 | 50.9 | 47.5 | 49.8 | 44.9 |
| 6 mismatches | 12.9 | 13.8 | 13.0 | 13.6 | 12.8 |
| Unknown | 0.5 | 0.3 | 0.3 | 1.1 | 1.3 |
| Donor type | ‡ | ‡ | ‡ | ‡ | |
| SCD | 40.8 | 47.6 | 39.6 | 51.4 | 40.0 |
| ECD | 8.5 | 10.1 | 9.7 | 6.8 | 9.9 |
| DCD | 6.1 | 10.0 | 9.2 | 10.8 | 7.7 |
| Living related | 26.9 | 16.5 | 21.9 | 17.4 | 24.9 |
| Living unrelated | 17.6 | 15.8 | 19.6 | 13.6 | 17.5 |
| Donor age, yrs | ‡ | † | ‡ | † | |
| <18 | 7.9 | 9.1 | 6.9 | 10.5 | 7.8 |
| 18–30 | 21.8 | 21.8 | 21.5 | 24.3 | 21.3 |
| 31–45 | 29.5 | 28.2 | 29.7 | 28.4 | 29.1 |
| 46–60 | 33.1 | 33.4 | 34.0 | 31.1 | 33.2 |
| >60 | 7.7 | 7.5 | 7.9 | 5.7 | 8.7 |
| Cold ischemia time, hrs | ‡ | ‡ | ‡ | ‡ | |
| Live donor | 9.2 | 6.2 | 9.6 | 4.6 | 9.7 |
| 0 to 12 | 52.4 | 44.6 | 45.6 | 48.1 | 46.3 |
| 13 to 24 | 28.0 | 33.2 | 30.6 | 35.6 | 22.9 |
| ≥24 | 9.0 | 13.6 | 12.7 | 10.4 | 12.6 |
| Missing | 1.3 | 2.5 | 1.5 | 1.3 | 8.4 |
| Delayed graft function | 13.1 | 18.9‡ | 15.5‡ | 21.0‡ | 15.4‡ |
| Transplant year | ‡ | ‡ | ‡ | ||
| 2005 to 2008 | 43.3 | 35.7 | 30.5 | 37.5 | 47.3 |
| 2008 to 2011 | 29.4 | 30.7 | 31.7 | 36.1 | 21.7 |
| 2012 to 2014 | 27.3 | 33.6 | 37.8 | 26.5 | 31.0 |
P-values:
0.02 ≤ p < 0.05;
0.0001 ≤ p < 0.01;
p < 0.0001
CsA, cyclosporine; DCD, donation after circulatory death; ECD, expanded criteria donor; ESRD, end-stage renal disease; IL2rAb, interleukin-2 receptor blocking antibodies; MPA, mycophenolate acid; mTORi, mammalian target of rapamycin inhibitor; PRA, panel-reactive antibody; Pred, prednisone; SCD, standard-criteria donor; Tac, tacrolimus.
Analyses
Observed Variation in Regimen Use across Centers
To visually assess unadjusted variation in induction use at the center level across the US, the observed proportion of patients receiving each induction regimen was determined and displayed as stacked bar plots. Plots were also stratified by immunological risk, wherein high risk was defined as black race, PRA >20, or retransplantation.
Combined Center and Case-Level modeling
Bi-level hierarchical models were constructed to adjust for clustering effects, similar to previous methods (14, 16–18). Level 1 comprised patient/donor and transplant (case) factors and level 2 represented the center, wherein use of each alternative regimen was compared individually to the reference regimen (pairwise). Empirical Bayes estimates (EBEs) provided the adjusted proportion (with 95% confidence intervals, CIs) of use of a regimen of interest compared with the reference regimen, incorporating case-mix adjustment from the hierarchical model. If the 95% CI for a center’s EBE of use of a regimen of interest did not include the median national rate of use, this indicated a prescribing pattern statistically significantly different from the expected rate of use for that regimen.
Heterogeneity in induction immunosuppression prescribing across centers was quantified using intraclass correlation (ICC) and median odds ratios (MOR). ICC is defined as the ratio of cluster variance (center impact) to the total observed variance in induction use, with contributions in our study framework defined as center-related, case-related, and other unmeasured effects. In this context, the ICC quantifies the proportion of total variance in induction use that is accounted for by center. The MOR provides the median of the odds that patients with identical characteristics will receive the induction regimen of interest when two centers are drawn at random (performed for all possible pairs of centers). For example, a MOR of 2.0 means that if centers are selected at random across all centers, a patient with a given set of characteristics has, on an average, twice the odds of receiving the induction regimen of interest at one of the randomly selected centers than at the other (19). The adjusted odds ratios of receiving an induction regimen other than the IL2rAb reference was determined for patient and donor factors, after accounting for the center effect using the hierarchical model. Data were analyzed using Stata 14, College Station, TX.
Contributions of Case-Level Factors to Variation in Induction Use
To quantify the degree to which variance in induction regimen use was explained by recipient and donor characteristics, we performed multivariate logistic regression modeling with induction regimen as the dependent variable and case factors as the predictors. Pairwise models were constructed to assess the relative likelihood of using each specific regimen (as outlined above) compared with IL2rAb induction.
RESULTS
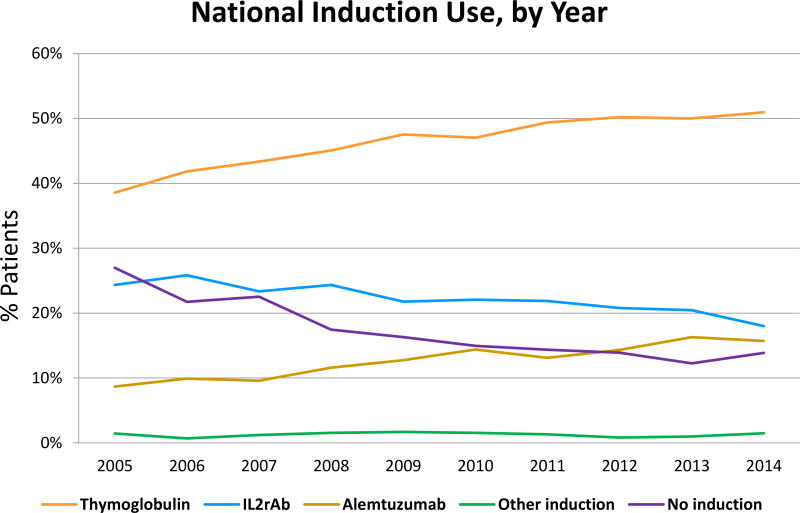
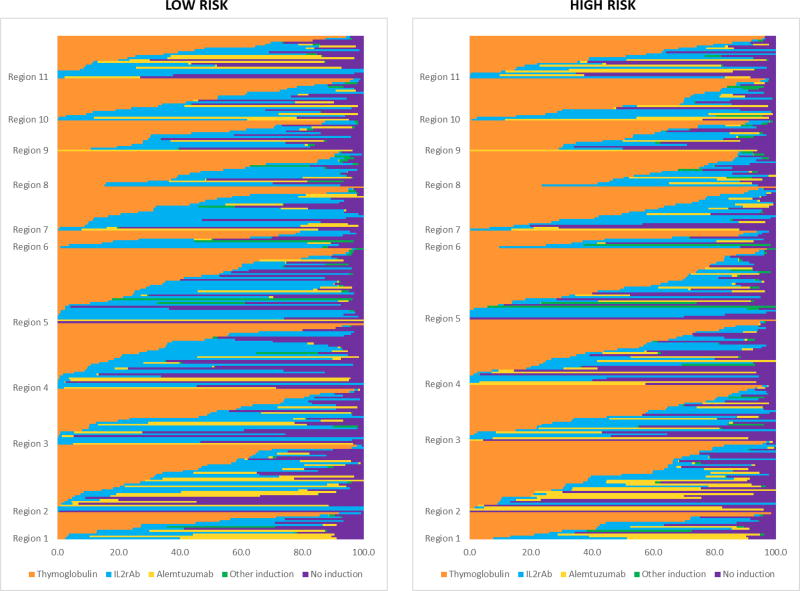
Among 166,776 kidney transplants performed from 2005 through 2014 at 266 US centers, 81.8% of recipients were treated with induction. TMG was the most commonly used induction agent (46.0%), followed by IL2R-Ab (21.9%), ALEM (12.5%), and other (1.3%) (Table 1). Other induction comprised ATGAM (84.3%), OKT3 (12.6%) and rituximab (4.6%). Nationally, ALEM use increased in recent years, while use of IL2rAb, other, and no induction decreased (Figure 1). More common use of TMG was apparent when center-level use was stratified by recipient immunologic risk (Appendix 1).
Figure 1.
National trends in kidney transplant induction over time. IL2rAb, interleukin-2 receptor blocking antibodies.
Patient and donor correlates with choice of regimen
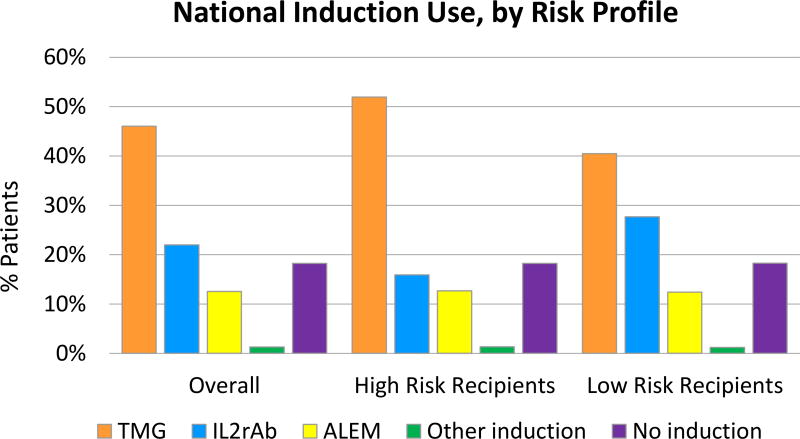
Choice of induction therapy was associated with certain patient and donor characteristics. Overall, TMG use was more common in higher- than in lower-risk recipients (52.0%% vs. 40.5%), while IL2rAb use was less common in higher- than in lower-risk recipients (15.9% vs. 27.7%) (Figure 2). In multi-level modeling considering center and case factors, IL2rAb use was more common in recipients who were children, white, and preemptively transplanted (Table 2). Conversely, recipients who were black, or highly sensitized, recipients, or recipients who experienced delayed graft function, or had longer pretransplant dialysis duration, were more likely to be treated with cell depleting agents (ALEM, TMG). Choice of induction regimen was also strongly correlated with posttransplant maintenance regimen. Patients discharged on triple therapy (Tac+MMF/MPA, Pred) were more likely to receive IL2rAb, compared with all other induction agents. ALEM (62.3%) administration was more common in steroid-free maintenance regimens (Tac+MMF/AZA).
Figure 2.
National trends in kidney transplant induction by recipient immunologic risk profile. ALEM, alemtuzumab; IL2rAb, interleukin-2 receptor blocking antibodies; TMG, thymoglobulin. High risk was defined as black race, PRA >20, or retransplantation.
Table 2.
Associations of recipient, donor and transplant case characteristics with induction regimen use compared to IL2rAb (reference regimen).
| Thymoglobulin | Alemtuzumab | Other Induction | No Induction | |
|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI) | ||||
| Maintenance regimen | ||||
| Tac+MPA + Pred | Reference | Reference | Reference | Reference |
| Tac+MPA | 2.03 (1.90,2.18) ‡ | 14.08 (12.83,15.44) ‡ | 2.98 (2.46,3.61) ‡ | 1.60 (1.47,1.74) ‡ |
| Tac alone or Tac + Pred | 0.80 (0.67,0.95) * | 14.82 (12.11,18.15) ‡ | 0.94 (0.59,1.49) | 2.06 (1.77,2.40) ‡ |
| mTORi-based | 1.05 (0.92,1.20) | 2.04 (1.62,2.56) ‡ | 0.69 (0.43,1.11) | 1.12 (0.99,1.27) |
| CsA-based | 0.54 (0.49,0.60) ‡ | 1.01 (0.83,1.22) | 0.82 (0.58,1.15) | 0.83 (0.75,0.92) ‡ |
| Other or unknown | 0.78 (0.70,0.87) ‡ | 3.48 (2.97,4.09) ‡ | 0.94 (0.65,1.35) | 5.05 (4.61,5.54) ‡ |
| Recipient age, yrs | ||||
| <18 | 0.62 (0.55,0.71) ‡ | 0.31 (0.25,0.39) ‡ | 0.67 (0.43,1.04) | 0.68 (0.59,0.77) ‡ |
| 18–30 | 1.02 (0.93,1.11) | 0.91 (0.79,1.04) | 1.12 (0.85,1.47) | 1.00 (0.92,1.10) |
| 31–45 | Reference | Reference | Reference | Reference |
| 46–60 | 0.92 (0.87,0.98) * | 0.79 (0.72,0.87) ‡ | 0.90 (0.74,1.08) | 0.90 (0.85,0.97) * |
| >60 | 0.63 (0.59,0.67) ‡ | 0.44 (0.40,0.49) ‡ | 0.44 (0.36,0.55) ‡ | 0.76 (0.71,0.82) ‡ |
| Female | 1.27 (1.21,1.32) ‡ | 1.22 (1.14,1.31) ‡ | 1.26 (1.09,1.45) † | 1.08 (1.03,1.13) † |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| Black | 1.51 (1.42,1.61) ‡ | 1.25 (1.14,1.38) ‡ | 1.63 (1.33,2.00) ‡ | 1.07 (1.00,1.14) * |
| Hispanic | 1.06 (0.99,1.13) | 1.11 (0.99,1.24) | 1.24 (1.00,1.54) | 1.01 (0.94,1.09) |
| Other | 1.07 (0.98,1.16) | 1.15 (0.99,1.33) | 1.10 (0.87,1.40) | 1.04 (0.94,1.14) |
| Body mass index, kg/m2 | ||||
| <18.5 | 0.83 (0.74,0.93) † | 0.78 (0.65,0.94) * | 1.01 (0.73,1.41) | 0.96 (0.86,1.09) |
| 18.5–24.9 | Reference | Reference | Reference | Reference |
| 25–29.9 | 1.04 (0.99,1.09) | 1.08 (0.99,1.17) | 0.95 (0.80,1.13) | 0.99 (0.94,1.05) |
| ≥30 | 1.14 (1.08,1.21) ‡ | 1.24 (1.13,1.35) ‡ | 1.06 (0.88,1.27) | 1.02 (0.96,1.08) |
| Unknown | 0.77 (0.67,0.89) ‡ | 0.74 (0.59,0.94) * | 2.01 (1.38,2.93) ‡ | 2.56 (2.28,2.87) ‡ |
| Cause of ESRD | ||||
| Diabetes | Reference | Reference | Reference | Reference |
| Glomerulonephritis | 1.07 (1.00,1.14) * | 1.03 (0.93,1.14) | 0.98 (0.80,1.21) | 0.95 (0.89,1.02) |
| Hypertension | 1.06 (0.99,1.12) | 1.03 (0.92,1.14) | 1.03 (0.83,1.28) | 1.04 (0.97,1.11) |
| Polycystic kidney disease | 1.05 (0.97,1.14) | 1.17 (1.03,1.33) * | 0.80 (0.61,1.05) | 0.98 (0.89,1.07) |
| Other or unknown | 0.92 (0.86,0.98) * | 0.93 (0.83,1.04) | 0.86 (0.69,1.07) | 0.93 (0.86,1.01) |
| Hypertension | 0.97 (0.92,1.03) | 1.23 (1.12,1.34) ‡ | 0.88 (0.73,1.07) | 0.80 (0.76,0.86)‡ |
| Previous transplant | 1.92 (1.79,2.06) ‡ | 1.10 (0.99,1.23) | 1.22 (0.99,1.51) | 1.09 (1.00,1.18)* |
| Dialysis duration, mos | ||||
| Preemptive | 0.94 (0.89,1.00) | 1.03 (0.94,1.13) | 1.05 (0.86,1.29) | 1.03 (0.96,1.10) |
| 0–24 | Reference | Reference | Reference | Reference |
| 25–60 | 1.10 (1.03,1.16) † | 1.08 (0.98,1.18) | 0.79 (0.66,0.95) * | 1.07 (1.00,1.14) * |
| >60 | 1.25 (1.16,1.34) ‡ | 1.19 (1.05,1.33) * | 0.92 (0.73,1.15) | 1.07 (0.99,1.16) |
| Missing | 0.80 (0.65,0.99) * | 1.08 (0.78,1.50) | 0.88 (0.45,1.75) | 2.14 (1.80,2.55) ‡ |
| Peak PRA level | ||||
| 0 to 9 | Reference | Reference | Reference | Reference |
| 10 to 79 | 1.90 (1.81,2.01) ‡ | 1.56 (1.43,1.70) ‡ | 1.93 (1.63,2.28) ‡ | 1.27 (1.20,1.35) ‡ |
| ≥80 | 4.82 (4.40,5.28) ‡ | 4.30 (3.74,4.96) ‡ | 6.83 (5.21,8.93) ‡ | 1.94 (1.75,2.16) ‡ |
| Missing | 0.95 (0.86,1.05) | 0.71 (0.58,0.87) † | 1.58 (1.07,2.35) * | 1.30 (1.15,1.47) ‡ |
| Primary payer | ||||
| Private | 1.09 (1.04,1.15) ‡ | 1.16 (1.07,1.26) ‡ | 1.14 (0.97,1.34) | 1.01 (0.96,1.07) |
| Public | Reference | Reference | Reference | Reference |
| Self/Other | 1.00 (0.70,1.42) | 2.05 (1.23,3.42) * | 0.99 (0.34,2.89) | 6.64 (5.18,8.51) ‡ |
| HLA mismatches | ||||
| Zero mismatch | 0.56 (0.52,0.61) ‡ | 0.38 (0.33,0.43) ‡ | 0.60 (0.46,0.77) ‡ | 1.10 (1.01,1.19) * |
| 1 to 3 mismatches | Reference | Reference | Reference | Reference |
| 4 to 5 mismatches | 0.99 (0.93,1.04) | 0.94 (0.86,1.02) | 0.99 (0.82,1.19) | 1.00 (0.94,1.06) |
| 6 mismatches | 1.00 (0.93,1.07) | 0.94 (0.83,1.06) | 1.06 (0.83,1.35) | 0.95 (0.88,1.04) |
| Unknown | 1.05 (0.71,1.55) | 0.95 (0.56,1.61) | 0.80 (0.34,1.87) | 0.97 (0.72,1.32) |
| Donor type | ||||
| SCD | Reference | Reference | Reference | Reference |
| ECD | 1.27 (1.16,1.39) ‡ | 1.20 (1.03,1.39) * | 0.95 (0.69,1.31) | 1.05 (0.95,1.17) |
| DCD | 1.49 (1.37,1.62) ‡ | 1.38 (1.21,1.57) ‡ | 1.34 (1.03,1.74) * | 1.21 (1.10,1.34) |
| Living related | 0.62 (0.57,0.68) ‡ | 0.75 (0.66,0.86) ‡ | 0.54 (0.42,0.71) ‡ | 0.96 (0.88,1.05) |
| Living unrelated | 0.80 (0.73,0.86) ‡ | 0.94 (0.83,1.08) | 0.64 (0.49,0.83) † | 0.86 (0.79,0.94) † |
| Donor age, yrs | ||||
| <18 | 1.01 (0.92,1.10) | 0.99 (0.86,1.15) | 1.12 (0.86,1.46) | 1.09 (0.99,1.20) |
| 18–30 | 0.91 (0.86,0.97) * | 1.01 (0.91,1.11) | 0.96 (0.79,1.16) | 0.98 (0.92,1.04) |
| 31–45 | Reference | Reference | Reference | Reference |
| 46–60 | 0.93 (0.88,0.98) * | 0.97 (0.89,1.06) | 0.95 (0.80,1.14) | 0.97 (0.91,1.03) * |
| >60 | 0.88 (0.80,0.98) * | 0.89 (0.76,1.04) | 0.74 (0.52,1.04) | 1.04 (0.93,1.16) |
| Cold ischemic time, hrs | ||||
| Live donor | 1.06 (0.97,1.16) | 1.17 (1.02,1.34) * | 1.48 (1.07,2.04) * | 1.40 (1.27,1.54) ‡ |
| 0 to 12 | Reference | Reference | Reference | Reference |
| 13 to 24 | 1.24 (1.16,1.32) ‡ | 1.16 (1.04,1.29) * | 1.16 (0.96,1.40) | 0.94 (0.87,1.01) |
| ≥24 | 1.38 (1.27,1.50) ‡ | 1.20 (1.05,1.38) * | 1.13 (0.87,1.47) | 0.93 (0.85,1.02) |
| Unknown | 1.59 (1.32,1.90) ‡ | 1.07 (0.80,1.44) | 1.23 (0.61,2.49) | 2.76 (2.36,3.23) ‡ |
| Delayed graft function | 1.79 (1.68,1.90) ‡ | 1.13 (1.02,1.25) * | 1.48 (1.22,1.80) ‡ | 1.07 (1.00,1.15) |
| Transplant year | ||||
| 2005 to 2008 | Reference | Reference | Reference | Reference |
| 2008 to 2011 | 1.05 (0.99,1.10) | 1.56 (1.43,1.71) ‡ | 0.98 (0.82,1.16) | 0.74 (0.70,0.79) ‡ |
| 2012 to 2014 | 1.09 (1.03,1.15) † | 3.63 (3.31,3.98) ‡ | 1.02 (0.84,1.23) | 0.76 (0.71,0.81) ‡ |
P-values:
0.02 ≤ p < 0.05;
0.0001 ≤ p < 0.01;
p < 0.0001
CsA, cyclosporine; DCD, donation after circulatory death; ECD, expanded criteria donor; ESRD, end-stage renal disease; IL2rAb, interleukin-2 receptor blocking antibodies; MPA, mycophenolate acid; mTORi, mammalian target of rapamycin inhibitor; PRA, panel-reactive antibody; Pred, prednisone; SCD, standard-criteria donor; Tac, tacrolimus.
Center Level Variation in Induction Regimen Use
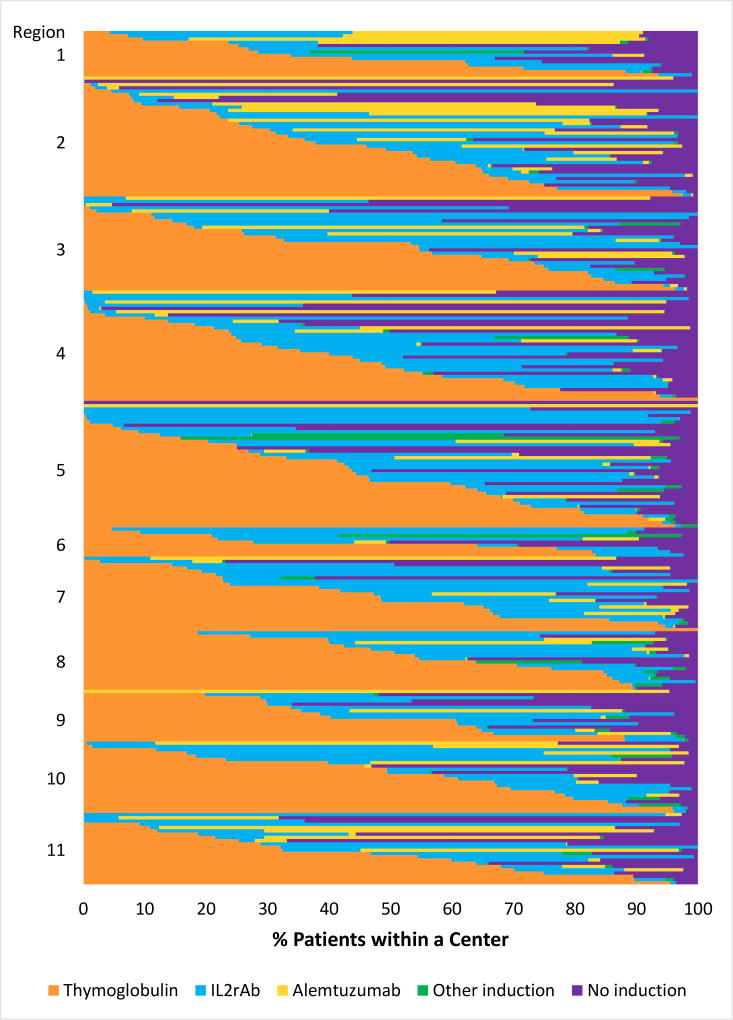
The proportion of patients treated with each induction agent varied widely across centers: IL2rAb (0%–98.8%), TMG (0%–100%), ALEM (0%–84%), none (0%–97%), and also varied within each of the 11 regions across the country (Figure 3).
Figure 3.
Proportion of patients receiving each induction immunosuppression option (including no induction) across US transplant centers (2005–2014). Each horizontal bar represents an individual center within US regions ordered by the proportion of patients receiving each regimen. Overall percentages of regimen use at patient level across centers: TMG, 46.0%; IL2rAb, 22.0%; ALEM, 12.5%; other induction, 1.3%; no induction, 18.2%.
ALEM, alemtuzumab; IL2rAb, interleukin-2 receptor blocking antibodies; TMG, thymoglobulin.
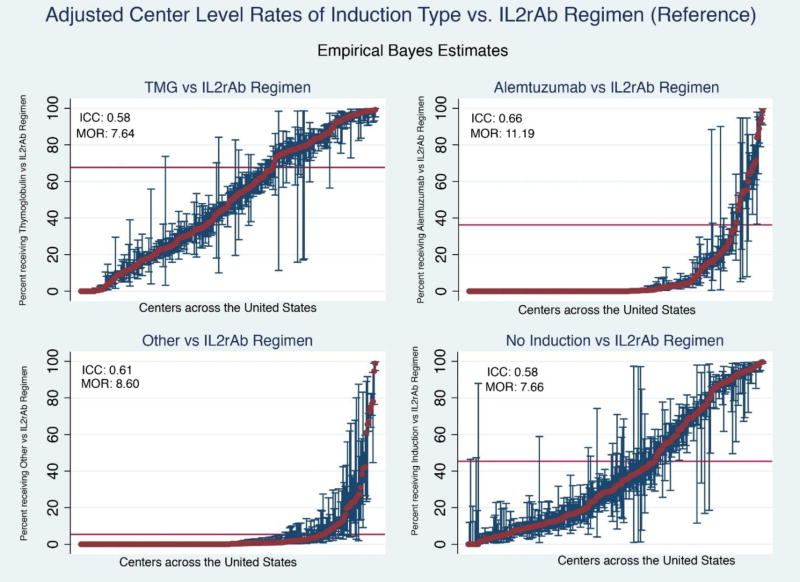
After adjustment for differences in donor and recipient characteristics using hierarchical logistic regression models, the observed between-center variation in use of specific induction regimens was significantly greater than what would be expected (Table 3Figure 4). Based on EBEs and pairwise comparison, comparing relative use of TMG with use of IL2rAb, use rates at 44.7% of centers were lower than expected. While only 46.0% of centers used any ALEM, 38.7% of those used it significantly more than the estimated national average ratio of ALEM to IL2rAb. Nearly 36% of centers used induction-free immunosuppression more commonly than expected. After adjustment for donor and recipient characteristics, the ICCs suggested that most variation in TMG (58%), ALEM (66%), other induction (61%), and no induction (58%) use reflected center practice, which is not explained by differences in the treated populations or the organs that were transplanted (Table 4).
Table 3.
Center-level empirical Bayes estimates adjusted for case-level characteristics. *
| Immunosuppression regimen (Reference: IL2rAb) |
No. of centers in pairwise comparison |
No. of centers significantly above reference probability |
No. of centers significantly below reference probability |
|---|---|---|---|
| Thymoglobulin | 253 | 105 (43.2%) | 116 (44.7%) |
| Alemtuzumab | 123 | 51 (38.7%) | 52 (10.5%) |
| Other | 132 | 40 (32.0%) | 38 (19.5%) |
| No induction | 256 | 89 (35.7%) | 115 (43.2%) |
Constructed from pairwise comparisons of regimen of interest versus reference regimen (IL2rAb).
IL2rAb, interleukin-2 receptor blocking antibodies.
Figure 4.
Empirical Bayes estimates for likelihood of induction regimen use compared with IL2rAb. Reference regimen based on current US Food and Drug Administration approval. Red bar demonstrates national average rate of use of each regimen (within pairwise regimen comparisons). Each red dot represents adjusted use at one center and the blue bars reflect 95% confidence intervals for use at the center determined by empirical Bayes estimates, adjusting for case factors of transplants at the center; exclusion of the national average by a 95% confidence interval reflects adjusted center use significantly above or below the national average.
ICC, intraclass correlation coefficient; IL2rAb, interleukin-2 receptor blocking antibodies; MOR, median odds ratio; TMG, thymoglobulin.
Table 4.
Heterogeneity across unadjusted and both adjusted models.*
| Immunosuppression Regimen (Reference: IL2rAb) |
Proportion of variance in hierarchical model explained by center characteristics (Unadjusted) |
MOR (Unadjusted) |
Proportion of variance in hierarchical model explained by center, adjusted for donor/recipient factors |
MOR (Adjusted) |
Proportion of variance in model explained by donor/recipient characteristics |
|---|---|---|---|---|---|
| Thymoglobulin | 0.55 | 6.76 | 0.58 | 7.64 | 0.11 |
| Alemtuzumab | 0.69 | 13.06 | 0.66 | 11.19 | 0.33 |
| Other | 0.58 | 7.69 | 0.61 | 8.60 | 0.10 |
| No induction | 0.56 | 7.00 | 0.58 | 7.66 | 0.12 |
Proportion of variance in hierarchical model is equal to the ICC.
ICC, intraclass correlation coefficient; IL2rAb, interleukin-2 receptor blocking antibodies; MOR, median odds ratio.
DISCUSSION
We examined the impact of center and case factors on induction regimen choice in contemporary US kidney transplant practice, and found that regimens varied widely across centers and also varied widely within each of the UNOS regions across the country. We confirmed that choice of induction regimen was associated with some donor and recipient factors; however, these factors explained only a minority of the variation observed nationally. After adjustment for clinical characteristics, most of observed variation in choice of a non-IL2rAb regimen reflected center practice patterns rather than patient or donor factors.
Two landmark trials established the efficacy of induction agents in reducing the risk of rejection in patients at high immunological risk for rejection. In 2006, Brennan et al compared the efficacy and safety of TMG vs. basiliximab (IL2rAb) (20). Among patients categorized as high risk for rejection and delayed graft function, incidence of acute rejection was lower in the TMG arm, and patient and graft survival were similar at 1 year. The overall risk of infections was significantly higher in the TMG arm, although the rate of cytomegalovirus infection was lower. A 10-year follow-up based on linkage of US study participants to the national transplant registry revealed similar patient and allograft survival in TMG- and basiliximab-treated patients over long-term follow-up, while the cumulative incidence of acute rejection remained lower in the TMG group (21). No difference was found in risk of other infections or posttransplant cancers. Notably, registry data may not identify serial changes in maintenance immunosuppression and patient compliance, and registry-based rejection and complications data may lack granularity.
In 2011, Hanaway et al reported a randomized comparison of TMG vs. ALEM among recipients at higher immunological risk and ALEM vs. IL2rAb (specifically basiliximab) among recipients at lower risk (“INTAC” Study) (22). All patients received Tac, MMF, and a rapid steroid withdrawal protocol. The study demonstrated that ALEM was superior to basiliximab even among recipients at lower immunological risk in preventing acute rejection; however, it found no difference in acute rejection with TMG vs. ALEM in higher-risk recipients. The overall risk of adverse events at 3 years was similar in all groups, although more serious infections were reported in the ALEM group (vs. basiliximab), and more cancers (vs. basilixmab and TMG combined). Perhaps driven by such trial results, we found increased use of cell depleting agents in recipients at higher immunological risk (e.g., black race, allosensitized, previous transplant) in our study.
In contrast to the benefits among high immunologic risk patients, the benefits of TMG in lower immunologic risk patients are less clear. A 2009 Cochrane meta-analysis examined 71 randomized clinical trials comparing different induction therapy (23). The reviewed trials dominantly enrolled low immunological risk recipients, with 72% being first-time transplant recipients. Eighteen of the 71 included studies compared TMG to IL2rAb, and found no differences in graft loss at any point or in the rate of clinically diagnosed acute rejection. However, TMG decreased the rate of biopsy-proven acute rejection.
Randomized clinical trials offer an unparalleled level of evidence, but inclusion criteria can be selective, follow up is short, and the care provided within the framework of a trial may not represent real world practice. While limited in granularity and lack of randomization, large database studies can provide increased power, better generalizability, and longer follow-up. One study of national registry data for US transplant recipients in 2001 to 2005 using exposure likelihood and outcome risk matching techniques to minimize the risk of confounding found lower risk of a 6-month composite of acute rejection, death or graft failure among patients who received TMG compared to basiliximab, across statistical approaches (24). More recently, a study by Koyawala et al. based on linking US registry data to Medicare claims (2003 to 2008) and matching patients based on many demographic, clinical and economic factors found that ALEM was associated with a 14% higher adjusted mortality, and 18% higher all cause graft failure and 31% higher acute rejection compared to TMG. IL2rAb (basiliximab) was associated with 8% increased mortality and 16% higher acute rejection but no increase in the risk of all cause graft failure compared to TMG (25). Results for the ALEM versus TMG comparisons were generally consistent among subgroups including elderly patients and those receiving prednisone. In contrast, this higher mortality observed with IL2rAb versus TMG was not confirmed in subgroup analyses. With regard to outcomes in specific sub-groups, a recent retrospective analysis of African American kidney transplant recipients captured in US registry data found that, compared to IL2rAb induction, depleting induction (including TMG, ALEM or OKT3) was associated with 32% reduction in acute rejection, 9% lower graft loss, and 12% lower mortality over up to 14 years of follow-up (9). Another registry-based study focused on retransplant recipients, and found that compared to patients induced with TMG, no induction was associated with 82% greater adjusted likelihood ratio of early acute rejection and IL2rAb induction was associated with more than twice the likelihood of early acute rejection (10). There were no differences in patient or graft survival with TMG versus IL2rAb treatment in the retransplant population.
Previous studies have suggested benefit of specific induction agents when combined with steroid-avoidance or MMF-sparing maintenance regimens. We found that ALEM use was almost 14-fold higher than IL2rAb use among patients receiving steroid-free regimens. Furthermore, ALEM use appeared to be higher among obese patients, possibly due to the desire to withdraw steroids once these patients were stable. The major differences between TMG and ALEM use appear to be the preference for using ALEM in rapid steroid protocols and greater use in recent years.
We demonstrated that center choice, rather than patient or donor characteristics, was the primary driver of induction immunosuppression regimen. The widest variation was in use of ALEM as induction therapy. ALEM was used at only 45.6% of centers analyzed. Among centers that used ALEM, variation in the proportions of patients who received it was wide. The MOR data demonstrate that if two centers were selected at random, the odds of a patient receiving ALEM vs. IL2rAb at one center was up to 11-fold higher than at the other, even after accounting for observed recipient, donor, and transplant characteristics. The MORs for other induction regimens demonstrated similarly high degrees of inter-center variation in use compared with IL2rAb: TMG (7.64), other induction (8.60), no Induction (7.66).
In concordance with prior national reports (13, 26), our data show an overall increase in use of induction agents over time. While use of IL2rAb has decreased, use of lymphocyte depleting agents (both TMG and ALEM) has increased. Economic factors may contribute the decision to use ALEM. Notably, in the US, ALEM was distributed for free for kidney transplant induction under the Campath® Distribution Program, and upfront cost savings may have been attractive giving recognition that reimbursement for kidney transplantation has not kept pace with the rising costs of the procedure (27), especially for under-insured patients. In fact, our analyses showed two-fold higher adjusted use of ALEM in self/other-pay patients, compared with IL2rAb use. Based on the absolute wholesale price, the cost of basiliximab is $6,490 per patient compared the approximate cost of $10,000–14,000 per patient (4.5 to 6.0 mg/kg dose for a 70 kg patient) for TMG(28). The Campath® Distribution Program is now restricted, which may limit further expansion of ALEM use.
Importantly, consideration of cost of an agent alone does not account for cost savings from reducing risks of rejection, graft loss, or other complications. The clinical benefit of appropriate induction therapy has been confirmed in recent analyses. In recipients of deceased donor kidney transplants, TMG and ALEM reduced acute rejection risks compared with IL2rAb, but only TMG has been correlated with better graft survival (29). In recipients of living donor transplants who were not treated with steroids, both ALEM and TMG reduced acute rejection risk compared with IL2rAb; however, ALEM has been associated with higher composite risk of graft failure or patient death, while TMG was not (30). Results with ALEM may improve over time as a center becomes more experienced with the drug (31).
The retrospective study by Koyawala et al found that one-year resource utilization was slightly lower among recipients treated with ALEM compared to TMG, but did not differ between those treated with basiliximab compared to TMG (25). When assessing cost-effectiveness of induction agents in deceased donor kidney recipients, Gharibi et al found that cell depleting regimens such as TMG and ALEM were more cost effective in both high and low immunologic risk groups. Only TMG was associated with graft survival benefit over no induction (32).
Limitations of our study include the binary definition of induction use in registry data. In addition, no information was available on induction regimen schedule or dosing, presence of donor-specific antibody, prior malignancy or infections, or other clinical factors that may have modified induction choice.
In conclusion, based on analyses of center-identified national transplant registry data, we found that kidney transplant induction therapy varies widely across US transplant centers, and that choice of regimen largely reflects center preference rather than patient or donor characteristics. The persistent variation in use of induction agents presents several unique opportunities. First, the observed variation can be used to analyze transplant outcomes to better target induction to patients who are expected to derive the best outcomes. Second, tools are needed to better guide clinicians to evidence-based selection of regimens. Centers with the best intermediate and long-term allograft and patient outcomes should be studied closely to understand how much of the improved performance is attributable to induction immunosuppression. Third, closer analyses of cumulative infection risk and posttransplant malignancies, and an economic analysis of overall costs attributed to induction immunosuppression, are needed. Further research including collaborative clinical trials and secondary data analyses of contemporary practice are needed to determine the relationship between center practice, post-transplant outcome, and patient selection to advance from a “one size fits all” to a personalized medicine approach to immunosuppression.
Acknowledgments
This work was conducted under the auspices of the Minneapolis Medical Research Foundation (MMRF), contractor for the Scientific Registry of Transplant Recipients (SRTR), as a deliverable under contract no. HHSH250201000018C (US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation). As a US Government-sponsored work, there are no restrictions on its use. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. The authors thank SRTR colleague Nan Booth, MSW, MPH, ELS, for manuscript editing.
This work was supported by a grant from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01-R01DK102981.
ABBREVIATIONS
- ALEM
Alemtuzumab
- AZA
Azathioprine
- CsA
Cyclosporine
- IL2rAb
IL-2 receptor antibody
- EBEs
Empirical Bayes estimates
- FDA
Food and Drug Administration
- HLA
human leukocyte antigen
- ICC
Intraclass correlation
- MMF/MPA
Mycophenolate Mofetil/Mycophenolate acid
- MOR
Median Odds Ratio
- OPTN
Organ Procurement and Transplantation Network
- OR
odds ratio
- PRA
Panel Reactive antibody
- Pred
prednisone
- RR
relative risk
- SRTR
Scientific Registry of Transplant Recipients
- Tac
Tacrolimus
- TMG
Thymoglobulin
Appendix 1
Proportion of patients receiving each induction immunosuppression option (including no induction) across US transplant centers (2005–2014), by clinical risk profile
Footnotes
Author Roles and Support:
Participated in study design, acquisition of data and regulatory approvals, data analysis, and writing of the paper. Support provided to the author’s institution by the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK
Participated in study design, interpretation, and writing of the paper
Participated in study design, interpretation, and writing of the paper. Support provided to the author’s institution by the NIH/NIDDK
Participated in data analysis and manuscript preparation. Support provided to the author’s institution by the NIH/NIDDK
DISCLOSURES/CONFLICTS OF INTEREST: The authors report no conflicts of interest.
An abstract describing this work was presented at the American Transplant Congress, May 2017, Chicago, IL.
References
- Halloran PF. Immunosuppressive Drugs for Kidney Transplantation. New England Journal of Medicine. 2004;351(26):2715–29. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation. 2003;75(6):844–51. doi: 10.1097/01.TP.0000056635.59888.EF. [DOI] [PubMed] [Google Scholar]
- Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80(9):1233–43. doi: 10.1097/01.tp.0000179639.98338.39. [DOI] [PubMed] [Google Scholar]
- Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87(7):1019–26. doi: 10.1097/TP.0b013e31819cc383. [DOI] [PubMed] [Google Scholar]
- Opelz G, Unterrainer C, Susal C, Dohler B. Efficacy and safety of antibody induction therapy in the current era of kidney transplantation. Nephrol Dial Transplant. 2016;31(10):1730–8. doi: 10.1093/ndt/gfw086. [DOI] [PubMed] [Google Scholar]
- Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1:CD004759. doi: 10.1002/14651858.CD004759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;(suppl 3):S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. The New England journal of medicine. 2006;355(19):1967–77. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- Taber DJ, McGillicuddy JW, Bratton CF, Rohan VS, Nadig S, Dubay D, et al. Cytolytic Induction Therapy Improves Clinical Outcomes in African-American Kidney Transplant Recipients. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schold J, Poggio E, Goldfarb D, Kayler L, Flechner S. Clinical outcomes associated with induction regimens among retransplant kidney recipients in the United States. Transplantation. 2015;99(6):1165–71. doi: 10.1097/TP.0000000000000507. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. [Accessed: July 7, 2017];1998 Approval Letter - Thymoglobulin. Available at: http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm117146.htm.
- Montgomery JR, Berger JC, Warren DS, James NT, Montgomery RA, Segev DL. Outcomes of ABO-incompatible kidney transplantation in the United States. Transplantation. 2012;93(6):603–9. doi: 10.1097/TP.0b013e318245b2af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A, Smith JM, Skeans MA, Gustafson SK, Stewart DE, Cherikh WS, et al. OPTN/SRTR 2015 Annual Data Report: Kidney. Am J Transplant. 2017;(17 Suppl 1):21–116. doi: 10.1111/ajt.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod DA, Naik AS, Schnitzler MA, Segev DL, Dharnidharka VR, Brennan DC, et al. National Variation in Use of Immunosuppression for Kidney Transplantation: A Call for Evidence-Based Regimen Selection. Am J Transplant. 2016;16(8):2453–62. doi: 10.1111/ajt.13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharnidharka VR, Schnitzler MA, Chen J, Brennan DC, Axelrod D, Segev DL, et al. Differential risks for adverse outcomes 3 years after kidney transplantation based on initial immunosuppression regimen: a national study. Transpl Int. 2016;29(11):1226–36. doi: 10.1111/tri.12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King EA, Kucirka LM, McAdams-DeMarco MA, Massie AB, Al Ammary F, Ahmed R, et al. Early Hospital Readmission After Simultaneous Pancreas-Kidney Transplantation: Patient and Center-Level Factors. Am J Transplant. 2016;16(2):541–9. doi: 10.1111/ajt.13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orandi BJ, James NT, Hall EC, Van Arendonk KJ, Garonzik-Wang JM, Gupta N, et al. Center-level variation in the development of delayed graft function after deceased donor kidney transplantation. Transplantation. 2015;99(5):997–1002. doi: 10.1097/TP.0000000000000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ML, Thomas AG, Shaffer A, Massie AB, Luo X, Holscher CM, et al. The National Landscape of Living Kidney Donor Follow-Up in the United States. Am J Transplant. 2017 doi: 10.1111/ajt.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. Journal of epidemiology and community health. 2006;60(4):290–7. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Thymoglobulin Induction Study G. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. The New England journal of medicine. 2006;355(19):1967–77. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- Lentine KL, Schnitzler MA, Xiao H, Brennan DC. Long-term safety and efficacy of antithymocyte globulin induction: use of integrated national registry data to achieve ten-year follow-up of 10-10 Study participants. Trials. 2015;16(1):365. doi: 10.1186/s13063-015-0891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. The New England journal of medicine. 2011;364(20):1909–19. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2010;(1):CD003897. doi: 10.1002/14651858.CD003897.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby LM, Schnitzler MA, Brennan DC, Pinsky BW, Dzebisashvili N, Buchanan PM, et al. Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: application of statistical approaches to reduce bias in observational comparisons. Transplantation. 2009;87(10):1520–9. doi: 10.1097/TP.0b013e3181a484d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyawala N, Silber JH, Rosenbaum PR, Wang W, Hill AS, Reiter JG, et al. Comparing Outcomes between Antibody Induction Therapies in Kidney Transplantation. Journal of the American Society of Nephrology. 2017 doi: 10.1681/ASN.2016070768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matas A, Smith J, Skeans M, Thompson B, Gustafson S, Stewart D, et al. OPTN/SRTR 2013 annual data report: kidney. American Journal of Transplantation. 2015;15(S2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- Axelrod DA, Schnitzler MA, Xiao H, Naik AS, Segev DL, Dharnidharka VR, et al. The Changing Financial Landscape of Renal Transplant Practice: A National Cohort Analysis. Am J Transplant. 2017;17(2):377–89. doi: 10.1111/ajt.14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A, Mannon RB. The Cost of Transplant Immunosuppressant Therapy: Is This Sustainable? Current transplantation reports. 2015;2(2):113–21. doi: 10.1007/s40472-015-0052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriover B, Jaikaransingh V, MacConmara MP, Parekh JR, Levea SL, Ariyamuthu VK, et al. Acute Rejection Rates and Graft Outcomes According to Induction Regimen among Recipients of Kidneys from Deceased Donors Treated with Tacrolimus and Mycophenolate. Clin J Am Soc Nephrol. 2016;11(9):1650–61. doi: 10.2215/CJN.13171215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriover B, Zhang S, MacConmara M, Gao A, Sandikci B, Ayvaci MU, et al. Induction Therapies in Live Donor Kidney Transplantation on Tacrolimus and Mycophenolate With or Without Steroid Maintenance. Clin J Am Soc Nephrol. 2015;10(6):1041–9. doi: 10.2215/CJN.08710814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano OK, Friedmann P, Ahsanuddin S, Millan C, Ben-Yaacov A, Kayler LK. Outcomes Associated with Steroid Avoidance and Alemtuzumab among Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2015;10(11):2030–8. doi: 10.2215/CJN.12161214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibi Z, Ayvaci M, Hahsler M, Giacoma T, Gaston RS, Tanriover B. Cost-Effectiveness of Antibody-Based Induction Therapy in Deceased Donor Kidney Transplantation in the United States. Transplantation. 2016 doi: 10.1097/TP.0000000000001310. [DOI] [PMC free article] [PubMed] [Google Scholar]