Highlights
-
•
Radiotherapy plus ipilimumab increases abscopal responses in metastatic melanoma patients.
-
•
Radiotherapy plus ipilimumab improves the OS in metastatic melanoma patients.
-
•
Radiotherapy plus ipilimumab does not increase toxicity in metastatic melanoma patients.
Keywords: Abscopal effect, Radiotherapy, Immunotherapy, CTLA-4, Ipilimumab
Abstract
Background
In the last years, limited studies have described that radiotherapy could produce important distant responses in unirradiated sites, the so-called “abscopal effect”. Recent evidence suggests that radiotherapy induces antigen release from tumor, in this way activating the immune system. However, radiotherapy alone is rarely enough to induce the systemic response requested for control of the metastases. With the advent of immunotherapy, the immune checkpoint inhibitors (ICI) have demonstrated impressive efficacy in various metastatic cancers. Currently, preclinical and clinical studies have reported a significant increase of abscopal responses in patients treated with the combination of radiotherapy and ICI. The purpose of this review was summarizing the clinical studies combining radiotherapy and ipilimumab (ipi), particularly focusing on abscopal responses.
Methods and Materials
Databases of Medline (via Pubmed) from 2009 to June 2, 2017 were reviewed to obtain English language studies reporting clinical abscopal effect in the combination of radiotherapy with exclusive ipi in metastatic melanoma cancers. Included studies reported the abscopal effect as a primary endpoint, and as secondary endpoint included overall survival and toxicity.
Results
A total of 16 studies met the inclusion criteria. These studies included a total of 451 patients, and in 5/16 studies the patients were treated on research protocols and followed-up prospectively. The median reported abscopal effect and OS were 26.5% and 19 months, respectively. The median toxicity ≥ Grade 3 was 18.3% ranged from 10% to 20%.
Conclusion
Early clinical outcomes reports suggest that the combination of ipilimumab and RT may improve survival in metastatic melanoma patients. The abscopal responses become a clinically relevant effect of such combination and should be studied in controlled randomized trials.
Introduction
Radiation therapy (RT) is one of the main pillars of cancer treatment and provides an effective and precise means for local tumor control and symptomatic relief. Several studies have shown that RT prompts an immunomodulating effect, which generates local immune system stimulation that can alter the immune-suppressive tumor microenvironment. This microenvironment alteration prompts reactivation of the immune response, thus increasing the potential for developing an abscopal effect, a phenomenon first described by Mole in 1953, in which localized radiation targeted at a neoplasm triggers systemic antitumor effects [1], [2]. Exploitation of the abscopal effect could allow for both local and systemic control of tumour burden. The abscopal effect with RT alone is a rare event [3]. However, with the development of new immunotherapies that further enhance the immune response, the abscopal response is likely to become more clinically meaningful [4]. Current challenges include optimizing radiation doses to maximize immune stimulation, determining the most favorable radiation sequence, defining the optimal combination of immune therapeutics to use alongside radiation, and further neutralizing the immunosuppressive elements involved [4].
Immune therapy includes several drugs that target immune checkpoints such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein PD-1, and its ligand PD-L1 [5]. Although the success of immune checkpoint inhibitors (ICI) has usually been associated with melanoma, it has recently resulted in auspicious clinical activity in an ample assortment of neoplasms, including non-small cell lung carcinoma (NSCLC) [6], [7], [8], small cell lung carcinoma [9], renal cell carcinoma [10], and Hodgkin’s lymphoma [11] among others. However, although ICIs have shown noteworthy activity against a variety of tumors, a group of patients do not respond to ICI in monotherapy or eventually develop resistant disease [2]. Therefore, it is of utmost importance to research additional treatment alternatives (such as combining ICI with other immunotherapies or conventional cancer treatment such as RT) that are aimed at improving this response rate.
Ipilimumab alone in unresectable or metastatic melanoma
Numerous recent clinical trials have sought to harness the stimulation to the immune system provided by ICI, which can be viewed as drugs that “remove the brake” of the immune system by blocking inhibitory receptors on T-cells.
Ipilimumab (ipi), an antibody against CTLA-4, was the first ICI approved for cancer treatment after it showed 20% long-term survival in a phase III trial for patients with metastatic melanoma [12].
In order to provide the baseline results using ipi alone and subsequently compare baseline values with outcomes from combined RT and ipi, we reviewed Schadendorf et al.’s pooled analysis of long-term survival data from phase II and III trials in unresectable or metastatic melanoma [12]. This analysis accrued 1861 patients with a median overall survival (OS) of 11.4 months. In addition, we assessed baseline ipi-induced toxicity by analyzing a retrospective review [13] comprising 1498 unresectable or metastatic melanoma patients with 84.8% of the subjects suffering any grade of toxicity. Some patients (25.3%) had Grade 3–4 and 0.9% had grade 5 toxicity. The most common symptomatology was gastrointestinal (diarrhea and/or colitis) and dermatological (rash, pruritus) with toxicities less frequently involving the liver and endocrine glands; neurological manifestations were rare. Inflammation-related adverse events, including uveitis, pneumonitis, pancreatitis, autoimmune nephritis, myasthenia gravis, and others that affect other organ systems appeared in <1% of patients. Most inflammation-related toxicities occurred during induction (initial four doses administered once every three weeks).
The combination of RT and immunotherapy is a rapidly developing research field with multiple preclinical studies striving to better understand its mechanisms and in vivo relevance [14]. On the contrary, the clinical results of such combinations in terms of abscopal responses, survival advantages, and toxicities are still under preliminary evaluation.
In this paper, we aimed to synthetize currently available studies concerning the use of ipi concurrently with RT regarding abscopal response, survival, and toxicity.
Materials and methods
Search strategy
MEDLINE (via PubMed) databases from 2009 to June 2, 2017 were reviewed in order to obtain English language studies reporting clinical abscopal effects with respect to the combination of RT with ipi in metastatic melanoma. Different terms were used, including “abscopal effect”, “immunotherapy”, “SBRT”, “SRS”, “radiotherapy”, “immune checkpoint inhibitors”, “ipilimumab”. Non-original articles were excluded.
Selection of studies and data compilation
All articles were evaluated based on title and abstract. Included studies relevant for this review met the following criteria:
-
a)
Abscopal effect and/or OS as primary endpoint
-
b)
External beam RT
-
c)
Exclusive use of ipi as ICI
-
d)
Study type included prospective or retrospective studies.
-
e)
Studies were published in English
Results
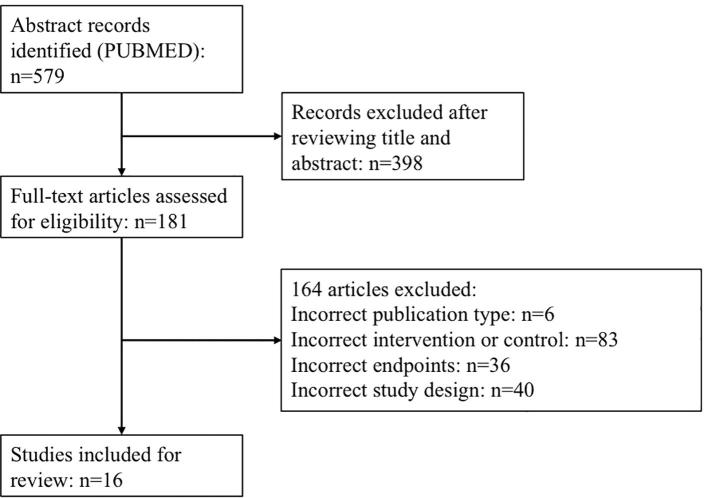
Our search generated a total of 579 results, and through a process of screening, 16 publications were selected for the review. Of 562 studies excluded for this review, 398 were excluded due to incorrect title or abstract (the articles did not conform to the specific inclusion criteria), 6 were excluded because they were not of the selected publication type, 83 were excluded for incorrect intervention or control, 36 not included the correct endpoints, and 40 were excluded by a incorrect study design. Therefore, 16 fulfilled the inclusion criteria and were included in our review. The flowchart of systematic literature search process is shown in Fig. 1.
Fig. 1.
Flow chart of systematic literature search process according to PRISMA statement.
Overall, a total of 16 eligible studies were included in this review. Those studies included a total of 451 patients, and in 5/12 of them, the patients were treated on research protocols and followed-up prospectively (Table 1).
Table 1.
Clinical outcomes and abscopal responses in clinical studies of melanoma with the combination of ipi and radiotherapy.
| Study type | N | Location | Modality | RT dose (Gy)/Fractions | ipi dosage | Median OS (months) | Abscopal response (%) | Toxicity ≥ Grade 3 | |
|---|---|---|---|---|---|---|---|---|---|
| Grimaldi [15] | Prospective | 21 | Various | SRS | 30/10; 20–24/1 | 3 mg/kg/3 w | 22,4 | 53 | NR |
| Chandra [16] | Prospective | 25 | Various | SRS | 26/4 | 3 mg/kg/3 w | 28 | 25 | NR |
| Theurich [17] | Prospective | 45 | Various | SBRT | Various | 3 mg/kg/3 w | 23,25 | 21 | 18,30% |
| Barker [20] | Prospective | 29 | Various | SBRT | 24/1 (SBRT); Various (EBRT) | 3–10 mg/kg/3 w | 39 | 28 | Not increased |
| Knisely [24] | Prospective | 27 | Brain | SRS | Not reported | NR | 21,3 | 10 | NR |
| Schoenfeld [18] | Retrospective | 16 | Brain | SRS | 36 (WBRT); 22 (SRS) | 3–10 mg/kg/3 w | 18 | 63 | Not increased |
| Koller [19] | Retrospective | 70 | Various | SBRT | NR | 3 mg/kg/3 w | 19 | 19,2 | Not increased |
| Gerber [21] | Retrospective | 13 | Brain | WBRT | 27–37.5/9–15 | 3–10 mg/kg/3 w | 4 | 31 | Not increased |
| Kropp [22] | Retrospective | 16 | Various | SBRT | Various | 3 mg/kg/3 w | 24 | NR | Not increased |
| Qin [23] | Retrospective | 44 | Various | SBRT | Various | NR | 21,8 | NR | Not increased |
| Silk [25] | Retrospective | 33 | Brain | SRS | 30–37/10–13 (WBRT); 14–24/1–5 (SRS) | 3 mg/kg/3 w | 18,3 | NR | Not increased |
| Mathew [26] | Retrospective | 25 | Brain | SRS | 15–20/1 | 3–10 mg/kg/3 w | 5,9 | NR | NR |
| Shoukat [27] | Retrospective | 11 | Brain | SRS | NR | NR | 28 | NR | Not increased |
| Kiess [28] | Retrospective | 46 | Brain | SRS | 15–24/1 | 3–10 mg/kg/3 w | 12,4 | NR | 20% |
| Tazi [29] | Retrospective | 10 | Brain | SRS | NR | NR | 18 | NR | 10% |
| Patel [30] | Retrospective | 20 | Brain | SRS | 15–21/1–5 | 3 mg/kg/3 w | 12 | NR | Not increased |
RT = radiation therapy; ipi = ipilimumab; SBRT = stereotactic body radiation therapy; SRS = stereotactic radio surgery; NR = not reported.
Radiotherapy plus ipilimumab increases abscopal responses in metastatic melanoma patients
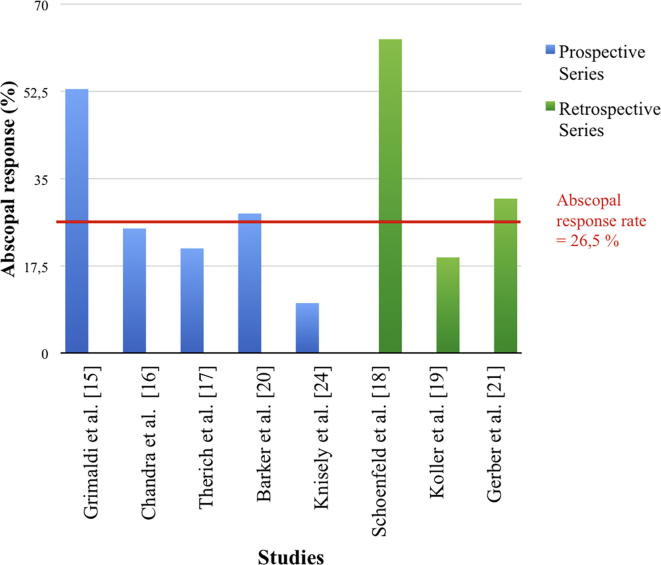
Eight out of the 16 studies included in this review [15], [16], [17], [18], [19], [20], [21], [24], quantified the abscopal responses observed. Overall, the median abscopal effect reported was 26.5% (10–63%). Abscopal responses were similarly reported in prospective and retrospective trials (23% and 31%, respectively) (Fig. 2). The most relevant studies evaluating abscopal effects are described below and summarized in Table 1.
Fig. 2.
Prospective and retrospective studies quantifying the abscopal responses.
Grimaldi et al. [15] analyzed 21 patients with advanced melanoma that was progressing even after ipi. Thirteen (62%) received RT for brain metastases, and eight received RT at extracranial sites. An abscopal response was observed in 11 patients (52%), nine of which had partial responses (43%). Two (10%) of these patients had stable disease. The median overall survival (OS) for all 21 patients was 13 months. The median OS for patients with abscopal responses increased to 22.4 months versus 8.3 months in subjects who did not manifest this event. Abscopal effects were only observed in patients exhibiting local responses.
Chandra et al. [16] analyzed 47 consecutive metastatic melanoma patients treated with ipi. Index lesion responses outside the radiation field were compared before and after radiotherapy. The median survival was 28 months with an estimated 20% 5-year survival. Index lesions shrank in seven (11%) instances prior to radiation therapy when compared with 16 (25%) cases after radiation therapy; in 11 of the latter occurrences (69%), the index lesion had been increasing in size prior to radiotherapy (p = 0.03). In 68% of the cases, radiotherapy was associated with an improved rate of index lesion response (p = 0.006). Dose per radiation fraction ≥3 Gy was the only parameter identified associated with favorable index lesion responses (p = 0.014).
Theurich et al. [17] analyzed data from 127 melanoma patients, including 45 with ipi and RT and 82 with ipi alone. The addition of RT to ipi significantly prolonged OS (median OS 93 versus 42 weeks, unadjusted HR, 0.46; p = 0.0028). Four (21%) out of the 19 local peripheral RT cases showed an abscopal response. In contrast, three (20%) out of 15 patients who received central nervous system (CNS) RT without local peripheral RT had measurable abscopal effects. Abscopal responses were mainly located at pulmonary metastatic sites. A multivariate Cox regression analysis shows that the effect of added RT on OS remained statistically significant (p = 0.05).
Schoenfeld et al. [18] reviewed 16 melanoma patients who received SRS to brain metastases and ipi and systematically assessed the abscopal responses by following the largest extra-cranial lesion. Index lesions decreased in size after brain-directed RT in 63 % of patients who received both radiotherapy (RT) and ipi within a three-month span. The median OS was 14 months among all patients, and 17 months in patients initially treated with stereotactic radiosurgery (SRS).
Overall, the reports about abscopal responses included in this review showed better clinical outcomes in patients treated with higher doses per fraction (>3 Gy) and ipilimumab [15], [16], [17], [18], [19], [20], [21], [24]. However, there are various limitations in the data that might reflect bias and complicate the comparison of results among studies due to heterogeneity in their study design. First, there are no randomized studies evaluating the abscopal responses in metastatic melanoma patients treated with the RT and ipi combination, but in most studies, the patients have been followed prospectively. Second, the number of patients in each study that evaluated abscopal responses was limited ranging from 16 to 47 patients [15], [16], [17], [18], [19], [20], [21], [24]. Extrapolation of results among different trials is difficult but shows a clearly tendency to obtain high abscopal response rates with the combination of RT and ipi. Finally, tumour localization in five studies included various sites, but in all cases the histology corresponded with melanoma. This fact has allowed analysis of the abscopal responses in the same disease and diminishes the probability of bias among trials. The median overall abscopal response rate from both prospective and retrospective trials was 26.5%. Originally, the abscopal response rates in retrospective studies appeared to be more pronounced as is shown in Fig. 1. Schoenfeld et al. [18] in a retrospective study reported 63% abscopal response. This study only included 16 patients, but all of them were treated with SRS and ipi. On the other hand, Grimaldi et al. [15] conducted a prospective study and reported similar results in 21 patients (52%) with abscopal response and including whole-brain RT or stereotactic body RT as RT treatments. In summary, the results reported in these trials evaluating abscopal responses show a positive trend with the combination of RT and ipilimumab.
Radiotherapy plus Ipilimumab improves the OS in metastatic melanoma patients
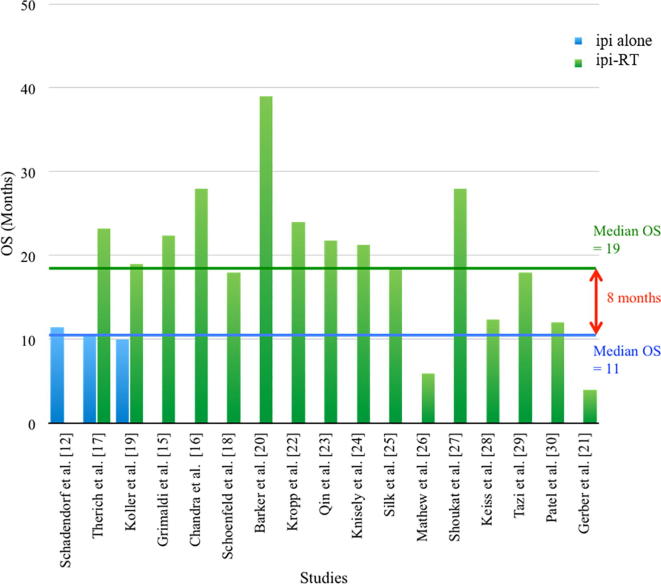
The median OS for the 486 patients included in the 16 reviewed trials was 19 months (4–39 months). Fig. 3 represents the reported OS in prospective and retrospective studies. A survival benefit of eight months in favor of the RT plus ipi combination was observed in comparison with the reported OS in the pooled analysis of phase II and III trials of ipi without RT of 11.4 months [11]. In the current analysis, a median OS of 10.5 months for the control arms (ipi alone) was reported only in two of the included studies [17], [19].
Fig. 3.
Overall survival outcomes reported in prospective and retrospective studies using ipi-RT versus ipi alone.
These outcomes show an impressive clinical benefit in terms of survival with the use of RT plus ipi in metastatic melanoma patients although it must be noted that the included studies provided data from heterogeneous backgrounds, and results could also be influenced by selection bias or publication bias. Most relevant studies included in the OS analysis are described below.
Barker’s et al. study [20] included 29 patients with melanoma who had undergone 33 courses of non-brain RT with concomitant ipi. Immune-related adverse effects were observed in 43% of patients receiving ipi at 10 mg/kg and in 22% of patients receiving 3 mg/kg; immune-related adverse effects were not significantly different compared with previous studies of ipi alone. The median OS was nine and 39 months in patients receiving RT during induction and maintenance with ipi, respectively.
Gerber et al. [21] conducted a retrospective analysis of 13 patients treated with whole-brain irradiation (WBRT) within 30 days of ipi administration. Four out of 13 patients (44%) experienced partial responses or stable central nervous system (CNS) disease as measured by WHO criteria, increasing to 5 when immune-related response criteria were used. The median OS was four months for the cohort with a 1-year survival rate of 15.4%. Treatment-related neurologic toxicity rates were low with one patient experiencing grade III-IV neurologic toxicity.
Kropp’s et al. study [22] included 16 patients who received RT to all sites of limited melanoma progression. Eight patients with an incomplete initial response to ipi received RT to new or progressive disease, whereas the remaining eight patients with a complete initial response to ipi received RT to sites of subsequent recurrence. The median local control with RT was 31.4 months. This cohort could not be directly analyzed for abscopal responses as all new or progressive lesions at the time of RT were treated. There were no reports of grade ≥3 toxicity. Seven patients (44%) had no evidence of melanoma at median follow-up of 29.5 months since completion of RT with no additional therapy.
Qin et al. [23] evaluated 88 patients with stage III or IV unresectable melanoma treated with ipi. At baseline, the ipi-RT group (n = 44) had more unfavorable characteristics. Despite this, OS, progression free survival (PFS), and both immune- and non-immune related toxicities were not statistically different (p = 0.67). Patients who received ipi before RT had an increase in duration of irradiated tumor responses compared to patients receiving ipi after RT (12 months 74.7% versus 44.8%, log-rank P = 0.01). Both ablative and conventionally fractionated radiation was administered with ipi without a clinically apparent increase in toxicity.
Koller et al. [19] assessed 101 patients treated with ipi. Seventy patients received ipi-RT, and 31 received ipi without concurrent radiotherapy. Median OS was significantly increased in the concurrent ipi-RT arm at 19 months versus 10 months for ipi alone (p = 0.01). The median progression-free survival (PFS) was marginally increased in the ipi-RT group compared to the ipi only group (five versus three months, p = 0.20). Rates of complete response (CR) were significantly increased in the ipi-RT group versus ipi alone (25.7% versus 6.5%; p = 0.04), and rates of overall responses in the groups were 37.1% versus 19.4% (p = 0.11). No increase in toxicity was observed in the ipi-RT group compared to ipi alone.
Knisely et al. evaluated a prospective cohort of 27 patients who received stereotactic radiosurgery (SRS) associated with ipi for brain melanoma metastases [24]. Using ipi as a supportive treatment for SRS for brain oligometastases was associated with an increased median survival from 4.9 to 21.3 months in patients who did not receive ipi (50 patients) with a 2-year survival rate of 47.2%. There were no statistically significant differences in survival according to log-rank analysis (p = 0.58). There was no excess toxicity related to RT or ipi.
Silk et al. [25] analyzed the clinical and radiographic records of melanoma patients with brain metastases who were treated with WBRT or SRS between 2005 and 2012. Thirty-three patients received ipi, and 37 did not. The patients who received ipi had a censored median OS of 18.3 months. The group that received SRS had a median OS of 19.3 months. Ipi and SRS were each significant predictors of improved OS (hazard ratio = 0.43 and 0.45, with p = 0.005 and 0.008, respectively). No increased toxicity was reported.
Mathew’s et al. [26] retrospective study assessed 58 patients with limited brain metastases from melanoma treated with SRS. Ipi was administered to 25 patients. The median local control (LC), time to progression in CNS, and OS for the entire group was 8.7, 4.3, and 5.9 months, respectively. Administration of ipi neither increased toxicity nor improved intracerebral disease control in patients with limited brain metastases who received SRS.
Shoukat et al. [27] compared a group of patients with melanoma brain metastases who underwent SRS (n = 176) to another who additionally received ipi (n = 38). The median OS for the cohort was nine months, and the median follow up was 41.2 months. Patients in the ipi group had a median OS of 28 versus seven months in the non-ipi group (p < .001). No differences were noted in LC or intracranial failures. There was no increased toxicity or need for repeated SRS in the ipi group.
Kiess et al. [28] included 46 patients with melanoma who had received single fraction SRS for brain metastases. Fifteen, 19, and 12 patients received SRS during, before, and after ipi, respectively. OS was significantly associated with the timing of SRS/ipi (p = 0.035) and melanoma-specific graded prognostic assessment (p = 0.013). Patients treated with SRS during or before ipi had better OS and less regional recurrence than those treated with SRS after ipi (1-yr OS 65% versus 56% versus 40%, p = 0.008; 1-yr RR 69% versus 64% versus 92%, p = 0.003). Grade III-IV toxicities were observed in 20% of patients.
Tazi et al. published a retrospective review [29], which analyzed a cohort of 10 subjects with brain metastases secondary to melanoma, who received ipi and SRS. The median OS was 29.3 months. The 3-year survival rate from the date of cycle one of ipi administration was 50%. There was no reported increase in toxicity.
Patel's et al. study [30] included 20 patients with melanoma brain metastases treated with ipi and SRS, observing one year LC 92.3%, (p = 0.40) and intracranial control 29.1% (p = 0.59). Patients administered ipi within 14 days of SRS had higher 1-year (42.9%) and 2-year OS (42.9%) relative to ipi delivered >14 days (33.8% and 16.9%, respectively) and SRS alone (38.5% and 25.7%, respectively). There was no excess RT- or ipi-related toxicity.
The median overall OS was 19 months (4–39 months) was obtained in the 16 analyzed studies (Fig. 2). This indicated that there was an increase of eight months in the OS in patients treated with RT plus ipi rather than ipi alone [11]. The heterogeneity among enrolled studies was important when describing the main factors that may contribute to the inadequate interpretation of the results. First, we found two studies of RT and ipi combination in which the OS was less than ipi alone. Gerber et al. [21] with 13 enrolled patients reported a median overall OS of four months. This might be justified in a more extended disease (brain and extracranial metastases), and most patients were treated with WBRT. Five patients underwent a craniotomy and tumour resection prior to WBRT, and four patients did not complete the ipi treatment due to disease progression. Mathew et al. [26] did not find an improvement in clinical results in patients treated with RT plus ipi. This study included 25 patients, but the majority of them (72–76%) had extracranial metastases in addition to the brain metastases; 74–90% died form intracranial disease progression [26]. Thus, the results of these studies might be related to inadequate selection and advanced disease.
Potentially, the improved overall OS with the RT and ipi combination might be compromised due to favorable prognostic factors. Among them are selection criteria, low metastases numbers, a good performance status, limited disease, and systemic maintenance treatment. Most studies included asymptomatic patients with Eastern Cooperative Oncology Group (ECOG) PS 0–1 and <3 metastatic lesions [22], [23], [24], [25], [26], [27], [30]. However, Silk et al. [25] found a significant improvement in OS with the use of SRS and ipi compared with those who received SRS alone with a median OS of 21.3 and 4.9 months, respectively. This study included ECOG PS 1–2 criteria and >3 metastatic lesions. Therefore, given the heterogeneity of studies at the present time, it is not possible to confirm whether the obtained results were the cause of a real RT and ipi combination effect in patients with metastatic melanoma or if the benefit in the OS was related to problems of confounding and bias. This treatment combination is promising, and it should be evaluated prospectively.
Radiotherapy plus Ipilimumab does not increase toxicity in metastatic melanoma patients
As reported in the literature, adverse events produced by the use of immune checkpoint blockade ipi in patients with metastatic melanoma includes immune-related adverse events (ir-AEs), which affect the skin, colon, endocrine organs, liver, and lungs with a 10–27% with severe grade 3 toxicity [31].
In the present review, most of the trials reported comparable toxicities with the combined approach versus ipi alone. The toxicity rate was detailed only in four trials [16], [18], [28], [29]. The prevalence of side effects ranged from 10% to 20 % as described in Table 1.
The most common complications of combined treatment described in these studies included colitis, local skin or mucosal effects, and/or diarrhea. These results were very similar to those obtained in the majority of trials evaluating ipi in monotherapy [11].
Conclusion and futures perspectives
Combined therapies open new possibilities in the field of cancer treatment. Several preclinical studies have allowed us to better understand the mechanisms involved in the tumor microenvironment and the effect of radiation therapy in the immune response [1]. Even so, several questions still remain unanswered. Dosage, fractionation, and timing of RT treatments apparently are important parameters determining the induction of an abscopal response and there are even more parameters when paired with ICI drugs. It seems that SRS/SBRT could be the most appropriate RT modality to be combined with ICI since it promotes immunogenic cell death and causes the release of several antigens that promote T CD4 cell proliferation and differentiation [32], [33].
In a review of combinations of immunotherapy and radiation in cancer therapy, Vatner et al. recommend that current challenges should include dose and fractionation, site of irradiation, the timing of RT, and defining the optimal combination of immune therapeutics to use in conjunction with RT. These variables are relevant to the evaluation and quantification of the abscopal effects in clinical practice [34].
Even though several trials involving RT and ICI have shown similar results in comparison to ICI alone, randomized and prospective controlled trials are needed to further evaluate the safety and efficacy of RT combined with ICI.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2017.12.004.
Appendix A. Supplementary data
References
- 1.Mole R.J. Whole body irradiation – Radiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. 13042090 PubMed. [DOI] [PubMed] [Google Scholar]
- 2.Formenti S.C., Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10(7):718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tubin S., Wolfgang R. Hunting for abscopal and bystander effects: clinical exploration of non-targeted effects induced by partial high-single-dose irradiation of the hypoxic tumour segment in oligometastatic patients. Act Oncol. 2017 doi: 10.1080/0284186X.2017.1346385. [DOI] [PubMed] [Google Scholar]
- 4.Weichselbaum R.R., Liang H., Deng L., Fu Y.X. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14(6):365–379. doi: 10.1038/nrclinonc.2016.211. [DOI] [PubMed] [Google Scholar]
- 5.Dummer R., Hauschild A., Lindenblatt N. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v126–v132. doi: 10.1093/annonc/mdv297. [DOI] [PubMed] [Google Scholar]
- 6.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J., Reckamp K.L., Baas P. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garon E.B., Rizvi N.A., Huli R. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 9.Antonia S.J., Lopez-Martin J.A., Bendell J. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 10.Motzer R.J., Escudier B., McDermott D.F. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ansell S.M., Lesokhin A.M., Borrello I. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schadendorf D., Hodi F.S., Robert C. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim R.A., Berman D.M., DePril V. Ipilimumab safety profile: summary of findings from completed trials in advanced melanoma. J Clin Oncol. 2011;29(suppl 15) 8583-8583. [Google Scholar]
- 14.Koo T., Kim I.A. Radiotherapy and immune checkpoint blockades: a snapshot in 2016. Radiat Oncol J. 2016;34(4):250–259. doi: 10.3857/roj.2016.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grimaldi A.M., Simeone E., Giannarelli D. Impact of radiotherapy in patients with advanced melanoma who progressed after ipilimumab 3 mg/kg. OncoImmunology. 2014;3 doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandra R., Wilhite T., Balboni T. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. OncoImmunology. 2015;4:11. doi: 10.1080/2162402X.2015.1046028. e1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theurich S., Rothschild S., Hoffmann M. Local tumor treatment in combination with systemic ipilimumab immunotherapy prolongs overall survival in patients with advanced malignant melanoma. cancer. Immunol Res. 2016;4(9) doi: 10.1158/2326-6066.CIR-15-0156. [DOI] [PubMed] [Google Scholar]
- 18.Schoenfeld J., Mahadevan A., Floyd S. Ipilmumab and cranial radiation in metastatic melanoma patients: a case series and review. J ImmunoTher Cancer. 2015;3:50. doi: 10.1186/s40425-015-0095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koller K., Mackley H., Liu J. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther. 2017;18(1):36–42. doi: 10.1080/15384047.2016.1264543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker C., Postow M., Khan S. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res. 2013;1(2):92–98. doi: 10.1158/2326-6066.CIR-13-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerber N.K., Young R.J., Barker C.A. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol. 2015;121(1):159–165. doi: 10.1007/s11060-014-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kropp L., De Los Santos J., McKee S. Radiotherapy to control limited melanoma progression following ipilimumab. J Immunother. 2016;39:373–378. doi: 10.1097/CJI.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin R., Olson A., Singh B. Safety and efficacy of radiation in advanced melanoma patients treated with ipilimumab. Int J Radiat Oncol Biol Phys. 2016 doi: 10.1016/j.ijrobp.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Knisely P., Yu J., Flanigan J. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117:227–233. doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silk A., Bassetti M., West B. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899–906. doi: 10.1002/cam4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew M., Tam M., Ott P. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23:191–195. doi: 10.1097/CMR.0b013e32835f3d90. [DOI] [PubMed] [Google Scholar]
- 27.Shoukat S, Marcus DM, Rizzo M, et al., Outcome with stereotactic radiosurgery (SRS) and ipilimumab (Ipi) for malignant melanoma brain metastases (mets). Abstract, ASCO 2014.
- 28.Kiess A., Wolchok J., Barker C. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368–375. doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tazi K., Hathaway A., Chiuzan C., Shirai K. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med. 2015;4(1):1–6. doi: 10.1002/cam4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel K., Shoukat S., Oliver D. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol. 2015 doi: 10.1097/COC.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 31.Haanen J.B.A.G., Carbonnel F., Robert C. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 32.Derer A., Deloch L., Rubner Y. Radio-immunotherapy induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses – preclinical evidence and ongoing clinical applications. Front Immunol. 2015;6:505. doi: 10.3389/fimmu.2015.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanpouille-Box C., Alard A., Aryankalayil M.J. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017 Jun;9(8):15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Silvia C Formenti. Combinations of immunotherapy and radiation in cancer therapy, doi: http://doi.org/10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.