Highlights
-
•
Most recurrences occurred within the primary tumor or initially affected lymph nodes.
-
•
All sites of loco-regional recurrence had received 92–106% of the prescribed dose.
-
•
No isolated nodal failure occurred, supporting the use of selective nodal irradiation.
Keywords: Small cell lung cancer, Recurrence, Radiotherapy, Selective node irradiation
Abstract
Objectives
Concurrent radiochemotherapy (RCHT) is standard treatment in locally advanced small cell lung cancer (SCLC) patients. Due to conflicting results on elective nodal irradiation (ENI) or selective node irradiation (SNI) there is no clear evidence on optimal target volumes. Therefore, the purposes of this study were to assess the sites of recurrent disease in SCLC and to evaluate the feasibility of SNI versus ENI.
Methods
A retrospective single-institution study of 43 consecutive patients treated with RCHT was performed. After state-of-the-art staging including FDG-PET/CT, all patients underwent three-dimensional conformal radiotherapy to a total dose of 45 Gy in twice-daily fractions of 1.5 Gy starting concurrently with the first or second chemotherapy cycle. All sites of loco-regional recurrences were correlated to the initial tumor and dose delivered. The impact of potential prognostic variables on outcome was evaluated using the Cox-regression model.
Results
13 patients (30%) relapsed locally or regionally: six within the initial primary tumor volume, five within the initially affected lymph nodes, one metachronously within primary tumor and initially affected lymph nodes, and one both inside and outside of the initial nodal disease. All sites of loco-regional recurrence had received 92–106% of the prescribed dose.
Conclusion
In our study most recurrences occurred within the primary tumor or initially affected lymph nodes, or distantly. We did not register any case of isolated nodal failure, supporting the use of selective nodal irradiation, possibly with the addition of supraclavicular irradiation in patients with nodal disease in the upper mediastinum.
Introduction
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung cancer cases [1]. Although the proportional incidence of SCLC has slightly decreased over the past three decades in Western countries, maybe due to the reduction in smoking habits, it remains a major public health problem [2], [3], [4], [5]. SCLC is an aggressive malignancy characterized by a rapid doubling time and early dissemination; approximately two-thirds of patients present with metastatic disease at diagnosis and so far there is no effective screening [6], [7]. The prognosis of the patients is still poor with an average 5 year overall survival of 10% [8].
For patients with limited stage disease and appropriate performance status (WHO 0–2) definitive radiochemotherapy (RCHT) is the mainstay of treatment [9]. While chemotherapy based on etoposide and cisplatin regimens continue to be considered as standard [10], [11], there is neither solid consensus on irradiation dose nor on radiotherapy (RT) target volume.
Selective nodal irradiation (SNI) has been proven to be adequate in NSCLC [12], [13], [14]. However, in a prospective study on omission of elective node irradiation (ENI) in patients with SCLC, based on CT scans only, a high rate of isolated nodal failures occurred in a small sample size [15]. The same study design transferred to irradiation on basis of FDG-PET/CT scans revealed a considerably lower rate of nodal failures [16]. Nevertheless, there is no clear evidence on optimal target volumes in SCLC.
The aim of curative-intent radiotherapy is permanent local and regional tumor control. In principle local tumor control might be improved by application of higher irradiation doses. In the study of Komaki et al. [17] SCLC patients were treated with different RT regimens and the 18-months survival rate was higher in patients who received 61.2 Gy (82%) compared to those receiving 50.4 Gy (25%). Nevertheless, higher irradiation doses imply a higher probability of toxicities, e.g., esophagitis or pneumonitis [18]. A reduction of irradiation dose to organs at risk may in principle be achieved by decreasing the irradiated volume; however, this might also increase the risk of local or regional recurrence.
Therefore, the aims of this study were the evaluation of sites of recurrent disease in patients with limited stage SCLC undergoing RCHT to assess the feasibility and safety of SNI versus ENI and, moreover, the extraction of prognostic factors for loco-regional control, freedom from distant metastases and overall survival.
Material and methods
Patient and tumor characteristics
This is a retrospective single institution study of 54 consecutive patients undergoing RCHT at the Department of Radiotherapy and Radiation Oncology of the University Hospital Carl Gustav Carus Dresden. The institutional ethics committee approved this retrospective analysis and all patients provided written informed consent for using their data before starting treatment.
The clinical stage was assessed by performing a chest X-ray (usually as a first radiological procedure), contrast-enhanced computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) or esophageal ultrasound guided fine needle aspiration (EUS-FNA). In our final analyses we only included all 43 patients with PET imaging to avoid a certain staging bias. Magnetic resonance imaging (MRI) of the brain was performed depending on the referring hospitals’ guidelines. Complete blood count, biochemical tests and electrocardiogram were performed to assess fitness for chemotherapy.
All patients were staged according to the latest TNM classification at diagnosis (UICC 6th or 7th edition). Only patients with limited stage disease (LS) were considered in our study, being defined as disease confined to one hemithorax ± mediastinal lymph node metastases ± bilateral supraclavicular node metastases. Patients with extensive disease (distant metastases at diagnosis) or previous resection of the primary tumor were excluded.
Radiation treatment planning
CT for treatment planning purposes was performed in supine position with both arms above the head; all FDG-PET studies were performed in the same position.
The treatment plans were generated using Oncentra Masterplan Version 4.3 (Elekta, Stockholm, Sweden). All patients underwent three-dimensional conformal radiotherapy (3DCRT) typically employing 6–15 MV photons; inhomogeneity correction algorithms integrated in the treatment planning system have been used.
Gross tumor volume (GTV) was defined as primary tumor and any suspect lymph nodes (LN) visualized on CT (>1 cm on short axial) or FDG-PET (FDG avid), or confirmed by positive cytology (EBUS, EUS) [19]. The clinical target volume (CTV) was obtained by expanding the GTV using a margin of 8 mm (9 mm cranio-caudally) and, after adjusting for anatomical boundaries, electively adding the supraclavicular lymph node stations in all patients. Thereafter, the CTV was expanded to a planning target volume (PTV) using institutional margins of 7 mm (6 mm cranio-caudally). The nodal classification was based on International Association for the Study of Lung Cancer (IASLC) Lymph Node Map [20].
Treatment schedules
All patients received the same radiotherapy regimen: 45 Gy in twice-daily fractions of 1.5 Gy according to Turrisi et al. [21] to the entire PTV to counteract repopulation of cancer stem cells during the course of radiotherapy [22]. Irradiation started concurrently with the first or second chemotherapy cycle. In all patients, the chemotherapy consisted of etoposide (intravenous administration of 80–120 mg/m2 on days 1–3) and cisplatin (intravenous administration of 60 mg/m2 on day 1) typically administered every 3 weeks for four cycles [23], [24]. Four to 12 weeks after completion of RCHT prophylactic whole-brain irradiation (PCI; 30 Gy in 15 fractions) was administered to patients with a complete or near-complete response and with favorable clinical condition [25].
Follow-up and evaluation of outcome
Follow-up (FU) consisted of a clinical examination 2–3 weeks after RCHT and a contrast-enhanced CT-thorax 6–12 weeks after completion of treatment, followed by a 3-monthly chest X-ray or CT-scan up to 2 years after RCHT. Thereafter, imaging intervals were extended to 6 months for the following 3 years. If recurrent disease was suspected (loco-regionally or distant), biopsy confirmation was generally performed, except in case of inaccessible tumor site or widespread disease.
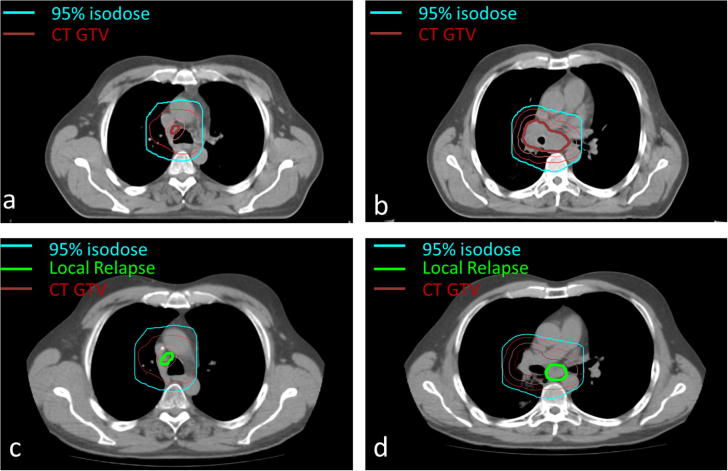
In this retrospective analysis all available imaging data (chest X-ray, CT, FDG-PET) were reassessed for the patterns of failure. In order to exactly evaluate sites of recurrences, the follow-up images were fused with the planning CT (see Fig. 1 as example). Local or regional relapse was scored if the recurrent tumor was located inside the original primary tumor or in the mediastinal lymph nodes, respectively. Recurrences were classified as in-field failures if more than 50% of the recurrent tumor volume was located in the PTV. In contrast, out-of-field failure was defined as recurrence outside the PTV but inside the hilum, mediastinum or ipsilateral lung lobe. Isolated nodal failure (INF) represented a single out-of-field nodal failure in the absence of other sites of recurrences.
Fig. 1.
Recurrence assessment. Geographical recurrence assessment in one exemplary SCLC patient. Original treatment planning CT slices (a and b) showing the gross tumor volume (inner, thick dark red contour), clinical target volume (orange contour) and planning target volume (outer, thin red contour), the latter being encompassed by the 95% isodose (sky blue contour). CT scan at time of locoregional recurrence (c and d) with fused initial contours and 95% isodose line showing the in-field local relapse (inner thick green contour). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Clinical endpoints and statistical analysis
Endpoints were loco-regional control (LRC), freedom from distant metastases (FFDM), and overall survival (OS). All endpoints were calculated from the first day of radiotherapy to the date of event or censoring. Corresponding survival curves were estimated by the Kaplan–Meier method. The impact of potential prognostic variables was evaluated using the Cox-regression model. All analyses were performed by the SPSS 23 software (IBM Corporation, Armonk, NY). Two-sided tests were performed and p-values < 0.05 were considered statistically significant.
Results
Patient characteristics
Between April 2004 and December 2013, 43 consecutive patients, eligible for this retrospective study, were treated in our center (see Table 1). Median age at diagnosis was 58 years (range 26–75 years) and 67% of patients were male. The majority (60%) of the patients had UICC stage IIIB SCLC; 26% had stage IIIA and 14% had stage IA to IIB disease. All patients underwent FDG-PET imaging during the staging procedure.
Table 1.
Patient, tumor and treatment characteristics.
| Variable | Absolute number of patients | Percentage (%) | |
|---|---|---|---|
| Gender | Male | 29 | 67 |
| Female | 14 | 33 | |
| WHO performance status | 0 | 18 | 42 |
| 1 | 14 | 33 | |
| 2 | 1 | 2 | |
| 3 | 1 | 2 | |
| Unknown | 9 | 21 | |
| Active smoker at diagnosis | Yes | 40 | 93 |
| No | 3 | 7 | |
| TNM staging | IA-IB | 1 | 2 |
| IIA-IIB | 5 | 12 | |
| IIIA | 11 | 26 | |
| IIIB | 26 | 60 | |
| Clinical tumor stage | Tx | 1 | 2 |
| T0-T1 | 2 | 5 | |
| T2 | 10 | 23 | |
| T3 | 6 | 14 | |
| T4 | 24 | 56 | |
| Clinical nodal stage | Nx | 2 | 5 |
| N0 | 7 | 16 | |
| N1 | 2 | 5 | |
| N2 | 14 | 32 | |
| N3 | 18 | 42 | |
| Location of primary tumor | Right lung | 25 | 58 |
| Left lung | 18 | 42 | |
| Affected nodal stations at diagnosis | Paratracheal | 26 | 60 |
| Hilar | 24 | 56 | |
| Subcarinal | 18 | 42 | |
| Para-aortic/sub-aortic | 9 | 21 | |
| Supraclavicular | 12 | 28 | |
| Concurrent chemotherapy | Yes | 43 | 100 |
| No | 0 | 0 | |
| Variable | Median (range) |
|---|---|
| Age | 58 (26–75) |
| GTV of primary tumor (ccm) | 34 (1–1341) |
| GTV of LN (ccm) | 68 (0–599) |
| Total GTV (ccm) | 120 (7–1341) |
Abbreviations: WHO, World Health Organization; LN, lymph nodes; GTV, gross tumor volume.
Nodal classification based on International Association for the Study of Lung Cancer (IASLC) Lymph Node Map.
Initial lymph nodal state
At diagnosis, 36 patients (84%) had nodal involvement. Paratracheal (stations 2 and/or 4), hilar (stations 10–14), sub-carinal (station 7), aortic (stations 5, 6) and supraclavicular (station 1) lymph nodes were involved in 26 (60%), 24 (56%), 18 (42%), 9 (21%), and 12 (28%) of the patients, respectively. Those patients with supraclavicular nodal disease had synchronous involvement of lymph node stations 2 and/or 4 in 75% of cases (9/12).
Treatment
Forty-two (98%) patients completed RCHT without interruption. One patient decided to terminate irradiation one day before the scheduled end (final dose: 43.5 Gy in 29 fractions). Five patients (12%) started radiotherapy with the second cycle of chemotherapy, the remaining patients started with both modalities simultaneously. PCI was applied in 27/43 (63%) patients after radical RCHT, whereas the remaining 16 patients did not receive PCI due to disease progression (n = 11, of whom five had cerebral progression) or refusal (n = 5). Twenty-six percent of the patients receiving PCI developed brain metastases over time.
Patterns of failure
Median follow-up of the entire patient cohort was 22.0 months (range 1–85 months) and 50.6 months (27–86 months) for those alive at the time of analysis. One patient died 1 month after RCHT unrelated to treatment and two patients were lost during follow-up (after 36 and 25 months, respectively). Median time to loco-regional and/or distant relapse was 12.5 months. 74% of patients had experienced loco-regional and/or distant relapse (see Table 2; actuarial data), which was treated with chemotherapy in most cases (57%).
Table 2.
Patterns of failure (actuarial data).
| Type of recurrence | Absolute number of patients | Percentage (%) |
|---|---|---|
| Total | 32 | 74 |
| Distant metastases alone | 19 | 44 |
| Loco-regional failure alone | 4 | 9 |
| Loco-regional and distant failure | 9 | 21 |
| Loco-regional failure | 13 | 41 |
| In-field | 11 | 34 |
| In-field and out-of-field (includes elective LNs) | 2 | 6 |
| Out-of-field (includes elective LNs) | 0 | 0 |
| Isolated nodal failure | 0 | 0 |
Abbreviation: LNs, lymph nodes. Nodal classification based on International Association for the Study of Lung Cancer (IASLC) Lymph Node Map.
In our patient cohort, loco-regional tumor control was 61% after 5 years. 13 patients (30%) had a local or regional relapse; in 8 cases this was the first failure event and in 3 patients distant metastases have been diagnosed simultaneously. The remaining two patients relapsed distantly first. Six patients had local relapse within the initial primary tumor volume, five patients had a regional relapse within the initially affected lymph node stations, one patient relapsed metachronously within the primary tumor and initially affected lymph node stations, and one patient relapsed within regional lymph nodes which were only partly initially affected. A total of 9 patients presented with local recurrences and distant metastases: in 3 cases both occurred simultaneously and in the other 6 cases sequentially after a time interval of 4–25 months.
All loco-regional relapses occurred within the high-dose volumes (92–106% of prescribed dose). There was no isolated nodal failure in our cohort.
Distant metastases were recorded in 65% of the population; mostly in brain (46%) and liver (39%), but also in distant lymph nodes (21%), bone (21%) or the contralateral lung (14%).
Outcome parameters
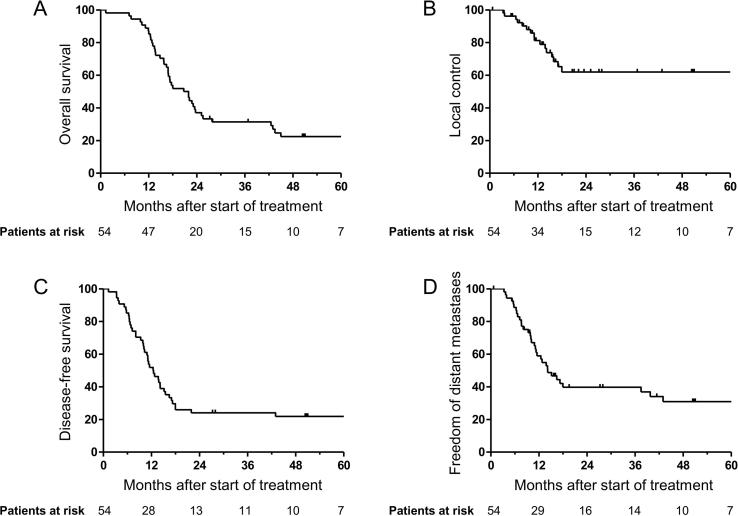
LRC after two years was 61%. Median time to distant metastases was 14.2 months (95% CI = 9.7–18.6 months) with a 2-year FFDM rate of 38%. Median time to death was 22.0 months (95% CI = 15.8–28.1 months) with a 2-year OS rate of 37% and a 3-year OS rate of 30% (see Fig. 2).
Fig. 2.
Clinical endpoints. Kaplan–Meier estimates of (A) overall survival, (B) local control, (C) disease-free survival and (D) freedom from distant metastases.
Analysis of prognostic factors on outcome
In univariate Cox analysis tumor- (T- and N-status, stage and different volume parameters) and patient-related factors (age, gender, WHO-PS and smoking status) were tested. The GTV of the primary tumor and the lymph nodes separately as well as the total GTV did not correlate with any of the outcome parameters. Other previously published prognostic factors affecting overall survival and metastases-free survival, e.g., smoking, WHO status or nodal involvement [37], [38], were also not confirmed in our analysis.
Discussion
The aim of this study was to analyze sites of recurrent disease and investigate prognostic factors in patients with SCLC treated with radiochemotherapy. Regarding overall survival and loco-regional tumor control the results in our patient cohort are in line with previously published literature [16], [21], [26], [27]. We did not observe any patient with isolated nodal failure in our cohort, in which all patients were irradiated selectively on the affected lymph nodes as well as electively on the supraclavicular regions. There was no significant correlation between any of the investigated patient- or tumor-related factors and patient outcome.
So far, there is no consensus on optimal irradiation dose and especially target volumes in SCLC patients. Recently published data may contribute to an international consensus on optimal dose [28]. Regarding target volumes the use of selective nodal irradiation increased during recent years in order to reduce radiation dose to organs at risk and potentially increase dose to the target [29]. The study of De Ruysscher et al. [15] was one of the first that attempted the omission of elective node irradiation in patients with LS–SCLC. In that prospective phase II study, target volume definition was based on CT images only and considered lymph nodes with a diameter >1 cm as pathologically enlarged. The authors found an unexpectedly high percentage of patients failing regionally (11%), all in the non-irradiated homolateral supraclavicular fossa. This underlines the natural spread of SCLC frequently recurring in the supraclavicular zone and probably justifies prophylactic irradiation of this volume, even in the absence of macroscopically evident disease as depicted by FDG-PET–CT imaging. probably due to the low sensitivity and specificity of CT in hilar/mediastinal lymph nodes in SCLC. A review of patterns of failure in two prospective clinical trials in 108 SCLC patients showed a lower rate of isolated nodal failures (4.6%) [27]. In contrast to these results, another prospective phase II study revealed no isolated nodal recurrence after CT-based omission of ENI in 38 SCLC patients [30]. Further retrospective data on SNI based on CT support these findings [31]. Summarizing, for CT-based treatment planning conflicting results on recurrences have been published so far.
SNI based on treatment planning with FDG-PET scans has been shown to be useful in the studies of van Loon et al. [16] and Shirvani et al. [32]. In the first, the authors applied 3DCRT and reported only 3% of isolated nodal recurrences and 8% of recurrences inside and outside the treatment field leading to a 2-year OS rate of 35% [16]. In the latter study assessing 60 SCLC patients treated with intensity-modulated radiotherapy (IMRT), one isolated elective nodal failure (2%) was reported along with three other cases (5%) of relapse inside and outside the treatment field [32]. The well-known limitations of FDG-PET staging for lung cancer, especially a relatively high amount of false negative lymph node metastases, have to be kept in mind when this imaging technique is included in target volume definition [33].
In our patient cohort staged by FDG-PET–CT and receiving SNI plus elective irradiation of the supraclavicular regions, no case of isolated nodal failure occurred, which, as in the studies of van Loon et al. [16] and Shirvani et al. [32], encourages omitting elective nodal irradiation. We observed only one case of combined relapse in the supraclavicular fossa (metachronously with relapse in the initially involved station 4L and novel station 4R) and in most cases nodal involvement of this region is synchronous with paratracheal nodal disease. Thus, the elective inclusion of the supraclavicular fossa in irradiation fields may be considered in those patients with affected lymph nodes in the upper mediastinal lymph node stations.
In our study all the loco-regional relapses occurred within high-dose regions (i.e., 92–106% of prescribed dose), even in the three cases of relapse outside the original GTV, but inside the PTV. This indicates higher radioresistance of SCLC than assumed and may call for dose escalation, which due to a smaller volume receiving a high dose is more appealing using SNI instead of ENI. One prospective phase II study on increased irradiation dose, even though in a small sample size, has recently been published with promising results [29]. The authors delivered a simultaneous integrated boost up to 57 Gy and reported a grade 3 esophagitis rate of 17% and no pneumonitis grade > 2. The median overall survival of 37.7 months and the corresponding 2-year survival rate of 68.5% in that trial are encouraging. The recently published CONVERT trial, a multicenter randomized phase III superiority trial, compared a higher once-daily radiation dose (66 Gy in fractions of 2 Gy) with concurrent chemotherapy to the currently used twice-daily schedule [28]. PET–CT was non-mandatory for both staging and radiation treatment planning, elective nodal irradiation not permitted and all patients were irradiated using a 3D-technique. The survival outcomes did not differ between both regimes (e.g., overall survival 25 months in the once-daily versus 30 months in the twice-daily cohort at a median follow-up of 45 months) and toxicity was similar and lower than expected. Since superiority of the escalated dose was not shown, the authors recommend that twice-daily radiotherapy to be continued as standard of care. Potentially, dose-escalation to a biologically defined target volume or combination with agents influencing the tumor’s oxygenation status or immune response may be of benefit [34], [35].
Our results also confirm the well-known high risk of metastatic spread in patients with SCLC, occurring often simultaneously with local recurrences. One current approach to overcome this is application of immunotherapy in combined treatment schedules. Clinical response to cytotoxic T-Lymphocyte antigen-4 (CTLA-4) antibodies and cell death-1 blockade (PD-1) has been shown in some SCLC trials, making these promising targets for more effective therapies, even if a first publication by Reck et al. [36] did not confirm prolonged overall survival rates after the addition of ipilimumab to chemotherapy compared to chemotherapy alone in patients with newly diagnosed extensive-stage SCLC [37], [38]. Use of specific biomarkers potentially predicting the risk of local recurrences or distant metastases would be promising as stratification criteria for further therapeutic approaches [39].
In our univariate analysis none of the investigated patient- and tumor-related co-factors significantly correlated with patient outcome (see Table 3). Conversely, Reymen et al. [40] showed a significant correlation between total GTV and OS in SCLC patients treated with FDG-PET based SNI. The fact that we did not observe an impact on OS in our patient cohort is unclear as the total GTVs in our study were larger and thus may have influenced OS more profoundly [median 120 cc (range 7–1341 cc) versus 93 cc (7.5–895 cc)] in Reymen et al. [40]. A higher rate of metastases may be explained by the correlation of larger amount of cancer stem cells with increasing tumor volume [41] and by the tumor volume itself. Also other previously published prognostic factors affecting overall survival and metastases-free survival, e.g., smoking, WHO status or nodal involvement [42], [43], were not confirmed in our analysis. One possible explanation could be missing variety in these co-factors in our patient cohort together with limited sample size.
Table 3.
Impact of prognostic factors on loco-regional control, freedom from distant metastases, and overall survival.
| Loco-regional control |
Freedom from distant metastases |
Overall survival |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| WHO-PS (0 vs 1, 2) | 1.96 (0.53–7.33) | 0.32 | 1.58 (0.68–3.70) | 0.29 | 1.89 (0.87–4.13) | 0.11 |
| T-status (1, 2 vs 3, 4) | 0.75 (0.22–2.48) | 0.63 | 1.01 (0.44–2.30) | 0.99 | 0.78 (0.38–1.60) | 0.49 |
| N-status (0, 1 vs 2, 3) | 0.28 (0.08–0.95) | 0.042 | 0.90 (0.34–2.38) | 0.83 | 0.76 (0.33–1.76) | 0.52 |
| Stage (I, II vs III) | 0.48 (0.13–1.75) | 0.27 | 1.34 (0.41–4.45) | 0.63 | 1.42 (0.50–4.05) | 0.51 |
| Gender | 0.99 (0.30–3.22) | 0.98 | 0.71 (0.38–1.95) | 0.71 | 0.96 (0.46–2.02) | 0.92 |
| Age | 1.03 (0.96–1.10) | 0.47 | 0.99 (0.95–1.03) | 0.67 | 1.02 (0.98–1.06) | 0.48 |
| Smoking | 0.31 (0.07–1.40) | 0.13 | 2.90 (0.39–21.4) | 0.30 | 1.43 (0.34–5.99) | 0.62 |
| Ln Volume GTV PT | 1.19 (0.78–1.81) | 0.42 | 1.12 (0.83–1.52) | 0.45 | 1.06 (0.82–1.37) | 0.68 |
| Ln Volume GTV LN | 0.74 (0.49–1.11) | 0.15 | 1.15 (0.86–1.53) | 0.34 | 1.05 (0.81–1.36) | 0.70 |
| Ln Total volume GTV | 0.88 (0.56–1.37) | 0.57 | 1.25 (0.91–1.72) | 0.17 | 1.12 (0.85–1.46) | 0.43 |
Abbreviations: WHO-PS, World Health Organization-Performance Status; Ln, natural logarithm; GTV, gross tumor volume; PT, primary tumor; LN, lymph nodes.
The major shortcomings of the current study include its retrospective nature and the limited patient number, which mainly resulted from the fact that patients ought to have been staged and planned incorporating FDG-PET–CT information. Moreover, all patients were treated with 3D-conformal radiotherapy, which may deliver a somewhat higher radiation dose to non-affected lymph node stations compared to more advanced techniques such as IMRT. However, this technique was also applied in the recently published CONVERT trial and should therefore still be regarded as one of the currently applied radiation techniques [28]. Finally, further prospective clinical trials including biomarker discovery are needed for final determination of irradiation fields and investigation of possible new treatment concepts in SCLC patients.
Conclusion
Our study showed that most recurrences in SCLC occur in the primary tumor volume or initially affected lymph nodes, and even more distantly. We did not observe any case of isolated nodal failure, suggesting the use of SNI, possibly with the addition of supraclavicular irradiation in patients with affected lymph nodes in the upper mediastinum instead of ENI. None of the investigated patient- and tumor-related co-factors significantly correlated with patient outcome. Further prospective clinical trials are needed for final determination of optimal irradiation fields in SCLC patients.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2017.09.010.
Appendix A. Supplementary data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Oberg K., Hellman P., Kwekkeboom D., Jelic S., ESMO Guidelines Working Group Neuroendocrine bronchial and thymic tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:220–222. doi: 10.1093/annonc/mdq191. [DOI] [PubMed] [Google Scholar]
- 3.Pesch B., Kendzia B., Gustavsson P., Jöckel K.H., Johnen G., Pohlabeln H. Cigarette smoking and lung cancer-relative risk estimates for the major histological types from a pooled analysis of case–control studies. Int J Cancer. 2012;131:1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Meerbeeck J.P., Fennell D.A., De Ruysscher D.K.M. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 5.Navada S., Lai P., Schwartz A.G., Kalemkerian G.P. Temporal trends in small cell lung cancer: analysis of the national Surveillance, Epidemiology, and End-Results (SEER) database. J Clin Oncol. 2006;24:7082. [Google Scholar]
- 6.Cuffe S., Moua T., Summerfield R., Roberts H., Jett J., Shepherd F.A. Characteristics and outcomes of small cell lung cancer patients diagnosed during two lung cancer computed tomographic screening programs in heavy smokers. J Thorac Oncol. 2011;6:818–822. doi: 10.1097/JTO.0b013e31820c2f2e. [DOI] [PubMed] [Google Scholar]
- 7.Jett J.R., Schild S.E., Kesler K.A., Kalemkerian G.P. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:400S–419S. doi: 10.1378/chest.12-2363. [DOI] [PubMed] [Google Scholar]
- 8.Früh M., De Ruysscher D., Popat S., Crinò L., Peters S., Felip E. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi99–vi105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- 9.Pignon J., Arriagada R., Ihde D.C., Johnson D.H., Perry M.C., Souhami R.L. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;237:1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 10.Jänne P.A., Freidlin B., Saxman S., Johnson D.H., Livingston R.B., Shepherd F.A. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer. 2002;95:1528–1538. doi: 10.1002/cncr.10841. [DOI] [PubMed] [Google Scholar]
- 11.Jackman D.M., Johnson B.E. Small-cell lung cancer. The Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 12.De Ruysscher D., Wanders S., Van Haren E., Hochstenbag M., Geeraedts W., Utama I. Selective mediastinal node irradiation based on FDG-PET scan data in patients with non-small-cell lung cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys. 2005;62:988–994. doi: 10.1016/j.ijrobp.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Rosenzweig K.E., Sura S., Jackson A., Yorke E. Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol. 2007;25:5557–5561. doi: 10.1200/JCO.2007.13.2191. [DOI] [PubMed] [Google Scholar]
- 14.Sulman E.P., Komaki R., Klopp A.H., Cox J.D., Chang J.Y. Exclusion of elective nodal irradiation is associated with minimal elective nodal failure in non-small cell lung cancer. Radiat Oncol. 2009;4:5. doi: 10.1186/1748-717X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ruysscher D., Bremer R.H., Koppe F., Wanders S., van Haren E., Hochstenbag M. Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: a phase II trial. Radiother Oncol. 2006;80:307–312. doi: 10.1016/j.radonc.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Van Loon J., De Ruysscher D., Wanders R., Boersma L., Simons J., Oellers M. Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2010;77:329–336. doi: 10.1016/j.ijrobp.2009.04.075. [DOI] [PubMed] [Google Scholar]
- 17.Komaki R., Swann R.S., Ettinger D.S., Glisson B.S., Sandler A.B., Movsas B. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: report of Radiation Therapy Oncology Group (RTOG) protocol 97–12. Int J Radiat Oncol Biol Phys. 2005;62:342–350. doi: 10.1016/j.ijrobp.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 18.NCCN Clinical Practice Guidelines in Oncology – Small Cell Lung Cancer– 1; 2016.
- 19.Senan S. Staging nodal disease in non-small cell lung cancer: implications for radiation therapy. J Thorac Oncol. 2007;2:585–587. doi: 10.1097/JTO.0b013e31807a2fbe. [DOI] [PubMed] [Google Scholar]
- 20.El-Sherief A.H., Lau C.T., Wu C.C., Drake R.L., Abbott G.F., Rice T.W. International Association for the Study of Lung Cancer (IASLC) lymph node map: radiologic review with CT illustration. Radiographics. 2014 doi: 10.1148/rg.346130097. [DOI] [PubMed] [Google Scholar]
- 21.Turrisi A.T., Kim K., Blum R., Sause W.T., Livingston R.B., Komaki R. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 22.Bütof R., Baumann M. Time in radiation oncology – keep it short! Radiother Oncol. 2013;106:271–275. doi: 10.1016/j.radonc.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Sundstrøm S., Bremnes R.M., Kaasa S., Aasebø U., Hatlevoll R., Dahle R. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol. 2002;20:4665–4672. doi: 10.1200/JCO.2002.12.111. [DOI] [PubMed] [Google Scholar]
- 24.Takada M., Fukuoka M., Kawahara M., Sugiura T., Yokoyama A., Yokota S. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–3060. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 25.Le Péchoux C., Sun A., Slotman B.J., De Ruysscher D., Belderbos J., Gore E.M. Prophylactic cranial irradiation for patients with lung cancer. Lancet Oncol. 2016;17:277–293. doi: 10.1016/S1470-2045(16)30065-1. [DOI] [PubMed] [Google Scholar]
- 26.Giuliani M.E., Lindsay P.E., Sun A., Bezjak A., Le L.W., Brade A. Locoregional failures following thoracic irradiation in patients with limited-stage small cell lung carcinoma. Radiother Oncol. 2012;102:263–267. doi: 10.1016/j.radonc.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Xia B., Chen G.Y., Cai X.W., Zhao J.D., Yang H.J., Fan M. Is involved-field radiotherapy based on CT safe for patients with limited-stage small-cell lung cancer? Radiother Oncol. 2012;102:258–262. doi: 10.1016/j.radonc.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Faivre-Finn C., Snee M., Ashcroft L., Appel W., Barlesi F., Bhatnagar A. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han D., Qin Q., Hao S., Huang W., Wei Y., Zhang Z. Feasibility and efficacy of simultaneous integrated boost intensity-modulated radiation therapy in patients with limited-disease small cell lung cancer. Radiat Oncol. 2014;9:280. doi: 10.1186/s13014-014-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colaco R., Sheikh H., Lorigan P., Blackhall F., Hulse P., Califano R. Omitting elective nodal irradiation during thoracic irradiation in limited-stage small cell lung cancer-evidence from a phase II trial. Lung Cancer. 2012;76:72–77. doi: 10.1016/j.lungcan.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Watkins J.M., Wahlquist A.E., Zauls A.J., Shirai K., Garrett-Mayer E., Aguero E.G. Involved-field radiotherapy with concurrent chemotherapy for limited-stage small-cell lung cancer: disease control, patterns of failure and survival. J Med Imaging Radiat Oncol. 2010;54:483–489. doi: 10.1111/j.1754-9485.2010.02201.x. [DOI] [PubMed] [Google Scholar]
- 32.Shirvani S.M., Komaki R., Heymach J.V., Fossella F.V., Chang J.Y. Positron emission tomography/computed tomography-guided intensity-modulated radiotherapy for limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:91–97. doi: 10.1016/j.ijrobp.2010.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shingyoji M., Nakajima T., Yoshino M., Yoshida Y., Ashinuma H., Itakura M. Endobronchial ultrasonography for positron emission tomography and computed tomography-negative lymph node staging in non-small cell lung cancer. Ann Thorac Surg. 2014;98:1762–1768. doi: 10.1016/j.athoracsur.2014.05.078. [DOI] [PubMed] [Google Scholar]
- 34.Kang J., Demaria S., Formenti S. Current clinical trials testing the combination of immunotherapy with radiotherapy. J Immunother Cancer. 2016;4:51. doi: 10.1186/s40425-016-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Löck S., Perrin R., Seidlitz A., Bandurska-Luque A., Zschaeck S., Zöphel K. Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging. Radiother Oncol. 2017;124(3):533–540. doi: 10.1016/j.radonc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Reck M., Luft A., Szczesna A., Havel L., Kim S.W., Akerley W. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34:3740–3748. doi: 10.1200/JCO.2016.67.6601. [DOI] [PubMed] [Google Scholar]
- 37.Arriola E., Wheater M., Galea I., Cross N., Maishman T., Hamid D. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J Thorac Oncol. 2016;11:1511–1521. doi: 10.1016/j.jtho.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chae Y.K., Pan A., Davis A.A., Mohindra N., Matsangou M., Villaflor V. Recent advances and future strategies for immune-checkpoint inhibition in small-cell lung cancer. Clin Lung Cancer. 2017;18(2):132–140. doi: 10.1016/j.cllc.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Baumann M., Krause M., Overgaard J., Debus J., Bentzen S.M., Daartz J. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16(4):234–249. doi: 10.1038/nrc.2016.18. [DOI] [PubMed] [Google Scholar]
- 40.Reymen B., Van Loon J., van Baardwijk A., Wanders R., Borger J., Dingemans A.M. Total gross tumor volume is an independent prognostic factor in patients treated with selective nodal irradiation for stage I to III small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;85:1319–1324. doi: 10.1016/j.ijrobp.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Bütof R., Dubrovska A., Baumann M. Clinical perspectives of cancer stem cell research in radiation oncology. Radiother Oncol. 2013;108:388–396. doi: 10.1016/j.radonc.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Chen J., Jiang R., Garces Y.I., Jatoi A., Stoddard S.M., Sun Z. Prognostic factors for limited-stage small cell lung cancer: a study of 284 patients. Lung Cancer. 2010;67:221–226. doi: 10.1016/j.lungcan.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Käsmann L., Janssen S., Rades D. Prognostic factors including the expression of thyroid transcription factor 1 (TTF1) in patients irradiated for limited-disease small cell lung cancer. Anticancer Res. 2016;36:3499–3503. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.