Abstract
Background and purpose
To compare chemoradiotherapy (CRT) with low-dose continuous 5-fluorouracil (5FU) to CRT with 5FU+cisplatin (CDDP) for esophageal squamous cell carcinoma (ESCC) in a retrospective cohort study.
Methods and materials
We reviewed the cases of Stage I–IV ESCC patients who underwent definitive CRT in 2000–2014. Concomitant chemotherapy was one of the three regimens: (1) high-dose intermittent 5FU and CDDP (standard-dose FP: SDFP), (2) low-dose continuous 5FU and CDDP (LDFP), or (3) low-dose continuous 5FU (LD5FU). The general selection criteria for chemotherapy were: SDFP for patients aged <70 yrs; LDFP for those aged 70–74 yrs; LD5FU for those aged ≥75 yrs or with performance status (PS) ≥3. Propensity scores were derived with chemotherapy (LD5FU vs. 5FU+CDDP) as the dependent variable.
Results
In a multivariate analysis, chemotherapy (LD5FU vs. SDFP, p = .24; LDFP vs. SDFP, p = .52) did not affect the overall survival (OS). LD5FU caused significantly less grade 3–4 leukopenia (9%) compared to SDFP (47%) and LDFP (44%) (p < .001). In a propensity-matched analysis, LD5FU affected neither OS (HR 1.06; 95%CI 0.55–2.05; p = .87) nor progression-free survival (HR 0.95, 95%CI 0.50–1.81; p = .87).
Conclusion
CRT with low-dose continuous 5FU may be a less toxic option for elderly ESCC patients.
Keywords: Esophageal cancer, Chemoradiotherapy, 5-Fluorouracil, Cisplatin, Hematological toxicity
Introduction
Definitive chemoradiotherapy (CRT) plays an important role in the management of esophageal cancer. The most widely used concomitant chemotherapy is the combination of 5-fluorouracil (5FU) and cisplatin (CDDP) [1], [2], [3], [4]. However, 30–50% of patients treated with this regimen suffer from severe acute toxicity [1], [5], [6]. CDDP is associated with various systemic side effects [7]. The routine use of CDDP thus tends to be avoided in elderly patients, who generally have impaired physiologic function [8].
CRT without CDDP has been studied as part of the effort to reduce CRT's toxicity in this patient subgroup. In a prospective trial of CRT with low-dose continuous 5FU for elderly patients with esophageal cancer, the complete response (CR) rate was 45%, and grade 3 or worse adverse events were observed in 11% of the patients [8]. Therefore, the CRT with low-dose continuous 5FU regimen can be considered effective for esophageal cancer. We have used this CRT regimen for esophageal squamous cell carcinoma (ESCC) patients who are not appropriate for CDDP treatment [9], [10], but its treatment outcomes have not been directly compared to those of the 5FU+CDDP regimens. Herein, we investigated whether CRT with low-dose continuous 5FU can reduce toxicity compared to regimens with 5FU and CDDP without compromising the survival outcomes.
Methods and materials
Patients
We performed a retrospective cohort study of ESCC patients who underwent definitive CRT at our institution in the 15-year period from 2000 January to 2014 December. The inclusion criteria were: (1) histological diagnosis of primary ESCC, and (2) concomitant chemotherapy consisting of either 5FU alone or 5FU plus CDDP. The three regimens of concomitant chemotherapy were as follows: two cycles of a bolus injection of 5FU and CDDP (SDFP; standard-dose 5FU+CDDP), or low-dose continuous 5FU and CDDP (LDFP: low-dose 5FU+CDDP), or low-dose continuous 5FU (LD5FU; low-dose 5FU). Staging was done with an upper gastrointestinal endoscopy and computed tomography (CT) of the neck, chest, and abdomen. 18F-fluorodeoxyglucose positron emission tomography was not used.
There was a major change in the American Joint Committee on Cancer (AJCC) tumor, node, metastasis (TNM) classification during the study period [11]. All patients in our study underwent individual treatment based on the 6th edition of the TNM classification. In the 7th edition, the N stage depends on the number of involved lymph nodes, but we do not consider this suitable for ESCC patients undergoing nonsurgical treatment. Pretreatment CT cannot accurately detect lymph node involvement [12]. In a retrospective study of definitive CRT for ESCC, the N stage determined based on the 7th edition did not correlate with the overall survival (OS) [13]. For these reasons, we used the 6th edition of the TNM classification. Our hospital's Institutional Review Board approved the study.
Radiotherapy
All patients underwent CT simulation and 3D conformal radiotherapy (RT). RT was delivered using a 10-MV linear accelerator with a daily fraction of 1.8–2 Gy. The gross tumor volume (GTV) included the esophageal primary tumor (primary GTV) and nodal metastases (nodal GTV). The clinical target volume (CTV) was created with a 2-cm margin in the superoinferior (SI) direction following the long axis of the esophagus and 5-mm margins in the anteroposterior (AP) and lateral directions around the primary GTV [14], [15]. The nodal GTV was expanded with a 5-mm CTV margin, and was unified with the CTV around the primary GTV. For elderly patients and those with a poor performance status (PS), the omission of elective nodal irradiation (ENI) was permitted.
The CTV was then expanded in the SI direction by 1–1.5 cm and in the AP and lateral directions by 0.5 cm to create the planning target volume (PTV). The first RT phase was delivered up to 40–44 Gy with 2–4 beams, to treat the PTV including the GTV and elective nodal regions. For tumors at the middle or lower third of the esophagus, four opposing AP and oblique beams were used to reduce the dose to heart [16]. The second RT phase was delivered with two opposing oblique beams to cover the PTV around the GTV, and to shield the spinal cord. The total dose was 50–70 Gy. The LDFP group and the LD5FU group tended to receive higher RT doses to compensate for the reduced chemotherapy doses. The upper limits of maximal dose were ≤54 Gy to the stomach and <50 Gy to the spinal cord. The recommended lung volume receiving ≥20 Gy (V20) was ≤30%.
Chemotherapy
The SDFP regimen consisted of CDDP 70 mg/m2/d on days 1 and 29 and 5FU 700 mg/m2/d on days 1–4 and 29–32, or CDDP 75 mg/m2/d and 5FU 1000 mg/m2/d on the same corresponding schedule. The LDFP consisted of CDDP 3 mg/m2/d and 5FU 250 mg/m2/d on all RT days. The LD5FU consisted of 5FU 250 or 300 mg/m2/d on all RT days. The general selection criteria for concurrent chemotherapy regimen were: SDFP for those aged <70 yrs; LDFP for those aged 70–74 yrs; LD5FU for those aged ≥75 yrs or with an Eastern Cooperative Oncology Group (ECOG) PS ≥3. All patients underwent a blood chemistry test and cell count once or twice a week during the CRT. For patients assigned to either SDFP or LDFP, 5FU 800 mg/m2/d on days 1–4 and CDDP 80 mg/m2/d on day 1 were given as consolidation chemotherapy when the treating oncologist considered these agents tolerable [17].
Response evaluation, follow-up, and assessment of toxicities
Each patient's tumor response was evaluated 1–2 months after the completion of CRT. The criteria for CR were: disappearance of the esophageal tumor on CT and endoscopy, and the absence of new metastatic lesions [18]. Follow-up CT and endoscopy were performed every 3–6 months. Either salvage surgery or endoscopic submucosal dissection (ESD) was considered upon locoregional failure (LRF) [19]. Adverse events were graded with the fourth version of the Common Terminology Criteria for Adverse Events (CTCAE).
Statistical analyses
We used the Kruskal-Wallis test to compare continuous variables, and the Χ2 test was used for the distribution of categorical variables among the CRT groups. The OS was calculated from the first day of CRT. The progression-free survival (PFS) was defined as the interval between the beginning of the CRT and the detection of tumor progression or death, whichever occurred first. We used the Cox proportional hazard model to determine the factors that increase the hazard ratio (HR) for all-cause mortality.
To reduce biases, we derived propensity scores with concurrent chemotherapy as the dependent variable [20]. In the KROSG0101/JROSG021 randomized trial, the OS did not differ significantly between the patients treated with CRT with low-dose continuous 5FU+CDDP and those treated with CRT with high-dose intermittent 5FU+CDDP [1]. Thus, we unified the LDFP and SDFP groups in the present study to create the 5FU+CDDP group. The LD5FU was the treatment of interest, and the 5FU+CDDP was control.
We defined the elderly patients as those aged ≥70 years [21]. All independent variables were transformed into binary covariates: age (<70 yrs vs. ≥70 yrs), PS (0–1 vs. 2–4), and stage (I, II vs. III, IV) [22]. Matching was done one-to-one with a caliper width of 0.2. P-values <0.05 were considered significant. All analyses were performed with R 3.0.2 (the R Foundation for Statistical Computing, Vienna, Austria) and EZR (a graphical user interface for R, Saitama Medical Center, Jichi Medical University, Saitama, Japan) [23].
Results
Patient characteristics and treatment details
We identified 226 consecutive patients who underwent definitive RT for esophageal cancer in the years 2000–2014 at our institution. Seventy-three of these patients were excluded: 59 patients with RT alone, three with non-ESCC histology, and 11 with other concomitant chemotherapies. The other 153 ESCC patients were considered suitable for the further analyses: 63 underwent SDFP, 43 underwent LDFP, and 47 underwent LD5FU.
The median age of the LD5FU group (75 yrs) was significantly higher than those of the LDFP (69 yrs) and SDFP (62 yrs) groups (Table 1; p < .001). The SDFP group had a significantly higher proportion of stage IV diseases compared to the other two groups (p = .013). There were 39 patients classified as M1 stage. Thirty-three of these patients had supraclavicular or lower abdominal lymph node involvement. The remaining six patients had distant organ metastases; one liver, four lungs, and one gastric wall. They underwent CRT on the same schedule as the M0 patients, and were included in the following analyses. The most frequently used dose fractionation was 60 Gy in 30 fractions. The median total dose was significantly higher for the LD5FU and LDFP groups (both 66 Gy) compared to the SDFP group (60 Gy) (p < .001).
Table 1.
Patient characteristics and treatment details (n = 153).
| Variable | LD5FU | LDFP | SDFP | p-value |
|---|---|---|---|---|
| (n = 47) | (n = 43) | (n = 63) | ||
| Age at diagnosis (yrs): | <0.001 | |||
| Median | 75 | 69 | 62 | |
| Range | 58–82 | 48–79 | 33–72 | |
| No. of elderly patients: | ||||
| <70, n (%) | 13 (28) | 22 (51) | 60 (95) | <0.001 |
| ≥70, n (%) | 34 (72) | 21 (49) | 3 (5) | |
| Gender, n (%): | 0.75 | |||
| Male | 40 (85) | 34 (79) | 52 (83) | |
| Female | 7 (15) | 9 (21) | 11 (17) | |
| PS, n (%): | ||||
| 0–1 | 34 (72) | 37 (86) | 56 (89) | 0.067 |
| 2–4 | 13 (28) | 6 (14) | 7 (11) | |
| Tumor location, n (%): | 0.044 | |||
| Ce | 5 (11) | 10 (23) | 6 (9) | |
| Ut | 7 (15) | 3 (7) | 18 (29) | |
| Mt | 21 (45) | 23 (54) | 24 (38) | |
| Lt | 13 (27) | 7 (16) | 15 (24) | |
| Ae | 1 (2) | 0 (0) | 0 (0) | |
| T stage, n (%): | 0.80 | |||
| 1 | 12 (26) | 11 (26) | 15 (24) | |
| 2 | 8 (17) | 4 (9) | 6 (10) | |
| 3 | 15 (31) | 13 (30) | 18 (28) | |
| 4 | 12 (26) | 15 (35) | 24 (38) | |
| N stage, n (%): | 0.032 | |||
| 0 | 25 (53) | 18 (42) | 18 (29) | |
| 1 | 22 (47) | 25 (58) | 45 (71) | |
| M stage, n (%): | 0.012 | |||
| 0 | 40 (85) | 35 (81) | 39 (62) | |
| 1 | 7 (15) | 8 (19) | 24 (38) | |
| Clinical stage, n (%): | 0.013 | |||
| I | 11 (23) | 6 (14) | 13 (21) | |
| II | 15 (32) | 11 (25) | 6 (9) | |
| III | 14 (30) | 18 (42) | 20 (32) | |
| IV | 7 (15) | 8 (19) | 24 (38) | |
| Total RT dose (Gy:) | <0.001 | |||
| Median | 66 | 66 | 60 | |
| Range | 10–70.2 | 50–70 | 50–70 | |
| ENI, n (%): | 0.010 | |||
| Yes | 24 (51) | 31 (72) | 49 (78) | |
| No | 23 (49) | 12 (28) | 14 (22) | |
| Consolidation CHT, n (%): | <0.001 | |||
| Yes | 0 (0) | 3 (7) | 31 (49) | |
| No | 47 (100) | 40 (93) | 32 (51) | |
5FU: 5-fluorouracil, Ae: abdominal esophagus, CDDP: cisplatin, Ce: cervical esophagus, CHT: chemotherapy, ENI: elective nodal irradiation, LD5FU: low-dose continuous 5FU, LDFP: low-dose continuous 5FU+CDDP, Lt: lower thoracic esophagus, Mt: middle thoracic esophagus, NA: not applicable, PS: performance status, RT: radiotherapy, SDFP: standard-dose 5FU+CDDP, Ut: upper thoracic esophagus.
Treatment response and pattern of failure
The primary treatment response was evaluated in 148 patients (97%). CR was documented in 22 patients in the LD5FU group (50%), 19 in the LDFP group (45%), and 28 in the SDFP group (45%) (p = .89). Sixty-three patients (16 in the LD5FU group, 21 LDFP, 26 SDFP) developed LRF, and 40 (11 LD5FU, 11 LDFP, 18 SDFP) developed distant organ failure. Nine (14%) of the patients with LRF underwent salvage ESD, and 11 (17%) underwent surgical removal.
Survival
The median follow-up period for the 153 patients was 70.6 months (mos.). At the time of analysis, 31 (20%) patients were still alive. The median survival time (MST) was 20.9 mos. (95%CI, 15.4–29.4); the 3- and 5-year survival rates were 37.6% (95%CI 29.7–45.4) and 29.6% (95%CI 22.3–37.4), respectively. The MST for each stage was as follows: stage I, 88.3 mos. (95%CI 32.4–∞); stage II, 48.3 mos. (95%CI 20.0–62.9); stage III, 13.0 mos. (95%CI 9.9–18.4); stage IV, 12.6 mos. (95%CI 9.2–16.1). The MST and 5-year survival rate of each CRT group were: LD5FU, 25.0 mos. (95%CI 11.1–48.3), 27.7% (95%CI 14.9–42.0); LDFP, 14.9 mos. (95%CI 9.9–24.1), 31.6% (95%CI 18.3–45.7); SDFP, 25.3 mos. (95%CI 15.4–39.1), 29.4% (95%CI 18.2–41.5) (p = .71), respectively. There were 114 deaths; 88 from primary ESCC, eight from treatment-related complications, and 18 from other causes.
In the multivariate analysis, PS (0–1 vs. 2–4, p < .001; Table 2) and stage (I, II vs. III, IV; p < .001) affected the OS, but chemotherapy regimen (LD5FU vs. SDFP, p = .24; LDFP vs. SDFP, p = .52) and total RT dose (≤60 Gy vs. >60 Gy, p = .69) did not have an impact on OS.
Table 2.
Multivariate analysis of overall survival.
| Variable | HR (95%CI) | p |
|---|---|---|
| PS: | ||
| 0–1 | Reference | |
| 2–4 | 2.43 (1.46–4.03) | <0.001 |
| Stage: | ||
| I, II | Reference | |
| III, IV | 2.75 (1.75–4.31) | <0.001 |
| Concurrent CHT: | ||
| SDFP | Reference | |
| LD5FU | 1.36 (0.81–2.28) | 0.24 |
| LDFP | 1.20 (0.69–2.06) | 0.52 |
| Total RT dose (Gy): | ||
| ≤60 | Reference | |
| >60 | 1.10 (0.69–1.73) | 0.69 |
Abbreviations are explained in the Table 1 footnote.
Adverse events
In the LD5FU group, there was significantly less grade 3–4 leukopenia (9%) compared to the SDFP (47%) and LDFP (44%) groups (Suppl. Appendix A, p < .001). LDFP was also associated with significantly more grade 3 increases in alanine aminotransferase (12%) compared to LD5FU and SDFP (p = .001). The frequencies of the other adverse events did not differ significantly among the three groups.
Propensity-matched analysis
In our univariate comparison of the patients' OS by concomitant chemotherapy, the 5-year survival rates of the SDFP group and LDFP group were identical. In the multivariate analysis, the LDFP regimen did not significantly affect the OS compared to the SDFP regimen. Although the median of the total RT doses in the LDFP group was significantly higher than that in the SDFP group, this difference did not affect the OS in the multivariate analysis, and we considered it appropriate to unify these two groups. A total of 78 patients (LD5FU, n = 39; LDFP, n = 27; SDFP, n = 12) were successfully matched, and the baseline characteristics were well balanced (Table 3).
Table 3.
Propensity-matched groups (n = 78).
| Variable | LD5FU | 5FU+CDDP | p |
|---|---|---|---|
| (n = 39) | (n = 39) | ||
| Concurrent CHT, n (%): | <0.001 | ||
| LD5FU | 39 (100) | 0 (0) | |
| LDFP | 0 (0) | 27 (69) | |
| SDFP | 0 (0) | 12 (31) | |
| Age (yrs), n (%): | |||
| <70 | 13 (33) | 15 (39) | 0.48 |
| ≥70 | 26 (67) | 24 (62) | |
| Median | 75 | 71 | |
| Range | 58–79 | 43–79 | |
| Stage, n (%): | |||
| I, II | 22 (56) | 18 (46) | 0.13 |
| III, IV | 17 (44) | 21 (54) | |
| PS, n (%): | |||
| 0–1 | 30 (77) | 28 (72) | 0.48 |
| 2–4 | 9 (23) | 11 (28) | |
Abbreviations are explained in the Table 1 footnote.
As illustrated in Fig. 1, Fig. 2, the OS (p = .78) and PFS (p = .26) were not significantly different between the LD5FU and 5FU+CDDP groups. The MST and the 5-year survival rate of each propensity-matched group were as follows. LD5FU: 22.9 mos. (95%CI 10.3–48.3) and 26.7% (95%CI 13.1–42.4); 5FU+CDDP: 15.4 mos. (95%CI 12.2–24.8) and 23.7% (95%CI 11.5–38.3) (p = .78), respectively. By Cox proportional hazard models, the LD5FU did not affect the patients' OS (HR 1.06, 95%CI 0.55–2.05, p = .87) or PFS (HR 0.95, 95%CI 0.50–1.81, p = .87).
Fig. 1.
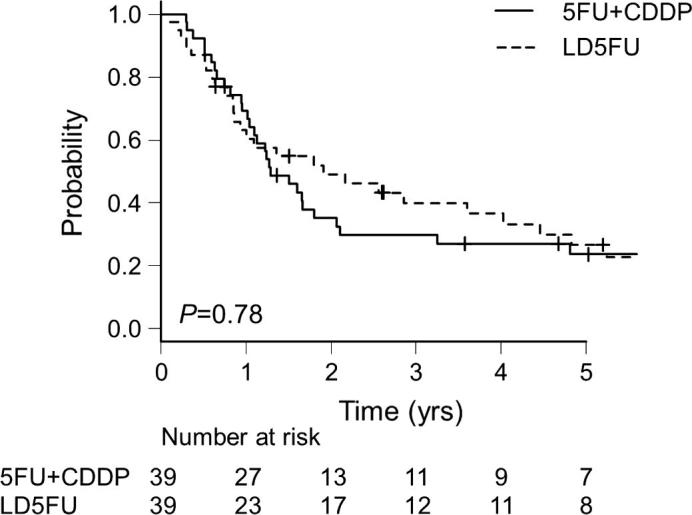
Kaplan-Meier estimates of overall survival (OS) for the propensity-matched patients. 5FU: 5-fluorouracil, CDDP: cisplatin, LD5FU: low-dose continuous 5FU.
Fig. 2.
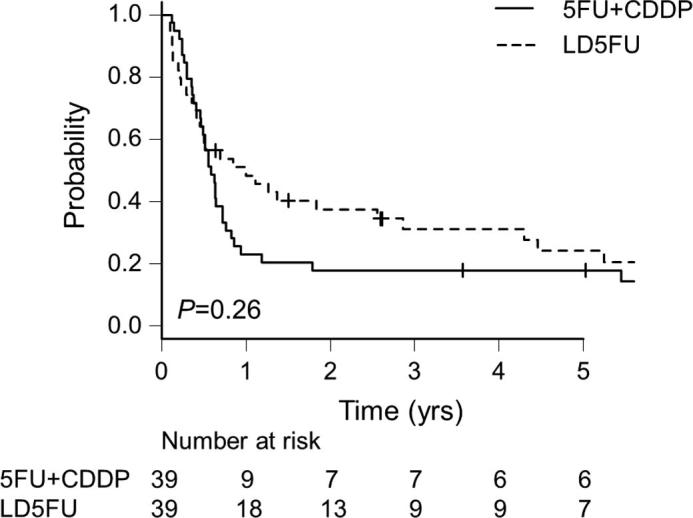
Progression-free survival (PFS) for the propensity-matched patients.
Discussion
We retrospectively compared CRT with low-dose continuous 5FU to CRT with 5FU+CDDP in ESCC patients. Our analyses revealed that the LD5FU regimen was associated with significantly less hematological toxicity compared to the 5FU+CDDP regimens. In the multivariate analysis, concomitant chemotherapy did not affect survival. The CR rate did not differ significantly among the treatment groups. In a propensity-matched analysis, the OS and PFS did not differ between the LD5FU and 5FU+CDDP groups.
We used the LD5FU regimen for elderly patients, and we explain the feasibility of this strategy as follows. In Japan, ESCC predominantly affects elderly people. In a nationwide survey organized by the National Cancer Center of Japan in 2012, the highest incidence of ESCC was observed in the individuals aged 75–79 years [24]. Age is an independent risk factor for perioperative cardiopulmonary complications [25] and mortality [26] after esophagectomy, and elderly patients may not be good surgical candidates. Definitive CRT has a potential for cure [17], [27]; therefore, it is an important treatment modality for elderly patients with ESCC.
Anticancer treatments with minimal toxicity are preferable for elderly patients [28]. However, the current evidence about such CRT regimens in esophageal cancer is still limited. The high-dose intermittent administration of 5FU and CDDP is regarded as the standard regimen [3], [5], [17]. Only two randomized trials compared the high-dose 5FU+CDDP regimen with other CRT regimens in esophageal cancer [1], [29]. The experimental regimens were 5FU+oxaliplatin and low-dose continuous 5FU+CDDP, respectively. Both failed to reduce grade 3–4 hematological and gastrointestinal toxicities compared to the high-dose 5FU+CDDP regimen.
Much of the hematological toxicity of CRT for esophageal cancer is attributable to CDDP. In a systematic review of the toxicity of concurrent CRT for uterine cervical cancer, CDDP significantly increased grade 3–4 leukopenia, grade 3–4 anemia and grade 1–2 thrombocytopenia [30]. In a meta-analysis of systemic chemotherapy regimens for elderly patients with metastatic non-small-cell lung cancer, the chemotherapies containing platinum agents significantly increased anemia and thrombocytopenia [21]. The platinum-based chemotherapies may not be tolerable for elderly patients.
We consider that the LD5FU regimen may be an alternative treatment for elderly patients with ESCC, for the following four reasons. First, in the present propensity-matched analysis, the OS and PFS did not significantly differ between the LD5FU group and the 5FU+CDDP group. Second, the MST and the 5-year survival rate of the LD5FU group were comparable to those of studies on CRT regimens containing both 5FU and CDDP (Table 4) [1], [3], [5], [6], [8]. Third, the CR rate in the LD5FU group was not significantly different from that in the 5FU+CDDP group. Finally, the LD5FU regimen was associated with significantly less hematological toxicity compared to the 5FU+CDDP regimens.
Table 4.
Precedent studies of definitive CRT for esophageal cancer.
| Author, Year | Histology | Stage | Treatment Arm | MST (mos.) | 5-yr SR (%) |
|---|---|---|---|---|---|
| al-Sarraf, 1997 [3] | SCC+AC | I–III | RT alone | 9.3 | 0 |
| RT+5FU+CDDP (high-dose intermittent) | 14.1 | 27 | |||
| Ishikura, 2003 [6] | SCC | I–IV | RT+5FU+CDDP (high-dose intermittent) | 21 | 29 |
| Burmeister, 2005 [8] | SCC+AC | I–III | RT+5FU (low-dose continuous) | 17 | NA |
| Nishimura, 2009 [1] | SCC | II–IV | RT+5FU+CDDP (high-dose intermittent) | NA | 35 |
| RT+5FU+CDDP (low-dose continuous) | NA | 24 | |||
| Kato, 2011[5] | SCC | II–III | RT+5FU+CDDP (high-dose intermittent) | 29 | 36.8 |
| Present study | SCC | I–IV | RT+5FU (low-dose continuous) | 25.0 | 27.7 |
| RT+5FU+CDDP (low-dose continuous) | 14.9 | 31.6 | |||
| RT+5FU+CDDP (high-dose intermittent) | 25.3 | 29.4 |
5FU: 5-fluorouracil, AC: adenocarcinoma, CDDP: cisplatin, CRT: chemoradiotherapy, MST: median survival time, NA: not available, RT: radiotherapy, SCC: squamous cell carcinoma, SR: survival rate.
Most of the SDFP group (78%) and the LDFP group (72%) underwent ENI. There have been few studies on the association between ENI and hematological toxicity in esophageal cancer. In a retrospective study of ESCC patients, ENI was not associated with increased hematological toxicity [31]. The hematological toxicity of CDDP is well established [21], [30], and we consider that the increased hematological toxicity in the present study’s 5FU+CDDP patients was attributable to CDDP rather than to ENI.
We speculate that the continuous administration of 5FU adequately enhanced the cytotoxicity of the 3D conformal radiotherapy against ESCC, and that its radiosensitizing potential was comparable to that of CDDP. An in vitro study demonstrated that the radiosensitizing effect of 5FU was maximized when the cells were continuously exposed to the drug after the X-irradiation [32]. In patients with rectal cancer, CRT with low-dose continuous 5FU resulted in a higher pathologic CR rate [33] and improved OS [34] compared to CRT with short-term bolus 5FU. Based on these findings, CRT with low-dose continuous 5FU is integrated into the preoperative management of rectal cancer [35].
The weak point of LD5FU is the patients’ discomfort due to the continuous intravenous administration of the drug, as well as the risk of catheter-related infection and thrombosis [8]. The outcomes of CRT regimens containing S1, an oral prodrug of 5FU, were published in 2015 [36]. The catheter-related complications of LD5FU might be overcome by this approach.
This retrospective study is not free from limitations. First, there were many differences in treatment details and patient characteristics among the three CRT groups; the total RT dose, the application of ENI, patient age, PS, stage, tumor location, etc. We used propensity-score matching to reduce these biases. Second, this study dealt only with ESCC patients in Japan. It is unclear whether LD5FU is effective for esophageal cancer patients in western countries, approximately half of whom have adenocarcinoma histology.
In conclusion, definitive CRT with low-dose continuous 5FU for ESCC provided equivalent survival and less hematological toxicity compared to that with 5FU and CDDP. CRT with low-dose continuous 5FU may thus be a less toxic option for elderly ESCC patients.
Conflict of interest
The authors have no conflicts of interest to declare.
Role of funding source
This work was partially supported by a JSPS KAKENHI grant, No. 15H04903.
Acknowledgement
Part of this work was presented at the 59th Annual Meeting of the American Society for Radiation Oncology (ASTRO) in September 2017, San Diego, CA.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2017.12.003.
Contributor Information
Hirotake Saito, Email: 1rtkcyte@med.niigata-u.ac.jp.
Hidefumi Aoyama, Email: h-aoyama@med.niigata-u.ac.jp.
Appendix A. Supplementary data
References
- 1.Nishimura Y., Mitsumori M., Hiraoka M., Koike R., Nakamatsu K., Kawamura M. A randomized phase II study of cisplatin/5-FU concurrent chemoradiotherapy for esophageal cancer: Short-term infusion versus protracted infusion chemotherapy (KROSG0101/JROSG021) Radiother Oncol. 2009;92:260–265. doi: 10.1016/j.radonc.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Bedenne L., Michel P., Bouche O., Milan C., Mariette C., Conroy T. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 3.al-Sarraf M., Martz K., Herskovic A., Leichman L., Brindle J.S., Vaitkevicius V.K. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15:277–284. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]
- 4.Herskovic A., Martz K., al-Sarraf M., Leichman L., Brindle J., Vaitkevicius V. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 5.Kato K., Muro K., Minashi K., Ohtsu A., Ishikura S., Boku N. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for Stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81:684–690. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Ishikura S., Nihei K., Ohtsu A., Boku N., Hironaka S., Mera K. Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:2697–2702. doi: 10.1200/JCO.2003.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Rabik C.A., Dolan M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burmeister B.H., Walpole E.T., Burmeister E.A., Thomas J., Thomson D.B., Harvey J.A. Feasibility of chemoradiation therapy with protracted infusion of 5-fluorouracil for esophageal cancer patients not suitable for cisplatin. Int J Clin Oncol. 2005;10:256–261. doi: 10.1007/s10147-005-0506-9. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchida E., Sakai K., Matsumoto Y., Sugita T., Sasamoto R., Yamanoi T. Concurrent chemoradiotherapy using low-dose continuous infusion of 5-fluorouracil for postoperative regional lymph node recurrence of esophageal squamous cell carcinoma. Esophagus. 2005;2:25–31. [Google Scholar]
- 10.Sakai K., Inakoshi H., Sueyama H., Oda J., Ito T., Tsuchida E. Concurrent radiotherapy and chemotherapy with protracted continuous infusion of 5-fluorouracil in inoperable esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 1995;31:921–927. doi: 10.1016/0360-3016(94)00415-3. [DOI] [PubMed] [Google Scholar]
- 11.Rice T.W., Rusch V.W., Ishwaran H., Blackstone E.H. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 12.van Vliet E.P., Heijenbrok-Kal M.H., Hunink M.G., Kuipers E.J., Siersema P.D. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer. 2008;98:547–557. doi: 10.1038/sj.bjc.6604200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nomura M., Shitara K., Kodaira T., Kondoh C., Takahari D., Ura T. Recursive partitioning analysis for new classification of patients with esophageal cancer treated by chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2012;84:786–792. doi: 10.1016/j.ijrobp.2011.12.069. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita H., Omori M., Takenaka R., Okuma K., Kobayashi R., Ohtomo K. Involved-field irradiation concurrently combined with nedaplatin/5-fluorouracil for inoperable esophageal cancer on basis of (18)FDG-PET scans: a phase II study. Radiother Oncol. 2014;113:182–187. doi: 10.1016/j.radonc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Button M.R., Morgan C.A., Croydon E.S., Roberts S.A., Crosby T.D. Study to determine adequate margins in radiotherapy planning for esophageal carcinoma by detailing patterns of recurrence after definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:818–823. doi: 10.1016/j.ijrobp.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 16.Crosby T., Hurt C.N., Falk S., Gollins S., Mukherjee S., Staffurth J. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627–637. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 17.Ohtsu A., Boku N., Muro K., Chin K., Muto M., Yoshida S. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–2921. doi: 10.1200/JCO.1999.17.9.2915. [DOI] [PubMed] [Google Scholar]
- 18.Di Fiore F., Lecleire S., Rigal O., Galais M.P., Ben Soussan E., David I. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12:4185–4190. doi: 10.3748/wjg.v12.i26.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeuchi M., Kobayashi M., Hashimoto S., Mizuno K., Kawaguchi G., Sasamoto R. Salvage endoscopic submucosal dissection in patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Scand J Gastroenterol. 2013;48:1095–1101. doi: 10.3109/00365521.2013.822092. [DOI] [PubMed] [Google Scholar]
- 20.Minniti G., Scaringi C., Lanzetta G., Terrenato I., Esposito V., Arcella A. Standard (60 Gy) or short-course (40 Gy) irradiation plus concomitant and adjuvant temozolomide for elderly patients with glioblastoma: a propensity-matched analysis. Int J Radiat Oncol Biol Phys. 2015;91:109–115. doi: 10.1016/j.ijrobp.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Santos F.N., Cruz M.R., Riera R. Chemotherapy for advanced non-small-cell lung cancer in elderly patients. JAMA Oncol. 2016;2:1645–1646. doi: 10.1001/jamaoncol.2016.2050. [DOI] [PubMed] [Google Scholar]
- 22.Austin P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Information Center, Japan National Cancer Center. Cancer Information Database.2017/3/2:http://gdb.ganjoho.jp/graph_db/index?changeLang=Submit.
- 25.Lester S.C., Lin S.H., Chuong M., Bhooshan N., Liao Z., Arnett A.L. A Multi-institutional analysis of trimodality therapy for esophageal cancer in elderly patients. Int J Radiat Oncol Biol Phys. 2017;98:820–828. doi: 10.1016/j.ijrobp.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Steyerberg E.W., Neville B.A., Koppert L.B., Lemmens V.E., Tilanus H.W., Coebergh J.W. Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. J Clin Oncol. 2006;24:4277–4284. doi: 10.1200/JCO.2005.05.0658. [DOI] [PubMed] [Google Scholar]
- 27.Sakai K., Sueyama H., Saito M., Sugita T., Tsuchida E., Matsumoto Y. Long-term results of concurrent chemoradiotherapy using low-dose continuous infusion of 5-fluorouracil for stage II-III esophageal cancer. J Jpn Soc Ther Radiol Oncol. 2001;13:97–101. [Google Scholar]
- 28.Stinchcombe T.E., Zhang Y., Vokes E.E., Schiller J.H., Bradley J.D., Kelly K. Pooled analysis of individual patient data on concurrent chemoradiotherapy for stage III non-small-cell lung cancer in elderly patients compared with younger patients who participated in US national cancer institute cooperative group studies. J Clin Oncol. 2017;35:2885–2892. doi: 10.1200/JCO.2016.71.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conroy T., Galais M.P., Raoul J.L., Bouche O., Gourgou-Bourgade S., Douillard J.Y. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305–314. doi: 10.1016/S1470-2045(14)70028-2. [DOI] [PubMed] [Google Scholar]
- 30.Kirwan J.M., Symonds P., Green J.A., Tierney J., Collingwood M., Williams C.J. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol. 2003;68:217–226. doi: 10.1016/s0167-8140(03)00197-x. [DOI] [PubMed] [Google Scholar]
- 31.Jing W., Zhu H., Guo H., Zhang Y., Shi F., Han A. Feasibility of elective nodal irradiation (ENI) and involved field irradiation (IFI) in radiotherapy for the elderly patients (Aged >/= 70 years) with esophageal squamous cell cancer: a retrospective analysis from a single institute. PLoS One. 2015;10:e0143007. doi: 10.1371/journal.pone.0143007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byfield J.E., Calabro-Jones P., Klisak I., Kulhanian F. Pharmacologic requirements for obtaining sensitization of human tumor cells in vitro to combined 5-Fluorouracil or ftorafur and X rays. Int J Radiat Oncol Biol Phys. 1982;8:1923–1933. doi: 10.1016/0360-3016(82)90451-5. [DOI] [PubMed] [Google Scholar]
- 33.Mohiuddin M., Regine W.F., John W.J., Hagihara P.F., McGrath P.C., Kenady D.E. Preoperative chemoradiation in fixed distal rectal cancer: dose time factors for pathological complete response. Int J Radiat Oncol Biol Phys. 2000;46:883–888. doi: 10.1016/s0360-3016(99)00486-1. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell M.J., Martenson J.A., Wieand H.S., Krook J.E., Macdonald J.S., Haller D.G. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 35.Aschele C., Cionini L., Lonardi S., Pinto C., Cordio S., Rosati G. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–2780. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 36.Yoon D.H., Jang G., Kim J.H., Kim Y.H., Kim J.Y., Kim H.R. Randomized phase 2 trial of S1 and oxaliplatin-based chemoradiotherapy with or without induction chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys. 2015;91:489–496. doi: 10.1016/j.ijrobp.2014.11.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


